Abstract
For decades, type I IFNs have been considered indispensable and unique antiviral mediators for the activation of rapid innate antiviral protection. However, the recent discovery of type III IFNs is challenging this paradigm. Since their identification in 2002/2003 by two independent groups, type III IFNs or IFN-λs, also known as IL-28/29, have been the subject of increased study with consequent recognition of their importance in virology and immunology. Initial reports suggested that IFN-λs functionally resemble type I IFNs. Although IFN-λs and classical type I IFNs (IFN-α/β) utilize distinct receptor complexes for signaling, both types of IFNs activate similar intracellular signaling pathways and biological activities, including the ability to induce antiviral state in cells, and both type I and type III IFNs are induced by viral infection. However, different antiviral potency, pattern of their induction and differential tissue expression of their corresponding receptor subunits suggest that the type I and type III IFN antiviral systems do not merely duplicate each other. Recent studies have started to reveal unique biological activities of IFN-λs in and beyond innate antiviral immunity.
Introduction
Interferons (IFNs) are defined by their ability to induce resistance to viral infection. Three distinct types of IFNs are distinguished (type I, type II and type III), based on their structural features, receptor usage and biological activities. Although all types of IFNs stimulate innate and adaptive immune mechanisms that contribute to the clearance of viral infections, only type I and type III IFNs are directly produced in response to virus infections. Until recently, it was widely accepted that type I IFNs played an exclusive role as early mediators of the innate response to viruses, as well as regulators of the subsequent responses from elements of the adaptive immune system. Surprisingly, a group of proteins functionally similar to type I IFNs was discovered in 2002/2003 [1;2]. These proteins, now collectively known as type III IFNs and first designated as IFN-λs [1] or IL-28/29 [2], share with type I IFNs similar expression patterns and trigger common signal transduction cascades and sets of stimulated genes. Consequently, both types of I and type III IFNs share many biological activities, including the ability to induce an antiviral state in cells.
In humans, three functional IFN-λ genes are clustered on human chromosome 19 and encode highly homologous IFN-λ1, IFN-λ2 and IFN-λ3 proteins [1], whereas the type I IFN family includes 13 IFN-α proteins, and one of each IFN-β, IFN-ω, IFN-κ and IFN-ε, all encoded in a gene cluster on chromosome 9 [3]. Although the type I IFN genes lack introns, the coding regions of the IFN-λ genes are interrupted by 4 introns, and the positions of the introns with respect to the protein reading frames are conserved for the IFN-λ genes and for genes encoding IL-10-related cytokines [3;4]. The amino acid identity between type I and type III IFNs is very low, ranging from 15 to 20%. All IFN types belong to a family of α-helical-bundle cytokines that share common functional and structural characteristics and a common evolutionary origin [3;4]. In addition to type I and type III IFNs, in humans this cytokine family also contains one type II IFN (IFN-λ), and six IL-10-related cytokines (IL-10, IL-19, IL-20, IL-22, IL-24 and IL-26). In other species, the family can be further expanded with several viral orthologs, as well as type I IFNs that are not represented in the human genome. All these cytokines are collectively designated CRF2 cytokines because they interact with receptors from a specific receptor family known as the class II cytokine receptor family (CRF2) that is defined by common structural and functional features [5].
Although, type I and type III IFNs all possess intrinsic antiviral activities, they engage IFN type-specific receptor complexes for signaling. Type III IFNs signal through a heterodimeric IFN-λ receptor complex composed of a unique IFN-λR1 chain and the IL-10R2 chain that is also the second subunit of the receptor complexes for IL-10, IL-22 and IL-26 [3]. In contrast, all type I IFNs signal through a common cellular IFN-α/β receptor complex composed of two unique subunits, IFN-αR1 (IFNAR1) and IFN-αR2 (IFNAR2) [6].
Recent studies have started to uncover a unique role of IFN-λs in antiviral defense, and there is emerging evidence that IFN-λs may have functional importance beyond innate antiviral protection. Although the overall biological significance of IFN-λs remains to be determined, this report summarizes current information about the IFN-λ ligand-receptor system focusing on advances in our understanding of the biological activities of type III IFNs, the differences between the type I and type III IFNs, and the therapeutic potential of the IFN-λs.
Expression patterns
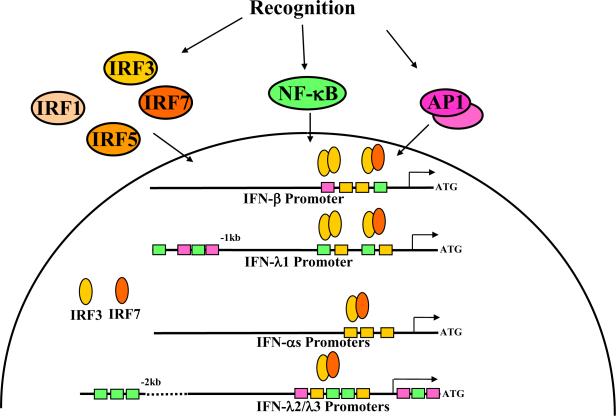
Co-expression of type I and type III IFNs in response to diverse viruses and various TLR agonists was reported in numerous in vitro and in vivo studies (reviewed in [7]), although differences in the expression of type I and type III IFNs have been documented. The similar expression patterns are due to the presence of common regulatory elements in the promoters of the type I and type III IFN genes (Fig. 1). Promoters of the IFN-λ genes contain predicted sites for binding of transcription factors AP1 (dimeric transcription factor containing members of the JUN, FOS, ATF and MAF protein families) and NF-κB (nuclear factor κB), and multiple virus response elements which are the sites for binding of various interferon regulatory factors (IRFs). All these factors are involved in the transcriptional regulation of the type I IFN genes [8]. The importance of the IRF and NF-κB pathways in the transcriptional regulation of the IFN-λ genes was also demonstrated [9;10]. It appears that the human IFN-λ1 and IFN-β genes have similar transcriptional regulation that is controlled by either IRF3 or IRF7, whereas IFN-λ2/3 genes, like most IFN-α genes, are more dependent on IRF7 [10]. This observation is important because IRF3 is constitutively and ubiquitously expressed in cells and, when activated upon viral entry, up-regulates expression of the IFN-β and IFN-λ1 genes. By contrast, IFN-α and IFN-λ2/3 genes are unresponsive to IRF3 alone and require IRF7 which is not constitutively expressed in most cell types but is induced in response to IFNs. In humans, both IFN-β and IFN-λ1 can prime cells for virus-induced IFN-α and IFN-λ2/3 production by up-regulating IRF7 expression. Thus, the expression pattern of the IFN-λ genes conforms to a similar positive feed-back mechanism that was first described for the type I IFN genes [11;12]. Similar to IFN-β, the IFN-λ1 gene represents an early response gene, whereas IFN-λ2/3 are likely to be expressed similar to IFN-αs, with delayed kinetics [13]. The regulation of type III IFNs may differ in mice, however, since there is no functional IFN-λ1 gene in the murine genome [14]. This multi-layered regulation allows the majority of nucleated cells to achieve finely tuned levels of IFN expression depending on the magnitude of viral infection. In contrast to most cell types, plasmacytoid dendritic cells (pDCs) constitutively express IRF7, enabling these cells to rapidly produce high levels of type I and type III IFNs upon stimulation [15;16]. Certain viruses or TLR agonists can induce IFN-λ production from several DC subsets, whereas other IFN-inducing stimuli act in a DC subset-restricted manner [16–19]. Thus, multiple cell types can co-produce type I and type III IFNs in response to viral infection.
Fig. 1. Model of the IFN expression.
A variety of sensors are employed by cells to recognize molecules associated with pathogens or the damage to the host cells caused by pathogens. When engaged, these sensors trigger several overlapping pathways leading to the activation of transcriptional factors that induce expression of the type I and type III IFN genes [69]. Two classes of transcription factors, nuclear factor κB (NF-κB) and interferon regulatory factors (IRFs), are crucially important for the induction of type I and type III IFN expression. AP1 transcription factor (dimeric transcription factor containing members of the JUN, FOS, ATF and MAF protein families) is involved in the regulation of transcription of the IFN-β gene [8]. AP1 binding sites are also predicted in the promoters of the IFN-λs genes, but their functions have not been studied.
Despite these similarities in the pattern and regulatory mechanisms of type I and type III IFN expression, potential differences have emerged indicating that our understanding of IFN expression is incomplete. For example, it was reported that murine macrophages express high levels of type I IFN mRNAs after HSV infection, but do not up-regulate IFN-λ mRNA [20]. Human alveolar type II cells produced high levels of IFN-λs but not IFN-β in response to influenza A virus infection [21]. Importantly, recent studies revealed that IFN-λs appear to be the major IFN type produced by both murine and human airway epithelial cells in response to various respiratory viruses [22–24]. There is also evidence that NF-κB alone is able to induce IFN-λ expression after LPS treatment, independent of IRFs [25]. This may be attributed to a cluster of NF-κB binding sites in the distal promoter of the human IFN-λ1 gene (Fig. 1). In support of this observation, inhibition of the NF-κB pathway in murine DCs and in mice has a stronger effect on the expression levels of IFN-λs than on type I IFNs [26]. These studies suggest important differences in the transcriptional regulation of the type I and type III IFN genes: whereas type I IFN expression is strongly dependent on the cooperative action of multiple transcription factors, particularly IRFs and NF-κB, expression of type III IFNs can be induced through the independent action of IRFs or NF-κB. One important implication is that it may be more difficult for viruses to interfere with type III IFN production because both IRF and NF-κB signaling pathways would need to be simultaneously inhibited, whereas blocking IRFs is sufficient for the suppression of type I IFN production. However, at least one virus has evolved a mechanism to inhibit both type I and type III IFNs: the Yaba-like disease virus produces a soluble IFN antagonist able to bind and neutralize not only all type I IFNs but also type III IFNs, despite the considerable structural and sequence differences among these cytokines [6]. The ability of many viruses to successfully target pathways leading to IRF activation [27] may underlie the high levels of type III IFNs, but not type I IFNs, detected in the lungs of mice infected with influenza A virus [24], and elevated levels of type III IFNs but not type I IFNs in liver biopsies from patients with chronic hepatitis C virus infection [28]. Similarly, Hantaan virus triggered an early and high level of expression of IFN-λ1 mRNA, followed by IFN-λ2 mRNA, and a delayed and low level of IFN-β mRNA, with no significant change in levels of IFN-α message [13].
Receptor complex and signaling
As previously mentioned, the IFN-λs interact with a unique heterodimeric receptor consisting of IFN-λR1 (also known as IL-28RA, LICR or CRF2-12), and IL-10R2 (also known as IL-10Rβ), originally identified as the second subunit of the IL-10 receptor, and now known to be used in specific receptor complexes for other members of the IL-10 cytokine family [3;4]. The genes encoding receptors for IFNs and IL-10-related cytokines share a similar intron/exon structure, with the coding regions of the receptor genes divided into seven exons [4]. The IFN-λ gene and the IL-10R2 gene are positioned on human chromosome 1 and chromosome 21, respectively.
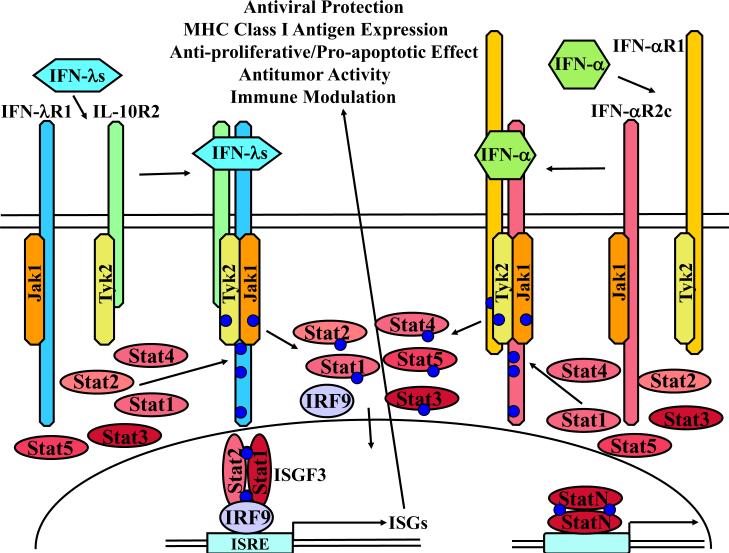
Crystal structures of human IFN-λ3 [29] and of human IFN-λ1 bound to the high affinity receptor subunit IFN-λR1 revealed a common topology with other CRF2 cytokines and a common mode of ligand-receptor interaction [30]. Similar to type I IFNs, IFN-λs are monomers in solution and interact with IFN-λR1 in a 1:1 ratio [1;29;30]. Thus, binding of a monomeric IFN-λ is likely to engage one molecule each of IFN-λR1 and IL-10R2 subunits (Fig. 2). Despite signaling through distinct receptor complexes, type I and type III IFNs trigger similar signaling pathways (Fig. 2), culminating in the activation of a transcriptional complex designated ISGF3 (IFN-stimulated gene factor 3) that is a unique and critical mediator of type I and type III IFN-induced biological activities. ISGF3 binds to the IFN-stimulated response element (ISRE) in the promoters of IFN-stimulated genes (ISGs) leading to gene transcription. Similar to the type I IFNs, IFN-λs also up-regulate expression of SOCS-3 providing the mechanism for negative regulation of IFN-λ signaling [31].
Fig. 2. Model of the IFN-λ receptor system, in relation to the type I IFN receptor system.
Type I and type III IFNs signal through unique receptor heterodimers. The functional IFN-λ receptor complex is composed of IFN-λR1 and IL-10R2 chains. IFN-λ-induced receptor engagement leads, via the activation of receptor-associated Jak kinases, Jak1 and Tyk2, to the tyrosine phosphorylation of the IFN-λR1 intracellular domain and subsequent activation of latent transcription factors of the STAT family: STAT1, STAT2, STAT3, STAT4, and STAT5 [1;37]. Phosphorylated STATs form various homo- and heterodimers, translocate to the nucleus, and bind to specific DNA elements in the promoters of IFN-stimulated genes (ISGs) leading to gene transcription and induction of IFN-λ-specific biological activities, such as upregulation of MHC class I antigen expression, activation of antiviral protection, anti-proliferative response and antitumor activities. STAT1-STAT2 heterodimers interact with a DNA-binding protein IRF9 to form IFN-stimulated gene factor 3 (ISGF3) complex that binds the IFN-stimulated response element (ISRE). Activated STAT1 can also homodimerize and bind to the GAS (gamma-activated sequence) element. Latent STAT2 is recruited to the IFN-λ receptor complexes through the interaction of STAT2 SH2 domain with two specific phosphotyrosine based motifs (Tyr343 or Tyr517) within the intracellular domain of IFN-λR1 that are similar to motifs found in the IFN-α receptors [37]. Activation of STAT2 requires the presence of either Tyr343 or Tyr517 of IFN-λR1, whereas STAT4 phosphorylation, and to some extent STAT1 and STAT3 phosphorylation, can proceed independently of IFN-λR1 tyrosine residues. The ability of IFN-λs to induce antiviral and antiproliferative activities is completely dependent on Tyr343 or Tyr517 of IFN-λR1, demonstrating that the activation of STAT2 is pivotal for these biological activities [37]. Not shown here, but mentioned in the text, are alternate signaling pathways, in addition to the Jak-Stat pathways illustrated here.
IFNs can also induce signaling through pathways other than the canonical Jak-STAT pathway [32]. Similar to type I IFNs, IFN-λs trigger signaling through three major mitogen-activated protein kinase (MAPK) cascades: the extracellular signal-regulated kinase (ERK)-1/2; stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK); and p38 kinase [31;33]. IFN-λs also induce phosphorylation of Akt though the phosphatidylinositol 3-kinase (PI3K) pathway [31]. Engagement of Akt-mTOR and MEK/ERK pathways by IFN-λ results in the activation of the downstream kinases, p70 ribosomal protein S6 kinase (p70S6K) and p90 ribosomal protein S6 kinase 1 (RSK1), and their targets, the translational repressor 4E-BP1 and eukaryotic translation-initiation factor 4B (eIF4B), which regulate the initiation of mRNA translation [34]. However, the ability of IFN-λs to trigger these alternative pathways could be cell-type specific or altered in cancer cells, because phosphorylation of Akt, ERK and SAPK/JNK in response to IFN-λ was not detected in a panel of melanoma cell lines [35]. IFN-λ-induced phosphorylation of ERK was also not detected in Raji cells [33], whereas levels of phosphorylated p38 did not significantly changed in colorectal adenocarcinoma HT-29 cells [31].
It should be noted that the intensity of STAT activation and subsequent biological activities in response to IFN-λs, particularly inhibition of cell proliferation, is generally weaker than in response to type I IFNs [1;2;36]. This may result from the low level of IFN-λR1 expression in cells, or from differential ability to recruit and/or activate components of the intracellular signaling system. Overexpression of IFN-λR1 or a chimeric receptor that recapitulates IFN-λ signaling enables type III IFNs to induce strong signaling, leading to the pronounced anti-proliferative and pro-apoptotic responses in the transfected cells [37;38]. Interestingly, overexpression of IFN-λR1 in cells resulted in decreased antiviral activity of IFN-α [2], suggesting that IFNs may compete for common downstream signaling components. It is important to note that receptor complexes for type I and type III IFNs act independently of each other: in vitro and in vivo experiments demonstrated that either type I or type III IFN antiviral systems are functional in cells lacking receptors from the opposite receptor complex [20;33;39].
Except for the conserved STAT2 docking sites, the intracellular domains of IFN-λR1 and IFN-αR2c, the receptor chains respectively responsible for STAT activation within the type III and type I IFN receptor complexes, are very different, providing the basis for the possible engagement of distinct signaling pathways by the each type of IFNs. These elusive type I and type III IFN-specific pathways and subsequent biological activities are still to be identified by future experiments.
Functional significance of type III IFNs
Signaling though common pathways enables type I and type III IFNs to induce similar biological activities, particularly antiviral resistence, in responsive cells, presumably mediated by the induction of nearly identical sets of more than 300 ISGs [33;40;41]. Consequently, the most prominent biological function of type I and type III IFNs resides in their ability to induce an antiviral state in cells. However, important differences between the two antiviral systems first emerged from in vitro and ex vivo experiments, which revealed that not all cell types respond to type III IFNs: whereas different epithelial-like cell lines and primary keratinocytes are responsive to both types of IFNs, splenocytes, fibroblasts and endothelial cells do not seem to respond to IFN-λ [14]. Subsequent in vivo experiments elegantly demonstrated that the primary targets of type III IFNs are epithelial cells of the respiratory, gastro-intestinal and reproductive tracts [20;39;42–44]. The unique functional tissue-specificity of IFN-λs is due to the cell type-restricted pattern of IFN-λR1 expression; although all cells express receptors for type I IFNs, IFN-λR1 is primarily expressed in epithelial cells and specific subsets of immune cells [14;20;33;39;40;42–46]. In vivo studies further demonstrated that both type I and type III IFN systems are capable of providing efficient, comparable, and independent antiviral protection in epithelial tissues where receptors for both types of IFNs are expressed [20;39;43;44;47]. However, the IFN-λ antiviral system alone cannot provide full protection against systemic virus infections, presumably because these viruses infect cells that are not responsive to type III IFNs; for systemic infections, the functional type I IFN antiviral system is required. In contrast, antiviral protection of intestinal epithelial cells against GI viruses mainly relies on the action of the type III IFN antiviral system [48]. This recent study demonstrates that mice lacking a functional IFN-λ receptor complex had impaired control of oral rotavirus infection; the type I IFN system alone was unable to protect against rotaviruses, which infect intestinal epithelial cells. Importantly, systemic administration of IFN-λ, but not type I IFN, was able to induce an antiviral state in intestinal epithelial cells resulting in the suppression of rotavirus replication in the gut. Thus, the type III IFN system has a unique function in antiviral protection of intestinal epithelium that is independent of, and not overlapping with, the type I IFN antiviral system. Because expression of IFN-λs may be triggered by various bacteria-associated molecules, type III IFNs may be involved in the maintenance of GI tract homeostasis.
Concluding remarks
It is now clear that IFN-λs are important mediators of antiviral responses in mucosal/epithelial tissues, and are critically important for the protection of GI epithelium. Nevertheless, important aspects of IFN-λ biology require further experimental exploration to advance our understanding of the complex role of type III IFNs in overall immunity.
For example, although specific sets of immune cells such as pDCs [49] clearly respond to type III IFNs, it remains controversial whether IFN-λs affect any aspect of T cell biology, and whether effects of IFN-λs on T cells are direct or mediated by DCs. Thus, the current, immunomodulatory activities of IFN-λs are poorly defined and include apparently opposing functions such as: DC-mediated stimulation of either T-reg proliferation [50] or skewing toward Th1 differentiation [51]; DC-independent inhibition of Th2 cytokine production from CD4+ T cells [52]; induction of apoptosis of CD3+ T cells [53]; or augmentation of CTL effector functions during vaccination [54]. Clarification of these effects and expansion to understand IFN-λ effects on other immune cells, particularly those found in epithelial tissues, is a clear part of the research agenda.
The roles of IFN-λs in pathology or the potential of either these cytokines or anti-cytokine therapeutics are new areas for investigation. IFN-λs may also have a specialized role in the etiology of some diseases of epithelial tissue, and in the treatment of viral infections of these and other responsive tissues. The tissue-restricted expression of the IFN-λ receptor has several implications, including the likelihood that IFN-λ therapy may cause fewer and/or milder side effects than IFN-α therapy which is accompanied by numerous side effects. In the airway and lung, for example, there is strong evidence that the type III IFN system play an important role in the pathology of asthma [51;55]. Also, because IFN-λs are active on lung epithelial cells and are important mediators in innate responses to respiratory viral infections [1;55], it is possible that intranasal delivery of IFN-λs could be effective treatment and/or preventive measure against numerous respiratory viruses, particularly against viruses which are poor IFN-inducers. Moreover, IFN-λ therapy may represent a novel approach to prevention and/or treatment of respiratory virus-triggered asthma exacerbations [51;55].
Pegylated IFN-λ1 is undergoing clinical trials for the treatment of chronic hepatitis C infection [56]. Human primary and cultured hepatocytes respond to type III IFNs, and IFN-λs exhibit antiviral activities against HCV and HBV in these cells [40;41;57;58]. Moreover, the importance of IFN-λs for antiviral immunity against HCV in humans is highlighted by recent reports about several single nucleotide polymorphisms (SNPs) near the IFN-λ3 gene, which seem to affect IFN-λ expression levels [59–61], were correlated with the spontaneous clearance of HCV [62], and were also associated with sustained virologic response (SVR) in patients with chronic HCV undergoing pegylated IFN-α/ribavirin (pegIFN-α/RBV) combination therapy [60,61,63]. On the other hand, the IFN-λ antiviral system appears to play minimal if any role in the protection of mice against hepatotropic viruses [39], so the results of the human clinical trials are of great interest.
The potential broad roles of IFN-λs in immune function also opens questions in autoimmunity and cancer therapy. By analogy with type I IFNs, it remains to be seen whether IFN-λs are involved in the development or can be used for the treatment of other inflammatory or autoimmune diseases such as systemic lupus erythematosus (SLE) [64], inflammatory bowl disease (IBD) [53], multiple sclerosis (MS) [65] or rheumatoid arthritis (RA). Furthermore, the finding that IFN-λs display potent antitumor activities in murine models of cancer [14;66–68], motivates an exploration of their potential as anti-cancer therapeutics. Thus, although the overall importance of the IFN-λs in host immune responses remains to be fully determined, accumulating evidence suggests that IFN-λs occupy a unique functional niche in the regulation of well-balanced immunity and may have strong and diverse therapeutic potential.
Highlights
-
>
The interferon (IFN) family was recently expanded with the discovery of type III IFNs
-
>
Type III IFNs or IFN-λs, also known as IL-28/29, are directly induced by viral infection
-
>
IFN-λs, together with type I IFNs, function as early mediators of the innate antiviral response
-
>
IFN-λs engage a specific receptor complex to induce antiviral state in cells
-
>
IFN-λs possess unique biological activities in and beyond innate antiviral immunity
Acknowledgments
I thank Dr. Jerome Langer for the critical reading of the review and helpful suggestions. This study was supported in part by the US Public Health Services Grants RO1 AI057468, R21 AI076937 and U01 AI082994 from the NIAID, and TIL Award from Alliance for Lupus Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
- 1.Kotenko SV, Gallagher G, Baurin VV, Lewis-Antes A, Shen M, Shah NK, Langer JA, Sheikh F, Dickensheets H, Donnelly RP. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat.Immunol. 2003;4:69–77. doi: 10.1038/ni875. [DOI] [PubMed] [Google Scholar]; **This report, along with Ref. [2], describes identification and initial characterization of IFN-λs, their receptors and biological activities.
- 2.Sheppard P, Kindsvogel W, Xu W, Henderson K, Schlutsmeyer S, Whitmore TE, Kuestner R, Garrigues U, Birks C, Roraback J, Ostrander C, Dong D, Shin J, Presnell S, Fox B, Haldeman B, Cooper E, Taft D, Gilbert T, Grant FJ, Tackett M, Krivan W, McKnight G, Clegg C, Foster D, Klucher KM. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat.Immunol. 2003;4:63–68. doi: 10.1038/ni873. [DOI] [PubMed] [Google Scholar]; **This report, along with Ref. [1], describes identification and initial characterization of IFN-λs, their receptors and biological activities.
- 3.Kotenko SV, Langer JA. Full house: 12 receptors for 27 cytokines. Int.Immunopharmacol. 2004;4:593–608. doi: 10.1016/j.intimp.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Kotenko SV. The family of IL-10-related cytokines and their receptors: related, but to what extent? Cytokine Growth Factor Rev. 2002;13:223–240. doi: 10.1016/s1359-6101(02)00012-6. [DOI] [PubMed] [Google Scholar]
- 5.Langer JA, Cutrone EC, Kotenko S. The Class II cytokine receptor (CRF2) family: overview and patterns of receptor-ligand interactions. Cytokine Growth Factor Rev. 2004;15:33–48. doi: 10.1016/j.cytogfr.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Huang J, Smirnov SV, Lewis-Antes A, Balan M, Li W, Tang S, Silke GV, Putz MM, Smith GL, Kotenko SV. Inhibition of type I and type III interferons by a secreted glycoprotein from Yaba-like disease virus. Proc Natl.Acad.Sci.U.S.A. 2007;104:9822–9827. doi: 10.1073/pnas.0610352104. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This study reveals the first viral defense mechanism that directly inhibits the action of type III IFNs.
- 7.Donnelly RP, Kotenko SV. Interferon-lambda: a new addition to an old family. J Interferon Cytokine Res. 2010;30:555–564. doi: 10.1089/jir.2010.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wathelet MG, Lin CH, Parekh BS, Ronco LV, Howley PM, Maniatis T. Virus infection induces the assembly of coordinately activated transcription factors on the IFN-beta enhancer in vivo. Mol.Cell. 1998;1:507–518. doi: 10.1016/s1097-2765(00)80051-9. [DOI] [PubMed] [Google Scholar]
- 9.Onoguchi K, Yoneyama M, Takemura A, Akira S, Taniguchi T, Namiki H, Fujita T. Viral infections activate types I and III interferon genes through a common mechanism. J.Biol.Chem. 2007;282:7576–7581. doi: 10.1074/jbc.M608618200. [DOI] [PubMed] [Google Scholar]; *This report, along with Ref. [10], characterizes transcriptional regulation of the expression of the IFN-λ genes.
- 10.Osterlund PI, Pietila TE, Veckman V, Kotenko SV, Julkunen I. IFN regulatory factor family members differentially regulate the expression of type III IFN (IFN-lambda) genes. J Immunol. 2007;179:3434–3442. doi: 10.4049/jimmunol.179.6.3434. [DOI] [PubMed] [Google Scholar]; *This report, along with Ref. [9], characterizes transcriptional regulation of the expression of the IFN-λ genes.
- 11.Sato M, Hata N, Asagiri M, Nakaya T, Taniguchi T, Tanaka N. Positive feedback regulation of type I IFN genes by the IFN-inducible transcription factor IRF-7. FEBS Lett. 1998;441:106–110. doi: 10.1016/s0014-5793(98)01514-2. [DOI] [PubMed] [Google Scholar]
- 12.Marie I, Durbin JE, Levy DE. Differential viral induction of distinct interferon-alpha genes by positive feedback through interferon regulatory factor-7. EMBO J. 1998;17:6660–6669. doi: 10.1093/emboj/17.22.6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stoltz M, Klingstrom J. Alpha/beta interferon (IFN-alpha/beta)-independent induction of IFN-lambda1 (interleukin-29) in response to Hantaan virus infection. J Virol. 2010;84:9140–9148. doi: 10.1128/JVI.00717-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lasfar A, Lewis-Antes A, Smirnov SV, Anantha S, Abushahba W, Tian B, Reuhl K, Dickensheets H, Sheikh F, Donnelly RP, Raveche E, Kotenko SV. Characterization of the mouse IFN-lambda ligand-receptor system: IFN-lambdas exhibit antitumor activity against B16 melanoma. Cancer Res. 2006;66:4468–4477. doi: 10.1158/0008-5472.CAN-05-3653. [DOI] [PubMed] [Google Scholar]; *This study reports antitumor activities of IFN-λs and characterizes the murine IFN-λ antiviral system.
- 15.Izaguirre A, Barnes BJ, Amrute S, Yeow WS, Megjugorac N, Dai J, Feng D, Chung E, Pitha PM, Fitzgerald-Bocarsly P. Comparative analysis of IRF and IFN-alpha expression in human plasmacytoid and monocyte-derived dendritic cells. J.Leukoc.Biol. 2003;74:1125–1138. doi: 10.1189/jlb.0603255. [DOI] [PubMed] [Google Scholar]
- 16.Coccia EM, Severa M, Giacomini E, Monneron D, Remoli ME, Julkunen I, Cella M, Lande R, Uze G. Viral infection and Toll-like receptor agonists induce a differential expression of type I and lambda interferons in human plasmacytoid and monocyte-derived dendritic cells. Eur.J.Immunol. 2004;34:796–805. doi: 10.1002/eji.200324610. [DOI] [PubMed] [Google Scholar]
- 17.Osterlund P, Veckman V, Siren J, Klucher KM, Hiscott J, Matikainen S, Julkunen I. Gene expression and antiviral activity of alpha/beta interferons and interleukin-29 in virus-infected human myeloid dendritic cells. J Virol. 2005;79:9608–9617. doi: 10.1128/JVI.79.15.9608-9617.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siren J, Pirhonen J, Julkunen I, Matikainen S. IFN-alpha regulates TLR-dependent gene expression of IFN-alpha, IFN-beta, IL-28, and IL-29. J.Immunol. 2005;174:1932–1937. doi: 10.4049/jimmunol.174.4.1932. [DOI] [PubMed] [Google Scholar]
- 19.Lauterbach H, Bathke B, Gilles S, Traidl-Hoffmann C, Luber CA, Fejer G, Freudenberg MA, Davey GM, Vremec D, Kallies A, Wu L, Shortman K, Chaplin P, Suter M, O'Keeffe M, Hochrein H. Mouse CD8alpha+ DCs and human BDCA3+ DCs are major producers of IFN-lambda in response to poly IC. J Exp.Med. 2010;207:2703–2717. doi: 10.1084/jem.20092720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ank N, Iversen MB, Bartholdy C, Staeheli P, Hartmann R, Jensen UB, gnaes-Hansen F, Thomsen AR, Chen Z, Haugen H, Klucher K, Paludan SR. An important role for type III interferon (IFN-lambda/IL-28) in TLR-induced antiviral activity. J.Immunol. 2008;180:2474–2485. doi: 10.4049/jimmunol.180.4.2474. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Oberley-Deegan R, Wang S, Nikrad M, Funk CJ, Hartshorn KL, Mason RJ. Differentiated human alveolar type II cells secrete antiviral IL-29 (IFN-lambda 1) in response to influenza A infection. J Immunol. 2009;182:1296–1304. doi: 10.4049/jimmunol.182.3.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheahan T, Morrison TE, Funkhouser W, Uematsu S, Akira S, Baric RS, Heise MT. MyD88 is required for protection from lethal infection with a mouse-adapted SARS-CoV. PLoS.Pathog. 2008;4:e1000240. doi: 10.1371/journal.ppat.1000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khaitov MR, Laza-Stanca V, Edwards MR, Walton RP, Rohde G, Contoli M, Papi A, Stanciu LA, Kotenko SV, Johnston SL. Respiratory virus induction of alpha-, beta- and lambda-interferons in bronchial epithelial cells and peripheral blood mononuclear cells. Allergy. 2009;64:375–386. doi: 10.1111/j.1398-9995.2008.01826.x. [DOI] [PubMed] [Google Scholar]
- 24.Jewell NA, Cline T, Mertz SE, Smirnov SV, Flano E, Schindler C, Grieves JL, Durbin RK, Kotenko SV, Durbin JE. Lambda interferon is the predominant interferon induced by influenza A virus infection in vivo. J Virol. 2010;84:11515–11522. doi: 10.1128/JVI.01703-09. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This work shows that intranasal influenza A virus infection leads to the robust type III IFN induction in the lungs of both WT and IFNAR-/- mice.
- 25.Thomson SJ, Goh FG, Banks H, Krausgruber T, Kotenko SV, Foxwell BM, Udalova IA. The role of transposable elements in the regulation of IFN-lambda1 gene expression. Proc.Natl.Acad.Sci.U.S.A. 2009;106:11564–11569. doi: 10.1073/pnas.0904477106. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This study identified a distal cluster of NF-κB binding sites in the IFN-λ1 promoter that regulate IFN-λ1 expression independently of IRF3/7 binding sites.
- 26.Iversen MB, Ank N, Melchjorsen J, Paludan SR. Expression of type III interferon (IFN) in the vaginal mucosa is mediated primarily by dendritic cells and displays stronger dependence on NF-kappaB than type I IFNs. J Virol. 2010;84:4579–4586. doi: 10.1128/JVI.02591-09. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This study reveals stronger dependence of type III IFN expression on NF-κB signaling than those of type I IFNs, which relies more on IRF activation.
- 27.Bowie AG, Unterholzner L. Viral evasion and subversion of pattern-recognition receptor signalling. Nat.Rev.Immunol. 2008;8:911–922. doi: 10.1038/nri2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mihm S, Frese M, Meier V, Wietzke-Braun P, Scharf JG, Bartenschlager R, Ramadori G. Interferon type I gene expression in chronic hepatitis C. Lab Invest. 2004;84:1148–1159. doi: 10.1038/labinvest.3700135. [DOI] [PubMed] [Google Scholar]
- 29.Gad HH, Dellgren C, Hamming OJ, Vends S, Paludan SR, Hartmann R. Interferon-lambda is functionally an interferon but structurally related to the interleukin-10 family. J Biol.Chem. 2009;284:20869–20875. doi: 10.1074/jbc.M109.002923. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Crystal structure of IFN-λ3 was solved.
- 30.Miknis ZJ, Magracheva E, Li W, Zdanov A, Kotenko SV, Wlodawer A. Crystal structure of human interferon-lambda1 in complex with its high-affinity receptor interferon-lambdaR1. J Mol.Biol. 2010;404:650–664. doi: 10.1016/j.jmb.2010.09.068. [DOI] [PMC free article] [PubMed] [Google Scholar]; **Structure of the complex between IFN-λ1 and soluble IFN-λR1 receptor is described.
- 31.Brand S, Beigel F, Olszak T, Zitzmann K, Eichhorst ST, Otte JM, Diebold J, Diepolder H, Adler B, Auernhammer CJ, Goke B, Dambacher J. IL-28A and IL-29 mediate antiproliferative and antiviral signals in intestinal epithelial cells and murine CMV infection increases colonic IL-28A expression. Am.J Physiol Gastrointest.Liver Physiol. 2005;289:G960–G968. doi: 10.1152/ajpgi.00126.2005. [DOI] [PubMed] [Google Scholar]
- 32.Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat.Rev.Immunol. 2005;5:375–386. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- 33.Zhou Z, Hamming OJ, Ank N, Paludan SR, Nielsen AL, Hartmann R. Type III interferon (IFN) induces a type I IFN-like response in a restricted subset of cells through signaling pathways involving both the Jak-STAT pathway and the mitogen-activated protein kinases. J.Virol. 2007;81:7749–7758. doi: 10.1128/JVI.02438-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kroczynska B, Joshi S, Eklund EA, Verma A, Kotenko SV, Fish EN, Platanias LC. Regulatory effects of ribosomal S6 kinase 1 (RSK1) in IFN{lambda} signaling. J Biol.Chem. 2011;286:1147–1156. doi: 10.1074/jbc.M110.183566. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This report demonstrates that IFN-λ activates the p90 ribosomal protein S6 kinase 1 (RSK1) and its downstream effector, initiation factor eIF4B to regulate the initiation of cap-dependent translation.
- 35.Guenterberg KD, Grignol VP, Raig ET, Zimmerer JM, Chan AN, Blaskovits FM, Young GS, Nuovo GJ, Mundy BL, Lesinski GB, Carson WE., III Interleukin-29 binds to melanoma cells inducing Jak-STAT signal transduction and apoptosis. Mol.Cancer Ther. 2010;9:510–520. doi: 10.1158/1535-7163.MCT-09-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meager A, Visvalingam K, Dilger P, Bryan D, Wadhwa M. Biological activity of interleukins-28 and -29: Comparison with type I interferons. Cytokine. 2005;31:109–118. doi: 10.1016/j.cyto.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 37.Dumoutier L, Tounsi A, Michiels T, Sommereyns C, Kotenko SV, Renauld JC. Role of the interleukin (IL)-28 receptor tyrosine residues for antiviral and antiproliferative activity of IL-29/interferon-lambda 1: similarities with type I interferon signaling. J.Biol.Chem. 2004;279:32269–32274. doi: 10.1074/jbc.M404789200. [DOI] [PubMed] [Google Scholar]; *Two Tyr residues in the IFN-λR1 intracellular domain were found to be responsible for STAT2 activation and induction of antiviral and antiproliferative activities by IFN-λs.
- 38.Li W, Lewis-Antes A, Huang J, Balan M, Kotenko SV. Regulation of apoptosis by type III interferons. Cell Prolif. 2008;41:960–979. doi: 10.1111/j.1365-2184.2008.00558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mordstein M, Kochs G, Dumoutier L, Renauld JC, Paludan SR, Klucher K, Staeheli P. Interferon-lambda contributes to innate immunity of mice against influenza A virus but not against hepatotropic viruses. PLoS.Pathog. 2008;4:e1000151. doi: 10.1371/journal.ppat.1000151. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This report describes type I IFN-independent action of type III IFNs in protecting mice against viral pathogens infecting the lung but not the liver.
- 40.Doyle SE, Schreckhise H, Khuu-Duong K, Henderson K, Rosler R, Storey H, Yao L, Liu H, Barahmand-pour F, Sivakumar P, Chan C, Birks C, Foster D, Clegg CH, Wietzke-Braun P, Mihm S, Klucher KM. Interleukin-29 uses a type 1 interferon-like program to promote antiviral responses in human hepatocytes. Hepatology. 2006;44:896–906. doi: 10.1002/hep.21312. [DOI] [PubMed] [Google Scholar]; *This study reports that IFN-λ1 (IL-29) stimulates nearly identical patterns of gene expression as analyzed by microarray analysis.
- 41.Marcello T, Grakoui A, Barba-Spaeth G, Machlin ES, Kotenko SV, MacDonald MR, Rice CM. Interferons alpha and lambda inhibit hepatitis C virus replication with distinct signal transduction and gene regulation kinetics. Gastroenterology. 2006;131:1887–1898. doi: 10.1053/j.gastro.2006.09.052. [DOI] [PubMed] [Google Scholar]; *This work reveals that type I and type III IFNs induced similar sets of genes in human hepatocytes, albeit with different kinetics.
- 42.Sommereyns C, Paul S, Staeheli P, Michiels T. IFN-lambda (IFN-lambda) is expressed in a tissue-dependent fashion and primarily acts on epithelial cells in vivo. PLoS.Pathog. 2008;4:e1000017. doi: 10.1371/journal.ppat.1000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mordstein M, Neugebauer E, Ditt V, Jessen B, Rieger T, Falcone V, Sorgeloos F, Ehl S, Mayer D, Kochs G, Schwemmle M, Gunther S, Drosten C, Michiels T, Staeheli P. Lambda interferon renders epithelial cells of the respiratory and gastrointestinal tracts resistant to viral infections. J Virol. 2010;84:5670–5677. doi: 10.1128/JVI.00272-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pulverer JE, Rand U, Lienenklaus S, Kugel D, Zietara N, Kochs G, Naumann R, Weiss S, Staeheli P, Hauser H, Koster M. Temporal and spatial resolution of type I and III IFN responses in vivo. J Virol. 2010 doi: 10.1128/JVI.00303-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Witte K, Gruetz G, Volk HD, Looman AC, Asadullah K, Sterry W, Sabat R, Wolk K. Despite IFN-lambda receptor expression, blood immune cells, but not keratinocytes or melanocytes, have an impaired response to type III interferons: implications for therapeutic applications of these cytokines. Genes Immun. 2009;10:702–714. doi: 10.1038/gene.2009.72. [DOI] [PubMed] [Google Scholar]
- 46.Wolk K, Witte K, Witte E, Proesch S, Schulze-Tanzil G, Nasilowska K, Thilo J, Asadullah K, Sterry W, Volk HD, Sabat R. Maturing dendritic cells are an important source of IL-29 and IL-20 that may cooperatively increase the innate immunity of keratinocytes. J Leukoc.Biol. 2008;83:1181–1193. doi: 10.1189/jlb.0807525. [DOI] [PubMed] [Google Scholar]
- 47.Ank N, West H, Bartholdy C, Eriksson K, Thomsen AR, Paludan SR. Lambda interferon (IFN-lambda), a type III IFN, is induced by viruses and IFNs and displays potent antiviral activity against select virus infections in vivo. J Virol. 2006;80:4501–4509. doi: 10.1128/JVI.80.9.4501-4509.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pott J, Mahlakoiv T, Mordstein M, Duerr CU, Michiels T, Stockinger S, Staeheli P, Hornef MW. IFN-{lambda} determines the intestinal epithelial antiviral host defense. Proc.Natl.Acad.Sci.U.S.A. 2011;108:7944–7949. doi: 10.1073/pnas.1100552108. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Results of this study identify the IFN-λ antiviral system as a critical component of the antiviral host defense in the intestinal epithelium.
- 49.Megjugorac NJ, Gallagher GE, Gallagher G. Modulation of human plasmacytoid DC function by IFN-lambda1 (IL-29) J Leukoc.Biol. 2009;86:1359–1363. doi: 10.1189/jlb.0509347. [DOI] [PubMed] [Google Scholar]
- 50.Mennechet FJ, Uze G. Interferon-lambda-treated dendritic cells specifically induce proliferation of FOXP3-expressing suppressor T cells. Blood. 2006;107:4417–4423. doi: 10.1182/blood-2005-10-4129. [DOI] [PubMed] [Google Scholar]
- 51.Koltsida O, Hausding M, Stavropoulos A, Koch S, Tzelepis G, Ubel C, Kotenko SV, Sideras P, Lehr HA, Tepe M, Klucher KM, Doyle SE, Neurath MF, Finotto S, Andreakos E. IL-28A (IFN-lambda2) modulates lung DC function to promote Th1 immune skewing and suppress allergic airway disease. EMBO Mol.Med. 2011 doi: 10.1002/emmm.201100142. [DOI] [PMC free article] [PubMed] [Google Scholar]; **This work reveals a critical role of IFN-λs in providing protection from allergic airway disease by inducing type 1 and suppressing Th2 and Th17 responses in vivo through the modulation of lung CD11c(+) DC function in experimental allergic asthma.
- 52.Dai J, Megjugorac NJ, Gallagher GE, Yu RY, Gallagher G. IFN-lambda1 (IL-29) inhibits GATA3 expression and suppresses Th2 responses in human naive and memory T cells. Blood. 2009;113:5829–5838. doi: 10.1182/blood-2008-09-179507. [DOI] [PubMed] [Google Scholar]
- 53.He SH, Chen X, Song CH, Liu ZQ, Zhou LF, Ma WE, Zhao LD, Li TL, Tang SG, Xing Z, Yang PC. Interferon-lambda Mediates Oral Tolerance and Inhibits Antigen-Specific, T-Helper 2 Cell-Mediated Inflammation in Mouse Intestine. Gastroenterology. 2011 doi: 10.1053/j.gastro.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 54.Morrow MP, Yan J, Pankhong P, Shedlock DJ, Lewis MG, Talbott K, Toporovski R, Khan AS, Sardesai NY, Weiner DB. IL-28B/IFN-lambda 3 drives granzyme B loading and significantly increases CTL killing activity in macaques. Mol.Ther. 2010;18:1714–1723. doi: 10.1038/mt.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Contoli M, Message SD, Laza-Stanca V, Edwards MR, Wark PA, Bartlett NW, Kebadze T, Mallia P, Stanciu LA, Parker HL, Slater L, Lewis-Antes A, Kon OM, Holgate ST, Davies DE, Kotenko SV, Papi A, Johnston SL. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat.Med. 2006;12:1023–1026. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]; **This study shows deficient induction of IFN-λs by rhinovirus in asthmatic primary bronchial epithelial cells and alveolar macrophages, which correlated with severity of rhinovirus-induced asthma exacerbation.
- 56.Ramos EL. Preclinical and clinical development of pegylated interferon-lambda 1 in chronic hepatitis C. J Interferon Cytokine Res. 2010;30:591–595. doi: 10.1089/jir.2010.0066. [DOI] [PubMed] [Google Scholar]
- 57.Robek MD, Boyd BS, Chisari FV. Lambda interferon inhibits hepatitis B and C virus replication. J Virol. 2005;79:3851–3854. doi: 10.1128/JVI.79.6.3851-3854.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lazaro CA, Chang M, Tang W, Campbell J, Sullivan DG, Gretch DR, Corey L, Coombs RW, Fausto N. Hepatitis C virus replication in transfected and serum-infected cultured human fetal hepatocytes. Am.J.Pathol. 2007;170:478–489. doi: 10.2353/ajpath.2007.060789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Langhans B, Kupfer B, Braunschweiger I, Arndt S, Schulte W, Nischalke HD, Nattermann J, Oldenburg J, Sauerbruch T, Spengler U. Interferon-lambda serum levels in hepatitis C. J Hepatol. 2011;54:859–865. doi: 10.1016/j.jhep.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 60.Suppiah V, Moldovan M, Ahlenstiel G, Berg T, Weltman M, Abate ML, Bassendine M, Spengler U, Dore GJ, Powell E, Riordan S, Sheridan D, Smedile A, Fragomeli V, Muller T, Bahlo M, Stewart GJ, Booth DR, George J. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat.Genet. 2009;41:1100–1104. doi: 10.1038/ng.447. [DOI] [PubMed] [Google Scholar]; **This study, along with Refs. [61, 63] identified genetic variants near or within the IFN-λ3 gene as being the strongest pretreatment predictors of SVR in patients with HCV genotype 1.
- 61.Tanaka Y, Nishida N, Sugiyama M, Kurosaki M, Matsuura K, Sakamoto N, Nakagawa M, Korenaga M, Hino K, Hige S, Ito Y, Mita E, Tanaka E, Mochida S, Murawaki Y, Honda M, Sakai A, Hiasa Y, Nishiguchi S, Koike A, Sakaida I, Imamura M, Ito K, Yano K, Masaki N, Sugauchi F, Izumi N, Tokunaga K, Mizokami M. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat.Genet. 2009;41:1105–1109. doi: 10.1038/ng.449. [DOI] [PubMed] [Google Scholar]; **See Ref. [60].
- 62.Thomas DL, Thio CL, Martin MP, Qi Y, Ge D, O'Huigin C, Kidd J, Kidd K, Khakoo SI, Alexander G, Goedert JJ, Kirk GD, Donfield SM, Rosen HR, Tobler LH, Busch MP, McHutchison JG, Goldstein DB, Carrington M. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461:798–801. doi: 10.1038/nature08463. [DOI] [PMC free article] [PubMed] [Google Scholar]; **This report describes association of genetic variants near or within the IFN-λ3 gene with spontaneous clearance of HCV infection.
- 63.Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, Heinzen EL, Qiu P, Bertelsen AH, Muir AJ, Sulkowski M, McHutchison JG, Goldstein DB. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]; **See Ref. [60].
- 64.Zahn S, Rehkamper C, Kummerer BM, Ferring-Schmidt S, Bieber T, Tuting T, Wenzel J. Evidence for a pathophysiological role of keratinocyte-derived type III interferon (IFNlambda) in cutaneous lupus erythematosus. J Invest Dermatol. 2011;131:133–140. doi: 10.1038/jid.2010.244. [DOI] [PubMed] [Google Scholar]
- 65.Rynda-Apple A, Huarte E, Maddaloni M, Callis G, Skyberg JA, Pascual DW. Active immunization using a single dose immunotherapeutic abates established EAE via IL-10 and regulatory T cells. Eur.J Immunol. 2011;41:313–323. doi: 10.1002/eji.201041104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sato A, Ohtsuki M, Hata M, Kobayashi E, Murakami T. Antitumor activity of IFN-lambda in murine tumor models. J Immunol. 2006;176:7686–7694. doi: 10.4049/jimmunol.176.12.7686. [DOI] [PubMed] [Google Scholar]
- 67.Numasaki M, Tagawa M, Iwata F, Suzuki T, Nakamura A, Okada M, Iwakura Y, Aiba S, Yamaya M. IL-28 elicits antitumor responses against murine fibrosarcoma. J Immunol. 2007;178:5086–5098. doi: 10.4049/jimmunol.178.8.5086. [DOI] [PubMed] [Google Scholar]
- 68.Abushahba W, Balan M, Castaneda I, Yuan Y, Reuhl K, Raveche E, de la TA, Lasfar A, Kotenko SV. Antitumor activity of type I and type III interferons in BNL hepatoma model. Cancer Immunol.Immunother. 2010;59:1059–1071. doi: 10.1007/s00262-010-0831-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ishii KJ, Koyama S, Nakagawa A, Coban C, Akira S. Host innate immune receptors and beyond: making sense of microbial infections. Cell Host.Microbe. 2008;3:352–363. doi: 10.1016/j.chom.2008.05.003. [DOI] [PubMed] [Google Scholar]