Abstract
Background
Confirmation of mild bites caused by Loxosceles reclusa with swab testing has not been previously documented, to our knowledge.
Methods
We report a case using an enzyme-linked immunosorbent assay (ELISA) test.
Results
A lesion lacking necrosis or other specific signs of loxoscelism was confirmed by identification of the Loxosceles venom and further confirmed by identification of a spider found in the patient’s bed.
Limitations
This is a pilot single-case report for this enzyme-linked immunosorbent assay test.
Conclusions
A sensitive and specific enzyme-linked immunosorbent assay designed to detect Loxosceles venom, using a specimen obtained by swabbing the lesion, can aid in diagnosis of loxoscelism.
Loxosceles reclusa and related arachnid species are indigenous US spiders that possess a venom capable of causing painful, disfiguring necrotic ulcers with surrounding dermal inflammation and, uncommonly, severe systemic effects.1–4 The diagnosis of a brown recluse spider bite is a clinical one made on the basis of the morphologic appearance of the cutaneous lesion.1–4 Definitive diagnosis is problematic because patients generally do not bring the offending spider to the clinician for identification. The appearance of significant envenomation with cutaneous necrosis is the usual basis for diagnosis but is not specific for Loxosceles species envenomation,3–5 with mimics including a variety of treatable illnesses.6–8 When significant necrosis is absent, as in the case presented here, the characteristic features of envenomation are lacking, and the diagnosis is more difficult. For this case, we used a sensitive and specific enzyme-linked immunosorbent assay (ELISA) designed to detect Loxosceles venom,9 using a specimen obtained by swabbing the lesion.
A 10-year-old girl came to our clinic in Rolla, Mo, with a 2-day history of a painful lesion in her left axilla. The child reported that she noticed the dermal discomfort 2 days earlier on awakening. During the initial period after the bite, the child’s mother found a dead spider (later identified) in the girl’s bed. On the day before presentation, the child developed a headache, severe nausea, vomiting, and a morbilliform exanthem.
Examination showed a quiet and mildly apprehensive girl with pulse of 96/min, blood pressure of 98/60 mm Hg, and a temperature of 37.1°C (98.7°F). A vesicle on the left axilla was surrounded by a tender, erythematous area with streaks (Fig 1). A fine morbilliform exanthem was present on the abdomen and back. The dead spider had been saved by the girl’s mother and was later identified by an arachnologist as a member of the species L reclusa (Fig 2).
Fig 1.
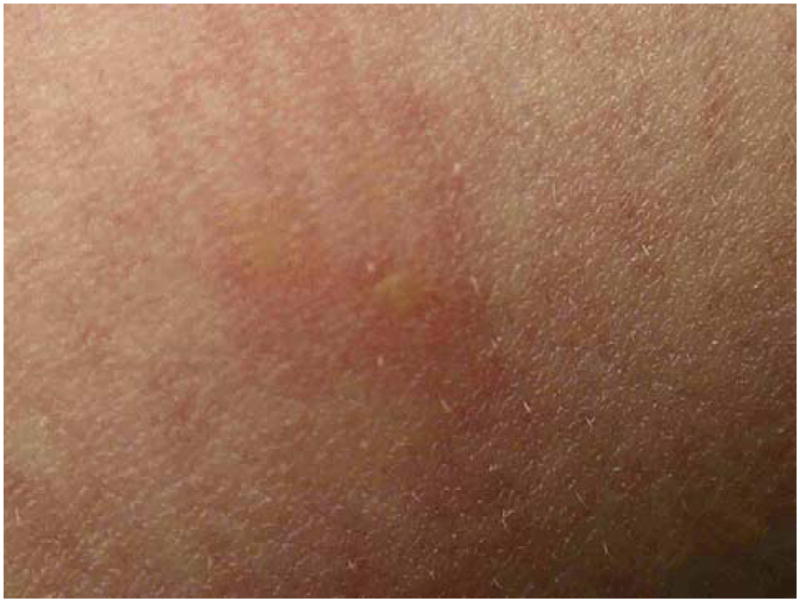
Small painful lesion of 10-year-old girl.
Fig 2.

Male Loxosceles reclusa, recovered by mother from girl’s bed.
On examination the next day, vital signs were unchanged except for the temperature of 36.2°C (97.2°F). The exanthem was present as on the previous day. A surface swab specimen was obtained noninvasively after the child’s mother signed informed consent per a protocol and consent form approved by our institutional review board. The specimen was taken from the vesicle and the surrounding inflamed area in the axilla, obtained by using a cotton swab moistened with normal saline. The venom was recovered by gently rubbing the area for 30 seconds. A serum sample was obtained by phlebotomy.
The swab specimen was flash frozen in liquid nitrogen and both specimens were maintained overnight in a frost-free freezer before moving to a −20°C freezer. The specimens were transported under ice to a state university. The swab was thawed, and the absorbent end was removed from the swab stick and placed in a 1.5-mL microcentrifuge tube. The swab was centrifuged at 10,000g for 10 minutes to recover the saline from the absorbent material. The presence of venom proteins in the solution was detected with an ELISA technique for detecting Loxosceles venom originally described by Gomez et al,9 with minor modifications noted herein.
Polyclonal antibodies for recognition of whole venom were raised in New Zealand white rabbits with unfractionated L reclusa venom. Antibodies were purified from serum by means of protein A column liquid chromatography.10 The concentration of blocking agents11 were increased and nonfat milk solids were added to the blocking buffer. The detection agent was changed from horseradish peroxidase to alkaline phosphatase after standard curves showed greater sensitivity with the alkaline phosphatase in the current assay design. Product generation was monitored at 405 nm on a microplate reader (model ELx808, BIO-TEK Inc, Winooski, Vt). With the modified methodology, a 24-pg venom standard consistently produced an absorbance that was greater than background plus 3SD, with the standard curve as noted in Fig 3. Necrotic and inflammatory lesions that were tested with ELISA had absorbance at the background level with this assay. The serum sample collected by phlebotomy also had an absorbance at the background level with this assay. The swab material from this case recovered 34.4 ± 4.3 pg Loxosceles venom protein/well (Fig 3).
Fig 3.
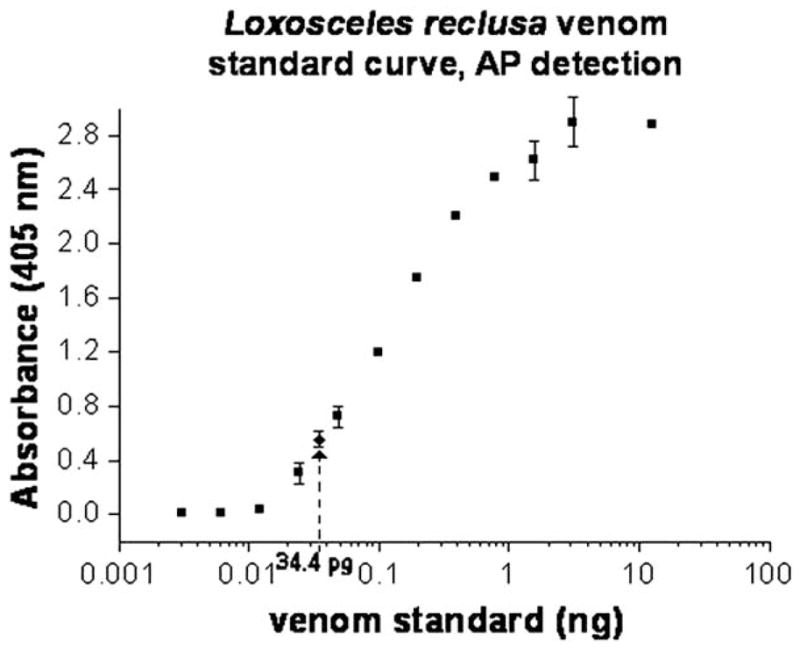
Alkaline phosphatase (AP) detection sensitivity curve for venom detection, with venom standards shown as squares and amount detected for case as diamond.
DISCUSSION
Physicians who practice within the L reclusa habitat in the central and southern areas of the Midwestern United States routinely see patients with suspected spider bites. Unfortunately, our experience has been that fewer than 10% of patients bring in the suspect spider for identification. This is consistent with the experience of Sams et al,12 who noted that 19 of 274 patients (7%) given a diagnosis of brown recluse spider bites between 1987 and 1993 brought in the spider. The spider may be found after a significant delay, leading to some uncertainty that the arachnid presented is the offending agent. Therefore, the diagnosis of most spider bites is generally dependent on analysis of the bite morphology. Severe bites may exhibit the red, white, and blue sign described by Sams et al3 or may show the sunken, bluish patch described by Anderson.4 Small bites such as the one described in this report lack these features and are not well characterized. They are likely more frequent in occurrence than the literature suggests. Small bites cannot be diagnosed definitively without the spider or a test that can unequivocally demonstrate the presence of spider venom.
There are pitfalls in diagnosing an unwitnessed bite and consequently there is the possibility that the dead spider that was found in the child’s bed was not responsible for the lesion. In addition, the lesion described here does not display the features of necrosis present in many case reports. However, some features of the case, including nausea, vomiting, headache, an exanthem, and significant pain, are seen with some bites. Clinical experience suggests that significant systemic findings are more common in children and can often be associated with small lesions.2,4 A flat, painful lesion, even when very small, when coupled with these systemic symptoms, can bring the possibility of loxoscelism to the fore in endemic areas even when no spider is available for examination. The finding of a brown recluse spider, confirmed by an arachnologist, makes the diagnosis likely. In the series of Sams et al,12 8 of the 19 documented cases, as in our case, had no necrosis.
The degree of specificity of the ELISA used here is an important concern. The specificity of an earlier implementation of the polyclonal ELISA used in this case was reported by Gomez et al.9 In that study, 17 North American arthropod venoms elicited no cross-reactivity when assayed at relevant venom amounts. Although neither the clinical case with a mild bite or the results of the ELISA assay offer complete proof of a brown recluse spider bite, the finding and identification of the spider after the bite and the demonstration of the presence of venom by an ELISA both support the diagnosis of loxoscelism.
The polyclonal swab assay presented here allowed identification of the Loxosceles venom on the skin 3 days after the bite. It is likely that venom may be detected in patients for an even greater period postspider bite. Krywko and Gomez13 reported detection of Loxosceles venom in dermal tissue 7 days after envenomation using a rabbit model. The ELISA assay coupled with a noninvasive means of specimen collection would allow confirmation of small, early, or atypical presentations of Loxosceles envenomation such as described in this case. Issues concerning optimal noninvasive sampling that need further investigation include determination of the optimal swab for collection (cotton vs Dacron), assessment of the maximal time before venom amounts on the skin surface decrease below the sensitivity of the assay, and possibly sources of false-positive assays.
A sensitive and specific Loxosceles species venom assay is clinically needed. In addition to the use of rapid clinical confirmation of loxoscelism in endemic areas, a venom-based ELISA could prove useful in excluding loxoscelism in the setting of unrelated and treatable illness in nonendemic areas, defining questionable lesions, and guiding development of appropriate clinical treatment.
The assistance of Tina Parks in protein preparations and ELISA determinations is gratefully acknowledged.
Acknowledgments
Supported by SpiderTech, Rolla, Missouri.
Footnotes
Disclosure: Dr Stoecker owns a controlling interest in SpiderTech, which develops tests for spider bites and spider traps. Drs Stoecker, Green, and Gomez have filed a provisional patent for a diagnostic test for Loxosceles envenomations.
References
- 1.Atkins JA, Wingo CW, Sodeman WA, Flynn JE. Necrotic arachnidism. Am J Trop Med Hyg. 1958;7:165–84. doi: 10.4269/ajtmh.1958.7.165. [DOI] [PubMed] [Google Scholar]
- 2.Wasserman GS, Anderson PC. Loxoscelism and necrotic arachnidism. J Toxicol Clin Toxicol. 1983–1984;21:451–72. doi: 10.3109/15563658308990434. [DOI] [PubMed] [Google Scholar]
- 3.Sams HH, Dunnick CA, Smith ML, King LE., Jr Necrotic arachnidism. J Am Acad Dermatol. 2001;44:561–73. doi: 10.1067/mjd.2001.112385. [DOI] [PubMed] [Google Scholar]
- 4.Anderson PC. Spider bites in the United States. Dermatol Clin. 1997;15:307–11. doi: 10.1016/s0733-8635(05)70438-1. [DOI] [PubMed] [Google Scholar]
- 5.Vetter RS. Envenomation by a spider, Agelenopsis aperta (family: Agelenidae), previously considered harmless. Ann Emerg Med. 1998;32:739–41. doi: 10.1016/s0196-0644(98)70076-9. [DOI] [PubMed] [Google Scholar]
- 6.Rosenstein ED, Kramer N. Lyme disease misdiagnosed as a brown recluse bite [letter] Ann Intern Med. 1987;107:782. doi: 10.7326/0003-4819-107-5-782_1. [DOI] [PubMed] [Google Scholar]
- 7.Rees R, Campbell D, Rieger E, King LE. The diagnosis and treatment of brown recluse spider bites. Ann Emerg Med. 1987;16:945–9. doi: 10.1016/s0196-0644(87)80738-2. [DOI] [PubMed] [Google Scholar]
- 8.Moaven LD, Altman SA, Newnham AR. Sporotrichosis mimicking necrotizing arachnidism. Med J Aust. 1999;171:865–8. doi: 10.5694/j.1326-5377.1999.tb123858.x. [DOI] [PubMed] [Google Scholar]
- 9.Gomez HF, Krywko DM, Stoecker WV. A new assay for the detection of Loxosceles species (brown recluse) spider venom. Ann Emerg Med. 2002;39:469–74. doi: 10.1067/mem.2002.122914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- 11.Gomez HF, Miller MJ, Waggener MW, Lankford HA, Warren JS. Antigenic cross-reactivity of venoms from medically important North American Loxosceles spider species. Toxicon. 2001;39:817–24. doi: 10.1016/s0041-0101(00)00212-9. [DOI] [PubMed] [Google Scholar]
- 12.Sams HH, Hearth SB, Long LL, Wilson DC, Sanders DH, King LE., Jr Nineteen documented cases of Loxosceles reclusa envenomation. J Am Acad Dermatol. 2001;44:603–8. doi: 10.1067/mjd.2001.112380. [DOI] [PubMed] [Google Scholar]
- 13.Krywko DM, Gomez HF. Detection of Loxosceles species venom in dermal lesions: a comparison of 4 venom recovery methods. Ann Emerg Med. 2002;39:475–80. doi: 10.1067/mem.2002.123551. [DOI] [PubMed] [Google Scholar]


