Abstract
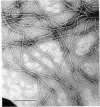
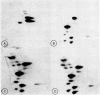
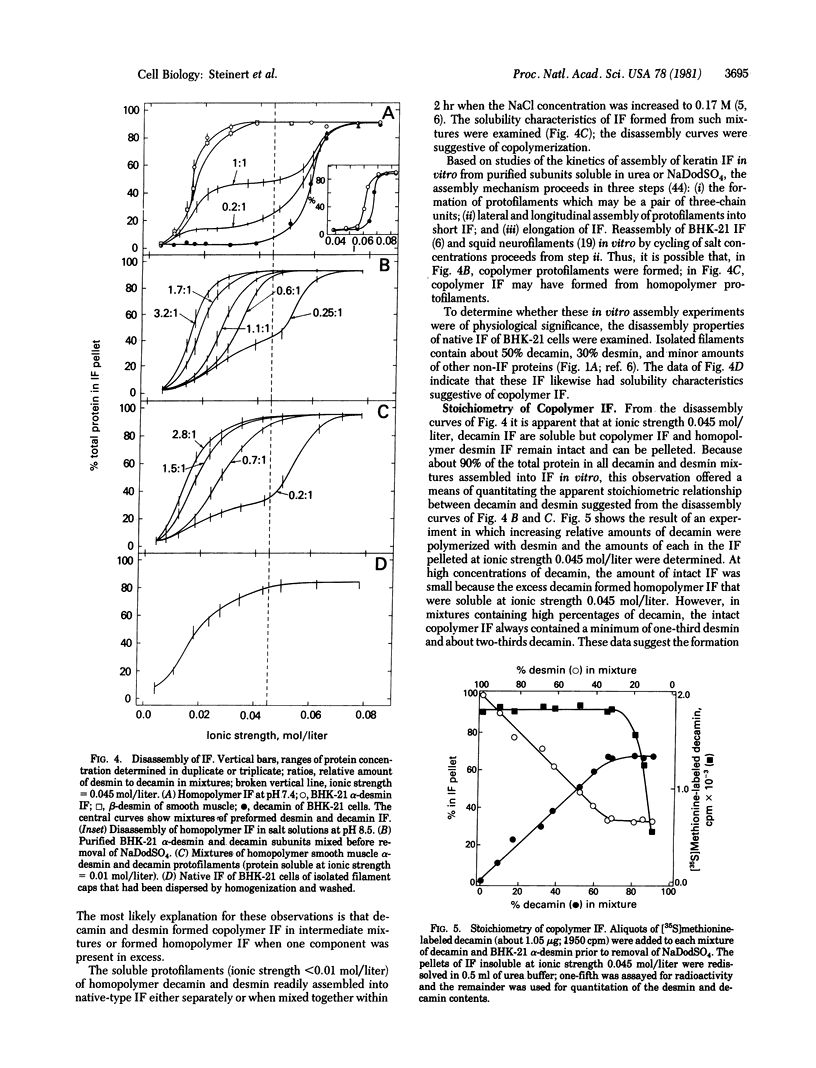
This paper presents evidence that the intermediate filament (IF) subunits of muscle cells (skeletin or desmin) and fibroblastic cells (decamin or vimentin) separately form homopolymer IF in vitro and, when mixed, prefer to form copolymer IF in vitro. Because they coexist in cells, they may also form copolymers in vivo. The IFs of baby hamster kidney fibroblasts (BHK-21) consist of a major subunit, decamin, and two minor subunits which, on the basis of two-dimensional gel and peptide mapping criteria, are identical to the alpha and beta subunits of smooth muscle desmin. The subunits differ only in their degrees of phosphorylation: alpha desmin contained 2 mol/mol of O-phosphoserine whereas beta desmin contained none. The decamin and desmin subunits assembled into homopolymer IF in vitro in high yield from purified denatured subunits under identical conditions of pH and ionic strength. However, homopolymer decamin IF disassembled into soluble protofilaments in solutions of ionic strength less than 0.05 mol/liter whereas homopolymer desmin IF disassembled at ionic strength less than 0.03 mol/liter. When decamin and desmin were mixed together as denatured subunits or as soluble protofilaments, the IF assembled in vitro had solubility properties intermediate between those of the homopolymer IFs, indicating that the two subunits had formed copolymer IF. The stoichiometry of copolymerization as determined in mixtures in which one subunit was present in excess was suggestive of the formation of three-chain units. The possibility of nonspecific aggregation was eliminated by isolation of stable three-chain alpha-helix-enriched particles from such IF. When tracer amounts of [35S]methionine-labeled decamin were mixed with desmin, labeled IFs were obtained under conditions in which homopolymer decamin IFs were soluble. These in vitro findings may be of physiological significance because native BHK-21 IF also had solubility properties similar to those of the copolymer IF.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borenfreund E., Schmid E., Bendich A., Franke W. W. Constitutive aggregates of intermediate-sized filaments of the vimentin and cytokeratin type in cultured hepatoma cells and their dispersal by butyrate. Exp Cell Res. 1980 May;127(1):215–235. doi: 10.1016/0014-4827(80)90428-0. [DOI] [PubMed] [Google Scholar]
- Bramhall S., Noack N., Wu M., Loewenberg J. R. A simple colorimetric method for determination of protein. Anal Biochem. 1969 Oct 1;31(1):146–148. doi: 10.1016/0003-2697(69)90251-6. [DOI] [PubMed] [Google Scholar]
- Cabral F., Gottesman M. M. Phosphorylation of the 10-nm filament protein from Chinese hamster ovary cells. J Biol Chem. 1979 Jul 25;254(14):6203–6206. [PubMed] [Google Scholar]
- Cabral F., Gottesman M. M., Zimmerman S. B., Steinert P. M. Intermediate filaments from Chinese hamster ovary cells contain a single protein. Comparison with more complex systems from baby hamster kidney and mouse epidermal cells. J Biol Chem. 1981 Feb 10;256(3):1428–1431. [PubMed] [Google Scholar]
- Cabral F., Willingham M. C., Gottesman M. M. Ultrastructural localization to 10 nm filaments of an insoluble 58K protein in cultured fibroblasts. J Histochem Cytochem. 1980 Jul;28(7):653–662. doi: 10.1177/28.7.7391554. [DOI] [PubMed] [Google Scholar]
- Day W. A., Gilbert D. S. X-ray diffraction pattern of axoplasm. Biochim Biophys Acta. 1972 Dec 28;285(2):503–506. doi: 10.1016/0005-2795(72)90342-x. [DOI] [PubMed] [Google Scholar]
- Douglas M., Finkelstein D., Butow R. A. Analysis of products of mitochondrial protein synthesis in yeast: genetic and biochemical aspects. Methods Enzymol. 1979;56:58–66. doi: 10.1016/0076-6879(79)56009-1. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Breitkreutz D., Lüder M., Boukamp P., Fusenig N. E., Osborn M., Weber K. Simultaneous expression of two different types of intermediate sized filaments in mouse keratinocytes proliferating in vitro. Differentiation. 1979;14(1-2):35–50. doi: 10.1111/j.1432-0436.1979.tb01010.x. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Osborn M., Weber K. Different intermediate-sized filaments distinguished by immunofluorescence microscopy. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5034–5038. doi: 10.1073/pnas.75.10.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Weber K., Osborn M. HeLa cells contain intermediate-sized filaments of the prekeratin type. Exp Cell Res. 1979 Jan;118(1):95–109. doi: 10.1016/0014-4827(79)90587-1. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Green H. The expression of keratin genes in epidermis and cultured epidermal cells. Cell. 1978 Nov;15(3):887–897. doi: 10.1016/0092-8674(78)90273-8. [DOI] [PubMed] [Google Scholar]
- Gard D. L., Bell P. B., Lazarides E. Coexistence of desmin and the fibroblastic intermediate filament subunit in muscle and nonmuscle cells: identification and comparative peptide analysis. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3894–3898. doi: 10.1073/pnas.76.8.3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard D. L., Lazarides E. The synthesis and distribution of desmin and vimentin during myogenesis in vitro. Cell. 1980 Jan;19(1):263–275. doi: 10.1016/0092-8674(80)90408-0. [DOI] [PubMed] [Google Scholar]
- Gilbert D. S., Newby B. J. Neurofilament disguise, destruction and discipline. Nature. 1975 Aug 14;256(5518):586–589. doi: 10.1038/256586a0. [DOI] [PubMed] [Google Scholar]
- Goldman R. D., Milsted A., Schloss J. A., Starger J., Yerna M. J. Cytoplasmic fibers in mammalian cells: cytoskeletal and contractile elements. Annu Rev Physiol. 1979;41:703–722. doi: 10.1146/annurev.ph.41.030179.003415. [DOI] [PubMed] [Google Scholar]
- Hearing V. J., Nicholson J. M., Montague P. M., Ekel T. M., Tomecki K. J. Mammalian tyrosinase. Structural and functional interraltionship of isozymes. Biochim Biophys Acta. 1978 Feb 10;522(2):327–339. doi: 10.1016/0005-2744(78)90067-0. [DOI] [PubMed] [Google Scholar]
- Henderson L. E., Oroszlan S., Konigsberg W. A micromethod for complete removal of dodecyl sulfate from proteins by ion-pair extraction. Anal Biochem. 1979 Feb;93(1):153–157. [PubMed] [Google Scholar]
- Huiatt T. W., Robson R. M., Arakawa N., Stromer M. H. Desmin from avian smooth muscle. Purification and partial characterization. J Biol Chem. 1980 Jul 25;255(14):6981–6989. [PubMed] [Google Scholar]
- Hynes R. O., Destree A. T. 10 nm filaments in normal and transformed cells. Cell. 1978 Jan;13(1):151–163. doi: 10.1016/0092-8674(78)90146-0. [DOI] [PubMed] [Google Scholar]
- Kemp D. J., Rogers G. E. Immunological and immuno-fluorescent studies on keratin of the hair follicle. J Cell Sci. 1970 Jul;7(1):273–283. doi: 10.1242/jcs.7.1.273. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Hubbard B. D. Immunological characterization of the subunit of the 100 A filaments from muscle cells. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4344–4348. doi: 10.1073/pnas.73.12.4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments as mechanical integrators of cellular space. Nature. 1980 Jan 17;283(5744):249–256. doi: 10.1038/283249a0. [DOI] [PubMed] [Google Scholar]
- MERCER E. H., ROGERS G. E., MUNGER B. L., ROTH S. I. A SUGGESTED NOMENCLATURE FOR FINE-STRUCTURAL COMPONENTS OF KERATIN AND KERATIN-LIKE PRODUCTS OF CELLS. Nature. 1964 Jan 25;201:367–368. doi: 10.1038/201367a0. [DOI] [PubMed] [Google Scholar]
- Osborn M., Franke W., Weber K. Direct demonstration of the presence of two immunologically distinct intermediate-sized filament systems in the same cell by double immunofluorescence microscopy. Vimentin and cytokeratin fibers in cultured epithelial cells. Exp Cell Res. 1980 Jan;125(1):37–46. doi: 10.1016/0014-4827(80)90186-x. [DOI] [PubMed] [Google Scholar]
- Rueger D. C., Huston J. S., Dahl D., Bignami A. Formation of 100 A filaments from purified glial fibrillary acidic protein in vitro. J Mol Biol. 1979 Nov 25;135(1):53–68. doi: 10.1016/0022-2836(79)90340-1. [DOI] [PubMed] [Google Scholar]
- Shih T. Y., Williams D. R., Weeks M. O., Maryak J. M., Vass W. C., Scolnick E. M. Comparison of the genomic organization of Kirsten and Harvey sarcoma viruses. J Virol. 1978 Jul;27(1):45–55. doi: 10.1128/jvi.27.1.45-55.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small J. V., Sobieszek A. Studies on the function and composition of the 10-NM(100-A) filaments of vertebrate smooth muscle. J Cell Sci. 1977 Feb;23:243–268. doi: 10.1242/jcs.23.1.243. [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Idler W. W., Goldman R. D. Intermediate filaments of baby hamster kidney (BHK-21) cells and bovine epidermal keratinocytes have similar ultrastructures and subunit domain structures. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4534–4538. doi: 10.1073/pnas.77.8.4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert P. M., Idler W. W. The polypeptide composition of bovine epidermal alpha-keratin. Biochem J. 1975 Dec;151(3):603–614. doi: 10.1042/bj1510603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert P. M., Idler W. W., Wantz M. L. Characterization of the keratin filament subunits unique to bovine snout epidermis. Biochem J. 1980 Jun 1;187(3):913–916. doi: 10.1042/bj1870913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert P. M., Idler W. W., Zimmerman S. B. Self-assembly of bovine epidermal keratin filaments in vitro. J Mol Biol. 1976 Dec 15;108(3):547–567. doi: 10.1016/s0022-2836(76)80136-2. [DOI] [PubMed] [Google Scholar]
- Steinert P. M. Structure of the three-chain unit of the bovine epidermal keratin filament. J Mol Biol. 1978 Jul 25;123(1):49–70. doi: 10.1016/0022-2836(78)90376-5. [DOI] [PubMed] [Google Scholar]
- Steinert P. M. The extraction and characterization of bovine epidermal alpha-keratin. Biochem J. 1975 Jul;149(1):39–48. doi: 10.1042/bj1490039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert P. M., Zimmerman S. B., Starger J. M., Goldman R. D. Ten-nanometer filaments of hamster BHK-21 cells and epidermal keratin filaments have similar structures. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6098–6101. doi: 10.1073/pnas.75.12.6098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T. T., Shih C., Green H. Keratin cytoskeletons in epithelial cells of internal organs. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2813–2817. doi: 10.1073/pnas.76.6.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuszynski G. P., Frank E. D., Damsky C. H., Buck C. A., Warren L. The detection of smooth muscle desmin-like protein in BHK21/C13 fibroblasts. J Biol Chem. 1979 Jul 10;254(13):6138–6143. [PubMed] [Google Scholar]
- Zackroff R. V., Goldman R. D. In vitro assembly of intermediate filaments from baby hamster kidney (BHK-21) cells. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6226–6230. doi: 10.1073/pnas.76.12.6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zackroff R. V., Goldman R. D. In vitro reassembly of squid brain intermediate filaments (neurofilaments): purification by assembly-disassembly. Science. 1980 Jun 6;208(4448):1152–1155. doi: 10.1126/science.7189605. [DOI] [PubMed] [Google Scholar]