Abstract
Aims: The aims were to provide proofs of mechanism and principle by establishing the ability of kynurenine metabolites to inhibit the liver mitochondrial low Km aldehyde dehydrogenase (ALDH) activity after administration and in vivo, and to induce aversion to alcohol. Methods: Kynurenic acid (KA), 3-hydroxykynurenine (3-HK) and 3-hydroxyanthranilic acid (3-HAA) were administered to normal male Wistar rats and ALDH activity was determined both in vitro in liver homogenates and in vivo (by measuring blood acetaldehyde following ethanol administration). Alcohol consumption was studied in an aversion model in rats and in alcohol-preferring C57 mice. Results: ALDH activity was significantly inhibited by all three metabolites by doses as small as 1 mg/kg body wt. Blood acetaldehyde accumulation after ethanol administration was strongly elevated by KA and 3-HK and to a lesser extent by 3-HAA. All three metabolites induced aversion to alcohol in rats and decreased alcohol preference in mice. Conclusions: The above kynurenine metabolites of tryptophan induce aversion to alcohol by inhibiting ALDH activity. An intellectual property covering the use of 3-HK and 3-HAA and derivatives thereof in the treatment of alcoholism by aversion awaits further development.
INTRODUCTION
It is generally accepted that the most effective relapse preventing drugs following detoxification of alcohol-dependent subjects are the N-methyl-D-aspartate modulator acamprosate, the μ-opioid receptor antagonist naltrexone and the aldehyde dehydrogenase (ALDH) inhibitor and alcohol aversion drug disulfiram (Mann, 2004). The superiority of disulfiram over acamprosate and naltrexone has been demonstrated (see, e.g. de Sousa and de Sousa, 2004, 2005; Laaksonen et al., 2008; Diehl et al., 2010). However, safety issues with disulfiram (Chick, 1999) suggest the need for developing safer alternative ALDH inhibitors for alcoholism treatment. One such alternative may be the essential amino acid L-tryptophan (Trp) and its metabolites.
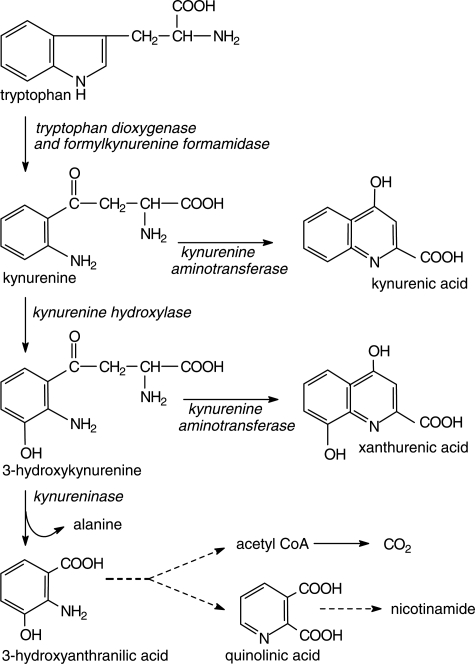
Of all amino acids, Trp has been the most extensively studied in relation to alcohol and alcoholism. While Trp metabolism and disposition are greatly influenced by acute and chronic alcohol (ethanol) consumption and subsequent withdrawal in both humans and experimental animals (for reviews, see Le Marquand et al., 1994a, b; Badawy, 2002, 2005), Trp itself can also influence alcohol consumption. Thus, alcohol consumption by rats is decreased by Trp and its 5-hydroxylated metabolite, although the Trp effect is controversial (for review and references, see Naranjo et al., 1986). The implication of serotonin (5-hydroxytryptamine or 5-HT) as a modulator of alcohol consumption has arisen from a variety of studies using treatments influencing the metabolism and function of this indolylamine, such as its Trp and 5-hydroxytryptophan precursors, the Trp hydroxylase inhibitor p-chlorophenylalanine, various serotonin reuptake inhibitors, serotonin postsynaptic receptor activators and neurotoxic destruction of serotonin neurons, (reviewed by Naranjo et al., 1986; Sellers et al., 1992). Accordingly, it has always been assumed that the decrease in alcohol consumption by Trp is mediated by serotonin, although no mechanistic studies were performed to confirm this assumption. An alternative or additional mechanism is that of Trp acting peripherally via its metabolites to induce aversion. Thus, we have previously reported (Badawy and Morgan, 2007) that a number of Trp metabolites of the quantitatively most important of the Trp-degradative pathways (the hepatic kynurenine pathway; Fig. 1), namely 3-hydroxykynurenine (3-HK), 3-hydroxyanthranilic acid (3-HAA) and kynurenic acid (KA), and also the Trp transamination product indol-3-ylpyruvic acid (IPA) strongly inhibit the activity of the mammalian (rat) liver mitochondrial low Km ALDH in vitro. At 2 μM, inhibition by these four metabolites was 55, 46, 40 and 30% respectively, against a 42% inhibition by a similar concentration of the classical AlDH inhibitor disulfiram. Less strong inhibition was also observed with the kynurenine metabolite xanthurenic acid and with 5-hydroxytryptophan and it is of interest that inhibition of alcohol consumption by this immediate serotonin precursor has been suggested to involve a peripheral, in addition to a central and component (Zabik et al., 1994). In the present paper, we demonstrate the ability of the former three kynurenine metabolites to: (a) inhibit ALDH activity after administration to rats; (b) elevate blood acetaldehyde concentration following acute ethanol administration; (c) induce aversion to alcohol in an experimental aversion model and (d) decrease alcohol preference in mice, and propose these metabolites as potential alcohol aversion drugs. The Trp transamination metabolite IPA was not included in the present work because of its back-conversion into Trp (Richards et al., 1972).
Fig. 1.
Diagram of the hepatic kynurenine pathway adapted from Badawy (2002) with kind permission.
MATERIALS AND METHODS
Chemicals and other materials
Bovine albumin, disulfiram (tetraethyl thiuram disulfide), ethanol (>99%), oxidized nicotinamide-adenine dinucleotide (NAD)+, Trp, 3-HK, 3-HAA, KA and other kynurenine metabolites were purchased from the Sigma-Aldrich Co Ltd. (Fancy Road, Poole, Dorset, UK) and were stored as directed by the manufacturer. Water and methanol [high-performance liquid-chromatographic (HPLC) grade] were purchased from either VWR International (Hunter Boulevard, Magna Park, Leicestershire, UK) or Fisher Scientific UK (Bishop Meadow Road, Loughborough, Leicestershire, UK). Acids and alkalis were purchased from VWR International, were of the purest commercially available grades and were made up in HPLC-grade water. Filtration, Eppendorf and other tubes were purchased from Fisher or other standard suppliers.
Animals and treatments
Adult normal male Wistar rats weighing between 150 and 170 g at the start of experiments were purchased from accredited animal suppliers and were acclimatized to our standard UK Home Office-approved housing conditions (21 ± 2°C, relative humidity of 55 ± 10% and a 12 h/12 h light: dark cycle) for at least one week before experiments. They were housed five per cage, unless stated otherwise, in conventional open-top cages with standard softwood bedding from accredited suppliers, and were allowed free access to standard laboratory RM1 diet and water. Adult male mice of the alcohol-preferring C57BL/6J strain, weighing between 20 and 22 g at the start of experiments were also investigated. This study was performed under the auspices of Cardiff University and approved and licensed (project licence No:PPL 30/2502) by the UK Home Office under the Animal (Scientific Procedures) Act 1986. All compounds were administered intraperitoneally in 0.9% (w/v) NaCl (physiological saline) or, in the case of disulfiram, in a mixture of dimethylformamide: saline (1:1). 3-HK, 3-HAA and KA were given in single doses of 1–10 mg/kg body wt, whereas disulfiram was given in a 100 mg/kg dose. When given repeatedly for up to 8 days, these compounds were given in the above single doses once daily.
Determination of ALDH activity
The mitochondrial low Km ALDH activity was determined by the method of Tottmar et al. (1973) in rat liver supernatants prepared as described by Mazzanti et al. (1989). Briefly, a 1 g piece of frozen liver was homogenized in 9 ml of an ice-cold homogenization buffer consisting of 0.25 M sucrose, 5 mM Tris–HCl and 0.5 mM ethylene-diamine tetra-acetic acid disodium salt (pH 7.2) for 1 min using an ultra-Turrax homogenizer. The homogenate was centrifuged at 500g for 15 min at 4°C. The decanted supernatant was treated with 0.4 ml of a 5% aqueous sodium deoxycholate solution, stirred gently and made up to 10 ml with the above homogenization buffer. For assay of the low Km ALDH activity, the 1 ml total incubation mixture contained the following components in their final concentrations: NAD+ (1 mM), pyrazole (0.1 mM), rotenone (2 μM), sodium pyrophosphate buffer, pH 8.8 (50 mM), acetaldehyde (5 μM) and 0.1 ml of the above liver supernatant. Before the addition of acetaldehyde, the mixture was preincubated for 10 min at 25°C. The reaction was then started by the addition of acetaldehyde followed by incubation at 25°C for 5 min with shaking, and was terminated by placing the incubation tubes on ice. The reduced nicotinamide-adenine dinucleotide formed was determined by measuring its absorption at 340 nm against a blank preincubated and incubated as above, but to which water was added instead of acetaldehyde. Blanks were performed in duplicates, whereas tests were in triplicates. ALDH activity was expressed in μmol of NADH formed/min per mg of protein. The latter was determined by the method of Lowry et al. (1951), using bovine serum albumin as standard. In initial experiments, the high Km ALDH activity was also determined in the presence of a 5 mM acetaldehyde concentration.
Determination of ALDH activity in vivo
ALDH activity in vivo was determined by measuring blood acetaldehyde concentration following acute ethanol administration. The recovery of acetaldehyde from rat blood is best achieved by haemolysis of the blood sample, rather than by acid precipitation (Eriksson et al., 1977; Eriksson, 1980). Rats received an intraperitoneal injection of kynurenine metabolites (10 mg/kg body wt each). One hour later, another injection of ethanol (2 g/kg body wt as a 25% v/v solution in physiological saline) was administered. Blood samples (100 μl each) were withdrawn at hourly intervals for 3 h after the ethanol injection from a tail vein under light isofluorane anaesthesia and immediately added to 0.9 ml of ice-cold water in a gas-chromatographic (GC) injection vial. The vial was immediately sealed using a crimper and the contents of the vial were mixed. The vials were subsequently stored in a refrigerator at 4°C overnight before analysis the following morning. Acetaldehyde and ethanol in the sealed vials were analyzed by head-space GC using a Perkin Elmer Clarus 500 gas chromatograph with a Turbo-Mix HS 40 autosampler. GC conditions were as follows: injection needle temperature: 85°C; transfer temperature: 70°C; oven temperature: 80°C; pressure: 13 psi; sample equilibration time: 22.3 min at 65°C. The system was operated by the associated Total Chrome software, which also controlled data handling and processing. A 100 μl portion of a standard mixture of ethanol and acetaldehyde (1 mg/ml each), prepared in the Laboratory by diluting suitable amounts of ethanol and acetaldehyde previously stored at 4°C with cold deionized water was diluted with 0.9 ml of cold water and run as calibrant at the start of each analytical run. Initially, this standard mixture was calibrated against a certified forensic analytical 6-component standard containing ethanol and acetaldehyde at 1 mg/ml each (Restek).
Pitfalls in acetaldehyde determination
One such pitfall is artifactual formation of acetaldehyde from ethanol in the presence of blood, by a process dependent on ethanol but not on its concentration (Truitt, 1970; Eriksson et al., 1977; Eriksson, 1980). This was confirmed in the present work in a control experiment in which ethanol was added in final concentrations of 10–100 mM to 0.1 ml portions of normal untreated rat blood. The mixture was diluted to 1 ml with water in GC vials, which were then sealed and analyzed as above. Acetaldehyde was formed in amounts (in μM) of 61, 63, 64, 69, 61, 65 and 73 when ethanol was present at concentrations of 10, 20, 30, 40, 50, 75 and 100 mM, respectively. The acetaldehyde formed was subtracted from the in vivo experimental values observed.
Another problem in acetaldehyde determination is its binding to haemoglobin and the consequently lower recovery. The above authors recommended that, for rat blood, this problem can be largely overcome by haemolysis of blood samples (by dilution of one part of blood with nine parts of water) prior to head-space GC analysis and by performing control experiments to assess acetaldehyde recovery. Acetaldehyde recovery from rat blood was reported by the above authors to be 90% when acetaldehyde was added at a final concentration of 100 μM. This was also confirmed in the present study, in which a recovery value (expressed as the mean percentage recovery ± SEM for three determinations) of 90.5 ± 3.8 was observed with the above acetaldehyde concentration. Recoveries at other concentrations of added acetaldehyde (25, 50, 150 and 200 μM) were 91.5 ± 2.8, 80.4 ± 2.7, 84.5 ± 2.4 and 85.4 ± 1.9%, respectively. The blood acetaldehyde concentration values in Fig. 2b were, however, not corrected for full recovery because of the simultaneous presence of ethanol under the experimental conditions of Fig. 2. Eriksson et al. (1977) found that, in the presence of ethanol, the 90% recovery value rose to 105.4% and it was therefore considered unnecessary to apply a recovery factor, which in any case would have little effect on the results in Fig. 3b.
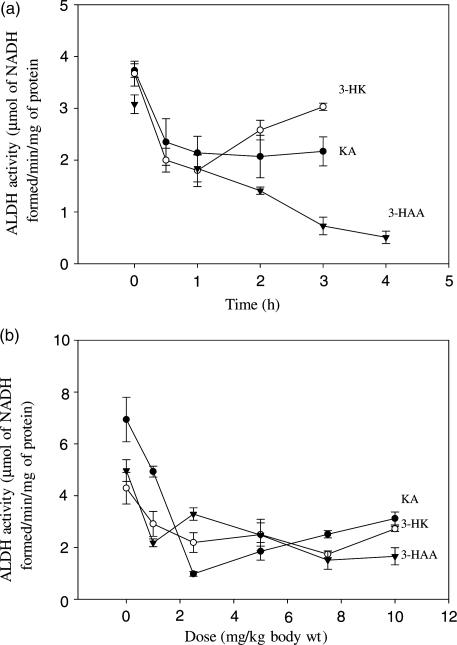
Fig. 2.
Time-course and dose–response effects of acute administration of kynurenine metabolites on activity of the rat liver mitochondrial low km ALDH activity was determined as described in the ‘Materials and Methods’ section at various times after intraperitoneal administration of a 10 mg/kg dose of kynurenine metabolites (a) or at 1 h after 1–10 mg/kg doses (b). Values are means ± SEM (bars) for each group of 4–5 rats. Values at the different time-intervals were compared statistically (t-test) with those at zero time, whereas those with various doses were compared with the zero-dose saline control, and the significance of the differences is expressed in the relevant text of the ‘Results’ section. Abbreviations used: ALDH, aldehyde dehydrogenase; KA, kynurenic acid; 3-HK, 3-hydroxykynurenine; 3-HAA, 3-hydroxyanthranilic acid.
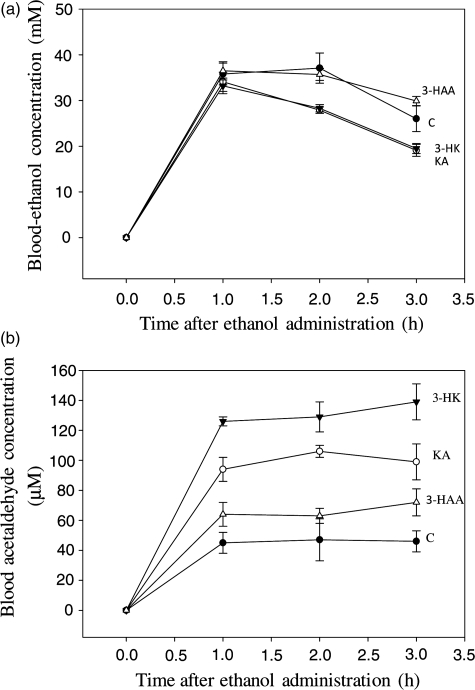
Fig. 3.
Effects of acute administration of kynurenine metabolites on rat blood-ethanol and acetaldehyde concentrations. Rats received an intraperitoneal injection (10 mg/kg body wt) of each kynurenine metabolite or an equal volume of saline. One hour later, a similar injection of ethanol (2 g/kg body wt as a 25% v/v solution in saline) was administered. Blood samples were analyzed at the times indicated for ethanol and acetaldehyde by head-space gas chromatography as described in the ‘Materials and Methods’ section. Values for blood-ethanol (a) and acetaldehyde (b) are means ± SEM (bars) for each group of four rats. Test groups were compared with the saline-pretreated control group (C) by the t-test. Abbreviations used are as in Fig. 2.
We have thus addressed the above pitfalls in detail, taken appropriate steps to circumvent them and are therefore confident of the validity of the blood acetaldehyde values reported in the present study.
Determination of liver Trp and kynurenine metabolite concentrations
Trp and its kynurenine metabolites were determined in liver by our newly developed rapid isocratic high-performance liquid-chromatographic procedure (Badawy and Morgan, 2010). Briefly, a Perkin Elmer LC200 system consisting of a quaternary pump, a column oven and a degasser was used with ultraviolet and fluorimetric detection in series. The mobile phase was a methanol: sodium dihydrogen phosphate mixture (27:73, by vol) at a final pH of 2.0 or 2.8. The system was run isocratically using a Synergi 4 μ reverse-phase Fusion-RP80 A column (250 × 4.6 mm) with guard column (Phenomenex). Operation of the system, data processing and handling were all performed by the associated Total Chrome software. A standard mixture of Trp and six of its kynurenine metabolites (1 μg/ml each) was used as calibrant at the start of each run. Results were corrected for full recovery.
Alcohol aversion test
Aversion to alcohol was assessed using the alcohol aversion model of Garver et al. (2000). Briefly, individually housed rats were allowed free access to food and water for 3 days. Disulfiram (100 mg/kg), kynurenine metabolites (10 mg/kg each) or vehicle were injected intraperitoneally once daily for 4 days. On the evening of the third day, water (but not food) was withdrawn for 18 h, but was then replaced by a 6% (v/v) ethanol solution, 2 h after the injection on the fourth day. Body wt was measured daily and alcohol consumption was monitored hourly for 4 h and levels were expressed cumulatively over this duration in g/kg body wt.
Alcohol consumption and preference in alcohol-preferring C57BL/6J mice
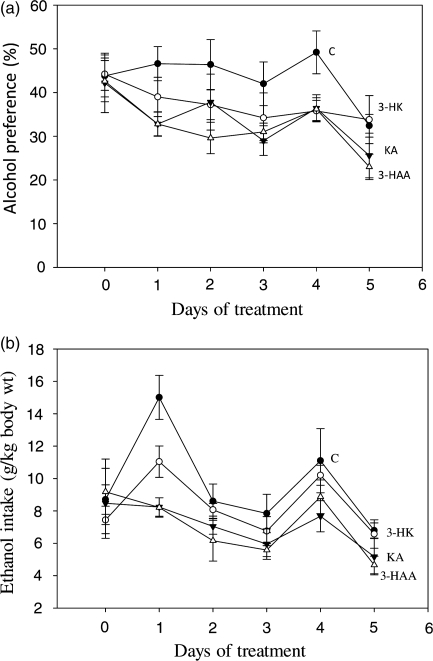
The potential effects of kynurenine metabolites on alcohol consumption and preference by male alcohol-preferring C57BL/6J mice were also studied. No attempt was made to enhance alcohol preference by acclimatizing the mice to increasing ethanol concentrations, as the purpose of our study was to investigate aversion, which is the proposed primary mechanism of action of kynurenine metabolites, rather than preference. Accordingly, four groups (n = 8 each) of individually housed mice were given free choice of drinking water and a 10% (v/v) ethanol solution for 3 weeks to establish their drinking patterns. Thereafter, mice received a single daily intraperitoneal injection of kynurenine metabolites (10 mg/kg body wt each) or an equal volume of saline for 5 days. Body wt and water and ethanol consumption were recorded daily throughout the whole study duration. Determined per kg body wt, daily alcohol consumption was expressed in absolute amounts (g) and as a percentage of total fluid intakes (% preference).
Statistical analysis
Enzymatic and other biochemical test results were compared with those of control groups by the unpaired t test, whereas alcohol consumption results were assessed initially by one-way analysis of variance (ANOVA) and additionally for within-group differences (time factor versus baseline values) by paired t-tests, using Sigma Plot (Systat, UK), version 11, with which graphics were prepared. For multiple group comparisons using this program, the Holm-Sidak test was applied, as it is more powerful than the Tukey or Bonferroni tests and can be used for both pairwise comparisons and those versus a control group. Where the data failed the normality (Shapiro–Wilk) test, Kruskal–Wallis one-way ANOVA on ranks was performed. A two-tailed level of significance (P) was set at 0.05.
RESULTS
Inhibition of the rat liver mitochondrial low Km ALDH activity by acute and chronic administration of kynurenine metabolites
Acute time-course (Fig. 2a) and dose–response (Fig. 2b) experiments were performed. Initially, we found that the high Km ALDH activity (assayed in the presence of 5 mM acetaldehyde) was not influenced by kynurenine metabolites. Consequently all results reported in this section concern the low Km activity. As shown in Fig. 2a, the mitochondrial low Km ALDH activity was inhibited by all three kynurenine metabolites after administration of a 10 mg/kg body wt dose. At 0.5 h after administration, ALDH activity was inhibited by 3-HK and KA by 45 and 37%, respectively (P = 0.0267–0.0024). Inhibition by all three metabolites was then maintained at 40–51% at 1 h. Thereafter, inhibition by KA remained at this latter level until 4 h, whereas that by 3-HAA continued to strengthen, reaching 84% at 4 h (P = 0.0000). With 3-HK, ALDH activity began to recover at 2 h, but remained significantly inhibited at 2 and 3 h (by 30 and 17%, respectively; P = 0.0128–0.0431).
As shown in Fig. 2b, significant inhibition of 32–56% (P = 0.0398–0.0008) of ALDH activity was observed at 1 h after administration of a 1 mg/kg body wt dose of kynurenine metabolites. Maximum inhibition at 1 h was observed with a 2.5 mg/kg dose of KA (86%) and with a 7.5 mg/kg dose of 3-HK (59%) and 3-HAA (70%) (P = 0.0035–0.0001).
ALDH activity was also inhibited when a 10 mg/kg body wt dose of the above 3 kynurenine metabolites was administered daily for 8 days (data not shown). At 2 h after the (final) injection on the 8th day, inhibition by KA, 3-HK and 3-HAA was 37%, 39% and 64% respectively (P = 0.0292–0.0007). Although this inhibition could very well be due to the acute effect of kynurenine metabolites, it suggests that no tolerance develops towards it after chronic treatment.
Inhibition of ALDH activity in vivo by acute administration of kynurenine metabolites
ALDH activity in vivo was determined by measuring the accumulation of acetaldehyde in blood following acute ethanol administration. The results in Fig. 3 show blood-ethanol (a) and acetaldehyde (b) concentrations after intraperitoneal administration of a 2 g/kg body wt dose of ethanol. In saline-pretreated control rats, ethanol concentration rose to 35.8 mM at 1 h and to 37.1 mM at 2 h before declining to 26.0 mM at 3 h. None of the three kynurenine metabolites exerted a significant effect on ethanol concentration at 1 h (P > 0.1). With 3-HAA, ethanol concentration resembled that in saline-treated controls at 2 and 3 h. By contrast, ethanol concentration at 2 and 3 h after ethanol administration was significantly decreased by pretreatment of rats with KA and 3-HK, by 24–27% (P = 0.05–0.0175).
Blood acetaldehyde concentration following ethanol administration (Fig. 3b) to saline-pretreated control rats remained at a constant level of 45–47 μM, suggesting a constant rate of ethanol and acetaldehyde oxidation over the 1–3 h observation period. Pretreatment of rats with KA increased acetaldehyde concentration by 109, 125 and 115%, respectively (P = 0.0104–0.0029). 3-HK induced a stronger elevation of acetaldehyde concentration, of 180, 174 and 202%, respectively at 1–3 h after ethanol administration (P = 0.0017–0.0000), whereas with 3-HAA, the elevation of acetaldehyde concentration was modest (42, 34 and 56%, respectively), and not significant (P > 0.1). The elevation of blood acetaldehyde concentration by 3-HK was significantly greater than that by KA at 1–3 h (P = 0.05–0.0095).
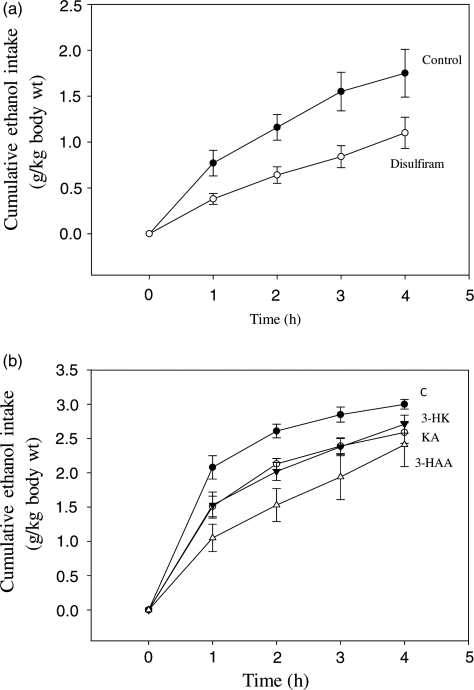
Demonstration of aversion to alcohol after administration of kynurenine metabolites and disulfiram
In the aversion model of Garver et al. (2000), rats treated with the classical ALDH inhibitor disulfiram consumed equal amounts of the ethanol drinking solution as control animals during the first hour of the test. Thereafter, alcohol consumption by disulfiram-treated rats remained static, unlike that by controls, which continued to increase cumulatively up to the fifth hour. The results in Fig. 4a, show that disulfiram actually inhibited alcohol consumption significantly and maximally during the first hour (by 51%; P 0.0351, paired t-test), thereafter the animals continued to drink the ethanol solution, but to a lesser degree than controls. Thus, the inhibition of ethanol consumption by disulfiram was maintained for two more hours, as 45–46% (P = 0.0158–0.0138), but, at 4 h, the 37% decrease was not significant. The results in Fig. 4b show that kynurenine metabolites also inhibited alcohol consumption in this model significantly (P = 0.05–0.005) over the first 3 h. Thus, as was the case with disulfiram, inhibition of alcohol consumption was strongest at 1 h after administration of KA, 3-HK and 3-HAA (by 28, 26 and 50%, respectively). Inhibition remained significant at 2 and 3 h, but, by 4 h, only that by KA was still significant.
Fig. 4.
Inhibition of alcohol consumption by disulfiram (a) and kynurenine metabolites (b) in a rat alcohol aversion model Experimental details are as described in the ‘Materials and Methods’ section. Ethanol consumption was monitored hourly for 4 h and is expressed in g/kg body wt cumulatively. Values are means ± SEM (bars) for each group of 4–6 rats. Abbreviations are as in Fig. 3.
It will be noted from the data in Fig. 4 that alcohol consumption by control rats in the disulfiram experiment (Fig. 4a) is lower than that of the control animals in the kynurenine metabolite experiment (Fig. 4b). This is almost certain to be due to the use of dimethylformamide, along with saline, to dissolve disulfiram, rather than to variations among different batches of animals, because, as will be seen in the accompanying paper (Badawy et al., 2011), the control data obtained in animals given saline only in two different experiments were broadly similar to those in the present experiment with kynurenines.
Alcohol consumption by C57 mice
As no attempt was made to enhance alcohol preference by acclimatizing the mice to increasing ethanol concentrations, there were wide variations in levels of consumption of the 10% (v/v) ethanol solution, with ∼37–50% of the mice in each group consuming >10 g/kg/day, and showing >50% preference, with the remainder consuming up to 7 g/kg/day with a level of preference <32%. To establish comparability between group at baseline (Day 0), six mice from each group were selected whose results are shown in Fig. 5. As shown, the % preference (Fig. 5a) and absolute ethanol intake in g/kg body wt (Fig. 5b) were broadly similar across groups at baseline, with no significant differences (P > 0.1). In the saline-treated control group, preference remained stable for 4 days and the decrease on Day 5 did not reach statistical significance (P = 0.095). By contrast, preference decreased in mice receiving the three kynurenine metabolites, with 3-HAA causing the largest decrease. Compared with baseline, the decrease in the % preference with 3-HAA (23–46%) was significant on all days (P = 0.043–0.009), except Day 4. With 3-HK and KA, only the decreases on Days 4 and/or 5 (19–39%) were significant (P = 0.05–0.011). When the % preference values with kynurenine metabolites were compared with those of the control group, significant decreases were also observed with 3-HK (37%) on Day 4, with KA (26–30%) on Days 1 and 4 and with 3-HAA (26–36%) on Days 1–5 (P = 0.05–0.0075).
Fig. 5.
Effects of repeated administration of kynurenine metabolites on alcohol consumption and preference in male alcohol-preferring C57BL/6J mice Experimental details are as described in the ‘Materials and Methods’ section. Alcohol consumption was monitored daily and is expressed (per kg body wt) for each group from Day 0 (before the injections) onwards, both as a preference % (a) and in g amounts (b). Values are means ± SEM (bars) for each group of six mice. For statistical comparisons, see the relevant text in the ‘Results’ section.
When alcohol consumption was expressed in absolute amounts (g/kg body wt) (Fig. 5b), a broadly similar pattern emerged, with 3-HAA causing the greatest decrease in alcohol intake. However, the only significant differences were those compared with the saline controls for KA on Days 1 and 4 and for 3-HAA on Days 1 and 5 (31–45%; P = 0.021–0.001).
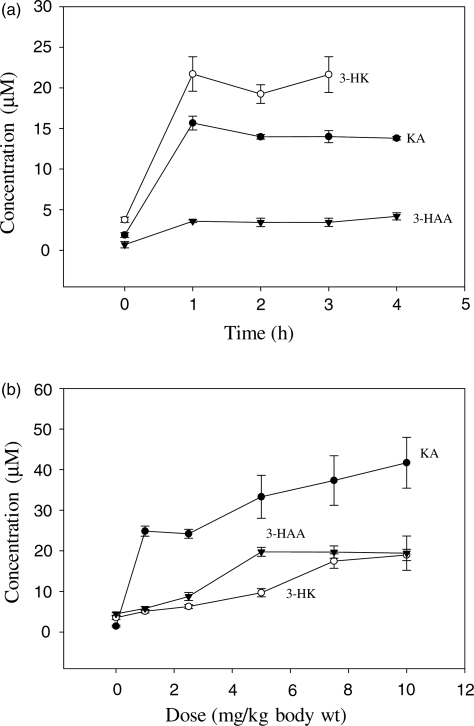
Hepatic concentrations of kynurenine metabolites after their administration
Hepatic concentrations of KA, 3-HK and 3-HAA were determined after acute administration of each individual compound in the same rats in which ALDH activity was assessed. Although concentrations of other kynurenine metabolites and of Trp were also determined, these are not reported here, as their relevance largely falls outside the scope of the present paper. When a 10 mg/kg body wt dose of the three kynurenine metabolites was administered (Fig. 6a), maximum increases (of 4.2–7.3-fold) in their concentrations were observed at 1 h (P = 0.0002) and were maintained for 3–4 h. Dose-dependent increases in liver kynurenine metabolite concentrations were observed at 1 h (Fig. 6b) in the 1–10 mg/kg dose-range with KA and 3-HK. However, with 3-HAA, the maximum increase was observed with the 5 mg/kg dose. Baseline [3-HAA] in the time-course experiment was lower than that in the dose–response one. This is most likely due to variations across different animal batches, as the relative increases in this metabolite concentration at 1 h after administration of the 10 mg/kg dose were similar in both experiments (2.67 and 2.97-fold, respectively).
Fig. 6.
Hepatic kynurenine metabolite concentrations after their acute administration Kynurenine metabolite concentrations were determined as described in the ‘Materials and Methods’ section in livers of the same rats undergoing the time-course (a) and dose–response (b) experiments with ALDH reported in Fig. 2. Values are means ± SEM (bars) for each group of five rats. Abbreviations and comparisons are as in Fig. 2.
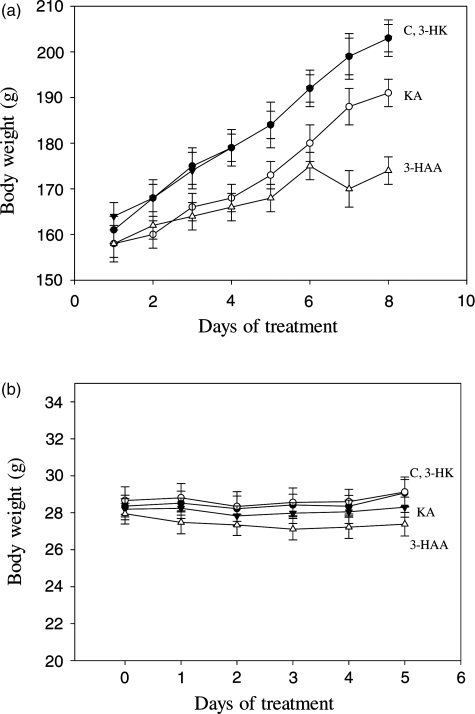
Animal body weights during repeated administration of kynurenine metabolites and disulfiram
Body wt was measured in chronic experiments, not only to determine daily dose levels, but also as a measure of animal welfare and safety of administered compounds. In the alcohol aversion study in which compounds were injected once daily for 4 days, both rats treated with kynurenine metabolites and their saline-treated controls gained weight at the normal rate during the first 3 days. However, small losses of 6–9% were observed in both control and test rats on the morning of the final (fourth) day, compared with body weights the day before, almost certainly due to introducing a water-deprivation regimen during the preceding 18 h. In the aversion experiments with disulfiram and its control rats receiving the vehicle (saline: dimethylformamide, 1:1), both control and test rats showed a small wt loss (2.8 and 4.2%) on Day 3, compared with Day 1, presumably due to this solvent, in addition to a 3–6% wt loss following the water-deprivation period, as was the case with kynurenine metabolites and their pure saline control.
In the chronic study of changes in rat liver ALDH activity in which kynurenine metabolites were administered daily for 8 days, changes in body weights were also recorded. As shown in Fig. 7a, all groups gained wt significantly (P = 0.001), with the gains by control rats reaching 26% on Day 8. Rats receiving KA and 3-HK also gained wt at a rate close to that of controls (respectively 21 and 24% on Day 8). 3-HAA-treated rats, however, gained wt less strongly, achieving only a 10% wt gain on Days 6–8. All animals appeared to tolerate kynurenine metabolites and showed no adverse reactions or unusual behaviours.
Fig. 7.
Body weights of rats and mice during chronic treatment with kynurenine metabolites on Day 1 (rats) or Day 0 (mice), animals received single daily intraperitoneal injections of saline or kynurenine metabolites (10 mg/kg each) for 8 (rats) or 5 (mice) days. Body wt was monitored daily each morning and is expressed in g for both rats (a) and mice (b). Values are means ± SEM (bars) for each group of six rats or eight mice. For abbreviations, see Fig. 5.
Body wt was also recorded for mice during the preference study. The body weights of all mice (n = 32) at the start of the study (mean ± SEM in g) (26.83 ± 0.25) rose by 5% during the 21-day free choice period to reach 28.09 ± 0.23 g (P = 0.003) on Day 0 of the 5-day drug administration experiment. As shown in Fig. 7b, body weights remained stable over the 5-day period and none of the changes in the control and test groups was significant when compared with the zero day value (P > 0.9). This is reflected in the small (not exceeding 2.5%), but insignificant, gains observed in the control group and those receiving 3-HK and KA and in the small (2%) and insignificant loss in mice of the 3-HAA group. As was the case with rats, mice appeared healthy and showed no adverse reactions or unusual behaviors.
DISCUSSION
Inhibition of ALDH activity after administration of kynurenine metabolites
The present results have established the ability of three Trp metabolites of the kynurenine pathway, namely KA, 3-HK and 3-HAA to inhibit the rat liver mitochondrial low Km activity of ALDH after acute administration (Fig. 2) and that no tolerance to this inhibition develops after repeated administration. Inhibition was significant with a dose as small as 1 mg/kg body wt (Fig. 2b) and was maximal at 1 h, except after 3-HAA (Fig. 2a). The hepatic concentrations of these metabolites also reached maximum values at 1 h (Fig. 5a) in line with the maximum inhibition. The hepatic concentrations observed (Fig. 5) are well in excess of the 2 μM concentration previously shown to cause 40–55% inhibition in vitro (Badawy and Morgan, 2007).
Inhibition in the present work lasted for at least 3–4 h (the longest durations studied). With 3-HK, ALDH inhibition, which was maximal at 1 h, began to lessen thereafter, suggesting that it is short-lived and hence reversible. However, with KA and 3-HAA, inhibition either remained at its maximal value or gained in strength, suggesting a longer duration and a possible irreversible nature. With disulfiram, its irreversible inhibition (Marchner and Tottmar, 1978) is characterized by a prolonged duration (7–10 days) after a single dose (see, Brien and Loomis, 1985). Kinetic studies are clearly required to establish the mechanism(s) of the ALDH inhibition by these kynurenine metabolites.
Inhibition of ALDH activity in vivo by administration of kynurenine metabolites
3-HK and KA also inhibited ALDH activity in vivo, as suggested by their strong elevation of blood acetaldehyde concentration after ethanol administration (Fig. 3b), whereas 3-HAA caused only a modest increase. As blood-ethanol concentration at 1 h was not influenced by any of the three kynurenine metabolites (Fig. 3a), we conclude that ethanol kinetics were not altered up to this time-point. Thereafter, blood-ethanol concentration was also little altered by 3-HAA, but was significantly decreased by KA and 3-HK, suggesting acceleration of ethanol metabolism by acting on alcohol dehydrogenase or other ethanol-oxidizing enzymes, or through other mechanisms. Further work is required to elucidate the nature of this effect. In relation to a potential acceleration of ethanol metabolism by KA, Lapin and Politi (1994) speculated as to whether the shortening of ethanol-induced sleep time by indol-3-ylpyruvic acid could be due to its conversion to KA. The elevation of blood acetaldehyde concentration by 3-HK and KA strongly suggests that both compounds inhibit AlDH activity in vivo, thus further corroborating their effects in vitro (Badawy and Morgan, 2007) and after administration (Fig. 2 in the present work).
Induction of aversion to alcohol by kynurenine metabolites
The alcohol aversion model of Garver et al. (2000) is a variant of the conditioned taste aversion paradigm in which a novel taste (that of alcohol introduced for the first time) is associated with a noxious condition (the disulfiram-ethanol reaction). That a robust conditioned taste aversion quickly develops under such conditions has been demonstrated (Nolan et al., 1997; Scalera et al., 1997; Barber et al., 1998; Yasoshima and Yamamoto, 1998). We have successfully confirmed the above authors’ findings with disulfiram and demonstrated the ability of our three kynurenine metabolites to induce aversion to alcohol (Fig. 4). Two differences from the findings by Garver et al. (2000) with disulfiram were, however, observed: (a) the inhibition of alcohol consumption by disulfiram was already significant and strongest at the 1 h observation period, whereas the above authors observed no inhibition at 1 h; (b) our disulfiram-treated rats continued thereafter to drink more fluid, though less than controls, whereas alcohol consumption by rats studied by the above authors remained static at the 1 h level. The earlier inhibition by disulfiram of alcohol consumption in our study may be due to the more rapid absorption of the drug solution after intraperitoneal, when compared with that of the drug suspension after oral, administration as reported by the above authors.
Both 3-HK and KA were equally effective, whereas 3-HAA caused a stronger inhibition of alcohol consumption. This is somewhat surprising, as this metabolite did not cause a significant increase in blood acetaldehyde concentration (Fig. 3b), even-though it caused the strongest inhibition of AlDH activity after administration (Fig. 2a). It is possible that additional factors are involved in the aversive effects of 3-HAA. Alternatively, it is possible that 3-HAA may also exert an inhibitory effect on ethanol oxidation, which, superimposed on its ALDH inhibition, may have led to the small insignificant elevation of blood acetaldehyde concentration. These possibilities require investigation.
Alcohol consumption was also decreased in alcohol-preferring C57 mice. In particular, the % preference, and to a lesser extent the absolute amount of alcohol consumed, were decreased, with 3-HAA causing the strongest decreases. However, due to the wide individual variations between mice and the small numbers used, these results, though complementary to the aversion results, can only be considered preliminary.
General safety of kynurenine metabolites
The present results have shown that a 10 mg/kg body wt dose of the three kynurenine metabolites studied exerted no undesirable side effects or toxicity in rats or mice when administered intraperitoneally once or once daily over a 4–8 day period. KA is the endogenous antagonist of the N-methyl-D-aspartate type of glutamate receptors (Stone, 1993) and doses of it as large as 500–1500 mg/kg body wt are tolerated by mice when administered subcutaneously; only sedation has been observed with these large doses (Rasmussen et al., 1991). Relatively smaller intraperitoneal doses of KA (up to 200 mg/kg) have been shown to be anxiolytic (Lapin, 1996, 1998). The potential toxicity of 3-HK and 3-HAA is a more controversial issue. 3-HAA has been reported (Morita et al., 2001) to induce apoptosis in monocyte-derived cells stimulated by interferon-γ, but at a concentration (200 μM) unlikely to be reached physiologically or even pathologically. Also this effect is limited to the THP-1 and U937, but not four other, cell lines. Under these conditions, the above authors could not detect an apoptotic effect for 3-HK at a similar concentration. In fact, a 250 mg/kg dose of 3-HK has been administered subcutaneously to rats with no reported side effects (Luthman et al., 1996). The fact that our rats treated daily with 3-HK for 8 days gained weight at a rate similar to controls suggests that this metabolite does not cause signs of toxicity at the dose level used. Only 3-HAA caused a significant weight loss, suggesting some degree of toxicity at the 10 mg/kg dose level used, although this was not visibly apparent.
3-HK, which can be taken-up by the brain (Speciale and Schwarcz, 1990; Fukui et al., 1991), has been suggested to be neurotoxic, as it can promote neuronal cell death by producing hydrogen peroxide (Okuda et al., 1996) (an effect that has not so far been duplicated in vivo) and in view of the earlier finding of its elevated levels in post-mortem brains of patients with Huntington's disease (Reynolds and Pearsons, 1993), although levels of 3-HK and 3-HAA in blood of living Huntington's patients (Stoy et al., 2005) and also those with chronic brain injury (Mackay et al., 2005) have been shown to be lower than in controls. Stoy et al. (2005) suggested that, although blood [3-HK] may not reflect that in brain, it is also possible that oxidative stress in Huntington's disease may trigger a secondary activation of the kynurenine pathway in the brain. Also, although the findings by Okuda et al. (1996) support a pro-oxidant effect of 3-HK, it has been reported (Goda et al., 1999) that, of all Trp and serotonin metabolites, both 3-HK and 3-HAA possess the highest antioxidant properties, with a radical-scavenging reactivity and inhibition of lipid peroxidation greater than those by α-tocopherol (vitamin E). Furthermore, as Morita et al. (2001) suggested that the apoptotic effect of 3-HAA is due to generation of H2O2, the failure of 3-HK to induce apoptosis in their study further suggests that this kynurenine metabolite does not generate H2O2 even at a 200 μM concentration. Nor are hydroxyl radicals involved in 3-HK oxidation by methaemoglobin with H2O2 (Ishii et al., 1992). Further evidence of an antioxidant role for 3-HK and 3-HAA has been obtained in vitro in rat cerebral cortex and cultured C6 glioma cells with the demonstration (Leipnitz et al., 2007) that both metabolites prevent lipid peroxidation in the brain, decrease peroxy radical induction, and prevent glutaric acid-induced free radical formation. Furthermore, the role of 3-HAA as a modulator of the immune system is increasingly recognized with, among others, its ability to decrease the release of cytokines, to exert direct antiproliferative effects, suppress the activation of pro-inflammatory transcription factor NFκB (nuclear factor kappa-light-chain-enhancer of activated B cells), and inhibit nitric oxide synthase (for review, see Darlington et al., 2010 and references cited therein).
General conclusions and comments
We have established proofs of mechanism (inhibition of the target enzyme ALDH after administration and in vivo) and principle (induction of aversion to alcohol) in the present preclinical developmental study with metabolites of the amino acid Trp of the kynurenine pathway. Previous studies (Lapin et al., 1991) have established a prior art for the use of KA in alcoholism treatment, whereas no prior art exists for the use of 3-HK or 3-HAA, for which a US patent is expected shortly. As the most potent AlDH inhibitor in vivo and the immediate precursor of 3-HAA, 3-HK is of particular interest, but its potential clinical use in alcoholism treatment requires further developmental studies by interested parties. A more immediate and near clinic-ready alternative is the use of its parent compound Trp under appropriate metabolic conditions, evidence for which is presented in the following paper (Badawy et al., 2011).
Funding
This work was funded by a project grant from the Wellcome Trust (069301) to AA-BB at Cardiff University's College of Medicine. S.B. was a Visiting Scholar and acknowledges financial support by the British Commonwealth Authority.
Acknowledgements
This study was initiated at the Cardiff & Vale NHS Trust's Biomedical Research Laboratory, Whitchurch Hospital, Cardiff, but was continued and completed at the School of Health Sciences, University of Wales Institute Cardiff (UWIC). We are grateful to Cardiff University for provision of facilities, Cambridge University Press for kind permission to reproduce here Fig. 1 published in Nutr Res Rev by Badawy (2002), and to C.J. Morgan and J.A. Turner for skilful technical assistance.
REFERENCES
- Badawy AA-B. Tryptophan metabolism in alcoholism. Nutr Res Rev. 2002;15:123–52. doi: 10.1079/NRR200133. [DOI] [PubMed] [Google Scholar]
- Badawy AA-B. Tryptophan metabolism and alcoholism. In: Preedy V, Watson RR, editors. Comprehensive Handbook of Alcohol-Related Pathology. Vol. 3. London: Elsevier; 2005. pp. 1303–22. chapter 99. [Google Scholar]
- Badawy AA-B, Morgan CJ. Tryptophan metabolites as potent inhibitors of aldehyde dehydrogenase activity and potential alcoholism-aversion therapeutic agents. Int Congress Series. 2007;1304:344–51. [Google Scholar]
- Badawy AA-B, Morgan CJ. Rapid isocratic liquid chromatographic separation and quantification of tryptophan and six kynurenine metabolites in biological samples with ultraviolet and fluorimetric detection. Int J Tryptophan Res. 2010;3:175–86. doi: 10.4137/IJTR.S6225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawy AA-B, Bano S, Steptoe A. Tryptophan in alcoholism treatment II. Inhibition of the rat liver mitochondrial low Km aldehyde dehydrogenase activity, elevation of blood acetaldehyde concentration and induction of aversion to alcohol by combined administration of tryptophan and benserazide. Alcohol Alcohol. 2011;46:661–71. doi: 10.1093/alcalc/agr135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber TA, Klunk AM, Howorth PD, et al. A new look at an old task: advantages and uses of sickness-conditioned learning in day-old chicks. Pharmacol Biochem Behav. 1998;60:423–30. doi: 10.1016/s0091-3057(97)00597-2. [DOI] [PubMed] [Google Scholar]
- Brien JF, Loomis CW. Aldehyde dehydrogenase inhibitors as alcohol-sensitizing drugs: a pharmacological perspective. Trends Pharmacol Sci. 1985;6:477–80. [Google Scholar]
- Chick J. Safety issues concerning the use of disulfiram in treating alcohol dependence. Drug Saf. 1999;20:427–35. doi: 10.2165/00002018-199920050-00003. [DOI] [PubMed] [Google Scholar]
- Darlington LG, Forrest CM, Mackay GM, et al. On the biological importance of the 3-hydroxyanthranilic acid: anthranilic acid ratio. Int J Tryptophan Res. 2010;3:51–9. doi: 10.4137/ijtr.s4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sousa A, de Sousa A. A one-year pragmatic trial of naltrexone versus disulfiram in the treatment of alcohol dependence. Alcohol Alcohol. 2004;39:528–31. doi: 10.1093/alcalc/agh104. [DOI] [PubMed] [Google Scholar]
- de Sousa A, de Sousa A. An open randomised study comparing disulfiram and acamprosate in the treatment of alcohol dependence. Alcohol Alcohol. 2005;40:545–8. doi: 10.1093/alcalc/agh187. [DOI] [PubMed] [Google Scholar]
- Diehl A, Ulmer L, Mutschler J, et al. Why is disulfiram superior to acamprosate in the routine clinical setting? A retrospective long-term study in 353 alcohol-dependent patients. Alcohol Alcohol. 2010;45:271–7. doi: 10.1093/alcalc/agq017. [DOI] [PubMed] [Google Scholar]
- Eriksson CJP. Problems and pitfalls in acetaldehyde determinations. Alcohol Clin Exp Res. 1980;4:22–9. doi: 10.1111/j.1530-0277.1980.tb04786.x. [DOI] [PubMed] [Google Scholar]
- Eriksson CJP, Sippel HW, Forsander OA. The determination of acetaldehyde in biological samples by head-space gas chromatography. Anal Biochem. 1977;80:116–24. doi: 10.1016/0003-2697(77)90631-5. [DOI] [PubMed] [Google Scholar]
- Fukui S, Schwarcz R, Rapoport SI, et al. Blood-brain barrier transport of kynurenines: implications for brain synthesis and metabolism. J Neurochem. 1991;56:2007–17. doi: 10.1111/j.1471-4159.1991.tb03460.x. [DOI] [PubMed] [Google Scholar]
- Garver E, Ross AD, Tu G-C, et al. Paradigm to test drug-induced aversion to ethanol. Alcohol Alcohol. 2000;35:435–8. doi: 10.1093/alcalc/35.5.435. [DOI] [PubMed] [Google Scholar]
- Goda K, Hamane Y, Kishimoto R, et al. Radical scavenging properties of tryptophan metabolites. Adv Exp Med Biol. 1999;467:397–402. doi: 10.1007/978-1-4615-4709-9_50. [DOI] [PubMed] [Google Scholar]
- Ishii T, Iwahashi H, Sugata R, et al. Oxidation of 3-hydroxykynurenine catalysed by methemoglobin with hydrogen peroxide. Free Radic Biol Med. 1992;13:17–20. doi: 10.1016/0891-5849(92)90160-i. [DOI] [PubMed] [Google Scholar]
- Laaksonen E, Koski-Jannes A, Salaspuro M, et al. A randomized, multicentre, open-label, comparative trial of disulfiram, naltrexone and acamprosate in the treatment of alcohol dependence. Alcohol Alcohol. 2008;43:53–61. doi: 10.1093/alcalc/agm136. [DOI] [PubMed] [Google Scholar]
- Lapin IP. Kynurenines and anxiety. Adv Exp Med Biol. 1996;398:191–4. doi: 10.1007/978-1-4613-0381-7_31. [DOI] [PubMed] [Google Scholar]
- Lapin IP. Antagonism of kynurenic acid to anxiogens in mice. Life Sci. 1998;63:PL231–6. doi: 10.1016/s0024-3205(98)00404-4. [DOI] [PubMed] [Google Scholar]
- Lapin IP, Politi V. Antiethanol effects of indol-3-ylpyruvic acid in mice. Alcohol Alcohol. 1994;29:265–8. [PubMed] [Google Scholar]
- Lapin IP, Mirzeav S, Prakhe’ EIB, et al. Using kynurenines to prevent ethanol-induced disorders in the hole reflex of mice and rats. Z Vys Nerv Deyatel'nosti Imeni I P Pavlova. 1991;41:551–7. [PubMed] [Google Scholar]
- Leipnitz G, Schumacher C, Dalcin KB, et al. In vitro evidence for an antioxidant role of 3-hydroxykynurenine and 3-hydroxyanthranilic acid in the rat brain. Neurochem Int. 2007;50:83–94. doi: 10.1016/j.neuint.2006.04.017. [DOI] [PubMed] [Google Scholar]
- LeMarquand D, Pihl RO, Benkelfat C. Serotonin and alcohol intake, abuse and dependence: clinical evidence. Biol Psychiat. 1994a;36:326–37. doi: 10.1016/0006-3223(94)90630-0. [DOI] [PubMed] [Google Scholar]
- LeMarquand D, Pihl RO, Benkelfat C. Serotonin and alcohol intake, abuse and dependence: findings of animal studies. Biol Psychiat. 1994b;36:395–421. doi: 10.1016/0006-3223(94)91215-7. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough HJ, Farr AL, et al. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- Luthman J, Vänerman E, Fredriksson G, et al. Regulation of quinolinic acid in the normal rat brain by kynurenine pathway precursors. Adv Exp Med Biol. 1996;398:229–39. doi: 10.1007/978-1-4613-0381-7_36. [DOI] [PubMed] [Google Scholar]
- Mackay GM, Forrest CM, Stoy N, et al. Tryptophan metabolism and oxidative stress in patients with chronic brain injury. Eur J Neurol. 2005;12:1–13. doi: 10.1111/j.1468-1331.2006.01220.x. [DOI] [PubMed] [Google Scholar]
- Mann K. Pharmacotherapy of alcohol dependence: a review. CNS Drugs. 2004;18:485–504. doi: 10.2165/00023210-200418080-00002. [DOI] [PubMed] [Google Scholar]
- Marchner H, Tottmar O. A comparative study on the effects of disulfiram, cyanamide and 1-aminocyclopropanol on the acetaldehyde metabolism in rats. Acta Pharmacol Toxicol. 1978;43:219–32. doi: 10.1111/j.1600-0773.1978.tb02258.x. [DOI] [PubMed] [Google Scholar]
- Mazzanti R, Moscarella S, Bensi G, et al. Hepatic lipid peroxidation and aldehyde dehydrogenase activity in alcoholic and non-alcoholic liver disease. Alcohol Alcohol. 1989;24:121–8. doi: 10.1093/oxfordjournals.alcalc.a044875. [DOI] [PubMed] [Google Scholar]
- Morita T, Saito K, Takemura M, et al. 3-Hydroxyanthranilic acid: an L-tryptophan metabolite, induces apoptosis in monocye-derived cells stimulated by interferon-γ. Ann Clin Biochem. 2001;38:242–51. doi: 10.1258/0004563011900461. [DOI] [PubMed] [Google Scholar]
- Naranjo CA, Sellers EM, Lawrin MO. Modulation of ethanol intake by serotonin uptake inhibitors. J Clin Psychiat. 1986;47(Suppl):16–22. [PubMed] [Google Scholar]
- Nolan LJ, McCaughey SA, Giza BK, et al. Extinction of a conditioned taste aversion in rats. 1. Behavioral effects. Physiol Behav. 1997;61:319–23. doi: 10.1016/s0031-9384(96)00411-8. [DOI] [PubMed] [Google Scholar]
- Okuda S, Nishiyama N, Saito H, et al. Hydrogen peroxide-mediated neuronal cell death induced by an endogenous neurotoxin, 3-hydroxykynurenine. Proc Natl Acad Sci USA. 1996;93:12553–8. doi: 10.1073/pnas.93.22.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen K, Krystal JH, Aghajanian GK. Excitatory amino acids and morphine withdrawal: differential effects of central and peripheral kynurenic acid administration. Psychopharmacol. 1991;105:508–12. doi: 10.1007/BF02244371. [DOI] [PubMed] [Google Scholar]
- Reynolds GP, Pearsons SJ. Neurochemical-clinical correlates in Huntongton's disease—applications of brain banking techniques. J Neural Transm. 1993;39(Suppl):207–14. [PubMed] [Google Scholar]
- Richards P, Brown CL, Lowe SM. Synthesis of tryptophan from 3-indolepyruvic acid by a healthy woman. J Nutr. 1972;102:1547–50. doi: 10.1093/jn/102.11.1547. [DOI] [PubMed] [Google Scholar]
- Scalera G, Grigson PS, Norgren R. Gustatory functions, sodium appetite, and conditioned taste aversion survive excitotoxic lesions of the thalamic taste area. Behav Neurosci. 1997;111:633–45. [PubMed] [Google Scholar]
- Sellers EM, Higgins GA, Sobell MB. 5-HT and alcohol abuse. Trends Pharmacol Sci. 1992;13:69–75. doi: 10.1016/0165-6147(92)90026-3. [DOI] [PubMed] [Google Scholar]
- Speciale C, Schwarcz R. Uptake of kynurenine into rat brain slices. J Neurochem. 1990;54:156–63. doi: 10.1111/j.1471-4159.1990.tb13296.x. [DOI] [PubMed] [Google Scholar]
- Stone TW. Neuropharmacology of quinolinic and kynurenic acids. Pharmacol Rev. 1993;45:309–85. [PubMed] [Google Scholar]
- Stoy N, Mackay GM, Forrest CM, et al. Tryptophan metabolism and oxidative stress in patients with Huntington's disease. J Neurochem. 2005;93:611–23. doi: 10.1111/j.1471-4159.2005.03070.x. [DOI] [PubMed] [Google Scholar]
- Tottmar SOC, Pettersson H, Kiessling K-H. The subcellular distribution and properties of aldehyde dehydrogenase in rat liver. Biochem J. 1973;135:577–86. doi: 10.1042/bj1350577a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truitt EB. Ethanol-induced release of acetaldehyde from blood and its effects on the determination of acetaldehyde. Q J Stud Alcohol. 1970;31:1–12. [PubMed] [Google Scholar]
- Yasoshima Y, Yamamoto T. Short-term and long-term excitability changes of the insular cortical neurons after the acquisition of taste aversion learning in behaving rats. Neurosci. 1998;84:1–5. doi: 10.1016/s0306-4522(97)00636-2. [DOI] [PubMed] [Google Scholar]
- Zabik JE, Sprague JE, Binkerd K. Central and peripheral components to the inhibitory actions of 5-HTP on ethanol consumption in the rat. Pharmacol Biochem Behav. 1994;47:547–51. doi: 10.1016/0091-3057(94)90157-0. [DOI] [PubMed] [Google Scholar]