Abstract
We examined whether a systemic marker of oxidative stress, F2-isoprostanes (F2-IP), was associated with total and regional adiposity, adipocytokines, and change in adiposity. Using data from 726 participants enrolled in the Health, Aging, and Body Composition study, F2-IP and adipocytokines were measured from baseline plasma samples. Total adiposity was measured by whole body DXA and regional adiposity by abdominal and thigh CT scans at baseline and 5-year follow-up. ANOVA models were estimated to examine associations between F2-IP tertiles and baseline adiposity and changes in body composition. Median F2-IP was 54.3 pg/ml; women had significantly higher levels than men (61.5 vs. 48.9 pg/ml, p<0.001). F2-IP was associated with higher levels of adiponectin, leptin, and TNF-α. Men in the highest F2-IP tertile had significantly higher total percent body fat than those in the lowest tertile. Positive associations were found between F2-IP and all measures of total and regional adiposity among women. In linear regression models, adipocytokines mediated associations among women. Over 5 years of follow up, women in the highest versus lowest F2-IP tertile exhibited significant loss of weight (lowest tertile: −1.1 kg, highest tertile: −2.7 kg, p<0.05). In conclusion, F2-isoprostanes were associated with measures of total and regional adiposity in women and with total body fat in men; associations for women were partially explained by adipocytokines. F2-isoprostanes predicted loss of total adiposity over time among women.
Keywords: Abdominal obesity, Adipokines, Adipose Tissue, Oxidative Stress, Weight Change
Introduction
Oxidative stress, a persistent imbalance between the production of highly reactive molecular species and antioxidant defenses, has been found to be associated with several inflammatory conditions.(1; 2) Highly reactive molecules, or free radicals, are essential for host defense mechanisms in cells of the immune system, but in excess quantities can cause tissue injury and cell death.(3) Obesity has been recognized as an inflammatory condition(4) with adipose tissue secreting several proinflammatory cytokines and hormones.(5; 6) These “adipocytokines” have been found to be associated with risk of metabolic and cardiovascular disease.(7–9) As a chronic inflammatory condition,(4) adiposity may be associated with oxidative stress either independently or through secretion of these adipocytokines.
A reliable marker of lipid peroxidation is 8-epi-prostaglandin F2α, also known as F2-isoprostanes,(10) which are prostaglandin-like products formed by free radical oxidation of phospholipids that contain arachidonic acid.(11) F2-isoprostanes are released from membrane phospholipids in response to cellular activation and circulate in plasma and are excreted in urine. Recent studies have found that F2-isoprostanes are the best index of oxidative injury in an animal model of oxidant stress.(12) High isoprostane levels have been associated with hypercholesterolemia,(13) diabetes,(14; 15) coronary artery disease,(16) and more recently with obesity(17–19) and other measures of body fat distribution(20; 21) in cross-sectional studies. However, few studies have examined associations between total and regional adiposity and adipocytokines with oxidative stress.(22) No studies have examined the association between oxidative stress and prospective change in adiposity.
We examined the relationship between total and regional adiposity and F2-isoprostane levels in a cohort of older adults. We further determined whether adipocytokines explained these associations. We also investigated the association between baseline F2-isoprostane levels and change in adipose tissue mass and area over 5 years of follow-up.
Methods and Procedures
Study Sample
Participants enrolled in the Health, Aging, and Body Composition (Health ABC) study were well-functioning White and Black men and women between the ages of 70 and 79 years who were recruited at two clinical sites, Pittsburgh, PA and Memphis, TN. To be eligible, participants had to report no difficulty in walking ¼ mile, climbing 10 steps, or performing basic activities of daily living.
We performed a nested subcohort study using stored plasma specimens, physical exam measurements, radiographic tests, and questionnaire data gathered at baseline (1997–1998) and repeat measures of adiposity measured at the sixth annual exam (2002–2003). We randomly sampled 740 participants (24% of the parent study sample) from the baseline examination, stratifying by race and sex (185 individuals from each of four race/sex subgroups). Fourteen participants with missing data on F2-isoprostanes were excluded, leaving a final sample of 726 individuals.
The study was approved by the institutional review boards of the University of California, San Francisco, University of Pittsburgh, and University of Tennessee. All of the study participants provided written informed consent to participate in the study.
F2-isoprostane measurement
Venous blood specimens were collected after an overnight fast. Plasma was separated by centrifugation and stored at −80°C at the Health ABC core laboratory. Specimens were shipped to the Molecular Epidemiology and Biomarker Research Laboratory (MEBRL) at the University of Minnesota, Minneapolis for F2-isoprostane analysis. In prior studies, F2-isoprostanes have been found to be completely stable under these conditions with no artifactual F2-isoprostanes formed during collection and handling. Plasma-free F2-isoprostanes were measured by a gas chromatography-mass spectrometry-based method.(23) The assay has an analytical variation of less than 10%. Blind duplicates were measured from 5% of subjects with a coefficient of variation (CV) of 6.96%.
Adiposity Measurements
The analysis included multiple measures of total adiposity, including total body fat (kg), total percent body fat, body mass index (BMI), and weight (kg). Total fat mass was measured by a whole body Dual X-ray Absorptiometry (DXA) scan (QDR 4500A, Hologic, Waltham, MA). Arm, leg, trunk, and total body fat were measured by DXA and total percent body fat was calculated. Body mass index (BMI) was calculated as weight in kg divided by height in meters squared. Weight was measured on a standard balance beam scale to the nearest 0.1 kg and height by a stadiometer to the nearest 0.1 cm.
Regional adiposity was measured by abdominal visceral and subcutaneous fat and thigh intramuscular and subcutaneous fat in centimeters squared. These measures were based on computed tomography (CT) scans using Somatom Plus 4 (Siemens, Erlangen, Germany), Picker PQ 2000S (Marconi Medical Systems, Cleveland, OH), or a 9800 Advantage scanner (General Electric, Milwaukee, WI) with standardized protocols. Visceral and subcutaneous abdominal fat were measured at the L4–L5 level. Visceral fat was manually distinguished from subcutaneous fat using the internal abdominal wall fascial plane. Fat areas were calculated with ILD software (RSI Systems, Boulder, CO). We also estimated abdominal fat by measuring waist circumference (cm2). Thigh intermuscular and subcutaneous fat area were measured by CT scan taken at mid-thigh level between the greater trochanter and the intercondyloid fossa. Intermuscular adipose tissue was distinguished from the subcutaneous adipose tissue by manual drawing of a line along the deep fascial plane surrounding the thigh muscles.(24)
The adiposity measures were collected at the sixth annual follow-up exam (2002–2003) using similar methods. For longitudinal analysis, variables were defined as the difference between total or regional adiposity at baseline and follow-up.
Covariates
Covariates included self-identified race, age, sex, clinical site, and use of aspirin or statin medications at baseline. Fasting lipoproteins (Johnson & Johnson, Vitros chemical methodology) and fasting and 2-hour post-challenge plasma glucose were measured (by automated glucose oxidase reaction, YSI 2300 Glucose Analyzer, Yellow Springs, OH). Baseline fasting serum insulin levels were measured by radioimmunoassay (Pharmacia, Uppsala, Sweden) among participants without known diabetes, and homeostatic assessment of insulin-resistance (HOMA-IR) was calculated as a surrogate measure of insulin resistance.(25) Adiponectin and leptin levels at baseline were measured in duplicate by radioimmunoassay (Linco Research, St. Charles, MO). The intra-assay CV was 1.78–3.59% for adiponectin and 3.7–7.5% for leptin. IL-6 and TNF-α were measured in duplicate by ELISA (R&D Systems, Minneapolis, MN). The lower limit of detection was <0.10 pg/ml for IL-6 and 0.18 pg/ml for TNF-α, with CV of 6.3% and 16%, respectively. PAI-1 was measured by a two-site ELISA (Collen laboratory) with a CV of 3.47%.
Statistical Analysis
Associations of baseline characteristics with F2-isoprostanes in tertiles were assessed using Kruskal-Wallis test, chi-square test or ANOVA as appropriate. Race and sex interactions were tested for F2-isoprostanes and each adiposity measure. The linearity of the association between F2-isoprostanes and adiposity was examined using splines from generalized additive models.
Sex-specific means and standard errors of each adiposity variable by F2-isoprostane tertiles were obtained using adjusted least squares means from ANOVA models. Models were adjusted for covariates found to be significantly associated with F2-isoprostanes for men or women in bivariate analysis (age, race, hypertension, and coronary heart disease (CHD)). We did not adjust for aspirin use because it was not associated with any measure of adiposity. To determine whether adipocytokines were mediators of F2-isoprostane-adiposity relationships, adipocytokines were sequentially added to linear regression models of these associations.
Finally, the association of baseline F2-isoprostane with change in adiposity over approximately 5 years was examined using ANOVA models adjusting for baseline adiposity, age, race, hypertension, CHD, and intentional weight loss.
Results
The median level of F2-isoprostanes in the sample was 54.3 pg/ml (interquartile range 41.6 to 72.8 pg/ml); women had significantly higher levels than men (61.5 vs. 48.9 pg/ml, p<0.001), and Whites had higher levels than Blacks (58.9 vs. 50.8 pg/ml, p<0.001). F2-isoprostanes were positively associated with HDL-cholesterol, adipocytokines (adiponectin, leptin, and TNF-α), and measures of adiposity (total body fat and abdominal subcutaneous fat). There was no significant association between F2-isoprostanes and any measure of glucose homeostasis. Because spline analysis suggested non-linearity between isoprostanes and adiposity measures, F2-isoprostanes were defined in tertiles. Based on adjusted models pooling men and women, interactions between F2-isoprostanes and sex were significant for most adiposity measures at p<0.05; exceptions were abdominal visceral fat (p for-interaction=0.09) and thigh intermuscular fat (p for-interaction=0.84). We thus proceeded with sex-stratified analysis. No significant interactions were found by race for men or women.
Table 1 presents sex-specific tertiles of F2-isoprostanes by demographic and health-related characteristics. Race differences in F2-isoprostane levels were evident among women only with a larger proportion of White women and a smaller proportion of Black women in higher tertiles. Among women, but not men, aspirin use was significantly more likely among those in higher tertiles of F2-isoprostanes. F2-isoprostanes were positively associated with adiponectin, leptin, and TNF-α in both men and women. Among men only, those in higher tertiles had significantly higher HDL-cholesterol and higher prevalence of hypertension and CHD. (F2-isoprostanes tertiles by glycemia traits are shown in supplemental table 1 online.)
Table 1.
Baseline associations between F2-isoprostanes tertiles and covariates by sex. Health ABC study (1997–98), N=726.
| MEN: | Tertile 1 n=119 <41.0 pg/ml |
Tertile 2 n=122 41.0–57.8 pg/ml |
Tertile 3 n=119 >57.8 pg/ml |
p-value |
|---|---|---|---|---|
|
Demographics:
| ||||
| Age, years b | 73.3 (72.8, 73.8) | 74.2 (73.7, 74.7) | 73.8 (73.3, 74.3) | 0.04 |
| Race, n (%): | ||||
| White | 57 (47.9) | 58 (47.5) | 64 (53.8) | 0.56 |
| Black | 62 (52.1) | 64 (52.5) | 55 (46.2) | |
| Clinic Site: | ||||
| Memphis | 59 (49.6) | 55 (45.1) | 65 (54.6) | 0.33 |
| Pittsburgh | 60 (50.4) | 67 (54.9) | 54 (45.4) | |
|
| ||||
|
Medication use:
| ||||
| Statin use, n(%) | 10 (8.4) | 16 (13.1) | 17 (14.3) | 0.32 |
| Aspirin use, n(%) | 47 (39.5) | 58 (47.5) | 52 (43.7) | 0.45 |
|
| ||||
|
Laboratory measures:
| ||||
| Total cholesterol, mg/dl b | 192.1 (186.2, 198.0) | 195.5 (189.5, 201.6) | 195.1 (189.0, 201.3) | 0.68 |
| HDL, mg/dl b | 44.5 (42.1, 46.8) | 49.5 (47.1, 51.9) | 50.2 (47.7, 52.6) | 0.002 |
| Triglycerides, mg/dla | 120.1 (110.6, 130.3) | 117.6 (108.1, 127.8) | 128.8 (118.3, 140.2) | 0.27 |
| CRP, mg/La | 1.8 (1.6, 2.1) | 1.8 (1.5, 2.1) | 1.6 (1.4, 1.9) | 0.72 |
| Adiponectin, μg/mla | 6.7 (6.0, 7.4) | 8.0 (7.2, 8.9) | 9.2 (8.2, 10.2) | <0.001 |
| PAI-1, ng/mla | 20.6 (18.2, 23.4) | 20.7 (18.1 23.5) | 22.1 (19.3, 25.2) | 0.71 |
| Leptin, ng/mla | 5.1 (4.4, 6.0) | 5.4 (4.6, 6.4) | 7.0 (5.9, 8.3) | 0.02 |
| Interleukin-6, pg/mla | 1.9 (1.7, 2.1) | 2.1 (1.9, 2.3) | 2.2 (2.0. 2.5) | 0.09 |
| TNF-α, pg/mla | 3.2 (2.9, 3.4) | 3.3 (3.0, 3.5) | 3.6 (3.4, 3.9) | 0.01 |
|
| ||||
|
Comorbid Conditions:
| ||||
| Diabetes (Type 2) | 24 (20.2) | 23 (18.9) | 20 (16.8) | 0.80 |
| Hypertension | 65 (54.6) | 74 (60.7) | 84 (70.6) | 0.04 |
| Coronary Heart Disease | 16 (13.5) | 31 (25.4) | 37 (31.1) | 0.005 |
| WOMEN: | Tertile 1 n=120 <50.6 pg/ml |
Tertile 2 n=125 50.6–75.5 pg/ml |
Tertile 3 n=121 >75.5 pg/ml |
p-value |
|---|---|---|---|---|
|
Demographics:
| ||||
| Age, years b | 73.1 (72.6, 73.7) | 73.6 (73.1, 74.1) | 73.9 (73.4, 74.5) | 0.11 |
| Race, n (%): | ||||
| White | 47 (39.2) | 59 (47.2) | 77 (63.6) | <0.001 |
| Black | 73 (60.8) | 66 (52.8) | 44 (36.4) | |
| Clinic Site: | ||||
| Memphis | 54 (45.0) | 64 (51.2) | 64 (52.9) | 0.43 |
| Pittsburgh | 66 (55.0) | 61 (48.8) | 57 (47.1) | |
|
| ||||
|
Medication use:
| ||||
| Statin use, n(%) | 10 (8.3) | 17 (13.6) | 17 (14.1) | 0.32 |
| Aspirin use, n(%) | 30 (25.0) | 51 (40.8) | 51 (42.2) | 0.009 |
|
| ||||
|
Laboratory measures:
| ||||
| Total cholesterol, mg/dl b | 213.8 (207.2, 220.5) | 212.4 (205.7, 219.1) | 210.7 (203.9, 217.4) | 0.86 |
| HDL, mg/dl b | 60.6 (57.5, 63.8) | 59.4 (56.3, 62.5) | 61.9 (58.7, 65.1) | 0.54 |
| Triglycerides, mg/dla | 112.1 (103.3, 121.7) | 123.3 (113.6, 133.8) | 122.1 (112.4, 132.7) | 0.20 |
| CRP, mg/La | 1.8 (1.6, 2.1) | 2.0 (1.7, 2.3) | 2.2 (1.9, 2.6) | 0.09 |
| Adiponectin, μg/mla | 10.5 (9.6, 11.6) | 10.1 (9.1, 11.1) | 13.1 (11.9, 14.5) | <0.001 |
| PAI-1, ng/mla | 20.1 (17.5, 23.1) | 23.7 (20.7, 27.2) | 21.0 (18.3, 24.2) | 0.22 |
| Leptin, ng/mla | 12.8 (11.2, 14.7) | 17.6 (15.3, 20.2) | 20.0 (17.4, 23.1) | <0.001 |
| Interleukin-6, pg/mla | 1.8 (1.6, 2.0) | 1.8 (1.6, 2.0) | 2.0 (1.8, 2.3) | 0.30 |
| TNF-α, pg/mla | 2.8 (2.6, 3.0) | 3.1 (2.8, 3.3) | 3.3 (3.0, 3.7) | 0.01 |
|
| ||||
|
Comorbid Conditions n (%):
| ||||
| Diabetes (Type 2) | 22 (18.3) | 30 (24.0) | 18 (14.9) | 0.18 |
| Hypertension | 73 (60.8) | 87 (69.6) | 79 (65.3) | 0.35 |
| Coronary Heart Disease | 10 (8.3) | 18 (14.4) | 15 (12.4) | 0.33 |
geometric mean (95% CI) presented due to skewness and resulting log transformation
regular mean (95% CI)
Table 2 presents adjusted associations between tertiles of F2-isoprostanes and adiposity measures by sex (unadjusted associations are available in a supplemental table 2 online). Models adjust for age, race, prevalent hypertension, and prevalent coronary heart disease. Among men, there was a positive association only for total percent body fat for those in T3 versus T1. In contrast, F2-isoprostanes were positively associated with all adjusted baseline measures of adiposity for women in T3 versus T1, and for those in T2 versus T1 for total percent body fat, abdominal subcutaneous fat, and thigh intermuscular fat. Next we examined whether baseline associations between F2-isoprostanes and selected measures of adiposity were explained by adipocytokines (leptin, adiponectin and TNF-α) considered separately (Table 3). We investigated these patterns among women only, given the general lack of association between F2-isoprostanes and adiposity among men. The coefficients in the table represent the amount of change in each measure for each standard deviation increase in F2-isoprostanes. Leptin explained a substantial proportion (more than 50%) of the association between F2-isoprostanes and each adiposity measure, although the relationship remained significant in each case. TNF-α also attenuated the association, although its effect was negligible in comparison to leptin. In contrast, adiponectin strengthened associations suggesting negative confounding. As a result, attenuation by all three adipocytokines taken together (model 2) was somewhat less than that of leptin alone for each association.
Table 2.
Adjusted baseline associations in body composition by F2-isoprostane tertiles by sex. Health ABC study (1997–98), N=726.
| MEN: | Tertile 1 n=119 <41.0 pg/ml |
Tertile 2 n=122 41.0–57.8 pg/ml |
Tertile 3 n=119 >57.8 pg/ml |
p-value (difference between T2 and T1) | p-value (difference between T3 and T1) |
|---|---|---|---|---|---|
| BMI, kg/m2 | 27.0 (26.2, 27.7) | 27.0 (26.3, 27.7) | 27.0 (26.3, 27.8) | 0.95 | 0.91 |
| Weight, kg | 81.2 (78.6, 83.7) | 80.4 (77.9, 82.9) | 81.5 (78.9, 84.0) | 0.68 | 0.87 |
| Waist circumference, cm | 100.8 (98.6, 102.9) | 98.6 (96.5, 100.7) | 101.1 (98.9, 103.2) | 0.16 | 0.85 |
| Total body fat, % | 28.6 (27.7, 29.6) | 28.8 (27.9, 29.8) | 29.7 (28.7, 30.7) | 0.78 | 0.13 |
| Total body fat, kg | 23.6 (22.2, 25.0) | 23.6 (22.3, 25.0) | 24.6 (23.3, 26.0) | 0.96 | 0.30 |
| Abdominal visceral fat, cm2 | 148.7 (137.7, 159.7) | 139.6 (128.7, 150.5) | 148.5 (137.5, 159.6) | 0.25 | 0.98 |
| Abdominal subcutaneous fat area, cm2 | 225.9 (208.7, 243.1) | 222.0 (204.5, 240.0) | 236.9 (219.5, 254.3) | 0.75 | 0.38 |
| Thigh intermuscular fat, cm2 | 10.2 (8.5, 11.9) | 9.9 (8.3, 11.6) | 12.0 (10.3, 13.7) | 0.86 | 0.14 |
| Thigh subcutaneous fat, cm2 | 47.0 (43.0, 50.9) | 48.4 (45.6, 52.3) | 49.9 (45.9, 53.8) | 0.60 | 0.31 |
| WOMEN: | Tertile 1 n=120 <50.6 pg/ml |
Tertile 2 n=125 50.6–75.5 pg/ml |
Tertile 3 n=121 >75.5 pg/ml |
p-value (difference between T2 and T1) | p-value (difference between T3 and T1) |
|---|---|---|---|---|---|
| BMI, kg/m2 | 26.6 (25.7, 27.5) | 27.2 (26.4, 28.1) | 29.1 (28.2, 30.0) | 0.35 | <0.001 |
| Weight, kg | 67.9 (65.5, 70.3) | 69.8 (67.5, 72.1) | 74.9 (72.7, 77.2) | 0.25 | <0.001 |
| Waist circumference, cm | 95.3 (92.9, 97.6) | 98.2 (95.9, 100.5) | 101.4 (99.0, 103.8) | 0.09 | 0.001 |
| Total body fat, % | 38.7 (37.7, 39.6) | 40.2 (39.2, 41.1) | 42.3 (41.4, 43.3) | 0.03 | <0.001 |
| Total body fat, kg | 26.8 (25.2, 28.3) | 28.6 (27.1, 30.1) | 32.4 (30.8, 33.9) | 0.09 | <0.001 |
| Abdominal visceral fat, cm2 | 116.8 (105.8, 127.8) | 128.7 (118.0, 139.3) | 138.6 (127.7, 149.5) | 0.13 | 0.007 |
| Abdominal subcutaneous fat area, cm2 | 309.2 (287.7, 330.8) | 339.6 (318.9, 360.3) | 378.7 (356.8, 400.6) | 0.05 | <0.001 |
| Thigh intermuscular fat, cm2 | 9.5 (8.5, 10.5) | 10.7 (9.7, 11.7) | 11.9 (10.9, 12.9) | 0.09 | 0.001 |
| Thigh subcutaneous fat, cm2 | 95.0 (87.0, 103.0) | 102.0 (94.4, 109.6) | 125.6 (117.7, 133.5) | 0.21 | <0.001 |
Data are adjusted means ± SE from general linear models. Adjusted baseline models control for age, race, prevalent hypertension, and prevalent coronary heart disease.
Table 3.
results for Men:
Linear regression models of association between baseline adiposity and F2-isoprostanes among women, adjusting sequentially for adipocytokines and HDL-cholesterol.
| MEN n=360 |
||
|---|---|---|
| β (95% CI) | p-value | |
| Baseline BMI model 1*: | 0.30 (−0.14, 0.73) | 0.18 |
| + leptin | −0.09 (−0.46, 0.29) | 0.65 |
| + adiponectin only | 0.44 (0.02, 0.86) | 0.04 |
| + TNF-α only | 0.19 (−0.24, 0.62) | 0.39 |
| + all three adipokines (model 2) | 0.04 (−0.34, 0.42) | 0.83 |
| Model 2 + HDL-cholesterol | 0.24 (−0.13, 0.61) | 0.21 |
| Baseline total body fat model 1*: | 0.96 (0.16, 1.75) | 0.004 |
| + leptin | 0.10 (−0.54, 0.75) | 0.75 |
| + adiponectin only | 1.15 (0.36, 1.94) | 0.005 |
| + TNF-α only | 0.79 (−0.003, 1.59) | 0.05 |
| + all three adipokines (model 2) | 0.29 (−0.36, 0.94) | 0.38 |
| Model 2 + HDL-cholesterol | 0.51 (−0.13, 1.16) | 0.12 |
| Baseline abdominal subQ fat model*: | 9.29 (−0.99, 19.56) | 0.08 |
| + leptin | −0.24 (−9.10, 8.61) | 0.96 |
| + adiponectin only | 11.46 (1.24, 21.68) | 0.03 |
| + TNF-α only | 7.51 (−2.82, 17.84) | 0.15 |
| + all three adipokines (model 2) | 2.34 (−6.57, 11.25) | 0.61 |
| Model 2 + HDL-cholesterol | 5.30 (−3.58, 14.18) | 0.24 |
| Baseline weight model 1*: | 1.24 (−0.23, 2.72) | 0.10 |
| + leptin | −0.03 (−1.33, 1.27) | 0.96 |
| + adiponectin only | 1.64 (0.18, 3.10) | 0.03 |
| + TNF-α only | 0.94 (−0.54, 2.41) | 0.21 |
| + all three adipokines (model 2) | 0.30 (−1.02, 1.63) | 0.65 |
| Model 2 + HDL-cholesterol | 0.96 (−0.34, 2.26) | 0.15 |
adjusted for age, race, prevalent coronary heart disease, prevalent hypertension
Lastly, we examined whether baseline F2-isoprostane levels were associated with change in adiposity over a 5-year period. Women in the highest F2-isoprostane tertile experienced a significantly greater loss of weight over time than those in the lowest tertile, and a marginal association was evident for total body fat (kg) (Figure 1). There was a marginally significant association for abdominal subcutaneous fat in T3 vs. T1 (p=0.07), but no significant associations were found for other regional adiposity measures. Among men (data not shown), higher baseline F2-isoprostanes were also associated with loss of BMI and weight, as well as for thigh subcutaneous fat; however, these associations were significant only for men in T2 versus T1, indicating a non-linear relationship. Based on pooled models, sex interactions were found for BMI only at p<0.05.
Figure 1.
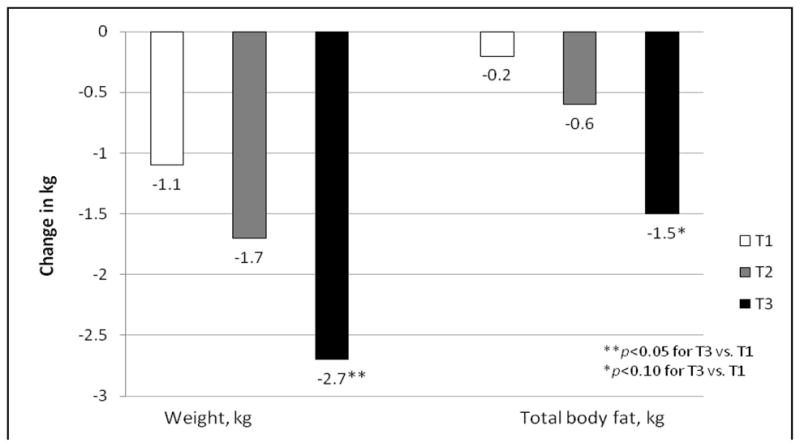
Adjusted longitudinal changes in adiposity: higher F2-isoprostanes tertile is associated with significant loss of weight and total body fat in women.
Discussion
In a diverse community-dwelling sample of older adults, a systematic marker of oxidative stress, F2-isoprostanes, was found to be positively associated with total and regional adiposity in women. Among men, a more limited pattern was evident, with significant relationships found only for total percent body fat. Significant sex interactions between F2-isoprostanes and most measures of adiposity confirmed that adiposity is more strongly associated with oxidative stress for older women than older men. Further, adipocytokines lie in the causal pathway between adiposity and F2-isoprostanes among women, given that a substantial proportion of associations were mediated by these adipose tissue hormones for both total and regional measures. Finally, higher baseline F2-isoprostane levels were associated with a loss of total adiposity over time among women.
In previous studies, oxidative stress, as measured either by isoprostanes or myeloperoxidase (an oxidative enzyme produced by macrophages), has been found to be positively associated with measures of total adiposity as well as regional adipose tissue deposits.(17; 18; 21; 22) This study confirms and extends these findings by examining a wide range of measures of both total and regional adiposity in men and women using a more sensitive measure of oxidative stress, plasma F2-isoprostanes. In addition, although some previous studies have found no sex differences in the association between oxidative stress and measures of adiposity,(18; 22) we found a significant sex interaction with stronger associations among women than men for most total and regional adiposity measures included in the analysis. Differences between the present and prior research may be due to our measurement of plasma, versus urinary, F2-isoprostanes or to age differences in study samples.
Sex differences found here may be due to greater adipose tissue volume in women than men. Women in the highest F2-isoprostane tertile had higher mean adiposity than their male counterparts for BMI and waist circumference, total body fat, and abdominal and thigh subcutaneous fat. This may indicate a threshold effect, with oxidative stress occurring only at the higher levels of adiposity found more commonly among women. However, a sex interaction was also found for weight and for abdominal visceral fat, both of which were lower on average among women than men by F2-isoprostanes tertile. More research is needed to identify the sources of sex differences in F2-isoprostane-adiposity associations.
Although there has been little consideration of mechanistic links between adiposity and oxidative stress, prior research has established associations between adipose tissue and both circulating inflammatory biomarkers and urinary isoprostane concentrations.(22) In the present analysis, leptin, a weight-regulatory hormone, was determined to play a significant explanatory role in relation to oxidative stress and both total and regional adiposity among women; we found a nominal mediating effect for TNF-α. Leptin is released into the circulatory system by adipose tissue, signaling the brain to decrease food intake and increase energy expenditure to maintain the size of the body fat stores.(26–28) Although leptin elevation may play an important role in energy balance at leaner weight levels, leptin insensitivity at higher levels of adiposity,(29) and subsequent excess leptin production, may have a detrimental effect on oxidative stress among women. Additionally, women have been found to have markedly higher leptin concentrations than men for any given degree of fat mass;(30) leptin may thus contribute to sex differences in associations between total and regional adiposity and F2-isoprostane levels.
We found that adiponectin, an anti-inflammatory adipocytokine, was positively associated with F2-isoprostanes in both men and women, a novel finding. Despite this positive association, adiponectin strengthened the association between total and regional adiposity and F2-isoprostane levels, implying negative confounding. The relationship between adiponectin and oxidative stress is less studied and more controversial. Some studies have found no association,(21; 31) and two studies observed an inverse correlation between adiponectin and urinary isoprostane levels.(20; 32) Interestingly, these latter studies were both conducted in middle-aged Japanese populations, and one showed significant inverse associations primarily among those with normal glucose tolerance.(32) Older age is associated with increased adiponectin levels, and studies in older populations have found a positive association between adiponectin and heart disease (9; 33) and mortality.(34). This finding of a positive association between adiponectin and F2-isoprostanes may be related to the theorized compensatory mechanism that may increase adiponectin levels as a response to age-related comorbidities such as insulin resistance and heart disease.
Because repeated measures of F2-isoprostanes were unavailable in the survey, we were unable to assess whether F2-isoprostane levels change over time relative to higher initial adiposity. However, the analysis found that higher F2-isoprostane levels among women at baseline were associated with loss of total adiposity over a 5-year period. This finding suggests a more complex relationship between the two factors than has previously been established, given that prior studies have been cross-sectional. This negative association may signal a physiological response to address excess adiposity, and/or a catabolic response to inflammation that causes adiposity to slowly diminish in older individuals. It is also possible that the pattern is due to unmeasured confounders, such as comorbidities that cause unintended loss of adipose tissue in individuals with elevated levels of oxidative stress, particularly among women in this age group.
Strengths of the study included a diverse, community-based sample; previous studies have focused mainly on Whites. The data included a wide range of measures of both total and regional adiposity, which enabled us to establish a consistent association between F2-isoprostanes and adiposity among women. However, this study was subject to important limitations. As stated earlier, measures of F2-ispoprostanes were available at baseline only, which restricted our ability to infer that adiposity results in greater oxidative stress over time. Given that the sample was limited to older adults, results may not generalize to the overall adult population or to younger age groups. The sample included only Whites and Blacks, so findings may not apply to other racial or ethnic populations.
In conclusion, F2-isoprostanes were associated with measures of total and regional adiposity in women, and with total percent body fat in men. Associations among women were partially explained by adipocytokines. F2-isoprostanes predicted loss of total adiposity over time among women. Higher adipose tissue volume in women may explain sex differences.
Supplementary Material
Acknowledgments
Funding sources: Alka M. Kanaya was funded by R21DK068608 and K23HL080026. The Health ABC study was funded via contracts with the National Institute on Aging contract #s: N01-AG-6-2101, N01-AG-6-2103, N01-AG-6-2106. This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging and included substantial involvement of NIA staff in data collection, analysis, interpretation, review, and approval of the manuscript.
Footnotes
Disclosures: none
References
- 1.Cracowski JL, Bonaz B, Bessard G, Bessard J, Anglade C, Fournet J. Increased urinary F2-isoprostanes in patients with Crohn’s disease. Am J Gastroenterol. 2002;97:99–103. doi: 10.1111/j.1572-0241.2002.05427.x. [DOI] [PubMed] [Google Scholar]
- 2.Basu S, Whiteman M, Mattey DL, Halliwell B. Raised levels of F(2)-isoprostanes and prostaglandin F(2alpha) in different rheumatic diseases. Ann Rheum Dis. 2001;60:627–631. doi: 10.1136/ard.60.6.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halliwell B. Antioxidants in human health and disease. Annu Rev Nutr. 1996;16:33–50. doi: 10.1146/annurev.nu.16.070196.000341. [DOI] [PubMed] [Google Scholar]
- 4.Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. Jama. 1999;282:2131–2135. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- 5.Vozarova B, Weyer C, Hanson K, Tataranni PA, Bogardus C, Pratley RE. Circulating interleukin-6 in relation to adiposity, insulin action, and insulin secretion. Obes Res. 2001;9:414–417. doi: 10.1038/oby.2001.54. [DOI] [PubMed] [Google Scholar]
- 6.Esposito K, Pontillo A, Di Palo C, Giugliano G, Masella M, Marfella R, Giugliano D. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. Jama. 2003;289:1799–1804. doi: 10.1001/jama.289.14.1799. [DOI] [PubMed] [Google Scholar]
- 7.Matsuzawa Y, Nakamura T, Shimomura I, Kotani K. Visceral fat accumulation and cardiovascular disease. Obes Res. 1995;3 (Suppl 5):645S–647S. doi: 10.1002/j.1550-8528.1995.tb00481.x. [DOI] [PubMed] [Google Scholar]
- 8.Fujimoto WY, Bergstrom RW, Boyko EJ, Chen KW, Leonetti DL, Newell-Morris L, et al. Visceral adiposity and incident coronary heart disease in Japanese- American men. The 10-year follow-up results of the Seattle Japanese- American Community Diabetes Study. Diabetes Care. 1999;22:1808–1812. doi: 10.2337/diacare.22.11.1808. [DOI] [PubMed] [Google Scholar]
- 9.Kanaya AM, Wassel Fyr C, Vittinghoff E, Havel PJ, Cesari M, Nicklas B, et al. Serum adiponectin and coronary heart disease risk in older Black and White Americans. J Clin Endocrinol Metab. 2006;91:5044–5050. doi: 10.1210/jc.2006-0107. [DOI] [PubMed] [Google Scholar]
- 10.Rosen P, Nawroth PP, King G, Moller W, Tritschler HJ, Packer L. The role of oxidative stress in the onset and progression of diabetes and its complications: a summary of a Congress Series sponsored by UNESCO-MCBN, the American Diabetes Association and the German Diabetes Society. Diabetes Metab Res Rev. 2001;17:189–212. doi: 10.1002/dmrr.196. [DOI] [PubMed] [Google Scholar]
- 11.Morrow JD, Roberts LJ., 2nd The isoprostanes. Current knowledge and directions for future research. Biochem Pharmacol. 1996;51:1–9. doi: 10.1016/0006-2952(95)02072-1. [DOI] [PubMed] [Google Scholar]
- 12.Kadiiska MB, Gladen BC, Baird DD, Germolec D, Graham LB, Parker CE, et al. Biomarkers of oxidative stress study II: are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radic Biol Med. 2005;38:698–710. doi: 10.1016/j.freeradbiomed.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 13.Davi G, Alessandrini P, Mezzetti A, Minotti G, Bucciarelli T, Costantini F, et al. In vivo formation of 8-Epi-prostaglandin F2 alpha is increased in hypercholesterolemia. Arterioscler Thromb Vasc Biol. 1997;17:3230–3235. doi: 10.1161/01.atv.17.11.3230. [DOI] [PubMed] [Google Scholar]
- 14.Gopaul NK, Anggard EE, Mallet AI, Betteridge DJ, Wolff SP, Nourooz-Zadeh J. Plasma 8-epi-PGF2 alpha levels are elevated in individuals with non-insulin dependent diabetes mellitus. FEBS Lett. 1995;368:225–229. doi: 10.1016/0014-5793(95)00649-t. [DOI] [PubMed] [Google Scholar]
- 15.Davi G, Ciabattoni G, Consoli A, Mezzetti A, Falco A, Santarone S, et al. In vivo formation of 8-iso-prostaglandin f2alpha and platelet activation in diabetes mellitus: effects of improved metabolic control and vitamin E supplementation. Circulation. 1999;99:224–229. doi: 10.1161/01.cir.99.2.224. [DOI] [PubMed] [Google Scholar]
- 16.Cipollone F, Ciabattoni G, Patrignani P, Pasquale M, Di Gregorio D, Bucciarelli T, et al. Oxidant stress and aspirin-insensitive thromboxane biosynthesis in severe unstable angina. Circulation. 2000;102:1007–1013. doi: 10.1161/01.cir.102.9.1007. [DOI] [PubMed] [Google Scholar]
- 17.Block G, Dietrich M, Norkus EP, Morrow JD, Hudes M, Caan B, Packer L. Factors associated with oxidative stress in human populations. Am J Epidemiol. 2002;156:274–285. doi: 10.1093/aje/kwf029. [DOI] [PubMed] [Google Scholar]
- 18.Keaney JF, Jr, Larson MG, Vasan RS, Wilson PW, Lipinska I, Corey D, Massaro JM, et al. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol. 2003;23:434–439. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- 19.Urakawa H, Katsuki A, Sumida Y, Gabazza EC, Murashima S, Morioka K, et al. Oxidative stress is associated with adiposity and insulin resistance in men. J Clin Endocrinol Metab. 2003;88:4673–4676. doi: 10.1210/jc.2003-030202. [DOI] [PubMed] [Google Scholar]
- 20.Fujita K, Nishizawa H, Funahashi T, Shimomura I, Shimabukuro M. Systemic oxidative stress is associated with visceral fat accumulation and the metabolic syndrome. Circ J. 2006;70:1437–1442. doi: 10.1253/circj.70.1437. [DOI] [PubMed] [Google Scholar]
- 21.Wu B, Fukuo K, Suzuki K, Yoshino G, Kazumi T. Relationships of systemic oxidative stress to body fat distribution, adipokines and inflammatory markers in healthy middle-aged women. Endocr J. 2009;56:773–782. doi: 10.1507/endocrj.k08e-332. [DOI] [PubMed] [Google Scholar]
- 22.Pou KM, Massaro JM, Hoffmann U, Vasan RS, Maurovich-Horvat P, Larson MG, et al. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation. 2007;116:1234–1241. doi: 10.1161/CIRCULATIONAHA.107.710509. [DOI] [PubMed] [Google Scholar]
- 23.Morrow JD, Roberts LJ., 2nd Mass spectrometric quantification of F2-isoprostanes in biological fluids and tissues as measure of oxidant stress. Methods Enzymol. 1999;300:3–12. doi: 10.1016/s0076-6879(99)00106-8. [DOI] [PubMed] [Google Scholar]
- 24.Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, et al. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol. 2001;90:2157–2165. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- 25.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 26.Weigle DS. Leptin and other secretory products of adipocytes modulate multiple physiological functions. Ann Endocrinol (Paris) 1997;58:132–136. [PubMed] [Google Scholar]
- 27.Klok MD, Jakobsdottir S, Drent ML. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. Obes Rev. 2007;8:21–34. doi: 10.1111/j.1467-789X.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- 28.Licinio J, Caglayan S, Ozata M, Yildiz BO, de Miranda PB, O’Kirwan F, et al. Phenotypic effects of leptin replacement on morbid obesity, diabetes mellitus, hypogonadism, and behavior in leptin-deficient adults. Proc Natl Acad Sci U S A. 2004;101:4531–4536. doi: 10.1073/pnas.0308767101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 30.Jequier E. Leptin signaling, adiposity, and energy balance. Ann N Y Acad Sci. 2002;967:379–388. doi: 10.1111/j.1749-6632.2002.tb04293.x. [DOI] [PubMed] [Google Scholar]
- 31.Shin MJ, Lee JH, Jang Y, Park E, Oh J, Chung JH, Chung N. Insulin resistance, adipokines, and oxidative stress in nondiabetic, hypercholesterolemic patients: leptin as an 8-epi-prostaglandin F2alpha determinant. Metabolism. 2006;55:918–922. doi: 10.1016/j.metabol.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 32.Nakanishi S, Yamane K, Kamei N, Nojima H, Okubo M, Kohno N. A protective effect of adiponectin against oxidative stress in Japanese Americans: The association between adiponectin or leptin and urinary isoprostane. Metabolism. 2005;54:194–199. doi: 10.1016/j.metabol.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 33.Laughlin GA, Barrett-Connor E, May S, Langenberg C. Association of adiponectin with coronary heart disease and mortality: the Rancho Bernardo study. Am J Epidemiol. 2007;165:164–174. doi: 10.1093/aje/kwk001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wannamethee SG, Whincup PH, Lennon L, Sattar N. Circulating adiponectin levels and mortality in elderly men with and without cardiovascular disease and heart failure. Arch Intern Med. 2007;167:1510–1517. doi: 10.1001/archinte.167.14.1510. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


