Abstract
The brain is the central organ of stress and adaptation to stress because it perceives and determines what is threatening, as well as the behavioral and physiological responses to the stressor. The adult, as well as developing brain, possess a remarkable ability to show reversible structural and functional plasticity in response to stressful and other experiences, including neuronal replacement, dendritic remodeling, and synapse turnover. This is particularly evident in the hippocampus, where all three types of structural plasticity have been recognized and investigated, using a combination of morphological, molecular, pharmacological, electrophysiological and behavioral approaches. The amygdala and the prefrontal cortex, brain regions involved in anxiety and fear, mood, cognitive function and behavioral control, also show structural plasticity. Acute and chronic stress cause an imbalance of neural circuitry subserving cognition, decision making, anxiety and mood that can increase or decrease expression of those behaviors and behavioral states. In the short term, such as for increased fearful vigilance and anxiety in a threatening environment, these changes may be adaptive; but, if the danger passes and the behavioral state persists along with the changes in neural circuitry, such maladaptation may need intervention with a combination of pharmacological and behavioral therapies, as is the case for chronic or mood anxiety disorders. We shall review cellular and molecular mechanisms, as well as recent work on individual differences in anxiety-like behavior and also developmental influences that bias how the brain responds to stressors. Finally, we suggest that such an approach needs to be extended to other brain areas that are also involved in anxiety and mood.
1. Introduction
The brain is the central organ of stress and adaptation to stress because it perceives and determines what is threatening, as well as the behavioral and physiological responses to the stressor. The adult, as well as developing brain, possess a remarkable ability to adapt and change with stressful, and other experiences. Structural changes - neuronal replacement, dendritic remodeling, and synapse turnover - are a feature of the adult brain's response to the environment. Nowhere is this better illustrated in the mammalian brain than in the hippocampus, where all three types of structural plasticity have been recognized and investigated using a combination of morphological, molecular, pharmacological, electrophysiological and behavioral approaches. At the same time, new data on the amygdala and the prefrontal cortex, brain regions involved in anxiety and fear, mood, cognitive function and behavioral control, have demonstrated that the adult brain is indeed a malleable and adaptable structure capable of reversible structural plasticity. Steroid hormones play an important role, acting via both genomic and non-genomic mechanisms. In addition, other intracellular mediators and neurotransmitter systems participate in structural plasticity.
The theme of this review is that stress causes an imbalance of neural circuitry subserving cognition, decision making, anxiety and mood that can increase or decrease expression of those behaviors and behavioral states. In the short term, such as for increased fearful vigilance and anxiety in a threatening environment, these changes may be adaptive; but, if the danger passes and the behavioral state persists along with the changes in neural circuitry, such maladaptation may need intervention with a combination of pharmacological and behavioral therapies, as is the case for chronic or mood anxiety disorders.
Besides reviewing cellular and molecular mechanisms, we shall discuss recent work on individual differences in anxiety-like behavior in the animals that we and others study, as well as possible developmental influences that may underlie those differences and bias how the brain responds to stressors. Finally, we shall note that multiple brain regions are involved and that investigations on amygdala, prefrontal cortex and hippocampus that have been very productive, need to be extended to other brain areas that are also involved in anxiety and mood.
2. Structural plasticity in the hippocampus, amygdala and prefrontal cortex
Hippocampus
Stress hormones modulate function within the brain by changing the structure of neurons. The hippocampus is one of the most sensitive and malleable regions of the brain. Within the hippocampus, the input from the entorhinal cortex to the dentate gyrus is ramified by the connections between the dentate gyrus and the CA3 pyramidal neurons. One granule neuron innervates, on the average, 12 CA3 neurons, and each CA3 neuron innervates, on the average, 50 other CA3 neurons via axon collaterals, as well as 25 inhibitory cells via other axon collaterals. The net result is a 600-fold amplification of excitation, as well as a 300 fold amplification of inhibition, that provides some degree of control of the system (McEwen 1999).
The circuitry of the dentate gyrus-CA3 system is believed to play a role in the memory of sequences of events, although long-term storage of memory occurs in other brain regions (Lisman & Otmakhova 2001). But, because the dentate gyrus DG -CA3 system is so delicately balanced in its function and vulnerability to damage, there is also adaptive structural plasticity, in that new neurons continue to be produced in the dentate gyrus throughout adult life, and CA3 pyramidal cells undergo a reversible remodeling of their dendrites in conditions, such as hibernation and chronic stress, including a combination of food restriction and increased physical activity (McEwen 2010). Whatever the physiological significance of these changes, be it protection (McEwen 2007) or increased vulnerability to damage (Conrad 2008), the hippocampus undergoes a number of adaptive changes in response to acute and chronic stress.
Replacement of neurons in dentate gyrus
One type of structural change that occurs in the hippocampus involves replacement of neurons (Altman & Das 1965; Kaplan & Bell 1984) (Cameron & Gould 1994). The subgranular layer of the dentate gyrus contains cells that have some properties of astrocytes, e.g. expression of glial fibrillary acidic protein which give rise to granule neurons (Seri et al 2001). After BrdU administration to label DNA of dividing cells, these newly born cells appear as clusters in the inner part of the granule cell layer, where a substantial number of them will go on to differentiate into granule neurons within as little as 7 days. In the adult rat, up to as many as 5-9000 new neurons are born per day and survive with a half-life of 28 days (Cameron & McKay 2001). There are many hormonal, neurochemical and behavioral modulators of neurogenesis and cell survival in the dentate gyrus, including estradiol, IGF-1, antidepressants, glucocorticoids, voluntary exercise and hippocampal-dependent learning (Kempermann & Gage 1999; McEwen 2010; van Praag et al 1999). With respect to stress, certain types of acute stress and many chronic stressors suppress neurogenesis or cell survival in the dentate gyrus, and the mediators of these inhibitory effects include excitatory amino acids acting via NMDA receptors and endogenous opioids (McEwen 2010).
Functional consequences
As to the functional consequences of structural remodeling in hippocampus, repeated stress can impair hippocampal-dependent behaviors in a manner that is reversible, along with dendritic shrinkage in the CA3 region, within days or weeks after the termination of the stressor. This supports the notion that the remodeling is not brain damage but a form of adaptive plasticity that may also protect the hippocampus from permanent excitotoxic damage (McEwen 2010). Yet, it is important to note that chronic stress causes other changes in the brain besides dendritic remodeling in CA3, e.g., prolonged stress can diminish the size of the dentate gyrus (Pham et al 2003) and also cause dentate gyrus dendritic remodeling (Sousa et al 2000) and dentate gyrus long-term potentiation LTP (Pavlides et al 2002). Moreover, 21d chronic restraint alters the ability of acute stress to affect hippocampal functions, such as spatial memory, and here an increase in sensitivity to glucocorticoids appears to be involved and to mediate at least some of the behavioral changes (Conrad 2006).
Prefrontal cortex and amygdala
Acute and repeated stress for 21days of CRS also causes functional and structural changes in other brain regions, such as the prefrontal cortex and amygdala (McEwen 2010). CRS and chronic immobilization caused dendritic shortening in medial prefrontal cortex (McEwen 2010), but produced dendritic growth in neurons in amygdala (Vyas et al 2002), as well as in orbitofrontal cortex (Liston et al 2006). Similarly, in the domain of substance abuse, different, and sometimes opposite, effects were seen on dendritic spine density in orbitofrontal cortex, medial prefrontal cortex and hippocampus CA1(Crombag et al 2005; Robinson & Kolb 1997).
Behavioral correlates of CRS-induced remodeling in the prefrontal cortex include impairment in attention set shifting, possibly reflecting structural remodeling in the medial prefrontal cortex (Liston et al 2006). Moreover, chronic restraint stress impairs extinction of a fear conditioning task (Miracle et al 2006) and the prefrontal cortex is involved in extinction of fear conditioning (Santini et al 2004).
Regarding the amygdala, chronic stress for 21 days or longer not only impairs hippocampal-dependent cognitive function, but it also enhances amygdala-dependent unlearned fear and fear conditioning (Conrad et al 1999). Chronic stress also increases aggression between animals living in the same cage, and this is likely to reflect another aspect of hyperactivity of the amygdala (Wood et al 2003). Moreover, chronic corticosterone treatment in the drinking water produces an anxiogenic effect that could be due to the glucocorticoid enhancement of CRF activity in the amygdala (Corodimas et al 1994; Makino et al 1995; McEwen 2010).
3. Cellular and molecular mechanisms involved in stress-related structural and functional plasticity
Adrenal steroids
Because the hippocampus was the first higher brain center that was recognized as a target of stress hormones (McEwen et al 1968), both the hippocampus and adrenal steroids have figured prominently in our understanding of how stress impacts brain structure and behavior. The hippocampus expresses both Type I mineralocorticoid, MR and Type II glucocorticoid, GR receptors, and these receptors mediate a biphasic response to adrenal steroids in the CA1 region, although not in the dentate gyrus (Joels 2006), which, nevertheless, shows a diminished excitability in the absence of adrenal steroids (Margineanu et al 1994) along with debranching of dentate granule neuron dendrites (Gould et al 1990). Other brain regions, such as the paraventricular nucleus, lacking in MR but having GR, show a monophasic response (Joels 2006).
Regarding the biphasic effects of adrenal steroids, these have been seen for excitability of hippocampal neurons in terms of long-term potentiation and primed burst potentiation (Diamond et al 1992; Pavlides et al 1994; Pavlides et al 1995), with parallel biphasic effects upon memory (Okuda et al 2004; Pugh et al 1997). As to mechanisms for the biphasic responses, the co-expression of MR and GR in the same neurons could give rise to heterodimer formation and a different genomic activation from that produced by either MR or GR homodimers (Joels 2006). Moreover, in addition, deletion of the Type I MR receptor by genetic means has revealed that MR is involved (Karst et al 2005) in corticosterone enhancement of extracellular levels of glutamate (Venero & Borrell 1999).
Another important non-genomic action of glucocorticoids is the rapid stimulation of endocannabinoid release (Hill et al 2010a; Tasker et al 2006), which is involved not only in HPA regulation (Hill et al 2010b), but also in negative regulation of glutamate, GABA and acetylcholine release (Hill & McEwen 2010).
Finally, although much of the work on MR and GR has been done on rat and mouse brains, it is important to note that the rhesus monkey hippocampus has a predominance of MR and relatively less GR compared to rodent species (Sanchez et al 2000). This finding may have important implications for the effects of adrenal steroids on learning and vulnerability to stress and excitotoxicity in primates and humans, as well as age-related changes, as discussed earlier.
Exploration of the underlying mechanisms for structural remodeling of dendrites and synapses reveals that it is not adrenal size or presumed amount of physiological stress per se that determines dendritic remodeling, but a complex set of other factors that modulate neuronal structure (McEwen 1999). Indeed, in species of mammals that hibernate, dendritic remodeling is a reversible process and occurs within hours of the onset of hibernation in European hamsters and ground squirrels, and it is also reversible within hours of wakening of the animals from torpor (Arendt et al 2003; Magarinos et al 2006; Popov & Bocharova 1992). This implies that reorganization of the cytoskeleton is taking place rapidly and reversibly and that changes in dendrite length and branching are not “damage” but a form of structural plasticity.
Importance of glutamate for remodeling in hippocampus
Adrenal steroids are important mediators of remodeling of hippocampal neurons during repeated stress, and exogenous adrenal steroids can also cause remodeling in the absence of an external stressor (Magarinos et al 1999; Sousa et al 2000). However, effects of chronic stress on dendritic remodeling are blocked by blocking NMDA receptors, as well as blocking adrenal steroid synthesis (Magarinos & McEwen 1995). A recent report indicates that NMDA receptors and glutamate are also involved in stress-induced shortening of dendrites in medial prefrontal cortex (Martin & Wellman 2011).
Further evidence for the importance of glutamate is the stress-induced elevation of extracellular glutamate levels, leading to induction of glial glutamate transporters, as well as increased activation of the nuclear transcription factor, phosphoCREB (Reagan et al 2004; Wood et al 2004). Excitatory amino acids released by the mossy fiber pathway play a key role in the remodeling of the CA3 region of the hippocampus, and regulation of glutamate release by adrenal steroids may play an important role (McEwen 1999). Moreover, 21d of CRS leads to depletion of clear vesicles from mossy fiber terminals and increased expression of presynaptic proteins involved in vesicle release (Grillo et al 2005; Magarinos et al 1997). Taken together with the fact that vesicles which remain in the mossy fiber terminal are near active synaptic zones and that there are more mitochondria in the terminals of stressed rats, this suggests that CRS increases the release of glutamate (Magarinos et al 1997).
Maintaining the balance between adaptation and excitotoxicity
Glucocorticoids also synergize with excitatory amino acids to promote excitotoxic damage and impairment of energy generation through inhibition of glucose uptake and energy metabolism appears to be one major mechanism (Sapolsky 1992). As another example of an inverted U shaped action of adrenal steroids, recent evidence indicates that glucocorticoid receptors translocate to mitochondria and, at physiological levels, reduce potentially damaging oxidative stress, whereas, at high levels, this mechanism fails after some hours and there is increased excitotoxicity (Du et al 2009). Therefore, mitochondria and their sensitivity to glucocorticoids (Roosevelt et al 1973) play an important role in maintaining the balance between adaptation and excitotoxicity.
In being able to protect and promote adaptation such as reversible stress-induced structural plasticity, on the one hand, and yet contribute to damage, on the other, glucocorticoids oppose some actions of stress and mediate others. For example, chronic stress induced induction of glutamate transporter Glt 1 in hippocampus (Reagan et al 2004) is biphasically modulated by glucocorticoids (Autry et al 2006), and kainate (KA1) receptor up-regulation by chronic stress is also biphasically modulated by glucocorticoids. See Table 1. At the same time, chronic stress induction of cocaine-amphetamine-regulated transcript (CART) in the hippocampus is mediated by glucocorticoids (Hunter et al 2007). In hippocampus, the up-regulation of CART is associated with a form of resistance of anxiety-generating effects of stress (Miller, M., Hunter, R., McEwen, B., unpublished). The subtlety and complexity of adrenal steroid actions that is revealed by these and others (Joels 2006; Joels et al 2006), are reminiscent of their role in modulation of the immune system (Sapolsky et al 2000), including apparent pro- as well as anti-inflammatory actions (Munhoz et al 2010).
Table 1. Glucocorticoid actions mediate or biphasically modulate actions of chronic stress.
|
Other mediators of hippocampal structural plasticity
Besides glutamate, the role of adrenal steroids in the hippocampus involves other interactions with neurochemical systems, including serotonin, endogenous opioids, calcium currents, and GABA-benzodiazepine receptors (McEwen 1999; McEwen & Chattarji 2004). Moreover, and beyond the scope of this article, there are other molecular players in the stress-induced remodeling of dendrites, which include extracellular molecules of the NCAM family, including PSA-NCAM; a transmembrane glycoprotein, M6a; corticotrophin releasing factor; tissue plasminogen activator (tPA), which is an extracellular protease and signaling molecule; and brain-derived neurotrophic factor, BDNF (McEwen 2010).
Mechanisms of structural plasticity in amygdala and prefrontal cortex
We know less about stress-related structural plasticity in the amygdala and prefrontal cortex. Yet, both amygdala and prefrontal cortex express adrenal steroid receptors and there is evidence that adrenal steroids play a role in structural plasticity, along with a role for tPA, CRF and BDNF (McEwen 2010). BDNF plays a particularly interesting role in amygdala plasticity, since over-expression of BDNF, without any applied stressor, enhances anxiety in an elevated plus maze and increases spine density on basolateral amygdala neurons and this occludes any further effect of immobilization stress on both anxiety and spine density (Govindarajan et al 2006). As noted, NMDA receptors and glutamate are also involved in stress-induced shortening of dendrites in medial prefrontal cortex (Martin & Wellman 2011) and, thus far, their role in structural plasticity in the amygdala is unclear.
Key role of BDNF
BDNF is particularly interesting because it appears to be a facilitator of plasticity directed by other cellular signals, as suggested originally by work in vitro (Horch & Katz 2002; Horch et al 1999). BDNF +/- mice show a less branched dendritic tree and do not show a further reduction of CA3 dendrite length with chronic stress, whereas wild-type mice show reduced dendritic branching after chronic stress (Magarinos et al 2011). Figure 1. At the same time, over-expression of BNDF prevents stress-induced reductions of dendritic branching in the CA3 hippocampus and results in anti-depressant-like effects in a Porsolt forced-swim task (Govindarajan et al 2006). Activation of neuronal activity by stress triggers increased BDNF synthesis to replace depletion of BDNF caused by stress (Marmigere et al 2003). Yet, BDNF and corticosteroids appear to oppose each other – with BDNF reversing reduced excitability in hippocampal neurons induced by stress levels of corticosterone (Hansson et al 2006). The occurrence of and timing of opposing effects of stress and glucocorticoids on BDNF may be the reason that chronic stress has been reported to deplete BDNF levels in some studies (Smith et al 1995) but not in others (Isgor et al 2004; Kuroda & McEwen 1998).
Figure 1. Effect of chronic restraint stress on the number of apical and basal (upper and lower panel on left) dendritic branch points and total dendritic length (upper and lower panel on right) of CA3 pyramidal neurons from wild type and haploinsufficient BDNF (BDNFBl/6) mice.
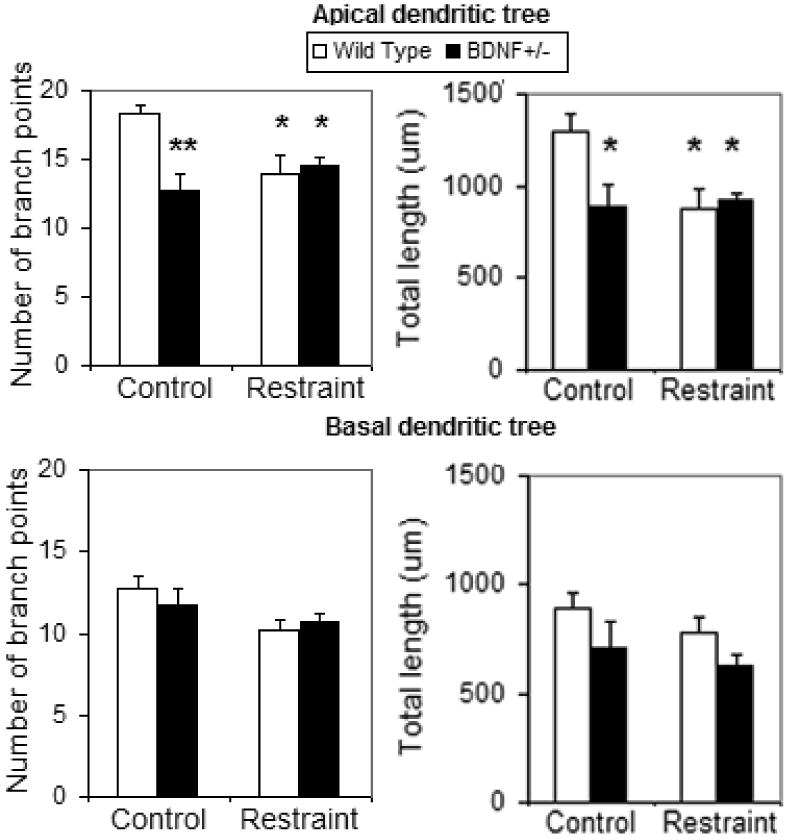
* * and *P < 0.01 and P < 0.05 respectively, compared with control wild types. One-way ANOVA, Tukey post hoc test. Bars represent means 1 SEM. From (Magarinos et al 2011).
At the same time, glucocorticoids appear to activate trk B signaling in a ligand-independent manner that has neuroprotective implications (Jeanneteau et al 2008), although this effect is transient and accommodates to chronic glucocorticoid exposure (Numakawa et al 2009). The authors of this study propose that TrkB-GR interaction plays a critical role in the BDNF-stimulated PLC-g pathway, which is required for glutamate release, and the decrease in TrkB-GR interaction caused by chronic exposure to glucocorticoids results in the suppression of BDNF-mediated neurotransmitter release via a glutamate transporter (Numakawa et al 2009). Thus, as is the case for glucocorticoid effects KA1 receptors and glutamate transporters (Table 1), the glucocorticoid actions on the BDNF-trkB system modulate this system and have a self-limiting (homeostatic) quality.
The role of BDNF as a facilitator of plasticity has led to expansion of the utility of BDNF in plasticity related to treatment of disease. Besides depression, where reduced BDNF may be a key feature of the depressive state and elevation of BDNF by diverse treatments ranging from antidepressant drugs, such as fluoxetine to regular physical activity (Duman & Monteggia 2006), there are other potential applications, such as the recently reported ability of fluoxetine to enhance recovery from stroke (Chollet et al 2011) and the previously reported effects of fluoxetine to ameliorate effects of monocular deprivation in the visual systems by enhancing visual stimulation-induced plasticity (Vetencourt et al 2008). However, a key aspect of this new view (Castren & Rantamaki 2010) is that the drug is opening a “window of opportunity” that may be capitalized by a positive behavioral intervention, e.g., behavioral therapy in the case of depression or the intensive therapy to promote neuroplasticity to counteract the effects of a stroke. In this connection, it is important to note that successful behavioral therapy, which is tailored to individual needs, can produce volumetric changes in both prefrontal cortex in the case of chronic fatigue (de Lange et al 2008), and in amygdala in the case of chronic anxiety (Holzel et al 2009). This implies at least 3 important lessons: i. that antidepressant drugs should not be given without such a positive intervention; ii. that there may be a limited window of opportunity, beyond which continued drug treatment may be ineffective; and iii. that negative experiences during the window may even make matters worse (Castren & Rantamaki 2010). In this connection, it should be noted that BDNF also has the ability to promote pathophysiology, as in seizures (Heinrich et al 2011).
4. Acute vs chronic stress effects
Responses to acute and chronic stress in both the amygdala and the prefrontal cortex present challenges to our understanding of the cellular and molecular mechanisms. In the amygdala, while chronic stress causes dendrites in the basolateral amygdala to increase in length along with increased spine density on dendrites (Vyas et al 2002), a single acute stress to a naïve rat causes increased spine density without increased dendritic branching or length after a 10d interval (Mitra et al 2005). The former increase in dendritic length after chronic stress can be mimicked by a single, acute injection of a large dose of glucocorticoids (Mitra & Sapolsky 2008). Yet, in relation to the effect of the single traumatic stressor, glucocorticoid presence before the traumatic stressor prevents the delayed increase in dendritic spines (Rao, McEwen, Chattarji, unpublished). This raises, again, the important issue that both glucocorticoid dose and timing are important for the outcome (Joels 2006; Joels et al 2006). Since actions of adrenal steroids can directly or indirectly affect gene expression through direct interactions with response elements or indirect signaling via second messenger pathways, the regulation of gene expression may provide some clues. So far the hippocampus has begun to provide some insights.
5. Epigenetic involvement in alterations due to stress
Adrenal steroids bind to MR and GR and end up in the cell nucleus (McEwen & Plapinger 1970), and thus the regulation of gene expression is a key aspect of their action, referred to now under the rubric of “epigenetic” influences. The subject of epigenetic influences on the function of the nervous system has been the subject of much recent research (Jiang et al 2008). Epigenetics are of interest with regard to disorders, such as anxiety and depression, because epigenetic change is a means to explain how life events, like stress, cause persistent changes in the brain and in behavior. Epigenetics also offers a possible explanation of how individuals sharing the same or similar genes might show large differences in disease susceptibility and resilience.
The term “epigenetics” encompasses a number of mechanisms by which biological information is transmitted above the level of the genetic code. Broadly speaking, this definition includes everything from DNA methylation to the transmission of culture and language, so we might more strictly define epigenetics as molecular events, other than the genetic code itself, which influence gene transcription over time. These include, DNA methylation, a variety of non-coding RNA mechanisms, and covalent modifications of histones. Histone modifications and DNA methylation have been the most thoroughly examined with regard to stress and the hippocampus. Histone proteins are subject to a variety of modifications at an ever growing number of residues. These modifications include acetylation, methylation (which has three valences) and phosphorylation, as well as a number of others (Gardner et al; Jiang et al 2008).
The stress sensitive hippocampus (McEwen 1999) expresses the enzymatic machinery of epigenetic change at levels as high or higher than other regions of the brain, as an afternoon spent on the Allan Brain Atlas will show. DNA methylation and histone H3 phospho-acetylation have been associated with hippocampal memory formation and the latter modification appears to be responsive to both stress and exercise (Collins et al 2009; Miller & Sweatt 2007; Reul et al 2009) and our own work (see below) has shown that histone methylation in the hippocampus is also dynamically responsive to stress. It is significant that the hippocampus is the site of one of the only examples of epigenetic action in the strict, heritable, sense; that is, the demonstration that differences in early life environment result in lasting, trans-generational changes in behavior, mediated in part by changes in epigenetic histone marks and DNA methylation of the 1-7 promoter of the GR in the hippocampus (Szyf et al 2005). The consequence of these modifications is a down, or up-regulation in the expression of the GR that persists across generations and produces significant differences in stress responsiveness and affective behaviors (Weaver et al 2004). Significantly, epigenetic changes in the DNA methylation status of both the GR and BDNF genes have since been shown to be associated with a history of childhood abuse in humans (McGowan et al 2009; Roth et al 2009).
Recent work in our laboratory has demonstrated that acute stress produces rapid decreases in histone H3 lysine 27 tri-methylation (a mark associated with transcriptional silencing and facultative heterochromatin) and equally rapid increases in levels of the heterochromatic H3 lysine 9 tri-methyl (H3K9me3) mark (associated with constitutive heterochromatin) (Hunter et al 2009b). See Figure 2. These effects attenuate over the course of 21 days of chronic stress, though the attenuation is partially blocked, in the case of H3K9, by co-administration of fluoxetine (Hunter et al 2009b). These results were surprising due to the magnitude and rapidity of the changes observed: less than two hours after the onset of stress H3K9me3 levels had doubled and the change persisted for at least 24 hours. Until recently, methyl marks, particularly constitutive heterochromatic marks like H3K9me3, were thought to be stable after cell fate was determined (Kubicek & Jenuwein 2004). Similar observations have been made in the nucleus accumbens after cocaine administration (Maze et al 2010). This change suggests that heterochromatin formation is much more dynamic than previously thought, and may play a role in the response of the brain to stress. More recent work suggests that this may constitute a genomic stress response, aimed at the protection of the genomic and transcriptomic stability required for the function of long lived, post-mitotic neurons via the control of “junk” transposable DNA elements (Hunter et al. unpublished data).
Figure 2. Changes in dentate gyrus histone H3K9 trimethylation after acute immobilization stress as well as 7 and 21 day repeated immobilization stress.
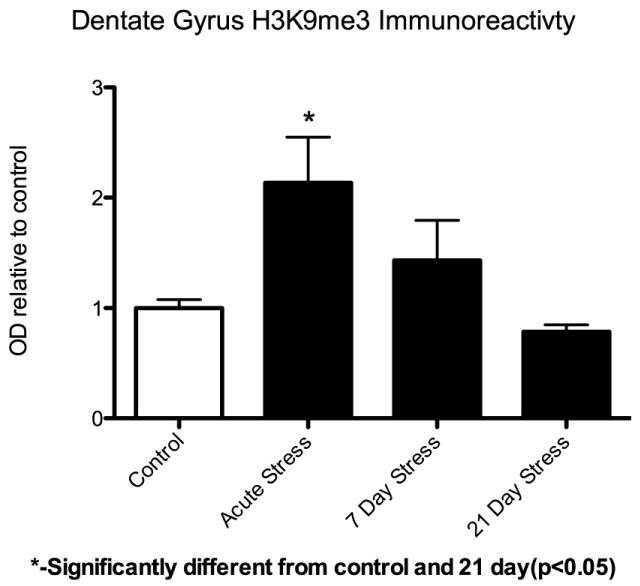
Acute stress induces a 2-fold increase in H3K9me3 levels. Adapted from (Hunter et al 2009b).
6. Individual differences in anxiety and their possible implications and origins
Whereas the amygdala and hippocampus have each provided challenging new information about the role of multiple mediators in acute and chronic stress effects upon neuronal structure and function, the prefrontal cortex has provided its own challenges when it comes to individual differences in anxiety related behavior. It is clear that the quite homogenous populations of laboratory rats show individual differences in anxiety related behaviors that can also influence lifespan, with more anxious rats having shorter lifespans (Cavigelli & McClintock 2003; Cavigelli et al 2006).
There is evidence pointing to a genetic component to these individual differences, as a number of rodent strains have been developed by crossbreeding individuals with particular anxiety phenotypes, such as novelty seeking high and low responders (Kabbaj et al 2000a) (Kabbaj et al 2000b), high and low fear conditioners (Bush et al 2007), and the Fischer and Lewis rat strains. Behavioral differences in these strains correspond with chemical, electrophysiological and structural differences in brain regions involved in the stress circuit.
Rats that were chosen based on their anxiety and locomotor response to novelty (Dellu et al 1996), have differences affecting the entire brain. High responders to novelty (i.e. those that have high locomotion and low freezing behavior in a novel environment) have increased expression of dopamine in the nucleus accumbens and decreased expression in the mPFC compared to low responders, as well as lower serotonergic expression overall (Piazza et al 1991). High responders also have a larger and longer lasting dopamine response to stress, which is dependent on corticosterone release (Rouge-Pont et al 1998). Yet high responders also have lower baseline and glucocorticoid receptor expression in the hippocampus, which appears to directly modulate novelty-induced exploratory behavior (Kabbaj et al 2000b). Furthermore, rats selected on the basis of their susceptibility or resistance to social defeat show differences in HPA reactivity in which differences in CRF actions are implicated (Wood et al 2010).
Specifically in the prefrontal cortex, rats bred for high anxiety also showed lower baseline activity and reduced tissue oxygenation, which corresponded to reduced post-stress mPFC activity compared to those bred for low anxiety (Kalisch et al 2004). Furthermore, high anxiety rats demonstrated impaired extinction learning and lower c-fos expression after extinction in both the mPFC and lateral amygdala (Muigg et al 2008). This is consistent with what has been found in our lab in the general rodent populations. Large cohorts of Sprague-Dawley and the more anxious Lewis strain were screened for baseline anxiety behavior. Those individuals that fell one standard deviation above and below the mean were grouped into high anxiety and low anxiety groups. In both strains, individuals that exhibited the highest amount of basal anxiety-like behavior in the open field had shortest apical dendrites in layer II/III pyramidal neurons of the prefrontal cortex compared to their low anxiety counterparts (Miller, et al. in press).
Individual variability in basal anxiety and locomotor behaviors has also been shown to correlate with serotonin levels (Antoniou et al 2008; Schwarting et al 1998), corticosteroid receptor concentrations (Herrero et al 2006), CART peptide expression (Miller, M. et al, unpublished), and cytokine expression (Pawlak et al 2003). A better understanding of how these different systems interact to create behavioral variation may help to elucidate why some individuals develop anxiety and mood disorders while others appear to be resistant.
7. Developmental effects on stress responsiveness and behavior
A contributing factor to individual differences in anxiety-related behaviors may be early life experiences that effect development of key brain structures. The quality and quantity of maternal care determines anxiety profiles via epigenetic mechanisms (Meaney & Szyf 2005) that can be transmitted across generations (Francis et al 1999). The consistency of maternal care is also important and exposure to novelty with good maternal care also improves cognitive and social development (Akers et al 2008; Tang et al 2006). Prenatal stress increases anxiety-like behaviors, but “postnatal handling” which elicits good maternal care can reverse those effects (Wakschlak & Weinstock 1990). However, prenatal stress, without good postnatal maternal care, causes abnormalities in hippocampal development and behavior (Maccari & Morley-Fletcher 2007).
Maternal separation, the separation of a litter of pups from the dam for 3 hours daily during the first two weeks of life (Plotsky & Meaney 1993), has long lasting effects on neural circuitry, cognition and behavior. Specifically, in adult rats exposed to maternal separation (MS rats), we have demonstrated baseline, as well as, stress-induced alterations in neuroanatomy, cognition, and emotionality (Eiland & McEwen). Given the importance of the hippocampus in stress responsiveness, we have focused on early life stress associated changes in hippocampal anatomy and hippocampal associated functions.
In regard to neuroanatomy, adult MS rats exhibit a baseline deficiency in apical dendritic length in CA3 region pyramidal neurons. This deficiency is evident distal to the cell soma, at distances of 390-480 μm. Further, this deficiency in apical dendritic length seems to impact the capacity for stress-induced dendritic remodeling. Specifically, MS rats exposed to 3 weeks of chronic restraint stress (CRS) do not exhibit significant apical dendritic remodeling, whereas normally reared rats exhibit significant shortening of apical dendrites with chronic stress exposure. A similar deficiency in CA3 apical dendritic length that is also coupled with failed stress-induced dendritic remodeling is seen in brain derived neurotrophic factor (BDNF) haploinsufficient mice (Magarinos et al 2011). Perhaps this similarity between MS rats and BDNF haploinsufficient mice stems from the reduced hippocampal levels of BDNF mature protein and mRNA exhibited in adult MS rats (Aisa et al 2009; Lippmann et al 2007). Future investigations examining the contribution of BDNF and other factors to the deficiency of CA3 apical dendritic material in MS rats, as well as the potential functional correlates of this deficiency, are needed. Such investigations may help clarify whether stress-induced dendritic remodeling is a protective mechanism or a process that increases vulnerability of pyramidal neurons.
Coincident with neuroanatomical changes in the MS rat are alterations in cognition and emotionality. Adult MS rats exhibit deficiency in hippocampal dependent spatial memory (Aisa et al 2009; Aisa et al 2007; Cannizzaro et al 2007; Huot et al 2002; Uysal et al 2005). We specifically used the object placement task to demonstrate longstanding baseline impairment of spatial memory in adult MS rats. Of note, we have demonstrated that this baseline impairment in spatial memory emerges early in development, as prepubescent MS rats (postnatal day 30) exhibit significant impairment (Eiland, unpublished data).
In regard to emotionality, we have demonstrated that early life stress exposure potentiates stress-induced anxiety. Using the elevated plus maze and open field, we do not find baseline differences between normally reared and MS rats. When MS rats are exposed to chronic restraint stress and given a 24-hour period of recovery, MS rats in the elevated plus maze exhibit significantly decreased open arm entry in comparison to normally reared CRS-exposed rats (Eiland and McEwen, 2011). See Figure 3. There is a significant interaction between early life stress and subsequent adult chronic stress in producing anxiety-like behavior. Similarly, MS rats that are exposed to an acute stress, such as forced swim demonstrate altered emotionality. During forced swim, MS rats demonstrate depressive-like behavior, as they exhibit significantly decreased latency to immobility in comparison to normally reared rats. Together these early life stress induced alterations in emotionality observed in the rat seem to parallel the increased vulnerability to depression and anxiety disorders observed in individuals with a history of early life adversity (Heim et al 2008). Early life stress seems to program longstanding changes in hippocampal anatomy and plasticity that may in turn serve to modulate cognition and emotionality across the lifespan.
Figure 3. Duration of time spent in open arms of elevated plus maze 24 h after termination of CRS.
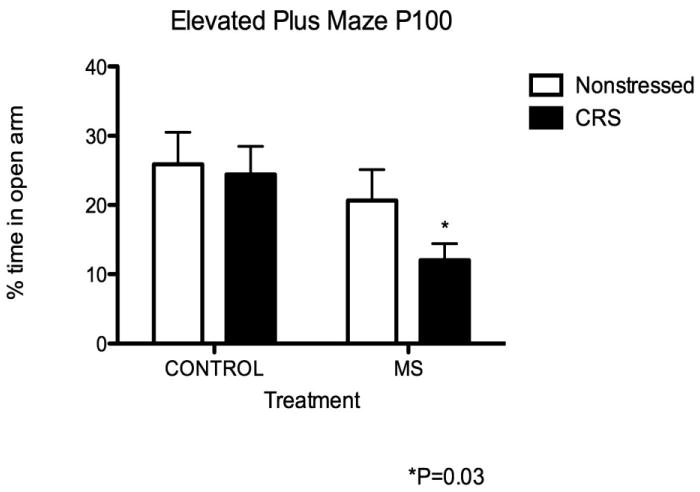
There was a significant effect of rearing on the percent time spent in the open arms of the EPM. MS-CRS spent significantly less time in the open arms than all other groups. NMS rats spent 25.98 6 4.64%, NMS-CRS 24.44 6 4.05%, MS 22.38 6 4.58%, and MS-CRS 12.03 6 2.40%. Two-way ANOVA rearing effect F1,42 5 4.83, *P 5 0.03, n 5 11–14 for all groups. From Eiland and McEwen, 2011.
8. Interactions among brain regions
Although there has been a major emphasis on brain regions such as the hippocampus, amygdala and, more recently, the prefrontal cortex, that have led to many new insights into brain involvement in stress and adaptation to stress, as well as pathophysiology, it is important to further understand the network of interactions in the whole brain. The prefrontal cortex, amygdala and hippocampus are interconnected and influence each other via direct and indirect neural activity (Petrovich et al 2001) (Ghashghaei & Barbas 2002; McDonald 1987; McDonald et al 1996), working together with the nucleus accumbens (Muschamp et al 2011) with regard to fear and hedonic behavior and with the periaqueductal gray region of the midbrain with respect to autonomic regulation, pain sensitivity and anxiety (Huber et al 2005) (Panksepp et al 1997; Panksepp & Watt 2011). For example, the periaqueductal gray is a target of anxiolytic actions of benzodiazepines and works with the amygdala in mediating fear and anxiety (Graeff et al 1993). Moreover, inactivation of the amygdala blocks stress-induced impairment of hippocampal LTP and spatial memory (Kim et al 2005) and stimulation of basolateral amygdala enhances dentate gyrus field potentials (Ikegaya et al 1995), while stimulation of medial prefrontal cortex decreases responsiveness of central amygdala output neurons (Quirk et al 2003). The processing of emotional memories with contextual information requires amygdala – hippocampal interactions (Phillips & LeDoux 1992; Richardson et al 2004), whereas the prefrontal cortex, with its powerful influence on amygdala activity (Quirk et al 2003), plays an important role in fear extinction (Milad & Quirk 2002; Morgan & LeDoux 1995). Because of these interactions, future studies need to address their possible role in the morphological and functional changes produced by single and repeated stress.
9. Conclusion: stress and brain plasticity over the life course
The brain responds to experiences with structural, as well as functional plasticity, and hormones play a role, along with neurotransmitters and other mediators via a complex collaborative network. Multiple brain regions are involved, including the amygdala, hippocampus, prefrontal cortex and nucleus accumbens; this list of brain regions needs to be expanded to include other parts of the brain, such as the periaqueductal gray matter and other components of the “separation distress circuit” (Northoff & Panksepp 2008).
Mediators of the plastic changes in the brain include glucocorticoids, excitatory and inhibitory neurotransmitters, neurotrophic factors and inter-cellular signaling molecules, including endocannabinoids. These mediators operate in a non-linear network in which there are biphasic actions of each mediator system and reciprocal interactions that have self-limiting (homeostatic) features that are also subject to disruption that may lead to pathophysiology (McEwen 1998; 2006).
Stressful experiences early in life can have long-lasting effects on brain development and the capacity to respond to later stressful challenges. Because of the central role of the brain in systemic physiology through its regulation of neuroendocrine, autonomic, metabolic and immune system function, adverse experiences in early life can have far-reaching effects for adult systemic and behavioral pathophysiology (Anda et al 2010). Individual differences that are evident in human beings and also in relatively homogeneous rodent populations in the laboratory (Cavigelli & McClintock 2003; Cavigelli et al 2006) are manifestations of early life experiences and genetic and epigenetic factors that are just beginning to be recognized.
These individual differences, along with the capacity of the brain for structural and functional plasticity, argue for a different way of understanding and using pharmacological agents in treatment of disorders as diverse as depression and stroke, namely, where necessary, to enhance the capacity for plasticity in combination with individualized therapies that recognize each individual's unique needs (Kirkengen 2010), including the differences between men and women (McEwen & Lasley 2005).
Highlights.
Structural plasticity in the hippocampus, amygdala and prefrontal cortex of adult rodents.
Role of glucocorticoids, excitatory amino acids and brain derived neurotrophic factor.
How histone modifications change with chronicity of stress in the dentate gyrus.
Individual differences in anxiety in rodent populations in the laboratory.
Maternal separation alters hippocampal development and exacerbates stress-induced anxiety.
Acknowledgments
Supported by research grants R01 MH41256 and P50 MH58911 to BMc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Aisa B, Elizalde N, Tordera R, Lasheras B, Del Rio J, Ramirez MJ. Effects of neonatal stress on markers of synaptic plasticity in the hippocampus: implications for spatial memory. Hippocampus. 2009;19:1222–31. doi: 10.1002/hipo.20586. [DOI] [PubMed] [Google Scholar]
- Aisa B, Tordera R, Lasheras B, Del Rio J, Ramirez MJ. Cognitive impairment associated to HPA axis hyperactivity after maternal separation in rats. Psychoneuroendocrinology. 2007;32:256–66. doi: 10.1016/j.psyneuen.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Akers KG, Yang Z, DelVecchio DP, Reeb BC, Romeo RD, et al. Social competitiveness and plasticity of neuroendocrine function in old age: influence of neonatal novelty exposure and maternal care reliability. PLoS ONE. 2008;3(7):e2840. doi: 10.1371/journal.pone.0002840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J, Das GD. Post-natal origin of microneurones in the rat brain. Nature. 1965;207:953–6. doi: 10.1038/207953a0. [DOI] [PubMed] [Google Scholar]
- Anda RF, Butchart A, Felitti VJ, Brown DW. Building a framework for global surveillance of the public health implications of adverse childhood experiences. Am J Prev Med. 2010;39:93–8. doi: 10.1016/j.amepre.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Antoniou K, Papathanasiou G, Papalexi E, Hyphantis T, Nomikos GG, et al. Individual responses to novelty are associated with differences in behavioral and neurochemical profiles. Behav Brain Res. 2008;187:462–72. doi: 10.1016/j.bbr.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Arendt T, Stieler J, Strijkstra AM, Hut RA, Rudiger J, et al. Reversible paired helical filament-like phosphorylation of tau is an adaptive process associated with neuronal plasticity in hibernating animals. J Neurosci. 2003;23:6972–81. doi: 10.1523/JNEUROSCI.23-18-06972.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autry AE, Grillo CA, Piroli GG, Rothstein JD, McEwen BS, Reagan LP. Glucocorticoid regulation of GLT-1 glutamate transporter isoform expression in the rat hippocampus. Neuroendocrinology. 2006;83:1–9. doi: 10.1159/000096092. [DOI] [PubMed] [Google Scholar]
- Bush DE, Sotres-Bayon F, LeDoux JE. Individual differences in fear: isolating fear reactivity and fear recovery phenotypes. J Trauma Stress. 2007;20:413–22. doi: 10.1002/jts.20261. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994;61:203–9. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RDG. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–17. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Cannizzaro C, Plescia F, Gagliano M, Cannizzaro G, Provenzano G, et al. Effects of pre-and postnatal exposure to 5-methoxytryptamine and early handling on an object-place association learning task in adolescent rat offspring. Neurosci Res. 2007;59:74–80. doi: 10.1016/j.neures.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Castren E, Rantamaki T. The role of BDNF and its receptors in depression and antidepressant drug action: Reactivation of developmental plasticity. Dev Neurobiol. 2010;70:289–97. doi: 10.1002/dneu.20758. [DOI] [PubMed] [Google Scholar]
- Cavigelli SA, McClintock MK. Fear of novelty in infant rats predicts adult corticosterone dynamics and an early death. Proc Natl Acad Sci USA. 2003;100:16131–6. doi: 10.1073/pnas.2535721100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavigelli SA, Yee JR, McClintock MK. Infant temperament predicts life span in female rats that develop spontaneous tumors. Horm Behav. 2006;50:454–62. doi: 10.1016/j.yhbeh.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Chollet F, Tardy J, Albucher JF, Thalamas C, Berard E, et al. Fluoxetine for motor recovery after acute ischaemic stroke (FLAME): a randomised placebo-controlled trial. Lancet Neurol. 2011;10:123–30. doi: 10.1016/S1474-4422(10)70314-8. [DOI] [PubMed] [Google Scholar]
- Collins A, Hill LE, Chandramohan Y, Whitcomb D, Droste SK, Reul JM. Exercise improves cognitive responses to psychological stress through enhancement of epigenetic mechanisms and gene expression in the dentate gyrus. PLoS One. 2009;4:e4330. doi: 10.1371/journal.pone.0004330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD. What is the functional significance of chronic stress-induced CA3 dendritic retraction within the hippocampus? Behav & Cogn Neurosci Rev. 2006;5:41–60. doi: 10.1177/1534582306289043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD. Chronic stress-induced hippocampal vulnerability: The glucocorticoid vulnerability hypothesis. Rev Neurosci. 2008;19:395–411. doi: 10.1515/revneuro.2008.19.6.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, Magarinos AM, LeDoux JE, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci. 1999;113:902–13. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- Corodimas KP, LeDoux JE, Gold PW, Schulkin J. Corticosterone potentiation of learned fear. Annals of the New York Acad Sci. 1994;746:392. doi: 10.1111/j.1749-6632.1994.tb39264.x. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Gorny G, Li Y, Kolb B, Robinson TE. Opposite effects of amphetamine self-administration experience on dendritic spines in the medial and orbital prefrontal cortex. Cerebral Cortex. 2005;15:341–8. doi: 10.1093/cercor/bhh136. [DOI] [PubMed] [Google Scholar]
- de Lange FP, Koers A, Kalkman JS, Bleijenberg G, Hagoort P, et al. Increase in prefrontal cortical volume following cognitive behavioural therapy in patients with chronic fatigue syndrome. Brain. 2008;131:2172–80. doi: 10.1093/brain/awn140. [DOI] [PubMed] [Google Scholar]
- Dellu F, Piazza PV, Mayo W, Le Moal M, Simon H. Novelty-seeking in rats--biobehavioral characteristics and possible relationship with the sensation-seeking trait in man. Neuropsychobiology. 1996;34:136–45. doi: 10.1159/000119305. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Bennett MC, Fleshner M, Rose GM. Inverted-U relationship between the level of peripheral corticosterone and the magnitude of hippocampal primed burst potentiation. Hippocampus. 1992;2:421–30. doi: 10.1002/hipo.450020409. [DOI] [PubMed] [Google Scholar]
- Du J, Wang Y, Hunter R, Wei Y, Blumenthal R, et al. Dynamic regulation of mitochondrial function by glucocorticoids. Proc Natl Acad Sci USA. 2009;106:3543–8. doi: 10.1073/pnas.0812671106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiat. 2006;59:1116–27. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Eiland L, McEwen BS. Early life stress followed by subsequent adult chronic stress potentiates anxiety and blunts hippocampal structural remodeling. Hippocampus. doi: 10.1002/hipo.20862. in press. [DOI] [PubMed] [Google Scholar]
- Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286:1155–8. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- Gardner KE, Allis CD, Strahl BD. Operating on chromatin, a colorful language where context matters. J Mol Biol. 2011;409:36–46. doi: 10.1016/j.jmb.2011.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghashghaei HT, Barbas H. Pathways for emotion: Interactions of prefrontal and anterior temporal pathways in the amygdala of the Rhesus monkey. Neuroscience. 2002;115:1261–79. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley C, McEwen BS. Short-term glucocorticoid manipulations affect neuronal morphology and survival in the adult dentate gyrus. Neuroscience. 1990;37:367–75. doi: 10.1016/0306-4522(90)90407-u. [DOI] [PubMed] [Google Scholar]
- Govindarajan A, Rao BSS, Nair D, Trinh M, Mawjee N, et al. Transgenic brain-derived neurotrophic factor expression causes both anxiogenic and antidepressant effects. Proc Natl Acad Sci USA. 2006;103:13208–13. doi: 10.1073/pnas.0605180103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeff FG, Silveira MC, Nogueira RL, Audi EA, Oliveira RM. Role of the amygdala and periaqueductal gray in anxiety and panic. Behav Brain Res. 1993;58:123–31. doi: 10.1016/0166-4328(93)90097-a. [DOI] [PubMed] [Google Scholar]
- Grillo CA, Piroli GG, Wood GE, Reznikov LR, McEwen BS, Reagan LP. Immunocytochemical analysis of synaptic proteins provides new insights into diabetes-mediated plasticity in the rat hippocampus. Neuroscience. 2005;136:477–86. doi: 10.1016/j.neuroscience.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Hansson AC, Sommer WH, Metsis M, Stromberg I, Agnati LF, Fuxe K. Corticosterone actions on the hippocampal brain-derived neurotrophic factor expression are mediated by Exon IV promoter. J Neuroendocrin. 2006;18:104–14. doi: 10.1111/j.1365-2826.2005.01390.x. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Heinrich C, Lahteinen S, Suzuki F, Anne-Marie L, Huber S, et al. Increase in BDNF-mediated TrkB signaling promotes epileptogenesis in a mouse model of mesial temporal lobe epilepsy. Neurobiol Dis. 2011;42:35–47. doi: 10.1016/j.nbd.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Herrero AI, Sandi C, Venero C. Individual differences in anxiety trait are related to spatial learning abilities and hippocampal expression of mineralocorticoid receptors. Neurobiol Learn Mem. 2006;86:150–9. doi: 10.1016/j.nlm.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Hill MN, Karatsoreos IN, Hillard CJ, McEwen BS. Rapid elevations in limbic endocannabinoid content by glucocorticoid hormones in vivo. Psychoneuroendocrinology. 2010a;35:1333–8. doi: 10.1016/j.psyneuen.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, McEwen BS. Involvement of the endocannabinoid system in the neurobehavioural effects of stress and glucocorticoids. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:791–7. doi: 10.1016/j.pnpbp.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, McLaughlin RJ, Bingham B, Shrestha L, Lee TT, et al. Endogenous cannabinoid signaling is essential for stress adaptation. Proc Natl Acad Sci U S A. 2010b;107:9406–11. doi: 10.1073/pnas.0914661107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzel BK, Carmody J, Evans KC, Hoge EA, Dusek JA, et al. Stress reduction correlates with structural changes in the amygdala. Soc Cogn Affect Neurosci. 2009 doi: 10.1093/scan/nsp034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horch HW, Katz LC. BDNF release from single cells elicits local dendritic growth in nearby neurons. Nature Neuroscience. 2002;5:1177–84. doi: 10.1038/nn927. [DOI] [PubMed] [Google Scholar]
- Horch HW, Kruttgen A, Portbury SD, Katz LC. Destabilization of cortical dendrites and spines by BDNF. Neuron. 1999;23:353–64. doi: 10.1016/s0896-6273(00)80785-0. [DOI] [PubMed] [Google Scholar]
- Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308:245–8. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- Hunter RG, Bellani R, Bloss E, Costa A, McCarthy K, McEwen BS. Regulation of kainate receptor subunit mRNA by stress and corticosteroids in the rat hippocampus. PLoS ONE. 2009a;4:e4328. doi: 10.1371/journal.pone.0004328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter RG, Bellani R, Bloss E, Costa A, Romeo RD, McEwen BS. Regulation of CART mRNA by stress and corticosteroids in the hippocampus and amygdala. Brain Res. 2007;1152:234–40. doi: 10.1016/j.brainres.2007.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter RG, McCarthy KJ, Milne TA, Pfaff DW, McEwen BS. Regulation of hippocampal H3 histone methylation by acute and chronic stress. Proc Natl Acad Sci USA. 2009b;106:20912–7. doi: 10.1073/pnas.0911143106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huot RL, Plotsky PM, Lenox RH, McNamara RK. Neonatal maternal separation reduces hippocampal mossy fiber density in adult Long Evans rats. Brain Res. 2002;950:52–63. doi: 10.1016/s0006-8993(02)02985-2. [DOI] [PubMed] [Google Scholar]
- Ikegaya Y, Abe K, Saito H, Nishiyama N. Medial amygdala enhances synaptic transmission and synaptic plasticity in the dentate gyrus of rats in vivo. J Neurophysiology. 1995;74:2201–3. doi: 10.1152/jn.1995.74.5.2201. [DOI] [PubMed] [Google Scholar]
- Isgor C, Kabbaj M, Akil H, Watson SJ. Delayed effects of chronic variable stress during peripubertal-juvenile period on hippocampal morphology and on cognitive and stress axis functions in rats. Hippocampus. 2004;14:636–48. doi: 10.1002/hipo.10207. [DOI] [PubMed] [Google Scholar]
- Jeanneteau F, Garabedian MJ, Chao MV. Activation of Trk neurotrophin receptors by glucocorticoids provides a neuroprotective effect. Proc Natl Acad Sci USA. 2008;105:4862–7. doi: 10.1073/pnas.0709102105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Langley B, Lubin FD, Renthal W, Wood MA, et al. Epigenetics in the nervous system. J Neurosci. 2008;28:11753–9. doi: 10.1523/JNEUROSCI.3797-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joels M. Corticosteroid effects in the brain: U-shape it. Trends Pharmacol Sci. 2006;27:244–50. doi: 10.1016/j.tips.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Joels M, Pu Z, Wiegert O, Oitzl MS, Krugers HJ. Learning under stress: how does it work? Trends Cogn Sci. 2006;10:152–8. doi: 10.1016/j.tics.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Kabbaj M, Devine DP, Savage VR, Akil H. Neurobiological correlates of individual differences in novelty-seeking behavior in the rat: Differential expression of stress-related molecules. J Neurosci. 2000a;20:6983–8. doi: 10.1523/JNEUROSCI.20-18-06983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabbaj M, Devine DP, Savage VR, Akil H. Neurobiological correlates of individual differences in novelty-seeking behavior in the rat: differential expression of stress-related molecules. J Neurosci. 2000b;20:6983–8. doi: 10.1523/JNEUROSCI.20-18-06983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch R, Salome N, Platzer S, Wigger A, Czisch M, et al. High trait anxiety and hyporeactivity to stress of the dorsomedial prefrontal cortex: a combined phMRI and Fos study in rats. Neuroimage. 2004;23:382–91. doi: 10.1016/j.neuroimage.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Kaplan MS, Bell DH. Mitotic neuroblasts in the 9 day old and 11 month old rodent hippocampus. J Neurosci. 1984;4:1429–41. doi: 10.1523/JNEUROSCI.04-06-01429.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karst H, Berger S, Turiault M, Tronche F, Schutz G, Joels M. Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc Natl Acad Sci USA. 2005;102:19204–7. doi: 10.1073/pnas.0507572102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Gage FH. New nerve cells for the adult brain. Sci Am. 1999;280:48–53. doi: 10.1038/scientificamerican0599-48. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Koo JW, Lee HJ, Han JS. Amygdalar inactivation blocks stress-induced impairments in hippocampal long-term potentiation and spatial memory. J Neurosci. 2005;25:1532–9. doi: 10.1523/JNEUROSCI.4623-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkengen AL. The Lived Experience of Violation: How Abused Children Become Unhealthy Adults. Bucharest, Romania: Zeta Books; 2010. [Google Scholar]
- Kubicek S, Jenuwein T. A crack in histone lysine methylation. Cell. 2004;119:903–6. doi: 10.1016/j.cell.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Kuroda Y, McEwen BS. Effect of chronic restraint stress and tianeptine on growth factors, GAP-43 and MAP2 mRNA expression in the rat hippocampus. Mol Brain Res. 1998;59:35–9. doi: 10.1016/s0169-328x(98)00130-2. [DOI] [PubMed] [Google Scholar]
- Lippmann M, Bress A, Nemeroff CB, Plotsky PM, Monteggia LM. Long-term behavioural and molecular alterations associated with maternal separation in rats. Eur J Neurosci. 2007;25:3091–8. doi: 10.1111/j.1460-9568.2007.05522.x. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Otmakhova NA. Storage, recall, and novelty detection of sequences by the hippocampus: Elaborating on the SOCRATIC model to account for normal and aberrant effects of dopamine. Hippocampus. 2001;11:551–68. doi: 10.1002/hipo.1071. [DOI] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–4. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccari S, Morley-Fletcher S. Effects of prenatal restraint stress on the hypothalamus-pituitary-adrenal axis and related behavioural and neurobiological alterations. Psychoneuroendo. 2007;32:S10–S5. doi: 10.1016/j.psyneuen.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, Deslandes A, McEwen BS. Effects of antidepressants and benzodiazepine treatments on the dendritic structure of CA3 pyramidal neurons after chronic stress. Eur J Pharm. 1999;371:113–22. doi: 10.1016/s0014-2999(99)00163-6. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, Li CJ, Gal Toth J, Bath KG, Jing D, et al. Effect of brain-derived neurotrophic factor haploinsufficiency on stress-induced remodeling of hippocampal neurons. Hippocampus. 2011;21:253–64. doi: 10.1002/hipo.20744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69:89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS, Saboureau M, Pevet P. Rapid and reversible changes in intrahippocampal connectivity during the course of hibernation in European hamsters. Proc Natl Acad Sci USA. 2006;103:18775–80. doi: 10.1073/pnas.0608785103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magarinos AM, Verdugo Garcia JM, McEwen BS. Chronic restraint stress alters synaptic terminal structure in hippocampus. Proc Natl Acad Sci USA. 1997;94:14002–8. doi: 10.1073/pnas.94.25.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S, Schulkin J, Smith M, Pacak K, Palkovits M, Gold P. Regulation of Corticotropin-Releasing Hormone Receptor Messenger Ribonucleic Acid in the Rat Brain and Pituitary by Glucocorticoids and Stress. Endocrinology. 1995;136:4517–25. doi: 10.1210/endo.136.10.7664672. [DOI] [PubMed] [Google Scholar]
- Margineanu DG, Gower AJ, Gobert J, Wulfert E. Long-term Adrenalectomy Reduces Hippocampal Granule Cell Excitability In Vivo. Brain Res Bull. 1994;33:93–8. doi: 10.1016/0361-9230(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Marmigere F, Givalois L, Rage F, Arancibia S, Tapia-Arancibia L. Rapid induction of BDNF expression in the hippocampus during immobilization stress challenge in adult rats. Hippocampus. 2003;13:646–55. doi: 10.1002/hipo.10109. [DOI] [PubMed] [Google Scholar]
- Martin KP, Wellman CL. NMDA Receptor Blockade Alters Stress-Induced Dendritic Remodeling in Medial Prefrontal Cortex. Cereb Cortex. 2011 doi: 10.1093/cercor/bhr021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maze I, Covington HE, 3rd, Dietz DM, LaPlant Q, Renthal W, et al. Essential role of the histone methyltransferase G9a in cocaine-induced plasticity. Science. 2010;327:213–6. doi: 10.1126/science.1179438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ. Organization of amygdaloid projections to the mediodorsal thalamus and prefrontal cortex: a fluorescence retrograde transport study in the rat. J Comp Neurol. 1987;262:46–58. doi: 10.1002/cne.902620105. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Guo L. Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience. 1996;71:55–75. doi: 10.1016/0306-4522(95)00417-3. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and Damaging Effects of Stress Mediators. New England J Med. 1998;338:171–9. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–22. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators: central role of the brain. Dial in Clin Neurosci: Stress. 2006;8:367–81. doi: 10.31887/DCNS.2006.8.4/bmcewen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress, sex, and neural adaptation to a changing environment: mechanisms of neuronal remodeling. Ann N Y Acad Sci. 2010;1204(Suppl):E38–59. doi: 10.1111/j.1749-6632.2010.05568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Chattarji S. Molecular mechanisms of neuroplasticity and pharmacological implications: the example of tianeptine. Eur Neuropsychopharm. 2004;14:S497–S502. doi: 10.1016/j.euroneuro.2004.09.008. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Lasley EN. Cerebrum The Dana Forum on Brain Science. Dana Press; 2005. The End of Sex as We Know It. [Google Scholar]
- McEwen BS, Plapinger L. Association of corticosterone-1,2 3H with macromolecules extracted from brain cell nuclei. Nature. 1970;226:263–4. doi: 10.1038/226263a0. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Weiss J, Schwartz L. Selective retention of corticosterone by limbic structures in rat brain. Nature. 1968;220:911–2. doi: 10.1038/220911a0. [DOI] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D'Alessio AC, Dymov S, Labonte B, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12:342–8. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M. Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues Clin Neurosci. 2005;7:103–23. doi: 10.31887/DCNS.2005.7.2/mmeaney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–4. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Miller CA, Sweatt JD. Covalent modification of DNA regulates memory formation. Neuron. 2007;53:857–69. doi: 10.1016/j.neuron.2007.02.022. [DOI] [PubMed] [Google Scholar]
- Miracle AD, Brace MF, Huyck KD, Singler SA, Wellman CL. Chronic stress impairs recall of extinction of conditioned fear. Neurobiol Learning & Memory. 2006;85:213–8. doi: 10.1016/j.nlm.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc Nat Acad Sci USA. 2005;102:9371–6. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R, Sapolsky RM. Acute corticosterone treatment is sufficient to induce anxiety and amygdaloid dendritic hypertrophy. Proc Natl Acad Sci USA. 2008;105:5573–8. doi: 10.1073/pnas.0705615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MA, LeDoux JE. Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav Neurosci. 1995;109:681–8. doi: 10.1037//0735-7044.109.4.681. [DOI] [PubMed] [Google Scholar]
- Muigg P, Hetzenauer A, Hauer G, Hauschild M, Gaburro S, et al. Impaired extinction of learned fear in rats selectively bred for high anxiety--evidence of altered neuronal processing in prefrontal-amygdala pathways. Eur J Neurosci. 2008;28:2299–309. doi: 10.1111/j.1460-9568.2008.06511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munhoz CD, Sorrells SF, Caso JR, Scavone C, Sapolsky RM. Glucocorticoids exacerbate lipopolysaccharide-induced signaling in the frontal cortex and hippocampus in a dose-dependent manner. J Neurosci. 2010;30:13690–8. doi: 10.1523/JNEUROSCI.0303-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschamp JW, Van't Veer A, Parsegian A, Gallo MS, Chen M, et al. Activation of CREB in the Nucleus Accumbens Shell Produces Anhedonia and Resistance to Extinction of Fear in Rats. J Neurosci. 2011;31:3095–103. doi: 10.1523/JNEUROSCI.5973-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Panksepp J. The trans-species concept of self and the subcortical-cortical midline system. Cell. 2008;12:259–64. doi: 10.1016/j.tics.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Numakawa T, Kumamaru E, Adachi N, Yagasaki Y, Izumi A, Kunugi H. Glucocorticoid receptor interaction with TrkB promotes BDNF-triggered PLC-g signaling for glutamate release via a glutamate transporter. Proc Natl Acad Sci USA. 2009;106:647–52. doi: 10.1073/pnas.0800888106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda S, Roozendaal B, McGaugh JL. Glucocorticoid effects on object recognition memory require training-associated emotional arousal. Proc Natl Acad Sci USA. 2004;101:853–8. doi: 10.1073/pnas.0307803100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp J, Nelson E, Bekkedal M. Brain systems for the mediation of social separation-distress and social-reward. Evolutionary antecedents and neuropeptide intermediaries. Ann N Y Acad Sci. 1997;807:78–100. doi: 10.1111/j.1749-6632.1997.tb51914.x. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Watt D. Why Does Depression Hurt? Ancestral Primary-Process Separation-Distress (PANIC/GRIEF) and Diminished Brain Reward (SEEKING) Processes in the Genesis of Depressive Affect. Psychiatry. 2011;74:5–13. doi: 10.1521/psyc.2011.74.1.5. [DOI] [PubMed] [Google Scholar]
- Pavlides C, Kimura A, Magarinos AM, McEwen BS. Type I adrenal steroid receptors prolong hippocampal long-term potentiation. NeuroReport. 1994;5:2673–7. doi: 10.1097/00001756-199412000-00067. [DOI] [PubMed] [Google Scholar]
- Pavlides C, Nivon LG, McEwen BS. Effects of chronic stress on hippocampal long-term potentiation. Hippocampus. 2002;12:245–57. doi: 10.1002/hipo.1116. [DOI] [PubMed] [Google Scholar]
- Pavlides C, Watanabe Y, Magarinos AM, McEwen BS. Opposing role of adrenal steroid Type I and Type II receptors in hippocampal long-term potentiation. Neuroscience. 1995;68:387–94. doi: 10.1016/0306-4522(95)00151-8. [DOI] [PubMed] [Google Scholar]
- Pawlak CR, Ho YJ, Schwarting RK, Bauhofer A. Relationship between striatal levels of interleukin-2 mRNA and plus-maze behaviour in the rat. Neurosci Lett. 2003;341:205–8. doi: 10.1016/s0304-3940(03)00184-8. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Canteras NS, Swanson LW. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res Rev. 2001;38:247–89. doi: 10.1016/s0165-0173(01)00080-7. [DOI] [PubMed] [Google Scholar]
- Pham K, Nacher J, Hof PR, McEwen BS. Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur J Neurosci. 2003;17:879–86. doi: 10.1046/j.1460-9568.2003.02513.x. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–85. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Rouge-Pont F, Deminiere JM, Kharoubi M, Le Moal M, Simon H. Dopaminergic activity is reduced in the prefrontal cortex and increased in the nucleus accumbens of rats predisposed to develop amphetamine self-administration. Brain Res. 1991;567:169–74. doi: 10.1016/0006-8993(91)91452-7. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- Popov VI, Bocharova LS. Hibernation-induced structural changes in synaptic contacts between mossy fibres and hippocampal pyramidal neurons. Neuroscience. 1992;48:53–62. doi: 10.1016/0306-4522(92)90337-2. [DOI] [PubMed] [Google Scholar]
- Pugh CR, Tremblay D, Fleshner M, Rudy JW. A selective role for corticosterone in contextual-fear conditioning. Behav Neurosci. 1997;111:503–11. [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23:8800–7. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagan LP, Rosell DR, Wood GE, Spedding M, Munoz C, et al. Chronic restraint stress up-regulates GLT-1 mRNA and protein expression in the rat hippocampus: Reversal by tianeptine. Proc Natl Acad Sci USA. 2004;101:2179–84. doi: 10.1073/pnas.0307294101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reul JM, Hesketh SA, Collins A, Mecinas MG. Epigenetic mechanisms in the dentate gyrus act as a molecular switch in hippocampus-associated memory formation. Epigenetics. 2009;4:434–9. doi: 10.4161/epi.4.7.9806. [DOI] [PubMed] [Google Scholar]
- Richardson MP, Strange BA, Dolan RJ. Encoding of emotional memories depends on amygdala and hippocampus and their interactions. Nature Neurosci. 2004;7:278–85. doi: 10.1038/nn1190. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J Neurosci. 1997;17:8491–7. doi: 10.1523/JNEUROSCI.17-21-08491.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roosevelt TS, Ruhmann-Wennhold A, Nelson DH. Adrenal corticosteroid effects upon rat brain mitochondrial metabolism. Endocrinology. 1973;53:619–25. doi: 10.1210/endo-93-3-619. [DOI] [PubMed] [Google Scholar]
- Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol Psychiatry. 2009;65:760–9. doi: 10.1016/j.biopsych.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouge-Pont F, Deroche V, Le Moal M, Piazza PV. Individual differences in stress-induced dopamine release in the nucleus accumbens are influenced by corticosterone. Eur J Neurosci. 1998;10:3903–7. doi: 10.1046/j.1460-9568.1998.00438.x. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Young LJ, Plotsky PM, Insel TR. Distribution of corticosteroid receptors in the rhesus brain: Relative absence of glucocorticoid receptors in the hippocampal formation. J Neurosci. 2000;20:4657–68. doi: 10.1523/JNEUROSCI.20-12-04657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Ge H, Ren K, Pena de Ortiz S, Quirk GJ. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J Neurosci. 2004;24:5704–10. doi: 10.1523/JNEUROSCI.0786-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky R. Stress, the Aging Brain and the Mechanisms of Neuron Death. Vol. 1. Cambridge MIT Press; 1992. p. 423. [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Schwarting RK, Thiel CM, Muller CP, Huston JP. Relationship between anxiety and serotonin in the ventral striatum. Neuroreport. 1998;9:1025–9. doi: 10.1097/00001756-199804200-00013. [DOI] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21:7153–60. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Makino S, Kvetnansky R, Post RM. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci. 1995;15:1768–77. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa N, Lukoyanov NV, Madeira MD, Almeida OFX, Paula-Barbosa MM. Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience. 2000;97:253–66. doi: 10.1016/s0306-4522(00)00050-6. [DOI] [PubMed] [Google Scholar]
- Szyf M, Weaver ICG, Champagne FA, Diorio J, Meaney MJ. Maternal programming of steroid receptor expression and phenotype through DNA methylation in the rat. Front Neuroendocrin. 2005;26:139–62. doi: 10.1016/j.yfrne.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Tang AC, Akers KG, Reeb BC, Romeo RD, McEwen BS. Programming social, cognitive, and neuroendocrine development by early exposure to novelty. Proc Natl Acad Sci USA. 2006;103:15716–21. doi: 10.1073/pnas.0607374103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasker JG, Di S, Malcher-Lopes R. Minireview: Rapid glucocorticoid signaling via membrane-associated receptors. Endocrinology. 2006;147:5549–56. doi: 10.1210/en.2006-0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uysal N, Ozdemir D, Dayi A, Yalaz G, Baltaci AK, Bediz CS. Effects of maternal deprivation on melatonin production and cognition in adolescent male and female rats. Neuro Endocrinol Lett. 2005;26:555–60. [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nature Neurosci. 1999;2:266–70. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Venero C, Borrell J. Rapid glucocorticoid effects on excitatory amino acid levels in the hippocampus: a microdialysis study in freely moving rats. Eur J Neurosci. 1999;1:2465–73. doi: 10.1046/j.1460-9568.1999.00668.x. [DOI] [PubMed] [Google Scholar]
- Vetencourt JFM, Sale A, Viegi A, Baroncelli L, De Pasquale R, et al. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science. 2008;320:385–8. doi: 10.1126/science.1150516. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Rao BSS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–8. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakschlak A, Weinstock M. Neonatal handling reverses behavioral abnormalities induced in rats by prenatal stress. Physiol Behav. 1990;48:289–92. doi: 10.1016/0031-9384(90)90315-u. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–54. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Wood GE, Young LT, Reagan LP, Chen B, McEwen BS. Stress-induced structural remodeling in hippocampus: Prevention by lithium treatment. Proc Natl Acad Sci USA. 2004;101:3973–8. doi: 10.1073/pnas.0400208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood GE, Young LT, Reagan LP, McEwen BS. Acute and chronic restraint stress alter the incidence of social conflict in male rats. Horm & Behav. 2003;43:205–13. doi: 10.1016/s0018-506x(02)00026-0. [DOI] [PubMed] [Google Scholar]
- Wood SK, Walker HE, Valentino RJ, Bhatnagar S. Individual differences in reactivity to social stress predict susceptibility and resilience to a depressive phenotype: role of corticotropin-releasing factor. Endocrinology. 2010;151:1795–805. doi: 10.1210/en.2009-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]


