Abstract
Supplemental oxygen contributes to the development of bronchopulmonary dysplasia (BPD) in premature infants. In this investigation, we tested the hypothesis that prenatal treatment of pregnant mice (C57BL/6J) with the cytochrome P450 (CYP)1A1 inducer, β-napthoflavone (BNF), will lead to attenuation of lung injury in newborns (delivered from these dams) exposed to hyperoxia by mechanisms entailing transplacental induction of hepatic and pulmonary CYP1A enzymes. Pregnant mice were administered the vehicle corn oil (CO) or BNF (40 mg/kg), i.p., once daily for 3 days on gestational days (17–19), and newborns delivered from the mothers were either maintained in room air or exposed to hyperoxia (> 95% O2) for 1–5 days. After 3–5 days of hyperoxia, the lungs of CO-treated mice showed neutrophil infiltration, pulmonary edema, and perivascular inflammation. On the other hand, BNF-pretreated neonatal mice showed decreased susceptibility to hyperoxic lung injury. These mice displayed marked induction of ethoxyresorufin O-deethylase (EROD) (CYP1A1) and methoxyresorufin O-demethylase (MROD) (CYP1A2) activities, and levels of the corresponding apoproteins and mRNA levels until PND 3 in liver, while CYP1A1 expression alone was augmented in the lung. Prenatal BNF did not significantly alter gene expression of pulmonary NAD(P)H quinone reductase (NQO1). Hyperoxia for 24–72 h resulted in increased pulmonary levels of the F2-isoprostane 8-iso-PGF2α, whose levels were decreased in mice prenatally exposed to BNF. In conclusion, our results suggest that prenatal BNF protects newborns against hyperoxic lung injury, presumably by detoxification of lipid hydroperoxides by CYP1A enzymes, a phenomenon that has implications for prevention of BPD in infants.
Keywords: Hyperoxia, CYP1A enzymes, Oxidant injury, lung injury, newborn, Bronchopulmonary dysplasia
INTRODUCTION
Supplemental oxygen is frequently used in the treatment of pulmonary insufficiency in preterm and term neonates (Northway and Rosan, 1968; Fisher, 1980). Considerable evidence links oxygen exposure to the development of neonatal diseases such as bronchopulmonary dysplasia (BPD), a major cause of morbidity and mortality in premature infants (Northway and Rosan, 1968; Bland and Coalson, 2000). It is well known that hyperoxia causes lung injury in animal models (Clark and Lambersten, 1971; Freeman and Crapo, 1981; Bryan and Jenkinson, 1988; Budinger et al., 2010), and similar lung injury may occur in infants undergoing supplemental oxygen therapy (Wagenaar et al., 2004; Bhandari, 2008; 2010). In fact, the prolonged exposure of newborn mice to hyperoxia creates pulmonary lesions that are very similar to human BPD (Warner et al., 1998; Ohki et al., 2009). Mechanical ventilation, which is almost always used in conjunction with supplemental oxygen in premature infants suffering from pulmonary insufficiency, also contributes to BPD in these patients (Gagliardi et al., 2011; Gupta et al., 2009). In utero viral infection may also contribute to BPD pathogenesis (Sawyer et al,, 1987; Couroucli et al., 2000). Thus, the development of BPD is multifactorial, with oxygen toxicity being one of the major factors,
The molecular mechanisms responsible for oxygen toxicity are not completely understood, but reactive oxygen species (ROS) such as superoxide anion, hydroxyl radical, and hydrogen peroxide are known to play a role (Moorthy et al., 1997; Morel and Barouki, 1998; Yang et al., 1999; Moorthy et al., 2000). The proposed mechanism of injury involves the oxidation/peroxidation of lung lipids, proteins, or other molecules (Freeman and Crapo, 1981).
The cytochrome P450 (CYP) system of enzymes is a superfamily of heme-containing proteins that is involved in the metabolism of a number of exogenous and endogenous compounds (Guengerich, 1990; 2004). The CYP1A1 isoform is classically induced by planar aromatic hydrocarbons, such as benzo[a]pyrene found in cigarette smoke, or 3-methylcholanthrene (MC) by Ah receptor (AHR)-dependent mechanisms (Whitlock et al., 1996; Whitlcok,1999; Nebert et al., 2004). Previous work in rodent models has shown that hyperoxia also induces CYP1A1 (Okamoto et al., 1993; Sindhu et al., 2000), but the mechanism involved is not clearly understood, although AHR-dependent mechanisms have been proposed (Okamoto et al., 1993; Couroucli et al., 2002; Jiang et al., 2004).
Numerous studies have implicated a role for P450 enzymes, including CYP1A1, in the formation and further reactions of ROS, and may play a role in the modulation of pulmonary oxygen toxicity (Gonder et al., 1985; Okamato et al., 1993; Moorthy et al., 1997; 2000; Sindhu et al., 2000; Couroucli et al., 2002; 2006a,b; Jiang et al., 2004; Sinha et al., 2005; Bhakta et al., 2008; Moorthy, 2008). The CYP enzymes are developmentally regulated (Omeicinski et al., 1990; Hakkola et al., 1998). It has been reported that 70% of the P450s studied are constitutively expressed at one stage or another during development of animals and humans and one third of these belong to the CYP1-CYP3 families (Hakkola et al., 1998). In rodents, CYP1A1 is detected only at embryonic day 7, CYP1B1 on days 11, 15, and 17, while CYP1A2 is not expressed at all during embryonic and fetal development (Hakkola et al., 1998). However, these enzymes are inducible during prenatal and postnatal stages by prototyope chemicals such as MC or β-naphthoflavone (BNF) (Hakkola et al., 1998).
Whereas induction of CYP has been implicated in the potentiation of hyperoxic lung injury (Hazinski et al., 1989; Hazinski et al., 1995), several groups have shown that CYP1A1 may play a protective role. Pretreatment of rats (Mansour et al., 1988a) or mice (Mansour et al., 1988b) with inducers of CYP1A enzymes attenuates hyperoxic lung injury. In lamb studies, pretreatment with cimetidine, a noncompetitive inhibitor of CYP activity, attenuates hyperoxic lung injury (Hazinski et al., 1989). It has been shown, however, that cimetidine inhibits CYP2A6 and CYP2C11, but not CYP1A1 (Levine et al., 1998). Additionally, work from our laboratory showed that pretreating rats with the inhibitor of CYP1A, aminobenzotriazole (ABT), followed by subsequent exposure to 95% O2 severely potentiates hyperoxic lung injury (Moorthy et al., 2000). We also reported that in adult rats, the CYP1A inducer BNF attenuates hyperoxic lung injury (Sinha et al., 2005). Further, we also showed that there exists an inverse correlation between pulmonary and hepatic CYP1A expression and the extent of lung injury (Sinha et al., 2005). In premature infants, cimetidine, a CYP inhibitor does not prevent against lung injury, and in fact, tracheal aspirates of infants exposed to cimetidine showed higher levels of F2-isoprostanes (Cotton et al., 2006). Taken together, these observations support the theory that CYP1A1 and CYP1A2 may play protective roles against oxygen-mediated lung injury.
In newborn rats, we showed previously that hyperoxia induces CYP1A1 and 1A2 enzymes in lung and liver, respectively (Couroucli et al., 2006a). Little is known regarding the role of these enzymes in hyperoxic lung injury in newborn mice. Moreover, it is not known if prenatal induction of CYP1A enzymes by inducers such as β-napthoflavone (BNF) would protect newborn mice against lung injury induced by oxygen. Because hyperoxic newborn mice are excellent models for BPD in premature infants, in this investigation, we tested the hypothesis that prenatal treatment of pregnant mice (C57BL/6J) with the cytochrome P450 (CYP)1A inducer, BNF, will lead to attenuation of lung injury in newborns exposed to hyperoxia by mechanisms entailing transplacental induction of hepatic and pulmonary CYP1A enzymes.
MATERIALS AND METHODS
Chemicals
Tris, sucrose, NADPH, bovine serum albumin, ethoxyresorufin, methoxyresourufin, and BNF were purchased from Sigma Chemical Co. (St. Louis, MO). Buffer components for electrophoresis and western blotting were obtained from Bio-Rad laboratories (Hercules, CA). The primary monoclonal antibody to CYP1A1 was a generous gift from Dr. P.E. Thomas. Goat anti-mouse IgG conjugated with horseradish peroxidase was from Bio-Rad laboratories (Richmond, CA). All Real Time RT-PCR reagents were from Applied Biosystems (Foster City, CA). 8-iso-PGF2α and 8-iso-PGF2α-d4 were purchased from Cayman Chemical Co. (Ann Arbor, MI). Butylated hydroxytoluene (BHT) and EDTA were obtained from Sigma Chemical Co. (St. Louis, MO).
Animals
Timed pregnant mice were purchased from Harlan Sprague-Dawley (Indianapolis, IN). Animals were maintained at the Feigin Center animal facility of Baylor College of Medicine, Houston, TX, and were permitted access to food and water ad libitum. A 12;12 h day-night cycle was maintained throughout the study. The animal protocols were approved by the Baylor College of Medicine Institutional animal care and use committee.
Animal experiments
Timed pregnant mice (C57BL/6J) were treated i.p., with the vehicle corn oil (CO) or BNF (40 mg/kg), dissolved in CO, once daily on gestational days 17–19, and newborns delivered from these mothers were maintained in room air or exposed to hyperoxia (> 95% oxygen) for 1, 3 or 5 days, after which the animals were sacrificed. Some pregnant mice were sacrificed on gestational day 20 (−1 PND) and some newborns were sacrificed at birth (0 PND) for determining BNF levels and CYP1A expression at these time points. Purified tap water and food [(Purina Rodent Lab Chow No. 5001 from Purina Mills, Inc. (Richmond, Indiana) were available ad libitum. The mothers were rotated every 24 h between room air and hyperoxia conditions, so that possible toxicity to the mothers during nursing was minimal. The newborns and the mothers were placed in plexi-glass chambers. Oxygen was delivered through a humidified circuit at a flow rate of 5 L/min. FiO2 was monitored continuously by means of in-line analyzers at the outport of the chambers (Nelin et al., 1996).
Perfusion and tissue harvesting
At the termination of their respective exposures, 9 newborns from each group were euthanized by exsanguination while under deep pentobarbital anesthesia. In 6 mice from each group, the lungs were perfused with phosphate buffered saline (PBS), and used for subsequent analyses of CYP1A-dependent activities and immunoreactive protein contents in individual animals. In each of the remaining 3 animals from each group, the left lungs were inflated through the intratracheal catheter and were fixed at constant pressure (20 cm H2O) with zinc formalin after which the lungs were embedded in paraffin for subsequent histological analyses of lung injury. The right lungs were snap-frozen at −80° C for subsequent RNA isolation and CYP1A1 mRNA analyses.
CYP1A1 and 1A2 enzyme assays
Lungs were perfused with ice-cold phosphate-buffered saline, pH 7.4. Ethoxyresorufin O-deethylase (EROD) (CYP1A1) activities in lung and liver tissues were assayed according to the method of Pohl and Fouts (1980), as we have described previously (Moorthy et al., 1997, 2000; Couroucli et al., 2000). Methoxyresorufin O-demethylase (MROD) (CYP1A2) were determined as reported earlier (Moorthy et al., 1997; 2000).
Western blotting
Liver and lung homogenates (20 µg of protein) prepared from individual animals were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) in 7.5% acrylamide gels. The separated proteins on the gels were transferred to polyvinylidene difluoride membranes, followed by Western blotting using monoclonal antibodies to CYP1A1, which cross-reacts with CYP1A2 (Moorthy et al., 2000; Couroucli et al., 2000; Jiang et al., 2004; 2010). For loading controls, the membranes were stripped and incubated with antibodies against β-actin, followed by electrochemical detection of bands.
Real time reverse transcriptase-polymerase chain reaction (RT-PCR) assays
Total RNA (50 ng) from livers and lungs of air-breathing or hyperoxic animals was subjected to one step real time quantitative TaqMan RT-PCR. ABI PRISM 7700 Sequence Detection System was used for the RT-PCR reactions, as we have described in our papers (Jiang et al, 2004; Bhakta et al., 2008). Serial dilutions of RNA were used to optimize and validate RT-PCR conditions for CYP1A1 and 18S genes. Each data point was repeated 3 times. Quantitative values were obtained from the threshold PCR cycle number (Ct) at which the increase in signal was associated with an exponential growth for PCR product starts to be detected. The relative mRNA levels for CYP1A1 and 1A2 were normalized to their 18S content. The relative expression levels of the target gene were calculated according to the equation, 2−ΔcT, where ΔcT = Ct target gene − Ct 18S gene. (Jiang et al., 2004; 2010). NQO1 mRNA levels were determined similarly, except that NQO1-specific primers and probes were used.
Tissue BNF levels
BNF levels in lung and liver tissues of mice prenatally exposed to BNF were determined by LC/MS/MS (Jiang et al., 2010). Briefly, mouse tissues were weighed to 0.1 mg and placed in polypropylene microvials. PBS was added in sufficient volume to bring materials to 20 mg tissue per mL of buffer for small tissue samples (<50 mg) and to 50 mg per mL for larger samples (>50 mg). Tissues were homogenized using ultrasound and the BNF extracted from the homogenized tissue using methyl tertiary butyl ether (MTBE). Samples were extracted three times using 2 mL of MTBE each time. The MTBE extracts were combined into a clean 13×100 mL glass culture tube and the MTBE evaporated to dryness under a flowing nitrogen gas stream at room temperature. The dried samples were reconstituted with 100 µL of 50:50 methanols: 0.2% formic acid (aq.) pH 3.0, and then analyzed by LC/MS/MS. The analysis was performed using a Waters QuattroUltima tandem mass spectrometer equipped with an Agilent 1100 HPLC system. A Phenomenex Luna C5, 50×2.0 mm 5µm analytical column was used to chromatographically resolve the BNF from background. BNF was resolved from background tissue extract using a linear methanol and 0.2% formic acid in water (pH 3.0) gradient. BNF was detected by selective reaction monitoring (SRM) mode using the transition m/z 272.4>115.1, with ionization in electrospray positive mode. Quantification of BNF in the samples was done by measuring the peak area for BNF in the tissue samples against a regression calibration curve prepared using the peak area for BNF reference standards and their respective concentration from 0.1 to 1000 ng/mL.
F2-Isoprostane levels
Levels of the F2-isoprostane, 8-iso-PGF2α, which is a reliable biomarker for oxidative stress in vivo (Minuz et al., 2006), were determined in lung tissues by LC-MS/MS. Lung tissues were extracted using the method described previously (Yang et al., 2006). Briefly, approximately 25 mg of frozen tissue was ground to a fine powder and homogenized in ice-cold PBS buffer containing 0.1% BHT and 1 mM EDTA by an Ultrasonic Processor (Misonix, Farmingdale, NJ). An aliquot (100 µl) of homogenate was subjected to extraction with hexane: ethyl acetate (1:1). The upper organic layer was collected and the organic phases from three extractions were pooled, and then evaporated to dryness under a stream of nitrogen at room temperature. All extraction procedures were performed at minimum light levels and under cold (4 °C) conditions. Samples were then reconstituted in 100 µl methanol: 10 mM ammonium acetate buffer, pH 8.5 (50:50, v:v) prior to LC/MS/MS analysis.
LC-MS/MS analyses were performed using a QuattroUltima mass spectrometer (Waters, Miford, MA) equipped with an Agilent 1100 binary pump HPLC system (Agilent, Wilmington, DE) using a modified version of the method of Yang et al (2006). 8-Iso-PGF2α and PGF2α were chromatographically separated using a Luna 3 µm phenyl-hexyl 4.6×100 mm analytical column (Phenomenex, Torrance, CA). The mobile phase consisted of 10 mM ammonium acetate, pH 8.5 and methanol. A linear methanol gradient from 50% to 60% for 10 min and then from 60% to 80% in 4 min and further increased methanol concentration to 100% in 6 min and kept at that level for additional 2 min was used to achieve chromatographic baseline resolution for the isoprostanes of interest. The mass spectrometer was operated in negative electrospray ionization mode with con voltage of 100 V. Source temperatures were 125°C with desolvation gas temperature 350°C. F2-Isoprostanes were detected and quantified using multiple reaction mode (MRM) monitoring the transitions m/z 357.3 → 313.4 for 8-iso-PGF2α-d4, m/z 353.25 → 309.5 for 8-iso-PGF2α.
Lung injury and Inflammation
Lung weight/body weight (LW/BW) ratios were calculated as an index of lung injury in animals whose lungs were not perfused for isolation of microsomes. Lung histology was performed by H&E staining of lung sections as described previously (Couroucli et al., 2006a; b). Neutrophil infiltration into lungs was determined by immunohistochemistry using anti-neutrophil antibodies, as reported previously (Jiang et al., 2004). Quantitation of neurophils was performed by averaging the number of counts using at least 10 random high power fields, as described previously (Ramsay et al., 1998).
Statistical analyses
Data are expressed as means ± SE. Two-way analyses of variance (ANOVA) (the effect of treatment and time), followed by modified t-tests, were used to assess significant differences arising from exposure to hyperoxia, BNF, or hyperoxia + BNF for different time points. P values < 0.05 were considered significant.
RESULTS
Effect of BNF and hyperoxia on LW/BW ratios
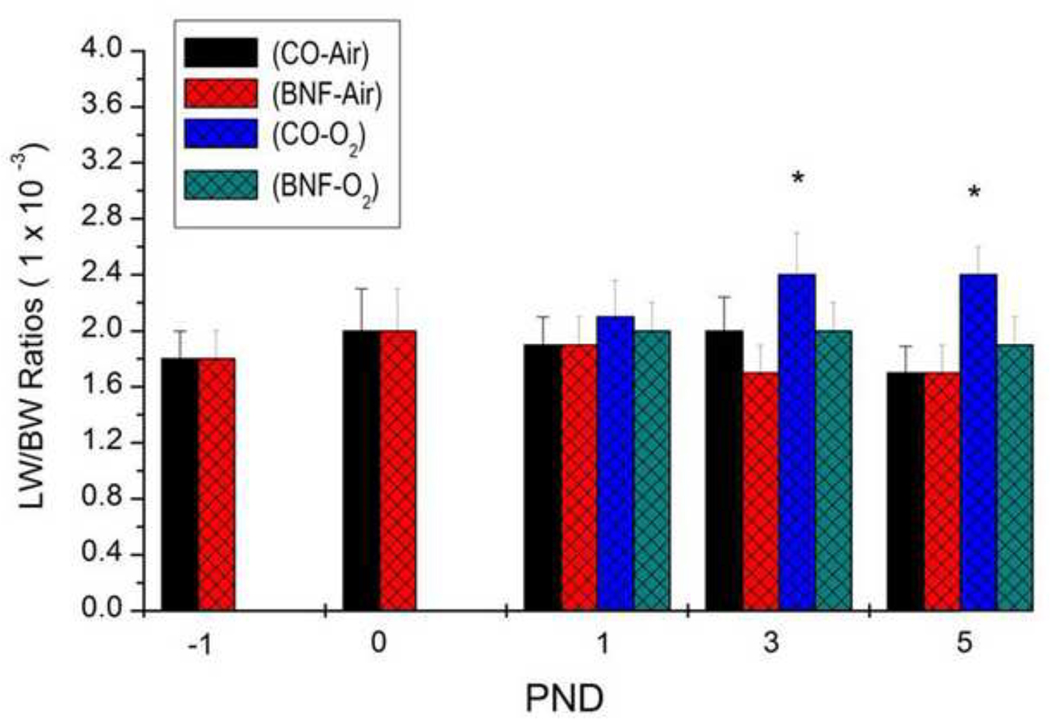
Because LW/BW ratios are considered as an index of lung injury, we determined the LW/BW ratios of newborn mice that were prenatally treated with BNF, followed by exposure to hyperoxia for 1, 3 or 5 days. As shown in figure 1, there was no significant increase in the LW/BW ratios of mice exposed to hyperoxia for 1 day, compared to those that were maintained in room air. But mice that were prenatally administered CO, followed by exposure of newborn mice to 3–5 days of hyperoxia showed significant increases in LW/BW ratios, compared to those that were maintained in room air. Mice that were prenatally treated with BNF did not show significant increases in LW/BW ratios even after exposure to hyperoxia for 3–5 days (Figure 1).
Figure 1. LW/BW ratios of newborn mice that were exposed to BNF prenatally and to hyperoxia postnatally.
Timed pregnant mice (C57BL/6J) were administered the vehicle corn oil (CO) or BNF (40 mg/kg), i.p., once daily on gestational days 17–19, and newborns delivered from timed pregnant mice, were maintained in room air or exposed to hyperoxia (> 95% oxygen) for 1, 3 or 5 days, after which the animals were sacrificed, and LW/BW ratios, which are indexes of lung injury, were determined as described under Materials and Methods. Values represent means ± SE of at least 5 mice from each group. *, Statistically significant differences between CO + O2 and CO + air-breathing mice at P < 0.05.
Attenuation of lung injury in newborn mice by prenatal administration of BNF
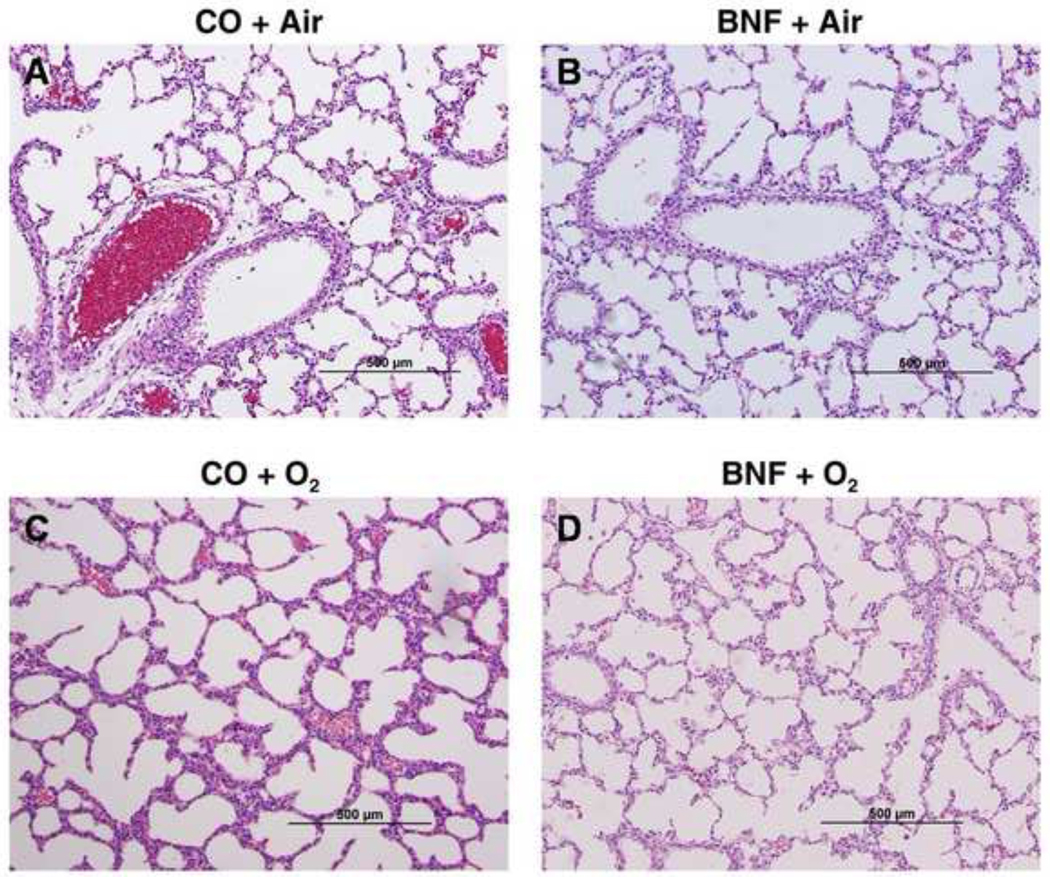
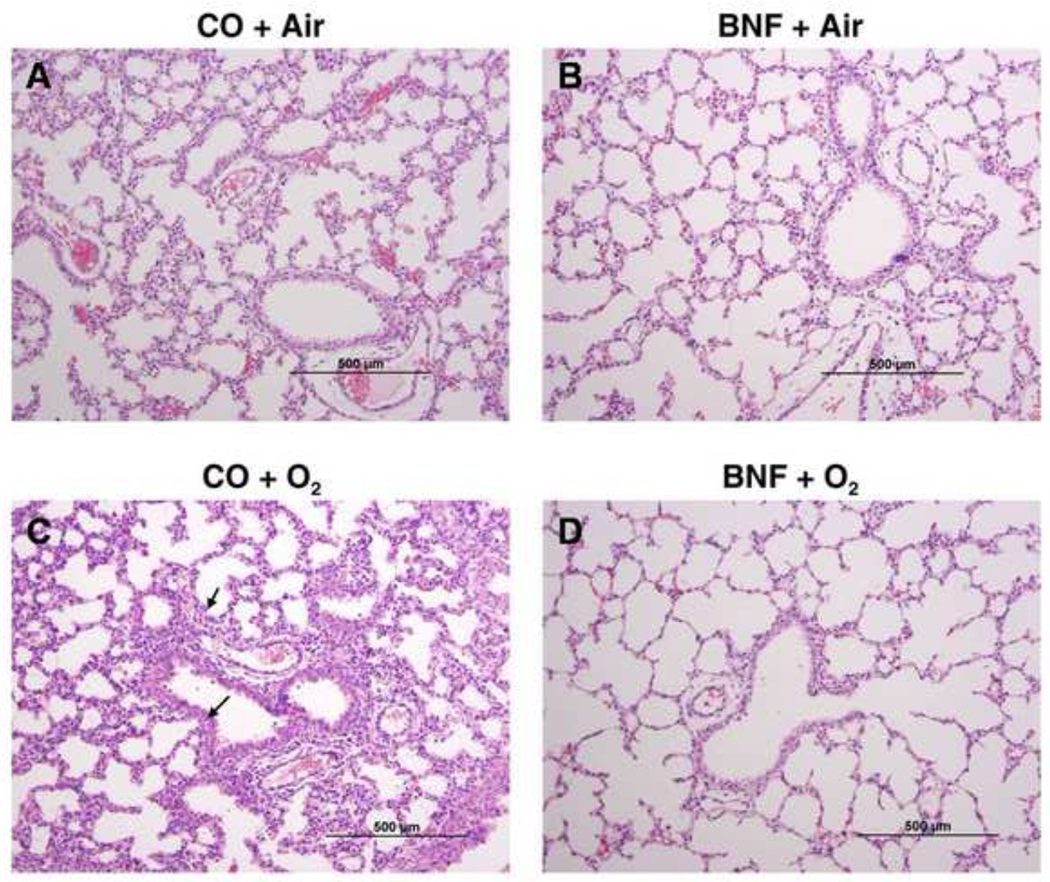
Lungs of newborn mice that were prenatally pre-treated with CO or BNF, followed by exposure of mice delivered from these dams were evaluated for lung injury by histology. Figures 2, 3, and 4 are lungs of mice prenatally exposed to CO or BNF, followed by exposure of newborn mice to room air or hyperoxia for 1, 3, and 5 days, respectively. Panels A, B, C, and D represent mice that were prenatally exposed to CO (A and C) or BNF (B and D), followed by maintenance in room air (A, B) or hyperoxia (C and D). Treatment of newborn mice in hyperoxia for 1 day did not show any appreciable lung injury (Figure 2). Exposure of newborn mice to 3 days of hyperoxia showed severe edema and perivascular inflammation (Figure 3C). Alveolar exudates and neutrophil infiltration were also observed. However, lungs of animals that were prenatally treated with BNF, followed by subsequent exposure to hyperoxia did not show appreciable injury and were, in fact, similar to room air controls (Figure 3D).
Figure 2. Lung histology of mice exposed to BNF prenatally and hyperoxia postnatally for 1 day.
Timed pregnant mice (C57BL/6J) were administered the vehicle corn oil (CO) or BNF (40 mg/kg), i.p., once daily on gestational days 17–19, and newborns delivered from timed pregnant mice, were maintained in room air or exposed to hyperoxia (> 95% oxygen) for 1 day. The animals were sacrificed and lungs were inflated at constant pressure with zinc formalin, and paraffin-embedded sections were stained using H& E. Panels A, B, C, and D are representative sections from mice (i) prenatally exposed to CO and postnally to room air (CO + air); (ii) prenatally to BNF and postnatally to room air (BNF + air); (iii) prenatally to CO and postnatally to hyperoxia (CO + O2); and (iv) prenatally to BNF and postnatally to hyperoxia (BNF + O2). As can be seen in the figure the sections in all the panels show normal lung architecture, and show no injury in hyperoxic mice.
Figure 3. Lung histology of mice exposed to BNF prenatally and hyperoxia postnatally for 3 days.
The experiments were conducted as described in the legend to Figure 2, except that the mice were exposed to hyperoxia for 3 days. The panels A, B, C, and D are defined in the legend to Figure 2. As shown in the figure, CO + O2 group showed perivascular edema and neutrophil infiltration (arrows). BNF + O2 group showed lung architecture that was similar to air-breathing controls.
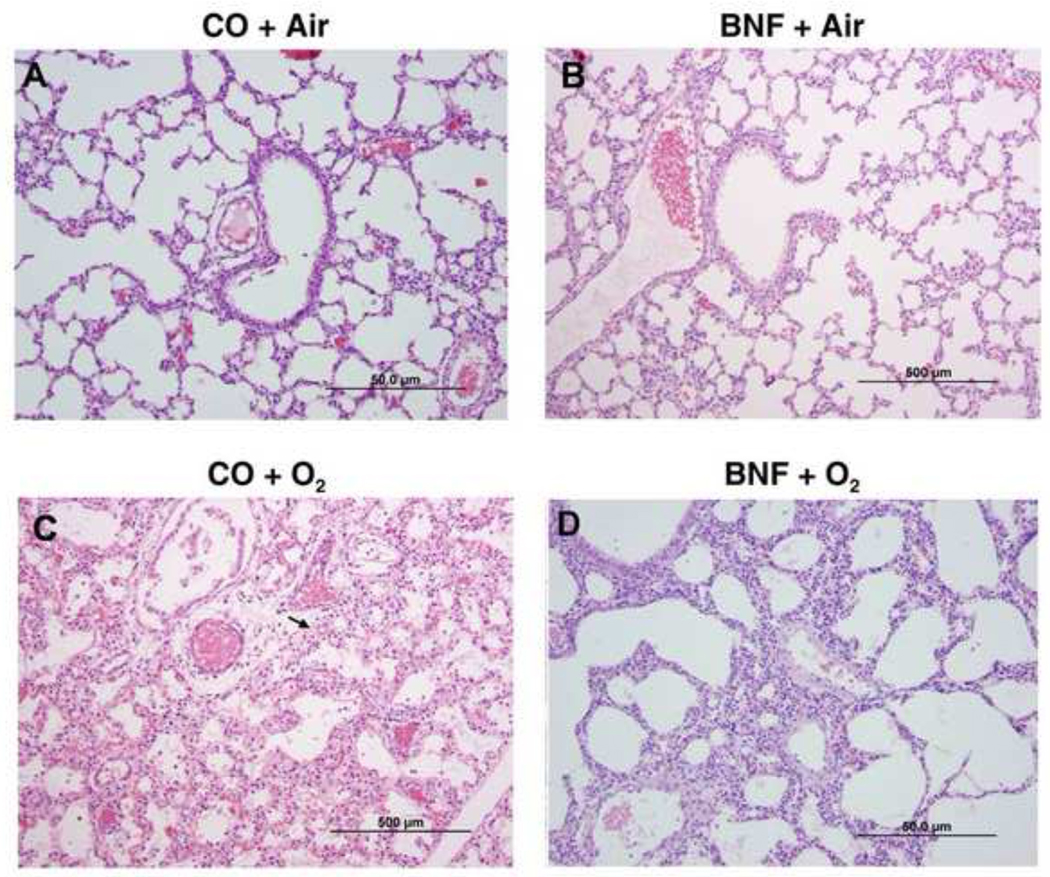
Figure 4. Lung histology of mice exposed to BNF prenatally and hyperoxia postnatally for 5 days.
The experiments were conducted as described in the legend to Figure 2, except that the mice were exposed to hyperoxia for 5 days. The panels A, B, C, and D are defined in the legend to Figure 2. As shown in the figure, CO + O2 group showed much more injury, perivascular edema and neutrophil infiltration (arrows), than that observed at 3 days. The BNF + O2 group showed improvement in lung injury, but the beneficial effects were not as pronounced as that seen at 3 days.
Lungs of mice prenatally exposed to CO, followed by exposure to hyperoxia for 5 days showed more alveolar injury and perivascular inflammation (Figure 4C) compared to those that were exposed to hyperoxia for 3 days (Figure 3C). Prenatal treatment of BNF attenuated lung injury in the mice that were subsequently exposed to hyperoxia for 5 days (Figure 4D). However, the beneficial effects of BNF were not as marked as seen at the 3 day time point.
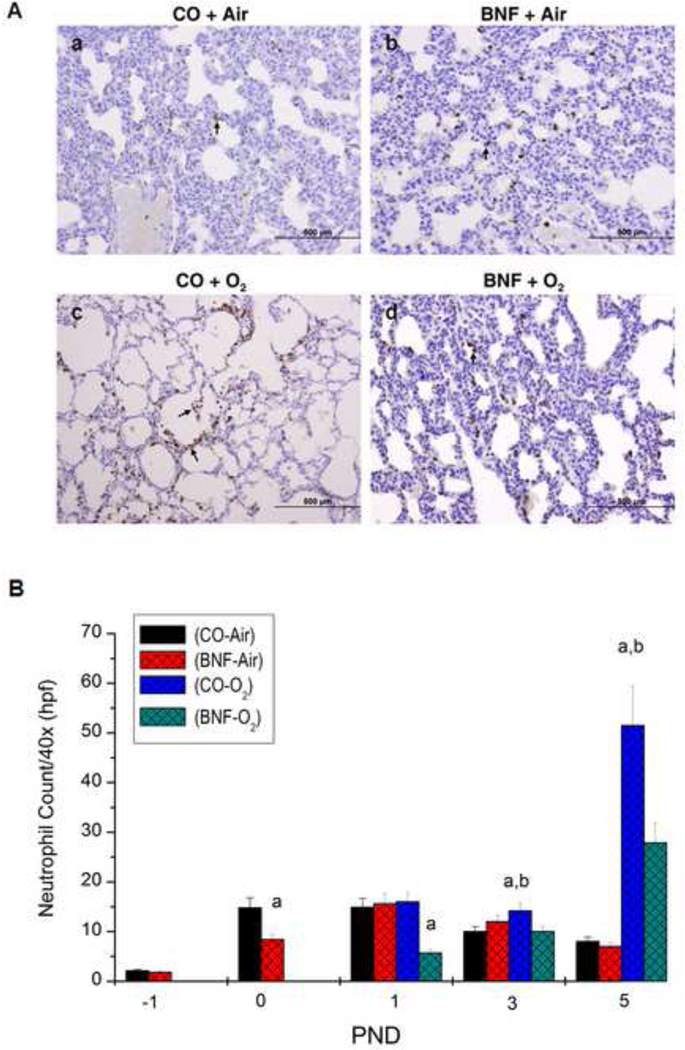
Effect of prenatal BNF treatment on neutrophil infiltration
Newborn exposed to hyperoxia for 5 days that were prenatally treated with the vehicle CO resulted in increased neutrophil infiltration compared to room air control, as determined by immunohistochemistry using anti-neutrophil antibodies (Figure 5A), In mice that were prenatally treated with BNF, followed by exposure of newborn mice to hyperoxia, there were lesser number of neutrophils (Figure 5A). Quantitative analyses revealed that hyperoxia for 120 h of mice prenatally treated with CO resulted in ~ 10-fold enhancement in the number of neutrophils, compared to room air controls (Figure 5B). BNF pretreatment resulted in significant attenuation (~ 55%) of neutrophil recruitment by hyperoxia (Figure 5B). Interestingly, even immediately after birth, which is hyperoxia for the mice compared to the situation in utero, mice that were prenatally exposed to BNF showed significantly decreased levels of neutrohils (Figure 5B).
Figure 5. Neutrophil infiltration in lungs of mice exposed to BNF prenatally and hyperoxia postnatally for 5 days (A).
The experiments were conducted as described in the legend to Figure 4, and except that the number of neutrophils were examined by immunohistochemistry, as described under Materials and Methods. The panels A, B, C, and D are defined in the legend to Figure 2. As shown in the figure, CO + O2 group showed much more number of neutrophils injury, compared to room air controls. The BNF + O2 group showed lesser number of neutrohils. Panel B shows the quantitative data of neutrophil infiltration at PND 1, 3, and 5. Data represent means ± SE of at least 3 mice from each group. a,and b depict statistically significant differences between each group versus the CO + air-breathing and BNF + O2, respectively, at P < 0.05.
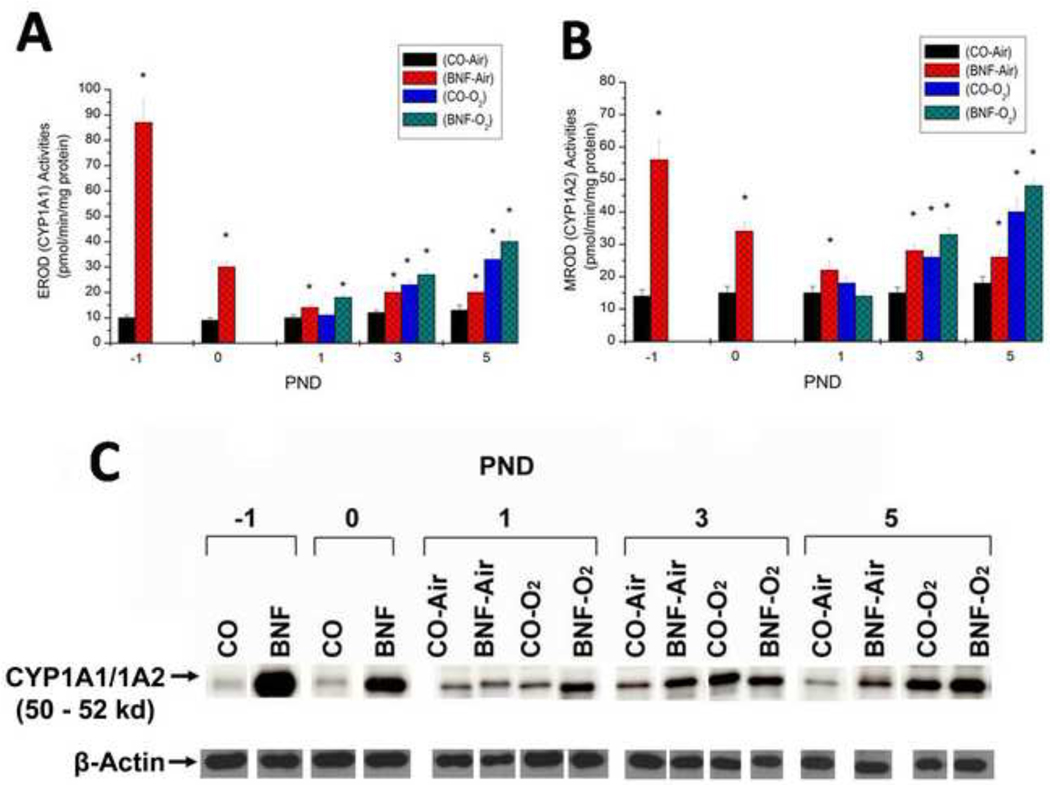
Effect of prenatal BNF administration on hepatic CYP1A1 and 1A2 expression
Prenatal treatment of mice with BNF displayed marked 9-fold induction of EROD (Figure 6A) and 3-fold induction of MROD (Figure 6B) activities in the fetuses at −1 PND. At birth (0 PND), the EROD activities, although significantly higher than controls were diminished compared to the −1 PND time point (Figure 6), In newborn mice exposed to hyperoxia for 3–5 days, there was significant induction of EROD, and synergistic induction was observed in mice that were prenatally exposed to BNF. MROD activities were induced by hyperoxia for 3 days, but induction declined by PND 6. (Figure 6B). There were statistically significant differences between CO + O2 and BNF + O2 in EROD activities at PND 1 and 5 (Figure 6A). There were also significant differences in MROD activities between these groups at PND 3 and 5 (Figure 6B). The enzyme activities correlated with apoprotein levels, as determined by western blotting (Figure 6C).
Figure 6. Effect of prenatal BNF and postnatal hyperoxia on hepatic EROD (A), MROD (B) activities and CYP1A1/1A2 apoprotein expression (C).
The prenatal treatment of timed pregnant mice was conducted as described in legend to Figure 2, and fetuses (−1 PND), and newborn mice were sacrificed at birth (0 PND), 1, 3, and 5 PND. Livers were excised and microsomes were prepared in individual mice, and EROD and MROD activities were measured as described under Materials and Methods. Values represent means ± SE of at least 5 mice from each group. *, Statistically significant differences between each group versus the CO + air-breathing group at each time point at P < 0.05. CYP1A1/1A2 apoprotein expression in individual microsomes was determined by Western blotting, and the figure shows a representative blot.
In order to determine if induction of CYP1A enzymes by BNF and hyperoxia were accompanied by similar alterations in the corresponding mRNA levels, we conducted real time RT-PCR experiments using total RNA from liver tissues of animals exposed to the different regimens described above. Prenatal treatment of mice with BNF displayed marked (110-fold) induction of CYP1A1 (Figure 7A) and 60-fold induction of CYP1A2 (Figure 7B) mRNAs in the fetuses (−1 PND), compared to those that were prenatally treated with CO (Figure 6), further suggesting that there was transplacental exposure of mice to BNF. At birth the induction of these mRNAs were still elevated, although to a lesser extent than that at −1 PND (Figure 7). By PND 1, the mRNA levels of CYP1A1 as well as CYP1A2 significantly dropped, but were significantly higher in the BNF and BNF + O2 groups, compared to room air controls (Figure 7).
Figure 7. Effect of prenatal BNF and postnatal hyperoxia on hepatic CYP1A1 (A) and CYP1A2 (B) mRNA levels.
The prenatal treatment of timed pregnant mice was conducted as described in legend to Figure 2, and fetuses (−1 PND), and newborn mice were sacrificed at birth (0 PND), 1, 3, and 5 PND. Livers were excised and total RNA was isolated, and CYP1A1 and 1A2 RNA levels were determined by real time RT-PCR, as described under Materials and Methods. Values represent means ± SE of at least 5 mice from each group. *, Statistically significant differences between each group versus the CO + air-breathing group at each time point at P < 0.05.
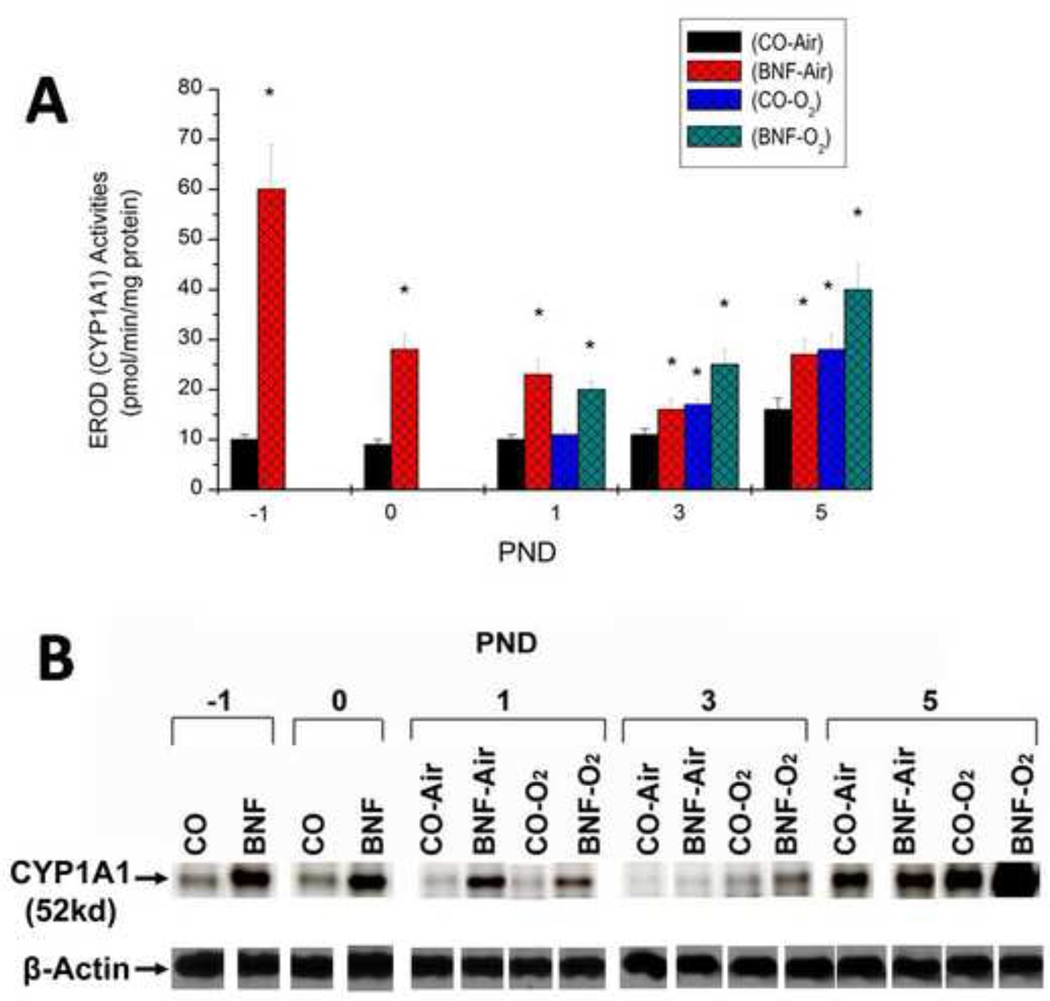
Effect of prenatal BNF administration on pulmonary CYP1A1 expression
Prenatal treatment of mice with BNF displayed marked 6-fold induction of pulmonary EROD (Figure 8A) activities in the fetuses (−1 PND) and at birth (0 PND), compared to those that were prenatally treated with CO (Figure 8A), suggesting that there was transplacental exposure of mice to BNF. The EROD activities dropped significantly by PND 1. In newborn mice exposed to hyperoxia for 3 days, there was significant induction of EROD after, and there was synergistic induction in mice that were prenatally exposed to BNF (Figure 8A). The induction of CYP1A1 by hyperoxia declined at PND 5, but in animals that were prenatally exposed to BNF, CYP1A1 expression was significantly augmented (Figure 8A). There were statistically significant differences in pulmonary EROD activities between CO + O2 and BNF + O2 at at each of the time points, i.e. PND 1, 3, and % (Figure 8A). The enzyme activities correlated with apoprotein levels (Figure 8B).
Figure 8. Effect of prenatal BNF and postnatal hyperoxia on pulmonary EROD (A), and CYP1A1 apoprotein expression (B).
The prenatal treatment of timed pregnant mice was conducted as described in legend to Figure 2, and fetuses (− 1 PND), and newborn mice were sacrificed at birth (0 PND), 1, 3, and 5 PND. Lungs were excised and microsomes were prepared in individual mice, and EROD activities were measured as described under Materials and Methods. Values represent means ± SE of at least 5 mice from each group. *, Statistically significant differences between each group versus the CO + air-breathing group at each time point at P < 0.05. CYP1A1 apoprotein expression in individual microsomes was determined by Western blotting, and the figure shows a representative Western blot.
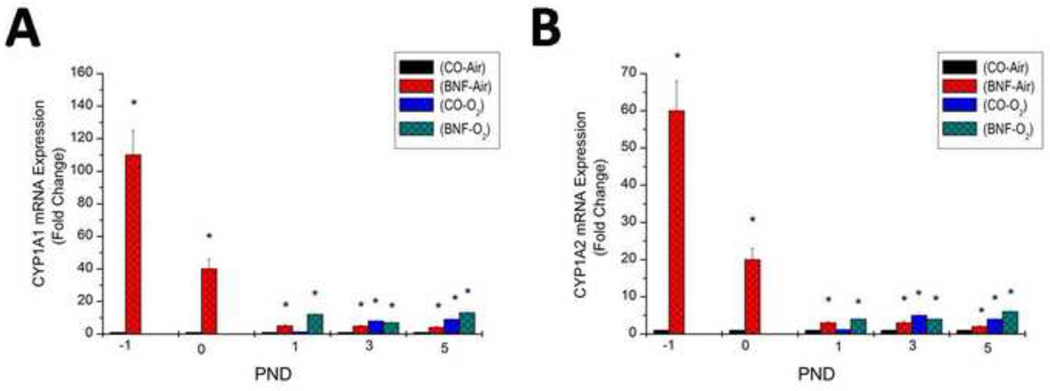
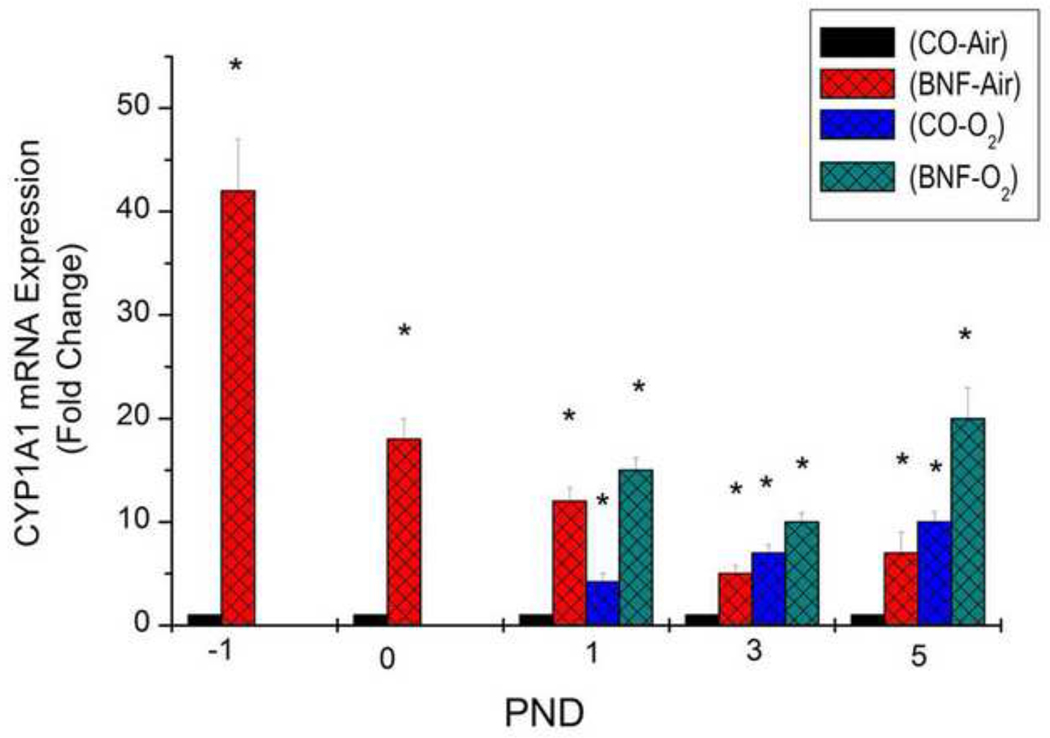
Real time RT-PCR analyses of total RNA from lungs of air-breathing and hyperoxic mice exposed given prenatal treatment of CO or BNF strongly revealed that induction of CYP1A1 enzyme and protein expresssion was accompanied by significant enhancement of CYP1A1 mRNA expression (Figure 9).
Figure 9. Effect of prenatal BNF and postnatal hyperoxia on pulmonary CYP1A1 mRNA levels.
The prenatal treatment of timed pregnant mice was conducted as described in legend to Figure 2, and fetuses (− 1 PND), and newborn mice were sacrificed at birth (0 PND), 1, 3, and 5 PND. Lungs were excised and total RNA was isolated, and CYP1A1 RNA levels were determined by real time RT-PCR, as described under Materials and Methods. Values represent means ± SE of at least 5 mice from each group. *, Statistically significant differences between each group versus the CO + air-breathing group at each time point at P < 0.05.
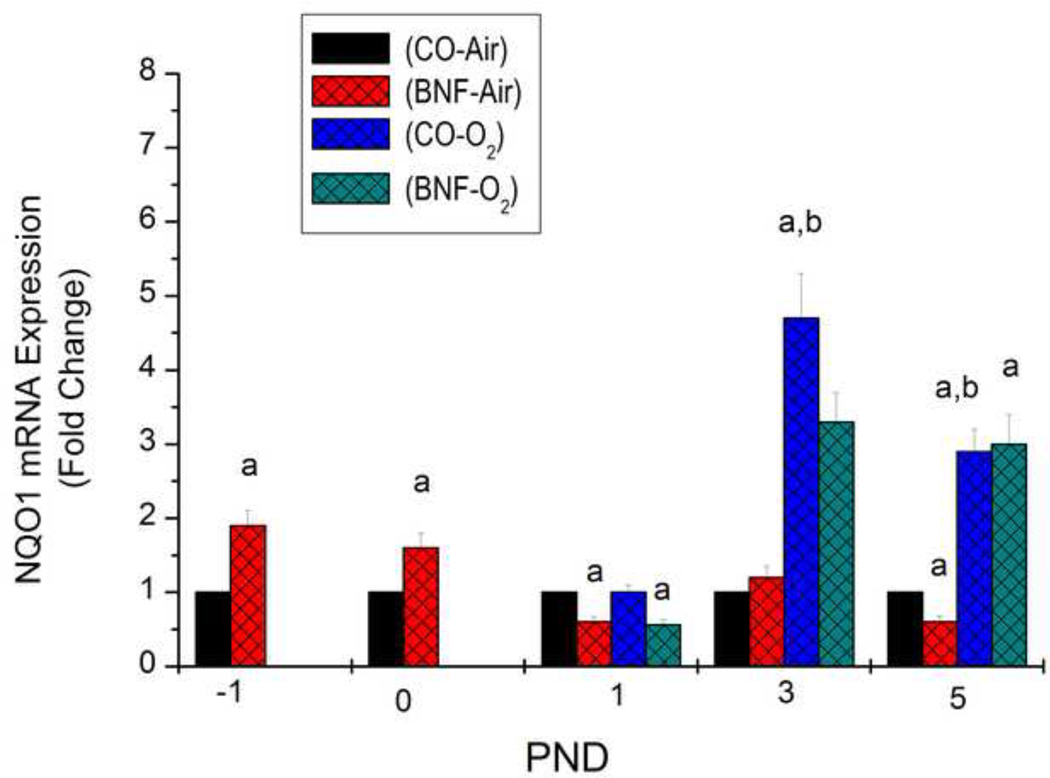
Effect of prenatal BNF treatment on pulmonary NQO1 gene expression
In order to determine if prenatal treatment with BNF will induce gene expression of the phase II enzyme, NQO1, we determined mRNA levels of NQO1 in lungs of mice prenatally treated with CO or BNF, followed by exposure of newborn mice to room air or hyperoxia for 24, 72, or 120 h. As shown in Figure 10, prenatal BNF induced NQO1 expression in fetal lung by 100%, compared to vehicle CO administration. Induction of NQO1 was maintained in newborn mice immediately after birth. At 24 h after hyperoxia mice that had been prenatally exposed to BNF showed decreased NQO1 gene expression (Figure 10). After 72 h of hyperoxia, the mice displayed a 4-fold induction of NQO1 expression compared to vehicle controls. Mice that had been prenatally exposed to BNF, followed by hyperoxia for 72 h, resulted in attenuation of NQO1 expression compared to CO-exposed mice (Figure 10). At 120 h, NQO1 expression in mice exposed prenatally to CO or BNF was comparable (Figure 10).
Figure 10. Effect of prenatal BNF and postnatal hyperoxia on pulmonary NQO1 mRNA levels.
The prenatal treatment of timed pregnant mice was conducted as described in legend to Figure 2, and fetuses (− 1 PND), and newborn mice were sacrificed at birth (0 PND), 1, 3, and 5 PND. Lungs were excised and total RNA was isolated, and NQO1 RNA levels were determined by real time RT-PCR, as described under Materials and Methods. Values represent means ± SE of at least 5 mice from each group. *, Statistically significant differences between each group versus the CO + air-breathing group at each time point at P < 0.05.
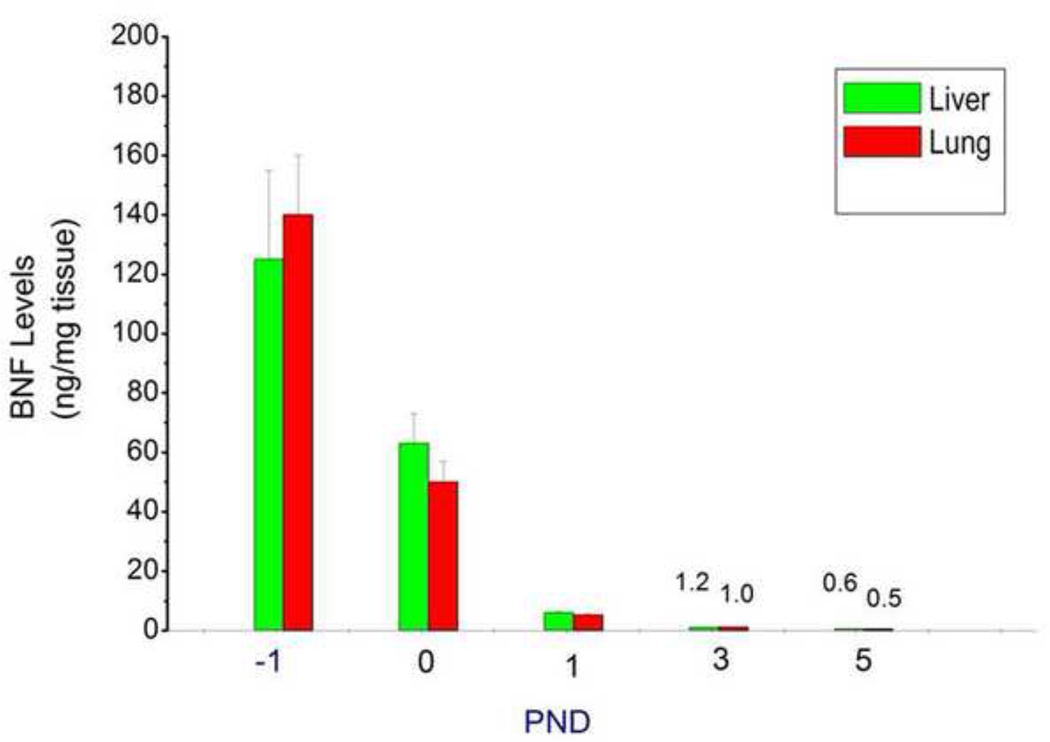
Tissue levels of parent BNF in fetuses and newborn mice following prenatal BNF treatment
In order to determine if prenatal BNF treatment results in transplacental transport of the inducer to hepatic and pulmonary tissues of newborn mice, livers and lungs of fetuses (−1 PND) and newborn mice at PND 0, 1, 3, and 5 were extracted and tissue levels of parent BNF were measured by LC-MS/MS. As shown in figure 9, high levels of BNF were observed in the fetuses. The tissue levels were detectable until PND 5 (Figure 11).
Figure 11. BNF levels in fetal and newborn liver and lung tissues.
The prenatal treatment of timed pregnant mice was conducted as described in legend to Figure 2, and fetuses (− 1 PND), and newborn mice were sacrificed at birth (0 PND), 1, 3, and 5 PND. Livers and lungs of mice exposed prenatally to BNF and postnatally to BNF were were excised and parent BNF levels were determined by LC-MS/MS, described under Materials and Methods. Values represent means ± SE of at least 5 mice from each group.
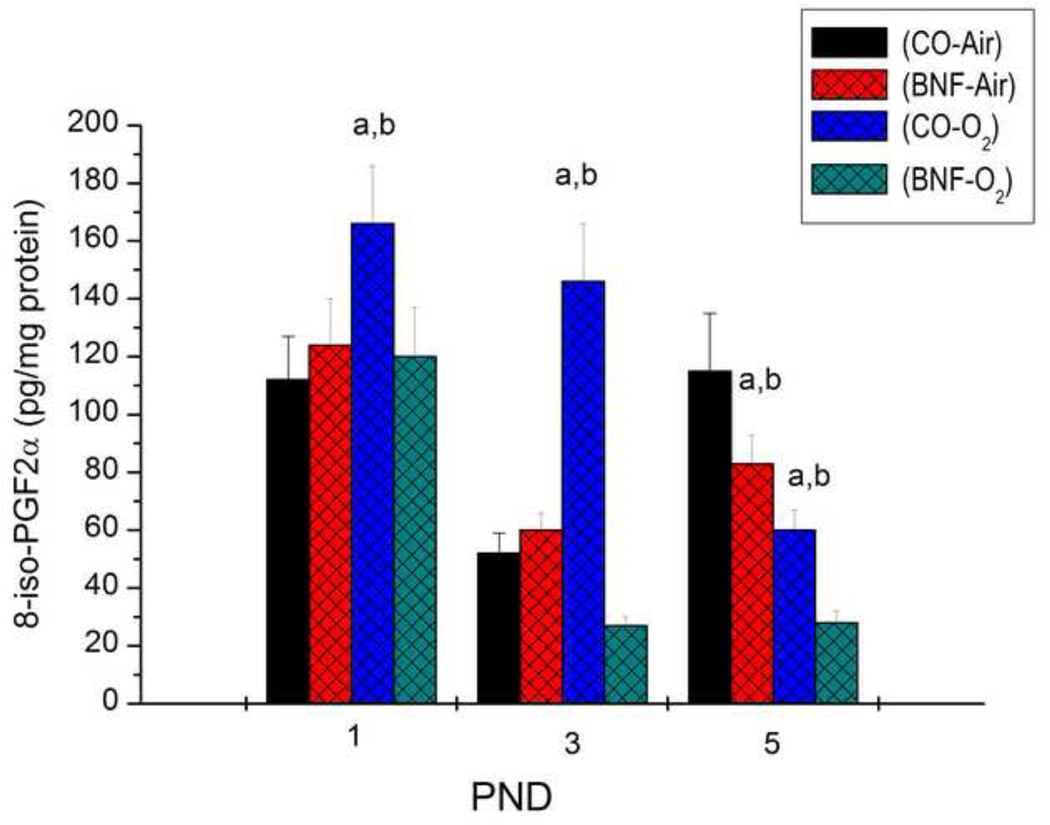
Pulmonary F2-isoprostane levels
To test if prenatal BNF attenuates hyperoxic lung injury by decreasing the levels of ROS-mediated lipid peroxidation products such as F2-isoprostanes, we determined levels of 8-iso-PGF2α in the lungs of newborn mice exposed to hyperoxia at different time points. As shown in Figure 12, hyperoxia for 24 h of newborn mice that were prenatally exposed to CO resulted in significantly increased levels of 8-iso-PGF2α, compared to mice that were maintained in room air. In mice that were prenatally treated with BNF, hyperoxia for 24 h did not elicit any changes in pulmonary levels of 8-iso-PGF2α (Figure 12). After 72 h of hyperoxia (PND 3), the levels of 8-iso-PGF2α were further increased (~ 3-fold), compared to room air controls, but in mice that had been prenatally treated with BNF, the 8-iso-PGF2α levels were markedly lower, and were in fact, about 45% lesser than those observed in room air controls (Figure 12). By PND 5, the levels of this compound in the hyperoxic mice significantly declined (Figure 12).
Figure 12. F2-isoprostane levels in lungs of newborn mice.
Newborn mice prenatally exposed to vehicle CO or BNF were maintained in room air or exposed to hyperoxia for 24, 72 or 120 h, and levels of iso-PGF2α were determined by LC-MS/MS, as described under Materials and Methods. Values represent means ± SE of at least 3 mice from each group. a,and b depict statistically significant differences between each group versus the CO + airbreathing and BNF + O2, respectively, at P < 0.05.
DISCUSSION
The major goal of this investigation was to test the hypothesis that treatment of pregnant mice with the CYP1A inducer, BNF, will protect newborn mice born to these dams from oxygen-mediated lung injury. The increases in LW/BW ratios in hyperoxic mice born to CO-treated mothers, compared to air-breathing controls (Figure 1) was most likely to lung injury caused by hyperoxia for 3–5 days. The decreases in the LW/BW ratios of hyperoxic mice born to dams that received BNF during pregnancy supported the hypothesis that prenatal BNF protects newborn mice against lung injury upon postnatal exposure to hyperoxia.
Histological analyses (Figures 2–4) confirmed our LW/BW data. The lung injury and inflammation in newborn mice exposed to >95% oxygen (Figure 3) for 3 days was in agreement with the findings of other investigators (Warner et al., 1998; Crapo, 1986; Bhandari, 2010). In fact, studies of hyperoxic chronic injury in newborn mice have shown morphologic changes similar to those seen in human BPD (Bhandari, 2010) and extremely premature baboons (Coalson et al., 1999).
The marked protection against hyperoxia-mediated lung damage in newborn mice that received BNF transplacentally supported the hypothesis that this compound protects against hyperoxic lung injury. These observations were also in agreement with our previous findings of beneficial effects of BNF against acute lung injury in adult rats (Sinha et al., 2005).
Our observations that neutrophil infiltration in the lungs was substantially augmented in newborn mice, which had been prenatally treated with the vehicle CO, and exposed to hyperoxia for 5 days (Figure 5A,B) was probably due to increased inflammation in these mice. The marked attenuation of neutrophils in hyperoxic mice that had been prenatally treated with BNF supported the hypothesis that BNF protects mice against lung inflammation induced by hyperoxia.
The robust induction of hepatic CYP1A1 and 1A2 expression at −1 PND (Figure 5) in the fetuses of mice treated with BNF indicated in utero exposure of the fetuses to BNF, resulting in induction of CYP1A1 and 1A2. These observations are highly significant, as there is a potential for future translational application in that women with the risk of preterm labor could receive BNF or other flavonoids, such that the premature babies born to these mothers can be protected from oxygen-mediated lung diseases such as BPD. Transplacental induction of CYP1A1 by MC has been shown previously (Anderson et al., 1989a,b; 1991; Miller, 1993; Xu et al., 2005). Also, BNF has been shown to modify transplacental tumorigenesis by MC, suggesting in utero induction of CYP1A1 by flavonoids (Anderson et al., 1989a).
The induction of hepatic EROD and MROD activities in the CO + O2 samples suggested that hyperoxia by itself induced CYP1A1 and 1A2 activities, probably via AHR-mediated mechanisms involving endogenous ligands for the AHR (Jiang et al. 2004; Couroucli et al., 2006a). The synergistic induction of CYP1A1 and 1A2 in mice treated prenatally with BNF, followed by exposure of newborns to hyperoxia for up to 5 days was probably due to the combined effect of hyperoxia and BNF, as CYP1A expression declined in air-breathing mice that were prenatally exposed to BNF (Figure 5). The robust induction of hepatic CYP1A1 and 1A2 mRNA expression by BNF at −1 PND and at birth (Figure 6) indicated that transplacental induction of CYP1A enzymes was mediated by transcriptional activation of CYP1A genes, presumably via AHR-dependent mechanisms. Hyperoxic induction of CYP1A mRNAs in newborn mice probably entailed similar mechanisms.
The observation that lungs of fetuses and newborn mice exposed prenatally to BNF displayed significant induction of EROD activities (Figure 7A), apoprotein levels (Figure 7B), and mRNA expression (Figure 8) supported the hypothesis that BNF elicited in utero induction of CYP1A1 in newborn mice. The increases of CYP1A1 expression in the control animals over time indicates developmental induction of CYP1A1 expression in the lung (Omeicinski et al., 1993). The induction of CYP1A1 gene expression by hyperoxia itself in the lungs of newborn mice was in agreement with previous findings by us in the newborn rat model (Couroucli et al., 2006a).
In order to determine if BNF does get transported to the livers and lungs of fetal and newborn mice exposed in utero, we determined the levels of parent BNF in these tissues by LC-MS/MS (Figure 9). Our results showed high levels of BNF at −1 PND and at birth, and very low, but detectable levels at later time points, suggesting rapid metabolism and elimination of BNF from the newborn mice. The data provide direct evidence that induction of CYP1A by BNF in utero occurs via transplacental transfer of BNF from the mothers to the fetuses. Furthermore, the fact that induction of CYP1A was maintained in livers and lungs of animals even at PND 5 in the BNF + O2 group, when most BNF had been metabolized and eliminated was probably due to long-term (imprinting) effect of BNF and hyperoxia on CYP1A expression. Similar imprinting effects on the metabolism of MC were reported in mice transplacentally exposed to MC or BNF (Anderson et al., 1991).
The mechanisms by which BNF protects mice against hyperoxic lung injury are not completely understood, but it is likely that CYP1A isoforms play a role in this phenomenon. Phase II antioxidant enzymes such as glutathione S-transferases, NAD(P)H qunione reductase (NQO1), and/or superoxide dismutase, which may have been induced by BNF, might have also contributed to the protection against lung injury. Our results on the effects of hyperoxia on pulmonary NQO1 mRNA expression showing lesser magnitude of induction in hyperoxic mice prenatally exposed to BNF, compared to those prenatally exposed to CO (Figure 10), suggests that the phase II enzyme NQO1 may not have played a major role in the attenuation of lung injury by BNF. The induction of NQO1 gene expression by BNF in fetal (−1 PND) and newborn mice (PND 0), compared to controls (Figure 10) was probably due to transcriptional activation of the NQO1 gene via AHR-mediated mechanisms. The marked augmentation of NQO1 mRNA expression by hyperoxia for 3 days was in agreement with previous reports shoowing induction of phase II enzymes by hyperoxia in newborn rats (Whitney and Frank, 1993).
In a recent study, we observed protection against oxygen injury in animals that had been pre-treated with MC 15 days prior to exposure to hyperoxia (W. Jiang, L. Wang, X.I. Couroucli, and B. Moorthy, unpublished observations). These animals displayed sustained pulmonary CYP1A1, but not phase II enzyme induction, suggesting that phase II enzymes may not have played a major role in attenuating hyperoxic lung injury. Furthermore, Anderson et al., (1989b) have shown that PAHs induce fetal phase II enzymes to much lower degree than phase I enzymes. Thus, it appears that CYP1A enzymes played a key role in the protection against lung injury in the BNF-treated rats described in the current study.
As mentioned previously, CYP enzymes are primarily involved in eliminating endogenous and exogenous ligands, molecules implicated in the pathogenesis of cellular injury. Recent studies have provided evidence for the involvement of lipid peroxidation products derived from arachidonic acid, i.e. F2-isoprostanes and isofurans in mediating lung injury mediated by hyperoxia (Fessel et al., 2002; Morrow and Roberts, 2002); (Janssen, 2000; 2001); Zahler and Becker, 1999). These molecules have also been implicated in the development of lung injury associated with BPD (Saugstad, 1997; Goil et al., 1998). Our observations (Figure 12) showing increased levels of the F2-isoprostane iso-PGF2α in lungs of newborn mice exposed to hyeproxia for 24–72 h, but not 120 h, supported tha hypothesis that iso-PGF2α plays a mechanistic role in the development of lung injury. The markedly decreased levels of iso-PGF2α in hyperoxic mice that been prenatally exposed to BNF was probably due to CYP1A-mediated detoxification of iso-PGF2α to non-toxic products. It is our supposition that members of the cytochrome P450 1A family may be intimately involved with the reduction or elimination of these compounds, either before or during the initial inflammatory event. Support for this idea stems from our recent observations showing increased susceptibility of Cyp1a1- or Cyp1a2-null mice to hyperoxic lung injury, a phenomenon that was also associated with increased levels of hepatic and pulmonary F2-isoprostanes and isofurans in these animals (W. Jiang, L. Wang, X.I. Couroucli, and B. Moorthy, unpublished observations).
On the other hand, earlier work on the role of CYP enzymes had implicated these enzymes in the potentiation of hyperoxic lung injury (Hazinski et al., 1989). In this study, the administration of cimetidine, a known inhibitor of the CYP system, to full-term, newborn lambs attenuated hyperoxic lung injury. Subsequent work from our laboratory has shown that induction of hepatic and pulmonary CYP1A1 and CYP1A2 attenuates hyperoxic lung injury (Moorthy et al., 1997; Moorthy et al., 2000; Jiang et al., 2004; Sinha, 2005; Sinha et al., 2005). In a human clinical trial with cimetidine, it has been demonstrated that, in critically ill, ventilator-dependent prematures at risk for developing BPD, this drug did not reduce early lung injury (Cotton et al., 2006). Interestingly, this group also found that the tracheal aspirate levels of F2-isoprostanes, markers of lipid peroxidation from free radical oxidant generation, were increased in the cimetidine group at 4 and 10 days after cimetidine treatment (Cotton et al., 2006), supporting the hypothesis that CYP enzymes may play a role in detoxification of F2-isoprostanes (Cotton et al., 2006). Involvement of other ROS such as eicasanoids and leukotrienes (Rogers et al., 2010), or oxidized phospholipids such as phosphatidyl serine and cardiolipin (Tyurina et al., 2010) in hyperoxic lung injury have not been excluded, however.
Regardless of the mechanisms, our observed benefical effects of BNF, a flavonoid, against cellular oxygen toxicity, is intriguing and warrants further in-depth studies. The flavonoids comprise a diverse family of chemicals that are commonly found in fruits and vegetables. A large number of these compounds, in addition to being potent modulators of expression and activity of various CYP enzymes as above, exert various effects on cell cycle regulation (Reiners et al., 1999).
In conclusion, in this manuscript, we report a novel finding, as we have demonstrated significant protection of newborn mice from hyperoxic lung injury upon in utero treatment with BNF. These studies have implications for hyperoxic lung injury in humans. In the clinical setting, antenatal administration of steroids is frequent in pregnant women with the risk of preterm labor, and studies have shown protective effects of antenatal steroids against BPD (Van Mater et al., 1990). Similarly, treating pregnant women having the risk of preterm delivery with BNF or other flavonoids could result in the newborn infants being better prepared to handle exposures to hyperoxia, due to transplacental induction of CYP1A enzymes. However, many more studies in animal models, including higher mammals (e.g., premature baboons) would be needed before clinical studies can be initiated. Further investigations into the role of CYP1A1 in modulating hyperoxic injury may lead to the development of rational strategies for the prevention of BPD in infants undergoing supplemental oxygen therapy.
Research Highlights.
Supplemental oxygen is routinely administered to premature infants.
Hyperoxia causes lung injury in experimental animals.
Prenatal treatment of mice with beta-naphthoflavone attenuates oxygen injury
Cytochrome P4501A enzymes play protective roles against lung injury
ACKNOWLEDGEMENTS
This work was in part supported by RO1 grants HL-088343 to X.I.C., and HL-070921, HL-087174, ES-019689, and ES 009132 to B.M. The study sponsors had no involvement in study design, data collection, analysis and interpretation, writing of the report or decision to submit the paper for publication. The authors thank Dr. Edward Felix of the M.D. Anderson Cancer Center in the carrying out LC-MS/MS analyses for determination of BNF levels in lung and liver tissues of fetal and newborn mice. We appreciate the assistance of Esther Inman in the preparation of this manuscript.
Abbreviations
- BPD
bronchopulmonary dysplasia
- CYP
cytochrome P450
- BNF
β-napthoflavone
- CO
corn oil
- EROD
ethoxyresorufin O-deethylase
- MROD
methoxyresorufin O-demethylase
- ROS
reactive oxygen species
- MC
3-methylcholanthrene
- AHR
Ah receptor
- RT-PCR
reverse transcriptase polymerase chain reaction
- PND
post-natal day
- ANOVA
analyses of variance
- LW/BW
lung weight/bodyweight
- NQO1
NAD(P)H quinone reductase
- PBS
phosphate buffered saline
- MTBE
methy tertiary butyl ether
- LC-MS/MS
liquid chromatography-Mass Spectrometry/Mass spectrometry
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Anderson LM, Jones AB, Riggs CW, Kovatch RM. Modification of transplacental tumorigenesis by 3-methylcholanthrene in mice by genotype at the Ah locus and pretreatment with beta-naphthoflavone. Cancer Res. 1989a;49:1676–1681. [PubMed] [Google Scholar]
- Anderson LM, Jones AB, Miller MS, Chauhan DP. Metabolism of transplacental carcinogens. IARC Sci Publ. 1989b;96:155–188. [PubMed] [Google Scholar]
- Anderson LM, Jones AB, Riggs CW. Long-term (imprinting) effects of transplacental treatment of mice with 3-methylcholanthrene or beta-naphthoflavone on hepatic metabolism of 3-methylcholanthrene. Pharmacol Toxicol. 1991;69:178–188. doi: 10.1111/j.1600-0773.1991.tb01294.x. [DOI] [PubMed] [Google Scholar]
- Barouki R, Morel Y. Repression of cytochrome P450 1A1 gene expression by oxidative stress: mechanisms and biological implications. Biochem Pharmacol. 2001;61:511–516. doi: 10.1016/s0006-2952(00)00543-8. [DOI] [PubMed] [Google Scholar]
- Bhakta KY, Jiang W, Couroucli XI, Fazili IS, Muthiah K, Moorthy B. Regulation of cytochrome P4501A1 expression by hyperoxia in human lung cell lines: Implications for hyperoxic lung injury. Toxicol Appl Pharmacol. 2008;233:169–178. doi: 10.1016/j.taap.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari V. Molecular mechanisms of hyperoxia-induced acute lung injury. Front Biosci. 2008;13:6653–6661. doi: 10.2741/3179. [DOI] [PubMed] [Google Scholar]
- Bhandari V. Hyperoxia-derived lung damage in preterm infants. Semin Fetal Neonatal Med. 2010;15:223–229. doi: 10.1016/j.siny.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland RD, Coalson JJ, editors. Chronic lung disease in early infancy. New York, NY: Marcel Dekker, Inc.; 2000. [Google Scholar]
- Bryan CL, Jenkinson SG. Oxygen toxicity. Clin Chest Med. 1988;9:141–152. [PubMed] [Google Scholar]
- Budinger GR, Mutlu GM, Urich D, Soberanes S, Buccellato LJ, Hawkins K, Chiarella SE, Radigan A, Eisenbart J, Agrawal H, Berkelhamer S, Hekimi S, Zhang J, Perlman H, Schumaker PT, Jain M, Chandel NS. Epithelial cell death is an important contributor to oxidant-mediated acute lung injury. Am J. Respir Crit Care Med. 2010;183:1043–1054. doi: 10.1164/rccm.201002-0181OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JM, Lambersten CJ. Pulmonary oxygen toxicity: a review. Pharmacol Rev. 1971;22:37–133. [PubMed] [Google Scholar]
- Coalson JJ, Winter VT, Siler-Khodr T, Yoder BA. Neonatal chronic lung disease in extremely immature baboons. Am J Respir Crit Care Med. 1999;160:1333–1346. doi: 10.1164/ajrccm.160.4.9810071. [DOI] [PubMed] [Google Scholar]
- Cotton RB, Hazinski TA, Morrow JD, Roberts LJ, Zeldin DC, Lindstrom DP, Lappalainen U, Law AB, Steele S. Cimetidine does not prevent lung injury in newborn premature infants. Pediatr Res. 2006;59:795–800. doi: 10.1203/01.pdr.0000219397.35473.5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couroucli XI, Welty SE, Ramsay PL, Wearden ME, Fuentes-Garcia FJ, Ni J, Jacobs TN, Towbin JA, Bowles NE. Detection of microorganisms in the tracheal aspirates of preterm infants by polymerase chain reaction: association of adenovirus infection with bronchopulmonary dysplasia. Pediatr Res. 2000;47:225–232. doi: 10.1203/00006450-200002000-00013. [DOI] [PubMed] [Google Scholar]
- Couroucli XI, Liang YW, Jiang W, Barrios R, Moorthy B. Attenuation of oxygen-induced abnormal lung maturation in rats by retinoic acid: possible role of cytochrome P4501A enzymes. J Pharmacol Exp Ther. 2006a;317:946–954. doi: 10.1124/jpet.105.100677. [DOI] [PubMed] [Google Scholar]
- Couroucli XI, Wei YH, Jiang W, Muthiah K, Evey LW, Barrios R, Moorthy B. Modulation of pulmonary cytochrome P4501A1 expression by hyperoxia and inhaled nitric oxide in the newborn rat: implications for lung injury. Pediatr Res. 2006b;59:401–406. doi: 10.1203/01.pdr.0000199909.96576.7f. [DOI] [PubMed] [Google Scholar]
- Couroucli XI, Welty SE, Geske RS, Moorthy B. Regulation of pulmonary and hepatic cytochrome P4501A expression in the rat by hyperoxia: implications for hyperoxic lung injury. Mol Pharmacol. 2002;61:507–515. doi: 10.1124/mol.61.3.507. [DOI] [PubMed] [Google Scholar]
- Crapo JD. Morphologic changes in pulmonary oxygen toxicity. Annu Rev Physiol. 1986;48:721–731. doi: 10.1146/annurev.ph.48.030186.003445. [DOI] [PubMed] [Google Scholar]
- Fessel JP, Porter NA, Moore KP, Sheller JR, Roberts LJ., 2nd Discovery of lipid peroxidation products formed in vivo with a substituted tetrahydrofuran ring (isofurans) that are favored by increased oxygen tension. Proc Natl Acad Sci U S A. 2002;99:16713–16718. doi: 10.1073/pnas.252649099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher AB. Oxygen therapy. Side effects and toxicity. Am Rev Respir Dis. 1980;122:61–69. doi: 10.1164/arrd.1980.122.5P2.61. [DOI] [PubMed] [Google Scholar]
- Freeman BA, Crapo JD. Hyperoxia increases oxygen radical production in rat lungs and lung mitochondria. J Biol Chem. 1981;256:10986–10992. [PubMed] [Google Scholar]
- Gagliardi L, Bellù R, Lista G, Zanini R. Network Neonatale Lombardo Study Group. Do differences in delivery room intubation explain different rates of bronchopulmonary dysplasia between hospitals? Arch Dis Child Fetal Neonatal Ed. 2011;96:F30–F35. doi: 10.1136/adc.2010.183905. [DOI] [PubMed] [Google Scholar]
- Goil S, Truog WE, Barnes C, Norberg M, Rezaiekhaligh M, Thibeault D. Eight-epi-PGF2alpha: a possible marker of lipid peroxidation in term infants with severe pulmonary disease. J Pediatr. 1998;132:349–351. doi: 10.1016/s0022-3476(98)70459-7. [DOI] [PubMed] [Google Scholar]
- Gonder JC, Proctor RA, Will JA. Genetic differences in oxygen toxicity are correlated with cytochrome P-450 inducibility. Proc Natl Acad Sci U S A. 1985;82:6315–6319. doi: 10.1073/pnas.82.18.6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guengerich FP. Enzymatic oxidation of xenobiotic chemicals. Crit Rev Biochem Mol Biol. 1990;25:97–153. doi: 10.3109/10409239009090607. [DOI] [PubMed] [Google Scholar]
- Guengerich FP. Cytochrome P450: what have we learned and what are the future issues? Drug Metab Rev. 2004;36:159–197. doi: 10.1081/dmr-120033996. [DOI] [PubMed] [Google Scholar]
- Gupta S, Sinha SK, Donn SM. Ventilatory management and bronchopulmonary dysplasia in preterm infants. Semin Fetal Neonatal Med. 2009;14:367–373. doi: 10.1016/j.siny.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Hakkola J, Tanaka E, Pelkonen O. Developmental expression of cytochrome P450 enzymes in human liver. Pharmacol Toxicol. 1998;82:209–217. doi: 10.1111/j.1600-0773.1998.tb01427.x. [DOI] [PubMed] [Google Scholar]
- Hazinski TA, France M, Kennedy KA, Hansen TN. Cimetidine reduces hyperoxic lung injury in lambs. J Appl Physiol. 1989;67:2586–2592. doi: 10.1152/jappl.1989.67.6.2586. [DOI] [PubMed] [Google Scholar]
- Hazinski TA, Noisin E, Hamon I, DeMatteo A. Sheep lung cytochrome P4501A1 (CYP1A1): cDNA cloning and transcriptional regulation by oxygen tension. J Clin Invest. 1995;96:2083–2089. doi: 10.1172/JCI118257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen LJ. Isoprostanes: generation, pharmacology, and roles in free-radical-mediated effects in the lung. Pulm Pharmacol Ther. 2000;13:149–155. doi: 10.1006/pupt.2000.0244. [DOI] [PubMed] [Google Scholar]
- Janssen LJ. Isoprostanes: an overview and putative roles in pulmonary pathophysiology. Am J Physiol Lung Cell Mol Physiol. 2001;280:L1067–L1082. doi: 10.1152/ajplung.2001.280.6.L1067. [DOI] [PubMed] [Google Scholar]
- Jiang W, Wang L, Kondraganti SR, Fazili IS, Couroucli XI, Felix EA, Moorthy B. Disruption of the gene for CYP1A2, which is expressed primarily in liver, leads to differential regulation of hepatic and pulmonary mouse CYP1A1 expression and augmented human CYP1A1 transcriptional activation in response to 3-methylcholanthrene in vivo. J Pharmacol Exp Ther. 2010;335:369–379. doi: 10.1124/jpet.110.171173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Welty SE, Couroucli XI, Barrios R, Kondraganti SR, Muthiah K, Yu L, Avery SE, Moorthy B. Disruption of the Ah receptor gene alters the susceptibility of mice to oxygen-mediated regulation of pulmonary and hepatic cytochromes P4501A expression and exacerbates hyperoxic lung injury. J Pharmacol Exp Ther. 2004;310:512–519. doi: 10.1124/jpet.103.059766. [DOI] [PubMed] [Google Scholar]
- Levine M, Law EY, Bandiera SM, Chang TK, Bellward GD. In vivo cimetidine inhibits hepatic CYP2C6 and CYP2C11 but not CYP1A1 in adult male rats. J Pharmacol Exp Ther. 1998;284:493–499. [PubMed] [Google Scholar]
- Mansour H, Brun-Pascaud M, Marquetty C, Gougerot-Pocidalo MA, Hakim J, Pocidalo JJ. Protection of rat from oxygen toxicity by inducers of cytochrome P-450 system. Am Rev Respir Dis. 1988a;137:688–694. doi: 10.1164/ajrccm/137.3.688. [DOI] [PubMed] [Google Scholar]
- Mansour H, Levacher M, Azoulay-Dupuis E, Moreau J, Marquetty C, Gougerot-Pocidalo MA. Genetic differences in response to pulmonary cytochrome P-450 inducers and oxygen toxicity. J Appl Physiol. 1988b;64:1376–1381. doi: 10.1152/jappl.1988.64.4.1376. [DOI] [PubMed] [Google Scholar]
- Minuz P, Fava C, Lechi A. Lipid peroxidation, isoprostanes and vascular damage. Pharmacol Rev. 2006;58:57–68. [PubMed] [Google Scholar]
- Miller MS. Transplacental lung carcinogenesis: a pharmacogenetic mouse model for the modulatory role of cytochrome P4501A1 on lung cancer initiation. Chem Res Toxicol. 1993;7:471–481. doi: 10.1021/tx00040a001. [DOI] [PubMed] [Google Scholar]
- Moorthy B, Nguyen UT, Gupta S, Stewart KD, Welty SE, Smith CV. Induction and decline of hepatic cytochromes P4501A1 and 1A2 in rats exposed to hyperoxia are not paralleled by changes in glutathione S-transferase-alpha. Toxicol Lett. 1997;90:67–75. doi: 10.1016/s0378-4274(96)03832-5. [DOI] [PubMed] [Google Scholar]
- Moorthy B, Parker KM, Smith CV, Bend JR, Welty SE. Potentiation of oxygen-induced lung injury in rats by the mechanism-based cytochrome P-450 inhibitor, 1-aminobenzotriazole. J Pharmacol Exp Ther. 2000;292:553–560. [PubMed] [Google Scholar]
- Moorthy B. The CYP1A subfamily. In: Ioannides E, editor. Cytochromes P450. Role in Drug Metabolism and Toxicity of Drugs and other Xenobiotics. Cambridge, UK: RSC Publishing; 2008. pp. 97–135. [Google Scholar]
- Morel Y, Barouki R. Down-regulation of cytochrome P450 1A1 gene promoter by oxidative stress. Critical contribution of nuclear factor 1. J Biol Chem. 1998;273:26969–26976. doi: 10.1074/jbc.273.41.26969. [DOI] [PubMed] [Google Scholar]
- Morrow JD, Roberts LJ. The isoprostanes: their role as an index of oxidant stress status in human pulmonary disease. Am J Respir Crit Care Med. 2002;166:S25–S30. doi: 10.1164/rccm.2206011. [DOI] [PubMed] [Google Scholar]
- Nebert DW, Dalton TP, Okey AB, Gonzalez FJ. Role of aryl hydrocarbon receptor-mediated induction of the CYP1 enzymes in environmental toxicity and cancer. J Biol Chem. 2004;279:23847–23850. doi: 10.1074/jbc.R400004200. [DOI] [PubMed] [Google Scholar]
- Nelin LD, Christman NT, Morrisey JF, Dawson CA. Electrochemical nitric oxide and nitrogen dioxide analyzer for use with inhaled nitric oxide. J Appl Physiol. 1996;81:1423–1429. doi: 10.1152/jappl.1996.81.3.1423. [DOI] [PubMed] [Google Scholar]
- Northway WH, Jr, Rosan RC. Radiographic features of pulmonary oxygen toxicity in the newborn: Bronchopulmonary dysplasia. Radiology. 1968;91:49–58. doi: 10.1148/91.1.49. [DOI] [PubMed] [Google Scholar]
- Okamoto T, Mitsuhashi M, Fujita I, Sindhu RK, Kikkawa Y. Induction of cytochrome P450 1A1 and 1A2 by hyperoxia. Biochem Biophys Res Commun. 1993;197:878–885. doi: 10.1006/bbrc.1993.2561. [DOI] [PubMed] [Google Scholar]
- Omeicinski CJ, Rediich CA, Costa P. Induction and developmental expression of cytochrome P4501A1 messenger RNA in rat and human tissues: detection by polymerase chain reaction. Cancer Res. 1990;50:4315–4321. [PubMed] [Google Scholar]
- Ohki Y, Mayuzumi H, Tokuyama K, Yoshizawa Y, Arakawa H, Mochizuki H, Morikawa A. Hepatocyte growth factor treatment improves alveolarization in a newborn murine model of bronchopulmonary dysplasia. Neonatology. 2009;95:332–338. doi: 10.1159/000187651. [DOI] [PubMed] [Google Scholar]
- Pohl RJ, Fouts JR. A rapid method for assaying the metabolism of 7-ethoxyresorufin by microsomal subcellular fractions. Anal Biochem. 1980;107:150–155. doi: 10.1016/0003-2697(80)90505-9. [DOI] [PubMed] [Google Scholar]
- Reiners JJ, Jr, Clift R, Mathieu P. Suppression of cell cycle progression by flavonoids: dependence on the aryl hydrocarbon receptor. Carcinogenesis. 1999;20:1561–1566. doi: 10.1093/carcin/20.8.1561. [DOI] [PubMed] [Google Scholar]
- Ramsay PL, Smith CV, Geske RS, Montgomery CA, Welty SE. Dexamethasone enhancement of hyperoxic lung inflammation in rats independent of adhesion molecule expression. Biochem Pharamcol. 1998;56:259–268. doi: 10.1016/s0006-2952(98)00138-5. [DOI] [PubMed] [Google Scholar]
- Rogers LK, Tipple TE, Britt RD, Welty SE. Hyperoxia exposure alters hepatic eicosanoid metabolism in newborn mice. Pediatr Res. 2010;67:144–149. doi: 10.1203/PDR.0b013e3181c2df4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saugstad OD. Bronchopulmonary dysplasia and oxidative stress: are we closer to an understanding of the pathogenesis of BPD? Acta Paediatr. 1997;86:1277–1282. doi: 10.1111/j.1651-2227.1997.tb14897.x. [DOI] [PubMed] [Google Scholar]
- Sawyer MH, Edwards DK, Spector SA. Cytomegalovirus infection and bronchopulmonary dysplasia in premature infants. Am J Dis Child. 1987;141:303–305. doi: 10.1001/archpedi.1987.04460030081030. [DOI] [PubMed] [Google Scholar]
- Sindhu RK, Sakai H, Kikkawa Y. Effect of hyperoxia on rat pulmonary and hepatic cytochrome P450 monooxygenases. Arch Toxicol. 2000;73:540–546. doi: 10.1007/s002040050006. [DOI] [PubMed] [Google Scholar]
- Sinha A, Muthiah K, Jiang W, Couroucli X, Barrios R, Moorthy B. Attenuation of hyperoxic lung injury by the CYP1A inducer beta-naphthoflavone. Toxicol Sci. 2005;87:204–212. doi: 10.1093/toxsci/kfi226. [DOI] [PubMed] [Google Scholar]
- Tyurina YY, Tyurin VA, Kaynar AM, Kapralova VI, Wasserloos K, Li J, Mosher M, Wright L, Wipf P, Watkins S, Pitt BR, Kagan VE. Oxidative lipidomics of hyperoxic acute lung injury: mass spectrometric characterization of cardiolipin and phosphatidylserine peroxidation. Am J Physiol Lung Cell Mol Physiol. 2010;299:L73–L85. doi: 10.1152/ajplung.00035.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Mater LJ, Leviton A, Kuban KC, Pagano M, Allred EN. Maternal glucocorticoid therapy and reduced bronchopulmonary dysplasia. Pediatrics. 1990;86:331–336. [PubMed] [Google Scholar]
- Wagenaar GT, ter Horst SA, van Gastelen MA, Leijser LM, Mauad T, van der Velden PA, de Heer E, Hiemstra PS, Poorthuis BJ, Walther FJ. Gene expression profile and histopathology of experimental bronchopulmonary dysplasia induced by prolonged oxidative stress. Free Radic Biol Med. 2004;36:782–801. doi: 10.1016/j.freeradbiomed.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Warner BB, Stuart LA, Papes RA, Wispe JR. Functional and pathological effects of prolonged hyperoxia in neonatal mice. Am J Physiol. 1998;275:L110–L117. doi: 10.1152/ajplung.1998.275.1.L110. [DOI] [PubMed] [Google Scholar]
- Whitlock JP., Jr Induction of cytochrome P4501A1. Annu Rev Pharmacol Toxicol. 1999;39:103–125. doi: 10.1146/annurev.pharmtox.39.1.103. [DOI] [PubMed] [Google Scholar]
- Whitlock JP, Jr, Okino ST, Dong L, Ko HP, Clarke-Katzenberg R, Ma Q, Li H. Cytochromes P450 5: induction of cytochrome P4501A1: a model for analyzing mammalian gene transcription. FASEB J. 1996;10:809–818. doi: 10.1096/fasebj.10.8.8666157. [DOI] [PubMed] [Google Scholar]
- Whitney PL, Frank L. Does lung NAD(P)H: quinone reductase (DT-diaphorase) play an antioxidant enzyme role in protection from hyperoxia? Biochim Biophys Acta. 1993;21:275–282. doi: 10.1016/0304-4165(93)90042-7. [DOI] [PubMed] [Google Scholar]
- Xu M, Nelson GB, Moore JE, McCoy TP, Dai J, Manderville RA, Rose JA, Miller MS. Induction of Cyp1a1 and Cyp1b1 and formation of DNA adducts in C57BL/Balb/c, and F1 mice following in utero exposure to 3-methylcholanthrene. Toxicol Appl Pharmacol. 2005;209:28–38. doi: 10.1016/j.taap.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Yang F, Coalson JJ, Bobb HH, Carter JD, Banu J, Ghio AJ. Resistance of hypotransferrinemic mice to hyperoxia-induced lung injury. Am J Physiol. 1999;277:L1214–L1223. doi: 10.1152/ajplung.1999.277.6.L1214. [DOI] [PubMed] [Google Scholar]
- Yang P, Chan D, Felix E, Madden T, Klein RD, Shureiqi I, Chen X, Dannenberg AJ, Newman RA. Determination of endogenous tissue inflammation profiles by LC/MS/MS: COX- and LOX-derived bioactive lipids. Prostaglandins, Leukotrienes and Essential Fatty acids. 2006;75:385–395. doi: 10.1016/j.plefa.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Zahler S, Becker BF. Indirect enhancement of neutrophil activity and adhesion to cultured human umbilical vein endothelial cells by isoprostanes (iPF2alpha-III and iPE2-III) Prostaglandins Other Lipid Mediat. 1999;57:319–331. doi: 10.1016/s0090-6980(98)00079-3. [DOI] [PubMed] [Google Scholar]