Abstract
Social rejection can create powerful changes in our brains and bodies. Here, we examined brain-based individual differences associated with buffering against cardiovascular threat responses to social rejection. Using electroencephalographic source localization techniques, we examined differences in intracortical asymmetry with the prediction that individuals with greater left relative to right dorsolateral prefrontal activity would show a more approach motivated response to social rejection. Eighty-four female participants were randomly assigned to stressful situations characterized by either social rejection, social evaluation without rejection, or self-evaluation. Among those assigned to social rejection, the greater the left prefrontal intracortical activity at baseline, the more participants had adaptive cardiovascular profiles and the more participants reported approach-oriented emotions. Social evaluation without rejection and self-evaluation did not show these relationships. These data are the first to show that social context matters when attempting to link individual neural differences in cortical asymmetry with approach-related cardiovascular and emotional outcomes.
Social rejection is a common, perhaps ubiquitous, outcome for social animals. We can be rejected by a romantic interest, passed over for a job, or ignored and belittled by others. Psychological research has demonstrated the powerful effects of social rejection on our minds, bodies, and experiences. Social rejection can result in negative emotional responses, including increased shame, sadness, and anxiety (Ayduk, Mischel, & Downey, 2002; Williams, 2001), physiological changes such as increased and sustained catabolic hormone levels, reduced immune function, malignant cardiovascular responses (Cacioppo, Hawkley, & Berntson, 2003; Stroud, et al., 2000; Mendes, Major, McCoy, & Blascovich, 2008) and neural responses, including recruitment of brain regions implicated in coding the emotional component of pain, such as the dorsal anterior cingulate cortex (Eisenberger, Lieberman, & Williams, 2003). While the psychological and neurobiological correlates of social rejection have been explored, few studies have investigated what enables some individuals to retain an approach motivation in the face of social scrutiny. Here we examine individual differences in intracortical asymmetry as a buffer to the psychological and physiological threat that typically follow social rejection.
Correlational research suggests that frontal cortical asymmetry in favor of the left hemisphere is related to approach motivation (which could reflect either positive affect or anger), better ability to regulate negative emotions, and increased well-being (Davidson, 1993; Harmon-Jones, Gable, & Peterson, in press; Jackson et al., 2003; Urry et al., 2004). More specifically, recent evidence implicates left dorsolateral prefrontal cortex (DLPFC) regions in some of these positive psychological outcomes (Berkman & Lieberman, 2009; Pizzagalli, Sherwood, Henriques, & Davidson, 2005). But how do individual differences in frontal cortical asymmetry influence these long term outcomes? We suggest that individuals with relatively greater left cortical asymmetry might respond to acute stressful and socially evaluative situations with a more resilient response profile. This psychological mettle against life’s stressors may then accumulate to better long term outcomes, including well-being and life satisfaction. We hypothesized that relatively increased left DLPFC resting activity would buffer against an intense psychologically stressful experience, specifically social rejection. To test this hypothesis, we examined how individual differences in resting frontal activity influenced autonomic nervous system responses to social rejection compared to social evaluation without rejection and self-evaluation.
Brain-to-body effects
As ANS functioning must largely be determined by activity in the brain, it is surprising how little work has managed to bridge the divide between neural and autonomic functioning and predict ANS responses from brain activity. To examine how individual differences in cortical asymmetry influence downstream ANS changes, we considered situations that were highly stressful and would activate the body’s two primary stress systems: the sympathetic-adrenal-medullary (SAM) and hypothalamic-pituitary-adrenocortical (HPA) axes. Prior research suggests that relative activation of these two systems can differentiate benign, positive stress states (challenge) from more damaging stress responses (threat) (Dienstbier, 1989; Blascovich & Mendes, 2010; Mendes et al., 2008). Although both challenge and threat occur during stressful situations, the two states differ in their appraisal process and downstream cardiovascular reactivity. Accordingly, challenge occurs when individuals appraise their resources as exceeding the demands of the task, whereas threat occurs when situational demands exceed resources. Cardiovascular responses linked to challenge are characterized by increases from baseline in cardiac output (CO, the volume of oxygenated blood), and decreases in total peripheral resistance (TPR)—vasodilation. Threat is characterized by little or no increase in CO and increases in TPR—vasoconstriction. Challenge states have been associated with better cognitive performance (Kassam, Koslov, & Mendes, 2009), more approach-oriented behavior (Mendes et al., 2008), and reduced risk of cellular aging (Mendes & Epel, 2010). Furthermore, one of the primary determinants of “challenge” states, increased cardiac output, has been linked to decelerated brain aging in the Framingham sample (Jefferson, et al., 2010). Individuals with greater cardiac output had increased brain volume and showed increased cognitive processing speed in older adulthood, leading these researchers to speculate that increased oxygenated blood produced by the heart can have long-term protective effects in the brain.
In the present study, we expected that individuals with greater left prefrontal asymmetry would experience stressful situations with more challenge physiological profiles. We anticipated that this relationship would especially emerge during situations that were associated with social evaluative threat—when an aspect of the self could be negatively judged by others (Dickerson & Kemeny, 2004) - compared to situations that were self-relevant or socially evaluative, but not threatening.
Method
Participants
We recruited 87 females (age: M = 22.2; SD = 1.9) via advertisements for a 3-hour study on physiological responses during various tasks. During a phone interview we administered a portion of the Structured Clinical Inventory for the DSM-IV (SCID; First, Spitzer, Gibbon & Williams, 2002) and scheduled women who were right-handed, reported no personal or first-degree family history of Axis I psychopathology, learning disorders, or neurological conditions. Furthermore, we prescreened participants for general health conditions and provided instructions to reduce factors that would influence neuroendocrine variables during testing. Participants were scheduled during the follicular stage of their menstrual cycle (Symonds, Gallagher, Thompson & Young, 2004) and compensated $10/hour.
Procedure
Upon arrival, participants were told that the experiment’s general purpose was to investigate physiological responses during rest versus active tasks. We did not initially describe the stress task to prevent anticipatory stress that might contaminate baseline assessments. We applied sensors for EEG and ANS response recording; then participants sat for an 8-min resting baseline. Approximately 30 minutes after arrival, the first saliva sample was obtained.
Social evaluation task
Next, the experimenter described the upcoming task and verbal consent was obtained. Participants were instructed that they would first prepare and then deliver a 5-minute speech, which would be followed by a 5-minute question and answer (Q&A) session in a mock job interview (Akinola & Mendes, 2008). Participants were randomly assigned to one of three conditions: no social evaluation (NSE; control); social evaluation (SE; with positive feedback), or social evaluative threat (SET; with negative feedback). These conditions were operationalized in terms of the presence or absence of interviewers during the speech (SE and SET vs. NSE) and then evaluation was further differentiated into SE and SET based on the type of non-verbal feedback given by interviewers. In the control condition, participants were told that they would deliver the speech alone in the room. The control condition was designed to require similar metabolic demands associated with speaking, but without social evaluation.
In the two social evaluation conditions, participants were informed that they would deliver the speech to two interviewers. Once the participants gave consent, two research assistants (one male, one female) entered the room to reiterate the instructions. Subsequently, participants were left alone for five minutes of speech preparation.
After preparation, the interviewers re-entered the room and participants began the speech. At this point the experimental conditions diverged into either social evaluation or social evaluative threat. The interviewers’ roles were scripted and coordinated so that all participants had a consistent experience. In SE, interviewers gave positive non-verbal feedback by smiling, nodding, leaning forward, and appearing to be actively engaged during the speech. In contrast, in the SET condition, interviewers shook their heads, frowned, leaned back, and appeared to dislike the participant’s performance. Prior to, as well as following, the speech all participants completed an appraisal and affect questionnaire.
Next, participants completed a 5-minute Q&A session during which the interviewers asked general questions (e.g., “Are you striving to be a jack of all trades or an expert in one field?”). During the Q&A, the feedback manipulations in the SE and SET conditions were maintained. In the NSE condition, the participant was handed index cards with one question per card and was instructed to read each question and then answer it aloud. Five minutes of cardiovascular data were collected during the speech and Q&A sessions.
After the Q&A, the interviewers left the room. A recovery period commenced after which the experimenter collected a second saliva sample that served as our reactivity sample (25 minutes from the start of the stressor). The participants then completed tasks not discussed here. Forty-five minutes after the start of the stressor, participants provided a third saliva sample that served as our recovery sample.
Physiological Responses
EEG measures
Resting EEG was recorded using a 128-channel Electrical Geodesic system (EGI Inc., Eugene, OR) during 8 alternating one-minute periods (four eyes closed, four eyes open; counterbalanced across participants). Data were sampled at 250 Hz (0.1-100 Hz analog filter) and referenced to the vertex. Impedances were kept below 45 kΩ.
ANS measures
Cardiac measures were recorded noninvasively using an ambulatory impedance cardiography recording device, AMS46 (Vrije University, Amsterdam). Cardiac impedance (Z0) and electrocardiogram (ECG) recordings were obtained from six electrodes placed on the neck and torso. In addition to these cardiac measures, blood pressure was measured throughout using tonometric technology (Biopac, Goleta, CA), which estimates blood pressure from the radial artery.
Data were scored in 1-minute segments to calculate cardiac output (CO), a measure of the blood being pumped from the heart, and pre-ejection period (PEP), a time-based measure of the force of the ventricle contractions. Total peripheral resistance (TPR) was estimated with the standard equation: (Mean Arterial Pressure/CO) × 80.
Neuroendocrine measures
Samples were obtained using the passive drool method and stored at −80° C. Upon completion of the study, samples were sent to Kirschbaum’s laboratory in Dresden, Germany, to be assayed for cortisol using commercial immunoassay kits (IBL). Intra- and inter-assay coefficients were less than 10%.
Self-report measures
We assessed demand and resources appraisals, affect states, and participants’ perceptions of how the interviewers perceived them (Akinola & Mendes, 2008). As in previous research, we created a ratio of perceived demands to personal resources to calculate a threat index, with higher scores reflecting greater threat states.
Self-reported affect was assessed using the Positive and Negative Affect Schedule (PANAS; Watson, Clark, & Tellegen, 1988). Participants rated their current feelings on 20 affect states (10 positive and 10 negative) using 5-point scales ranging from 1 (not at all) to 5 (a great deal). The positive and negative affect scales were calculated for each time point and had high reliability (αs: .85-.91). We also calculated an a priori “approach” scale using the items strong, alert, determined, and active (αs: .72-.76).
As a manipulation check, participants assigned to the evaluation conditions rated how well they believed each of the interviewers thought they performed (e.g., “She thought I performed well on the task”). Responses for the male and female judges were highly correlated (α = .91) so we averaged these responses into a single score.
Data Reduction and Scoring
EEG data
EEG data were re-referenced off-line to an average reference. Eye-movement (e.g., blinks) and ECG artifacts were removed using Independent Component Analysis (ICA) performed using Brain Vision Analyzer software (Brain Products GmbH, Germany). Data were scored manually to eliminate artifacts, and all available artifact-free 2048-ms EEG epochs were extracted. Low Resolution Electromagnetic Tomography (LORETA, Pascual-Marqui et al., 1999) was used to estimate current density for various EEG frequency bands; in light of the extensive frontal EEG asymmetry literature, analyses focused on the alpha1 (8.5-10.0 Hz) and alpha2 (10.5-12.0 Hz) band (Pizzagalli et al., 2005). The LORETA solution space included 2,394 cubic elements (“voxels,” 7 mm3) and was restricted to cortical gray matter and hippocampi, as defined by the MNI305 template (Montreal Neurologic Institute, Montreal, Quebec, Canada). Before statistical analyses, overall current density for each band was intensity-normalized to 1.
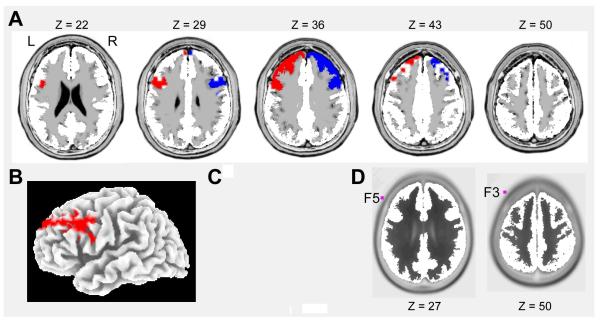
In light of a priori hypotheses about the role of alpha activity within DLPFC regions, a region-of-interest (ROI) approach was used to minimize the number of statistical tests performed. Specifically, the left and right Brodmann Areas (BA) 9 and 46 were anatomically defined (Lancaster et al., 2000; Petrides & Pandya, 1999; Rajkowska & Goldman-Rakic, 1995a,b) (Figure 1). The left and right BA9 contained 35 (12.01 cm3) and 38 (13.03 cm3) voxels, respectively; the left and right BA46 each included 12 voxels (4.12 cm3).
Figure 1.
(a) Axial slices (in 7-mm increments) showing the location and extent of the left (red) and right (blue) Brodmann areas 9, which were defined using the Talairach Daemon (Lancaster et al., 2000) as well as anatomical landmarks (Petrides & Pandya, 1999; Rajkowska & Goldman-Rakic, 1995a,b). (b) 3-D cortical surface rendering of Brodmann area 9; (c) Cytoarchitectonic maps of the lateral surface of the human frontal lobe according to Petrides & Pandya (1999) [reproduced with permission]; (d) Axial slices showing the 3-dimensional location of the scalp electrodes F3 and F5 with respect to underlying neuroanatomy. Coordinates in MNI space. L = left, R = right.
The extracted alpha1 and alpha2 current density was averaged across voxels and log-transformed, and then frontal asymmetry was calculated by taking the current density in the right and subtracting current density in the left. Because alpha activity is inversely correlated with brain activation (Coan & Allen, 2004, Davidson, Jackson & Larson, 2000; Oakes et al., 2004), a positive frontal intracortical asymmetry index reflects relatively higher activity in the left DLPFC. The four variables (2 sub-bands x 2 ROIs) were highly correlated (r = .93, p < .0001); accordingly, analyses focused on a composite of alpha1 and alpha2 extracted from BA9. BA9 was prioritized because it was spatially closer than BA46 to the location of F3 and F4 (Figure 1d), the scalp electrodes most widely probed in frontal EEG studies. Similar, albeit statistically less robust, findings emerged when considering BA46.
Results
Participant attrition
Of the original 87 participants, one was lost because of illness, and two were excluded due to protocol deviations. The remaining 84 participants were used in all analyses and varying degrees of freedom below represent missing values for physiological or self-report data.
Sympathetic nervous system responses
We first compared the NSE condition to the average of the two evaluation conditions with respect to changes in sympathetic activation, PEP, during the stress task. Average changes in PEP from the interview task yielded a significant difference by evaluation condition, F (1, 73) = 17.34, p < .0001. As expected, the NSE condition resulted in significantly less sympathetic activation (ΔPEP: M = −2.0, S.D. = 7.0) than the evaluation conditions (ΔPEP: M = −10.2, S.D. = 8.5). Importantly, only the evaluation conditions resulted in a significant decrease from baseline (evaluation, t (50) = −8.47, p < .0001; no evaluation, t (25) = −1.41, ns).
Subjective experience
Next, we examined if the manipulations were perceived and experienced as intended. We operationalized social evaluative threat as the extent to which participants believed that they were performing poorly and experienced the interview task as more threatening. To confirm this manipulation, we first examined participants’ responses to how they believed the interviewers perceived their speech. As intended, participants in the SET condition perceived the evaluators as disliking their interview more than those in the SE condition, F (1, 51) = 19.5, p < .0001 (Table 1).
Table 1.
Means and SDs of perceptions of judges, affect, and appraisals by feedback condition
| Feedback Condition | |||
|---|---|---|---|
| Social Evaluation | Non-Social | ||
| Social evaluation |
Social evaluative threat |
Control | |
| “[Judges] thought I performed well”1 |
4.77 (1.0)a | 3.34 (1.4)b | -- |
| Negative affect2 | 1.6 (.5) b | 1.9 (.9) a | 1.4 (.4) b |
| Positive affect 2 | 2.7 (.9)a | 2.3 (.8)b | 2.4 (1.) ab |
| Threat ratio | .73 (.3)a | .91 (.4)b | .73 (.2)a |
Note.
Ratings for the male and female judges were averaged.
Means are adjusted for prespeech affect ratings. Different subscript letters across rows indicate significant differences by feedback condition.
We then compared all conditions on appraisals and changes in affect. The SET condition resulted in participants appraising the situation as more threatening than those in the SE or NSE condition, F (2, 81) = 3.82, p < .02. We then examined negative and positive affect and observed significant differences by condition. Controlling for pretask affect, negative affect was significantly greater in the SET condition than the other conditions, F (2, 81) = 6.50, p < .01. Similarly, positive affect was higher in SE than the other conditions, F (2, 81) = 4.69, p < .02, driven primarily by higher positive affect in SE compared to SET. Altogether, these findings indicate that we successfully manipulated the subjective experience of different types of social evaluation.
Neuroendocrine responses
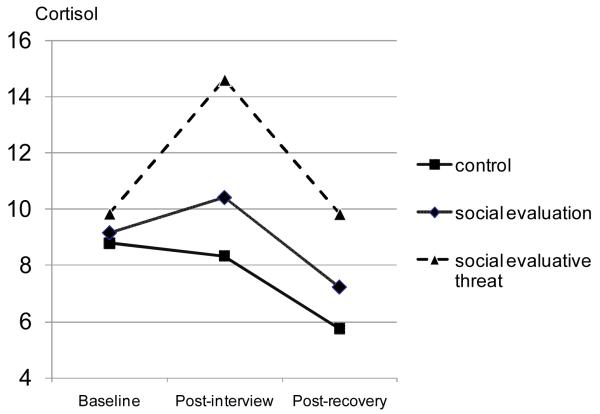
To evaluate neuroendocrine data, we conducted a mixed model analysis of variance (ANOVA) with condition as between subjects, time (baseline, reactivity, and recovery) as within subjects, and number of hours since waking as the covariate. This model produced a significant effect for condition, F (2, 79) = 4.48, p < .014, which was qualified by a significant time by condition interaction, F (4, 158) = 2.58, p < .04 (Figure 2). Simple effects tests within each time period showed that there were no differences between conditions at baseline, F (2, 79) = 0.48, ns, but there were significant condition effects at time 2 (reactivity), F (2, 79) = 4.29, p < .02, and time 3 (recovery), F (2, 79) = 4.77, p < .01. Orthogonal simple comparisons from time 2 confirmed that cortisol reactivity was greater in the SET relative to SE condition, F (1, 79) = 3.91, p < .05, which in turn elicited greater cortisol reactivity than the control condition, F (1, 79) = 4.67, p < .04. Simple comparisons between the conditions at time 3 yielded similar findings (SET > SE; F (1, 79) = 4.05, p < .05).
Figure 2.
Cortisol levels (nmol/L) in the control, social evaluation and social evaluative threat conditions at baseline (Time 1), reactivity (Time 2) and recovery (Time 3).
As cortisol increases tend to be psychologically non-specific—many different psychological states are associated with increased cortisol responses—we did not expect cortisol reactivity to be associated with resting BA9 activity. We examined correlations among cortisol reactivity and resting BA9 responses and even though the direction of the relationship was consistent with more adaptive profiles associated with more left cortical asymmetry none of the condition effects were significant: no social evaluation, r = −.16; social evaluation, r = −.15; social evaluative threat, r = −.36.
Left asymmetric intracortical activity as a buffer to social threat
Our primary prediction was context-specific, and suggested relations between EEG asymmetry and autonomic activation only in the SET condition. Before testing this prediction, we examined whether asymmetric activation was related to any of the cardiovascular responses at rest. We tested the bivariate correlation between our asymmetry variable and resting CV responses, specifically CO, PEP, and TPR. None of the CV responses at baseline were significantly correlated with asymmetric activity, all rs < |0.12|.
We then tested the primary prediction that greater left asymmetric activity would be associated with buffered cardiovascular reactivity to the SET condition but not NSE or SE conditions. We first calculated bivariate correlations of asymmetric activity and cardiovascular reactivity data by condition. As shown in Table 2, significant correlations emerged between relative left frontal cortical activity and cardiovascular and emotional indicators of threat/challenge, but only in the SET condition. These correlations show that the greater the relative left frontal activity at rest, the higher the CO, the lower the TPR reactivity, and the greater the self-reported, approach affect during SET, all indicating greater challenge responses.
Table 2.
Bivariate correlations among relative left frontal cortical activation and cardiovascular indices of challenge and threat and self-reported “approach” states by feedback condition
| No Social Evaluation |
|||
|---|---|---|---|
| Δ CO | Δ TPR | Approach | |
| Relative left frontal cortical activity |
−.30 | .31 | .22 |
|
| |||
| Social Evaluation | |||
| Relative left frontal cortical activity |
.21 | −.05 | .24 |
|
| |||
| Social Evaluative Threat | |||
| Relative left frontal cortical activity |
.56** | −.45* | .42* |
p < .05
p < .01.
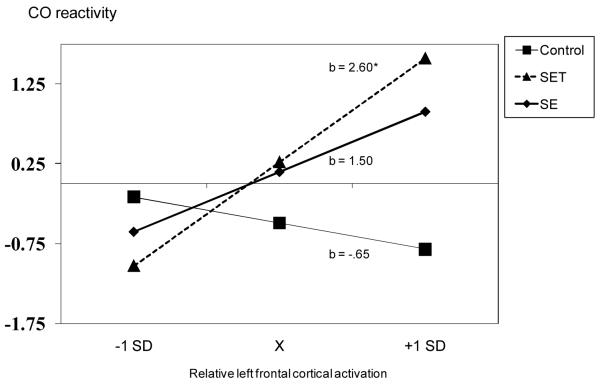
Using regression analyses, we then formally tested whether the effects we observed in SET were significantly different from those in the other conditions. When predicting CO, the first step, which included the asymmetry variable and effect-coded condition main effects, produced a non-significant model, R2 = .11, n.s. The second step included the initial predictors plus the interaction terms (condition by asymmetry interactions). As expected, the inclusion of the interaction terms significantly increased model fit, ΔR2 = .07, p < .02. Supporting the threat buffering hypothesis, the more participants displayed relatively greater left asymmetry during rest the greater the CO increase during the social evaluative threat task (b = 2.60, p < .01) (Figure 3a). The relations between asymmetry and CO changes were not significant during the SE (b = 1.50, ns) or the control (b = −0.65, ns) conditions.
Figure 3a.
Participants’ left frontal asymmetry predicting their CO reactivity (L) in the control, social evaluation and social evaluative threat conditions. Predicted regression values are plotted at the mean and +/− SD of asymmetry.
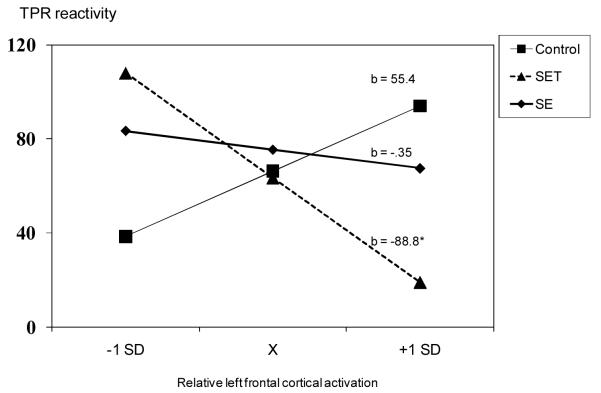
We re-ran this model predicting changes in TPR. The initial model was not significant, R2 = .11, n.s, however, the addition of the asymmetry x condition terms significantly increased model fit, ΔR2 = .06, p < .022 (Figure 3b). Similar to the CO analyses, among participants assigned to the SET condition, the greater the left frontal asymmetry the lower the TPR (b = −.88, p < .05). Asymmetric activity was not related to TPR changes during the SE (b = −35.0, ns) or NSE (b = 55.4, ns) conditions.
Figure 3b.
Participants’ left frontal asymmetry predicting their TPR reactivity in the control, social evaluation and social evaluative threat conditions. Predicted regression values are plotted at the mean and +/− SD of asymmetry.
We then used this model to predict self-reported approach emotions. Although the bivariate correlations showed significant relations between asymmetry and self-reported approach affect in the SET condition and not in the other conditions, the effects in SET were not significantly different than the other conditions.
Discussion
The goal of the present study was to examine individual differences in frontal resting asymmetry as a predictor of approach motivation in stressful situations involving social rejection. We hypothesized that during socially evaluative situations resting left DLPFC asymmetry would buffer against threat responses as indexed by cardiovascular reactivity. We observed significant associations between left frontal asymmetry and cardiovascular stress responses, but only when participants were exposed to social rejection. Specifically, under social threat conditions, left frontal asymmetry (measured as an average of alpha1 and alpha2 current density in Brodmann area 9) predicted increased cardiac output - a sign of cardiac efficiency - and decreased total peripheral pressure - an indication of dilation in the arterioles, both of which have been linked to a challenge or approach stress states. Conversely, the less left frontal asymmetry was associated with maladaptive, or threat, cardiovascular response. Collectively, these findings indicate that participants with higher resting activity in the left relative to right prefrontal cortex exhibited more adaptive, approach-oriented cardiovascular stress responses when confronted with social evaluative threat.
These data highlight the importance of taking into account environmental and contextual factors when seeking the putative impact of brain-based traits on physiological and emotional outcomes. We know of no previous studies which have examined relationships between frontal cortical asymmetry and cardiovascular responding, and this work indicates that such relationships may only emerge when examined in relevant contexts. In this study, that context was social evaluation, operationalized as a motivated performance situation with two interviewers, where either the interviewers gave positive feedback - which was itself a protective factor - or negative feedback, leading to a situation of social evaluative threat. The beneficial effects of left prefrontal asymmetry emerged only in the condition where participants were without environmental protective factors, in short, when they were most vulnerable to experiencing social stress.
In spite of these findings, it is important to emphasize that it is unclear from our data what affective states are associated with approach-motivated physiology. While challenge states are often associated with positive affect, these states have also been associated with anger (Mendes et al., 2008). Furthermore, left prefrontal asymmetry has been associated with anger responses (Harmon-Jones, 2003; Harmon-Jones & Allen, 1998), a negatively valenced, approach-related emotion. Given this prior data highlighting both positive and negative affective correlates of left frontal asymmetry, we must be cautious in interpreting these left frontal asymmetry relationships in a purely positive light. Individuals with relatively higher left frontal activity likely experienced a blend of affective responses in the social threat condition - anger and challenge. Importantly, we did not find any evidence that participants were angrier using PANAS items, but we would consider this an open question. The careful conclusion to draw from this work is that left frontal asymmetry was associated with approach motivation, and future research should attempt to disambiguate the valence components of this response.
One could draw parallels between this work and research highlighting associations between specific genetic traits and emotional disorders emerging exclusively when considering life stressors (e.g. Caspi et al., 2003). In an acute setting, we found that right prefrontal asymmetry might represent a disposition to experiencing exacerbated threat to social rejection. As researchers continue to search for biological differences in the etiology of physical and mental diseases, we think the present findings represent a simple but powerful example that context matters -reactions to an acute stressor can reveal relationships that do not exist during resting states or “positive” stress experiences.
There may be important physical and psychological health outcomes which are dependent on both an individual’s trait frontal asymmetry and the types of social stressors s/he encounters in life. Since individuals with right prefrontal asymmetry demonstrated malignant acute reactivity to a social threat this may accumulate over time to vulnerabilities such as coronary disease or hypertension. In addition, increased sensitivity to and vigilance for social threat could contribute to the development and maintenance of social anxiety or depression. Within this framework, it is interesting to note that both depression (Pizzagalli et al., 2002) and social anxiety disorders (Davidson, et al, 2002) have been associated with increased right frontal asymmetry. In sum, our findings demonstrate that left resting prefrontal asymmetry can act as a protective factor for individuals in a threatening situational context, while right prefrontal asymmetry may be an important vulnerability factor to consider in stress-diathesis models of disease etiology and progression.
Acknowledgements
This research was supported by a NHLB Institute grant (RO1 HL079383) to WBM, and an NIMH grant (R01 MH68376) to DAP. Inquiries can be directed to Wendy Berry Mendes (wendy.mendes@ucsf.edu).
References
- Akinola M, Mendes WB. The dark side of creativity: Biological vulnerability and negative mood leads to greater artistic creativity. Personality and Social Psychology Bulletin. 2008;34:1677–1686. doi: 10.1177/0146167208323933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayduk O, Mischel W, Downey G. Attentional mechanisms linking rejection to hostile reactivity: The role of “hot” vs. “cool” focus. Psychological Science. 2002;13:443–448. doi: 10.1111/1467-9280.00478. [DOI] [PubMed] [Google Scholar]
- Berkman ET, Lieberman MD. Approaching the bad and avoiding the good: Lateral prefrontal cortical asymmetry distinguishes between action and valence. Journal of Cognitive Neuroscience. 2010;22:1970–1979. doi: 10.1162/jocn.2009.21317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blascovich J, Mendes WB. Social psychophysiology and embodiment. In: Fiske ST, Gilbert DT, Lindzey G, editors. The Handbook of Social Psychology. 5th Edition Wiley; New York: 2010. pp. 194–227. [Google Scholar]
- Cacioppo JT, Hawkley LC, Berntson GG. The anatomy of loneliness. Current Directions in Psychological Science. 2003;12:71–74. [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Coan JA, Allen JJB. Frontal EEG asymmetry as a moderator and mediator of emotion. Biological Psychology. 2004;67:7–49. doi: 10.1016/j.biopsycho.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Cerebral asymmetry and emotion: Conceptual and methodological conundrums. Cognition & Emotion. 1993;7:115–138. [Google Scholar]
- Davidson RJ, Jackson DC, Larson CL. Human electroencephalography. In: Cacioppo JT, Bernston GG, Tassinary LG, editors. Handbook of Psychophysiology. 2nd Edition Cambridge University Press; New York: 2000. pp. 27–52. [Google Scholar]
- Davidson RJ, Marshall JR, Tomarken AJ, Henriques JB. While a phobic waits: regional brain electrical and autonomic activity in social phobics during anticipation of public speaking. Biological Psychiatry. 2000;47:85–95. doi: 10.1016/s0006-3223(99)00222-x. [DOI] [PubMed] [Google Scholar]
- Dienstbier RA. Arousal and physiological toughness: Implications for mental and physical health. Psychological Review. 1989;96:84–100. doi: 10.1037/0033-295x.96.1.84. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute Stressors and Cortisol Responses: A Theoretical Integration and Synthesis of Laboratory Research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An fMRI study of social exclusion. Science. 2003;302:290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version. Biometrics Research, New York State Psychiatric Institute; New York: 2002. Patient Edition. (SCID-I/P) [Google Scholar]
- Harmon-Jones E. Clarifying the emotive functions of asymmetrical frontal cortical activity. Psychophysiology. 2003;40:838–848. doi: 10.1111/1469-8986.00121. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Allen JJB. Anger and frontal brain activity: EEG asymmetry consistent with approach motivation despite negative affective valence. Journal of Personality and Social Psychology. 1998;74(5):1310–1316. doi: 10.1037//0022-3514.74.5.1310. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Gable PA, Peterson CK. The role of asymmetric frontal cortical activity in emotion-related phenomena: A review and update. Biological Psychology. doi: 10.1016/j.biopsycho.2009.08.010. in press. 2009 Sep 4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Jackson DC, Mueller CJ, Dolski I, Dalton KM, Nitschke JB, Urry HL, Rosenkranz MA, Ryff CD, Singer BH, Davidson RJ. Now you feel it, now you don’t: Frontal Brain Electrical Asymmetry and Individual Differences in Emotion Regulation. Psychological Science. 2003;14:612–617. doi: 10.1046/j.0956-7976.2003.psci_1473.x. [DOI] [PubMed] [Google Scholar]
- Jefferson AL, Himali JJ, Beiser AS, Au R, Massaro JM, Seshadri S, Gona P, et al. Cardiac index is associated with brain aging. Circulation. 2010;122:690–697. doi: 10.1161/CIRCULATIONAHA.109.905091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassam KS, Koslov K, Mendes WB. Decisions under distress: Stress profiles influence anchoring and adjustment. Psychological Science. 2009;20:1394–1399. doi: 10.1111/j.1467-9280.2009.02455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH. Salivary cortisol in psychoneuroendocrine research: Recent developments and applications. Psychoneuroendocrinology. 1994;19:313–333. doi: 10.1016/0306-4530(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov DN, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Human Brain Mapping. 10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes WB, Epel ES. Lower levels of telomerase predict acute threat reactivity during stressful laboratory tasks. 2010 Manuscript in preparation. [Google Scholar]
- Mendes WB, Major B, McCoy S, Blascovich J. How attributional ambiguity shapes physiological and emotional responses to social rejection and acceptance. Journal of Personality and Social Psychology. 2008;94:278–291. doi: 10.1037/0022-3514.94.2.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes TR, Pizzagalli DA, Hendrick AM, Horras KA, Larson CL, Abercrombie HC, Schaefer SM, Koger JV, Davidson RJ. Functional coupling of simultaneous electrical and metabolic activity in the human brain. Human Brain Mapping. 2004;21:257–270. doi: 10.1002/hbm.20004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Marqui RD, Lehmann D, Koenig T, Kochi K, Merlo MC, Hell D, Koukkou M. Low resolution brain electromagnetic tomography (LORETA) functional imaging in acute, neuroleptic-naive, first-episode, productive schizophrenia. Psychiatry Research: Neuroimaging. 1999;90:169–179. doi: 10.1016/s0925-4927(99)00013-x. [DOI] [PubMed] [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Echallier JF. Spherical splines for scalp potential and current density mapping. Electroencephalography and clinical neurophysiology. 1989;72:184–197. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya D. Dorsolateral prefrontal cortex: Comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. European Journal of Neuroscience. 1999;11:1011–1036. doi: 10.1046/j.1460-9568.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Nitschke JB, Oakes TR, Hendrick AM, Horras KA, Larson CL, Abercrombie HC, Schaefer SM, Koger JV, Benca RM, Pascual-Marqui RD, Davidson RJ. Brain electrical tomography in depression: the importance of symptom severity, anxiety, and melancholic features. Biological Psychiatry. 2002;52:73–85. doi: 10.1016/s0006-3223(02)01313-6. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Oakes TR, Fox AS, Chung MK, Larson CL, Abercrombie HC, Schaefer SM, Benca RM, Davidson RJ. Functional but not structural subgenual prefrontal cortex abnormalities in melancholia. Molecular Psychiatry. 2004;9:393–405. doi: 10.1038/sj.mp.4001501. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Sherwood RJ, Henriques JB, Davidson RJ. Frontal Brain Asymmetry and Reward Responsiveness: A Source-Localization Study. Psychological Science. 2005;16:805–813. doi: 10.1111/j.1467-9280.2005.01618.x. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Goldman-Rakic P. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: I. Remapping of Areas 9 and 46 using quantitative criteria. Cerebral Cortex. 1995a;5:307–322. doi: 10.1093/cercor/5.4.307. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Goldman-Rakic P. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: II. Variability in locations of Areas 9 and 46 and relationship to the Talairach coordinate system. Cerebral Cortex. 1995;5:323–337. doi: 10.1093/cercor/5.4.323. [DOI] [PubMed] [Google Scholar]
- Stroud LR, Tanofsky-Kraff M, Wilfley DE, Salovey P. The Yale Interpersonal Stressor (YIPS): Affective, physiological, and behavioral response to a novel interpersonal rejection paradigm. Annals of Behavioral Medicine. 2000;22:1–11. doi: 10.1007/BF02895115. [DOI] [PubMed] [Google Scholar]
- Symonds CS, Gallagher P, Thompson JM, Young AH. Effects of the menstrual cycle on mood, neurocognitive and neuroendocrine function in healthy premenopausal women. Psychological Medicine. 2004;34:93–102. doi: 10.1017/s0033291703008535. [DOI] [PubMed] [Google Scholar]
- Urry HL, Nitschke JB, Dolski I, Jackson DC, Dalton KM, Mueller CJ, Rosenkranz MA, Ryff CD, Singer BH, Davidson RJ. Making a life worth living: Neural correlates of well-being. Psychological Science. 2004;15:367–372. doi: 10.1111/j.0956-7976.2004.00686.x. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and Validation of Brief Measures of Positive and Negative Affect: The PANAS Scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Williams KD. Ostracism: The power of silence. Guilford Publications; New York, NY: 2001. [Google Scholar]