Abstract
Functional neuroimaging studies in healthy adults demonstrate involvement of a left-lateralized network of frontal, temporal, and parietal regions during a variety of semantic processing tasks. While these areas are believed to be fundamental to semantic processing, it is unclear if task performance is correlated with differential recruitment of these or other brain regions. The objective of this study was to identify the structures underlying improved accuracy on a semantic decision task. We also investigated whether extra-scanner performance on the Boston Naming Test (BNT) and Semantic Fluency Test (SFT), neuropsychological measures of semantic retrieval, is correlated with specific areas of activation during the semantic decision/tone decision (SDTD) fMRI task. Fifty-two healthy, right-handed individuals performed a block-design SDTD task. Regression analyses revealed that increased performance on this task was associated with activation in the right inferior parietal lobule. Higher SFT performance resulted in greater recruitment of right frontal regions; improved performance on BNT was associated with more widespread activation in prefrontal, temporal, and parietal cortex bilaterally, although this activation appeared to be stronger in the right hemisphere. Overall, our results suggest that improved performance on both intra- and extra-scanner measures of semantic processing are associated with increased recruitment of right hemispheric regions.
Keywords: fMRI, language, semantic decision, performance, semantic fluency, naming
1. Introduction
Language comprehension involves both speech perception and lexical-semantic processing, functions often associated with the left temporal lobe (Indefrey & Levelt, 2004; Wernicke, 1874). While the significance of this area continues to be recognized, functional magnetic resonance imaging (fMRI) studies of speech comprehension in healthy adults have improved our understanding of the functional anatomy of language and suggest the presence of a more complicated system (Karunanayaka et al., 2007, 2010, 2011; Kim et al., 2011; Price, 2010).
Processing speech sounds activates the superior temporal gyrus bilaterally and relatively symmetrically (Binder et al., 2000; Schlosser et al., 1998). However, similar activation has been shown to occur during passive listening to non-words as well as complex non-speech sounds (Binder et al., 2008). While the superior temporal gyrus is known to play an important role in linguistic comprehension, particularly prelexical processing, converging evidence from neuroimaging and lesion studies suggests that semantic processing involves cortical areas beyond the superior temporal gyrus, including temporal (inferior and middle temporal cortices), inferior parietal (angular gyrus), and prefrontal regions (Bates et al., 2003; Dapretto & Bookheimer, 1999; D’Esposito et al., 1997; Hickok & Poeppel, 2007; Price, 2000, 2010). In addition, although language processing is believed to take place primarily in the left hemisphere, there is increasing evidence that the role of the right hemisphere has been largely overlooked (Bookheimer, 2002; Karunanayaka et al., 2010; Kim et al., 2011). Clearly, the right hemisphere is involved at the least in non-propositional speech and its involvement in language production may increase with left-handedness, presence or absence of preexisting insults (e.g., stroke or epilepsy) or other biologic (e.g., hormonal) factors (Code, 1997; Geschwind & Galaburda, 1985; Searleman, 1977; Vigneau et al., 2011). This picture is further complicated by potential sex differences in language abilities. It has been shown that women outperform men on verbal tasks, particularly fluency measures (Weiss et al., 2003a). However, it remains unclear whether these behavioral differences are associated with sex differences in the underlying language networks. It has been proposed that language functions are more strongly lateralized in males compared to females (Shaywitz et al., 1995). Yet, fMRI studies of verbal fluency revealed that, when matched for performance, males and females show similar patterns of activation (Weiss et al., 2003b; Allendorfer et al., in press). Although this issue will continue to be debated, possible sex differences need to be explored in neuroimaging studies of language before generalizing the findings to both sexes.
More recently, researchers have begun to explore the influence of performance on fMRI activation during language tasks. There is evidence that task performance directly correlates with levels of brain activation in task related cortical regions (Vannest et al., 2010). Further, Yeatman et al. (2010) found that frontal regions are recruited in response to increasing task demands in children with superior language skills, suggesting that children with strong language abilities are activating higher-order brain functions with increased task complexity. It has also been shown that higher verbal IQ is associated with increased right-hemispheric involvement on a language comprehension task (Lidzba, in press). Thus, it is currently unclear whether improved language performance is associated with stronger activation of regions known to be involved in language functioning or recruitment of more widespread regions.
Many fMRI studies attempt to isolate the cortical areas associated with a specific language process by contrasting a language related task (active condition) with a non-language related task (control condition). In doing so, general cognitive processes thought to be common to both tasks can be subtracted out, revealing the brain regions specifically involved in the language process of interest (Binder et al., 2008). Using this subtraction method, Binder et al. (1995) designed a semantic decision/tone decision task (SDTD) to identify the cortical regions specific to semantic processing. Contrasting the semantic decision condition with a tone decision condition results in elimination of activation associated with both tasks, namely low-level auditory processing, sustained attention, executive systems, and response production. In studies utilizing standard analysis methods (e.g., t-test, correlation analysis, or general linear modeling) the semantic decision condition is known to produce blood oxygenation level-dependent (BOLD) signal increases in several left hemispheric regions including prefrontal portions of the inferior, middle, and superior frontal gyri; anterior portions of the superior temporal sulcus and middle temporal gyrus spreading ventrally to include the inferior temporal gyrus; fusiform and parahippocampal gyri; angular gyrus; and posterior cingulate cortex (Binder et al., 1995, 1997, 2008; Szaflarski et al., 2002, 2008; Springer et al., 1999). More advanced data-driven analysis methods, such as independent component analysis (ICA), that do not assume an a priori hemodynamic response have shown a much more complicated picture of the SDTD task (Kim et al., 2011; Karunanayaka et al., 2011). As we have recently shown, ICA has the capability of examining the activations specific to each decision condition (semantic or tone) rather than making inferences based on the contrast between those conditions (Kim et al., 2011); in this study we have shown that semantic processing is a complex task that includes several sequential steps including auditory input and perceptual processing, verbal encoding and mental imagery, and semantic decision with many of these processes involving bilateral albeit asymmetric networks (see Figure 4 in Kim et al., 2011).
Several functional neuroimaging studies in healthy adults demonstrate activation in anterior temporal, inferior parietal, and prefrontal regions during a variety of semantic processing tasks (Cappa et al., 1998; Roskies et al., 2001; Scott et al., 2003; Spitsyna et al., 2006). However, there is conflicting evidence regarding the relative importance of these areas to the semantic processing. Impairments of semantic cognition can arise from a variety of disorders that differentially affect these brain regions. For example, individuals with semantic dementia suffer from bilateral atrophy of the anterior temporal lobes, while those with semantic aphasia following a stroke generally have damage to left prefrontal and temporoparietal regions, with a relative sparing of the anterior temporal regions (Alexander et al., 1989; Bozeat et al., 2000; Jefferies & Lambon Ralph, 2006; Kertesz et al., 1982). Both types of patients display deficits in comprehension and naming. Yet, upon closer examination, their deficits are qualitatively different (Corbett et al., 2009; Jefferies & Lambon Ralph, 2006). Individuals with semantic dementia perform poorly across a variety of semantic tasks, while aphasic patients show more variability in their performance depending on the amount of executive control required by the task. For example, in the study by Jefferies & Lambon Ralph (2006), both groups displayed poor picture naming ability; the aphasic patients benefited from phonemic cueing while those with semantic dementia did not. These authors concluded that in those with semantic dementia, storage of semantic knowledge is impaired as opposed to being unavailable as is the case in those with post-stroke aphasia. Thus, a comparison of these two syndromes suggests that the anterior temporal lobes store semantic information, and the left prefrontal cortex and temporo-parietal regions contribute to semantic control processes needed to work flexibly with previously stored semantic knowledge (Corbett et al., 2009; Jefferies & Lambon Ralph, 2006).
Neuropsychological studies further support the position that distinct cortical regions subserve various aspects of semantic processing. For example, the Boston Naming Test (BNT), a measure of confrontation naming and semantic retrieval, is sensitive to lesions in the anterior temporal regions (Bell et al., 2001; Kaplan et al., 1983; Wilson et al., 1996) while verbal fluency tasks, which also measure semantic retrieval, are often used as an index of frontal lobe function (Baldo & Shimamura, 1998; Stuss et al., 1998). Verbal fluency tasks have long been used in neuroimaging studies to investigate the cortical areas involved in word generation (e.g., Frith et al, 1991; McCarthy et al, 1993). The fact that they are used as a measure of frontal lobe function in neuropsychology is not surprising given the executive control needed to successfully retrieve and produce words associated with either an identified category (semantic fluency) or those beginning with a defined letter (letter fluency) over a limited period of time. Given the increased demand on semantic memory, category fluency has been shown to rely more heavily on temporal regions than letter fluency (Mummery et al., 1996). However, both tasks are impaired in individuals with frontal lobe lesions (Baldo & Shimamura, 1998). Using these lesion studies one can begin to make assumptions about the areas of activation seen during neuroimaging studies of semantic processing.
Functional neuroimaging studies using the SDTD task consistently show similar patterns of activation, emphasizing the reliability of the paradigm (Eaton et al., 2008). However, no study to date has examined the effect of task performance on this pattern of activation. The neuropsychological literature suggests that frontal, temporal, and parietal regions make varying contributions to semantic cognition. Thus, it is reasonable to believe that while this pattern of activation is fundamental to semantic processing, one’s performance on the SDTD task will determine to what extent each of these regions is recruited. Therefore, the purpose of this study was to identify the cortical areas associated with increased accuracy on the semantic decision condition of the SDTD task. Further, we wanted to investigate whether performance on extra-scanner neuropsychological measures is correlated with specific areas of activation during the SDTD task. We expected that higher performance on the Semantic Fluency Test (SFT) would be associated with greater recruitment of left prefrontal cortex during the SDTD task. In other words, we expected that individuals who perform well on the SFT are better able to strategically access previously stored information and would increasingly utilize left frontal regions to successfully retrieve this information when performing the SDTD task. Conversely, it was expected that higher performance on the BNT would be associated with greater involvement of temporal cortex, which is thought to play an important role in the long-term storage of semantic knowledge (Chao et al., 1999). Those individuals who have encoded and consolidated more information to semantic memory would show enhanced activation in these temporal regions as subjects attempt to make semantic decisions during the SDTD task.
To test our hypotheses we used three regression models to examine the effect of intra- and extra-scanner performance on activation during the SDTD fMRI task. First, to explore the relationship between cortical activation and intra-scanner performance, accuracy during the semantic decision condition was used as a predictor of group activation in the regression model. Next, two separate models were created using BNT and SFT scores as predictors to determine how performance on these tests affects the pattern of activation during the SDTD task. In addition, we examined which cortical areas are associated with increased tone decision performance in order to determine whether the areas associated with increased semantic decision accuracy truly reflect improved semantic retrieval as opposed to improved attention and executive processes that are similar to both tasks.
2. Results
2.1 Task Performance
Performance scores for the neuropsychological tests as well as the SDTD task are presented in Table 1. On the BNT, subjects achieved a mean score of 57 (range: 48–60). Subjects generated between 32 and 79 words on the SFT with an average score of 55. As shown in Table 1, most subjects performed well above chance levels on both the semantic decision (M = 68.5% correct) and tone decision (M = 94.3% correct) conditions. These performance rates are similar to those reported previously (Karunanayaka et al., 2011; Kim et al., 2011; Szaflarski et al., 2008). Although few individuals performed at or below chance on the semantic decision condition, they performed above chance on the tone condition suggesting that their relatively poor semantic decision performance was not related to lack of concentration or cooperation. These individuals were included in all analyses as the main goal of this study was to evaluate the cortical underpinnings of improved performance. Neither BNT nor SFT scores were significantly correlated with accuracy on the semantic decision condition. However, BNT scores were more strongly correlated (r = 0.21, p = 0.12) than SFT scores (r = 0.05, p = 0.72). Performances on BNT and SFT were positively correlated (r = 0.39, p = 0.004).
Table 1.
Demographic and performance characteristics for study subjects
| M | SD | Range | |
|---|---|---|---|
| Age (years) | 36.5 | 13.1 | 18–62 |
| EHI score | 89.1 | 12.5 | 60–100 |
| Semantic decision, % accuracy | 68.5 | 11.1 | 34–84 |
| Tone decision, % accuracy | 94.3 | 10.8 | 55–100 |
| Boston Naming Test | 57 | 3.1 | 48–60 |
| Semantic Fluency Test | 55.4 | 11.7 | 32–79 |
2.2 Functional MRI results
When contrasted with the non-language tone decision condition, the semantic decision condition elicited clusters of activation similar to those found in previous studies that used this task (Binder et al., 1995, 1997, 2008; Springer et al., 1999; Szaflarski et al., 2002, 2008). As shown in Figure 1a these regions were located primarily in the left hemisphere and right cerebellum. Significant clusters included prefrontal portions of the inferior, middle, and superior frontal gyri, posterior cingulate gyrus and retrosplenial cortex, anterior superior temporal gyrus, parahippocampal and fusiform gyri, angular gyrus and precuneus, and posterior cerebellum (see Table 2). Activation that was greater for the tone decision condition compared to the semantic decision condition was much more extensive, involving large temporal and parietal regions symmetrically as well as a smaller area of activation in the right prefrontal region (data not shown).
Fig 1.
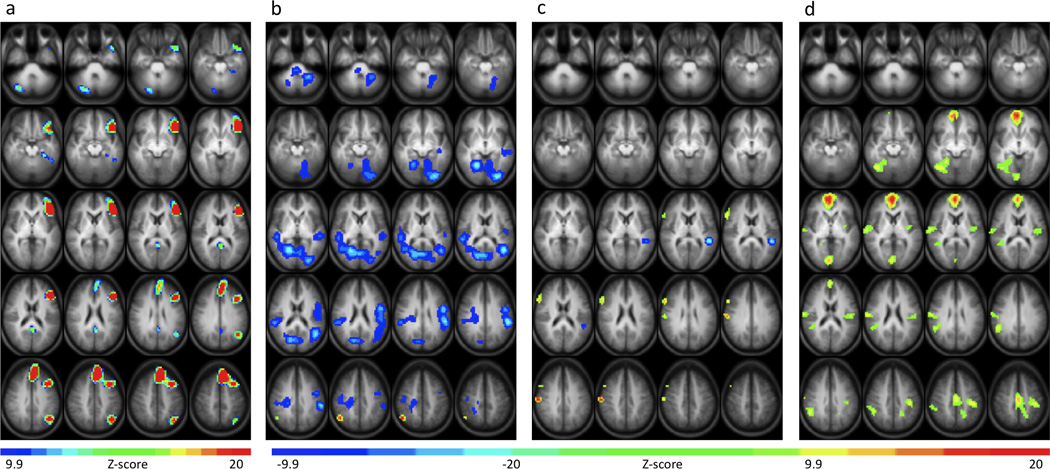
Group z-score maps showing areas of increased BOLD signal during the semantic decision condition compared to the tone decision conditiona and clusters of significant positive and negative activation associated with increased performance on the semantic decision condition of the SDTD taskb, Semantic Fluency Testc, and Boston Naming Testd. Maps are presented in radiological convention with left on the picture corresponding to the right hemisphere. The 20 axial slices selected range in Talairach coordinates from z = −29 mm to z = +47 mm.
Table 2.
Brain regions showing significantly greater activation during the semantic condition compared to tone condition
| Anatomical Location | Brodmann's Areas | Talairach coordinates (center of mass) |
Volume (mm3) |
|---|---|---|---|
| L. Superior temporal gyrus | 38 | −41, 19, −21 | 2,496 |
| L. Parahippocampal gyrus | 36 | −28, −34, −13 | 1,344 |
| L. Fusiform gyrus | 37 | −34, −45, −9 | 896 |
| L. Inferior/Middle frontal gyrus | 10, 45, 46, 47 | −47, 29, 15 | 41,344 |
| L. Superior frontal gyrus, Cingulate gyrus | 6, 8, 9, 32 | −2, 38, 35 | 34,240 |
| L. Posterior cingulate, Precuneus | 30, 31 | −2, −49, 15 | 3,840 |
| L. Angular gyrus, Precuneus, Superior parietal lobule | 7, 39 | −34, −69, 47 | 8,192 |
| R. Cerebellum | 9, −79, −21 | 4,992 |
Increased accuracy on the semantic decision condition of the SDTD task was positively correlated with activation in the right inferior parietal lobule, extending into portions of the angular and supramarginal gyri (Fig. 1b). Significant negative correlations between semantic decision performance and BOLD response were noted in superior and middle temporal gyri bilaterally corresponding to bilateral primary auditory cortices (Heschl’s gyri) and adjacent cortical areas. However, this negative BOLD signal was somewhat greater in the right hemisphere. Additional BOLD signal decreases were present in occipital, sensory-motor, and cerebellar regions, bilaterally (see Table 3).
Table 3.
Areas of significant activation for the various performance regressions
| Performance | Anatomical Location | Brodmann's Areas |
Talairach coordinates (center of mass) |
Volume (mm3) |
|
|---|---|---|---|---|---|
| SD | − | R. Superior temporal gyrus, Insula, Postcentral gyrus | 2, 13, 41, 42 | 58, −34, 19 | 15,424 |
| − | R. Middle/Superior temporal gyrus | 22 | 39, −53, 7 | 6,464 | |
| − | L. Superior/Middle temporal gyrus (anterior) | 21, 22 | −58, −26, −1 | 5,568 | |
| − | L. Superior/Middle temporal gyrus (posterior) | 22, 39 | −43, −53, 11 | 12,032 | |
| + | R. Inferior parietal lobule, Angular gyrus, Supramarginal gyrus | 39, 40 | 47, −56, 43 | 1,984 | |
| − | L. Inferior parietal lobule, Postcentral gyrus | 2, 40 | −58, −38, 35 | 11,968 | |
| − | L. Precentral gyrus | 6 | −43, 0, 35 | 8,256 | |
| − | R. Cingulate gyrus | 31 | 28, −26, 35 | 8,832 | |
| − | R. Cuneus, Lingual gyrus, Posterior cingulate | 18, 19 | 2, −79, 27 | 24,576 | |
| − | L. Cuneus, Lingual gyrus, Posterior cingulate | 18, 19 | −2, −60, 7 | 15,616 | |
| − | L. Inferior/Middle occipital gyrus, Lingual gyrus | 18, 19 | −36, −79, −5 | 13,504 | |
| − | L. Cerebellum | −21, −41, −41 | 16,000 | ||
| − | R. Cerebellum | 13, −53, −37 | 3,200 | ||
| − | Midbrain | 2, −26, −29 | 3,776 | ||
| SFT | − | L. Superior temporal gyrus | 41 | −43, −34, 11 | 3,776 |
| + | R. Inferior/Middle frontal gyrus | 9, 44 | 58, 15, 11 | 4,032 | |
| + | R. Precentral/Postcentral gyrus | 3, 4 | 58, −19, 39 | 2,386 | |
| BNT | + | R. Superior temporal gyrus, Precentral gyrus | 4, 22 | 58, −19, 23 | 6,592 |
| + | L. Insula | 13 | −47, −19, 15 | 4,096 | |
| + | R./L. Medial frontal, Paracentral lobule, Cingulate gyrus | 6 | 6, −26, 67 | 11,072 | |
| + | R./L. Superior frontal gyrus, Anterior cingulate, Medial frontal | 10, 24, 32 | 13, 60, 3 | 19,200 | |
| + | R. Inferior parietal lobule, Supramarginal gyrus | 13, 40 | 54, −30, 43 | 7,360 | |
| + | L. Inferior parietal lobule, Postcentral gyrus | 3, 40 | −43, −30, 43 | 5,184 | |
| + | R. Fusiform gyrus, Parahippocampal gyrus | 19 | 43, −60 −5 | 8,448 | |
| + | R./L. Lingual gyrus | 18 | 2, −79, 3 | 4,160 | |
| + | L. Precuneus | 7 | −17, −56, 47 | 2,432 | |
Note: SD (Accuracy on the semantic decision condition of SDTD task); SFT (Semantic Fluency Test); BNT (Boston Naming Test); "+" indicates positive correlation between performance and BOLD signal change; "−" indicates negative correlation between performance and BOLD signal change
Improved extra-scanner performance on the SFT was associated with increased activation in the right hemisphere during the SDTD task (Fig. 1c). These regions included significant portions of the inferior and middle frontal gyri as well as sensory-motor cortex (Table 3). Performance on the SFT was negatively correlated with activation in the left superior temporal gyrus while positive correlations between SFT performance and BOLD responses were noted in the right inferior/middle frontal gyri and in the precentral/postcentral gyri. Finally, improved performance on the BNT was associated with increased BOLD signal changes in several cortical regions (Fig. 1d). The regions associated with higher BNT scores consist of a large prefrontal region (right > left) including medial portions of the superior frontal gyrus, extending into the cingulate gyrus as well as several right temporal areas located in portions of the superior temporal gyrus, fusiform gyrus, and parahippocampal gyrus. Additional clusters of positive activation occurred bilaterally in occipital and parietal regions (Table 3).
Given the potential for sex related differences in cortical activation patterns associated with language processing (Harrington & Farias, 2008; Shaywitz et al., 1995), we performed the above analyses again while treating both age and sex as covariates. A direct comparison of males and females revealed that males show greater deactivation in temporal and occipital areas during the SDTD task. Overall, the three performance regressions have not changed substantially when age and sex were used in the analysis. The only difference was noted in the regression model using SFT performance as a predictor of SDTD activation. When sex was treated as a covariate only the positive correlations remained (right inferior/middle frontal gyri and precentral/postcentral gyri). The original area of decreased activation in the left superior temporal gyrus was no longer present indicating that this area was likely utilized less by males given their overall pattern of deactivation on the SDTD task compared to females.
Lastly, increased performance on the tone decision condition was correlated with increased activation in right frontal cortex, including portions of the inferior and middle frontal gyri as well as a smaller area of medial frontal activation. Similar to semantic decision accuracy, tone decision accuracy was positively correlated with increased BOLD signal in the right inferior parietal lobule. This positive parietal activation was also seen in the left hemisphere, although to a lesser extent. Significant negative correlations between tone decision accuracy and BOLD signal were found in occipital and temporal cortex as well as the precuneus as shown in Figure 2.
Fig 2.
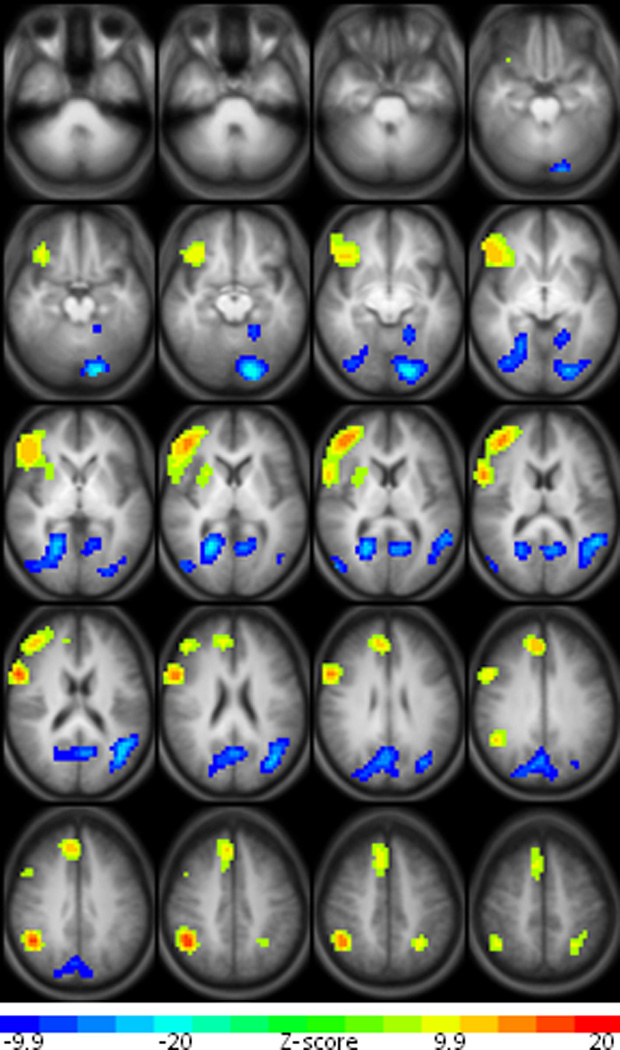
Clusters of significant positive and negative activation associated with increased performance on the tone decision condition of the SDTD task. Maps are presented in radiological convention with left on the picture corresponding to the right hemisphere. The 20 axial slices selected range in Talairach coordinates from z = −29 mm to z = +47 mm.
3. Discussion
A major finding of the current study was that after controlling for age and sex improved intra-scanner performance on the semantic decision condition of the SDTD task was associated with increased activation in the right inferior parietal lobule. Further, higher performance on extra-scanner neuropsychological measures of semantic retrieval was also associated with increased recruitment of right hemisphere regions during the SDTD task. In contrast to these findings, standard GLM analysis of the fMRI data contrasting the semantic decision condition with the tone decision condition revealed strongly left lateralized activation in areas known to be involved in semantic processing. Thus, we attribute the improvements in task performance to the increased involvement of right hemispheric regions. Further support of this finding is the fact that increased tone decision accuracy was also positively correlated with BOLD signal in the right inferior parietal lobule, indicating that this region is activated by a cognitive demand similar to both tasks and is not necessarily a reflection of one’s semantic abilities.
In addition to the positive correlation between semantic decision performance and right parietal activation, we also noted negative correlations between semantic decision performance and BOLD signal changes in bilateral temporal, occipital, and cerebellar regions (Table 3). The decreased activation in occipital cortex occurred mainly in the cuneus and lingual gyrus, areas that are related to mental imagery (Balsamo et al., 2006; Gardini et al., 2006). This likely reflects the decreased need for the retrieval of stored mental images related to presented animal names. In other words, if one can quickly generate a correct response based on semantic memory, there is less need for access to a visual representation of the animal. Similarly, individuals that perform well on the semantic decision task may rely less on superior and middle portions of the temporal lobe because they are able to recognize and comprehend the animal names quickly and more efficiently. Finally, the cerebellum, typically activated with this linguistic task (e.g., Szaflarski et al., 2002), is thought to play a role in a variety of cognitive functions, including verbal working memory, problem-solving, and attention (Baillieux et al., 2008; Durisko & Fiez, 2010). While the specific role of the cerebellum in the SDTD task remains somewhat unclear, it possibly reflects its involvement in semantic discrimination, which may be modulated by the level of task difficulty (Xiang et al., 2003).
Recruitment of bilateral networks during language tasks is often viewed as a compensatory function. For example, increased bilateral activity in older adults is associated with maintenance or increase in performance in a variety of cognitive domains, including naming accuracy (Cabeza et al., 2002; Grady, 2000; Wierenga et al., 2008). It has been proposed that this additional recruitment of right hemisphere regions is implemented to counteract age-related neurocognitive decline (Cabeza et al., 2002; Szaflarski et al., 2006). However, others argue that this increased activation reflects impairment in older adults’ ability to inhibit processing of irrelevant information (Milham et al., 2002) and that this may be related to disconnection of widespread cortical networks as examined with diffusion tensor imaging (O’Sullivan et al., 2001). While it is unclear whether this additional involvement is compensatory or related to decreases in effective processing, right-hemisphere involvement in language functioning does not only occur subsequent to pathology associated with aging. For example, while language-related activation in healthy right-handed individuals is predominantly left hemispheric, right hemisphere areas are often activated to some extent (Bookheimer, 2002; Knecht et al., 2003; Price, 2000; Springer et al., 1999). While our study did not show right hemisphere activation during the SDTD task, other studies using this task have shown activation in right-hemispheric regions when using less stringent cluster size thresholds or when including patients with atypical language representation due to disease state (Binder et al., 1997; Lee et al., 2008; Szaflarski et al., 2008). Taken together, these results suggest that the right hemisphere participates in language comprehension, although to a lesser extent than the left hemisphere. Thus, it is reasonable to speculate that as task demands increase, secondary to either task difficulty or damage to left hemispheric language regions, greater recruitment of right hemisphere occurs.
A growing number of studies report right hemispheric brain activity during higher-level language tasks, such as comprehending metaphors and jokes (Coulson & Wu, 2005; Mashal et al., 2007). For example, during a test of sentence comprehension, Just et al. (1996) found increased activation in right hemispheric areas with increasing linguistic complexity of the sentences. Similarly, an fMRI study investigating the neural circuitry of word retrieval revealed that increased difficulty in word retrieval leads to activation of right hemispheric parietal regions (Dräger et al., 2004). Lastly, a recent study assessing language lateralization in healthy subjects demonstrated a correlation between increased right hemispheric involvement and better performance on a variety of language tasks, including reading, verbal fluency, and naming (van Ettinger-Veenstra et al., 2010). Our results are in line with these studies and provide evidence to support a relationship between improved performance on a semantic decision language task and increased right hemispheric involvement. However, our ability to compare performance on a semantic decision task with a non-language tone decision task allows us to make assumptions about the role of this right hemispheric activation. The importance of right hemispheric regions to the semantic decision task has been previously demonstrated using ICA (Kim et al., 2011). Consistent with GLM analyses of this task, ICA captured a left-lateralized network of frontal, temporal, and parietal regions. However, these authors proposed an integrated model of semantic decision to account for the more bilateral, although asymmetric, areas of activation including auditory/perceptual processes, verbal encoding/mental imagery, semantic decision, and maintaining attention (Fig 4 in Kim et al., 2011).
It is impossible to ascribe a particular cognitive function to areas of activation in functional neuroimaging studies (Price & Friston, 2002). However, we can begin to explore possibilities through combining neuropsychological and neuroimaging studies. Our results suggest that the right parietal activation associated with increased performance on the SDTD task is due to higher-order cognitive functioning rather than language processing. BOLD signal in the right parietal lobe was positively correlated with performance on both, the semantic decision task and the tone decision task indicating that this activation is associated with cognitive functions similar to both tasks rather than a demand on semantic processing. Interestingly, this parietal region has been implicated in decision making as well as sustained attention and executive control (Daniels et al., 2003; Shaywitz et al., 2001; Vickery & Jiang, 2009), cognitive systems integral to both tasks. Another study found similar parietal activation with increasing word retrieval difficulty, but these authors did not specifically attribute this activation to language processing (Dräger et al., 2004). Rather, they proposed that increasing task difficulty is associated with a greater need for attention, working memory, and executive systems. These authors suggested that if the left-hemispheric language areas are overtaxed by the complexity of the language tasks, the right hemisphere will show increased activation associated with these additional cognitive systems. In the current study, we did not specifically assess task difficulty, but a similar interpretation can be applied to our results especially that a ceiling effect for the intra-scanner performance was not observed. We propose that higher accuracy on the semantic decision condition is related to improved attention and executive control rather than improved semantic processing. It is possible that the activation associated with these cognitive systems may be lateralized to the right hemisphere due to the left hemisphere’s extensive involvement in semantic processing given that tone decision performance is positively correlated with activation in the inferior parietal lobule of both hemispheres. However, the tone related activation is much stronger in the right hemisphere providing further support that this right hemisphere region is integral to improved performance on both tasks. Thus, we cannot propose that the right parietal activation seen with increased accuracy on the semantic decision task is a result of the left hemisphere being overtaxed by the language task. Instead, we believe our results suggest that the right inferior parietal lobule plays an important role in performance on decision making tasks and may reflect sustained attention or executive control in those individuals putting forth good effort throughout the SDTD task. Performance on both tasks was negatively correlated with BOLD signal in posterior temporal and occipital cortex. However, only semantic decision performance was negatively correlated with bilateral activation in the middle and superior temporal gyri as well as the cerebellum, perhaps reflecting less recruitment of these regions in individuals who are able to perform the semantic decision task more easily and efficiently.
As expected, individuals who performed higher on the SFT increasingly recruited prefrontal regions during the SDTD task. This frontal activation likely reflects executive processes that are involved in both the SFT and SDTD task, such as attention and working memory (Vigneau et al., 2011). However, the lateralization of this activity to the right hemisphere in the current study may also represent an increased demand on episodic memory in addition to semantic memory as individuals determine whether animals are “native to the United States” and “used by humans.” If an individual is unable to make a decision based on general word knowledge, it seems likely that they would begin to retrieve particular experiences with those animals to determine whether or not these animals are used by humans and native to the United States. Right prefrontal cortical regions are involved in retrieval of episodic information in contrast to the retrieval of information from semantic memory carried out by left prefrontal regions (Tulving et al., 1994; Wagner et al., 2001). Further, both neuroimaging and lesional studies confirm the importance of the left inferior and middle frontal gyri to verbal fluency performance (Abrahams et al., 2003; Baldo & Shimamura, 1998; Stuss et al., 1998). These regions are also believed to be involved in semantic processing; particularly semantic decision tasks (Gernsbacher & Kaschak, 2003). The decreases in BOLD signal in left temporal cortex may suggest decreased need for auditory language processing given that semantic information is likely retrieved quickly in individuals known to perform well on the SFT. It is important to note that given the relatively weak correlation between SFT scores and semantic decision accuracy, it is possible that the increased activation in right frontal regions associated with improved SFT performance does not improve accuracy on the semantic decision condition. Perhaps this pattern of activation is associated with spontaneous selection and decision making at the cost of more in-depth processing and retrieval. Interestingly, the area of decreased activation in the left superior temporal gyrus is not present when sex is treated as a covariate in the regression model. A direct comparison of males and females revealed that males show greater deactivation on the SDTD task. Therefore, the decreased activation associated with increased SFT performance may be a result of different patterns of activation for males and females.
In Alzheimer’s disease and temporal lobe epilepsy impaired performance on the BNT is directly related to the extent of temporal lobe pathology highlighting the importance of temporal lobe structures to object-naming ability (Bell et al., 2001; Wilson et al., 1996). Therefore, we hypothesized that increased performance on the BNT would be correlated with enhanced activation in temporal cortex during the SDTD task. The results of our analyses confirmed the original hypothesis that higher performance on the BNT was associated with activation in temporal regions, namely the superior temporal gyrus, fusiform gyrus, and parahippocampal gyrus in the right hemisphere. Further, BNT performance was most strongly correlated with signal change in the right superior temporal gyrus, extending into portions of the precentral gyrus. Increased performance on the BNT was also associated with increased medial prefrontal activation. Object naming requires the ability to select an intended word from a competing set of words. This goal-directed selection and response inhibition is generally associated with prefrontal cortex (Ridderinkhof et al., 2004). Thus, the prefrontal activation in high BNT performers is likely related to an enhanced ability to use these skills during the SDTD task. Additional increases in activation were observed in occipital and parietal regions, including the lingual gyrus and precuneus, which are known to be involved in visual imagery (Cavanna & Trimble, 2006; D’Esposito et al., 1997) and are thought to be a part of the language circuit associated with semantic decision (Kim et al., 2011). While the BNT requires subjects to name visually presented objects, the SDTD task likely involves generating visual representations of animals presented aurally. Our results suggest that the connections between cortex involved in visual imagery and that involved in storage of semantic knowledge are stronger in individuals that perform well on the BNT. Activation associated with increased BNT performance was seen bilaterally. However, this activation was once again greater in the right hemisphere. Confrontation naming, or retrieving words that denote concrete entities, is often believed to rely on the left hemisphere. Conversely, retrieving conceptual knowledge about these entities depends on regions located predominantly in the right hemisphere (Damasio et al., 2004). Therefore, given that the SDTD task relies more on retrieval of conceptual information about animals (i.e. “native to the United States & used by humans”) rather than naming ability, the presence of BOLD signal increases in right hemispheric brain regions is logical.
This study has its limitations. The subtraction methodology used in the SDTD task attempts to isolate brain areas associated with semantic processing by eliminating activation associated with both tasks (e.g. low-level auditory processing, sustained attention, and motor response). However, this method only allows one to make assumptions about what areas are important for semantic processing by identifying regions that are activated more for the semantic decision condition relative to the tone decision condition. It cannot be concluded that these areas are necessary for semantic comprehension. The study was further limited by the fact that there was a small range of scores for the semantic decision condition, with only eight individuals being less than 60% accurate. Similarly, 90% of the subjects answered less than five items incorrectly on the BNT. The restriction of range for both of these performance measures could have masked performance related differences. We chose to include ambiguous items in the semantic decision task in order to increase the difficulty. However, accuracy may reflect differences in opinion rather than a correct or incorrect answer. For example, animals that are used by humans for food can vary greatly depending on regions within the United States. Lastly, given that we did not measure education or overall intelligence, we cannot rule out the potential role of general intellectual ability in the relationship between performance and cortical activation. However, the fact that the semantic decision condition and the tone decision condition, which relies less on intellectual abilities, both showed increased BOLD signal in the right inferior parietal lobule with increased performance indicates that this region is positively correlated with performance regardless of IQ or education. Despite these limitations, to our knowledge this is the first study to examine the performance related neural substrates of semantic decision making in healthy adults.
In contrast to the traditional view that language processing is primarily dependent on the left superior temporal gyrus (Wernicke, 1874), functional neuroimaging studies suggest that a distributed left-lateralized network of frontal, temporal, and parietal regions is responsible for our linguistic abilities (Bookheimer, 2002; Gernsbacher & Kaschak, 2003; Hickok, 2009). Further, studies that utilize data-driven rather than hypothesis-driven neuroimaging analysis methods (Karunanayaka et al., 2007, 2010) paint a much more complicated picture of the language network. These studies implicate bilateral networks in language processing, but the dominant node appears to be the left fronto-temporal region, whether it is verb generation (Karunanayaka et al., 2010), story listening (Karunanayaka et al., 2007) or semantic decision (Karunanayaka et al., 2011; Kim et al., 2011). Finally, lesions in the primary left hemispheric language network nodes produce deficits on tasks of language comprehension and semantic retrieval (Alexander et al., 1989; Corbett et al., 2009; Hart & Gordon, 1990; Noppeney et al., 2007). Taken together, there is strong evidence to support the importance of left hemispheric regions in language processing. However, previous studies have not explored the cortical underpinnings of increased performance on language related measures. A unique contribution of the current study is our ability to correlate intra- and extra-scanner performance on tests of semantic processing with BOLD response during a semantic decision task to reveal the brain regions that are associated with improved language performance. Interestingly, we found that improved performance on these measures of semantic retrieval was associated with increased activation in the right hemisphere.
4. Experimental Procedure
4.1 Subjects
Fifty-two right-handed, healthy volunteers (29 females) took part in the study. Subjects were 18 years of age or older (range: 18–62), spoke English as their first language, and had no history of neurological or psychiatric disease. Edinburgh Handedness Inventory (EHI) laterality quotients ranged from 60 to 100, indicating right-handed preference for all subjects (Oldfield, 1971). Recruitment consisted of local advertisement and word of mouth. This research was part of a larger study (R01 NS048281), which was approved by the University of Cincinnati Institutional Review Board. Each participant provided written informed consent prior to participation in the study. Demographic information for all participants is summarized in Table 1.
4.2 Neuropsychological measures
Neuropsychological tests were administered to each subject prior to scanning. The 60-item BNT is a measure of confrontation naming (Kaplan et al., 1983). Subjects are presented with pictures of objects (ranging from high frequency to rare objects), which they are required to name. The number of correct responses without phonemic cueing was used as a score for each subject. The SFT is a measure of verbal fluency (Lezak, 1995). Subjects were asked to provide as many words as possible within 60 seconds from each of three different categories. The categories were randomized among subjects and included animals, fruits and vegetables, things that are hot, things that are cold, food, and cities. SFT requires semantic knowledge as well as executive control and effective search processes. The total number of responses given across three categories, excluding perseverations and errors, was used as a performance score for each subject. The BNT and SFT were chosen to examine different aspects of semantic retrieval (i.e. naming and speeded fluency, respectively), language skills believed to be necessary for successful performance on the SDTD task. The SDTD task, similar to the BNT and SFT, requires activation of the semantic system including access to stored semantic information (i.e. verbal concepts and mental images) and executive control needed to work flexibly with this information in order to generate an appropriate motor response (Binder et al., 1995; Kim et al., 2011).
4.3 Semantic Decision—Tone Decision functional MRI task (SDTD)
As previously, the block-design SDTD task consisted of alternating active (semantic decision) and control (tone decision) conditions (Karunanayaka et al., 2011; Kim et al., 2011; Szaflarski et al, 2008). Each condition lasted 30 seconds with stimuli presented every 6 seconds. Responses consisted of a thumb press to a hand-held button device placed in each subject’s left hand. During the tone decision condition, subjects heard five brief sequences of four to seven 500 and 750-Hz pure tones. Subjects were instructed to respond by pressing the button on the left for any sequence containing two 750-Hz tones and the button on the right for all other sequences. In the semantic decision condition, subjects heard five spoken English nouns designating animals and pressed the button on the left for animals they considered to be both “native to the United States” and “used by humans.” For all other animals they pushed the button on the right. The task begins with a control condition (30 seconds; data discarded), followed by alternating active and control conditions (5 blocks of 30 second duration each) for the total duration of the task of 5 minutes and 30 seconds. The SDTD task was presented and responses were recorded using DirectRT (Version 2008; Empirisoft, www.empirisoft.com). Instructions and brief practice trials on each task were given before scanning. Subjects completed two runs of the SDTD task. Performance was defined as the percentage of accurate semantic decisions out of 50 animals or 50 tone sequences presented.
4.4 Magnetic resonance imaging
All MRI data were acquired on a 3.0 Tesla Phillips MRI system at the Imaging Research Center at the Cincinnati Children’s Hospital Medical Center. The scanner is equipped with an audiovisual system for presentation of task stimuli (Avotech Systems Inc., www.avotecinc.com). Echo planar imaging (EPI) fMRI scans were performed using thirty-two 4 mm thick axial slices covering the entire brain. EPI images were obtained using a T2*-weighted gradient-echo EPI pulse sequence (TR/TE = 2000/38 ms, FOV = 24.0 × 24.0 cm, matrix = 64 × 64, slice thickness = 4 mm, voxel size = 4×4×4). Two runs of the SDTD task were performed. For each run, whole brain images were sampled at 165 time points. Prior to the fMRI sessions, a multi-echo reference scan (MERS) was acquired for use during fMRI image reconstruction to correct for geometric distortions and Nyquist ghost artifacts (Schmithorst et al., 2001). In addition, a high-resolution T1-weighted three-dimensional anatomical scan was obtained (TR/TE = 8.1/3.7 ms, FOV 25.0 × 21.1 × 18.0 cm, matrix 252 × 211, flip angle 8°, slice thickness = 1 mm) for localization of brain regions.
4.5 Image processing and data analysis
Image analysis was performed using CCHIPS (Cincinnati Children’s Hospital Image Processing System; https://irc.cchmc.org/software/cchips.php), a software package developed by the Imaging Research Center in the IDL environment (www.ittvis.com). Following MERS reconstruction of fMRI datasets, the first 15 time points during each run of the SDTD task were removed to allow for T1 equilibration. Next, the two runs were concatenated into a single dataset followed by motion correction using a pyramid iterative algorithm (Thevenaz et al., 1998). This step was followed by affine transformation into Talairach space (Talairach & Tournoux, 1988) without resampling based on methods described previously (Muzik et al., 2000; Wilke et al., 2002).
Single subject fMRI statistical analysis was performed using GLM to determine differences in the magnitude of the BOLD response for the active versus control conditions of the SDTD task. The hemodynamic response was modeled as 30-second blocks of the active condition (semantic decision) and 30-second blocks of the control condition (tone decision). Rather than excluding subjects for motion, the estimated motion parameters were included as covariates in the single subject regression analysis, which was shown to be more advantageous than performing motion correction alone (Evans et al., 2010). Low frequency (i.e., quadratic) signal drift was also included as a covariate of no interest in the first level analysis. Individual Z-score maps resulting from this analysis were combined for group-level analysis and a one-sample t-test was performed to create a Z-score map representing regions of significant group activation. As a final step, a 6 mm Gaussian filter was applied to the composite dataset for better between-subject overlap of activation. Three models were then created to examine the effect of intra- and extra-scanner performance on activation during the task. First, to explore the relationship between cortical activation and intra-scanner performance, accuracy during the semantic decision condition was used as a predictor of group activation in the regression model. Next, two separate models were created using BNT and SFT scores as predictors to determine how performance on these tests affects the pattern of activation during the SDTD task. All Z-score maps were generated using a threshold Z score of 9.9. This nominal Z-score value, combined with a cluster size of at least 30 contiguous voxels, resulted in a corrected p < 0.005 for all images, as determined via Monte-Carlo simulation (Forman et al, 1995). Age was controlled for in the three performance regressions given that certain aspects of language functioning have been shown to decline with age, including semantic fluency and confrontation naming (Kozora & Cullum, 1995; Mackay et al., 2002; Wierenga et al., 2008) and that language lateralization may be age dependent (Springer et al., 1999; Szaflarski et al., 2002, 2006). Further, another fMRI study revealed age-related differences in activation during a word retrieval task (Wierenga et al., 2008). Thus, we performed the above analyses again while treating both age and sex as covariates to assess potential sex related differences in cortical activation patterns (Harrington & Farias, 2008; Shaywitz et al., 1995). Lastly, a follow-up analysis explored the relationship between cortical activation and intra-scanner performance on the tone decision task. We created a model using tone decision accuracy as a predictor of group activation in the regression model
Highlights.
We examined the cortical substrates of semantic processing.
Semantic decision accuracy is associated with activation in right parietal cortex.
The right hemisphere is important to improved semantic decision performance.
Acknowledgments
This work was part of the first author’s Major Qualifying Exam in the Department of Psychology, University of Cincinnati, Cincinnati, Ohio. The authors acknowledge the assistance of Paula Shear, PhD and Krista Medina, PhD who provided valuable comments.
Support for this study was provided by a grant from the National Institutes of Health (NIH R01 NS048281 to JPS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This study was presented in part at the 16th Annual Meeting of the Organization for Human Brain Mapping, Barcelona, Spain and the 39th Annual Meeting of the International Neuropsychological Society, Boston, Massachusetts.
Contributor Information
Kiely M. Donnelly, Email: donnelky@mail.uc.edu.
Jane B. Allendorfer, Email: allendjb@ucmail.uc.edu.
Jerzy P. Szaflarski, Email: szaflaj@ucmail.uc.edu.
References
- Abrahams S, Goldstein LH, Simmons A, Brammer MJ, Williams SCR, Giampietro VP, Andrew CM, Leigh PN. Functional magnetic resonance imaging of verbal fluency and confrontation naming using compressed image acquisition to permit overt responses. Hum Brain Mapp. 2003;20:29–40. doi: 10.1002/hbm.10126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander MP, Hiltbrunner B, Fischer RS. Distributed anatomy of transcortical sensory aphasia. Arch of Neurol. 1989;46:885–892. doi: 10.1001/archneur.1989.00520440075023. [DOI] [PubMed] [Google Scholar]
- Allendorfer JB, Lindsell CJ, Siegel M, Banks CL, Vannest J, Holland SK, Szaflarski JP. Females and males are highly similar in language performance and cortical activation patterns during verb generation. Cortex. doi: 10.1016/j.cortex.2011.05.014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillieux H, De Smet HJ, Paquier PF, De Deyn PP, Mariën P. Cerebellar neurocognition: Insights into the bottom of the brain. Clin Neurol Neurosurg. 2008;110:763–773. doi: 10.1016/j.clineuro.2008.05.013. [DOI] [PubMed] [Google Scholar]
- Balsamo LM, Xu B, Gaillard WD. Language lateralization and the role of the fusiform gyrus in semantic processing in young children. Neuroimage. 2006;31:1306–1314. doi: 10.1016/j.neuroimage.2006.01.027. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Shimamura AP. Letter and category fluency in patients with frontal lobe lesions. Neuropsychology. 1998;12:259–267. doi: 10.1037//0894-4105.12.2.259. [DOI] [PubMed] [Google Scholar]
- Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, Dronkers NF. Voxel-based lesion-symptom mapping. Nat Neurosci. 2003;6:448–450. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- Bell BD, Hermann BP, Woodard AR, Jones JE, Rutecki PA, Sheth R, Dow CC, Seidenberg M. Object naming and semantic knowledge in temporal lobe epilepsy. Neuropsychology. 2001;15:434–443. doi: 10.1037//0894-4105.15.4.434. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PSF, Springer JA, Kaufman JN, Possing ET. Human temporal lobe activation by speech and nonspeech sounds. Cereb Cortex. 2000;10:512–528. doi: 10.1093/cercor/10.5.512. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Cox RW, Rao SM, Prieto T. Human brain language areas identified by functional magnetic resonance imaging. J Neurosci. 1997;17:353–362. doi: 10.1523/JNEUROSCI.17-01-00353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Rao SM, Hammeke TA, Frost JA, Bandettini PA, Jesmanowicz A, Hyde JS. Lateralized human brain language systems demonstrated by task subtraction functional magnetic resonance imaging. Arch Neurol. 1995;52:593–601. doi: 10.1001/archneur.1995.00540300067015. [DOI] [PubMed] [Google Scholar]
- Binder JR, Swanson SJ, Hammeke TA, Sabsevitz DS. A comparison of five FMRI protocols for mapping speech comprehension systems. Epilepsia. 2008;49:1980–19978. doi: 10.1111/j.1528-1167.2008.01683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer S. Functional MRI of language: New approaches to understanding the cortical organization of semantic processing. Annu Rev Neurosci. 2002;25:151–188. doi: 10.1146/annurev.neuro.25.112701.142946. [DOI] [PubMed] [Google Scholar]
- Bozeat S, Lambon Ralph MA, Patterson K, Garrard P, Hodges JR. Non-verbal semantic impairment in semantic dementia. Neuropsychologia. 2000;38:1207–1215. doi: 10.1016/s0028-3932(00)00034-8. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: Compensatory brain activity in high-performing older adults. Neuroimage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Cappa SF, Perani D, Schnur T, Tettamanti M, Fazio F. The effects of semantic category and knowledge type on lexical-semantic access: A PET study. Neuroimage. 1998;8:350–359. doi: 10.1006/nimg.1998.0368. [DOI] [PubMed] [Google Scholar]
- Cao Y, Vikingstad EM, George KP, Johnson AF, Welch KMA. Cortical language activation in stroke patients recovering from aphasia with functional MRI. Stroke. 1999;30:2331–2340. doi: 10.1161/01.str.30.11.2331. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: A review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chao LL, Haxby JV, Martin A. Attribute-based neural substrates in temporal cortex for perceiving and knowing about objects. Nat Neurosci. 1999;2:913–919. doi: 10.1038/13217. [DOI] [PubMed] [Google Scholar]
- Code C. Can the right hemisphere speak? Brain Lang. 1997;57:38–59. doi: 10.1006/brln.1997.1833. [DOI] [PubMed] [Google Scholar]
- Corbett F, Jefferies E, Ehsan S, Lambon Ralph MA. Different impairments of semantic cognition in semantic dementia and semantic aphasia: Evidence from the non-verbal domain. Brain. 2009;132:2593–2608. doi: 10.1093/brain/awp146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson S, Wu YC. Right hemisphere activation of joke-related information: An event-related brain potential study. J Cogn Neurosci. 2005;17:494–506. doi: 10.1162/0898929053279568. [DOI] [PubMed] [Google Scholar]
- Damasio H, Tranel D, Grabowski T, Adolphs R, Damasio A. Neural systems behind word and concept retrieval. Cognition. 2004;92:179–229. doi: 10.1016/j.cognition.2002.07.001. [DOI] [PubMed] [Google Scholar]
- Daniels C, Witt K, Wolff S, Jansen O, Deuschl G. Rate dependency of the human cortical network subserving executive functions during generation of random number series – a functional magnetic resonance imaging study. Neurosci Lett. 2003;345:25–28. doi: 10.1016/s0304-3940(03)00496-8. [DOI] [PubMed] [Google Scholar]
- Dapretto M, Bookheimer SY. Form and content: dissociating syntax and semantics in sentence comprehension. Neuron. 1999;24:427–432. doi: 10.1016/s0896-6273(00)80855-7. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Detre JA, Aguirre GK, Stallcup M, Alsop DC, Tippet LJ, Farah MJ. A functional MRI study of mental image generation. Neuropsychologia. 1997;35:725–730. doi: 10.1016/s0028-3932(96)00121-2. [DOI] [PubMed] [Google Scholar]
- Dräger B, Jansen A, Bruchmann S, Förster AF, Pleger B, Zwitserlood P, Knecht S. How does the brain accommodate to increased task difficulty in word finding? A functional MRI study. Neuroimage. 2004;23:1152–1160. doi: 10.1016/j.neuroimage.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Durisko C, Fiez JA. Functional activation in the cerebellum during working memory and simple speech tasks. Cortex. 2010;46:896–906. doi: 10.1016/j.cortex.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton KP, Szaflarski JP, Altaye M, Ball AL, Kissela BM, Banks C, Holland SK. Reliability of fMRI for studies of language in post-stroke aphasia subjects. Neuroimage. 2008;41:311–322. doi: 10.1016/j.neuroimage.2008.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JW, Todd RM, Taylor MJ, Strother SC. Group specific optimization of fMRI processing steps for child and adult data. Neuroimage. 2010;50:479–490. doi: 10.1016/j.neuroimage.2009.11.039. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Frith CD, Friston TKJ, Liddle PF, Frackowiak RSJ. A pet study of word finding. Neuropsychologia. 1991;29:1137–1148. doi: 10.1016/0028-3932(91)90029-8. [DOI] [PubMed] [Google Scholar]
- Gardini S, Cornoldi C, De Beni R, Venneri A. Left mediotemporal structures mediate the retrieval of episodic autobiographical mental images. Neuroimage. 2006;30:645–655. doi: 10.1016/j.neuroimage.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Gernsbacher MA, Kaschak MP. Neuroimaging studies of language production and comprehension. Annu Rev Psychol. 2003;54:91–114. doi: 10.1146/annurev.psych.54.101601.145128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind N, Galaburda M. Cerebral lateralization. Biological mechanisms, associations, and pathology: I. A hypothesis and a program for research. Arch Neurol. 1985;42:428–459. doi: 10.1001/archneur.1985.04060050026008. [DOI] [PubMed] [Google Scholar]
- Grady CL. Functional brain imaging and age-related changes in cognition. Biol Psychol. 2000;54:259–281. doi: 10.1016/s0301-0511(00)00059-4. [DOI] [PubMed] [Google Scholar]
- Harrington GS, Farias ST. Sex differences in language processing: Functional MRI methodological considerations. J Magn Reson Imaging. 2008;27:1221–1228. doi: 10.1002/jmri.21374. [DOI] [PubMed] [Google Scholar]
- Hart J, Gordon B. Delineation of single-word semantic comprehension deficits in aphasia with anatomical correlation. Ann Neurol. 1990;27:226–231. doi: 10.1002/ana.410270303. [DOI] [PubMed] [Google Scholar]
- Hickok G. The functional neuroanatomy of language. Phys Life Rev. 2009;6:121–143. doi: 10.1016/j.plrev.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Indefrey P, Levelt WJM. The spatial and temporal signatures of word production components. Cognition. 2004;92:101–144. doi: 10.1016/j.cognition.2002.06.001. [DOI] [PubMed] [Google Scholar]
- Jefferies E, Lambon Ralph MA. Semantic impairment in stroke aphasia versus semantic dementia: A case-series comparison. Brain. 2006;129:2132–2147. doi: 10.1093/brain/awl153. [DOI] [PubMed] [Google Scholar]
- Just MA, Carpenter PA, Keller TA, Eddy WF, Thulborn KR. Brain activation modulated by sentence comprehension. Science. 1996;274:114–116. doi: 10.1126/science.274.5284.114. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Philadelphi: Lea & Febiger; 1983. [Google Scholar]
- Karunanayaka PR, Holland SK, Schmithorst VJ, Solodkin A, Chen EE, Szaflarski JP, Plante E. Age-related connectivity changes in fMRI data from children listening to stories. Neuroimage. 2007;34:349–360. doi: 10.1016/j.neuroimage.2006.08.028. [DOI] [PubMed] [Google Scholar]
- Karunanayaka P, Kim KK, Holland SK, Szaflarski JP. The effects of left or right hemispheric epilepsy on language networks investigated with semantic decision fMRI task and independent component analysis. Epilepsy Behav. 2011;20:623–632. doi: 10.1016/j.yebeh.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunanayaka P, Schmithorst VJ, Vannest J, Szaflarski JP, Plante E, Holland SK. A group independent component analysis of covert verb generation in children: A functional magnetic resonance imaging study. Neuroimage. 2010;51:472–487. doi: 10.1016/j.neuroimage.2009.12.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz A, Sheppard A, MacKenzie R. Localization in transcortical sensory aphasia. Arch Neurol. 1982;39:475–478. doi: 10.1001/archneur.1982.00510200017002. [DOI] [PubMed] [Google Scholar]
- Kim KK, Karunanayaka P, Privitera MD, Holland SK, Szaflarski JP. Semantic association investigated with functional MRI and independent component analysis. Epilepsy Behav. 2011;20:613–622. doi: 10.1016/j.yebeh.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht S, Jansen A, Frank A, van Randenborgh J, Sommer J, Kanowski M, Heinze HJ. How atypical is atypical language dominance? Neuroimage. 2003;18:917–927. doi: 10.1016/s1053-8119(03)00039-9. [DOI] [PubMed] [Google Scholar]
- Kozora E, Cullum CM. Generative naming in normal aging: Total output and qualitative changes using phonemic and semantic constraints. Clin Neuropsychol. 1995;9:313–320. [Google Scholar]
- Lee D, Swanson SJ, Sabsevitz DS, Hammeke TA, Winstanley FS, Possing ET, Binder JR. Functional MRI and Wada studies in patients with interhemispheric dissociation of language functions. Epilepsy Behav. 2008;13:350–356. doi: 10.1016/j.yebeh.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak M. Neuropsychological assessment. New York: Oxford University Press; 1995. [Google Scholar]
- Lidzba K, Schwilling E, Grodd W, Krägeloh-Mann I, Wilke M. Language comprehension vs. language production: Age effects on fMRI activation. Brain Lang. doi: 10.1016/j.bandl.2011.02.003. in press. [DOI] [PubMed] [Google Scholar]
- Mackay AJ, Connor LT, Albert ML, Obler LK. Noun and verb retrieval in healthy aging. J Int Neuropsychol Soc. 2002;8:764–770. doi: 10.1017/s1355617702860040. [DOI] [PubMed] [Google Scholar]
- Mashal N, Faust M, Hendler T, Jung-Beeman M. An fMRI investigation of the neural correlates underlying the processing of novel metaphoric expressions. Brain Lang. 2007;100:115–126. doi: 10.1016/j.bandl.2005.10.005. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Blamire AM, Rothman DL, Gruetter R, Shulman RG. Echo-planar magnetic resonance imaging studies of frontal cortex activation during word generation in humans. P Natl Acad Sci USA. 1993;90:4952–4956. doi: 10.1073/pnas.90.11.4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milham MP, Erickson KI, Banich MT, Kramer AF, Webb A, Wszalek T, Cohen NJ. Attentional control in the aging brain: Insights from an fMRI study of the Stroop task. Brain Cogn. 2002;49:277–296. doi: 10.1006/brcg.2001.1501. [DOI] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Hodges JR, Wise RJS. Generating ‘tiger’ as an animal name or a word beginning with T: Differences in brain activation. Proc Biol Sci. 1996;263:989–995. doi: 10.1098/rspb.1996.0146. [DOI] [PubMed] [Google Scholar]
- Muzik O, Chugani DC, Juhasz C, Shen C, Chugani HT. Statistical parametric mapping: Assessment of application in children. Neuroimage. 2000;12:538–549. doi: 10.1006/nimg.2000.0651. [DOI] [PubMed] [Google Scholar]
- Noppeney U, Patterson K, Tyler LK, Moss HE, Stamatakis EA, Bright P, Mummery C, Price CJ. Temporal lobe lesions and semantic impairment: A comparison of herpes simplex virus encephalitis and semantic dementia. Brain. 2007;130:1138–1147. doi: 10.1093/brain/awl344. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- O’Sullivan M, Jones DK, Summers PE, Morris RG, Williams SCR, Markus HS. Evidence for cortical “disconnection” as a mechanism of age-related cognitive decline. Neurology. 2001;57:632–638. doi: 10.1212/wnl.57.4.632. [DOI] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: Contribution from functional neuroimaging. J Anat. 2000;197:335–359. doi: 10.1046/j.1469-7580.2000.19730335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: A review of 100 fMRI studies published in 2009. Ann N.Y. Acad Sci. 2010;1191:62–88. doi: 10.1111/j.1749-6632.2010.05444.x. [DOI] [PubMed] [Google Scholar]
- Price CJ, Friston KJ. Degeneracy and cognitive anatomy. Trends Cogn Sci. 2002;6:416–421. doi: 10.1016/s1364-6613(02)01976-9. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, van den Wildenberg WPM, Segalowitz SJ, Carter CS. Neurocognitive mechanisms of cognitive control: The role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cogn. 2004;56:129–140. doi: 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Roskies AL, Fiez JA, Balota DA, Raichle ME, Petersen SE. Task-dependent modulation of regions in the left inferior frontal cortex during semantic processing. J Cogn Neurosci. 2001;13:829–843. doi: 10.1162/08989290152541485. [DOI] [PubMed] [Google Scholar]
- Schlosser MJ, Aoyagi N, Fulbright RK, Gore JC, McCarthy G. Functional MRI studies of auditory comprehension. Hum Brain Mapp. 1998;6:1–13. doi: 10.1002/(SICI)1097-0193(1998)6:1<1::AID-HBM1>3.0.CO;2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Dardzinski BJ, Holland SK. Simultaneous correction of ghost and geometric distortion artifacts in EPI using a multiecho reference scan. IEEE Trans Med Imaging. 2001;20:535–539. doi: 10.1109/42.929619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SK, Leff AP, Wise RJS. Going beyond the information given: A neural system supporting semantic interpretation. Neuroimage. 2003;19:870–876. doi: 10.1016/s1053-8119(03)00083-1. [DOI] [PubMed] [Google Scholar]
- Searleman A. A review of right hemisphere linguistic capabilities. Psychol Bull. 1977;84:503–528. [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Pugh KR, Constable RT, Skudlarski P, Fulbright RK, Bronen RA, Fletcher JM, Shankweiler DP, Katz L, Gore JC. Sex differences in the functional organization of the brain for language. Nature. 1995;373:607–609. doi: 10.1038/373607a0. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Pugh KR, Fulbright RK, Skudlarski P, Mencl WE, Constable RT, Marchione KE, Fletcher JM, Klorman R, Lacadie C, Gore JC. The functional neural architecture of components of attention in language-processing tasks. Neuroimage. 2001;13:601–612. doi: 10.1006/nimg.2000.0726. [DOI] [PubMed] [Google Scholar]
- Spitsyna G, Warren JE, Scott SK, Turkheimer FE, Wise RJS. Converging language streams in the human temporal lobe. J Neurosci. 2006;26:7328–7336. doi: 10.1523/JNEUROSCI.0559-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer JA, Binder JR, Hammeke TA, Swanson SJ, Frost JA, Bellgowan PSF, Brewer CC, Perry HM, Morris GL, Mueller WM. Language dominance in neurologically normal and epilepsy subjects: A functional MRI study. Brain. 1999;122:2033–2045. doi: 10.1093/brain/122.11.2033. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP, Hamer L, Palumbo C, Dempster R, Binns M, Levine B, Izukawa D. The effects of focal anterior and posterior brain lesions on verbal fluency. J Int Neuropsychol Soc. 1998;4:265–278. [PubMed] [Google Scholar]
- Szaflarski JP, Binder JR, Possing ET, McKiernan KA, Ward BD, Hammeke TA. Language lateralization in left-handed and ambidextrous people: fMRI data. Neurology. 2002;59:238–244. doi: 10.1212/wnl.59.2.238. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Holland SK, Jacola LM, Lindsell C, Privitera MD, Szaflarski M. Comprehensive presurgical functional MRI language evaluation in adult patients with epilepsy. Epilepsy Behav. 2008;12:74–83. doi: 10.1016/j.yebeh.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski JP, Holland SK, Schmithorst VJ, Byars AW. fMRI study of language lateralization in children and adults. Hum Brain Mapp. 2006;27:202–212. doi: 10.1002/hbm.20177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System: An Approach to Cerebral Imaging. Stuttgart: Thieme Medical Publishers; 1988. [Google Scholar]
- Thevenaz P, Ruttimann UE, Unser M. A pyramid approach to subpixel registration based on intensity. IEEE Trans Image Process. 1998;7:27–41. doi: 10.1109/83.650848. [DOI] [PubMed] [Google Scholar]
- Tulving E, Kapur S, Craik FIM, Moscovitch M, Houle S. Hemisphereic encoding/retrieval asymmetry in episodic memory: Positron emission tomography findings. Proc Natl Acad Sci U S A. 1994;91:2016–2020. doi: 10.1073/pnas.91.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ettinger-Veenstra HM, Ragnehed M, Hällgren M, Karlsson T, Landtblom AM, Lundberg P, Engström M. Right-hemispheric brain activation correlates to language performance. Neuroimage. 2010;49:3481–3488. doi: 10.1016/j.neuroimage.2009.10.041. [DOI] [PubMed] [Google Scholar]
- Vannest J, Rasmussen J, Eaton KP, Schmithorst V, Karunanayaka P, Plante E, Byars A, Holland S. fMRI activation in language areas correlates with verb generation performance in children. Neuropediatrics. 2010;41:235–239. doi: 10.1055/s-0030-1267982. [DOI] [PubMed] [Google Scholar]
- Vickery TJ, Jiang YV. Inferior parietal lobule supports decision making under uncertainty in humans. Cereb Cortex. 2009;19:916–925. doi: 10.1093/cercor/bhn140. [DOI] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Herve PY, Jobard G, Petit L, Crivello F, Mellet E, Zago L, Mazover B, Tzourio-Mazoyer N. What is right-hemisphere contribution to phonological, lexioc-semantic, and sentence processing? Insights from a meta-analysis. Neuroimage. 2011;54:577–593. doi: 10.1016/j.neuroimage.2010.07.036. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Pare-Blagoev EJ, Clark J, Poldrack RA. Recovering meaning: Left prefrontal cortex guides controlled semantic retrieval. Neuron. 2001;31:329–338. doi: 10.1016/s0896-6273(01)00359-2. [DOI] [PubMed] [Google Scholar]
- Weiss EM, Kemmler G, Deisenhammer EA, Fleischhacker WW, Delazer Sex differences in cognitive functions. Pers Individ Dif. 2003a;35:863–875. [Google Scholar]
- Weiss EM, Siedentopf C, Hofer A, Deisenhammer EA, Hoptman MJ, Kremser C, Golaszewski S, Felber S, Fleischhacker WW, Delazer M. Brain activation pattern during a verbal fluency test in healthy male and female volunteers: a functional magnetic resonance imaging study. Neurosci Lett. 2003b;352:191–194. doi: 10.1016/j.neulet.2003.08.071. [DOI] [PubMed] [Google Scholar]
- Wernicke C. Der aphasische Symptomenkomplex. Breslau: Cohn & Weigert; 1874. [Google Scholar]
- Wierenga CE, Benjamin M, Gopinath K, Perlstein WM, Leonard CM, Rothi LJG, Conway T, Cato MA, Briggs R, Crosson B. Age-related changes in word retrieval: Role of bilateral frontal and subcortical networks. Neurobiol Aging. 2008;29:436–451. doi: 10.1016/j.neurobiolaging.2006.10.024. [DOI] [PubMed] [Google Scholar]
- Wilke M, Schmithorst VJ, Holland SK. Assessment of spatial normalization of whole-brain magnetic resonance images in children. Hum Brain Mapp. 2002;17:48–60. doi: 10.1002/hbm.10053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Sullivan M, de Toledo-Morrell L, Stebbins GT, Bennett DA, Morrell F. Association of memory and cognition in Alzheimer’s disease with volumetric estimates of temporal lobe structures. Neuropsychology. 1996;10:459–463. [Google Scholar]
- Xiang H, Lin C, Ma X, Zhang Z, Bower JM, Weng X, Gao JH. Involvement of the cerebellum in semantic discrimination: An fMRI study. Hum Brain Mapp. 2003;18:208–214. doi: 10.1002/hbm.10095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman JD, Ben-Shachar M, Glover GH, Feldman HM. Individual differences in auditory sentence comprehension in children: An exploratory event-related functional magnetic resonance imaging investigation. Brain Lang. 2010;114:72–79. doi: 10.1016/j.bandl.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


