Abstract
Treatment of patients with adoptive T cell therapy requires expansion of unique tumor-infiltrating lymphocyte (TIL) cultures from single cell suspensions processed from melanoma biopsies. Strategies which increase the expansion and reliability of TIL generation from tumor digests are necessary to improve access to TIL therapy. Prior work evaluated artificial antigen presenting cells (aAPCs) for their antigen-specific and costimulatory properties. We investigated engineered cells for co-stimulatory enhancement (ECCE) consisting of K562 cells which express 4-1BBL in the absence of artificial antigen stimulation. ECCE accelerated TIL expansion and significantly improved TIL numbers (p=0.001) from single cell melanoma suspensions. TIL generated with ECCE contain significantly more CD8+CD62L+ and CD8+CD27+ T cells then comparable IL-2-expanded TIL and maintained anti-tumor reactivity. Moreover, ECCE improved TIL expansion from non-melanoma cell suspensions similar to that seen with melanoma tumors. These data demonstrate that ECCE addition to TIL production will enable treatment of patients ineligible using current methods.
Keywords: Co-stimulation, tumor-infiltrating lymphocytes, adoptive immunotherapy
Introduction
Melanoma is the sixth leading cancer in both men and women (1). Metastatic melanoma has a poor prognosis with a five-year survival of less than 5%. FDA-approved treatments for metastatic melanoma include aldesleukin, ipilimumab, and dacarbazine chemotherapy. Aldesleukin has an objective clinical response rate of about 16% and a complete response rate of 6% (2). Ipilimumab was recently approved by the FDA based on its ability to increase overall survival in a large multicenter trial (3). Dacarbazine-based chemotherapy has a clinical response rate of up to 20% but few complete responders or long-term survivors (4). Treatment with tumor-infiltrating lymphocytes (TIL) is still an experimental approach yet highly effective for patients with advanced melanoma. Ninety-three patients with measurable metastatic melanoma were treated with autologous TIL following lymphopenic conditioning on three consecutive clinical trials with objective response rates reaching up to 72%. 20 of the 93 patients (22%) achieved complete tumor regressions and 19 have ongoing regressions beyond three years (5). Due to the personalized nature of TIL treatment, dissemination is limited by a lack of a standardized technique to rapidly generate TIL from every patient regardless of disease progression or histology.
In the initial summary of the treatment of melanoma patients with adoptive transfer of TIL in the absence of lymphodepletion, it was noted that patients treated with TIL from younger cultures had a higher frequency of clinical response to treatment than patients treated with TIL from older cultures (p = 0.0001) (6). More recent studies in patients receiving rapidly expanded TIL after lymphodepletion support these early observations (7). Additional experiments examining the role of telomere length of the TIL cultures in the 93 patients who received TIL support this hypothesis. TIL which persisted in vivo (p<0.001) were associated with objective clinical responses in treated patients and had longer telomere lengths than TIL that were not associated with clinical responses (p < 0.01) (8). Surface expression of CD27 and CD28 was associated with T cell proliferative and survival capacity and were used as markers of less-differentiated T cells. T cell clonotypes with long-term in vivo persistence expressed higher levels of CD27 and CD28 than clonotypes with short-term persistence (9). In a murine model of adoptive immunotherapy, the differentiation of effector T cells in culture had a negative effect on their capacity to function in vivo, and T cells expressing higher levels of CD27 and CD62L had superior in vivo antitumor activity than cells lacking CD27 and CD62L (10).
Other investigators have used artificial antigen presenting cells (aAPCs) comprised of K562 engineered to provide T cell receptor (TCR) and 4-1BB signaling to expand CD8 T cells (11–15). K562 cells express ICAM-1 (CD54), LFA-1 (CD58), and B7-H3 molecules (11, 12) which improve the interaction and stimulation of T cells (16, 17). An absence of endogenously expressed HLA molecules (12), with the possible exception of HLA-C, reduces the possibility of unintended allogeneic T cell responses. The tumor necrosis factor family member, 4-1BB (CD137), is a co-stimulatory molecule that is preferentially expressed on activated T cells (18). Engagement of 4-1BB inhibits activation-induced cell death (19) and enhances CD8 T cell survival and development of a memory phenotype (20–23). 4-1BB preferentially expand CD8+ T cells compared to CD4+ T cells (13, 15, 24–27), and impacts natural killer (NK) cell proliferation and function (28, 29). Murine models using agonistic antibodies have demonstrated the therapeutic effects of 4-1BB in the treatment of tumors (30–32).
Multiple T cell receptor stimulations drive T cell differentiation toward an exhausted terminal effector state associated with impaired anti-tumor function in mouse models (10, 33, 34). In an attempt to limit effector T cell differentiation yet apply an off-the-shelf product to improve TIL generation, we engineered K562 cells to deliver an agonistic 4-1BB signal in the absence of additional T cell receptor stimulation. These 4-1BBL (CD137L)-expressing K562 cells were designated engineered cells for costimulatory enhancement (ECCE) to distinguish them from other K562 aAPC systems which deliver a TCR signal together with costimulation. In this report we examined the contribution of ECCE to expand TIL for patient therapy. The impact on speed of TIL generation, reliability, and the nature of the T cell product was evaluated in TIL derived from melanoma and other cancer types.
Materials and Methods
Development of Engineered cells for costimulatory enhancement (ECCE)
PCR cassettes expressing either human Fc fragment of IgG, high-affinity Ia, receptor (FCGR1A)(CD64) (NM_000566), human tumor necrosis factor (ligand) superfamily, member 9 (TNFSF9)(CD137L)(4-1BBL)(NM_003811), or human CD80 molecule (CD80)(NM_005191) were cloned from a human cDNA library. These were ligated into the mouse stem cell virus-based splice-gag vector (MSGV1) (35) to create MSGV1-CD64, MSGV1-CD137L, and MSGV1-CD80 retroviral vector backbones. Transient retroviral supernatents were collected after co-transfection of HEK-293GP packaging cells (36) with the RD114 envelope protein (37) and either MSGV1-CD64, MSGV1-CD80, or MSGV1-CD137L. A single ECCE clone with strong surface expression of both CD64 and 4-1BBL was obtained after retroviral transduction and limiting dilution. A line of ECCE expressing CD80 (ECCE.CD80) was obtained after retroviral transduction. All ECCE lines were subjected to 100Gy of γ-irradiation prior to use. CD64 expression affords the opportunity to load ECCE with IgG as done by others (13, 15). In this report, CD64 was only used for identifying ECCE and not for decorating with antibodies including anti-CD3.
Generation of ECCE and standard TIL
Patients with metastatic disease underwent resection of a metastasis and the sample was processed to a single cell suspension for generation of minimally cultured ‘young’ TIL as previously described (38, 39). Briefly, tumor single cell suspensions were obtained by enzymatic digestion or mechanical dissociation in media containing collagenase and DNAse. TIL were expanded from single cell suspensions in complete media (CM: RPMI1640 with 10% human AB serum, 25 mM HEPES, 10 ug/mL gentamicin, and 5.5×10−5 M β-mercaptoethanol) and interleukin-2 (IL-2, Chiron). To generate ECCE TIL, 10-fold fewer ECCE were added to cultures when they were initiated. Media was replaced 5 days later with fresh CM supplemented with IL-2 and every 2–3 days thereafter. Growth positive TIL cultures were identified using an inverted light microscope and were characterized as achieving a single confluent layer of lymphocytes with simultaneous elimination of accompanying tumor, fibroblasts, and other stromal cells. The number of TIL was recorded periodically during the culture period and each pair of ECCE and standard TIL was harvested simultaneously.
Characterization of ECCE TIL and standard TIL
Cellular phenotypes were determined by flow cytometric analysis after staining with antibodies specific for CD3, CD4, CD8, CD56, CD62L, CD57, CD279, CD27 and CD28 (BD Pharmingen). Tumor-specific recognition was determined after overnight culture with autologous HLA-matched, or HLA-mismatched tumor cells at a ratio of 1:1. Supernatant from each coculture was then assayed for IFN-γ secretion by enzyme-linked immunosorbent assay (ELISA) (Peirce/Endogen) according to the manufacturer's recommendations.
Secondary rapid expansion (REP) of ECCE and standard ‘young’ TIL
Standard TIL and ECCE-generated TIL were rapidly expanded as previously described (38, 40). Briefly TIL were expanded in media containing IL-2 and anti-CD3 (Orthoclone OKT3, Ortho Biotech Products) with a 200-fold excess of γ-irradiated peripheral blood mononuclear cells (PBMC). Cultures were maintained for 14 days before harvest and the total number of TIL evaluated.
Statistics
Data were analyzed using paired two-tailed t tests. Statistically significant differences were judged as p values ≤0.05.
Results
Addition of ECCE improves the establishment of TIL cultures from melanoma tumor cell suspensions
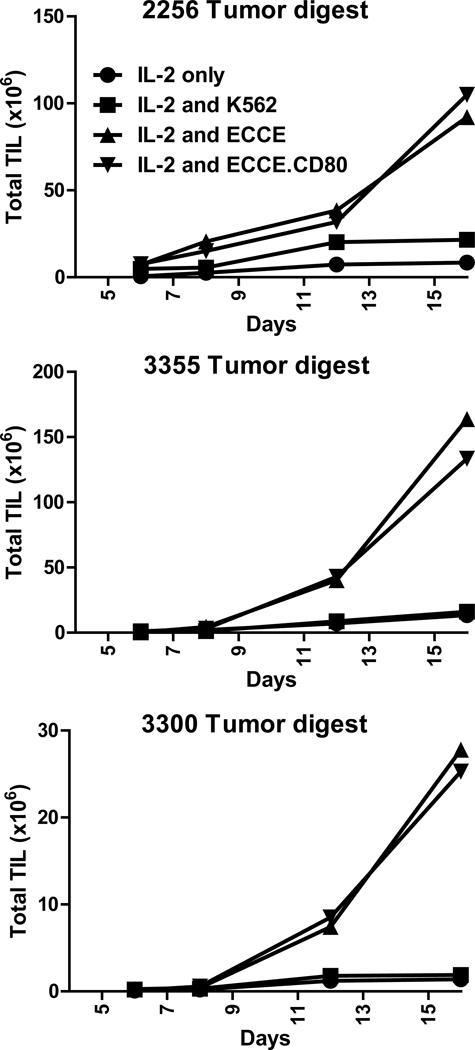
Tumor infiltrating lymphocytes fail to expand from some melanoma single cell suspensions even when plated in media containing 6000 IU/mL interleukin-2 (IL-2). Typically the tumors that fail to generate TIL cultures start with a low frequency of infiltrating lymphocytes (41). We initially examined if ECCE could enhance TIL generation from these melanoma tumors. Twenty-five melanoma tumor cell suspensions from which a TIL culture initially failed to grow were chosen for investigation. Cryopreserved tumors were thawed and TIL generation was examined in the presence or absence of ECCE. In these studies, TIL establishment was defined as the elimination of the adherent tumor cells within culture wells. This typically occurred concurrently with growth of lymphocytes to confluence in the well. When cultured with IL-2 alone, 9 of 25 tumor cultures (29%; Table 1) expanded enough TIL to achieve tumor clearance and confluent growth. The remaining cultures failed to expand or exhibited poor expansion. In contrast, 18 of the 25 same tumor cell suspensions (67%, p=0.02; Table 1) cultured with ECCE resulted in robust TIL expansion. Three representative examples are shown in Figure 1a which demonstrates that 3–5×106 tumor cells can produce 1.9–14.4 ×107 TIL in over 18 days of culture. TIL growth from the twenty-five tumor cell cultures containing only IL-2 resulted in 13.9±4.9×106 TIL in 11–24 days while the addition of ECCE produced 101.3±36×106 TIL in the same culture period.
Table 1.
ECCE significantly increase TIL production from melanoma single-cell suspensions
| Tumor digests1 | IL-2 Only2 | IL-2 and ECCE2 | Significance3 |
|---|---|---|---|
| Cryopreserved | 9/25 (29%) | 18/25 (67%) | p=0.02 |
| Fresh | 4/12 (33%) | 12/12 (100%) | p=0.001 |
| Total | 13/37 (30%) | 30/37 (79%) | p=0.0001 |
Melanoma single-cell suspensions were either cultured immediately after dissociation (fresh) or after thaw from cryopreservation (cryopreserved). All cultures were performed in parallel and for the identical amount of time. Data shown is the number of cultures which expanded TIL per the total number of cultures initiated.
Cultures initiated in 24-well plate in 6000 IU/mL IL-2 with or without 105 ECCE/well
p values from a paired students t test evaluating the effect of ECCE of TIL growth
Figure 1. ECCE significantly improve TIL production from melanoma single-cell suspensions.
3–5×106 viable cells from cryopreserved (A) or freshly prepared (B) tumor single-cell suspensions were cultured in CM supplemented with IL-2 in the presence or absence of ECCE. Total viable TIL were evaluated throughout the culture. (C) The time required to achieve confluent lymphocyte growth (see material and methods) was determined.
The effect of ECCE on TIL culture initiated from fresh tumor cell suspensions, without cryopreservation, was evaluated next. Tumors with a low frequency of lymphocytes (1 – 24% of tumor cell suspensions) were again selected for analysis. When cultured in media containing only IL-2, 4 of 12 fresh cultures (33%, Table 1) produced TIL cultures while all 12 of the same digests generated TIL when ECCE were added (100%, Table 1). Standard TIL cultures (in IL-2 only) resulted in 4.9±1.7×106 TIL in 12–24 days, while ECCE TIL cultures resulted in 91.1±31.2×106 lymphocytes over the same culture period. Representative examples of TIL expansion from freshly prepared tumor cell suspensions are shown in Figure 1b. Overall, these results indicated that the addition of ECCE significantly increased TIL generation from 37 examined cryopreserved and freshly prepared melanoma cell suspensions selected based on low lymphocyte infiltration (Table 1, p=0.001).
We next extended the analysis of ECCE to tumors with substantial lymphocytic infiltrates, which typically generate TIL cultures with IL-2 only. 3–6×106 cells from eleven melanoma tumors containing 24–93% lymphocytes were initiated in the presence and absence of ECCE. Tumor cell suspensions were cultured at 106/well in 24-well plates and the earliest time when lymphocyte cultures became confluent was recorded. Melanoma cell suspensions cultured with ECCE reached TIL confluence significantly faster compared to IL-2 alone (Figure 1c, p=0.0002). These results indicate that ECCE significantly accelerated TIL growth from melanoma cell suspensions containing substantial TIL infiltration.
4-1BBL expression by ECCE is necessary for augmented TIL production
ECCE endogenously express ICAM-1 (CD54) and LFA-1 (CD58) (12), involved in T cell adhesion and co-stimulation (16, 17), as well as the transfected costimulatory ligand, 4-1BBL (CD137L). We investigated if the expression of 4-1BBL was necessary for improving TIL generation. Nine cryopreserved and four freshly prepared melanoma cell suspensions, all with low lymphocytic infiltration associated with poor TIL production, were selected for analysis. Tumor cell suspensions cultured with ECCE produced TIL in 13 of 13 cultures (100%, Table 2). Representative examples seen in figure 2 demonstrated that 2–20 ×107 TIL can be obtained over 16 days of culture. In contrast, only 3 of 13 (23%) of the same tumors generated TIL cultures with K562 cells lacking 4-1BBL (p=0.0001; Table 2). Thus, 4-1BBL expression on the ECCE was required for improving TIL production.
Table 2.
4-1BBL expression by ECCE is necessary for augmented TIL production
| Tumor digests1 | IL-2 and K5622 | IL-2 and ECCE3 | Significance4 |
|---|---|---|---|
| Cryopreserved | 3/9 (33%) | 9/9 (100%) | p=0.009 |
| Fresh | 0/4 (0%) | 4/4 (100%) | p=0.03 |
| Total | 3/13 (23%) | 13/13 (100%) | p=0.0001 |
Melanoma single-cell suspensions were either cultured immediately after dissociation (fresh) or after thaw from cryopreservation (cryopreserved). All cultures were performed in parallel and for the identical amount of time. Data shown is the number of cultures which expanded TIL per the total number of cultures initiated.
Cultures initiated in 24-well plate in 6000 IU/mL IL-2 with 105 K562/well
Cultures initiated in 24-well plate in 6000 IU/mL IL-2 with 105 ECCE/well
p values from a paired students t test evaluating the effect of ECCE of TIL growth
Figure 2. 4-1BBL but not CD80 expression is necessary for augmented TIL production.
3–5×106 viable cells from cryopreserved tumor single-cell suspensions were cultured in CM supplemented with IL-2 only, ECCE, K562 cells or ECCE transduced to express CD80 (ECCE.CD80). Total viable TIL were evaluated throughout the culture.
Co-stimulatory molecules can synergize to deliver an agonistic effect on T cell proliferation and survival. In particular, CD28 stimulation can synergize with 4-1BB signaling to enhance CD8 immunity in some systems (42, 43). To determine if CD28 signaling synergizes with 4-1BB to enhance TIL production from melanoma cell suspensions, ECCE were transduced with CD80, the CD28 ligand to generate the line, ECCE.CD80. TIL expansion from thirteen tumor cell suspensions with limited lymphocytic infiltration was evaluated with ECCE or ECCE.CD80. Examples of TIL expansion are shown in Figure 2. The kinetics of TIL expansion was not affected by CD80 expression in these cultures. Overall, CD80 expression did not either significantly improve or impair TIL production from melanoma cell suspensions (Table 3). Taken together, CD80 expression did not augment ECCE TIL generation.
Table 3.
CD80 expression does not impair TIL production
| Tumor digests1 | IL-2 and ECCE2 | IL-2 and ECCE.803 |
|---|---|---|
| Cryopreserved | 9/9 (100%) | 9/9 (100%) |
| Fresh | 4/4 (100%) | 4/4 (100%) |
| Total | 13/13 (100%) | 13/13 (100%) |
Melanoma single-cell suspensions were either cultured immediately after dissociation (fresh) or after thaw from cryopreservation (cryopreserved). All cultures were performed in parallel and for the identical amount of time. Data shown is the number of cultures which expanded TIL per the total number of cultures initiated.
Cultures initiated in 24-well plate in 6000 IU/mL IL-2 with 105 ECCE/well
Cultures initiated in 24-well plate in 6000 IU/mL IL-2 with 105 ECCE.80/well
ECCE increase the natural killer and CD8 composition in TIL
The effect of ECCE on the CD4+, CD8+ and natural killer (NK) cellular composition of TIL cultures was examined next. 31 pairs of age-matched TIL cultures generated with or without ECCE were compared by flow cytometry at the end of culture. The addition of ECCE significantly increased the frequency of CD8+ T cells (Figure 3b, p<0.0001). CD4+ T cells were almost eliminated in some ECCE-initiated cultures compared to standard IL-2 TIL cultures. A dramatic example demonstrating the precipitous drop in CD4+ T cell frequency is shown in Figure 3a (right panel). In this example, IL-2-cultured TIL contained 62.4% CD4+ T cells compared to 2.1% CD4+ T cells when expanded with ECCE. The frequency of NK cells in TIL was highly variable between patients and between cultures from a single patient. The addition of ECCE to tumor cell suspensions increased the frequency of NK cells compared to TIL grown in IL-2 only (p=0.01, Figure 3b).
Figure 3. ECCE enhance CD8+ T cell and natural killer cell expansion.
(A) Age-matched TIL expanded with or without ECCE were evaluated for the frequency of CD3 and CD56 to determine natural killer (NK) cell frequency (left) or CD4+ and CD8+ T cells (right) by flow cytometry. (B) Pooled results from independent experiments showing frequency of CD3−CD56+ NK cells (n=27, left) or CD8+ of CD3+ T cells (n=31, right).
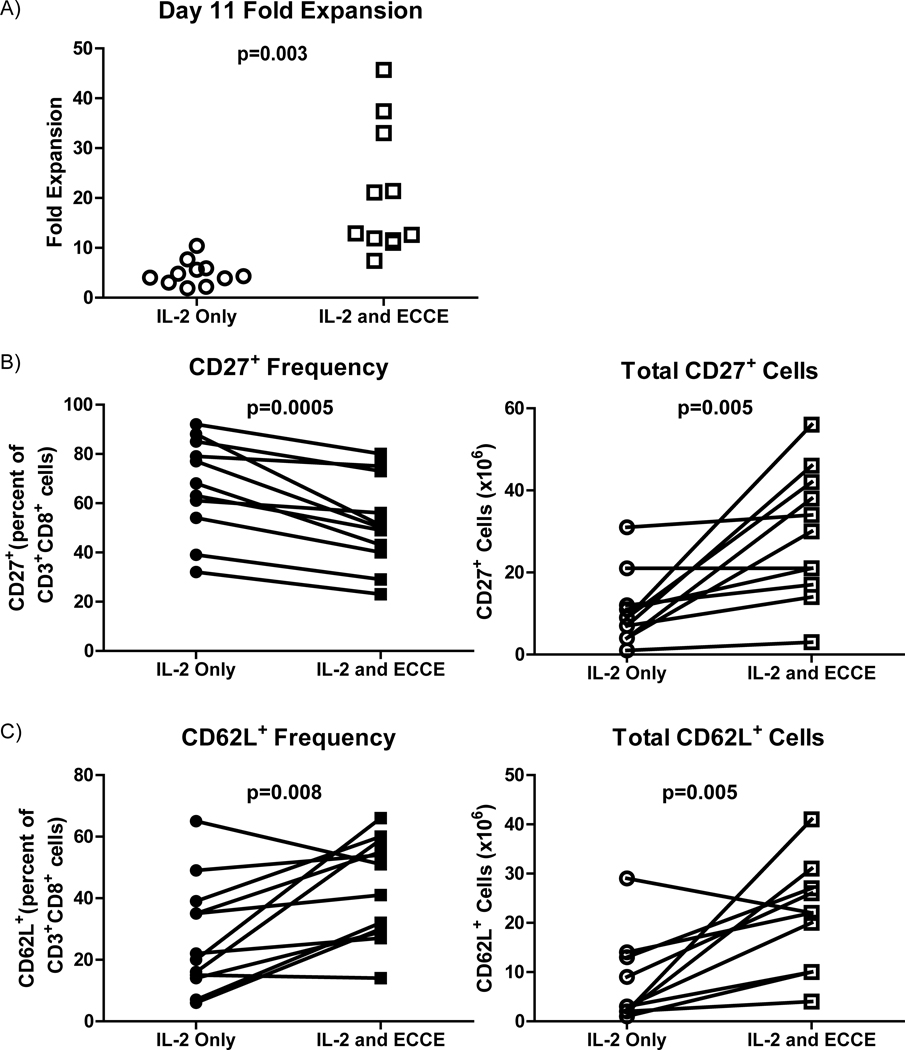
The effect of ECCE-driven expansion on TIL phenotype was examined next. TIL from eleven tumor cell suspensions expanded with and without ECCE for eleven days were compared. In all pairs, ECCE TIL had expanded to significantly greater total cell numbers than standard IL-2 TIL cultures (p=0.003, Figure 4a). Culture of TIL in media containing IL-2 reduces the expression of CD27 (9). Thus, TIL were rested for 48 hours prior to analysis in media lacking IL-2 to mitigate this effect. TIL were analyzed with flow cytometry by gating on CD8+ cells and evaluating surface expression of CD27 and CD62L. ECCE-cultured CD8+ TIL exhibited significantly reduced CD27 expression compared to IL-2-cultured TIL (p=0.0005, Figure 4b). Despite a 1.4-fold reduction in CD27 expression, the 4.2-fold increase in TIL expansion resulted in a net gain of CD8+CD27+ lymphocytes in the ECCE TIL culture (p=0.005, Figure 4b). CD28 expression was variable between TIL cultures but no consistent change between ECCE and standard TIL was observed (p=0.3, data not shown). CD62L expression on ECCE and IL-2-expanded CD8 TIL was examined next. After 48-hour IL-2 withdrawal, ECCE-expanded TIL exhibited a significantly higher frequency and total number than IL-2-expanded TIL (p=0.008 and p=0.005, respectively, Figure 4c). Similar trends were obtained for CD27 frequency and CD62L frequency without prior IL-2 withdrawal (data not shown).
Figure 4. ECCE increase the absolute number of CD27+ and CD62L+ CD8+ T cells.
(A) Fold expansion of TIL expanded with or without ECCE in media containing IL-2 for eleven days (n=11). Frequency (left, closed symbols) and total number (right, open symbols) of CD3+CD8+CD27+ (B) and CD3+CD8+CD62L+ (C) cells after 48 hours in media lacking IL-2.
ECCE-expanded TIL recognize tumor and lack evidence of proliferative exhaustion
We next compared the tumor recognition exhibited by ECCE-expanded TIL and standard TIL. Samples were chosen for this experiment if both cryopreserved melanoma cell suspensions and an autologous tumor cell line were available. TIL were generated with ECCE and with IL-2 only; ECCE TIL expanded faster and grew to a greater numbers of cells than standard TIL (not shown), but matched pairs of TIL were tested for anti-tumor function at the same age. TIL were tested for specific tumor recognition by cytokine secretion after overnight coculture with autologous and HLA-mismatched tumors (see Materials and Methods). Both standard and ECCE TIL from all five tumor digests exhibited tumor-specific recognition (Table 4). ECCE-generated TIL did not significantly differ in the amount of IFN-γ secreted compared to cultures generated solely with IL-2 (p=0.2).
Table 4.
ECCE-generated TIL exhibit tumor recognition
| TIL | HLA-A | No Tumor Line |
HLA Mismatched Tumor Line |
Autologous Tumor Line |
|||
|---|---|---|---|---|---|---|---|
| None | ECCE | None | ECCE | None | ECCE | ||
| IFN-γ (pg/mL) | |||||||
| 3018 | 02,33 | 5 | 0 | 26 | 35 | 217 | 502 |
| 3289 | 29,32 | 3 | 3 | 55 | 58 | 789 | 410 |
| 3282 | 02,24 | 2 | 3 | 7 | 19 | 341 | 363 |
| 3084 | 02,24 | 0 | 0 | 0 | 12 | 1760 | 6040 |
| 3281 | 03,11 | 8 | 15 | 0 | 0 | 522 | 2290 |
TIL grown in 6000 IU/mL IL-2 with or without ECCE were co-cultured with the same HLA-mismatched allogeneic and autologous tumor cell line. Data shown is the amount of IFN-γ secreted after 24-hours.
Values underlined indicate tumor-specific reactivity identified as greater than 200pg/mL IFN-γ release and twice that to HLA-mismatched tumor line
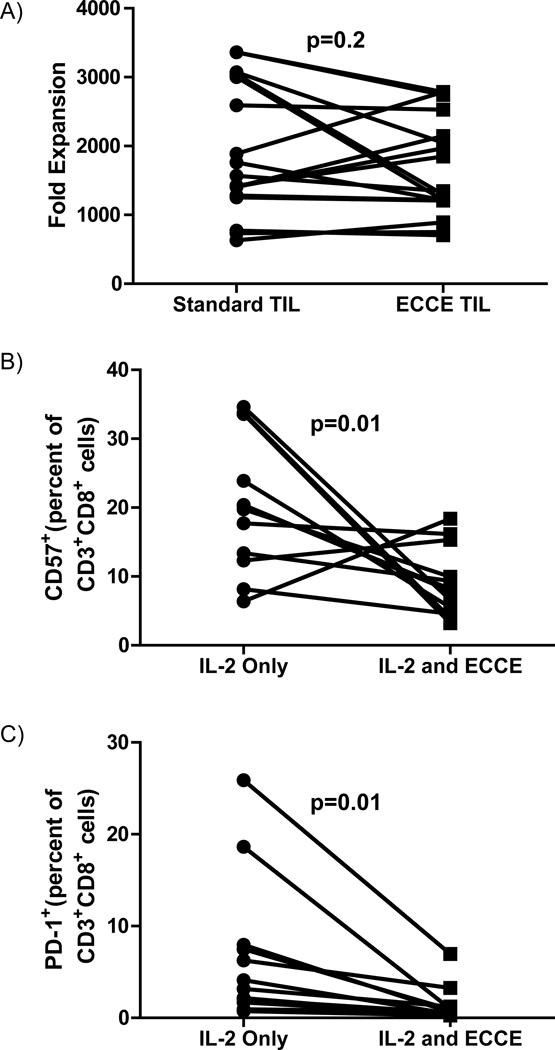
To test whether ECCE-expanded TIL exhibited signs of terminal differentiation and proliferative exhaustion, seventeen ECCE TIL were compared to matched standard TIL in a rapid expansion protocol (REP). The REP uses anti-CD3 antibody, IL-2, and irradiated peripheral blood mononuclear feeder cells to drive lymphocyte proliferation. ECCE TIL reached 700–2800 fold expansion in 14 days in the REP, and expanded equivalently to standard TIL (p=0.2, Figure 5a). TIL expansion in a REP is not affected by the initial frequency of NK cells. The cellular composition after a REP is greater than 99% T lymphocytes and there is no correlation between initial NK frequency and fold expansion (data not shown). This suggested that despite substantially increased numerical expansion prior to the REP (16.5±3.2×106 standard TIL versus 82.4±14.5×106 ECCE TIL, p=0.0001), ECCE TIL were not proliferatively exhausted compared to standard TIL from the same tumors.
Figure 5. ECCE-generated TIL vigorously expand with rapid expansion protocol (REP).
(A) Standard and ECCE-generated TIL were rapidly expanded with irradiated peripheral blood mononuclear feeder cells in media containing IL-2 and anti-CD3 (See materials and methods). Pooled results from independent experiments (n=17). Age-matched standard and ECCE-generated TIL were evaluated for frequency of (B) CD57+ or (C) CD279 (PD-1) after gating on CD3+CD8+.
To further test for terminal differentiation, expression of CD57 and CD279 (PD-1) were examined on ECCE and standard TIL. Expression of CD57 and PD-1 on CD8 T cells are associated with terminal differentiation and functional exhaustion (44, 45). Twelve pairs of ECCE and standard TIL were evaluated by flow cytometry after gating on the CD8 T cells. The ECCE-expanded TIL achieved significantly greater fold expansion (6.1±1.4 vs 33.2±13.1, p=0.05) and had a significantly greater composition of CD8 T cells (49.7±6.5 vs 76.4±5.0, p=0.0005). While there was substantial variability between TIL cultures, a paired analysis revealed the addition of ECCE significantly reduced the frequency of CD57 (p=0.01, Figure 5b) and PD-1 (p=0.01, Figure 5c) expression.
ECCE generate TIL from multiple disease histologies
The encouraging results from melanoma clinical trials with TIL have not been seen in other disease histologies. A major hurdle has been a lack of reliable generation of TIL cultures in sufficient numbers from non-melanoma tumors. We examined if the addition of ECCE to tumor cell suspensions from tumor histologies other than melanoma would result in enhanced TIL production from these tumors. Single cell enzymatic digests were generated from resected deposits of metastatic colorectal carcinoma, lung adenocarcinoma, neuroendocrine tumor, clear cell renal carcinomas, synovial sarcomas, or mesothelioma, and cultured in the presence or absence of ECCE. Cultures were monitored for lymphocyte expansion over time, and after 14 days the TIL were evaluated for cellular composition and phenotype. One sample, from a parotid adenocarcinoma which started with no detectable lymphocytic infiltrate in the single cell tumor digest, failed to generate TIL under either condition. Other samples, initiated with between 0.01 and 0.3 × 106 lymphocytes per well, expanded TIL cultures preferentially with ECCE (Figure 6a). In nine of 11 ECCE TIL samples and 3 of 11 standard TIL samples (p=0.03) TIL expanded more than 5 fold – a level considered clinically relevant - only with ECCE but not under standard conditions. The cellular composition of 8 pairs of non-melanoma TIL samples was compared (Figure 6b–e). The quality as well as the quantity of ECCE TIL was better than standard TIL from most tumor digests. Visual inspection of standard IL-2 cultures with minimal growth typically revealed mixed cell populations with remnant tumor cells interspersed with granular pleomorphic cells, fibroblasts, and debris, as well as lymphocytes. Rapidly growing ECCE TIL cultures appeared relatively homogeneous with predominantly or exclusively activated lymphocytes. A semi-quantitative measurement of this observation was obtained with the application of standard gating in the FACS analysis. A standard forward scatter by side scatter (FSC × SSC) gate for lymphocytes captured a significantly higher percent of cells at day 14 of culture from ECCE cultures than from standard TIL cultures (Figure 6b, p=0.003). The composition of the lymphocyte populations in standard TIL and ECCE TIL from non-melanoma tumors appeared significantly different. As with melanoma, the fraction of CD4+ cells was significantly reduced (p=0.002, Figure 6e) and the fraction of NK cells was significantly increased in ECCE TIL from non-melanoma tumors compared with standard TIL (p=0.008, Figure 6c). The fraction of CD8 cells was highly variable between tumors and between samples, but there was no consistent trend in the direction of variation.
Figure 6. ECCE and IL-2 expand TIL from non-melanoma tumor single-cell suspension.
(A) Fold expansion was examined after a 14 day culture of 11 non-melanoma tumor digests with and without ECCE. (B–E) Flow cytometric analysis of expanded TIL: (B) Percentage of cells contained in forward-scatter versus side-scatter lymphocyte gate, (C) CD3−CD56+ NK cells, (D) CD3+CD8+ cells, and (E) CD3+CD4+cells.
Discussion
Obtaining tumor reactive T cells is required for adoptive T cell therapy of cancer. Lymphocytic infiltrates from tumors have been a valuable source of tumor reactive T cells that can mediate therapeutic responses when infused into patients. Procuring tumor-infiltrating lymphocytes (TIL) for treatment requires establishing unique cultures derived from the patient’s tumor resection. Due to the personalized nature of the treatment, increasing access to TIL therapy requires improvement in speed and reliability of TIL generation. We demonstrated that provision of ECCE significantly augments TIL production from tumor cell suspensions including cultures which did not generate TIL with IL-2 alone. The augmented TIL production and accelerated TIL expansion were mediated by 4-1BBL expression on ECCE. Despite the augmented expansion, ECCE-expanded TIL contained a higher number of CD27+ and CD62L+-CD8+ T cells, markers associated with T cells prior to extensive cell division and differentiation toward terminal exhaustion. These observations are consistent with the reported effects of 4-1BB signaling to increase T cell survival (15, 20, 23, 46).
ECCE are the latest addition to iterative advancements in over two decades of producing TIL for treating patients. A retrospective analysis of patient treatment records found that between 1997 and 2002 only 27 percent of patients who underwent tumor resection with the intent to treat with TIL therapy were actually administered a TIL product (107/402 patients) (47). Stringent eligibility requirements dictated that only tumor reactive TIL cultures were selected for use in treatment. Patients became ineligible for TIL therapy when none were available. Elimination of the prohibitive selection criteria resulted in an increased number of patients eligible to receive TIL. From 2007 to 2009, 122 consecutive patients were resected with intent to treat; 43 percent (53/122) patients were infused with unselected TIL (41). 21 patients (17%) failed to generate adequate TIL for therapy. Interrogation of these samples demonstrated that patients whose TIL did not grow in vitro had a low level of lymphocytic infiltration in their tumors, with a median of only 8% lymphocytes in the initial single cell suspension. A similar rate - 89% of patients (24/27) generated sufficient TIL from infusion - has been reported by other investigators (48). We found that provision of ECCE to TIL generation yielded tumor-reactive T cell cultures from tumors digests. When ECCE was added to previously unproductive, cryopreserved tumor digests or fresh, non-cryopreserved digests selected based on poor lymphocytic infiltration, robust and reliable expansion was observed compared to IL-2 alone.
Another source of attrition involves patients for whom TIL were obtained but therapy was not administered due to disease progression or protocol specific ineligibilities subsequent to tumor resection. This category included 62 of 386 patients (16%) set up for selected TIL through 2007, and 20 of the 122 patients (16%) patients resected for generation of unselected TIL between 2007 and 2009. The provision of ECCE reduced the requisite culture time for TIL generation potentially facilitating treatment of patients with rapidly progressing disease. Moreover, shorter in vitro TIL culture was associated with tumor regression in multiple independent clinical trials examining the response of patients treated with unselected TIL (6, 41, 48). Besser et al found that all responding patients had TIL cultures of less than 20 days prior to initiation of rapid expansion protocols using anti-CD3 and irradiated PBMC feeders (48). These observations indicated that ECCE could enhance the accrual to TIL trials and accelerate the production of patient therapies potentially generating TIL with increased therapeutic efficacy.
Tumor antigen-specific CD8+ T cells expanded from peripheral blood using 4-1BB and TCR signaling provided by aAPCs were shown to be safe and capable of in vivo persistence when administered to advanced melanoma patients (49). However, in this study 8 of 9 patients had only two-fold increase in tumor antigen-specific cells after two months in the peripheral blood. The repetitive stimulations used in this and other studies with aAPCs may compromise in vivo persistence. Multiple T cell receptor stimulations will eventually induce a state of terminal differentiation characterized by proliferative exhaustion, loss of telomeres, and reduced surface expression of CD27 and CD28. These qualities have been associated in mouse models with poor functionality and hampered capacity for tumor regression (10). Tumor-reactive T cells can be expanded from TIL in IL-2 without further T cell sensitization methods. We hypothesized that pro-survival 4-1BB signaling in the absence of additional TCR stimulation would mediate T cell expansion with minimal differentiation and potentially result in enhanced persistence when infused into patients. Indeed, TIL with prolonged in vivo persistence and enhanced therapeutic efficacy in patients express CD27 (9) and contain longer telomeres (8). 4-1BBL signaling delivered in “trans” was demonstrated to cause improved T cell proliferation in a mouse model when transduced into T cells (43). This current report represents the first use of 4-1BB signaling delivered in “trans” to human lymphocytes for potential tumor therapy without additional TCR or anti-CD3 stimulation. Two strategies were employed to examine the function of ECCE-expanded TIL. First by evaluating markers associated with T cell differentiation we found that ECCE-expanded cultures contained a higher number of CD27+ and CD62L+ TIL suggesting that they contain less differentiated T cells despite the profound expansion. A reduction in CD57 and PD-1 expression compared to standard TIL further suggests that ECCE TIL have not reached a state of terminal differentiation or proliferative exhaustion. Moreover, preliminary data indicates that ECCE maintain TIL telomeres compared to standard TIL cultures (data not shown). To corroborate these observations, standard and ECCE-expanded TIL were evaluated for expansion after an additional TCR stimulation. Both exhibited equivalent expansion suggesting that ECCE TIL have not progressed farther towards proliferative exhaustion compared to standard TIL. When these data are considered together it suggests that ECCE significantly improve TIL generation without causing terminal differentiation.
This report represents a detailed examination of 4-1BBL stimulation for human lymphocytes derived from solid tissue. Others have demonstrated that aAPCs expressing 4-1BBL together with TCR stimulation promotes CD8 and NK expansion from peripheral blood (11, 15, 50) or lymph nodes (51). However, solid tissues contain a substantially different progenitor population than peripheral blood. Examination of ECCE impact on TIL revealed a potential to skew and manipulate the composition of the expanded lymphocytes. ECCE-generated TIL contained a higher frequency of CD8+ T cells and NK cells compared to culture with IL-2 alone. We have reported CD8-enriched TIL are sufficient to mediate tumor regression in patients (41). However, enrichment of CD8 TIL for patient treatment is an expensive and time-consuming prospect. ECCE-enhanced CD8 TIL expansion could obviate an enrichment step to obtain therapeutic CD8 TIL. Augmented CD8 expansion was also accompanied by an elevated frequency of natural killer (NK) cells. Yet the NK composition in the TIL prior to the REP did not affect the number of cells acquired after the REP. NK cells exhibit poor expansion compared to the T cells during an anti-CD3-driven REP. However, if NK cells are suspected to cause T cell expansion or clinical complications, an enrichment for CD8 T cells remains possible (7). The enhanced CD8 TIL expansion observed with ECCE is dependent on 4-1BBL expression. Attempts to improve expansion with CD80 co-expression were not successful. Co-expression of other co-stimulatory or cytokine molecules may influence the cell product generated. Using K562 cells as an ancillary product to access and manipulate T cell therapies has been suggested by others (13, 52) and the data expanding TIL with ECCE support this notion.
These data demonstrated that ECCE increase the speed and reliability of TIL generation from tumor digests and could facilitate treatment of patients ineligible with current TIL production methods. This includes patients with disease histologies other than melanoma. ECCE TIL can be tumor reactive and contain significantly more lymphocytes expressing markers associated with therapeutic efficacy. The anti-tumor effect can be evaluated in a clinical trial using a cGMP compliant ECCE to expand TIL for patient treatment and these data support efforts to engineer and validate the clinical use of ECCE.
Acknowledgements
The authors would like to thank the Surgery Branch TIL lab for all of their contributions and helpful discussions. We also thank Drs Carl June and Bruce Levine for their help with initial protocols and reagents.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: All authors have declared there are no financial conflicts of interest in regards to this work.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Atkins MB, Kunkel L, Sznol M, Rosenberg SA. High-dose recombinant interleukin-2 therapy in patients with metastatic melanoma: long-term survival update. Cancer J Sci Am. 2000;6 Suppl 1:S11–S14. [PubMed] [Google Scholar]
- 3.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gogas HJ, Kirkwood JM, Sondak VK. Chemotherapy for metastatic melanoma: time for a change? Cancer. 2007;109:455–464. doi: 10.1002/cncr.22427. [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg SA, Yang JC, Sherry RM, et al. Durable Complete Responses in Heavily Pretreted Patients with Metastatic Melanoma Using T Cell Transfer Immunotherapy. Clin Cancer Res. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenberg SA, Yannelli JR, Yang JC, et al. Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. J Natl Cancer Inst. 1994;86:1159–1166. doi: 10.1093/jnci/86.15.1159. [DOI] [PubMed] [Google Scholar]
- 7.Prieto PA, Durflinger KH, Wunderlich JR, Rosenberg SA, Dudley ME. Enrichment of CD8+ cells from melanoma tumor-infiltrating lymphocyte cultures reveals tumor reactivity for use in adoptive cell therapy. J Immunother. 33:547–556. doi: 10.1097/CJI.0b013e3181d367bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou J, Shen X, Huang J, Hodes RJ, Rosenberg SA, Robbins PF. Telomere length of transferred lymphocytes correlates with in vivo persistence and tumor regression in melanoma patients receiving cell transfer therapy. J Immunol. 2005;175:7046–7052. doi: 10.4049/jimmunol.175.10.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang J, Kerstann KW, Ahmadzadeh M, et al. Modulation by IL-2 of CD70 and CD27 expression on CD8+ T cells: importance for the therapeutic effectiveness of cell transfer immunotherapy. J Immunol. 2006;176:7726–7735. doi: 10.4049/jimmunol.176.12.7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gattinoni L, Klebanoff CA, Palmer DC, et al. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115:1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butler MO, Lee JS, Ansen S, et al. Long-lived antitumor CD8+ lymphocytes for adoptive therapy generated using an artificial antigen-presenting cell. Clin Cancer Res. 2007;13:1857–1867. doi: 10.1158/1078-0432.CCR-06-1905. [DOI] [PubMed] [Google Scholar]
- 12.Maus MV, Thomas AK, Leonard DG, et al. Ex vivo expansion of polyclonal and antigen-specific cytotoxic T lymphocytes by artificial APCs expressing ligands for the T-cell receptor, CD28 and 4-1BB. Nat Biotechnol. 2002;20:143–148. doi: 10.1038/nbt0202-143. [DOI] [PubMed] [Google Scholar]
- 13.Suhoski MM, Golovina TN, Aqui NA, et al. Engineering artificial antigen-presenting cells to express a diverse array of co-stimulatory molecules. Mol Ther. 2007;15:981–988. doi: 10.1038/mt.sj.6300134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas AK, Maus MV, Shalaby WS, June CH, Riley JL. A cell-based artificial antigen-presenting cell coated with anti-CD3 and CD28 antibodies enables rapid expansion and long-term growth of CD4 T lymphocytes. Clin Immunol. 2002;105:259–272. doi: 10.1006/clim.2002.5277. [DOI] [PubMed] [Google Scholar]
- 15.Zhang H, Snyder KM, Suhoski MM, et al. 4-1BB is superior to CD28 costimulation for generating CD8+ cytotoxic lymphocytes for adoptive immunotherapy. J Immunol. 2007;179:4910–4918. doi: 10.4049/jimmunol.179.7.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaw AS, Dustin ML. Making the T cell receptor go the distance: a topological view of T cell activation. Immunity. 1997;6:361–369. doi: 10.1016/s1074-7613(00)80279-4. [DOI] [PubMed] [Google Scholar]
- 17.Watts TH, DeBenedette MA. T cell co-stimulatory molecules other than CD28. Curr Opin Immunol. 1999;11:286–293. doi: 10.1016/s0952-7915(99)80046-6. [DOI] [PubMed] [Google Scholar]
- 18.Vinay DS, Kwon BS. Role of 4-1BB in immune responses. Semin Immunol. 1998;10:481–489. doi: 10.1006/smim.1998.0157. [DOI] [PubMed] [Google Scholar]
- 19.Hurtado JC, Kim YJ, Kwon BS. Signals through 4-1BB are costimulatory to previously activated splenic T cells and inhibit activation-induced cell death. J Immunol. 1997;158:2600–2609. [PubMed] [Google Scholar]
- 20.Lee HW, Park SJ, Choi BK, Kim HH, Nam KO, Kwon BS. 4-1BB promotes the survival of CD8+ T lymphocytes by increasing expression of Bcl-xL and Bfl-1. J Immunol. 2002;169:4882–4888. doi: 10.4049/jimmunol.169.9.4882. [DOI] [PubMed] [Google Scholar]
- 21.Lin GH, Sedgmen BJ, Moraes TJ, Snell LM, Topham DJ, Watts TH. Endogenous 4-1BB ligand plays a critical role in protection from influenza-induced disease. J Immunol. 2009;182:934–947. doi: 10.4049/jimmunol.182.2.934. [DOI] [PubMed] [Google Scholar]
- 22.Pulle G, Vidric M, Watts TH. IL-15-dependent induction of 4-1BB promotes antigen-independent CD8 memory T cell survival. J Immunol. 2006;176:2739–2748. doi: 10.4049/jimmunol.176.5.2739. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi C, Mittler RS, Vella AT. Cutting edge: 4-1BB is a bona fide CD8 T cell survival signal. J Immunol. 1999;162:5037–5040. [PubMed] [Google Scholar]
- 24.Shuford WW, Klussman K, Tritchler DD, et al. 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J Exp Med. 1997;186:47–55. doi: 10.1084/jem.186.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu Y, Zhu G, Luo L, Flies AS, Chen L. CD137 stimulation delivers an antigen-independent growth signal for T lymphocytes with memory phenotype. Blood. 2007;109:4882–4889. doi: 10.1182/blood-2006-10-043463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bukczynski J, Wen T, Ellefsen K, Gauldie J, Watts TH. Costimulatory ligand 4-1BBL (CD137L) as an efficient adjuvant for human antiviral cytotoxic T cell responses. Proc Natl Acad Sci U S A. 2004;101:1291–1296. doi: 10.1073/pnas.0306567101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin GH, Liu Y, Ambagala T, Kwon BS, Ohashi PS, Watts TH. Evaluating the cellular targets of anti-4-1BB agonist antibody during immunotherapy of a pre-established tumor in mice. PLoS One. 5:e11003. doi: 10.1371/journal.pone.0011003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imai C, Iwamoto S, Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood. 2005;106:376–383. doi: 10.1182/blood-2004-12-4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Melero I, Johnston JV, Shufford WW, Mittler RS, Chen L. NK1.1 cells express 4-1BB (CDw137) costimulatory molecule and are required for tumor immunity elicited by anti-4-1BB monoclonal antibodies. Cell Immunol. 1998;190:167–172. doi: 10.1006/cimm.1998.1396. [DOI] [PubMed] [Google Scholar]
- 30.Melero I, Shuford WW, Newby SA, et al. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat Med. 1997;3:682–685. doi: 10.1038/nm0697-682. [DOI] [PubMed] [Google Scholar]
- 31.Miller RE, Jones J, Le T, et al. 4-1BB-specific monoclonal antibody promotes the generation of tumor-specific immune responses by direct activation of CD8 T cells in a CD40-dependent manner. J Immunol. 2002;169:1792–1800. doi: 10.4049/jimmunol.169.4.1792. [DOI] [PubMed] [Google Scholar]
- 32.Kim JA, Averbook BJ, Chambers K, et al. Divergent effects of 4-1BB antibodies on antitumor immunity and on tumor-reactive T-cell generation. Cancer Res. 2001;61:2031–2037. [PubMed] [Google Scholar]
- 33.Wang LX, Huang WX, Graor H, et al. Adoptive immunotherapy of cancer with polyclonal, 108-fold hyperexpanded, CD4+ and CD8+ T cells. J Transl Med. 2004;2:41. doi: 10.1186/1479-5876-2-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sussman JJ, Parihar R, Winstead K, Finkelman FD. Prolonged culture of vaccine-primed lymphocytes results in decreased antitumor killing and change in cytokine secretion. Cancer Res. 2004;64:9124–9130. doi: 10.1158/0008-5472.CAN-03-0376. [DOI] [PubMed] [Google Scholar]
- 35.Hughes MS, Yu YY, Dudley ME, et al. Transfer of a TCR gene derived from a patient with a marked antitumor response conveys highly active T-cell effector functions. Hum Gene Ther. 2005;16:457–472. doi: 10.1089/hum.2005.16.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burns JC, Friedmann T, Driever W, Burrascano M, Yee JK. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci U S A. 1993;90:8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Porter CD, Collins MK, Tailor CS, et al. Comparison of efficiency of infection of human gene therapy target cells via four different retroviral receptors. Hum Gene Ther. 1996;7:913–919. doi: 10.1089/hum.1996.7.8-913. [DOI] [PubMed] [Google Scholar]
- 38.Dudley ME, Wunderlich JR, Shelton TE, Even J, Rosenberg SA. Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J Immunother. 2003;26:332–342. doi: 10.1097/00002371-200307000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tran KQ, Zhou J, Durflinger KH, et al. Minimally cultured tumor-infiltrating lymphocytes display optimal characteristics for adoptive cell therapy. J Immunother. 2008;31:742–751. doi: 10.1097/CJI.0b013e31818403d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riddell SR, Greenberg PD. The use of anti-CD3 and anti-CD28 monoclonal antibodies to clone and expand human antigen-specific T cells. J Immunol Methods. 1990;128:189–201. doi: 10.1016/0022-1759(90)90210-m. [DOI] [PubMed] [Google Scholar]
- 41.Dudley ME, Gross CA, Langhan MM, et al. CD8+ enriched "young" tumor infiltrating lymphocytes can mediate regression of metastatic melanoma. Clin Cancer Res. doi: 10.1158/1078-0432.CCR-10-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rudolf D, Silberzahn T, Walter S, et al. Potent costimulation of human CD8 T cells by anti-4-1BB and anti-CD28 on synthetic artificial antigen presenting cells. Cancer Immunol Immunother. 2008;57:175–183. doi: 10.1007/s00262-007-0360-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stephan MT, Ponomarev V, Brentjens RJ, et al. T cell-encoded CD80 and 4-1BBL induce auto- and transcostimulation, resulting in potent tumor rejection. Nat Med. 2007;13:1440–1449. doi: 10.1038/nm1676. [DOI] [PubMed] [Google Scholar]
- 44.Ahmadzadeh M, Johnson LA, Heemskerk B, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gattinoni L, Powell DJ, Jr, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6:383–393. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hernandez-Chacon JA, Li Y, Wu RC, et al. Costimulation through the CD137/4-1BB pathway protects human melanoma tumor-infiltrating lymphocytes from activation-induced cell death and enhances antitumor effector function. J Immunother. 34:236–250. doi: 10.1097/CJI.0b013e318209e7ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goff SL, Smith FO, Klapper JA, et al. Tumor infiltrating lymphocyte therapy for metastatic melanoma: analysis of tumors resected for TIL. J Immunother. 33:840–847. doi: 10.1097/CJI.0b013e3181f05b91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Besser MJ, Shapira-Frommer R, Treves AJ, et al. Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin Cancer Res. 16:2646–2655. doi: 10.1158/1078-0432.CCR-10-0041. [DOI] [PubMed] [Google Scholar]
- 49.Butler MO, Friedlander P, Milstein MI, et al. Establishment of Antitumor Memory in Humans Using in Vitro-Educated CD8+ T Cells. Sci Transl Med. 3:80ra34. doi: 10.1126/scitranslmed.3002207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gong W, Xiao W, Hu M, et al. Ex vivo expansion of natural killer cells with high cytotoxicity by K562 cells modified to co-express major histocompatibility complex class I chain-related protein A, 4-1BB ligand, and interleukin-15. Tissue Antigens. 76:467–475. doi: 10.1111/j.1399-0039.2010.01535.x. [DOI] [PubMed] [Google Scholar]
- 51.Sluijter BJ, van den Hout MF, Stam AG, et al. 4-1BB-mediated expansion affords superior detection of in vivo primed effector memory CD8+ T cells from melanoma sentinel lymph nodes. Clin Immunol. 137:221–233. doi: 10.1016/j.clim.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 52.Paulos CM, Suhoski MM, Plesa G, et al. Adoptive immunotherapy: good habits instilled at youth have long-term benefits. Immunol Res. 2008;42:182–196. doi: 10.1007/s12026-008-8070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]