Abstract
We investigated the origin of spontaneous transient inward current (STIC) oscillations in descending vasa recta (DVR) pericytes. In cells clamped at −80 mV, angiotensin II (ANG II; 10 nmol/l) induced oscillations with mean amplitude and frequency of −65.5 pA and 1.2 Hz. Simultaneous recording of cytoplasmic calcium ([Ca2+]CYT) and membrane current oscillations verified their synchrony and the correlation of their amplitudes. Confocal recording in fluo-4-loaded DVR showed that ANG II can induce either stable pericyte [Ca2+]CYT elevation or oscillations, while decreasing adjacent endothelial [Ca2+]CYT. Oscillating currents reversed sign at −30.2 mV and were blocked by niflumic acid, implicating charge transfer via Cl− ion. Removal of extracellular Ca2+, blockade of Ca2+ influx with SKF96365 (30 μmol/l), ryanodine (30 μmol/l), or caffeine (10 mmol/l) inhibited oscillations. In contrast, they were insensitive to removal of extracellular Na+ and exposure to either nifedipine (1 μmol/l) or 2-aminoethoxydiphenyl borate (10 μmol/l). Ouabain (100 nmol/l) increased basal pericyte [Ca2+]CYT and the frequency of resting STICs but did not affect the larger oscillations that followed ANG II stimulation. We conclude that [Ca2+]CYT oscillations stimulate Cl− currents. The former are most likely maintained by repetitive cycles of ryanodine-sensitive SR Ca2+ release and SKF96365-sensitive store refilling.
Keywords: terminal resistance vessels, blood flow, renal medulla
descending vasa recta (DVR) are terminal resistance vessels that arise from juxtamedullary efferent arterioles to distribute blood flow to the renal outer and inner medulla. Specialized smooth remnants (pericytes) surround DVR and impart contractility (37). We previously showed that stimulation of DVR pericytes by angiotensin II (ANG II) induces voltage-gated Ca2+ entry and activates Ca2+-dependent chloride channels (ClCa) (35, 49). High concentrations of ANG II (10 nmol/l) also inhibit K+ conductance so that the combination of CaCl activation and K+ channel suppression shifts the membrane potential toward the Cl− ion equilibrium potential (ECl) to depolarize the cells (5, 34). A sizable fraction of DVR pericytes respond to ANG II stimulation with membrane potential oscillations. Similarly, during voltage clamp at −80 mV, spontaneous transient inward current (STIC) oscillations occur (35). Given that −80 mV is below the threshold for activation of voltage-gated Ca2+ channels (CaV), we hypothesized that the STIC oscillations do not require CaV-mediated Ca2+ entry but are nonetheless dependent on underlying cytoplasmic Ca2+ ([Ca2+]CYT) oscillations. In this study, we tested that hypothesis by determining whether [Ca2+]CYT oscillations synchronize with STIC oscillations. We also tested the relative roles of store release and Ca2+ entry in their generation. The results show that [Ca2+]CYT oscillations occur, are temporally associated with STIC oscillations, and that the major charge carrier is Cl− ion. Blockade of ClCa channels, ryanodine receptors, or nonselective cation channels reversibly eliminates their occurrence.
METHODS
Isolation of DVR.
Investigations involving animal use were performed according to protocols approved by the Institutional Animal Care and Use Committee of the University of Maryland. Kidneys were harvested from Sprague-Dawley rats (100–150 g; Harlan) that had been anesthetized by an intraperitoneal injection of ketamine/xylazine (80 mg/kg; 10 mg/kg). Tissue slices were stored at 4°C in a physiological saline solution (PSS; in mM: 155 NaCl, 5 KCl, 1 MgCl2, 1 CaCl2, 10 HEPES, and 10 glucose, pH 7.4). Small wedges of renal medulla were dissected and transferred to Blendzyme 1 (Roche) at 0.27 mg/ml in high-glucose DMEM media (Invitrogen), incubated at 37°C for 45 min whereupon they were transferred to PSS and stored at 4°C. At intervals, DVR were isolated from the enzyme-digested renal tissue and transferred to a perfusion chamber for patch-clamp recording.
Whole cell patch-clamp recording.
Patch pipettes were made from borosilicate glass capillaries (PG52151-4, World Precision Instruments, Sarasota, FL), using a two-stage vertical pipette puller (Narshige PP-830) and heat polished. For whole cell patch-clamp recording, the pipette solution was (in mM) 120 Kaspartate, 20 KCl, 10 NaCl, 10 HEPES, pH 7.2, with or without nystatin (100 μg/ml with 0.1% DMSO) in ultrapure water. Measurements were obtained as previously described (35). Briefly, most membrane currents were measured using a CV201AU headstage and Axopatch 200 amplifier (Axon Instruments, Foster City, CA) at 10 Hz using 8- to 10-MΩ pipettes and nystatin patches. In experiments where simultaneous recording of [Ca2+]CYT variation and whole cell current was performed, the ruptured patch method was substituted for nystatin so that fluo-4 could diffuse into the pericyte cytoplasm.
Fluorescent recording of [Ca2+]CYT.
To record [Ca2+]CYT variations of DVR pericytes, the Ca2+-sensitive fluorescent probe, fluo-4 (Molecular Probes), was either included in the electrode buffer as the pentapotassium salt (50 μM, patch-clamp recordings) or loaded into isolated DVR by incubation with the AM ester (10 μmol/l, 20 min, 37°C, confocal microscopy). During patch-clamp experiments, the probe was excited at 485 nm (DeltaRam, PTI) and emissions were captured at 530 nm (B-2E/C filter cube, Nikon) using a ×40 CF fluor oil immersion objective, 1.3 numerical aperture. The image of the patched fluorescent pericyte was directed to a D104B photon counting photomultiplier detection assembly (PTI) set to operate in analog mode. In other experiments, to simultaneously visualize [Ca2+]CYT transients in pericytes and endothelia and to test for their occurrence in the absence of voltage clamp, isolated, fluo-4-loaded DVR adherent to the bottom of a recording chamber were visualized by laser-scanning confocal microscopy (BioRad MRC600). That system excites fluo-4 with the 488 line of an argon gas laser and captures 8-bit, 256 gray scale images at a maximum rate of 1 Hz.
STIC quantification and analysis.
We quantified oscillating inward currents using the “threshold search” function of Clampfit (Axon Instruments). All negative deviations of current from baseline were identified over a specified time interval (T). The amplitude of each STIC was measured as the greatest deviation from baseline. The number of oscillations from baseline (Nc) during the interval was counted to calculate frequency (equal to Nc/T). An experimental maneuver can alter charge transfer across the cell membrane by changing amplitude and/or frequency. To account for both, the time average current was calculated. The integral of the current from its beginning to its return to baseline was calculated by the software, yielding the total charge transferred by the ith STIC (Qi). The time average current (I) for the interval of interest is thus
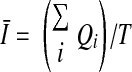 |
Vasoconstriction of isolated DVR.
As previously described, we quantified constriction of DVR by measuring changes in diameter of isolated, perfused vessels (33). Briefly, DVR were isolated from renal outer medullary vascular bundles and transferred to a perfusion chamber. With the use of concentric pipettes, the vessels were cannulated, perfused, and bathed in the following buffer at 37°C (in mmol/l: 5 HEPES, 140 NaCl, 10 NaAcetate, 5 KCl, 1.2 MgCl2, 1.71 Na2HPO4, 0.29 NaH2HPO4, 1 CaCl2, 5 alanine, 5 glucose, pH 7.4). Vessels were recorded with a video camera (Dage-MTI, CCD model 72) using a ×40 objective to yield a final screen magnification of ×1,300. During video playback, internal diameters were quantified with calipers at the point of greatest constriction. Changes were calculated as percent constriction, calculated from basal diameter in the absence of hormones (Do) and the experimental diameter (D) using % Constriction = (1 − D/Do) × 100.
Reagents.
ANG II, nifedipine, SKF96365, caffeine, and ryanodine were from Sigma (St. Louis, MO). Niflumic acid, nifedipine, fluo-4 (Molecular Probes) and 2-aminoethoxydiphenyl borate (2-APB; Calbiochem) were prepared in DMSO. SKF96365 (1-{β-[3-(4-Methoxyphenyl)propoxy]-4-methoxyphenethyl}-1H-imidazole hydrochloride) and ANG II were dissolved in water. Stock reagents were stored at −20°C. Reagents were thawed and diluted 1:1,000 on the day of the experiment. Blendzyme (Roche) was stored in 40-μl aliquots of 4.5 mg/ml in water and diluted on the day of the experiment.
Statistics.
Data in the text and figures are reported as means ± SE. The significance of differences was evaluated with SigmaStat 3.11 (Systat Software, Point Richmond, CA) using parametric or nonparametric tests as appropriate for the data. Comparisons between two groups were performed with Student's t-test (paired or unpaired, as appropriate) or the rank sum test (nonparametric). Comparisons between multiple time points within a group were examined with repeated-measures ANOVA. Post hoc comparisons were performed using Tukey's or Holm-Sidak tests. P < 0.05 was used to reject the null hypothesis.
RESULTS
Summary of ANG II-stimulated STIC oscillations.
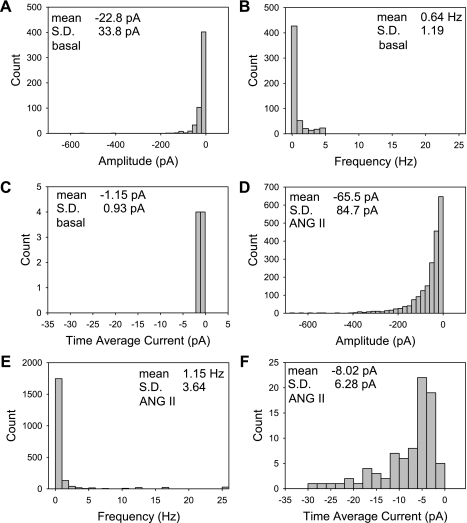
Figure 1, A –C, summarizes the current and frequency of 563 STICs that occurred in 8 cells held near the K+ ion equilibrium potential at −80 mV. In the absence of ANG II stimulation, low-amplitude spontaneous inward currents were present. By comparison, STIC oscillations after ANG II (10 nmol/l) stimulation were generally larger and of higher frequency. Their characteristics are summarized in the histograms of Fig. 1, D–F, for 2,062 occurrences in 73 cells. The data summarized in Fig. 1, D–F, are that of the control periods of the experiments presented below. To follow up our prior studies (34, 35) and to generate robust currents amenable to analysis, we performed the majority of these studies in ANG II (10 nmol/l). Most post-ANG II STICs carried less than −100 pA of current; however, there was wide variation (means ± SD = −65.5 ± 84.7). A few cells exhibited amplitudes greater than −500 pA. The frequency was 1.2 ± 3.6 Hz (means ± SD) and the time average current accounted for by STIC oscillations was −8.0 ± 6.3 pA (means ± SD).
Fig. 1.
Summary of ANG II-stimulated spontaneous transient inward current (STIC) oscillations. A–C: amplitude, frequency, and time average current histograms of 563 STICs recorded in 8 cells in their resting state. E–F: amplitude, frequency, and time average current histograms of 2,062 STICs recorded and analyzed during control periods of 73 cells exposed to ANG II (10 nmol/l) during these studies.
ANG II-induced STICs and [Ca2+]CYT oscillations.
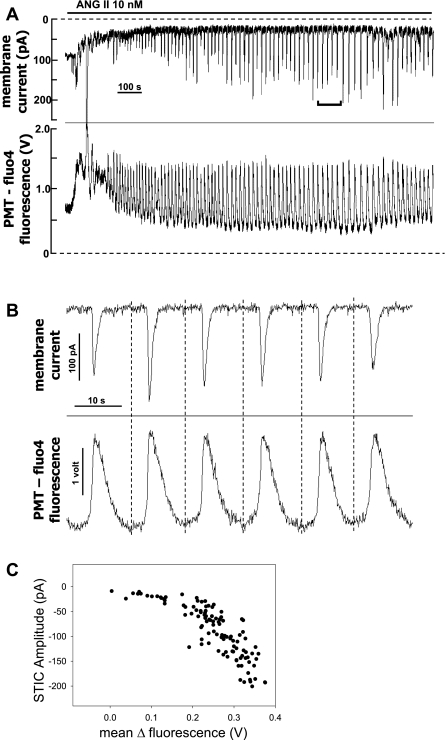
We tested whether [Ca2+]CYT oscillations occur in ANG II (10 nmol/l)-stimulated pericytes and whether they are temporally associated with the STICs. In those experiments, pericytes were subjected to ruptured patch whole cell recording during voltage clamp at −80 mV using electrode buffer that contained fluo-4 (50 μmol/l). As shown in the example in Fig. 2A concurrent recording of fluorescence, quantified as photomultiplier (PMT) output in volts, and membrane current verified that [Ca2+]CYT and STIC oscillations were synchronous. The region indicated on Fig. 2A is expanded in Fig. 2B to show that the threshold rise in [Ca2+]CYT precedes the onset of a measurable inward current, consistent with causality. The relationship between inward current amplitude and the corresponding mean fluorescence of the associated fluo-4 peak is shown in Fig. 2C. They correlated in a nonlinear manner. The relationship shown in Fig. 2C was similar to that of 11 cells wherein fluo-4 fluorescence and STIC oscillations were simultaneously recorded.
Fig. 2.
Cytoplasmic calcium ([Ca2+]CYT) oscillations are synchronous with ANG II-induced STICs. A–C: results of simultaneous membrane current and fluo-4 [Ca2+]CYT variations in a single cell exposed to ANG II (10 nmol/l). A: full record shows initial ANG II application that elicits both an increase in inward current (top) and rise of [Ca2+]CYT (fluo-4 fluorescence; bottom) followed by stabilization to persistent oscillations. Fluorescence was quantified as photomultiplier (PMT) output in volts. The inverted bracket in the top shows the region expanded in B. B: time-expanded region from A shows synchronous oscillations of current (top) and fluo-4 [Ca2+]CYT (bottom). Vertical broken lines were drawn by eye at the time of onset of the rise of [Ca2+]CYT. [Ca2+]CYT elevations precede onset of inward current variations. C: plot of STIC amplitude vs. maximal change in fluo-4 fluorescence for the record in A. Results are similar to 10 other cells subjected to simultaneous current and fluorescent recordings.
ANG II-induced [Ca2+]CYT oscillations by confocal recording.
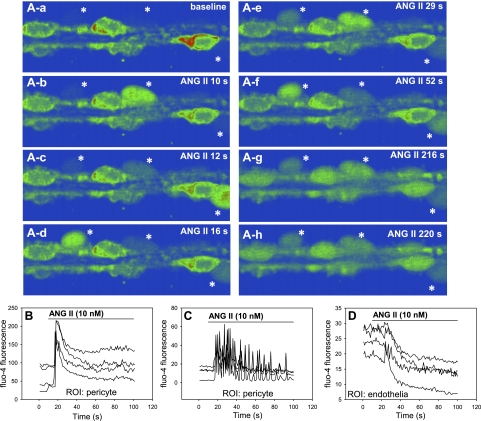
The [Ca2+]CYT oscillations in Fig. 2 were measured during voltage clamp at −80 mV. To determine whether similar oscillations occur in vessels not subjected to voltage clamp and cytoplasmic dialysis, we fixed isolated vessels to coverslips (n = 7), loaded them with fluo-4 by incubation with the AM-ester, and captured sequential fluorescent images (1 Hz) during exposure to ANG II (10 nmol/l). As anticipated, ANG II increased pericyte [Ca2+]CYT. In some pericytes (16 of n = 27), [Ca2+]CYT rose in a stable “peak-plateau” manner while others (11 of n = 27) oscillated. Figure 3A shows sequential pseudocolored confocal images of an oscillating vessel at baseline (Fig. 3, A-a) and at various times thereafter during ANG II application (Fig. 3, A–b to A–h). Baseline fluorescence of abluminal pericyte cell bodies (3 cells, location denoted by *) was too low to detect (Fig. 3A-a) but became measurable during the oscillations. The oscillations of individual pericytes on a vessel were asynchronous. A corresponding movie file is provided as a supplement to this communication: ANG IICaOsc.avi (the online version of this article contains supplemental data). Regions of interest (ROI) placed over the cell bodies of nonoscillating and oscillating pericytes yielded results typified by the examples shown in Fig. 3, B and C, respectively. Image acquisition rates were too slow to determine whether the rise in [Ca2+]CYT in the nonoscillating pericytes resulted from the syncytium of rapidly occurring intracellular “Ca2+ sparks” (32). Using fura-2-loaded vessels, we previously showed that endothelial [Ca2+]CYT declines during ANG II stimulation (36, 39). This was again observed during confocal recordings in fluo-4-loaded vessels (Fig. 3D). These data show that the decline of endothelial [Ca2+]CYT can occur while nearby pericyte [Ca2+]CYT rises or oscillates.
Fig. 3.
Confocal recording of ANG II-induced [Ca2+]CYT oscillations in descending vasa recta (DVR) pericytes. Confocal recording of fluo-4 fluorescence in a DVR fixed to a coverslip and exposed to ANG II. Aa–Ah: pseudocolor serial images recorded at times of ANG II exposure indicated in the top right corner of each panel. Concentration of [Ca2+]CYT increases from blue to green to red. Asterisks indicate the locations of 3 pericyte cell bodies that only become visible in some panels during asynchronous oscillations. Also note the progressive decline of fluo-4 fluorescence of endothelial cells that line the lumen of the sequential images. The vessel is ∼12 μm in diameter. B: examples of recordings in which [Ca2+]CYT oscillations do not occur. Regions of interest (ROI) were set to quantify fluorescence of pericyte cell bodies. C: ROI corresponding to pericyte cell bodies in a recording where [Ca2+]CYT oscillations take place. D: quantification of ANG II-induced suppression of endothelial [Ca2+]CYT. ROI were set to measure fluo-4 fluorescence from endothelial cell bodies.
Ion movements underlying ANG II-induced STIC oscillations.
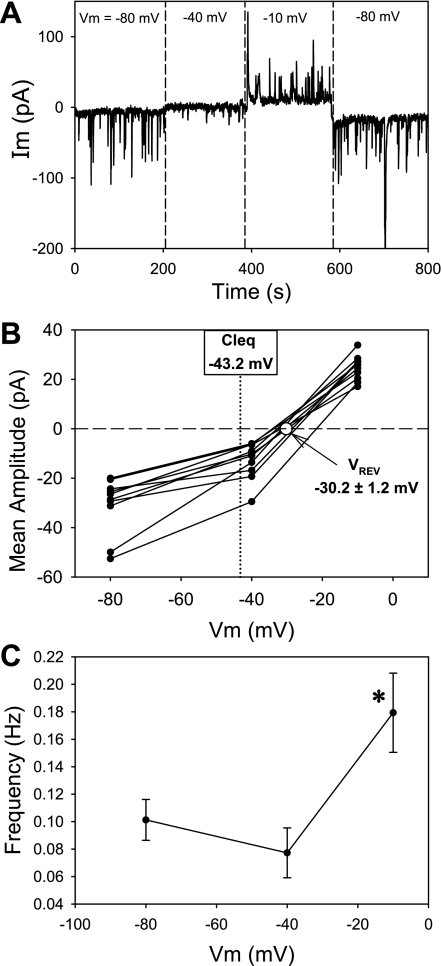
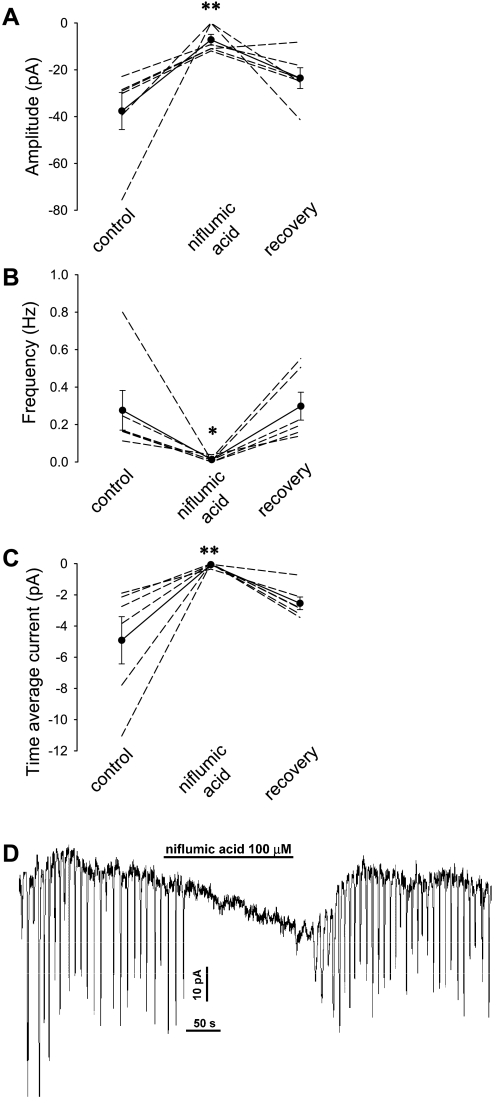
To study the charge carrier responsible for STICs, we measured the reversal potential (Vrev) and sensitivity to the Cl− channel blocker, niflumic acid. As shown in Fig. 4, the oscillating inward currents reversed to outward currents (Fig. 4A) when holding potential was sequentially raised in n = 11 cells. Interpolation by linear regression yielded a mean ± SE for Vrev = −30.2 ± 1.2 mV (Fig. 4B) which is close to the equilibrium potential for Cl− ion in our buffers. Spontaneous transient outward currents (STOCs), generally attributed to Ca2+-dependent K+ channel activation (26, 50), were not detected near ECl where they should be observed if activated. DVR pericytes express a ClCa conductance that is activated by both [Ca2+]CYT and depolarization. Consistent with a role for those channels, the frequency of STIC oscillations increased with membrane potential (Fig. 4C), nearly doubling from 0.10 ± 0.01 to 0.18 ± 0.03 Hz as holding potential was increased from −80 to −10 mV (n = 10, P < 0.05). Niflumic acid, a potent inhibitor of whole cell ClCa currents of DVR pericytes (35), reversibly blocked the STIC oscillations (Fig. 5).
Fig. 4.
Membrane potential dependence of STIC amplitude and frequency. A: example in which STIC oscillations were recorded in ANG II (10 nmol/l) as membrane potential was changed from −80 to −40 (near ECl) to −10 and then back to −80 mV. B: summary of mean amplitudes of 11 records similar to A and the corresponding reversal potential (Vrev) calculated from interpolation of the records to zero current. The Cl− equilibrium potential (ECl) for the extracellular and electrode buffers is −43.2 mV. C: mean STIC frequency in serial records as a function of holding potential. The frequency of STICs increased with holding potential. *P < 0.05 vs. frequency at −80 mV.
Fig. 5.
Inhibition of STICs by niflumic acid. A–C: exposure to niflumic acid (100 μmol/l) during ANG II (10 nmol/l) stimulation inhibited STIC activity. D: example record showing the reversible inhibition by niflumic acid. *P < 0.05; **P < 0.01 vs. control period.
Plasmalemmal Ca2+ entry and DVR pericyte STIC oscillations.
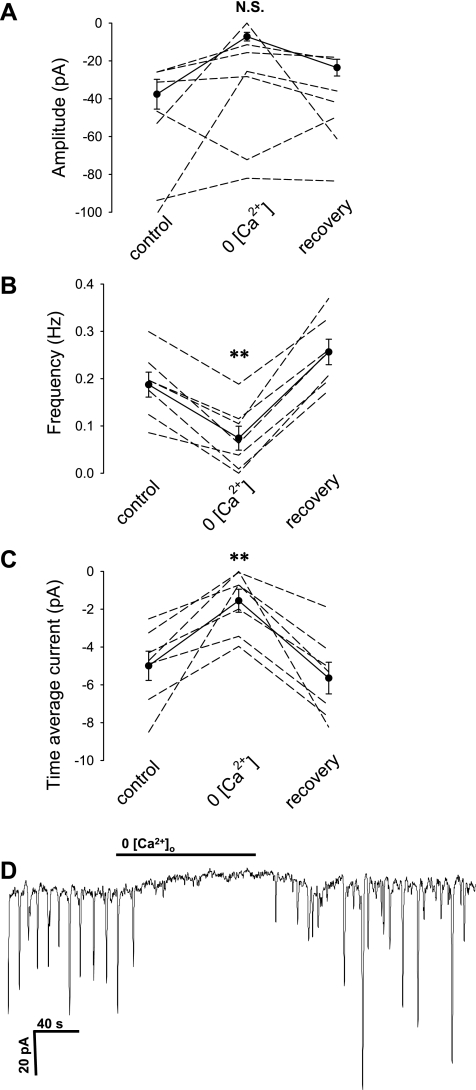
The results in Figs. 2 and 3 point to [Ca2+]CYT oscillations as the stimulus for the ClCa conductance changes that generate the STIC oscillations of DVR pericytes. To examine roles for entry of Ca2+ via channels or Na+/Ca+ exchange, we tested the effects of removing extracellular Na+ or Ca2+ ions. As shown in Fig. 6, removal of Ca2+ markedly reduced STIC frequency and current. In contrast, complete substitution of Na+ with N-methyl-d-glucamine (NMDG+) had no effect; time average current in NMDG+ was −11.1 ± 5 pA compared with a baseline value of −8.9 ± 2.3 pA (n = 6, not significant, data not shown).
Fig. 6.
Inhibition of STICs by removal of extracellular Ca2+. A–C: exposure to nominally zero Ca2+ buffer during ANG II (10 nmol/l) stimulation inhibited STIC activity. D: example record showing the reversible inhibition in zero Ca2+ buffer. *P < 0.05; **P < 0.01 vs. control period.
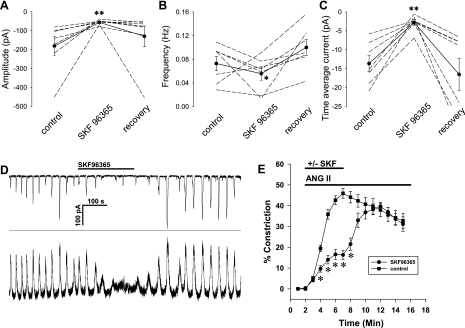
Since prolonged removal of extracellular Ca2+ can lead to its depletion from cellular stores, the results in Fig. 6 support but cannot confirm that entry of external Ca2+ is an essential event. Thus we tested whether blocking L-type channels (CaV1.x) or nonselective cation channels with nifedipine or SKF96365, respectively, affected oscillations. In those studies, STICs were measured in pericytes voltage clamped to −80 mV, well below the activation threshold for high- or low-voltage-activated CaV isoforms. Hence, it seemed unlikely that CaV played a role in their generation. Consistent with that expectation, nifedipine (1 μmol/l) failed to block oscillations. Time average current was −3.5 ± 0.58 vs. −2.1 ± 0.81 pA before and during exposure to nifedipine, respectively (means ± SE, n = 6, P > 0.05). In contrast, the nonselective cation channel blocker, SKF96365 (30 μmol/l), was very effective to eliminate both current and [Ca2+]CYT oscillations (Fig. 7). We also verified that SKF96365 partially blocks ANG II-induced constriction of isolated, microperfused vessels (Fig. 7E). That experiment supports a role for Ca2+ entry into pericytes via nonselective cation channels during ANG II stimulation.
Fig. 7.
Inhibition of STICs by SKF96365 (SKF). A–C: exposure to SKF (30 μmol/l) during ANG II (10 nmol/l) stimulation inhibited STIC activity. D: example record showing the reversible inhibition of membrane current (top) and [Ca2+]CYT oscillations (bottom). E: partial inhibition of ANG II-induced vasoconstriction of isolated, perfused DVR by SKF. *P < 0.05; **P < 0.01 vs. control period.
Ca2+ release from internal stores.
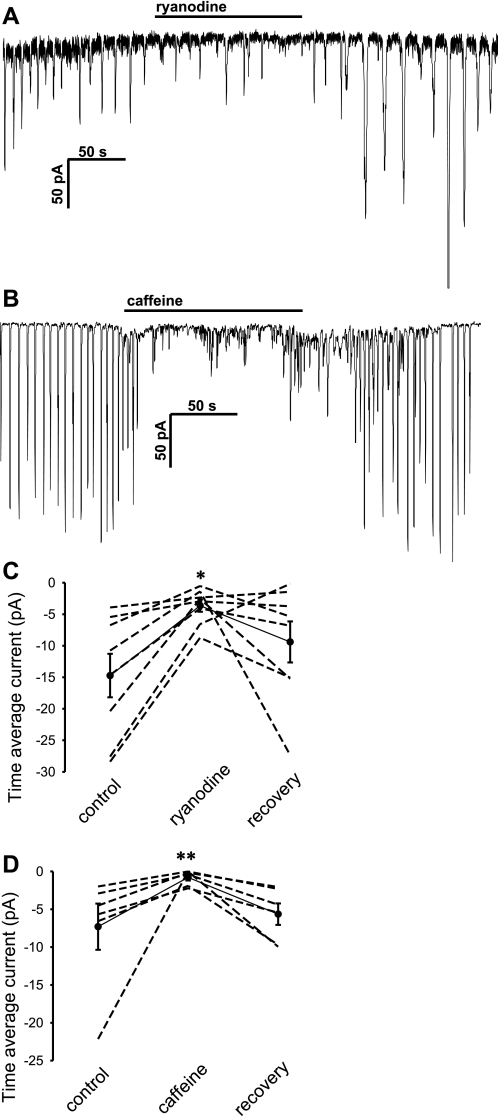
To test whether oscillations in DVR pericytes involve repetitive depletion and refilling of Ca2+ stores, we examined the ability of ryanodine (30 μmol/l), caffeine (10 mmol/l), and 2-APB (10 μmol/l) to block them. The ryanodine receptor (RyR) agonists/antagonists, ryanodine and caffeine (Fig. 8), were both effective to reduce the frequency of STIC oscillations and the associated time averaged current. In both cases, the mean changes in frequency of STICs, but not the changes in their amplitude, reached significance (not shown). The inositol trisphosphate receptor (IP3R) blocker, 2-APB, was without effect on amplitude, frequency, or time average current (data not shown).
Fig. 8.
Inhibition of STICs by ryanodine or caffeine. A–B: example records show reversible inhibition of STICs by ryanodine (30 μmol/l) or caffeine (10 mmol/l) during ANG II (10 nmol/l) stimulation. C–D: summary of time average current inhibition in DVR pericytes exposed to ryanodine or caffeine. *P < 0.05; **P < 0.01 vs. control period.
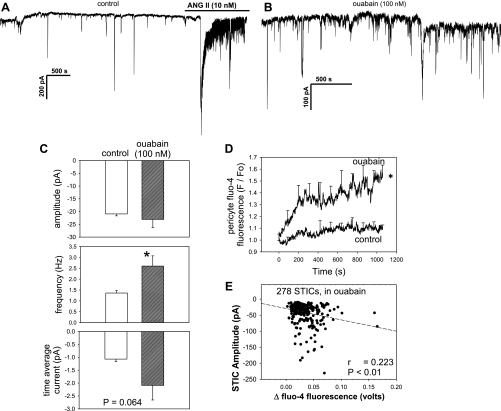
Enhancement of spontaneous STICs by ouabain.
The above results are most consistent with the interpretation that [Ca2+]CYT oscillations and STIC oscillations arise from events that involve Ca2+ entry via nonselective cation channels (Fig. 7) and intermittent release of Ca2+ from ryanodine-sensitive intracellular stores (Fig. 8). Ouabain has been hypothesized to increase the load of Ca2+ within internal stores and enhance the release of Ca2+ by agonists (2, 3, 38). Based on those considerations, we tested the hypothesis that ouabain would increase the current carried by STICs. Addition of ouabain during ANG II stimulation had no significant effects on frequency, current, or amplitude; time average current was −6.9 ± 1.4 pA before and −6.3 ± 1.2 pA after ouabain (n = 10). In contrast, ouabain did affect the low-amplitude spontaneous inward currents present in resting cells that had not been subject to ANG II stimulation (Fig. 9, A –C). Its primary effect was to increase their frequency. In some cells, fluo-4 was included in the pipette to enable recording of [Ca2+]CYT-sensitive fluorescence during exposure to ouabain or vehicle. Compared with the vehicle-treated time controls, ouabain significantly increased basal fluo-4 fluorescence (Fig. 9D). It also tended to lower holding current to more negative values, −31.1 ± 7.8 (control) vs. −47.2 ± 3.3 pA (ouabain), P = 0.09 (data not shown). A scatter plot of changes in fluo-4 fluorescence (as PMT output in volts) vs. STIC amplitude during ouabain exposure yielded the significant correlation illustrated in Fig. 9E.
Fig. 9.
Effect of nanomolar ouabain on STICs. A–B: example records of basal STICs in DVR pericytes in vehicle or ouabain (100 nmol/l). A: ANG II (10 nmol/l) was introduced near the end of the record to demonstrate responsiveness and the increase in amplitude of STICs. C, top, middle, and bottom: group comparison of STIC amplitude, frequency, and time average current in cells preexposed to either ouabain or vehicle. STICs were significantly more frequent in ouabain. D: averaged tracings of the change in fluo-4 fluorescence in n = 7 and 6 cells exposed to ouabain or vehicle, respectively. Compared with control cells, ouabain slowly and significantly increased pericyte [Ca2+]CYT. E: plot of all STIC amplitudes vs. maximal change in fluo-4 fluorescence (PMT output in volts) of cells in ouabain yields a significant correlation. *P < 0.05; **P < 0.01 vs. control period; N.S., not significant.
DISCUSSION
We previously showed that DVR pericytes sometimes undergo oscillations of membrane potential during current clamp and oscillations of membrane current during voltage clamp (35). In this study, the relationship of the latter to synchronous variations of [Ca2+]CYT was confirmed (Fig. 2). Fluorescence of the Ca2+-sensitive probe, fluo-4, rises before the onset of inward currents and the magnitude of the rise correlates with the amplitude of STIC oscillations (Fig. 2C). Oscillations are not an artifact related to the cellular dialysis necessitated by patch clamp because they also exist in the absence of electrophysiological recording. Examination of post-ANG II [Ca2+]CYT transients in DVR pericytes and endothelia with confocal microscopy confirmed the presence of oscillations in the former as well as reduction of [Ca2+]CYT in the latter (Fig. 3) (36, 39). These observations expand on our prior studies and clarify several issues. First, in individual pericytes of an intact vas rectum, ANG II can stimulate either a stable increase in [Ca2+]CYT or oscillations (Fig. 3, B and C). Second, the [Ca2+]CYT oscillations of adjacent pericytes on an individual vessel need not be synchronous (Fig. 3, A and C). Finally, the rise in pericyte [Ca2+]CYT can be accompanied by a simultaneous fall of [Ca2+]CYT in adjacent DVR endothelia (Fig. 3D). We showed that the DVR endothelium is an electrical syncytium and that connexins are expressed by both pericytes and endothelial cells (46). Figure 3 shows that asynchronous [Ca2+]CYT changes occur between individual pericytes and between pericytes and adjacent endothelium despite putative interendothelial and myoendothelial coupling. It is possible that ANG II helps to uncouple cells by closing gap junctions, as has been observed after ANG II stimulation of pericytes in the retinal circulation (25).
Microvessel contraction is tied to elevation of smooth muscle [Ca2+]CYT. The increase of [Ca2+]CYT can be partially mediated through voltage-gated Ca2+ channels and receptor- or store-operated nonselective cation channels (NSCC). Study of the Ca2+ influx pathways in smooth muscle of the renal microcirculation yielded varied results. Signature L- and T-type CaV currents have been elicited during patch clamp of preglomerular smooth muscle (15) and inhibitors of the former are effective blockers of either afferent constriction or the [Ca2+]CYT increase evoked by KCl depolarization (7, 17, 21, 31). Similarly, application of agonists such as ANG II, endothelin, and ATP consistently elicits preglomerular smooth muscle [Ca2+]CYT responses that are sensitive to L-type antagonists (1, 6, 18, 22, 23, 29). In the split hydronephrotic kidney preparation, ANG II constricts both afferent and efferent arterioles but depolarizes only the former (30). A possible role for efferent L- and T-type channel activation has been recognized in juxtaglomerular efferent smooth muscle (12, 17) and vasa recta (17, 47, 49).
We previously observed that CaV blockade reduces the plateau phase of [Ca2+]CYT elevation in DVR pericytes stimulated with ANG II (49). In contrast, NSCC-mediated Ca2+ entry is required for the maintenance of [Ca2+]CYT and STIC oscillations, when they occur. Evidence for the latter is that NSCC blockade with SKF96365 blocked both STIC and [Ca2+]CYT oscillations (Fig. 7). Given that the pericytes in this study were voltage clamped below the threshold for CaV activation, we cannot rule out a role for CaV in naturally occurring oscillations where membrane potential is free to swing above and below the CaV activation threshold. On the other hand, the slowly occurring depolarizations that characterize oscillations would probably tend to inactivate CaV rendering them unavailable to conduct significant Ca2+ entry. We conclude that participation of CaV is not essential to the maintenance of [Ca2+]CYT oscillations.
Particularly in renal efferent smooth muscle, pathways other than CaV participate in the agonist-activated Ca2+ entry. Both receptor-operated and store-operated NSCC have been implicated that are most likely transient receptor potential (TRP) channel isoforms. Using SKF96365 as a blocker of NSCC, it has been shown that ANG II-induced [Ca2+]CYT responses (29) and vasoconstriction (42) of efferent arterioles probably involve such channels. This study extends those observations by demonstrating that SKF96365 partially blocks ANG II-induced vasoconstriction of isolated, perfused DVR as well as ANG II-induced [Ca2+]CYT oscillations of DVR pericytes (Fig. 7). In high concentrations, SKF96365 can block voltage-gated channels. Our results cannot be fully accounted for by such nonspecific blockade because we used this reagent at an appropriately low concentration and because it was effective in cells whose membrane potential was clamped below the threshold for CaV activation, at −80 mV.
An important role for release of Ca2+ from intracellular stores in the maintenance of oscillations is also favored by our results. In this study, ryanodine and caffeine were very effective to block STIC oscillations (Fig. 8), pointing to a role for Ca2+ release from RyR-sensitive stores in their generation. This is similar to findings in other preparations (13, 19, 24, 32). 2-APB is frequently used to inhibit IP3 receptors but is known to have other effects, particularly to block store-operated cation channels. We previously showed that 2-APB suppresses DVR endothelial [Ca2+]CYT responses to ouabain (38). In this study, however, it did not affect STIC oscillations. Blockade of STICs by 2-APB in other preparations has been variable. It effectively blocked them in rabbit corpus cavernosum (8) while reducing spatial spread but not frequency in interstitial cells of Cajal (40). Wakui et al. (43) concluded that variation of intracellular IP3 is not required for oscillations to occur. The effectiveness of ryanodine and caffeine (Fig. 8) contrasted with the ineffectiveness of 2-APB may support a physical separation of RyR and IP3 releasable stores in DVR pericytes.
This work adds to the accumulating evidence that ANG II stimulation partially exerts its effects on pericytes through activation of Ca2+-dependent Cl− channels. In prior studies, we showed that blockade of those channels with niflumic acid or anthracene-9-carboxylic acid (A9C) reverses ANG II depolarization (35) and inhibits ANG II-mediated vasoconstriction (48). The identity of Cl− as the charge carrier was favored by the demonstration that a reversal potential shift of membrane current occurs when the concentration of extracellular Cl− ion is reduced (35). Similarly, at the functional level, ANG II-mediated DVR vasoconstriction intensifies when pericyte membrane potential depolarization is augmented by lowering extracellular Cl− concentration (48). In cells clamped below the equilibrium potential for Cl−, Ca2+-dependent Cl− channels transport Cl− ions from the interior to the exterior of the cell. That current is the major source of charge transfer during the STICs that synchronize with [Ca2+]CYT oscillations (Fig. 2), a conclusion that is favored by both reversal potential experiments (Fig. 4) and their sensitivity to niflumic acid (Fig. 5) (20, 26). The reversal potential we identified (Vrev = −30.2 ± 1.2 mV) is distant from the K+ equilibrium potential (EK = −84.7 mV) and close to, but clearly greater than, the equilibrium potential of Cl− ion (ECl = −43.2 mV) for our buffers. That difference between Vrev and ECl is most readily explained if a component of cation entry (Ca2+ and/or Na+) accompanies Cl− efflux to generate the post-ANG II STICs of DVR pericytes. STOCs are generally observed when quantum Ca2+ release events (sparks) stimulate Ca2+-dependent K+ channels (50). Both Vrev > ECl and failure to observe STOCs in cells clamped near ECl (Fig. 4) are consistent with possible inhibition of those channels by ANG II as previously surmised (34).
The use of fenamates such as niflumic acid as probes to identify Cl− conductance has come under increasing scrutiny. This is due to the fact that they can nonspecifically activate large-conductance Ca2+-dependent K+ channels (11, 14, 16). Despite this, it seems unlikely that K+ currents contaminated the recordings of this study to alter our conclusions. The holding potential for these experiments (−80 mV) was close to EK (−84.7 mV) and KCa activity is low in such hyperpolarized cells. Moreover, activation of K+ currents would tend to draw Vrev to values that are below ECl, not to higher values, as was observed (Fig. 4). We previously showed that ANG II inhibits classes of K+ channels in DVR pericytes and it is possible that KCa channels are suppressed as part of its action (5, 34).
Taken together, our data favor repetitive cycles of Ca2+ entry via NSCC and Ca2+-induced Ca2+ release (CICR) from RyR-sensitive stores as integral components of the generation of the [Ca2+]CYT oscillations that underlie STICs. A plausible scenario is that store-operated NSCC conduct Ca2+ into the cells where it both recharges SR stores and helps to raise [Ca2+]CYT to eventually trigger CICR. A slow rise in [Ca2+]CYT might also be favored by IP3R- and RyR-mediated leak of Ca2+ into the cytoplasm to reach the CICR threshold. This sequence predicts that NSCC current will tend to fill SR stores even as Ca2+-ATPases and, possibly, Na+/Ca2+ exchange (NCX) are concomitantly clearing the cytoplasm of Ca2+. That seems possible, particularly if the NSCC-mediated SR refilling occurs via microdomains that are spatially separate from the bulk cytoplasm. Preferential abutments of the SR with the plasmalemma, i.e., are recognized (4, 9, 27, 28).
In rodents, nanomolar concentrations of ouabain inhibit specific isoforms of Na-K-ATPase that are targeted to subplasmalemmal microdomains without inhibiting the predominant α1, housekeeping isoform (10, 27, 28, 41). That inhibition has been hypothesized to elevate subplasmalemmal Na+ and Ca2+ concentrations through reduction of NCX activity, resulting in an increase in Ca2+ content of ER/SR stores and bulk cytoplasm (4). Functional studies showed that this favors an increase in microvessel contractility and a rise in the Ca2+ released by agonists (3, 38, 45). Given the dependence of STICs on Ca2+ store release, we tested whether ouabain would affect their occurrence in DVR pericytes. An effect on post-ANG II currents was not found; however, ouabain did increase basal [Ca2+]CYT and STIC frequency (Fig. 9). The resting amplitude of STICs (means ± SE = 20.9 ± 0.8 pA; Fig. 9C) contrasts with higher values in ANG II-stimulated cells (means ± SE = 65.6 ± 1.9 pA; Fig. 1). Ouabain has also been shown to stimulate other signaling cascades through its ability to activate Src kinase (44) and we cannot rule out participation of those mechanisms in its modulation of pericyte [Ca2+]CYT.
In summary, we investigated the events that underlie STIC oscillations in DVR pericytes. Evidence favors the following interpretations. STICs are largely comprised of Cl− currents that arise from [Ca2+]CYT oscillations that stimulate ClCa channels. [Ca2+]CYT oscillations of pericytes on an isolated vessel can be asynchronous and ANG II suppresses endothelial [Ca2+]CYT even when adjacent pericyte [Ca2+]CYT rises or oscillates. The [Ca2+]CYT oscillations arise from repetitive refilling and release of Ca2+ from RyR-sensitive SR stores. The refilling partially occurs from the extracellular space via SKF96365-sensitive nonselective cation channels. Low-amplitude STICs occur in the absence of agonist stimulation and are increased along with resting [Ca2+]CYT by nanomolar concentrations of ouabain.
GRANTS
These studies were supported by National Institutes of Health Grants R37-DK-42495, R01-DK-67621, R01-DK-53775, and P01-HL-78870.
Supplementary Material
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Arendshorst WJ, Brannstrom K, Ruan X. Actions of angiotensin II on the renal microvasculature. J Am Soc Nephrol 10, Suppl 11: S149–S161, 1999. [PubMed] [Google Scholar]
- 2.Arnon A, Hamlyn JM, Blaustein MP. Na+ entry via store-operated channels modulates Ca2+ signaling in arterial myocytes. Am J Physiol Cell Physiol 278: C163–C173, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Arnon A, Hamlyn JM, Blaustein MP. Ouabain augments Ca2+ transients in arterial smooth muscle without raising cytosolic Na+. Am J Physiol Heart Circ Physiol 279: H679–H691, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Blaustein MP, Zhang J, Chen L, Hamilton BP. How does salt retention raise blood pressure? Am J Physiol Regul Integr Comp Physiol 290: R514–R523, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Cao C, Lee-Kwon W, Silldorff EP, Pallone TL. KATP channel conductance of descending vasa recta pericytes. Am J Physiol Renal Physiol 289: F1235–F1245, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Carmines PK, Morrison TK, Navar LG. Angiotensin II effects on microvascular diameters of in vitro blood-perfused juxtamedullary nephrons. Am J Physiol Renal Fluid Electrolyte Physiol 251: F610–F618, 1986. [DOI] [PubMed] [Google Scholar]
- 7.Conger JD, Falk SA. KCl and angiotensin responses in isolated rat renal arterioles: effects of diltiazem and low-calcium medium. Am J Physiol Renal Fluid Electrolyte Physiol 264: F134–F140, 1993. [DOI] [PubMed] [Google Scholar]
- 8.Craven M, Sergeant GP, Hollywood MA, McHale NG, Thornbury KD. Modulation of spontaneous Ca2+-activated Cl− currents in the rabbit corpus cavernosum by the nitric oxide-cGMP pathway. J Physiol 556: 495–506, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edwards A, Pallone TL. Modification of cytosolic calcium signaling by subplasmalemmal microdomains. Am J Physiol Renal Physiol 292: F1827–F1845, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Edwards A, Pallone TL. Ouabain modulation of cellular calcium stores and signaling. Am J Physiol Renal Physiol 293: F1518–F1532, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Farrugia G, Rae JL, Szurszewski JH. Characterization of an outward potassium current in canine jejunal circular smooth muscle and its activation by fenamates. J Physiol 468: 297–310, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng MG, Li M, Navar LG. T-type calcium channels in the regulation of afferent and efferent arterioles in rats. Am J Physiol Renal Physiol 286: F331–F337, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Feng Z, Wei C, Chen X, Wang J, Cheng H, Zhang X, Hong Q, Shi S, Fu B, Wei R. Essential role of Ca2+ release channels in angiotensin II-induced Ca2+ oscillations and mesangial cell contraction. Kidney Int 70: 130–138, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Gogelein H, Dahlem D, Englert HC, Lang HJ. Flufenamic acid, mefenamic acid and niflumic acid inhibit single nonselective cation channels in the rat exocrine pancreas. FEBS Lett 268: 79–82, 1990. [DOI] [PubMed] [Google Scholar]
- 15.Gordienko DV, Clausen C, Goligorsky MS. Ionic currents and endothelin signaling in smooth muscle cells from rat renal resistance arteries. Am J Physiol Renal Fluid Electrolyte Physiol 266: F325–F341, 1994. [DOI] [PubMed] [Google Scholar]
- 16.Greenwood IA, Large WA. Comparison of the effects of fenamates on Ca-activated chloride and potassium currents in rabbit portal vein smooth muscle cells. Br J Pharmacol 116: 2939–2948, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansen PB, Jensen BL, Andreasen D, Skott O. Differential expression of T- and L-type voltage-dependent calcium channels in renal resistance vessels. Circ Res 89: 630–638, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Helou CM, Marchetti J. Morphological heterogeneity of renal glomerular arterioles and distinct [Ca2+]i responses to ANG II. Am J Physiol Renal Physiol 273: F84–F96, 1997. [DOI] [PubMed] [Google Scholar]
- 19.Hogg RC, Wang Q, Helliwell RM, Large WA. Properties of spontaneous inward currents in rabbit pulmonary artery smooth muscle cells. Pflügers Arch 425: 233–240, 1993. [DOI] [PubMed] [Google Scholar]
- 20.Hogg RC, Wang Q, Large WA. Action of niflumic acid on evoked and spontaneous calcium-activated chloride and potassium currents in smooth muscle cells from rabbit portal vein. Br J Pharmacol 112: 977–984, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inscho EW, Mason MJ, Schroeder AC, Deichmann PC, Stiegler KD, Imig JD. Agonist-induced calcium regulation in freshly isolated renal microvascular smooth muscle cells. J Am Soc Nephrol 8: 569–579, 1997. [DOI] [PubMed] [Google Scholar]
- 22.Inscho EW, Schroeder AC, Deichmann PC, Imig JD. ATP-mediated Ca2+ signaling in preglomerular smooth muscle cells. Am J Physiol Renal Physiol 276: F450–F456, 1999. [DOI] [PubMed] [Google Scholar]
- 23.Iversen BM, Arendshorst WJ. ANG II and vasopressin stimulate calcium entry in dispersed smooth muscle cells of preglomerular arterioles. Am J Physiol Renal Physiol 274: F498–F508, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Janssen LJ, Sims SM. Spontaneous transient inward currents and rhythmicity in canine and guinea-pig tracheal smooth muscle cells. Pflügers Arch 427: 473–480, 1994. [DOI] [PubMed] [Google Scholar]
- 25.Kawamura H, Kobayashi M, Li Q, Yamanishi S, Katsumura K, Minami M, Wu DM, Puro DG. Effects of angiotensin II on the pericyte-containing microvasculature of the rat retina. J Physiol 561: 671–683, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Large WA, Wang Q. Characteristics and physiological role of the Ca2+-activated Cl− conductance in smooth muscle. Am J Physiol Cell Physiol 271: C435–C454, 1996. [DOI] [PubMed] [Google Scholar]
- 27.Lee CH, Kuo KH, Dai J, Leo JM, Seow CY, Breemen C. Calyculin-A disrupts subplasmalemmal junction and recurring Ca2+ waves in vascular smooth muscle. Cell Calcium 37: 9–16, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Lee MY, Song H, Nakai J, Ohkura M, Kotlikoff MI, Kinsey SP, Golovina VA, Blaustein MP. Local subplasma membrane Ca2+ signals detected by a tethered Ca2+ sensor. Proc Natl Acad Sci USA 103: 13232–13237, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loutzenhiser K, Loutzenhiser R. Angiotensin II-induced Ca2+ influx in renal afferent and efferent arterioles: differing roles of voltage-gated and store-operated Ca2+ entry. Circ Res 87: 551–557, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Loutzenhiser R, Chilton L, Trottier G. Membrane potential measurements in renal afferent and efferent arterioles: actions of angiotensin II. Am J Physiol Renal Physiol 273: F307–F314, 1997. [DOI] [PubMed] [Google Scholar]
- 31.Loutzenhiser R, Hayashi K, Epstein M. Divergent effects of KCl-induced depolarization on afferent and efferent arterioles. Am J Physiol Renal Fluid Electrolyte Physiol 257: F561–F564, 1989. [DOI] [PubMed] [Google Scholar]
- 32.Miriel VA, Mauban JR, Blaustein MP, Wier WG. Local and cellular Ca2+ transients in smooth muscle of pressurized rat resistance arteries during myogenic and agonist stimulation. J Physiol 518: 815–824, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pallone TL Microdissected perfused vessels. Methods Mol Med 86: 443–456, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Pallone TL, Cao C, Zhang Z. Inhibition of K+ conductance in descending vasa recta pericytes by ANG II. Am J Physiol Renal Physiol 287: F1213–F1222, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Pallone TL, Huang JM. Control of descending vasa recta pericyte membrane potential by angiotensin II. Am J Physiol Renal Physiol 282: F1064–F1074, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Pallone TL, Silldorff EP, Zhang Z. Inhibition of calcium signaling in descending vasa recta endothelia by ANG II. Am J Physiol Heart Circ Physiol 278: H1248–H1255, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Pallone TL, Zhang Z, Rhinehart K. Physiology of the renal medullary microcirculation. Am J Physiol Renal Physiol 284: F253–F266, 2003. [DOI] [PubMed] [Google Scholar]
- 38.Pittner J, Rhinehart K, Pallone TL. Ouabain modulation of endothelial calcium signaling in descending vasa recta. Am J Physiol Renal Physiol 291: F761–F769, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Rhinehart K, Handelsman CA, Silldorff EP, Pallone TL. ANG II AT2 receptor modulates AT1 receptor-mediated descending vasa recta endothelial Ca2+ signaling. Am J Physiol Heart Circ Physiol 284: H779–H789, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Sergeant GP, Johnston L, McHale NG, Thornbury KD, Hollywood MA. Activation of the cGMP/PKG pathway inhibits electrical activity in rabbit urethral interstitial cells of Cajal by reducing the spatial spread of Ca2+ waves. J Physiol 574: 167–181, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song H, Lee MY, Kinsey SP, Weber DJ, Blaustein MP. An N-terminal sequence targets and tethers Na+ pump alpha2 subunits to specialized plasma membrane microdomains. J Biol Chem 281: 12929–12940, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Takenaka T, Suzuki H, Okada H, Inoue T, Kanno Y, Ozawa Y, Hayashi K, Saruta T. Transient receptor potential channels in rat renal microcirculation: actions of angiotensin II. Kidney Int 62: 558–565, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Wakui M, Potter BV, Petersen OH. Pulsatile intracellular calcium release does not depend on fluctuations in inositol trisphosphate concentration. Nature 339: 317–320, 1989. [DOI] [PubMed] [Google Scholar]
- 44.Xie Z, Cai T. Na+-K+-ATPase-mediated signal transduction: from protein interaction to cellular function. Mol Interv 3: 157–168, 2003. [DOI] [PubMed] [Google Scholar]
- 45.Zhang J, Lee MY, Cavalli M, Chen L, Berra-Romani R, Balke CW, Bianchi G, Ferrari P, Hamlyn JM, Iwamoto T, Lingrel JB, Matteson DR, Wier WG, Blaustein MP. Sodium pump alpha2 subunits control myogenic tone and blood pressure in mice. J Physiol 569: 243–256, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Q, Cao C, Mangano M, Zhang Z, Silldorff EP, Lee-Kwon W, Payne K, Pallone TL. Descending vasa recta endothelium is an electrical syncytium. Am J Physiol Regul Integr Comp Physiol 291: R1688–R1699, 2006. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Z, Cao C, Lee-Kwon W, Pallone TL. Descending vasa recta pericytes express voltage operated Na+ conductance in the rat. J Physiol 567: 445–457, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Z, Huang JM, Turner MR, Rhinehart KL, Pallone TL. Role of chloride in constriction of descending vasa recta by angiotensin II. Am J Physiol Regul Integr Comp Physiol 280: R1878–R1886, 2001. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Z, Rhinehart K, Pallone TL. Membrane potential controls calcium entry into descending vasa recta pericytes. Am J Physiol Regul Integr Comp Physiol 283: R949–R957, 2002. [DOI] [PubMed] [Google Scholar]
- 50.ZhuGe R, Sims SM, Tuft RA, Fogarty KE, Walsh JV Jr. Ca2+ sparks activate K+ and Cl− channels, resulting in spontaneous transient currents in guinea-pig tracheal myocytes. J Physiol 513: 711–718, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.