Abstract
Botulism is a disease characterized by neuromuscular paralysis and is produced from botulinum neurotoxins (BoNTs) found within the Gram positive bacterium Clostridium botulinum. This bacteria produces the most deadliest toxin known, with lethal doses as low as 1 ng/kg. Due to the relative ease of production and transport, the use of these agents as potential bioterrorist weapons has become of utmost concern. No small molecule therapies against BoNT intoxication have been approved to date. However, 3,4-diaminopyridine, (3,4-DAP), a potent reversible inhibitor of voltage-gated potassium channels, is an effective cholinergic agonist used in the treatment of neuromuscular degenerative disorders that require cholinergic enhancement. 3,4-DAP has also been shown to facilitate recovery of neuromuscular action potential post botulinum intoxication by blocking K+ channels. Unfortunately, 3,4-DAP displays toxicity largely due to blood-brain-barrier (BBB) penetration. As a dual-action prodrug approach to cholinergic enhancement we have designed carbamate and amide conjugates of 3,4-DAP. The carbamate prodrug is intended to be a slowly reversible inhibitor of acetylcholinesterase (AChE) along the lines of the stigmines thereby allowing increased persistence of released acetylcholine within the synaptic cleft. As a secondary activity, cleavage of the carbamate prodrug by AChE will afford the localized release of 3,4-DAP, which in turn, will enhance the pre-synaptic release of additional acetylcholine. Being a competitive inhibitor with respect to acetylcholine, the activity of the prodrug will be greatest at the synaptic junctions most depleted of acetylcholine. Here we report upon the synthesis and biochemical characterization of three new classes of prodrugs intended to limit previously reported stability and toxicity issues. Of the prodrugs examined, compound 32, demonstrated the most clinically relevant half-life of 2.76 h, while selectively inhibiting AChE over butyrylcholinesterase – a plasma-based high activity esterase. Future in vivo studies could provide validation of prodrug 32 as a potential treatment against BoNT intoxication as well as other neuromuscular disorders.
Keywords: Botulinum neurotoxins; Acetylcholinesterase inhibitors; 3,4-DAP; prodrug
1. Introduction
Botulinum neurotoxins (BoNTs) produced from the Gram positive bacterium Clostridium botulinum are some of the deadliest toxins known to man1 with lethal doses as low as 1 ng per kg body weight being 10 million times more toxic than cyanide.2 Intoxication by BoNT produces generalized muscle weakness, and in severe cases flaccid paralysis leading to impaired respiration and autonomic function. In many cases rapid intubation and mechanical respiration are required to prevent greater risk and even death.3 BoNT intoxication is primarily caused by the consumption of poisoned canned food products; however, the use of these agents as potential bioterorrist weapons has become of concern. As such, the US Centers for Disease Control (CDC) have classified BoNTs as one of the six highest-risk agents for bioterrorism (Class A).
The induction of neuromuscular paralysis by BoNTs requires three biochemical steps. First, BoNT protein binds to gangliosides on the presynaptic cholinergic nerve terminal through interactions with the heavy chain. These interactions allow subsequent endocytosis into the neuron through several possible mechanisms involving synaptotagmins I and II (BoNT/B and BoNT/G) and SV2 (BoNT/A).4 The toxin is then translocated into the cytosol where its light chain (LC), a metalloprotease, binds to, and cleaves soluble N-ethylmaleimide-sensitive factor attachment protein receptor proteins (SNAREs).5 This action halts the release of acetylcholine (ACh) at the neuromuscular junction, leading to the cessation of neurotransmission.
Currently, the only approved therapies against BoNT intoxication include pre-exposure prophylaxis with a vaccine and post-exposure administration of sera containing anti-BoNT antibodies.6 Upon cellular intoxication, however, it is imperative to provide fast acting neuro-modulatory drugs to recover neurotransmission through ACh release, to at least restore partial muscle function. Thus, a potential small molecule pharmacological treatment could provide many benefits over these antibody-based approaches. Most small molecule research efforts have targeted the metallo-proteolytic properties of the BoNT LC protease, however, no small molecule therapeutics have been approved to date.7 Yet, clinically approved cholinergic enhancing drugs have been implemented to treat similar neurological disorders including Lambert-Eaton myasthenic syndrome (LEMS),8 multiple sclerosis (MS),9 and Alzheimer’s disease.10
Of these classes of drugs, aminopyridines, in particular 3,4-diaminopyridine (3,4-DAP), has shown promise due to its agonistic effect on neurotransmitter release through Kv channel blockade.11 The aminopyridines, 3,4-DAP along with 4-aminopyridine (4-AP) facilitate recovery of neuromuscular action potential post botulism intoxication by reversibly blocking voltage-dependent K+ channels.12 This action promotes Ca2+ influx, driving signal transduction and ACh release at the synapse. The mechanism by which aminopyridines inactivate the Kv channel is unknown; however, using molecular modeling Caballero and colleagues hypothesized that two putative receptor sites found within the tetrameric channel are important in this overall process.13 It has also been hypothesized that once aminopyridines cross the membrane, the molecules become protonated intracellularly, thereby allowing the molecule to interact with receptors within the pore of the channel.
Recently, our laboratory initiated studies toward deciphering the pKa/hydrogen-bonding properties of 3,4-DAP in conjunction with the Kv channel.14 By synthesizing structural analogues, we hoped to discover more potent, yet, less toxic forms of the pharmacophore for treatment against BoNT intoxication. Previous reports indicate that 3,4-DAP and 4-AP, when administered at high doses, demonstrate toxicity issues such as seizures due to the molecules’ ability to penetrate the blood-brain barrier (BBB). As such, the development of new compounds that alleviate BBB penetration without hampering the necessary molecular dynamics between the compound and the Kv channel would provide a path forward for treating BoNT/A intoxication. Exploring this possibility, a series of compounds were synthesized, and 3,4,5-triaminopyridine and 3,4-diamino-1-(prop-2-ynl)pyridinium were found to exhibit therapeutic potential, as characterized by paralysis reversal in mouse phrenic nerve-hemidiaphragm assays post BoNT/A induced paralysis. Yet, a shortcoming with these molecules is that they possessed modest plasma half-lives of 0.31 h and 1.2 h, respectively. Drug candidates with limited plasma lifetimes would require repeated dose regimen or a continuous iv infusion to systemically provide paralytic extinction.
While many research programs have focused on inhibition of the BoNT LC protease itself, therapies directed toward the inhibition of acetylcholinesterase (AChE) post BoNT intoxication remain sparse.15
The stigmines, a class of carbamate-based AChE inhibitors, provide effective treatment of multiple sclerosis,16 myasthenia gravis,17 and Alzheimer’s disease.18 Mechanistically, carbamates produce reversible covalent inhibition via transesterification between the carbamoyl-moiety and the active site serine. Hydrolysis of the serine-carbamate intermediate is considerably slower (seconds to tens of minutes) than that of the acetyl-serine adduct formed during substrate hydrolysis. Clinically approved stigmines such as Rivastigmine (Exelon) are characterized as pseudo-substrate inhibitors of both AChE and serum-based butyrylcholinesterase (BChE), a highly active esterase with largely overlapping substrate specificity that must be avoided for therapeutic efficacy.19 Although obtaining selectivity for AChE over BChE is a challenging task, the success of the stigmines suggests that prodrug-type molecules could be similarly designed with sufficient selectivity towards AChE over BChE.
From the structure activity relationship found within the stigmines, carbamate conjugates of 3,4-DAP could act as potential pseudo-substrate inhibitors of AChE thereby providing the enhanced benefit of selectively unmasking 3,4-DAP proximal to its site of action. Also of note, carbamate inhibitors are competitive with respect to ACh meaning that inhibition of AChE and liberation of 3,4-DAP will occur in those synaptic junctions most depleted (and in need) of ACh. AChE is homeostatically occupied by ACh (Km = 0.4 mM) within the neuromuscular junction.20 Interestingly, studies by Smart and McCammon have found that the deactivation of AChE increases ACh lifetime in the synaptic cleft from 200 to 900 µs.21 Acetyl choline depletion could produce up to a 25-fold inhibition selectivity for AChE at BoNT/A intoxicated neuromuscular junctions over undamaged junctions. Therefore, there could be a therapeutically relevant directed inhibition of AChE and 3,4-DAP liberation in those synaptic clefts most depleted of ACh by BoNT intoxication.
Herein, we describe our studies regarding the design, synthesis and in vitro study of 3,4-DAP-based dual-acting prodrugs against BoNT intoxication.
1. Materials and Methods
2.1 Materials
Acetylthiocholine iodide (AChI), S-Butyrylthiocholine iodide (BChI), 5,5’-Dithiobis(2-nitrobenzoic acid) (DTNB), and sera from clotted mouse blood were purchased from Sigma Aldrich (St. Louis, MO). 100 mM stocks of AChI, BChI, and DTNB were prepared in 100 mM sodium phosphate pH 8.0. All other chemicals used were of analytical grade and highest chromatographic purity available. AChE purified from Electrophorus electricus (Electric eel) – Type V-S and BChE from equine serum were purchased from Sigma Aldrich and reconstituted in 1% gelatin/100 mM sodium phosphate pH 8.0 at 500 U/mL each.
2.2 Enzyme inhibition assays
The activities of class I, II, and III inhibitors were measured according to the method of Ellman et al.22 using AChI or BChI as the respective substrates for each enzyme. In brief, 100 µM AChI or BChI was added to 100 mM sodium phosphate pH 8.0 containing 360 µM DTNB and a range of prodrug concentrations from 500 µM to 2 µM. To initiate the reaction, 0.5 U of enzyme was added to each sample in a final volume of 200 µl per well in Costar 3904 96 well plates (Corning Inc., Corning, NY). Enzyme rates were measured at 37°C over the linear range using a Spectromax 250 spectrophotometer with 412 nm absorbance readings. (All reagent stocks were prepared in 100 mM sodium phosphate pH 8.0 unless stated otherwise).
In order to determine the kinetic mechanism of inhibition, inhibitors and substrate(s) were run in varying concentrations bracketing their Kis and Kms respectively.
2.3 Enzyme-specific drug release assays
To determine enzyme-mediated drug release, 500 µM of prodrug was incubated in 100 mM sodium phosphate pH 8.0 containing 1.0 U enzyme (100 µL total volume) for 3 h at 37°C. Post incubation, samples were quenched with 17.4 M glacial acetic acid and analyzed by analytical reverse-phase HPLC using a Vydac C18 218TP54 column (Western Analytical Products, Lake Elsinore, CA) monitored at 254 and 320 nm. A linear gradient of 0.5–50% (v/v) of solvent B (0.09% v/v TFA/acetonitrile) in solvent A (0.1% v/v TFA/water) from 5 to 15 min, then 50–90% (v/v) B over 5 min was used for all compounds. Peak area and linear dynamic range used to calculate 3,4-DAP was by use of authentic standards. Prodrugs demonstrating greater 3,4-DAP release by AChE over BChE were further analyzed for half-life in mouse sera.
2.4 Sera half-life assays
15 µl of a 10 mM prodrug stock was added to 85 µL sera, pre-warmed to 37°C. Samples were then incubated at the following fixed time intervals 0, 5, 10, 20, 40, 60, and 120 min. Upon extraction with 300 µL 0 °C acetonitrile, precipitated proteins were removed by centrifugation (20,817 rcf) and the liquid phase was subsequently analyzed by analytical reverse-phase HPLC as described above. Stability of prodrug was determined based on presence of breakdown product 3,4-DAP. Half-life values were calculated using the 1-hour endpoint samples.
2.5 Statistical analysis
The prodrug concentration producing the 50% AChE and BChE activity inhibition (IC50), along with inhibition constant Ki values were calculated by non-linear regression analysis using GraphPad Prism v.5.0b software (GraphPad Software Inc., CA).
3. Results and Discussion
3.1 Design of Prodrugs
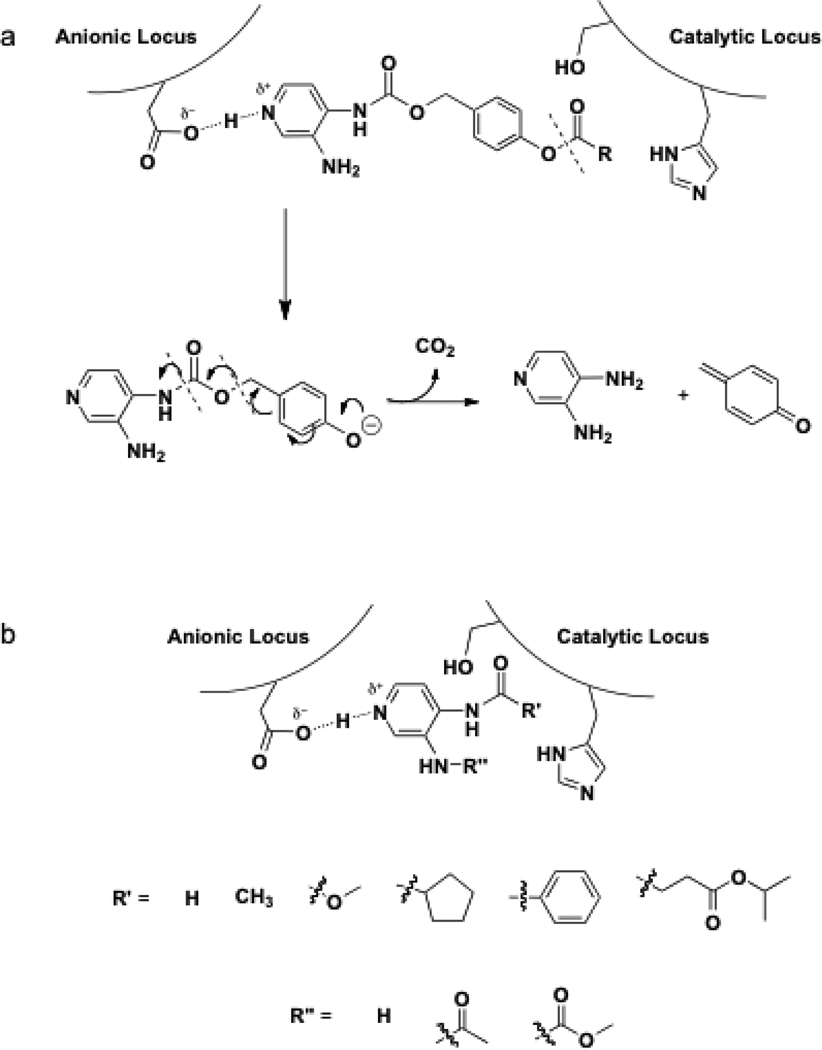
Based on previous reports of SAR and plasma stability, three prodrug models were designed with the hope of discovering dual action AChE inhibitors providing the selective release of 3,4-DAP at the site of intoxication. Class I molecules were designed from substrate specificity studies and modeled such that the anionic binding site of AChE would be occupied by 3,4-DAP while the active site would be occupied by an ester (Figure 1). Upon ester catalyzed hydrolysis, the prodrug would degrade to release 3,4-DAP into the synaptic cleft to act on nearby Kv channels (Figure 1). Class II prodrugs, on the other hand, were designed to contain a carbamoyl scissile linker like the stigmines. Lastly, class III prodrugs were designed to test whether non-specific esterases/peptidases in sera would allow the release of 3,4-DAP slowly over time.
Figure 1.
Topa. Proposed mechanism of class I 3,4-DAP prodrugs. Bottomb. Representative carbamate and amide class II and III inhibitors and AChE active site mechanism of action.
3.2 Synthesis of Class I prodrugs
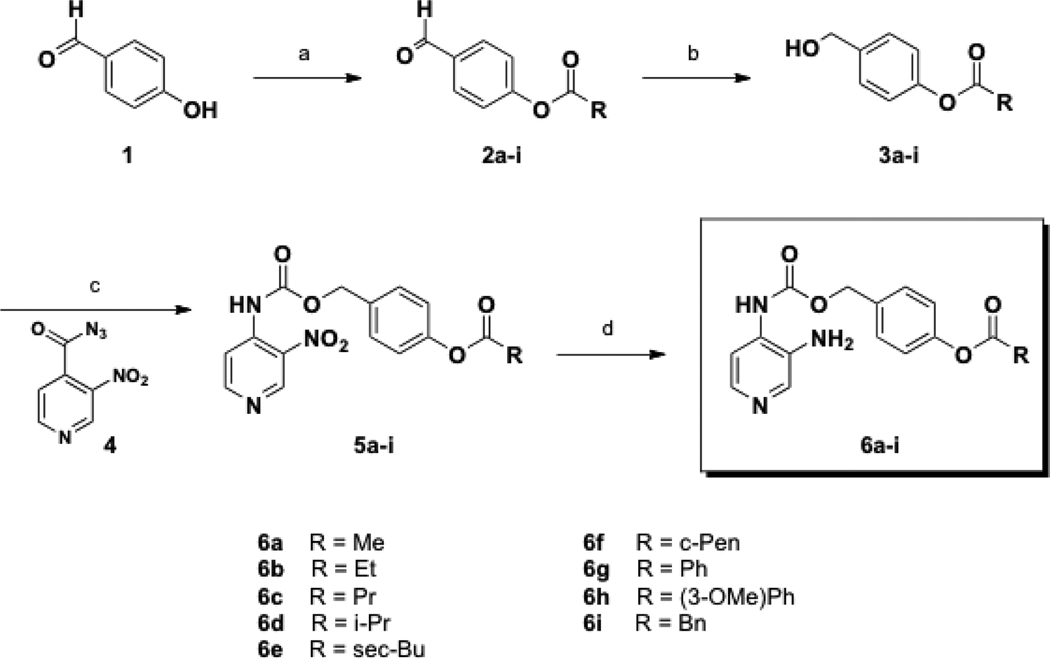
The synthesis of Class I prodrugs followed one of two paths. Class Ia prodrugs (6a–i) began from 4-hydroxybenzaldehyde (1), which was treated with various alkyl acid chlorides affording compounds 2a-i, (Scheme 1). Subsequent reduction with NaBH4 resulted in benzyl alcohols 3a–i. Reaction of aryl azide 4,23 and subsequently its Curtius rearrangement; the resulting isocyanate was trapped by alcohols 3a–i to provide carbamates 5a-i. Finally, reduction of the nitro group with stannous chloride afforded DAP prodrugs 6a–i. Here yields ranged from 4% to 32% over four steps.
Scheme 1.
Synthesis of Class Ia prodrugs.
Conditions: (a) RCOCl, Et3N, THF, 0 °C to rt, 3 h; (b) NaBH4, THF, 0 °C to rt, 0.5 – 2.5 h; (c) benzene, reflux, 0.5 – 1 h; (d) SnCl2•2H2O, MeCN, reflux, 1 h.
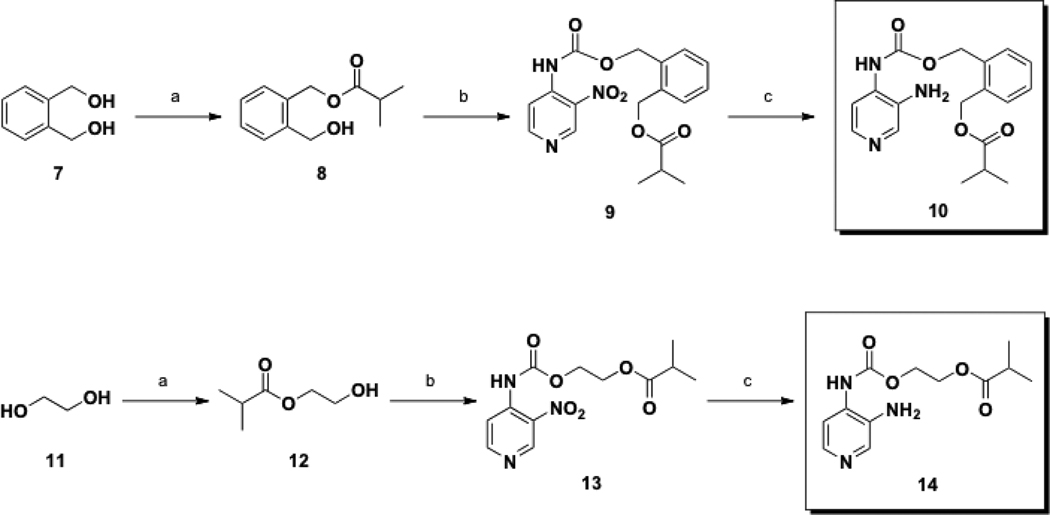
The general synthesis of Class Ib prodrugs followed a similar sequence. For compound 10, 1,2-phenylenedimethanol, (7), was treated with isobutyryl chloride affording 8, (Scheme 2a). Next, alcohol 8 underwent reaction with 4 providing carbamate 9. The nitro moiety was subsequently reduced yielding 10 in an overall yield of 9% over three steps.
Scheme 2.
Synthesis of Class Ib prodrugs 10 (Top)a and 14 (Bottom)b.
aConditions: (a) isobutyryl chloride, Et3N, CH2Cl2, rt, 16 h; (b) 4, benzene, reflux, 0.5 h; (c) SnCl2•2H2O, MeCN, reflux, 1.5 h.
bConditions: (a) isobutyryl chloride, Et3N, CH2Cl2, rt, 1 h; (b) benzene, reflux, 20 min; (c) H2 (50 psi), Pd/C, EtOAc, rt, 14 h.
On the other hand, the synthesis of carbamate 14 began from ethylene glycol (11), which was converted to ester 12 using isobutyryl chloride. Conversion to nitrocarbamate 13, vide supra, followed by its reduction, (Scheme 2b), afforded 14 in 12% yield over three steps.
3.3 Synthesis of Class II prodrugs
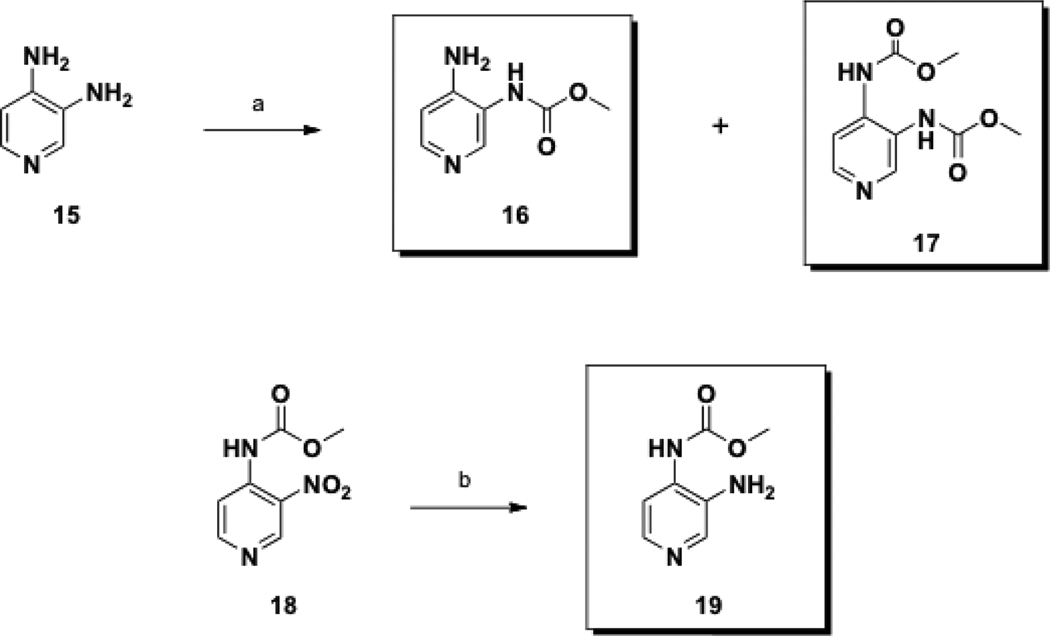
The construction of the class II prodrugs began with the reaction of commercially available 3,4-DAP (15) and methyl chloroformate to provide carbamates 16 and 17 in one pot, (Scheme 3), which upon separation resulted in yields of 10% and 22% respectively. The 3,4-DAP prodrug 19, in which the carbamate is located at the 4-position, was prepared via palladium-catalyzed hydrogenation of the known precursor 18,24 with a yield of 36%.
Scheme 3.
Synthesis of Class II prodrugs.
Conditions: (a) ClCO2Me, DMA, rt, 5 h, 10; (b) H2 (50 psi), Pd/C, EtOAc, rt, 48 h.
3.4 Synthesis of Class III prodrugs
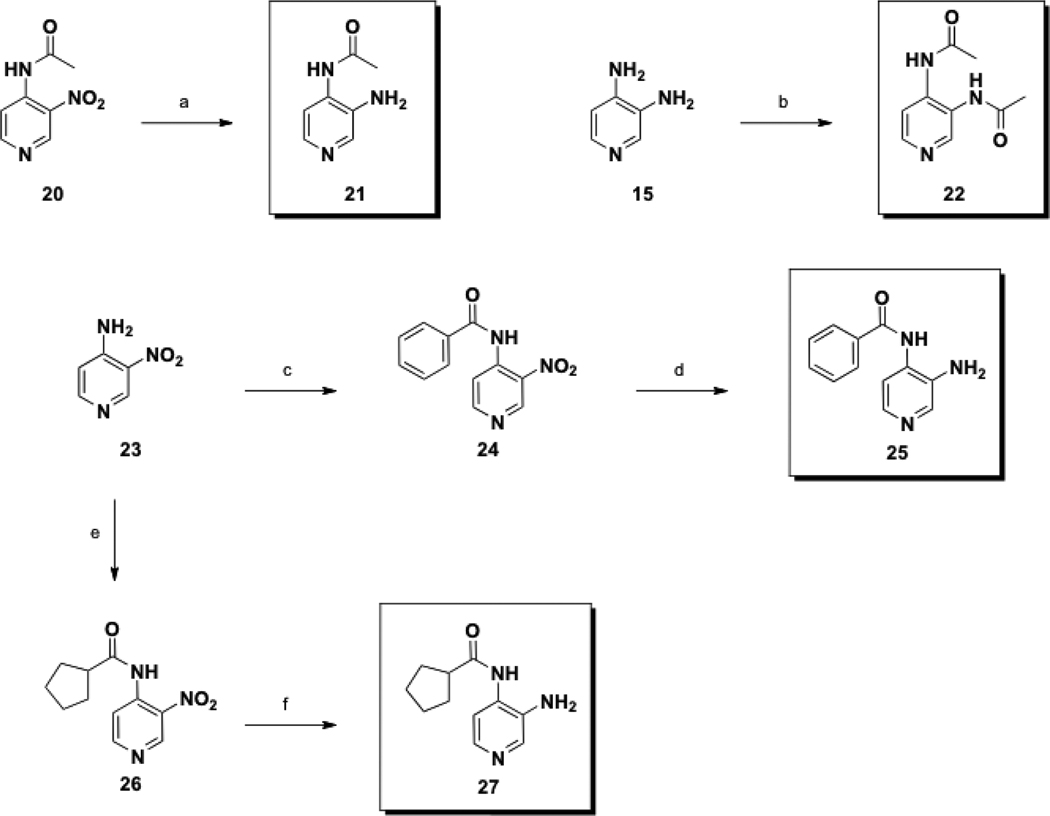
The synthesis of class III prodrugs are depicted in Scheme 4 and Scheme 5. Amide 21 was obtained via palladium-catalyzed hydrogenation of the known aminopyridine 20,25 (Scheme 4), 68% yield. Diamide 22 was prepared in one step from 3,4-DAP (15) using acetyl chloride.
Scheme 4.
Synthesis of Class III prodrugs 21, 22, 25, and 27.
Conditions: (a) H2 (60 psi), Pd/C, MeOH, rt, 16 h; (b) AcCl, DMA, rt, 14 h; (c) BzCl, Et3N, CH2Cl2, 0 °C to rt, 14 h; (d) H2 (50 psi), Pd/C, EtOAc, rt, 60 h; (e) cyclopentanecarbonyl chloride, Et3N, CH2Cl2, 0 °C to rt, 60 h; (f) H2 (50 psi), Pd/C, EtOAc, rt, 13 h.
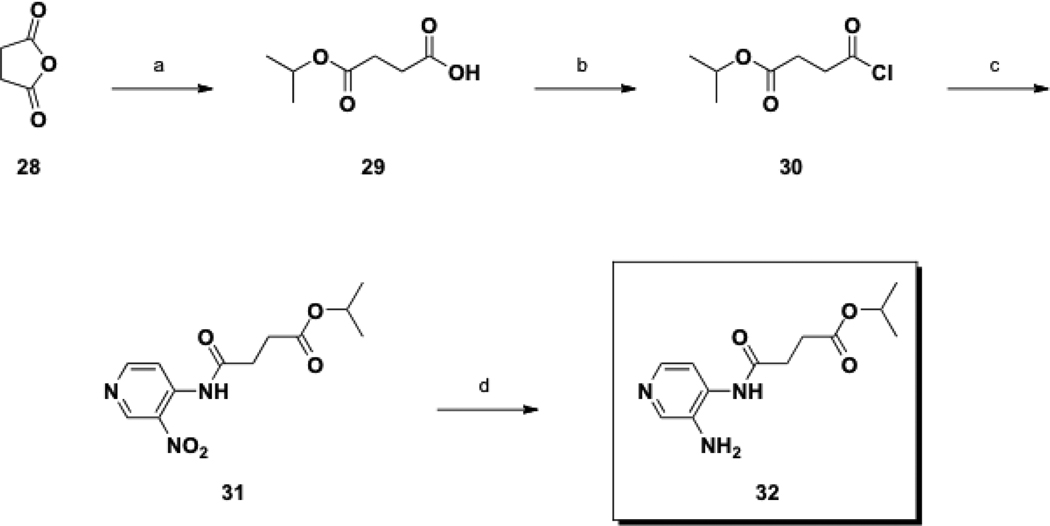
Scheme 5.
Synthesis of Class III prodrug 32.
Conditions: (a) iPrOH, reflux, 14 h, then 150 °C, 3 h; (b) (COCl)2, CH2Cl2, DMF, rt, 0.5 h; (c) 23, Et3N, CH2Cl2, rt, 15 h; (d) H2 (50 psi), Pd/C, EtOAc, rt, 3 h.
The synthesis of amides 25 and 27 proceeded from a common, commercially available starting material, 3-nitro-4-aminopyridine (23). In pursuit of 25, amide bond formation with benzoyl chloride yielded 24, followed by reduction of the nitro group to provide 25, with a yield of 18% over two steps. Synthesis of 27 began with the amide union between 23 and cyclopentanecarbonyl chloride, followed by reduction to provide 27 with a yield of 48% over two steps.
The final Class III prodrug, 32, started from commercially available succinic anhydride (28), which was treated with isopropyl alcohol to form the ester 29, followed by conversion to acyl chloride 30 (Scheme 5). The reaction of 30 with 23 in the presence of base provided 31, which in turn was reduced to the desired primary amine 32 via palladium-catalyzed hydrogenation. The overall yield over this four-step synthesis was 17%.
3.5 Inhibition, release profile, and stability of prodrugs
From screens of candidate compounds against AChE and BChE, only compounds 6b and 6d, of the substrate-like class I prodrugs, demonstrated moderate selectivity of AChE over BChE, as well as AChE-dependent release of 3,4-DAP (Tables 1 and 2). Both compounds bound to AChE 100-fold more tightly than Km for ACh. Progress curves of substrate consumption in the presence of the inhibitors were essentially linear indicating no significant buildup of acyl-enzyme intermediates (which would give time-dependent inhibition). While inhibition was relatively potent, turnover and release of 3,4-DAP was modest (Table 2). Incubation studies with AChE and BChE at saturating levels of 6b and 6d gave the expected zero order kinetics with apparent kcats that were approximately 10−4 slower than the natural substrates ACh and BCh. Given the higher concentration of 3,4-DAP release by AChE, these two prodrugs were further tested for their respective half-lives in mouse sera. Unfortunately compounds 6b and 6d were unstable to incubation in sera, with half-lives of just 1.2 and 1.9 min respectively. The extremely short half-lives of these molecules are due to unidentified esterases of the labile esters found within the prodrug molecules. The structural similarity of all class I substrate-like prodrugs meant that no other compounds from this class were likely to be stable in mouse sera.
Table 1.
In vitro inhibition profiles of 6b, 6d, and 32.
| Compd. | Kia | |
|---|---|---|
| AChE | BChE | |
| 6b | 5.7 ± 0.7 | 25 ± 3 |
| 6d | 3.9 ± 0.6 | 42 ± 5 |
| 32 | 3.2 ± 0.6 | 62 ± 11 |
Calculated Ki values in µM. All data are represented as average ± S.E.M.
Table 2.
In vitro kinetic profiles of 6b, 6d, and 32.
Concentration of 3,4-DAP in µM post 3 h enzymatic hydrolysis.
Calculated half-life values in min.
The carbamates most representative of our intended dual action strategy, compounds 16, 17, and 19 from class II prodrugs all produced moderate to poor inhibition profiles (IC50s: 36– 660 µM) but failed as pseudosubstrates to generate 3,4-DAP enzymatically (Supporting Information Table S2). Simple methyl carbamates of 3,4-DAP are not recognized by the cholinergic esterases as stigmine-like substrates. As such, these carbamates were not analyzed for half-lives in sera.
Amide 21 from the class III prodrugs demonstrated competitive inhibition of AChE (32 µM Ki). Not surprisingly, the less labile amide bond did not undergo internal release upon incubation with AChE for 3 hours. Amide 32, while potently inhibiting AChE (3 µM Ki) also demonstrated negligible release of 3,4-DAP over the 3 h incubation period (Table 2). The greater stability of the amide-conjugated 3,4-DAP gave this prodrug an appreciable half-life of 2.8 h in mouse sera, providing a path forward for in vivo analysis, as well as elucidating the core functionality necessary in the design and synthesis of additional 3,4-DAP prodrugs.
4. Conclusion
The design and synthesis of small molecule inhibitors of BoNT have been of great interest over the past decade.26 While most compounds have focused on the direct inhibition of the BoNT protease, few have been designed to symptomatically relieve neuromuscular paralysis by blocking voltage-dependent potassium channels. This report took an alternative approach by investigating the possibility of dual action toward cholinergic enhancement at BoNT damaged synapses. We attempted to mask the Kv channel blocker 3,4-DAP as a carbamate or amide conjugated pseudo-substrate inhibitor of AChE thus allowing its directed delivery to afflicted synapses. The combined effect of the prodrug is to enhance the action potential for the neuron pre-synaptically as well as increase the lifetime of ACh post-synaptically. Although simple methylcarbamate conjugates to 3,4-DAP were not recognized by AChE as pseudo-substrates, the carbamoyl-linked 4-benzylalcohol-phenol esters 6b and 6d were. These compounds bound relatively tightly to AChE (low uM) but were sluggish in their turnover by AChE having only 10−4 the kcat of ACh. The esters within these compounds rendered them unstable to mouse sera.
Alternatively, amide conjugation of 3,4-DAP exemplified by 32, resulted in compounds that inhibited AChE selectively over BChE with good potency but did not liberate 3,4-DAP through the intended internal release mechanism. The greater stability of the amide bond gave 32 a reasonable half-life (2.8 hours) for the release of 3,4-DAP in mouse sera. Although such compounds lack the dual action originally intended, masking 3,4-DAP for controlled release holds therapeutic promise. On its own, 3,4-DAP generates muscle action potential and muscle contraction in vivo, yet its ability to cross the BBB and produce seizures has questioned the potential therapeutic relevance of this molecule.27 As such, the design of prodrugs of 3,4-DAP that limit toxicity would be pertinent as a means to effectively treat neuro-paralytic disorders including BoNT induced paralysis.
Three classes of prodrugs were designed, synthesized, and evaluated in vitro with the goal of elucidating structures that act as pseudosubstrates for AChE and release 3,4-DAP at affected neurons, via enzyme mediated catalysis. Localized 3,4-DAP would act to increase vesicular ACh release at intoxicated junctions, allowing the recovery of neuromuscular transmission while reduced acetylcholinesterase activity via AChE inhibition will allow the limited ACh present to persist. We anticipate that continual 3,4-DAP in circulation, released slowly overtime, would nullify the previous inefficacies of concentrated, repeated dosing, as well as BBB penetration.
Of the 19 compounds synthesized, two demonstrated inhibition and selectivity between enzyme classes, as well as a drug-release profile necessary for half-life determination in mouse sera (6b and 6d). Unfortunately, further testing revealed that these two class I prodrugs were unstable in sera limiting their usefulness in vivo. Prodrug 32 on the other hand, demonstrated inhibition and selectivity for AChE, but did not act as a pseudosubstrate for the enzyme. Regardless, 32 was tested for half-life in sera to determine if unidentified esterases/peptidases present could degrade the prodrug while in circulation, ultimately releasing 3,4-DAP over time. Prodrug 32 in fact had the best half-life of 2.76 h. The stability of 32 in sera may possibly be due to its amide bond, which would not be readily cleaved within the active site of AChE, but may be hydrolyzed by non-specific peptidases in sera. Modeling studies may help to clarify the chemical properties necessary for binding and release via AChE. Insum, 32 could be used as a lead structure to elucidate more potent inhibitors. However, future in vivo experiments will be necessary to validate prodrug 32 as an effective treatment for BoNT intoxication and other neuromuscular disorders.
Supplementary Material
Acknowledgments
This project was supported by a postdoctoral fellowship from the German Academic Exchange Service (DAAD) to D.G., as well as The Skaggs Institute for Chemical Biology, and federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under award number AI080671 (K.D.J.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
Supplementary data associated with this article can be found, in the online version, at doi:.
References and notes
- 1.Johnson EA, Bradshaw M. Toxicon. 2001;39:1703. doi: 10.1016/s0041-0101(01)00157-x. [DOI] [PubMed] [Google Scholar]
- 2.Schantz EJ, Johnson EA. Microbiol Rev. 1992;56:80. doi: 10.1128/mr.56.1.80-99.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turton K, Chaddock JA, Acharya KR. Trends Biochem Sci. 2002;27:552. doi: 10.1016/s0968-0004(02)02177-1. [DOI] [PubMed] [Google Scholar]
- 4.Dong M, Yeh F, Tepp WH, Dean C, Johnson EA, Janz R, Chapman ER. Science. 2006;312:592. doi: 10.1126/science.1123654. [DOI] [PubMed] [Google Scholar]
- 5.Simpson LL. Annu Rev Pharmacol Toxicol. 2004;44:167. doi: 10.1146/annurev.pharmtox.44.101802.121554. [DOI] [PubMed] [Google Scholar]
- 6.Arnon SS, Schechter R, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Fine AD, Hauer J, Layton M, Lillibridge S, Osterholm MT, O'Toole T, Parker G, Perl TM, Russell PK, Swerdlow DL, Tonat K. JAMA. 2001;285:1059. doi: 10.1001/jama.285.8.1059. [DOI] [PubMed] [Google Scholar]
- 7.(a) Dickerson TJ, Janda KD. ACS Chem Biol. 2006;1:359. doi: 10.1021/cb600179d. [DOI] [PubMed] [Google Scholar]; (b) Capkova K, Salzameda NT, Janda KD. Toxicon. 2009;54:575. doi: 10.1016/j.toxicon.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) McEvoy KM, Windebank AJ, Daube JR, Low PAN. Engl J Med. 1989;321:1567. doi: 10.1056/NEJM198912073212303. [DOI] [PubMed] [Google Scholar]; (b) Oh SJ, Claussen GG, Hatanaka Y, Morgan MB. Muscle Nerve. 2009;40:795. doi: 10.1002/mus.21422. [DOI] [PubMed] [Google Scholar]
- 9.Schwid SR, Petrie MD, McDermott MP, Tierney DS, Mason DH, Goodman AD. Neurology. 1997;48:817. doi: 10.1212/wnl.48.4.817. [DOI] [PubMed] [Google Scholar]
- 10.(a) Davidson M, Zemishlany Z, Mohs RC, Horvath TB, Powchik P, Blass JP, Davis KL. Biol Psychiatry. 1988;23:485. doi: 10.1016/0006-3223(88)90020-0. [DOI] [PubMed] [Google Scholar]; (b) Youdim MB, Buccafusco JJ. J Neural Transm. 2005;112:519. doi: 10.1007/s00702-004-0214-z. [DOI] [PubMed] [Google Scholar]
- 11.(a) Flet L, Polard E, Guillard O, Leray E, Allain H, Javaudin L, Edan G. J Neurol. 2010;257:937. doi: 10.1007/s00415-009-5442-6. [DOI] [PubMed] [Google Scholar]; (b) Ionno M, Moyer M, Pollarine J, van Lunteren E. Respir Physiol Neurobiol. 2008;160:45. doi: 10.1016/j.resp.2007.08.003. [DOI] [PubMed] [Google Scholar]; (c) Smith DT, Shi R, Borgens RB, McBride JM, Jackson K, Byrn SR. Eur J Med Chem. 2005;40:908. doi: 10.1016/j.ejmech.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 12.(a) Adler M, Capacio B, Deshpande SS. Toxicon. 2000;38:1381. doi: 10.1016/s0041-0101(99)00231-7. [DOI] [PubMed] [Google Scholar]; (b) Adler M, Macdonald DA, Sellin LC, Parker GW. Toxicon. 1996;34:237. doi: 10.1016/0041-0101(95)00127-1. [DOI] [PubMed] [Google Scholar]
- 13.Caballero NA, Melendez FJ, Nino A, Munoz-Caro C. J Mol Model. 2007;13:579. doi: 10.1007/s00894-007-0184-9. [DOI] [PubMed] [Google Scholar]
- 14.Mayorov AV, Willis B, Di Mola A, Adler D, Borgia J, Jackson O, Wang J, Luo Y, Tang L, Knapp RJ, Natarajan C, Goodnough MC, Zilberberg N, Simpson LL, Janda KD. ACS Chem Biol. 2010;5:1183. doi: 10.1021/cb1002366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiou CY. Arch Int Pharmacodyn Ther. 1973;201:170. [PubMed] [Google Scholar]
- 16.Judge SI, Bever CT., Jr Pharmacol Ther. 2006;111:224. doi: 10.1016/j.pharmthera.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Henze T. Fortschr Neurol Psychiatr. 1996;64:110. doi: 10.1055/s-2007-996377. [DOI] [PubMed] [Google Scholar]
- 18.(a) Verheijen JC, Wiig KA, Du S, Connors SL, Martin AN, Ferreira JP, Slepnev VI, Kochendorfer U. Bioorg Med Chem Lett. 2009;19:3243. doi: 10.1016/j.bmcl.2009.04.089. [DOI] [PubMed] [Google Scholar]; (b) Zheng H, Youdim MB, Fridkin M. J Med Chem. 2009;52:4095. doi: 10.1021/jm900504c. [DOI] [PubMed] [Google Scholar]; (c) Darvesh S, Darvesh KV, McDonald RS, Mataija D, Walsh R, Mothana S, Lockridge O, Martin E. J Med Chem. 2008;51:4200. doi: 10.1021/jm8002075. [DOI] [PubMed] [Google Scholar]; (d) Toda N, Tago K, Marumoto S, Takami K, Ori M, Yamada N, Koyama K, Naruto S, Abe K, Yamazaki R, Hara T, Aoyagi A, Abe Y, Kaneko T, Kogen H. Bioorg Med Chem. 2003;11:1935. doi: 10.1016/s0968-0896(03)00091-9. [DOI] [PubMed] [Google Scholar]
- 19.Bar-On P, Millard CB, Harel M, Dvir H, Enz A, Sussman JL, Silman I. Biochemistry. 2002;41:3555. doi: 10.1021/bi020016x. [DOI] [PubMed] [Google Scholar]
- 20.(a) Colquhoun D. Neuromuscular Transmission: Basic and Applied Aspects. Chapter 7. New York: Elsevier; 1990. [Google Scholar]; (b) Shen T, Tai K, Henchman RH, McCammon JA. Acc Chem Res. 2002;35:332. doi: 10.1021/ar010025i. [DOI] [PubMed] [Google Scholar]
- 21.Smart JL, McCammon JA. Biophys J. 1998;75:1679. doi: 10.1016/S0006-3495(98)77610-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ellman GL, Courtney KD, Andres V, Jr, Feather-Stone RM. Biochem Pharmacol. 1961;7:88. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 23.Holt J, Andreassen T, Bakke JM, Fiksdahl A. J Heterocyclic Chem. 2005;42:259. [Google Scholar]
- 24.Bakke JM, Gautun HSH, Svensen H. J Heterocyclic Chem. 2003;40:585. [Google Scholar]
- 25.Bakke JM, Riha J. J Heterocyclic Chem. 1999;36:1143. [Google Scholar]
- 26.Willis B, Eubanks LM, Dickerson TJ, Janda KD. Angew Chem Int Ed Engl. 2008;47:8360. doi: 10.1002/anie.200705531. [DOI] [PubMed] [Google Scholar]
- 27.(a) Uchiyama T, Lemeignan M, Lechat P. Jpn J Pharmacol. 1985;38:329. doi: 10.1254/jjp.38.329. [DOI] [PubMed] [Google Scholar]; (b) Plewa MC, Martin TG, Menegazzi JJ, Seaberg DC, Wolfson AB. Ann Emerg Med. 1994;23:499. doi: 10.1016/s0196-0644(94)70069-9. [DOI] [PubMed] [Google Scholar]; (c) Damsma G, Biessels PT, Westerink BH, De Vries JB, Horn AS. Eur J Pharmacol. 1988;145:15. doi: 10.1016/0014-2999(88)90343-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.