Abstract
Background
Chronic rhinosinusitis (CRS) occurs at high frequency in patients with cystic fibrosis, suggesting that the cystic fibrosis transmembrane conductance regulator (CFTR) chloride (Cl) ion channel might be involved in the development of chronic sinusitis in the general population. CFTR Cl ion transport controls the hydration of mucosal surfaces and promotes effective mucociliary clearance. Altered ion transport, and hence disrupted mucociliary function, could play a role in the pathogenesis of sinus disease. L-ascorbate is a metabolically active component of the nasal and tracheobronchial airway lining fluids and appears to serve as an important biological effector of CFTR-mediated chloride secretion. The purpose of this study was to determine the effects of L-ascorbate on Cl ion transport in freshly excised sinonasal epithelia from normal controls and patients with CRS.
Methods
Four different types of sinonasal tissue (normal sinus mucosa, sinus mucosa from CRS, normal nasal mucosa, nasal mucosa from CRS) were obtained during endoscopic sinus surgery and mounted on sliders with open areas of 0.03 to 0.71cm2 between Ussing hemichambers. Short-circuit current (Isc) was continuously recorded, and a serosa-to-mucosa-directed Cl gradient was applied to increase the electrochemical driving force.
Results
L-ascorbate (500µM) stimulated Cl currents (ΔICl, µA/cm2) across sinonasal epithelia from normal and CRS patients. The Cl secretory response to L-ascorbate was effectively blocked by the Cl ion transport inhibitors glibenclamide and bumetanide. A maximal dose of L-ascorbate (at 1 mM) stimulated 53–70% of Cl currents elicited by the cAMP agonist forskolin. CRS sinonasal tissue was characterized by impaired Cl secretory responses to L-ascorbate that were reduced by 33% in sinus epithelial tissue and by 70% in nasal epithelial tissue when compared to normal subjects. In nasal epithelial tissue from normal subjects, Cl secretion was approximately 2-fold increased when compared to sinus epithelial tissue. In contrast, nasal versus sinus epithelial tissue from CRS patients showed no differences.
Conclusion
Topical administration of L-ascorbate to freshly excised sinus and nasal mucosa enhances chloride secretion. Given that decreased CFTR-mediated Cl secretion may contribute to the development of CRS, L-ascorbate may offer potential as a therapeutic agent for the improvement of mucociliary clearance.
Keywords: ascorbate, vitamin C, chloride channel, CFTR, ion transport, chronic rhinosinusitis, Ussing chamber, epithelium
Introduction
Epithelial ion transport controls the hydration of mucosal surfaces in the airway and promotes effective mucociliary clearance. The cystic fibrosis transmembrane conductance regulator (CFTR) functions as a chloride (Cl) and bicarbonate (HCO3) channel and is critical for the normal height of the airway surface liquid (ASL) which promotes effective mucociliary clearance. L-ascorbate is a metabolically active component of the nasal and tracheobronchial airway lining fluids and appears to serve as an important biological effector of CFTR-mediated chloride secretion.(1–4) Altered ion transport, and hence disrupted mucociliary function, could play a role in the pathogenesis of sinus disease.(5)
A prospective trial showed that serum vitamin C (L-ascorbate) levels are lower in chronic sinusitis compared to controls, and a recent study showed that L-ascorbate is depleted in the fluid lining the respiratory tract as a consequence of oxidative stress.(6;7) We hypothesized that low levels of L-ascorbate in chronic sinusitis may be associated with thickened mucus and impaired mucociliary clearance. The purpose of this study was to compare Cl transport in normal and diseased sinonasal mucosa and to determine the effect of L-ascorbate on CFTR-mediated Cl secretion. Whereas previous electrophysiologic studies of sinonasal epithelium have utilized a cell culture model, we utilized an ex vivo model examining freshly excised sinonasal mucosa.
Methods
Study Population
This study was approved by the institutional review boards at Stanford University and Children’s Hospital Oakland Research Institute. Normal sinonasal mucosa was obtained intraoperatively from patients undergoing endoscopic surgery for pituitary tumor, benign sinonasal tumor or lacrimal obstruction. Sinonasal mucosa was also taken from patients with medically refractory CRS undergoing endoscopic sinus surgery (ESS). All CRS patients fulfilled diagnostic criteria endorsed by the American Academy of Otolaryngology. (8) At the time of ESS, biopsy specimens were divided into nasal and sinus tissue based on the site of harvest. Specimens from nasal septum and turbinates were assigned as nasal tissue and those from uncinate process and ethmoid sinus were assigned as sinus tissue. Four groups of sinonasal specimens were collected: 1) normal sinus epithelia (SE) (n = 4); 2) sinus epithelia from CRS (CRS SE) (n = 10); 3) normal nasal epithelia (NE) (n = 5); and 4) nasal epithelia from CRS (CRS NE) (n = 5).
Ussing Chamber Studies
Tissue specimens were immediately stored in an ice cold Hanks buffer or LHC-9 medium (Invitrogen, USA) and transferred to the laboratory. The mean transfer times from harvest to experiment were 6.6 ± 2.3 hours (NE), 6.3 ± 1.0 hours (SE), 7.8 ± 2.4 hours (CRS NE), and 6.2 ± 2.3 hours (CRS SE), respectively and there was no statistical significance between the four groups ( p> 0.05). A thin layer of epithelial tissue was dissected from the surgical specimen, placed on a slider with an open area of 0.031 – 0.71cm2 and mounted between Ussing-type hemichambers (Easy Mount, Physiologic Instruments Inc. CA. USA). Short-circuit current (Isc) was continuously recorded and at 50-second intervals transepithelial voltage was clamped from 0 to 1 mV to monitor transepithelial resistance (Rte) using Ohm’s law. At the beginning of the experiments tissue specimens were perfused with warmed (37°C) Krebs bicarbonate solution containing (in mM) 120 NaCl, 20 NaHCO3, 5 KHCO3, 1.2 NaH2PO4, 5.6 glucose, 2.5 CaCl2, and 1.2 MgCl2. After blocking electrogenic Na+ absorption with amiloride (10uM, mucosal), half of the Cl salts were exchanged for gluconate salts in the mucosal side of the chamber and tissue specimens were equilibrated at least for 30 minutes. In our first protocol, 500µM L-ascorbate was added to the mucosal side of the chamber to quantify the magnitude of the L-ascorbate stimulated Cl current (ΔICl, µA/cm2). In another set of experiments, a second dose of L-ascorbate (1mM final concentration) was subsequently added to determine the dose-dependency of L-ascorbate stimulated Cl currents in sinus and nasal epithelia from CRS. Then the cAMP elevating agonist (20µM forskolin, bilateral) was added to maximally stimulate CFTR-mediated Cl secretion. To prevent misinterpretation of flux measurement by edge damage of freshly excised specimens, CFTR blocker (1mM glibenclamide, mucosal) and NaKCC inhibitor (500µM bumetanide, serosal) were used at the end of all experiments to block transepithelial Cl secretion by inhibiting mucosal CFTR Cl channel and Na-K-2Cl cotransporter, respectively. Low-resistance tissues (R<20Ω·cm2) were excluded from the analysis.
Statistical Analysis
Statistical analyses were performed using SPSS software (version 15.0) and the significant statistical difference was set at P < 0.05. Statistical analyses were performed using paired and unpaired Student t tests, the Mann-Whitney rank sum test, or the Kruskal-Wallis 1-way analysis of variance of ranks, followed by the Dunn multiple comparison test as appropriate.
Results
Stimulation of transepithelial Cl secretion by L-ascorbate in sinonasal tissue
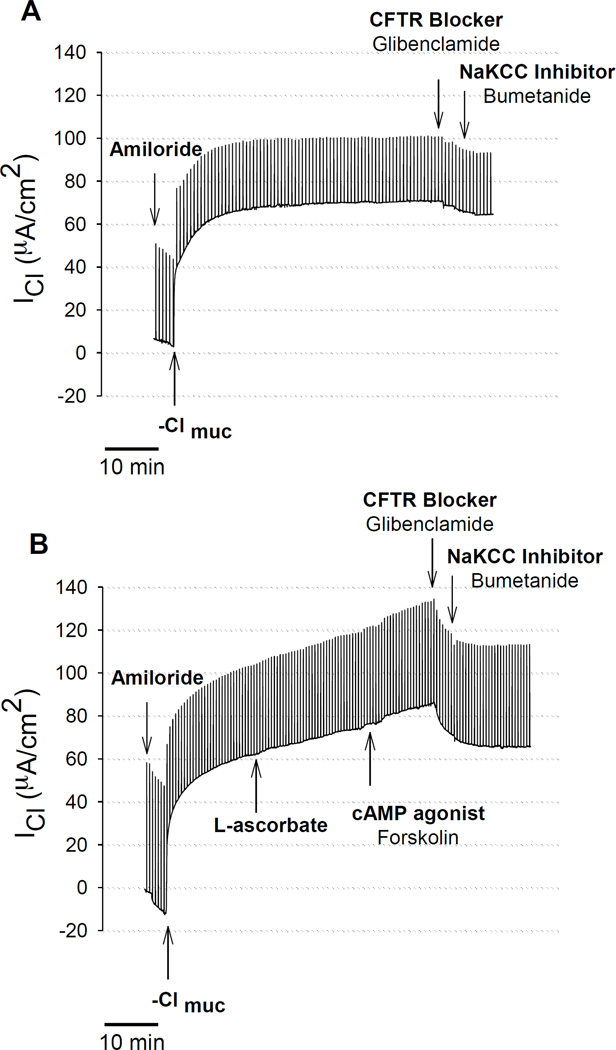
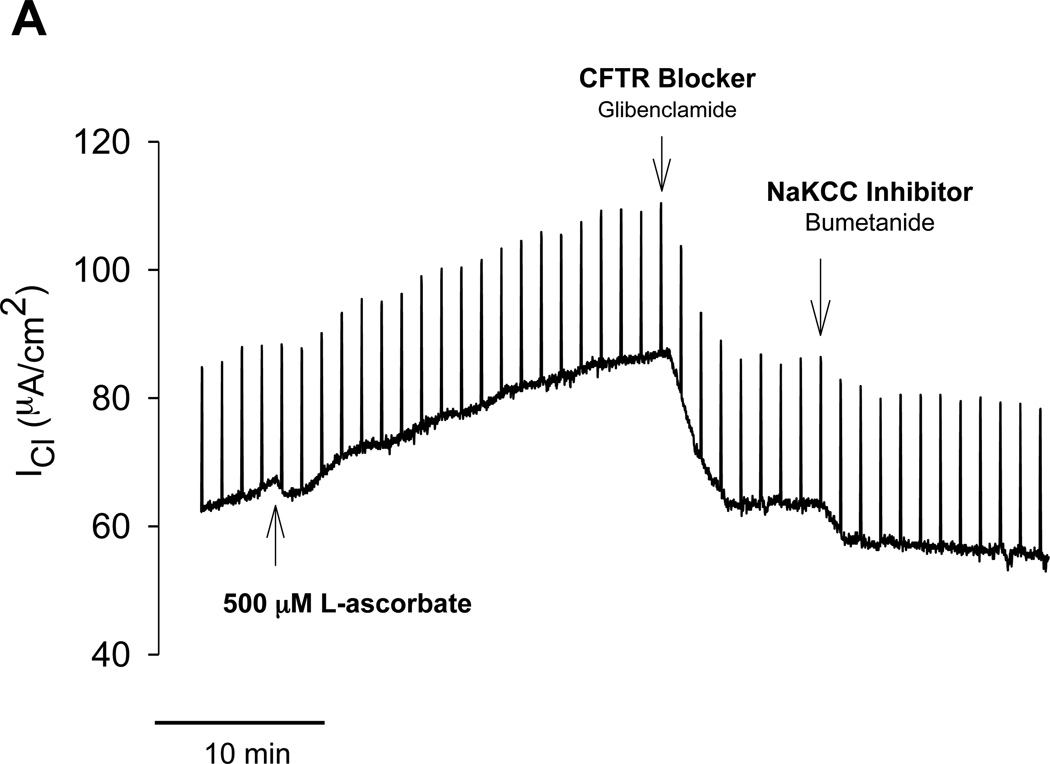
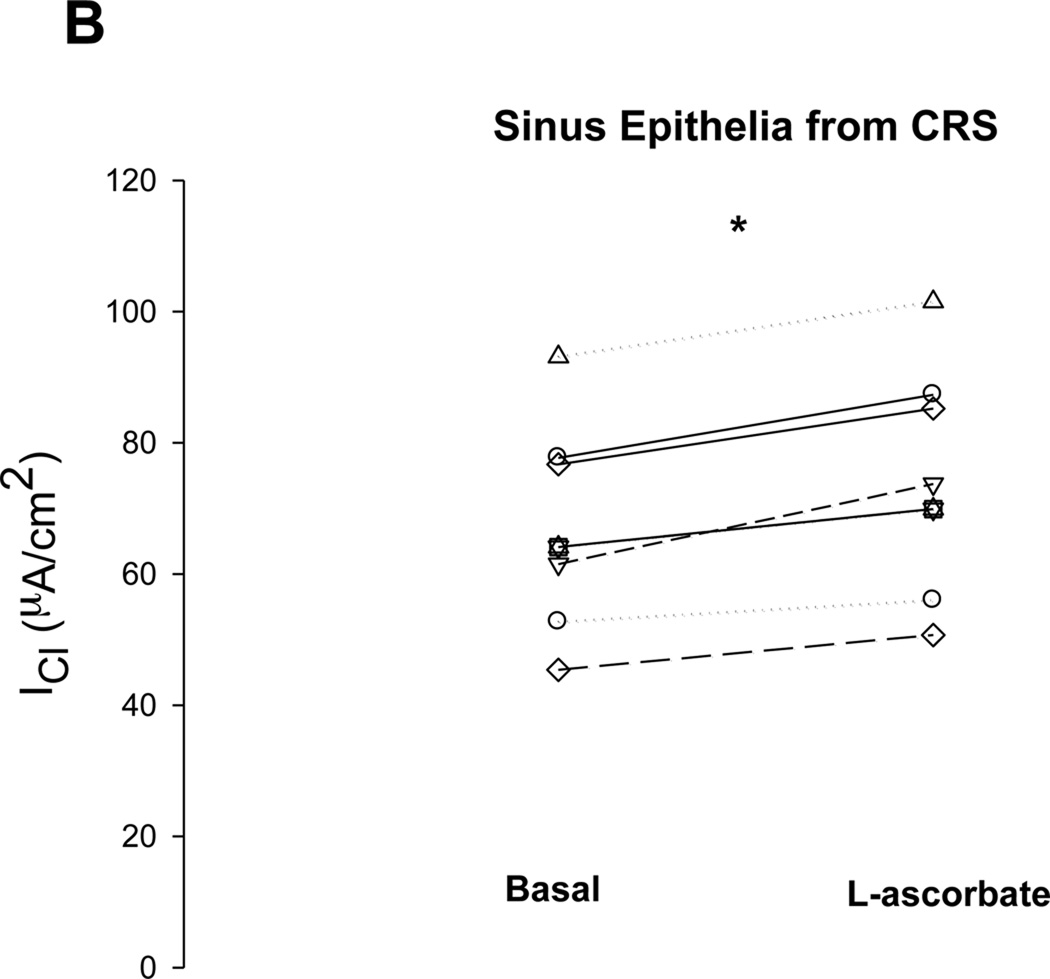
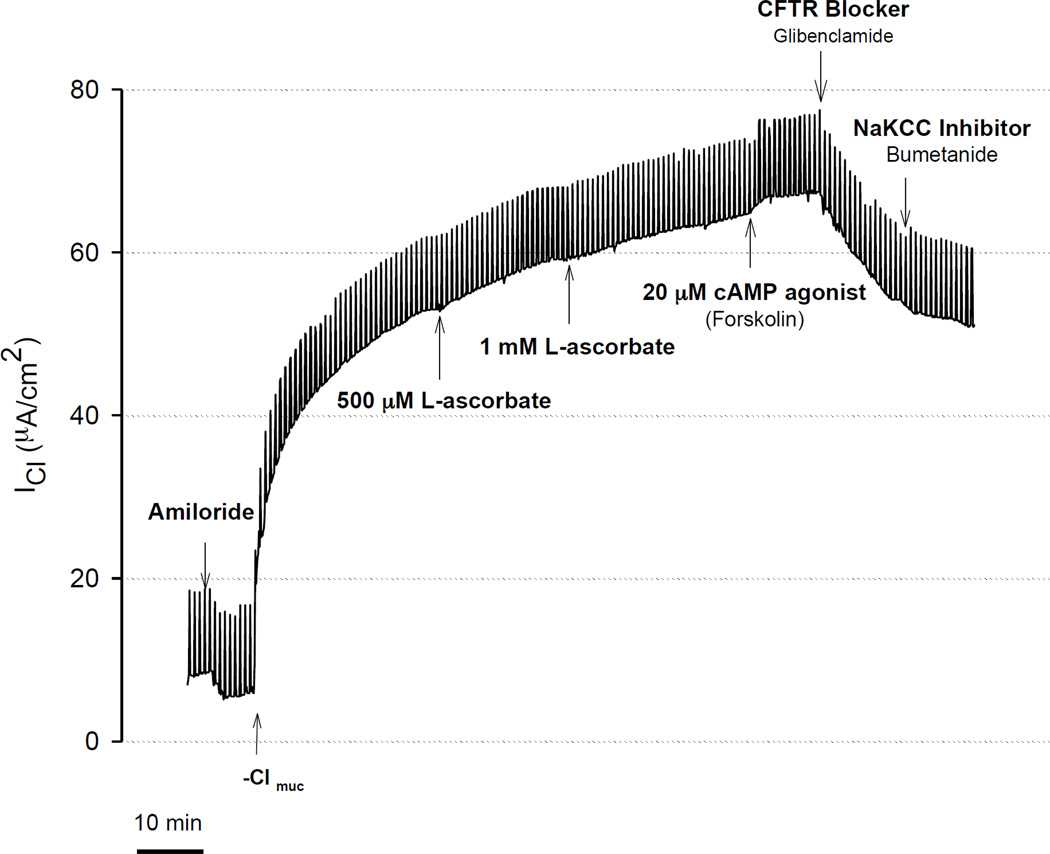
Without exposure of the apical surface to L-ascorbate, there was no stimulation of transepithelial Cl current (ICl) in freshly excised sinus epithelia. The time control experiment in figure 1A shows that gradient-driven ICl remained stable upon removal of chloride for more than one hour. Exposure of the apical surface to L-ascorbate (500µM) gradually stimulated ICl in a sustained fashion over the course of 15–30 minutes (Figure 1B). L-ascorbate was an effective stimulator in sinus epithelia obtained from healthy subjects (Figure 1B) as well as in patients with chronic rhinosinusitis (CRS) (Figure 2A). Subsequent additions of the Cl transport inhibitors glibenclamide and bumetanide significantly reduced ascorbate-stimulated ICl to baseline levels indicating that L-ascorbate stimulated Cl transport across the cell. Figure 2B shows original ICl values before and after addition of 500 µM L-ascorbate from seven different CRS sinus epithelia. Changes in ascorbate-stimulated ICl (ΔICl) are plotted as individual data points and average values in Figure 3 and compared between normal and CRS subjects.
Figure 1.
Stimulation of transepithelial Cl secretion by L-ascorbate in sinus tissue excised from normal human subject. Transepithelial Cl currents (ICl) across sinus epithelia were measured in the presence of the sodium channel blocker Amiloride (10 µM) and a serosal-to-mucosal directed Cl gradient (−Clmuc). A: Time control experiment. Without adding L-ascorbate, there was no increase in ICl. B: L-ascorbate (500 µM) applied to the mucosal surface stimulated ICl in a sustained fashion and subsequent addition of the cAMP agonist forskolin (20 µM) further stimulated ICl, whereas Cl transport inhibitors (Glibenclamide, Bumetanide) significantly reduced the ascorbate- and forskolin stimulated ICl.
Figure 2.
Stimulation of transepithelial Cl secretion by L-ascorbate in sinus tissue excised from patients with chronic rhinosinusitis (CRS). A: Effective stimulation of gradient-driven Cl current (ICl) by 500 µM L-ascorbate in amiloride-treated CRS sinus tissue and subsequent inhibition by Cl transport inhibitors (Glibenclamide, Bumetanide). B: Summary of data obtained from seven CRS tissue specimens. ICl before and after exposure to L-ascorbate (500 µM) for 15–30 minutes. Each paired specimen is connected by a line (P < 0.05, Paired Sample t test).
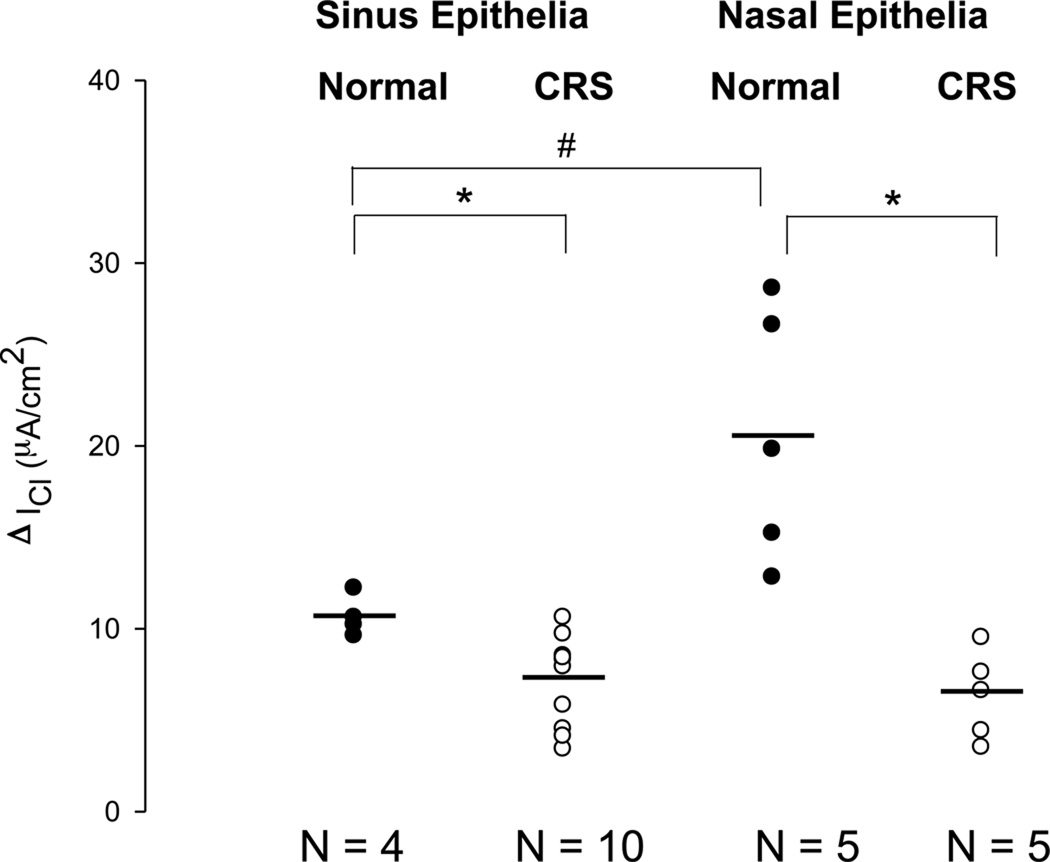
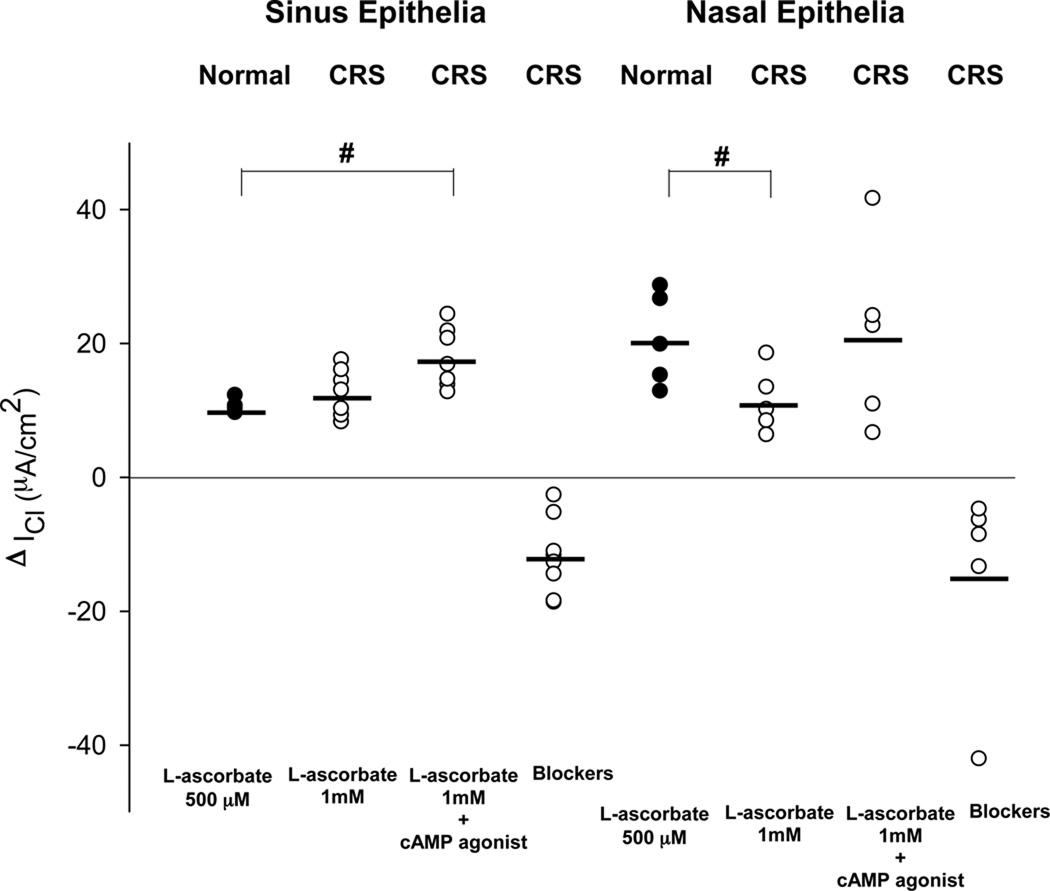
Figure 3.
Changes in basal Cl currents after stimulation with L-ascorbate (ΔICl) in freshly excised sinus and nasal epithelial tissue from normal subjects (Normal) and patients with chronic rhinosinusitis (CRS). L-ascorbate stimulated Cl currents are plotted from individual experiments and average values are shown as horizontal bars. The largest Cl secretory response to L-ascorbate was noted in nasal epithelia from normal subjects. Both sinus and nasal epithelia from CRS patients showed lower Cl secretory responses to L-ascorbate compared to their normal counterparts. #: denotes a significant difference between nasal and sinus epithelial tissue from normal subjects (p<0.05). *: denotes a significant difference between sinus epithelia obtained from normal and CRS subjects (p<0.05). ●: Normal epithelia. ○: Diseased epithelia.
Sinus vs. Nasal Tissue from Normal Subjects
The magnitude of the chloride secretory response to 500 µM L-ascorbate was significantly reduced by a factor of two in sinus tissue when compared to nasal tissue that was excised from normal subjects. The ascorbate-stimulated ICl (ΔICl) averaged 10.7 ± 1.1µA/cm2 in sinus epithelia (n = 4) and ΔICl = 20.6 ± 6.9µA/cm2 in nasal epithelia (n = 5, p = 0.015).
Normal versus CRS Sinonasal Tissue
Although stimulated by L-ascorbate, the Cl secretory response in CRS was diminished compared to normals. This was observed in both nasal mucosa and sinus mucosa. The ascorbate-stimulated increase in Cl secretion in CRS sinus mucosa was 33.3% less than the increase observed in normal sinus epithelia (ΔICl = 7.1 ± 2.5 µA/cm2 vs. 10.7 ± 1.1µA/cm2, p = 0.037). In CRS nasal mucosa, the increase in Cl secretion was 69.4% less than the increase observed in normal nasal epithelia (ΔICl = 6.3 ± 2.2 µA/cm2 vs. 20.6 ± 6.9 µA/cm2, p = 0.03). Therefore, both sinus and nasal epithelia from CRS showed lower Cl secretory responses compared to their normal counterparts. There was no significant difference in ΔICl between sinus and nasal epithelium from CRS (p > 0.05).
Dose-Dependency of Ascorbate-Stimulated Cl Currents
Exposure of the mucosal side to an additional dose of L-ascorbate (1mM total concentration) further stimulated ICl to 12.7 ± 3.3 µA/cm2 in sinus epithelia from CRS (n = 8) and to 11.3 ± 4.8 µA/cm2 in nasal epithelia from CRS (n = 5) (Figure 4). At this double dose of L-ascorbate, the ΔICl of sinus mucosa from CRS equaled the ΔICl observed in normal sinus epithelia at a dose of 500 µM (p > 0.05). In nasal epithelia from CRS, the ΔICl was two-fold increased at 1mM total concentration of L-ascorbate.
Figure 4.
Stimulation of transepithelial Cl secretion by L-ascorbate and cAMP agonist (forskolin) in freshly excised sinus tissue from CRS patient. Transepithelial Cl currents (ICl) were measured in the presence of the sodium channel blocker amiloride (10 µM) and a serosal-to-mucosal directed Cl gradient (−Clmuc). Gradient driven Cl currents (ICl) stabilized after ~30 minutes. Addition of increasing concentrations of L-ascorbate (500 µM, 1 mM) to the mucosal surface stimulated ICl in a dose-dependent fashion. L-ascorbate stimulated ICl was further activated by the cAMP agonist forskolin (20 µM) and sequentially blocked by the CFTR blocker glibenclamide (1 mM) and NaKCC inhibitor bumetanide (200 µM). In this experiment, Cl secretion was stimulated by ascorbate to 80.6% of the currents elicited by forskolin.
Maximal Stimulation with Forskolin
L-ascorbate-induced Cl secretion was further increased by forskolin (20µM, bilateral) (Figure 4). At maximal stimulation with a combination of 1mM L-ascorbate and forskolin, the ΔICl of nasal mucosa from CRS equaled the ΔICl observed in normal nasal epithelia (p > 0.05) (Figure 5). On average, Cl secretion in the sinus (n = 8) and nasal epithelia (n = 5) from CRS was stimulated by L-ascorbate to 70% and 54% of the currents elicited by forskolin, respectively.
Figure 5.
Changes in chloride currents (ΔICl) in normal and CRS sinonasal tissue. The magnitude of ascorbate-stimulated Cl currents and Cl currents blocked after addition of Cl transport inhibitors (glibenclamide, bumetanide) are plotted from individual experiments and average values are shown as horizontal bars. At 1 mM L-ascorbate, the ΔICl of sinus mucosa from CRS equaled the ΔICl observed in normal sinus epithelia at 500 µM L-ascorbate (p > 0.05). However, the ΔICl of nasal mucosa from CRS at 1 mM L-ascorbate was still decreased to the ΔICl observed in normal nasal epithelia at 500 µM L-ascorbate (p < 0.05). At maximal stimulation with a combination of 1 mM L-ascorbate and forskolin, the ΔICl of nasal mucosa from CRS was increased when compared to the response observed in normal nasal epithelia at 500 µM L-ascorbate (p > 0.05). #: denotes a significant difference (p < 0.05). ●: Normal epithelia. ○: Diseased epithelia.
Discussion
This study was designed to investigate the role of L-ascorbate in enhancing Cl secretion in sinonasal mucosa from control and CRS patients in an ex-vivo model, and to clarify the potential involvement of CFTR-mediated Cl secretion in the pathogenesis of CRS. We used transepithelial short-circuit current measurements in Ussing chambers to investigate the effect of L-ascorbate on transepithelial Cl secretion. Freshly excised human sinonasal epithelia are easily accessible in the clinic or during ESS, yielding satisfactory surface area for analysis (exposure area 0.71cm2). Good tissue viability is critical for quantitative assessment of bioelectric responses and is best maintained by storing tissue specimens on ice in an appropriate medium and by conducting functional measurements immediately.(9) Despite the challenge of edge damage in freshly excised tissue, we demonstrated that sinonasal tissue can be analyzed successfully in Ussing chambers. We therefore believe that analysis of freshly excised sinonasal epithelium offers a novel and effective platform for electrophysiologic studies of the upper respiratory epithelium.
This study showed that freshly excised sinus and nasal epithelial tissue from CRS patients exhibited increased chloride secretion in response to external administration of L-ascorbate. Although the magnitude of chloride stimulation was not as great in CRS tissue as compared to normals at the same dose of L-ascorbate, the difference could be overcome by higher doses of L-ascorbate, giving evidence of a dose-response relationship. At doses of 1mM of L-ascorbate, the magnitude of ascorbate-induced chloride stimulation in sinus tissue from CRS equaled that of normal tissue at a dose of 500µM. At 1 mM L-ascorbate Cl secretion was two-fold increased in nasal tissue from CRS.
The use of CFTR-specific blockers after ascorbate stimulation allows one to confirm that the ascorbate-response is indeed modulated by CFTR(1). The stimulatory effect of L-ascorbate on transepithelial Cl secretion was effectively blocked by glibenclamide, an inhibitor of CFTR and other chloride channels, and further reduced by bumetanide, an inhibitor of Cl uptake across the basolateral membrane via the Na-K-2Cl-cotransporter. A maximal dose of L-ascorbate (at 1 mM) stimulated 53–70% of Cl currents elicited by the cAMP agonist forskolin. In nasal epithelial tissue from normal subjects, Cl secretion was approximately 2-fold increased when compared to sinus epithelial tissue. In contrast, nasal and sinus epithelial tissue from CRS patients showed no differences. In summary, CRS sinonasal tissue was characterized by impaired Cl secretory responses to L-ascorbate that were reduced by 33% in sinus epithelial tissue and by 70% in nasal epithelial tissue when compared to normal subjects.
Epithelial ion transport regulates hydration of airway mucosal surfaces and promotes effective mucociliary clearance. Thus failure to clear bacteria or toxins from sinus surfaces could play a role in the pathogenesis of sinus disease.(5) The source of supplying water to maintain the homeostasis of the height of the ASL in the nasal cavity has not been elucidated. Yasuda et al. mentioned that epithelium of paranasal sinus mucosa would secrete water and maintain ASL covering upper airway using cultured nasal epithelium.(10) We demonstrated that application of L-ascorbate to the surface of freshly excised sinonasal tissue stimulated Cl secretion in a sustained fashion to 70% (in sinus epithelium from CRS) and 53.6% (in nasal epithelium from CRS) of Cl currents elicited at maximally stimulated cAMP levels using 20 µM forskolin. Our data using freshly excised human tissue supports that the source of moisture in the nasal cavity may come from the epithelium of paranasal sinuses. Normal sinus and nasal mucosa, mostly from inferior turbinates and septum, showed the largest L-ascorbate stimulated Cl secretions compared to sinus and nasal mucosa from CRS.
Abnormal ion transport in epithelial cells could contribute to the pathogenesis of CRS. For example, altered expression of ClC-2 and ClC-3 chloride channels (11) and upregulation of the transient receptor potential channel vanilloid type 4 (TRP4) cation channel (12) were specifically detected in nonpolypoid CRS patients. In the ethmoid mucosa ClC-2 and ClC-3 mRNA expresssion levels correlated with cytokine levels for transforming growth factor (TGF)-β and interleukin (IL)-4 suggesting a modulatory role of inflammation.(11) Our study measured functional CFTR-mediated Cl transport activity, and detected a decrease in the stimulatory effect of L-ascorbate on CFTR-mediated Cl secretion across the sinonasal epithelia from CRS compared to normal controls. Decreased CFTR-mediated Cl secretion across the mucosal surface may contribute to the development of CRS by depletion of ASL which, in turn, may hinder effective sinonasal mucociliary clearance. The resulting stasis of sinonasal secretions is an important element for subsequent infection, and persistent inflammation in CRS.(13) Decreased CFTR-mediated Cl secretion may also be linked to increased sodium absorption and the development of submucosal edema.(5)
L-ascorbate, commonly known as vitamin C, is an essential nutrient most notably for its role as a powerful antioxidant working to lower oxidative stress on cells.(3) Unlike most animals, humans must take it through diet because we do not synthesize it from D-glucose via the glucuronic pathway.(14) Insufficient dietary intake of vitamin C, environmental pollutants, and a number of inflammatory disorders of the airways are known to severely deplete the pools of vitamin C in ASL or plasma.(15) Because plasma vitamin C concentration does not exceed 90µM even at higher oral doses, oral supplementation of vitamin C may not be sufficient to enhance transepithelial chloride secretion.(16) Topical delivery of vitamin C to paranasal sinuses yields higher concentration at the target tissues and may be an effective therapeutic modality for loosening thick mucous secretions and improving mucociliary clearance of paranasal sinuses.(1;16)
There are some shortcomings of this study. We have small number of tissue samples from normal controls in our present data. A much larger group of patients would have been necessary to study the possibility that there is a correlation between cAMP activated Cl transport and other clinical characteristics, such as environmental factors or clinical outcomes.
Conclusions
Freshly excised human sinonasal epithelial tissue is easily accessible, and its ion transport characteristics can be readily studied. L-ascorbate increased Cl currents across sinonasal epithelial tissue from both normal subjects and CRS patients. L-ascorbate stimulated Cl currents were significantly lower in sinonasal epithelia from CRS patients than epithelia from normal controls. Notably, this difference could be overcome by higher pharmacological doses of L-ascorbate, giving evidence of a dose-response relationship. Decreased CFTR-mediated Cl secretion into the mucosal surface may contribute to the development of CRS by depletion of the ASL. The pool of L-ascorbate in the sinonasal airway provides a potential target for the improvement of sinonasal mucociliary clearance and treatment of thickened secretions in CRS.
Acknowledgements
This work was supported by National Institutes of Health [P01 AT002620], Cystic Fibrosis Foundation (ILLEK08G0).
Footnotes
Presented at the American Rhinologic Society’s 54th Annual Meeting, Chicago, Illinois, September 20th, 2008
The authors had no conflicts of interest or conflicting financial interests to disclose.
The study protocol has been approved by Stanford University and CHORI Institutional Review Board.
References
- 1.Fischer H, Schwarzer C, Illek B. Vitamin C controls the cystic fibrosis transmembrane conductance regulator chloride channel. Proc.Natl.Acad.Sci.U.S.A. 2004;101:3691–3696. doi: 10.1073/pnas.0308393100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schock BC, Koostra J, Kwack S, et al. Ascorbic acid in nasal and tracheobronchial airway lining fluids. Free Radic.Biol.Med. 2004;37:1393–1401. doi: 10.1016/j.freeradbiomed.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 3.Slade R, Crissman K, Norwood J, et al. Comparison of antioxidant substances in bronchoalveolar lavage cells and fluid from humans, guinea pigs, and rats. Exp.Lung Res. 1993;19:469–484. doi: 10.3109/01902149309064358. [DOI] [PubMed] [Google Scholar]
- 4.Fischer H, Illek B. Vitamin C and flavonoids potentiate Cl secretion across airway epithelia. In: Schultz C, editor. Defects of Secretion in Cystic Fibrosis. Kluwer Academic/Plenum Publisher; 2005. pp. 129–143. [Google Scholar]
- 5.Dejima K, Randell SH, Stutts MJ, et al. Potential role of abnormal ion transport in the pathogenesis of chronic sinusitis. Arch.Otolaryngol.Head Neck Surg. 2006;132:1352–1362. doi: 10.1001/archotol.132.12.1352. [DOI] [PubMed] [Google Scholar]
- 6.Mudway IS, Krishna MT, Frew AJ, et al. Compromised concentrations of ascorbate in fluid lining the respiratory tract in human subjects after exposure to ozone. Occup.Environ.Med. 1999;56:473–481. doi: 10.1136/oem.56.7.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Unal M, Tamer L, Pata YS, et al. Serum levels of antioxidant vitamins, copper, zinc and magnesium in children with chronic rhinosinusitis. J.Trace Elem.Med.Biol. 2004;18:189–192. doi: 10.1016/j.jtemb.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Benninger MS, Ferguson BJ, Hadley JA, et al. Adult chronic rhinosinusitis: definitions, diagnosis, epidemiology, and pathophysiology. Otolaryngol.Head Neck Surg. 2003;129:S1–S32. doi: 10.1016/s0194-5998(03)01397-4. [DOI] [PubMed] [Google Scholar]
- 9.Mall M, Hirtz S, Gonska T, et al. Assessment of CFTR function in rectal biopsies for the diagnosis of cystic fibrosis. J.Cyst.Fibros. 2004;3 Suppl 2:165–169. doi: 10.1016/j.jcf.2004.05.035. [DOI] [PubMed] [Google Scholar]
- 10.Yasuda M, Niisato N, Miyazaki H, et al. Epithelial ion transport of human nasal polyp and paranasal sinus mucosa. Am.J.Respir.Cell Mol.Biol. 2007;36:466–472. doi: 10.1165/rcmb.2006-0064OC. [DOI] [PubMed] [Google Scholar]
- 11.Li H, Jiang H, Cheng L, et al. Possible role of transforming growth factor beta and interleukin-4 in the up-regulation of CLC-2 and CLC-3 in chronic rhinosinusitis. Am.J.Rhinol. 2007;21:389–394. doi: 10.2500/ajr.2007.21.3045. [DOI] [PubMed] [Google Scholar]
- 12.Bhargave G, Woodworth BA, Xiong G, et al. Transient receptor potential vanilloid type 4 channel expression in chronic rhinosinusitis. Am.J.Rhinol. 2008;22:7–12. doi: 10.2500/ajr.2008.22.3125. [DOI] [PubMed] [Google Scholar]
- 13.Chen B, Shaari J, Claire SE, et al. Altered sinonasal ciliary dynamics in chronic rhinosinusitis. Am.J.Rhinol. 2006;20:325–329. doi: 10.2500/ajr.2006.20.2870. [DOI] [PubMed] [Google Scholar]
- 14.Nishikimi M, Fukuyama R, Minoshima S, et al. Cloning and chromosomal mapping of the human nonfunctional gene for L-gulono-gamma-lactone oxidase, the enzyme for L-ascorbic acid biosynthesis missing in man. J.Biol.Chem. 1994;269:13685–13688. [PubMed] [Google Scholar]
- 15.Kodavanti UP, Costa DL, Richards J, et al. Antioxidants in bronchoalveolar lavage fluid cells isolated from ozone--exposed normal and ascorbate-deficient guinea pigs. Exp.Lung Res. 1996;22:435–448. doi: 10.3109/01902149609046034. [DOI] [PubMed] [Google Scholar]
- 16.Levine M, Conry-Cantilena C, Wang Y, et al. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc.Natl.Acad.Sci.U.S.A. 1996;93:3704–3709. doi: 10.1073/pnas.93.8.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]