Abstract
Successful thymocyte maturation is essential for normal, peripheral T cell function. Vasoactive intestinal peptide (VIP) is a neuropeptide which is highly expressed in the thymus that has been shown to modulate thymocyte development. VIP predominantly binds two G protein coupled receptors, termed vasoactive intestinal peptide receptor 1 (VPAC1) and VPAC2, but their expression profiles in CD4−/CD8− (double negative, DN) thymocyte subsets, termed DN1–4, have yet to be identified. We hypothesized that a high VPAC1:VPAC2 ratio in the earliest thymocyte progenitors (ETP cells) would be reversed during early lymphopoiesis as observed in activated, peripheral Th2 cells, as the thymus is rich in Th2 cytokines. In support of this hypothesis, high VPAC1 mRNA levels decreased 1000-fold, accompanied with a simultaneous increase in VPAC2 mRNA expression during early thymocyte progenitor (ETP/DN1) → DN3 differentiation. Moreover, arrested DN3 cells derived from an Ikaros null mouse (JE-131 cells) failed to completely reverse the VIP receptor ratio compared to wild type DN3 thymocytes. Surprisingly, VPAC2−/− mice did not show significant changes in relative thymocyte subset numbers. These data support the notion that both VPAC1 and VPAC2 receptors are dynamically regulated by Ikaros, a master transcriptional regulator for thymocyte differentiation, during early thymic development. Moreover, high VPAC1 mRNA is a novel marker for the ETP population making it enticing to speculate that the chemotactic VIP/VPAC1 signaling axis may play a role in thymocyte movement. Also, despite the results that VPAC2 deficiency did not affect thymic subset numbers, future studies are necessary to determine whether downstream T cell phenotypic changes manifest themselves, such as a propensity for a Th1 vs Th2 polarization.
Keywords: vasoactive intestinal peptide (VIP), neuropeptide, thymus, thymocyte maturation, hematopoietic stem cell, Ikaros
1. Introduction
Successful maturation of thymocytes is of central importance for the proper development and function of the immune system [2]. Mouse peripheral T cells are derived from a pluripotent hematopoietic stem cell (HSC) population (CD34+, Sca-1+ Thy1.1lo c-kit+) within bone marrow that migrates to the thymus to complete T cell development [39]. Although not well understood, postnatal HSC thymic homing is characterized by transient windows of receptivity to developmental cues, thought to be mediated by molecules such as selectins, integrins, cytokines and chemokines [21, 37]. Migrating HSC enter the inner cortex of the thymus from post-capillary venules and initiate a maturation program beginning with double negative (DN) cells (CD4−/CD8−), which are further subdivided into four populations (DN1–4) based on their differential expression of CD44 and CD25. DN cells differentiate into double positive (DP) cells ( CD4+/CD8+) that give rise to functionally mature, single positive (SP) CD4 or CD8 T cells that egress from the thymus to enter peripheral circulation [2, 21, 30].
DN cells (DN1–DN4) undergo extensive changes in gene expression, some of which ultimately determine T lineage commitment, yet the identity of these genes remains elusive [49]. Early lymphopoiesis is marked by a progressive loss of pluripotency with increasing commitment to the T cell lineage. Therefore, it follows that stem cell genes, and genes that would give rise to alternative lineages are downregulated. A recent study by Yui et al. demonstrated that c-kit+ expression (CD117) distinguishes authentic early T cell precursors (ETP) from stromal thymic epithelial cells and other non-T cell progenitors found within the thymus. A number of receptor transcription factor and T cell specific genes are altered during ETP→DN3 maturation, but the molecular underpinnings driving T cell commitment have not yet been defined.
The neuropeptide, vasoactive intestinal peptide (VIP), has been shown to modulate the maturation of thymocytes [25]. Peptidergic and noradrenergic nerve fibers innervate the thymic cortex and medulla, where they bathe proximal thymocyte populations with the VIP ligand [1, 6, 7, 29]. VIP binds two G-protein coupled receptors, termed vasoactive intestinal peptide receptor 1 (VPAC1) and VPAC2, with high affinity. Both receptors signal through several pathways including Gαs, Gαi/o, and Gαq[29]. VIP binding to VPAC2 induces a cellular program that skews differentiation of thymocytes towards the CD4+/CD8− phenotype without changes in proliferation or apoptosis [25]. In contrast, VPAC1 is expressed on HSCs and induces chemotaxis of peripheral T cells trafficking to the Peyer’s Patches [24]. During peripheral T cell activation, the VIP receptors have been reported to undergo a receptor switch from a high to low VPAC1:VPAC2 ratio [16, 46]. To provide a basis for understanding the role of VIP-induced signals in thymopoiesis, we proposed to chart the expression of VPAC1 and VPAC2 during early stages of thymopoiesis (ETP→DN4). These data would be the first steps to eventually allow us to determine whether the VPAC1:VPAC2 ratio in ETP undergoes a reversal similar to that seen in the periphery, and whether this could play a role in HSC homing within the thymus or influence early T cell development and/or lineage commitment.
This study maps the expression of VPAC1 and VPAC2 in total DN cells and in individual DN subpopulations (ETP, DN1–4). To our knowledge, this is the first quantitative report of VPAC1 and VPAC2 expression in mouse DN subsets. In these experiments, we demonstrate that VPAC1 is the exclusive VIP receptor expressed in earliest thymic progenitor (ETP) cells. Furthermore, we show a radical receptor reversal between VPAC1 and VPAC2 that peaks at the DN3 stage. Although mice lacking VPAC2 expression showed similar thymic subset numbers compared to wild type mice, DN3 cells derived from Ikaros null mice (JE131 cells) [14] failed to reverse the VPAC1:VPAC2 ratios. Collectively, we identify VPAC1 as the sole VIP receptor in the ETP population, and show that VIP receptor reversal is coordinately regulated at the DN-2→ DN3 differentiation stage, potentially mediated by Ikaros DNA binding. These studies establish that both VIP receptors are oppositely regulated during early lymphopoiesis. Future studies are now warranted to determine whether this signaling axis is involved in HSC homing to and within the thymus microenvironment and/or could influence the development and lineage commitment towards the T cell phenotype.
2. Materials and Methods
2.1 Reagents
The following anti-mouse antibodies (Ab) (clone #) and rat isotype controls (clone #) were purchased from eBioscience (San Diego, CA) or Biolegend (San Diego, CA): CD16/CD32 (93), CD4 PE-Cy5 (GK1.5 or clone RM4-4), CD4 FITC (GK1.5), IgG2b PE-Cy5 (RTK4530), CD8 FITC (eBioH3H-17.2 or clone 53–6.7), CD8a PE (53–6.7), CD8b (clone H3H-17.2), IgG2b FITC (eB149/10H5), CD25 PE-Cy5 (PC61.5 or clone 3C7, Biolegend), CD25 Pacific Blue (PC61), IgG1 PE-Cy5 (eBRG1), CD44 FITC (IM7), CD44 Alexa 700 (IM7), IgG2b FITC (eB149/10H5), and CD117 APC (clone 2B8, Biolegend). Anti-mouse CD4 and CD8 magnetic bead conjugated Abs and anti-biotin magnetic beads were from Miltenyi Biotec (Auburn, CA). RNeasy kits were purchased from Qiagen (Valencia, CA). Reagents for cDNA synthesis were purchased from Promega (Madison, WI) or the QuantiTect Reverse Transcription Kit from Qiagen (Valencia, CA) was utilized. The DNA-free kit and glycogen were from Ambion (Austin, TX). The Onestep Taqman mastermix was purchased from ABI (Foster City, CA). All other chemicals were purchased from Sigma (St. Louis, MO).
2.2 Mice
For these studies, we used VPAC2 knockout mice (kind gift from Dr. Harmar, University of Edinburgh) or wild type C57Bl/6 mice (Jackson Laboratories, Bar Harbor, ME) bred in the facility at North Dakota State University or housed in the Animal Care Facility at Loma Linda University (LLU). Mice were housed in a ventilated Nalgene Armadio cabinet (VWR) or Opti-Cage system (Animal Care Systems), and mice were euthanized by CO2 narcotization followed by rapid cervical dislocation [45]. All mouse protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at NDSU or LLU and met all federal guidelines.
2.3 Thymocyte isolation, Antibody staining, cell sorting and flow cytometry
Harvested thymi were passed through a screen mesh followed by a 70 μm sieve to obtain a single cell suspension. Cells were pelleted and erythrocytes were lysed by resuspension 3 mL of 1× ammonium solution (155mM NH4CL, 10mM KHCO3, 1.0mM EDTA) for 1 minute, diluted with 1× PBS and centrifuged at 300 × g for 10 minutes. On ice, total thymocyte populations were blocked with 1 μg CD16/CD32 antibody for 10 minutes in 0.5%BSA/PBS, followed by 1 μg/1×106 cells CD4 PE-Cy5 and CD8 FITC, or equivalent amounts of isotype control antibodies for 30 minutes in the dark and assessed by a FACSCalibur (Becton Dickinson, San Jose, CA), an Accuri C6 flow cytometer (Ann Arbor, MI) or a 7-color MACSQuant Analyzer (Miltenyi Biotec, Auburn, CA). Double negative (DN; CD4−/CD8−) thymocytes were negatively enriched by AutoMACS magnetic bead technology (Miltenyi; Auburn, CA) through sequential depletions using CD8a and CD4 magnetic beads as described by the manufacturer. Alternatively CD8 and CD4 cells were depleted by magnetic separation using anti-PE and anti-FITC microbeads (Miltenyi Biotec) following staining with CD4 FITC and CD8 PE. In some experiments, single cell suspensions of thymocytes were frozen prior to DN isolation. Similar results were achieved with fresh and frozen cells. Enriched double negative cells were stained as above with CD25 PE/Cy5, CD44 FITC, or CD25 Pacific Blue, CD44 Alexa 700 and CD117 APC, to further define four distinct DN subpopulations (DN1–DN4). Early thymocyte precursors (ETP) were defined as CD117+ DN1 cells. SP, DP, DN, ETP and DN1–DN4 populations were sorted to ≥95% purity by fluorescence activated cell sorting (FACSAria cell sorter, Becton Dickinson Immunocytometry Systems, San Jose, CA). Purified cell populations were assayed immediately or frozen at −80°C in RNA lysis buffer (Qiagen) for subsequent analysis.
2.4 RNA isolation and qRT-PCR
Total RNA isolation from enriched thymocyte populations (≤3×106 cells) was performed as previously described [44]. Briefly, sorted DN subsets were isolated by sequential passes through a QIAshredder spin column followed by an RNeasy Micro column or RNeasy Mini column with on-column DNase I treatment as described by the manufacturer (Qiagen, 2010 protocol). Following total RNA elution, a second DNAse treatment (gDNA wipeout) was performed. Some total RNA isolations were further purified by phenol chloroform isoamyl extraction (25:24:1 v/v/v) and ethanol precipitation using 0.5 M ammonium acetate, 0.02 mg/mL glycogen and diluting with 2.5 volumes of 100% ethanol with similar results. cDNA synthesis was performed using the QuantiTect Reverse Transcription Kit as described by the manufacturer (Qiagen, 2010 protocol), or as previously described [44]. RNA was used immediately for cDNA synthesis or stored at −80°C until needed. Real time PCR reactions contained the following: 1× SybrGreen Master Mix (Applied Biosystem, Inc.), 250nM mVPAC1 (forward, 5′-GATATGGCCCTCTTCAACAACG-3′; reverse, 5′-GAAGTTGGCCATGACGCAAT-3′) or mHPRT (forward, 5′-CTGGTGAAAAGGACCTCTCG-3′; reverse, 5′-TGAAGTACTCATTATAGCAAGGGCA-3′) or 400nM mVPAC2 (forward, 5′-CCAGATGTTGGTGGCAATGA-3′, reverse, 5′-GTATGTGGATGAGATGCCAATAGG-3′) primers and ten (10) microliters of serially diluted cDNA (Neat, 1:4, 1:16) were used as template. Hypoxanthine-guanine phosphoribosyltransferase (HPRT) primers were used as normalizing controls as previously described [44]. Comparative relative changes were calculated by the ΔΔCt method as previously described [9].
2.5 VPAC2−/− DN thymocyte analysis
Single cell thymocytes suspensions were obtained from C57Bl/6J wild type or VPAC2−/− mice as described above followed by depletion by staining with biotinylated (1ug/5.0 × 107 cells/0.5 ml) Abs to CD4, CD8a, CD11c, TCRγδ, NK1.1, TCRβ, CD122, Gr1, Ter119 for 30 minutes on ice. Thymocytes were depleted by incubating with anti-biotin magnetic beads, and passed through a magnetic column once (Automacs, Auburn, CA) as described by the manufacturer. Negatively purified DN cells were subsequently stained as described above using CD25 PE (3C7), CD44 Pe/Cy7 (IM7) and CD117 APC (2B8).
2.6 Cell Culture
The Ikaros null JE131 thymocyte cell line [14] was a kind gift from Dr. Susan Winandy (Boston University). Cells were cultured using 89% RPMI supplemented with 10% FBS and 1% penicillin/streptomycin. Cells were seeded at 3 × 105 cells/ml on Monday/Wednesday and 1 × 105 cells/ml on Friday. Four million cells were used for total RNA isolation as described above.
2.7 Statistical analysis
All data are presented as means ± SEM of two or three independent experiments unless otherwise noted in the figure legend. Two way t-test analyses were performed by Origin® graphical software program (OriginLab, Northampton, MA) to determine statistical significance between data sets. Statistical significance values (p≤0.05) are noted in the figure legends by asterisk symbols. Flow cytometry data were collected using Cellquest Pro (Becton Dickinson, San Jose, CA) or MACSQuantify software (Miltenyi Biotec) and analyzed using FlowJo software (Ashland,OR).
3. Results
3.1 VIP receptors are differentially expressed in thymic subsets
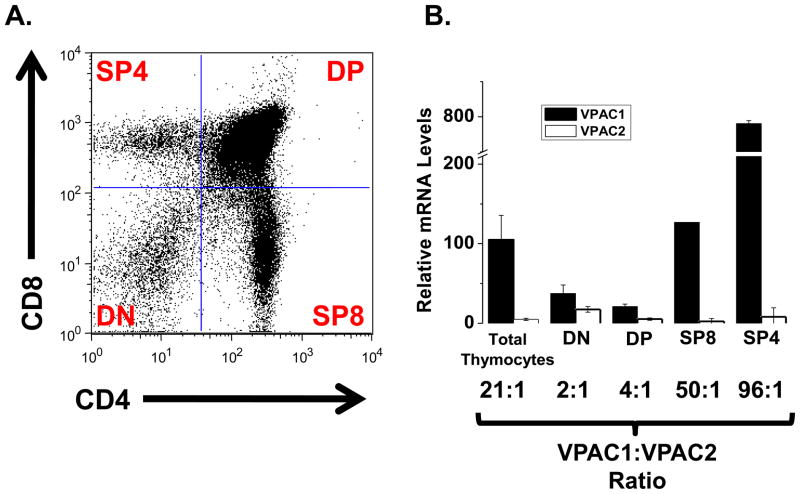
Previous studies are in disagreement regarding whether VIP receptors are expressed in mouse double negative thymocytes (DN, CD4−/CD8−) [25, 47]. Due to this controversy, our first goal was to confirm the expression of VPAC1 and VPAC2 mRNA in total DN cells. To this end, cells were isolated from mouse thymocytes (Fig, 1A) [38, 41] and VIP receptor expression was assessed by qRT-PCR. These data revealed high VPAC1:VPAC2 ratios in total thymocytes (21:1), with much higher VPAC1:VPAC2 ratios in the mature single positive CD4 (SP4; 96:1) and SP8 (50:1) developmental stages. In contrast, more equivalent expression of VIP receptors was observed in earlier developmental stages, as double positive cells (CD4+/CD8+) and double negative cells (CD4−/CD8−) possessed a 4:1 and 2:1 VPAC1:VPAC2 ratios, respectively (Fig. 1B). These data indicate that SP4 and SP8 thymocytes are the predominant VPAC1 expressing subsets in C57Bl/6J mice, while the greatest expression of VPAC2 mRNA is present within the early thymocyte subsets representing DP and DN cells.
Figure 1. Differential VIP receptor expression in thymocytes.
Thymi were harvested from C57BL/6J mice to obtain single-cell suspensions (Materials and Methods). Experiments were conducted at least three independent times using four to six mice per experiment. A. Thymocytes were stained for surface CD4 and CD8 and a representative dot plot is shown denoting the four major thymic subpopulations: DN, DP, SP8 and SP4. B. VPAC1 and VPAC2 mRNA expression levels in indicated thymic subpopulations as detected by qRT-PCR are shown in the bar graph +/− SEM. Expression levels were normalized to HPRT. The ratios of VPAC1:VPAC2 are reported below the graph.
3.2 A transient VIP receptor reversal is observed at the mRNA level during early thymocyte development
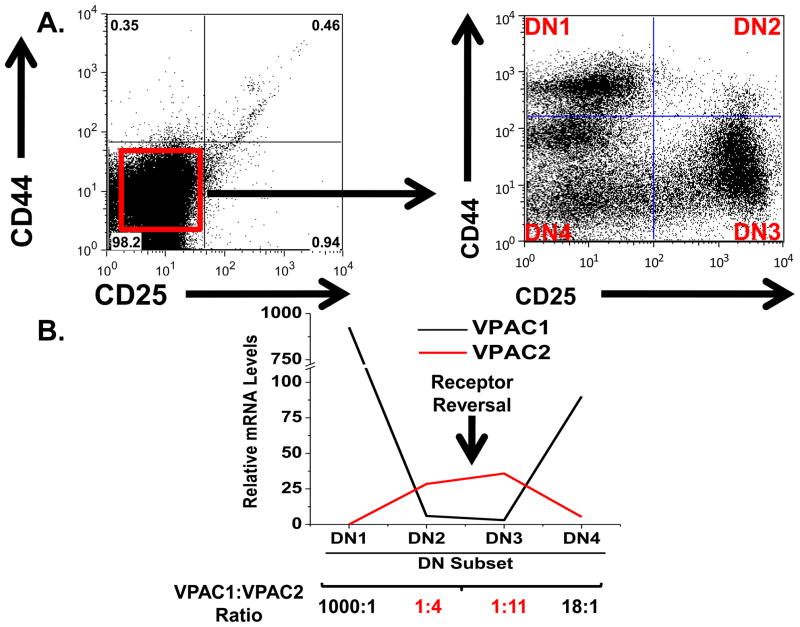
The VIP ligand is highly expressed within the thymic microenvironment by VIP+ nerves innervating this organ, as well as, by thymocytes themselves [1, 6, 7, 29]. Our data confirmed VIP receptor mRNA expression in DN thymocytes, and revealed that VPAC1 and VPAC2 mRNA expression levels were closest [VPAC1:VPAC2 (2:1)] in this subset. Since T cell commitment and β chain selection also take place during DN differentiation [49], changes in the dynamics of VIP receptor expression in this compartment could indicate involvement of the VIP signaling axis in T cell development. To determine if the VPAC1:VPAC2 expression ratio reverses during early lymphopoiesis in a manner similar to that observed during activation of peripheral Th2 cells [43], we measured the expression profile of VPAC1 and VPAC2 in all four known DN subpopulations (DN1–DN4) [28, 49]. Figure 2A shows magnetically isolated DN cells stained with CD44 and CD25 DN markers to obtain DN1–DN4 subsets (Fig 2B), which were subsequently isolate by FACS. In support of our hypothesis, qRT-PCR analysis revealed high VPAC1:VPAC2 ratios in DN1 (1000:1) and DN4 (18:1) thymocytes, which were reversed in DN2 and DN3 populations showing a low VPAC1:VPAC2 ratio of 1:4 and 1:11, respectively (Fig. 2C). Taken together, these results show a VIP receptor reversal during early lymphopoiesis coinciding with T cell commitment and β chain selection.
Figure 2. VIP receptor reversal in early T cell development.
A. Thymocytes were depleted of CD4 and CD8 expressing cells to yield the DN population. B. DN cells were stained for surface CD44 and CD25 expression, and the four subsets were sorted. C. VPAC1 and VPAC2 mRNA levels for all four DN subsets were measured by qRT-PCR analysis and normalized to HPRT. Data from individual subsets is representative of two to four independent experiments using six to eight mice per experiment.
3.3 VPAC1 is the exclusive VIP receptor expressed in the earliest thymic precursor (ETP) population
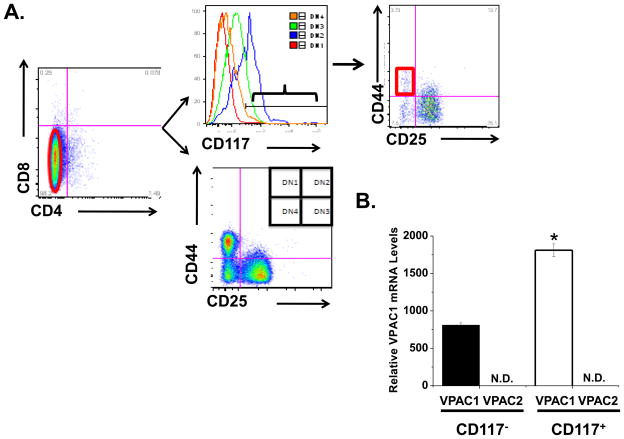
DN1 thymocytes (CD4−/CD8−/CD25−/CD44+) are a heterogeneous subpopulation, consisting of at least five distinct subsets identifiable by CD24 and CD117 expression [28]. The c-kit receptor (CD117) differentiates between authentic T cell precursors (DN1 CD117+) and stromal cells (DN1 CD117−) that also express CD44 [28, 49]. We further differentiated the DN1 population using CD117 to isolate the ETP population (Fig. 3A–C). Analysis of the DN1 CD117+ population (0.07% of all thymocytes) by qRT-PCR showed high levels of VPAC1 mRNA, but no detectable expression of VPAC2 (Fig. 3D). These data provide evidence that VIP acts on ETP solely through VPAC1. CD117− DN1 cells also expressed high levels of VPAC1 with no VPAC2 mRNA detected (Fig. 3D). This is consistent with our observation that the total DN1 population expressed high VPAC1, but little VPAC2 (Fig. 2C). Table 1 summarizes the relative VIP receptor mRNA levels shown in Figures 1–3. Taken together, these data suggest that VPAC1 is the more likely candidate to transmit VIP effects to both early thymic precursors (ETP) and thymic stromal cells, as it is the exclusive VIP receptor expressed in these thymic cell populations. In contrast, VPAC2 is the likely candidate receptor mediating VIP effects during DN2 and DN3 stages of early thymopoiesis.
Figure 3. Exclusive VPAC1 expression in authentic DN1 CD117+ T cell progenitors (ETP cells).
A. Total thymocytes were co-stained for CD4-FITC, CD8-PE, CD25 Pacific Blue, CD44 Alexa 700 and CD117 APC. CD4 and CD8 expressing cells were depleted by magnetic separation to obtain purified DN cells (left panel). The right panel shows CD44/CD25 co-staining on the purified DN cells. B. A histogram plot of CD117 expression gated on D1, D2, D3 and D4 subsets is shown. C. Coexpression of CD44 and CD25 on gated CD117+ purified DN thymocytes identifies DN1 ETP cells (red gate) for FACS. D. Sorted DN1 CD117+ (ETP cells) and DN1 CD117− cells were analyzed for VPAC1 and VPAC2 mRNA expression by qRT-PCR and normalized to HPRT mRNA levels. N.D. = not detected. Data is shown as the mean +/− SEM of two independent experiments using four mice each, with an * indicating a p ≤ 0.05.
Table 1.
| Thymocyte Subset | VPAC1 | VPAC2 |
|---|---|---|
| Total thymoctyes | 35 | 2 |
| Double Negative (DN) | 13 | 6 |
| Double Positive (DP) | 7 | 2 |
| CD4 (Single Positive) | 44 | 1* |
| CD8 (Single Positive) | 266 | 4 |
| DN1 | 312 | N.D. |
| DN2 | 2 | 10 |
| DN3 | 1* | 13 |
| DN4 | 30 | 1 |
| CD117− DN1 | 273 | N.D. |
| CD117+ DN1 | 607 | N.D. |
The amount of mRNA detected in this sample is set to 1, and other values within that column are relative fold differences over this value.
N.D. = Not detected. No VPAC2 mRNA was detected in these thymocyte populations.
3.4 Ikaros deficiency fails to completely reverse the VPAC1:VPAC2 receptor mRNA ratio at the DN3 developmental stage
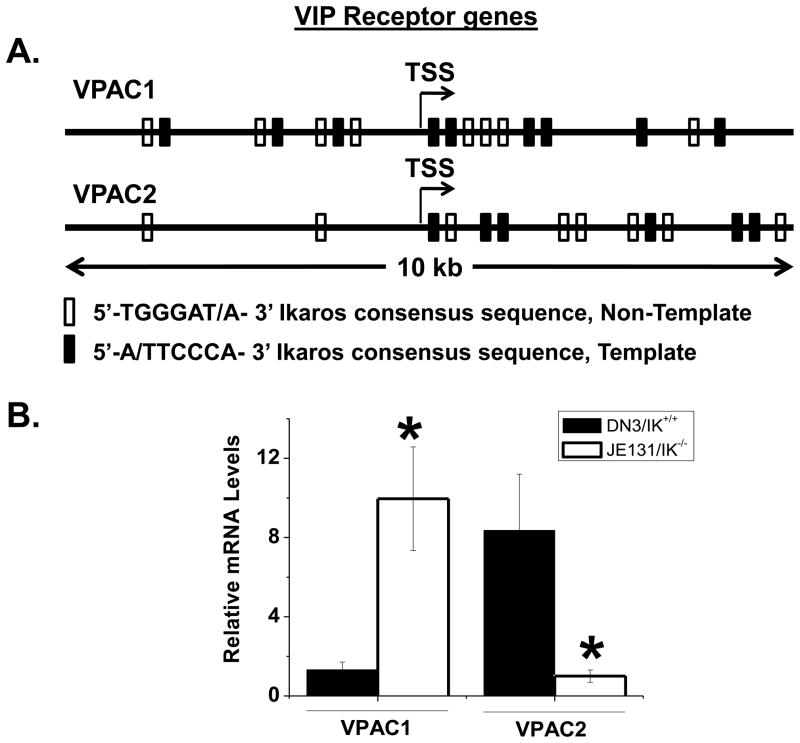
We have previously reported that Ikaros binds the VPAC1 promoter in human peripheral activated CD4 T cells as demonstrated by electrophoretic mobility shift assays (EMSA) and chromatin immunoprecipitation (ChIP) assays [32]. As Ikaros is a master regulator of lymphoid development [11], we hypothesized that Ikaros deficiency would negate the observed VPAC receptor reversal in DN3 thymocytes. Inspection of the transcriptional start site (TSS) of the mouse VPAC2 promoter revealed 14 putative Ikaros consensus sequences (Fig. 4A) [22]. The JE131 mouse cell line is a thymocyte population from IK−/− mice that is arrested at the DN3 stage [14]). Using JE131 cells, we showed that in the absence of Ikaros, VPAC2 induction is not observed, and there is higher VPAC1 mRNA levels compared to wild type DN3 cells (Fig. 4B). Said another way, Ikaros deficiency fails to bring about the VPAC1:VPAC2 receptor reversal to the same extent as wild type cells. These observations now warrant in vivo comparison of the VIP receptor expression profile using IK knockout mice, which is a major future goal. Nevertheless, these data provide evidence that Ikaros may play a role in the coordinate regulation of both VIP receptors during early T cell development.
Figure 4. Ikaros deficiency negates VPAC2 upregulation at the DN3 stage.
A. Frequency of putative Ikaros binding sites in the VPAC2 promoter. The Ikaros consensus binding sequences are shown in the forward and reverse directions. B. qRT-PCR measurement of VPAC2 levels in DN3 wild type thymocytes versus JE-131 IK−/− cells. Data is represented as the mean +/− SEM from four independent experiments using four mice each.
3.5 VPAC2−/− knockout mice show similar thymic subset distribution compared to littermate controls
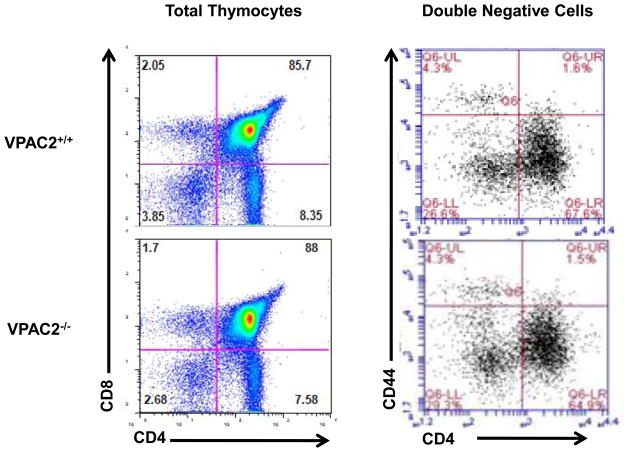
To identify potential functional consequences of the transient VPAC2 upregulation during early DN2 and DN3 stages of T cell development, we compared VPAC2−/− DN thymocyte subsets to age-matched VPAC2+/+ controls. As shown in Figure 5, the distribution of thymocyte subsets within the DN, DP and SP populations, as well as in the DN1–DN4 subsets were similar in VPAC2−/− mice and VPAC2+/+ controls. These data provide evidence showing that the loss of the VPAC2 receptor does not appear to significantly alter the dynamics of thymocyte maturation and suggests that any VIP effects on thymocyte development are mediated though VPAC1. Thymopoiesis is steeped with molecular redundancy, and thus it is not uncommon for T lineage gene knockout mice to fail to show a developmental phenotype. Thus, it will be important to investigate additional knockout mouse models, including VIP−/− and VPAC1−/− mice, to identify the role of VIP/VPAC1 signals and redundancies in this pathway that control thymocyte development.
Figure 5. VPAC2 −/− mice show similar cell distribution within thymocyte populations as compared to wild type control.
Analytical flow cytometry analysis of VPAC2+/+ versus VPAC2−/− thymocytes. The left panel shows total thymocytes stained with both anti-CD4 and anti-CD8. The right panel shows DN cells stained with anti-CD44 and anti-CD25. Percentage of cells in each DN subpopulation in wild type and VPAC2 −/− cells is reported.
4. Discussion
Here we use quantitative RT-PCR to provide a comprehensive analysis of the dynamic changes in VPAC1 and VPAC2 expression during thymopoiesis. Our data show that the earliest T cell progenitor population in the thymus expresses VPAC1 mRNA exclusively. During ETP→DN3 differentiation, there is a radical VIP receptor reversal, which results in a 1000-fold decrease in VPAC1 expression, and a concomitant induction of VPAC2 from undetectable levels in ETP cells to eleven-fold greater than VPAC1 at the DN3 stage. JE-131 IK−/− cells, failed to show this receptor reversal resulting in a lack of VPAC2 induction and elevated VPAC1 mRNA expression. These data support the requirement for Ikaros binding at the VPAC1/2 loci regulating its coordinate, yet opposite, transcriptional expression. Finally, mice lacking the VPAC2 receptor did not show major differences in thymocyte subset numbers compared to age-matched controls, although phenotypic differences cannot be ruled out at this time.
VIP receptors and their functions have been investigated in rat, mouse and human thymocytes. The results of these studies have shown both correlations and discrepancies. In the rat, thymocytes express functionally active VPAC1 as the predominant VIP receptor [12]. Exogenously added VIP blocked TCR- and PHA-induced chemotaxis in a cAMP-dependent mechanism that was suppressed by a VPAC1 antagonist [4]. VIP also protects thymocytes from dexamethosone-induced apoptosis, and VPAC1 antagonists enhanced proliferation of cell cycle arrested cells [5, 42]. Since VIP did not modulate apoptosis or proliferation when added alone, it was suggested that VIP acts as a thymic “tonic” to influence cellular outcomes based on its specific microenvironment [42]. In mouse thymocyte studies, which almost exclusively used the Balb/c strain, VIP binding sites (8000 sites/cell) were detected by 125I-VIP measurements [23]. These VIP binding sites represent functionally active receptors as VIP has been shown to inhibit cytokine expression (IL-2/IL-4) in activated thymocytes through a VPAC2-mediated mechanism [47, 48]. Using NMRI mice, VIP rescues thymocytes from prednisolone induced cell death [10]. The greatest discrepancy regarding VPAC1 and VPAC2 expression profiles is found between rat and human. Although most studies agree on the presence of both VPAC1 and VPAC2 expression in mice, there are discrepancies in the expression profiles. For example, one study showed high VPAC1 levels in total DN thymocytes from BALB/C mice [25]. Conversely, a second study showed undetectable levels of VPAC1 in total DN thymocytes using the same mouse strain [47]. In the present study utilizing C57BL/6 mice, there is agreement with the above two studies with respect to the VPAC1 and VPAC2 profile in some, but not all, thymic subpopulations. This could be due to mouse strain differences as well as different PCR techniques employed. Xin et al. utilized semi-quantitative RT-PCR, Pankhaniya et al. used a more sensitive radioactive nucleotide RT-PCR strategy, while the current study employed real time RT-PCR [25, 47]. In human thymocytes, VPAC2 is believed to be the predominant VIP receptor, in contrast to studies using rat thymocytes [17], and the results of this study. These findings further substantiate inter- and intra-species differences in VPAC1 and VPAC2 expression profiles. In agreement with research conducted on BALB/C thymocytes, VIP also protects human thymocytes from prednisolone induced apoptosis [10]. As humans are far more genetically diverse than inbred mice, measuring VIP receptor expression levels in thymocytes from a greater number of patients may be warranted. Based on this collective research in murine and human species, we propose that the VPAC2 induction observed in this study could serve to protect thymocytes from cell death during early lymphopoiesis and/or regulate thymocytes movement.
As ETP cells differentiate into DN2 cells, VPAC1 is essentially silenced (Fig. 2), which suggests that signaling from this receptor may need to be turned off for ETP→DN2 transition. Also, VPAC1 silencing might be critical for allowing ETP cells to migrate from the corticomedullary junction (CMJ) into the thymic cortex, a necessary prerequisite for ETP→DN2 differentiation [18, 27]. In support of this, exogenously added VIP has been shown to increase T cell adhesion to the extracellular matrix protein, fibronectin, which is highly expressed in the CMJ [19, 36, 40]. Therefore, VPAC1 silencing could facilitate the detachment of ETP cells from the CMJ and allow for their migration into the cortex. Indeed, a developmental arrest of DN1 cells has been seen with conditional knockouts of CXCR4, which is critical for intrathymic migration of DN1 cells to the cortex. This result demonstrated that maturation to the DN2 stage is dependent on migration from the CMJ to the cortex [26]. It is not surprisingly to us that CXCR4 is a direct gene target of VIP/VPAC1 signaling [9]. Moreover, using an Aspergillus allergic mouse model, VPAC2−/− mice showed a significant temporal and magnitude delay in VIP ligand induction, premature elevation in VPAC1 expression and either delays or retention of leukocytes within the pulmonary environment [33–35]. Development of a VPAC1 transgenic mouse is now underway to ascertain the extent to which VPAC1 signaling may impede ETP→DN2 differentiation and intrathymic migration.
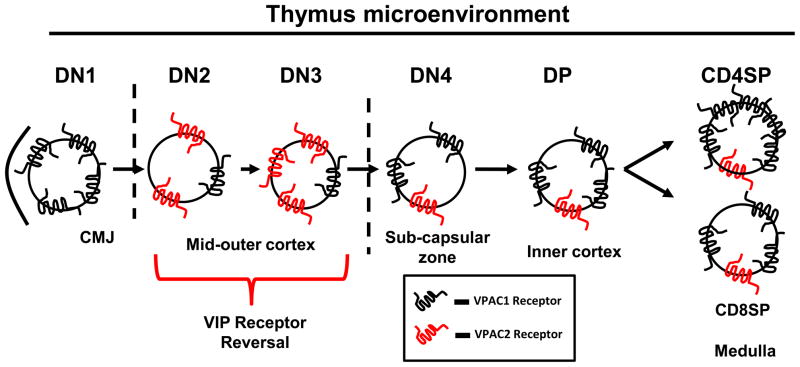
High VPAC1 mRNA expression in ETP thymocytes suggests a role for this GPCR in thymic precursor homing as summarized in Fig. 6. Several lines of direct evidence from this study, as well as established observations by our laboratory and others, support such a model. In 2002, it was discovered that CD34+ bone marrow derived HSC expressed the VPAC1 receptor [31]. Kawakami et al. more recently showed similar VPAC1 expression in primitive and mature CD34+CD38− and CD34+CD38+ human cord blood HSC [15]. These studies indicated that VPAC1 is expressed as a functional receptor in the hematopoietic compartment. VPAC1 has also been shown to assist peripheral CD4 T cell trafficking to Peyer’s patches [24]. Johnston et al. showed that VIP is a chemoattractant to VPAC1 expressing naïve T cells by in vitro matrigel analysis [13]. In addition, our recently published research shows that peripheral blood T cells express 2-fold higher levels of VPAC1 than splenic T cells [44]. It could follow that trafficking HSC may elevate VPAC1 expression to assist in thymic homing. Finally, the VPAC1 ligand, VIP, is secreted by both nerves innervating the thymus [3, 20] and thymocytes themselves [7, 8, 17]. This ligand could act as a “molecular beacon” for VPAC1+ thymic progenitor cells. Based on this collective evidence, it is enticing to hypothesize that VPAC1 acts as a candidate GPCR chemokine receptor that contributes to HSC thymic homing.
Figure 6. Hypothetical model for VIP receptor axis in the thymus.
Once recruited to the CMJ, ETP cells show exclusive, high VPAC1 levels, which are downregulated upon DN2 differentiation. DN2 and DN3 cells show a coordinate upregulation of VPAC2 expression. VIP receptor levels are restored during later differentiation stages, with VPAC1 levels predominating.
Summarizing the major findings of this study, we conclude that: 1.) DN cells from C57BL6/J mice do indeed express VIP receptors at the mRNA level, which until now was inconclusive in the VIP field, and therefore supports a mouse strain (C57BL6 and BalB/c) and species (mouse, rat and human) effect with respect to VIP receptor mRNA expression in developing thymocytes, 2.) The earliest thymic precursors (ETP) express VPAC1 mRNA, with no detectable VPAC2 mRNA, supporting it as a novel marker for ETPs in C57BL/6 mice, 3.) A coordinate VIP receptor reversal between VPAC1 and VPAC2 mRNA as thymocytes mature from ETP → DN3 stages, 4.) Ikaros null JE131 cells that have a DN3 phenotype fail to show the same receptor reversal supporting the idea that Ikaros may induce VPAC2, while silencing VPAC1 to its wild type DN3 levels and 5.) there is little difference in thymocyte subset numbers due to VPAC2 deficiency, but phenotypic differences cannot be ruled out at this time.
Highlights.
VPAC1 is the exclusive VIP receptor expressed on early thymoctye progenitors.
VPAC1 and VPAC2 undergo a transitory reversal in expression during thymocyte development.
The transcription factor, Ikaros, may play a role in VPAC2 induction.
VPAC2 knockout mouse and wild type controls show similar DN percentages.
VPAC2 induction may protect thymocytes from cell death during early lymphopoiesis.
Acknowledgments
We would like to thank the Dorsam Lab, and Drs. Sheri Dorsam, Jodie Haring and Stuart Haring for thoughtful critiques. We are indebted to Dr. Jodie Haring for her expertise in post flow cytometry data analysis. This study was supported by a NIH/NIDDK career award 1KO1 DK064828 to GPD, and 2P20 RR015566 (Co-PIs are GPD and JMS) and P20 RR016741 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and by Award Number R25GM060507 and 3R25GM060507-09S1 from the National Institute Of General Medical Sciences (Center for Health Disparities and Molecular Medicine, Loma Linda University). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ader R, Felten D, Cohen N. Interactions between the brain and the immune system. Annu Rev Pharmacol Toxicol. 1990;30:561–602. doi: 10.1146/annurev.pa.30.040190.003021. [DOI] [PubMed] [Google Scholar]
- 2.Anderson G, Jenkinson EJ. Lymphostromal interactions in thymic development and function. Nat Rev Immunol. 2001;1:31–40. doi: 10.1038/35095500. [DOI] [PubMed] [Google Scholar]
- 3.Bellinger DL, Lorton D, Horn L, Brouxhon S, Felten SY, Felten DL. Vasoactive intestinal polypeptide (VIP) innervation of rat spleen, thymus, and lymph nodes. Peptides. 1997;18:1139–49. doi: 10.1016/s0196-9781(97)00075-2. [DOI] [PubMed] [Google Scholar]
- 4.Delgado M, De la Fuente M, Martinez C, Gomariz RP. Pituitary adenylate cyclase-activating polypeptides (PACAP27 and PACAP38) inhibit the mobility of murine thymocytes and splenic lymphocytes: comparison with VIP and implication of cAMP. J Neuroimmunol. 1995;62:137–46. doi: 10.1016/0165-5728(95)00105-6. [DOI] [PubMed] [Google Scholar]
- 5.Delgado M, Garrido E, Martinez C, Leceta J, Gomariz RP. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptides (PACAP27) and PACAP38) protect CD4+CD8+ thymocytes from glucocorticoid-induced apoptosis. Blood. 1996;87:5152–61. [PubMed] [Google Scholar]
- 6.Delgado M, Martinez C, Leceta J, Garrido E, Gomariz RP. Differential VIP and VIP1 receptor gene expression in rat thymocyte subsets. Peptides. 1996;17:803–7. doi: 10.1016/0196-9781(96)00070-8. [DOI] [PubMed] [Google Scholar]
- 7.Delgado M, Martinez C, Leceta J, Gomariz RP. Vasoactive intestinal peptide in thymus: synthesis, receptors and biological actions. Neuroimmunomodulation. 1999;6:97–107. doi: 10.1159/000026369. [DOI] [PubMed] [Google Scholar]
- 8.Delgado M, Pozo D, Ganea D. The significance of vasoactive intestinal Peptide in immunomodulation. Pharmacol Rev. 2004;56:249–90. doi: 10.1124/pr.56.2.7. [DOI] [PubMed] [Google Scholar]
- 9.Dorsam ST, Vomhof-Dekrey E, Hermann RJ, Haring JS, Van der Steen T, Wilkerson E, et al. Identification of the early VIP-regulated transcriptome and its associated, interactome in resting and activated murine CD4 T cells. Mol Immunol. 47:1181–94. doi: 10.1016/j.molimm.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ernstrom U, Gafvelin G, Mutt V. Rescue of thymocytes from cell death by vasoactive intestinal peptide. Regul Pept. 1995;57:99–104. doi: 10.1016/0167-0115(95)00023-5. [DOI] [PubMed] [Google Scholar]
- 11.Georgopoulos K, Bigby M, Wang JH, Molnar A, Wu P, Winandy S, et al. The Ikaros gene is required for the development of all lymphoid lineages. Cell. 1994;79:143–56. doi: 10.1016/0092-8674(94)90407-3. [DOI] [PubMed] [Google Scholar]
- 12.Gomariz RP, Garrido E, Leceta J, Martinez C, Abalo R, Delgado M. Gene expression of VIP receptor in rat lymphocytes. Biochem Biophys Res Commun. 1994;203:1599–604. doi: 10.1006/bbrc.1994.2369. [DOI] [PubMed] [Google Scholar]
- 13.Johnston JA, Taub DD, Lloyd AR, Conlon K, Oppenheim JJ, Kevlin DJ. Human T lymphocyte chemotaxis and adhesion induced by vasoactive intestinal peptide. J Immunol. 1994;153:1762–8. [PubMed] [Google Scholar]
- 14.Kathrein KL, Lorenz R, Innes AM, Griffiths E, Winandy S. Ikaros induces quiescence and T-cell differentiation in a leukemia cell line. Mol Cell Biol. 2005;25:1645–54. doi: 10.1128/MCB.25.5.1645-1654.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawakami M, Kimura T, Kishimoto Y, Tatekawa T, Baba Y, Nishizaki T, et al. Preferential expression of the vasoactive intestinal peptide (VIP) receptor VPAC1 in human cord blood-derived CD34+CD38− cells: possible role of VIP as a growth-promoting factor for hematopoietic stem/progenitor cells. Leukemia. 2004;18:912–21. doi: 10.1038/sj.leu.2403330. [DOI] [PubMed] [Google Scholar]
- 16.Lara-Marquez M, O’Dorisio M, O’Dorisio T, Shah M, Karacay B. Selective gene expression and activation-dependent regulation of vasoactive intestinal peptide receptor type 1 and type 2 in human T cells. J Immunol. 2001;166:2522–30. doi: 10.4049/jimmunol.166.4.2522. [DOI] [PubMed] [Google Scholar]
- 17.Lara-Marquez ML, O’Dorisio MS, Karacay B. Vasoactive intestinal peptide (VIP) receptor type 2 (VPAC2) is the predominant receptor expressed in human thymocytes. Ann N Y Acad Sci. 2000;921:45–54. doi: 10.1111/j.1749-6632.2000.tb06950.x. [DOI] [PubMed] [Google Scholar]
- 18.Lind EF, Prockop SE, Porritt HE, Petrie HT. Mapping precursor movement through the postnatal thymus reveals specific microenvironments supporting defined stages of early lymphoid development. J Exp Med. 2001;194:127–34. doi: 10.1084/jem.194.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lombardi G, Burzyn D, Mundinano J, Berguer P, Bekinschtein P, Costa H, et al. Cathepsin-L influences the expression of extracellular matrix in lymphoid organs and plays a role in the regulation of thymic output and of peripheral T cell number. J Immunol. 2005;174:7022–32. doi: 10.4049/jimmunol.174.11.7022. [DOI] [PubMed] [Google Scholar]
- 20.Mignini F, Streccioni V, Amenta F. Autonomic innervation of immune organs and neuroimmune modulation. Auton Autacoid Pharmacol. 2003;23:1–25. doi: 10.1046/j.1474-8673.2003.00280.x. [DOI] [PubMed] [Google Scholar]
- 21.Misslitz A, Bernhardt G, Forster R. Trafficking on serpentines: molecular insight on how maturating T cells find their winding paths in the thymus. Immunol Rev. 2006;209:115–28. doi: 10.1111/j.0105-2896.2006.00351.x. [DOI] [PubMed] [Google Scholar]
- 22.Molnar A, Wu P, Largespada DA, Vortkamp A, Scherer S, Copeland NG, et al. The Ikaros gene encodes a family of lymphocyte-restricted zinc finger DNA binding proteins, highly conserved in human and mouse. J Immunol. 1996;156:585–92. [PubMed] [Google Scholar]
- 23.Nguyen TT, Krco CJ, Gores A, Go VL. Analysis of the immunomodulatory properties of the secretin-glucagon family of peptides on mouse lymphoid cell functions and the demonstration of specific receptors on T cells. Immunol Invest. 1987;16:555–77. doi: 10.3109/08820138709087102. [DOI] [PubMed] [Google Scholar]
- 24.Ottaway CA, Cheng HP, Bjerknes ML. Migration of individual lymphocytes into Peyer’s patches in vivo. Adv Exp Med Biol. 1987;216A:295–303. doi: 10.1007/978-1-4684-5344-7_34. [DOI] [PubMed] [Google Scholar]
- 25.Pankhaniya R, Jabrane-Ferrat N, Gaufo GO, Sreedharan SP, Dazin P, Kaye J, et al. Vasoactive intestinal peptide enhancement of antigen-induced differentiation of a cultured line of mouse thymocytes. FASEB J. 1998;12:119–27. doi: 10.1096/fasebj.12.1.119. [DOI] [PubMed] [Google Scholar]
- 26.Plotkin J, Prockop SE, Lepique A, Petrie HT. Critical role for CXCR4 signaling in progenitor localization and T cell differentiation in the postnatal thymus. J Immunol. 2003;171:4521–7. doi: 10.4049/jimmunol.171.9.4521. [DOI] [PubMed] [Google Scholar]
- 27.Porritt HE, Gordon K, Petrie HT. Kinetics of steady-state differentiation and mapping of intrathymic-signaling environments by stem cell transplantation in nonirradiated mice. J Exp Med. 2003;198:957–62. doi: 10.1084/jem.20030837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Porritt HE, Rumfelt LL, Tabrizifard S, Schmitt TM, Zuniga-Pflucker JC, Petrie HT. Heterogeneity among DN1 prothymocytes reveals multiple progenitors with different capacities to generate T cell and non-T cell lineages. Immunity. 2004;20:735–45. doi: 10.1016/j.immuni.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 29.Pozo D, Delgado M, Martinez M, Guerrero JM, Leceta J, Gomariz RP, et al. Immunobiology of vasoactive intestinal peptide (VIP) Immunol Today. 2000;21:7–11. doi: 10.1016/s0167-5699(99)01525-x. [DOI] [PubMed] [Google Scholar]
- 30.Prockop S, Petrie HT. Cell migration and the anatomic control of thymocyte precursor differentiation. Semin Immunol. 2000;12:435–44. doi: 10.1006/smim.2000.0267. [DOI] [PubMed] [Google Scholar]
- 31.Rameshwar P, Gascon P, Oh HS, Denny TN, Zhu G, Ganea D. Vasoactive intestinal peptide (VIP) inhibits the proliferation of bone marrow progenitors through the VPAC1 receptor. Exp Hematol. 2002;30:1001–9. doi: 10.1016/s0301-472x(02)00875-5. [DOI] [PubMed] [Google Scholar]
- 32.Ronni T, Payne KJ, Ho S, Bradley MN, Dorsam G, Dovat S. Human Ikaros function in activated T cells is regulated by coordinated expression of its largest isoforms. J Biol Chem. 2007;282:2538–47. doi: 10.1074/jbc.M605627200. [DOI] [PubMed] [Google Scholar]
- 33.Samarasinghe AE, Hoselton SA, Schuh JM. The absence of the VPAC(2) receptor does not protect mice from Aspergillus induced allergic asthma. Peptides. 31:1068–75. doi: 10.1016/j.peptides.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Samarasinghe AE, Hoselton SA, Schuh JM. The absence of VPAC2 leads to aberrant antibody production in Aspergillus fumigatus sensitized and challenged mice. Peptides. 32:131–7. doi: 10.1016/j.peptides.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Samarasinghe AE, Hoselton SA, Schuh JM. Spatio-temporal localization of vasoactive intestinal peptide and neutral endopeptidase in allergic murine lungs. Regul Pept. 164:151–7. doi: 10.1016/j.regpep.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Savino W, Cotta-de-Almeida V, van Buul-Offers SC, Koster JG, Dardenne M. Abnormal thymic microenvironment in insulin-like growth factor-II transgenic mice. Neuroimmunomodulation. 2005;12:100–12. doi: 10.1159/000083582. [DOI] [PubMed] [Google Scholar]
- 37.Sendo F, Araki Y. Regulation of leukocyte adherence and migration by glycosylphosphatidyl-inositol-anchored proteins. J Leukoc Biol. 1999;66:369–74. doi: 10.1002/jlb.66.3.369. [DOI] [PubMed] [Google Scholar]
- 38.Shi YF, Bissonnette RP, Parfrey N, Szalay M, Kubo RT, Green DR. In vivo administration of monoclonal antibodies to the CD3 T cell receptor complex induces cell death (apoptosis) in immature thymocytes. J Immunol. 1991;146:3340–6. [PubMed] [Google Scholar]
- 39.Shortman K, Wu L. Early T lymphocyte progenitors. Annu Rev Immunol. 1996;14:29–47. doi: 10.1146/annurev.immunol.14.1.29. [DOI] [PubMed] [Google Scholar]
- 40.Smaniotto S, de Mello-Coelho V, Villa-Verde DM, Pleau JM, Postel-Vinay MC, Dardenne M, et al. Growth hormone modulates thymocyte development in vivo through a combined action of laminin and CXC chemokine ligand 12. Endocrinology. 2005;146:3005–17. doi: 10.1210/en.2004-0709. [DOI] [PubMed] [Google Scholar]
- 41.Smith CA, Williams GT, Kingston R, Jenkinson EJ, Owen JJ. Antibodies to CD3/T-cell receptor complex induce death by apoptosis in immature T cells in thymic cultures. Nature. 1989;337:181–4. doi: 10.1038/337181a0. [DOI] [PubMed] [Google Scholar]
- 42.Trejter M, Warchol JB, de Caro R, Brelinska R, Nussdorfer GG, Malendowicz LK. Studies on the involvement of endogenous neuropeptides in the control of thymocyte proliferation in the rat. Histol Histopathol. 2001;16:155–8. doi: 10.14670/HH-16.155. [DOI] [PubMed] [Google Scholar]
- 43.Voice J, Donnelly S, Dorsam G, Dolganov G, Paul S, Goetzl EJ. c-Maf and JunB mediation of Th2 differentiation induced by the type 2 G protein-coupled receptor (VPAC2) for vasoactive intestinal peptide. J Immunol. 2004;172:7289–96. doi: 10.4049/jimmunol.172.12.7289. [DOI] [PubMed] [Google Scholar]
- 44.Vomhof-Dekrey EE, Dorsam GP. Stimulatory and suppressive signal transduction regulates vasoactive intestinal peptide receptor-1 (VPAC-1) in primary mouse CD4 T cells. Brain Behav Immun. 2008 doi: 10.1016/j.bbi.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vomhof-Dekrey EE, Hermann RJ, Palmer MF, Benton KD, Sandy AR, Dorsam ST, et al. TCR signaling and environment affect vasoactive intestinal peptide receptor-1 (VPAC-1) expression in primary mouse CD4 T cells. Brain Behav Immun. 2008 doi: 10.1016/j.bbi.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vomhof-DeKrey EE, Hermann RJ, Palmer MF, Benton KD, Sandy AR, Dorsam ST, et al. TCR signaling and environment affect vasoactive intestinal peptide receptor-1 (VPAC-1) expression in primary mouse CD4 T cells. Brain Behav Immun. 2008;22:1032–40. doi: 10.1016/j.bbi.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xin Z, Jiang X, Wang HY, Denny TN, Dittel BN, Ganea D. Effect of vasoactive intestinal peptide (VIP) on cytokine production and expression of VIP receptors in thymocyte subsets. Regul Pept. 1997;72:41–54. doi: 10.1016/s0167-0115(97)01028-8. [DOI] [PubMed] [Google Scholar]
- 48.Xin Z, Tang H, Ganea D. Vasoactive intestinal peptide inhibits interleukin (IL)-2 and IL-4 production in murine thymocytes activated via the TCR/CD3 complex. J Neuroimmunol. 1994;54:59–68. doi: 10.1016/0165-5728(94)90231-3. [DOI] [PubMed] [Google Scholar]
- 49.Yui MA, Feng N, Rothenberg EV. Fine-scale staging of T cell lineage commitment in adult mouse thymus. J Immunol. 185:284–93. doi: 10.4049/jimmunol.1000679. [DOI] [PMC free article] [PubMed] [Google Scholar]