Abstract
West Nile virus (WNV), from the Flaviviridae family, is a re-emerging zoonotic pathogen of medical importance. In humans, WNV infection may cause life-threatening meningoencephalitis or long-term neurologic sequelae. WNV is transmitted by Culex spp mosquitoes and both the arthropod vector and the mammalian host are equipped with antiviral innate immune mechanisms sharing a common phylogeny. As far as the current evidence is able to demonstrate, mosquitoes primarily rely on RNA interference, Toll, Imd and JAK-STAT signaling pathways for limiting viral infection, while mammals are provided with these and other more complex antiviral mechanisms involving antiviral effectors, inflammatory mediators, and cellular responses triggered by highly specialized pathogen detection mechanisms that often resemble their invertebrate ancestry. This mini-review summarizes our current understanding of how the innate immune systems of the vector and the mammalian host react to WNV infection and shape its pathogenesis.
Molecular virology and epidemiology of West Nile Virus
West Nile virus (WNV) is an enveloped, single stranded, positive-sense RNA virus. It belongs to the Flaviviridae family, which includes a number of medically important human pathogens such as Dengue virus (DENV), hepatitis C virus, and Japanese encephalitis virus. The WNV genome is translated into a single polyprotein, which is cleaved by host and viral proteases into 3 structural proteins (Envelope E, Pre-membrane /membrane prM/M, and Capsid C), and 7 nonstructural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5). WNV E protein mediates viral entry and assembly, C binds to viral RNA, and prM prevents premature viral fusion. Nonstructural proteins play important roles in viral transcription, translation, replication, maturation, and immune evasion (Diamond et al., 2009). The WNV virion is ~500 Å in diameter and its surface comprises 180 copies of highly glycosylated E and M proteins (Mukhopadhyay et al., 2003).
Although it was first isolated in Uganda as a distinct viral species in 1937 and has caused sporadic and mild infections in the Old World ever since, WNV has been more widely recognized since 1999 when it was first introduced into North America and was accountable for 7 deaths (Nash et al., 2001). WNV can now be found all over the mainland of the United States, and has been associated with 12,727 encephalitis/meningitis cases and 1,208 deaths (mortality rate 9.5%) according to the US Centers for Disease Control and Prevention. WNV is maintained in an enzootic life cycle between mosquitoes and birds (Kramer et al., 2008). A large number of mosquito species are competent for WNV, predominantly Culex species. Birds, primarily American crows (Brault et al., 2007, Komar et al., 2003) and house sparrows are also good reservoirs for WNV in nature (Komar et al., 2001). WNV is transmitted to mammals including humans and horses when an infected female mosquito takes a blood meal. Mammals seem to be the dead end of WNV transmission cycle as their viremia is insufficient to infect a feeding mosquito. Infection in humans is usually asymptomatic and self-limiting, but occasionally elicits fever and neurological symptoms, including acute flaccid paralysis, meningitis and encephalitis. These neurological symptoms may be life-threatening in susceptible individuals such as the elderly (Debiasi et al., 2006).
Based on its complete or partial genome sequence, WNV is grouped into 5 lineages, with lineage I and II being best understood. Lineage II was initially considered an Africa-confined zoonotic pathogen, but it now appears in Europe (Gubler, 2007). Lineage I is epidemic worldwide and was the culprit of the New York outbreak in 1999 (NY99 strain). The NY99 strain shares the highest identity with an isolate from a dead goose in Israel in 1998 (Lanciotti et al., 1999), suggesting an Old World origin of NY99. However, a distinct genotype denoted WN02 has dominated the United States since 2002 (Davis et al., 2005), likely due to the increased vector competence and transmission efficiency in Culex spp. mosquitoes (Moudy et al., 2007).
Innate immunity control of WNV in mosquitoes
Our understanding of mosquito innate immunity started to progress with the sequencing of Anopheles gambiae and Aedes aegypti genomes (Christophides et al., 2002, Holt et al., 2002, Nene et al., 2007, Waterhouse et al., 2007). The current knowledge about the mosquito immune system has been derived primarily from the work of Plasmodium infection in Anopheles. Recently there have been promising advances in investigations of the antiviral mechanisms of Aedes mosquitoes, the primary vectors for DENV. However, our current understanding of the immune response of Culex mosquitoes against WNV is just about to start (Brackney et al., 2009). A recent study revealed that the Culex quinquefasciatus gene family members share the greatest similarities with Aedes aegypti (Bartholomay et al., 2010), suggesting that common antiviral mechanisms exist across insect species. Indeed, the major antiviral mechanisms of Drosophila melanogaster are fairly conserved in Anopheles gambiae mosquitoes as well (Fragkoudis et al., 2009). Thus the antiviral mechanisms in the established models like Aedes, Anopheles and Drosophila may be well adapted to WNV-Culex system.
Midgut barrier, a physical firewall
In nature, WNV is acquired by female mosquitoes when they feed on a viremic bird. The virus replicates in the midgut epithelia and disseminates via the hemolymph throughout the body, including salivary glands, being secreted into the mosquito saliva, where it is present in high concentrations and ready for transmission to mammalian hosts. The midgut epithelium serves as a physical and immune barrier to microorganisms by producing antimicrobial peptides (Tzou et al., 2000), and a peritrophic matrix composed of chitin proteins and proteoglycans (Shao et al., 2001). Certain Aedes aegypti populations/ Culex species are more resistant to DENV-2 / WNV infection than others (Black et al., 2002, Ebel et al., 2005, Reisen et al., 2008, Reisen et al., 2005). Possible mechanisms of the resistant populations include either that the virus cannot effectively infect the midgut epithelium, or, even after successfully establishing infection, that the virus may be unable to escape out from midgut cells (Black et al., 2002).
RNA interference (RNAi), the primary antiviral mechanism
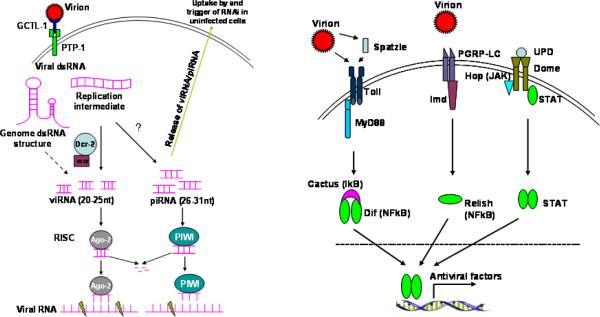
Small interfering RNA-mediated RNAi is an evolutionarily conserved mechanism of gene regulation and viral control in plants and invertebrates. To activate the RNAi pathway, virus-derived long dsRNA -either genomic dsRNA structures or replicative dsRNA intermediates (Sabin et al., 2009, Siu et al., 2011)- are cleaved by an RNase III Dicer-2 (Dcr-2) into a 20–25bp long siRNA, termed viRNA (virus induced siRNA). Dicer, aided by the dsRNA-binding protein R2D2 and Ars2 (Sabin et al., 2009), integrates one strand of a viRNA (guide strand) into the multiprotein complex RISC (RNA-Induced Silencing Complex) containing the slicer Argonaute-2 (Ago-2) among others. The RISC then targets viRNA-complementary sequences on the viral single stranded RNA for degradation (Figure 1a). The other strand of viRNA (passenger strand) is degraded. An siRNA-mediated antiviral mechanism has been shown to be crucial for controlling Alphavirus infection in Anopheles gambiae or Aedes aegypti mosquitoes (Campbell et al., 2008b, Cirimotich et al., 2009, Keene et al., 2004, Myles et al., 2008), DENV infection in Aedes aegypti (Sanchez-Vargas et al., 2009, Sanchez-Vargas et al., 2004) and WNV infection in Drosophila melanogaster (Chotkowski et al., 2008). The siRNA pathway is also induced by WNV infection in Culex pipiens (Brackney et al., 2009) but it fails to control WNV replication in C6/36 (Aedes albopictus) cells due to a premature codon in Dcr-2 (Brackney et al., 2010). While Dcr-2 is the key molecule of the siRNA pathway, upon binding of dsRNA it may also act as a RIG-I-like receptor to initiate a robust antiviral signaling cascade in Drosophila (Deddouche et al., 2008). Another RNAi pathway, PIWI (P-element Induced Wimpy testis in Drosophila) -interacting RNA (piRNA) (Aravin et al., 2007, Campbell et al., 2008a), may participate in mosquito antiviral defense via RISC. Deficiency of the PIWI pathway in Drosophila increased susceptibility to WNV infection (Chotkowski et al., 2008), and silencing of an Anopheles gambiae PIWI protein, Ago-3, enhanced dissemination of O'nyong-nyong virus (ONNV; Togaviridae, Alphavirus) (Keene et al., 2004). More recently, piRNA was also implicated in anti-DENV2 defense in Aedes aegypti (Hess et al., 2011).
Figure 1.
Proposed anti-WNV signaling pathways in mosquitoes. There is very little information on WNV-mosquito interactions. The model is drawn based on known antiviral pathways in Drosophila and Anopheles. a) siRNA-mediated antiviral signaling. Extracellular WNV virions are recognized by a secreted C-type lectin, mosGCTL-1, which then interacts with a cell surface membrane-bound protein tyrosine phosphatase homologue, mosPTP-1, to enable receptor-mediated endocytosis of virions. The dsRNA structures of viral genome or dsRNA intermediates resulted from replication are processed by RNase III, Dcr-2, into 20–25 nt long viRNAs. viRNAs are then integrated into RISC complex containing Ago-2 and several other proteins. One strand of viRNA is then degraded, the remainder is targeted to a complementary viral RNA, which is subsequently cleaved by RISC clipper Ago-2. piRNA-mediated RNAi is less understood, but could resemble viRNA. Free siRNAs or viral RNA released by secretory transport or lysis of infected cells could be internalized by uninfected cells, stimulating an antiviral state. b) Toll, Imd and Jak-STAT antiviral pathways. Virions can be directly sensed by Toll receptors or viral infection and can activate a serine protease cascade, leading to activation of Spatzle, a Toll ligand. Engagement of Toll leads to MyD88-dependent signaling cascade and eventually NF-κB mediated-transcriptional induction of numerous antiviral factors. Similarly, virus (glycosylated envelope proteins?) acting via peptidoglycan recognition protein (PGRP) or other unknown receptors, may activate Imd-dependent antiviral gene induction. The Jak-STAT pathway is initiated by the binding of Unpaired (UPD) to its transmembrane receptor, Domeless (Dome), which is then phosphorylated by Hopscotch (Hop). Subsequent phosphorylation, dimerization and nuclear translocation of STAT activates antiviral gene expression.
Toll, Imd and JAK/STAT pathways, activators of antiviral gene expression
Experiments in Drosophila melanogaster are revealing that the production of antimicrobial factors results primarily from the immune signaling of the Toll, Immune Deficiency (Imd), and JAK/STAT pathways. Although mainly implicated in anti-bacterial or fungus responses (Valanne et al., 2011) in insects, the Toll and Imd pathways have also been shown to play an important role in antiviral immune responses (Figure 1b). Both pathways eventually lead to mosquito NF-kB -mediated transcription of antimicrobial genes. Toll signaling plays a role in the control of Drosophila X virus infection in Drosophila (Zambon et al., 2005) and DENV-2 in Aedes aegypti (Ramirez et al., Xi et al., 2008). The Imd pathway also contributes to the Drosophila immune response to several RNA viruses (Avadhanula et al., 2009, Costa et al., 2009). The engagement of the cytokine receptor Domeless (Dome) by Unpaired (Upd) or other unknown cytokines leads to activation of STAT-mediated transcription of antiviral genes (Souza-Neto et al., 2009), which resembles the vertebrate type I interferon (IFN) signaling. Indeed, the JAK-STAT pathway is required for restricting RNA virus infection in Drosophila by inducing a number of antiviral genes (Dostert et al., 2005), and is crucial for controlling DENV infection in Aedes aegypti (Souza-Neto et al., 2009).
Antimicrobial peptides (AMPs), potent antiviral humoral factors
AMPs are usually cationic, short (usually less than 100 amino acids) and structurally diverse peptides that kill pathogens by disintegrating the cytoplasmic membranes (Imler et al., 2005). They are produced in the fat body (equivalent to the mammalian liver), epithelia, the genital tract and malpighian tubules, and are secreted into the hemolymph where they act as humoral factors to restrict pathogen dissemination (Bulet et al., 2005). In Anopheles, there are at least 4 families of AMPs: 4 defensins, 4 cecropins, one attacin and one gambicin (Yassine et al., 2010). Although little is known about the function of AMPs in mosquito antiviral immunity, they are generally involved in resistance to parasites and bacteria (Yassine et al., 2010), and, like their mammalian counterparts, mosquito AMPs are likely active against viral pathogens.
C-type lectins, cellular receptors for WNV
C-type lectin receptors (CLRs) are a family of pattern recognition receptors (PRR) that detect specific carbohydrate patterns in pathogens. They are able to elicit innate immune responses such as activation of the complement cascade, phagocytosis, induction of antimicrobial gene expression, and antigen presentation (Robinson et al., 2006). The mosquito genome encodes ~20 CLRs, several of which have been recently shown to facilitate WNV and DENV infection of Aedes aegypti and Culex quinquefasciatus (Cheng et al., 2010). A galactose-specific binding C-type lectin, designated mosGCTL-1, serves as a soluble receptor for WNV in the extracellular milieu. A mammalian CD45 phosphatase homologue, mosPTP-1 tethers on the cell membrane the mosGCTL-1-WNV complex and initiates endocytosis of viruses. Thus, moGCTL-1 is a potential target for blocking mosquito the acquisition of WNV. Indeed, delivery of anti-mosGCTL-1 antibodies via blood-feeding to mosquito midgut significantly reduced WNV infectivity (Cheng et al., 2010).
Innate immune control of WNV in mammals
Innate responses play a vital role during the early stages of infection, and are particularly relevant in the case of viruses presenting with acute/subacute pathogenic time courses such as WNV. Moreover, the activation of certain innate immune pathways specialized in pathogen detection is necessary not only for early control of viral replication (mainly mediated by the IFN response) but also for triggering and sustaining the adaptive response in the mammalian host. Ample experimental evidence indicates that the IFN response is a key innate defense mechanism: WNV replication is inhibited when cells are pretreated with type I IFN (Anderson et al., 2002, Samuel et al., 2005). In vivo, IFN-α treatment protects mice against lethal WNV infection (Morrey et al., 2004) and mice deprived of IFNα/βR rapidly succumb to WNV infection (Samuel et al., 2005). Antiviral effectors induced during the second wave of the IFN response such as PKR and RNAseL have also been shown to play a significant role in early WNV pathogenesis (Samuel et al., 2006b). Of note, several strains of WNV are able to antagonize the IFN response at multiple levels (Daffis et al., 2009), including inhibiting the JAK/Stat pathway in human immune cells (add Kong Viral Immun. 2008), and resistance to α/β IFN has been shown to be a key determinant of WNV replication fitness and virulence (Keller et al., 2006).
Sensing WNV infection: pattern recognition receptors (PRRs)
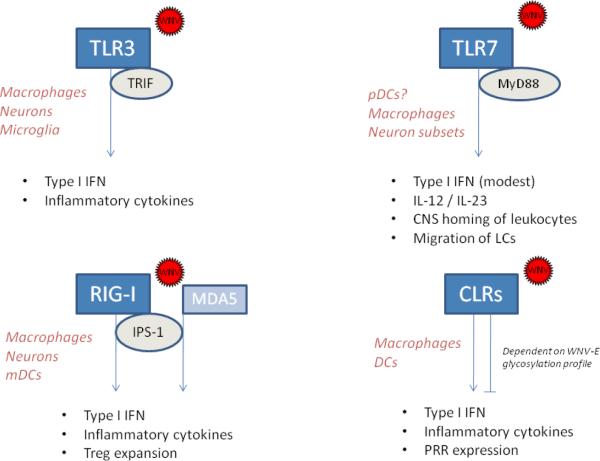
Mammalian Toll-like receptors (TLRs) constitute a group of at least 11 evolutionarily conserved type-I transmembrane glycoproteins (homologues of the Drosophila Toll gene) expressed either on the cell surface or intracellularly on endosomal membranes. Each receptor is comprised of a leucine rich repeat motif in the pathogen-binding ectodomain and a cytoplasmic Toll/IL-1R homology domain (TIR domain) responsible for signal transduction. Upon receptor engagement, the TIR domain signals through specific adaptor molecules such as myeloid differentiation factor 88 (MyD88) or TIR-containing adaptor-inducing IFN-β (TRIF), triggering signaling events that lead to activation of the transcription factors NF-κB and IRF-3, production of proinflammatory mediators including type I IFNs, and induction of costimulatory molecules (Kawai et al., 2006). Among the virus-sensing TLRs, TLR3 senses dsRNA (Alexopoulou et al., 2001), a replication derivative that is produced during the life cycle of many viruses, including WNV (Samuel, 2002). Murine infection models have yielded substantial evidence demonstrating that TLR3 is involved in WNV immunopathogenesis. Initially Wang and colleagues showed that TLR3-deficient mice present with an increased peripheral viral load, reduced production of antiviral and proinflammatory cytokines, and reduced mortality rate upon intraperitoneal WNV challenge (Wang et al., 2004). The authors postulated that due to the detrimental effect of TNF-α on blood-brain barrier (BBB) permeability, the reduced level of peripheral inflammatory responses in the absence of TLR3 rendered mice more resistant to viral neuroinvasion and accordingly, TLR3-deficient mice showed enhanced survival. A later study using mosquito cell-passaged WNV inoculated subcutaneously showed, however, that a lack of TLR3 enhanced viral replication in neurons in culture and in vivo. Thus, TLR3 appears to limit WNV infection in a cell type-restricted manner (Daffis et al., 2008). Indeed, results obtained from in vitro studies indicate that TLR3 may be dispensable for WNV recognition in certain cell types (Fredericksen et al., 2006). Other TLRs involved in viral recognition include TLR7 and the phylogenetically related TLR8, both of which have the ability to detect ssRNA and ribonucleic acid homologs (Kawai et al., 2006). A recent report has shown that TLR7-deficient mice and macrophages had reduced IL-12 and IL-23 responses after WNV infection and presented with increased susceptibility to lethal WNV encephalitis. Mechanistically, TLR7 and IL-23-dependent responses modulate immune cell homing to infected target cells (Town et al., 2009). In addition, TLR7-mediated responses following cutaneous infection have been shown to promote the migration of Langerhans cells (LCs) (Welte et al., 2009). Unlike TLR3, which uses TRIF as adaptor molecule, TLR7 signaling depends on the adaptor molecule MyD88. Mice lacking MyD88 show increased WNV-induced lethality and elevated viral burden primarily in the brain, albeit only a modest effect was observed in the systemic type I IFN response. In vitro, increased WNV replication was observed in MyD88-deficient macrophages and subsets of neurons but not in myeloid dendritic cells. MyD88 also modulated the expression of chemokines that regulate immune cell migration into the central nervous system (Szretter et al., 2010), which is in line with the phenotype reported for TLR7-deficient mice (Town et al., 2009).
Another important family of viral PRRs are the cytoplasmic RNA helicases, retinoic acid-inducible gene I (RIG-I) and melanoma differentiation-associated gene 5 (MDA5). RIG-I and MDA5 have a C-terminal DExD/H box RNA helicase domain (required for the interaction with dsRNA) and two N-terminal caspase activation and recruitment domains, which allow interaction with the adaptor protein interferon beta promoter stimulator protein 1 (IPS-1) and transmission of the anti-viral signal (Saito et al., 2007). In WNV-infected mouse embryonic fibroblasts (MEFs) lacking RIG-I, IRF-3 activation is significantly delayed, and this phenomenon was correlated with an increase in viral titers and cytopathic effects (Fredericksen et al., 2006). On the other hand, it has been shown recently that the IPS-1-dependent induction of IFN-β and the innate antiviral response in myeloid cells does not require IRF-3 and IRF-7 factors (Daffis et al., 2009). The WNV-induced type I IFN production detected in MDA5-deficient dendritic cells (DCs) and macrophages was only slightly reduced when compared to that seen in control cells (Gitlin et al., 2006). It is therefore possible that MDA5 has a complementary role in sensing WNV dsRNA, particularly once productive infection has been established in non-immune cell types, as MDA5 levels were significantly increased in neurons 48 hours after WNV infection (Daffis et al., 2007). Suthar et al. have recently reported that WNV-infected IPS-1-deficient mice display uncontrolled inflammatory responses associated with a lack of regulatory T cell expansion that normally occurs during acute WNV infection. This enhanced inflammatory response in the absence of IPS-1 was associated with increased susceptibility to WNV infection, positing IPS-1-dependent signaling as a modulator of the innate/adaptive balance in the overall immune response to WNV infection (Suthar et al., 2010).
As occurs in mosquitoes, mammalian CLRs bind to various sugar moieties that decorate many microbial products, including viral proteins (Takahashi et al., 2006). In the case of WNV, the mammalian DC-SIGN and DC-SIGNR have been involved in cellular attachment and infection via their recognition of the envelope glycoprotein (Davis et al., 2006a, Davis et al., 2006b). As the engagement of DC-SIGN and other DC-SIGN-like molecules by several bacterial pathogens has been shown to modulate immune cell activation and cytokine production (Caparros et al., 2006, Geijtenbeek et al., 2003), detection of WNV envelope glycoprotein (WNV-E) by DC-SIGN and possibly other CLRs may constitute the first WNV-derived signal for triggering host innate immune responses. In fact, Kong et al. have shown that binding of the glycosylated WNV-E to DC-SIGN leads to a reduction in the expression of TLR3 in macrophages via the STAT1-mediated pathway. This signaling is impaired in the elderly, and the elevated levels of TLR3 result in an elevation of cytokine levels upon WNV-infection in vitro of macrophages from older donors. The authors proposed that this age-associated alteration of the innate immune response may contribute to increased BBB permeability, which would partially explain the increased severity of WNV infection in older individuals (Kong et al., 2008a).
Cytokines involved in the innate immune response to WNV
Cytokines other than type IFNs also contribute significantly to WNV pathogenesis. In the mouse model, WNV infection has been shown to increase macrophage migration inhibitory factor (MIF) levels during the early stage of infection, and WNV-infected patients also present with increased MIF levels in plasma and in cerebrospinal fluid (CSF) (Arjona et al., 2007a). Abrogation of MIF by three independent mechanisms all rendered mice more resistant to WNV challenge. Although wild-type and MIF-deficient mice presented with comparable viral titers in the periphery, WNV neuroinvasion was delayed and reduced overall in animals lacking MIF (Arjona et al., 2007a). Although MIF promoted WNV neuroinvasion by increasing BBB permeability via inflammatory cytokines, it cannot be ruled out that MIF also increases BBB breaching during WNV infection by inducing the synthesis of matrix metalloproteinases (MMPs). In fact, MMP-9 facilitates WNV entry into the brain (Wang et al., 2008).
Mice deficient in IFN-γ or in the IFN-γ receptor infected with WNV showed increased mortality rates and decreased survival time when compared to control mice. The survival pattern of mice lacking IFN-γ production or signaling correlated with higher viremia and greater viral replication in peripheral tissues, which led to early and overall increase of WNV neuroinvasion (Shrestha et al., 2006). In addition, it has been shown that treatment of primary dendritic cells (DCs) with IFN-γ greatly reduces WNV replication (Shrestha et al., 2006). Absence of IFN-γ does not significantly alter adaptive immune responses, and there is a cell-specific requirement for IFN-γ as γδ T cells, but not CD8 T cells, require this cytokine to limit WNV dissemination (Shrestha et al., 2006, Wang et al., 2003). Thus, it appears that the dominant antiviral role of IFN-γ falls within the innate response.
Other innate inflammatory cytokines are induced during WNV infection. For example, IL-1β expression is upregulated in WNV-infected murine macrophages as soon as 12 hours post-infection (Shirato et al., 2006). Upregulation of IL-1β was evident in neural tissue from WNV encephalitis patients as well as in WNV-infected cultured human glia (van Marle et al., 2007). Although it is not known whether the role of IL-1β in the CNS is protective or detrimental, peripheral IL-1β appears to be necessary for LC migration, accumulation in the draining lymph nodes, and the initiation of lymph node shut-down in response to a cutaneous WNV infection (Byrne et al., 2001).
Immune cells involved in the innate response to WNV
In WNV patients, macrophages constitute an important fraction of the inflammatory infiltrate seen in the CNS (Kelley et al., 2003). Mouse studies have shown that, in microglia, the antiviral response is partially dependent on TLR3 (Town et al., 2006, Wang et al., 2004). Macrophages can function as effective WNV antigen-presenting cells (APCs), promoting WNV-specific T cell proliferation (Kulkarni et al., 1991). Consistent with a protective role, macrophage depletion in WNV-infected mice results in increased viremia, even allowing a non-neurotropic WNV strain to cross the BBB in the absence of macrophages (Ben-Nathan et al., 1996). Experimental data also indicate that macrophages require constitutive expression of key host defense molecules (e.g., RIG-I, MDA5, PKR and RNase L) to control WNV infection, in ways both dependent and independent of type I IFN production (Daffis et al., 2007, Samuel et al., 2006b). Interestingly, murine macrophages deficient in IRF-3 lacked basal expression of some of these host defense genes and supported increased WNV infection as well as increased production of IFNs (Daffis et al., 2007). Macrophages may also control WNV infection by direct effector mechanisms such as the production of nitric oxide intermediates (Garcia-Tapia et al., 2006, Kreil et al., 1996, Lin et al., 1997, Samuel et al., 2006a) or production of type I IFN, which can act to promote anti-viral mechanisms by neutrophils (Bai et al., 2010). Infection with WNV of primary human macrophages induced production of IL-8 but inhibited that of IL-1β and type I IFN due to interference with the downstream JAK/STAT pathway, which is important for macrophage activation (Kong et al., 2008b).
DCs express DC-SIGN-like attachment molecules (Davis et al., 2006b), which positions them as initial targets for WNV replication after skin inoculation (Pierson et al., 2005). LCs, the resident DC type of the skin, carry WNV to local lymph nodes in murine models of infection in an IL-1β-dependent manner (Byrne et al., 2001, Johnston et al., 2000). CD4+ T cell proliferation studies have demonstrated that DCs are able to present WNV antigens (Kulkarni et al., 1991), although they may do so to a lesser extent than macrophages (Pisarev et al., 2003). In vitro studies revealed that WNV triggers type I IFN production in human pDCs and monocyte-derived DCs and this depends on whether virus was produced in a mammalian or insect host. pDCs produce IFN-α when stimulated with WNV grown in mammalian (host) cells but not when stimulated with WNV derived from mosquito cells (Silva et al., 2007). Similarly, WNV-E protein is able to block dsRNA-induced cytokine production in macrophages only when carrying a mosquito-derived glycosylation profile (Arjona et al., 2007b). However, the viral source may not have a significant effect on WNV pathogenesis after all, as tissue tropism, infectivity, clinical disease, and mortality did not differ for mice inoculated with WNV grown in mosquito or mammalian cells (Lim et al., 2010b). Studies conducted with human DCs have identified that the production of type I IFN is significantly lower in DCs from older donors compared to that of younger donors, a phenomenon correlated with a diminished induction of late-phase responses (Qian et al., 2011).
The γ/δ T cell population expands significantly after WNV infection, and mice deficient in γ/δ T cells develop higher viral load and have increased mortality (Wang et al., 2003). Soon after WNV infection γ/δ T cells produce high amounts of IFN-γ, which correlates with an increase in the cytotoxic effector perforin expression in splenic T cells. Bone marrow chimera reconstitution experiments have further demonstrated that IFN-γ derived from γ/δ T is required for the early control of WNV dissemination (Shrestha et al., 2006). In addition, γ/δ T cells promote protective adaptive immune responses to WNV infection by facilitating the maturation of DCs (Fang et al., 2010). Thus, γδ T cells constitute an important functional link between innate and adaptive immune responses to WNV infection.
Finally, Bai and colleagues have shed light on the role of polymorphonuclear leukocytes (PMNs) in WNV infection. They found that PMNs are rapidly recruited to the site of infection in mice and support efficient replication of WNV. Mice depleted of PMNs after WNV inoculation developed higher viremia and experienced earlier death. On the other hand, when PMNs were depleted prior to infection with WNV, and in mice deficient in the chemokine receptor CXCR2, viremia was reduced and survival was enhanced. These data indicate that PMNs serve as an early reservoir for replication and dissemination, hampering their putative protective role during WNV infection (Bai et al., 2010). Of note, WNV meningoencephalitis is frequently associated with substantial CSF neutrophilia (Rawal et al., 2006, Tyler et al., 2006).
Conclusions
The current framework for arthropod innate immune mechanisms is mostly derived from studies in Drosophila melanogaster or Anopheles gambiae. At present we have very limited knowledge about the specific antiviral immune signaling in the most relevant vector host, Culex spp. With the recent unraveling of the Culex genome sequence (Arensburger et al., 2010); it is likely that some of the most important questions can now start to be addressed. For example, which PRRs are involved in arthropods and how do they recognize viruses? What is the exact role of secreted cytokines like Spatzle? What viral components are sensed? Which virus-induced cytokines stimulate JAK-STAT signaling via Dome? Understanding of the antiviral mechanisms in mosquitoes should provide us with new prophylactic approaches, such as virus-resistant trans-genetic mosquitoes (Alphey, 2009), for the control of WNV transmission.
On the mammalian host side, much has been learned in the past years about the innate immune responses that determine resistance to WNV, and it has been valuable to mine the remarkable conservation of immune recognition and effector mechanisms between arthropods and mammals. Many of these innate responses, like those mediated by type I IFNs as well as the γδ T cells/IFN-γ response, are effective not only for restraining initial WNV dissemination but also for inducing the adaptive responses responsible for WNV clearance. However, other WNV-induced innate responses, due to their inflammatory nature, may end up favoring WNV neuroinvasion. Because these deleterious responses appear during the early stages of infection, they are promising targets for therapeutic intervention. It is clear, on the other hand, that canonical viral recognition receptors such as TLR3, TLR7, RIG-I and MDA5 play important roles in sensing WNV infection in a cell type-dependent manner (Fig. 2). It is also critical to examine the contribution of non-conventional PRRs, such as carbohydrate-discriminating receptors (e.g., CLRs) which, due to their bona-fide interaction with WNV structural proteins, may modulate innate cell activation well before WNV genomic products such are exposed to recognition. In a broader sense, PRRs include any PAMP-recognizing molecule capable of triggering any type of antiviral response in leukocytes, including immediate, non-genomic effector functions such as phagocytosis or degranulation. The activation of non-conventional PRRs may trigger innate immune responses that could be critical for the control of WNV infection. In addition, investigating whether genetic polymorphisms in PRRs and inflammatory mediators (Lim et al., 2010a) influence the susceptibility to WNV encephalitis may elucidate a valuable set of therapeutic and prognostic biomarkers.
Figure 2.
Depiction of the mammalian WNV-sensing innate mechanisms mediated by pattern-recognition receptors (TLR3, TRL7, RIG-I, MDA-5 and C-type lectine receptors) along with their corresponding adaptor molecules. Cell type specific pattern recognition receptors and adaptors have been implicated in WNV immunopathogenesis and are shown with the main innate responses they modulate according to the current available data. The cell types in which each pattern-recognition receptor have been shown to play a significant role are listed in red. TLRs, Toll-like receptors; CLRs, C-type lectin receptors.
We are also now beginning to understand how aging affects WNV-induced innate immune signaling (Kong et al., 2008a, Qian et al., 2011), and further research in this direction will be necessary for effectively preventing and treating WNV infection in this susceptible population. Expanding our knowledge on how innate immune cells react to WNV and modulate subsequent adaptive responses will be critical to guide the design of future immunoprophylactic strategies. Finally, although much progress has been made in understanding at the molecular level how WNV infection is detected and which innate immunity mechanisms are activated upon WNV recognition, there is a need for a better understating of how each cell type (with its specific repertoire of PRRs) influences WNV pathogenesis.
Acknowledgements
This work is supported by NIH grants, AI-055749 and AI-50031. E.F is an Investigator of the Howard Hughes Medical Institute. P.W is supported by a Career Development Award from Northeast Biodefense Center U54-AI057158-Lipkin.
References
- Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- Alphey L. Natural and engineered mosquito immunity. J Biol. 2009;8:40. doi: 10.1186/jbiol143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JF, Rahal JJ. Efficacy of interferon alpha-2b and ribavirin against West Nile virus in vitro. Emerg Infect Dis. 2002;8:107–108. doi: 10.3201/eid0801.010252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravin AA, Hannon GJ, Brennecke J. The Piwi-piRNA pathway provides an adaptive defense in the transposon arms race. Science (New York, N.Y. 2007;318:761–764. doi: 10.1126/science.1146484. [DOI] [PubMed] [Google Scholar]
- Arensburger P, Megy K, Waterhouse RM, Abrudan J, Amedeo P, Antelo B, et al. Sequencing of Culex quinquefasciatus establishes a platform for mosquito comparative genomics. Science (New York, N.Y. 2010;330:86–88. doi: 10.1126/science.1191864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arjona A, Foellmer HG, Town T, Leng L, McDonald C, Wang T, et al. Abrogation of macrophage migration inhibitory factor decreases West Nile virus lethality by limiting viral neuroinvasion. J Clin Invest. 2007a;117:3059–3066. doi: 10.1172/JCI32218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arjona A, Ledizet M, Anthony K, Bonafe N, Modis Y, Town T, Fikrig E. West Nile virus envelope protein inhibits dsRNA-induced innate immune responses. J Immunol. 2007b;179:8403–8409. doi: 10.4049/jimmunol.179.12.8403. [DOI] [PubMed] [Google Scholar]
- Avadhanula V, Weasner BP, Hardy GG, Kumar JP, Hardy RW. A novel system for the launch of alphavirus RNA synthesis reveals a role for the Imd pathway in arthropod antiviral response. PLoS pathogens. 2009;5:e1000582. doi: 10.1371/journal.ppat.1000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai F, Kong KF, Dai J, Qian F, Zhang L, Brown CR, et al. A paradoxical role for neutrophils in the pathogenesis of West Nile virus. J Infect Dis. 2010;202:1804–1812. doi: 10.1086/657416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomay LC, Waterhouse RM, Mayhew GF, Campbell CL, Michel K, Zou Z, et al. Pathogenomics of Culex quinquefasciatus and meta-analysis of infection responses to diverse pathogens. Science (New York, N.Y. 2010;330:88–90. doi: 10.1126/science.1193162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Nathan D, Huitinga I, Lustig S, van Rooijen N, Kobiler D. West Nile virus neuroinvasion and encephalitis induced by macrophage depletion in mice. Archives of virology. 1996;141:459–469. doi: 10.1007/BF01718310. [DOI] [PubMed] [Google Scholar]
- Black W.C.t., Bennett KE, Gorrochotegui-Escalante N, Barillas-Mury CV, Fernandez-Salas I, de Lourdes Munoz M, et al. Flavivirus susceptibility in Aedes aegypti. Arch Med Res. 2002;33:379–388. doi: 10.1016/s0188-4409(02)00373-9. [DOI] [PubMed] [Google Scholar]
- Brackney DE, Beane JE, Ebel GD. RNAi targeting of West Nile virus in mosquito midguts promotes virus diversification. PLoS pathogens. 2009;5:e1000502. doi: 10.1371/journal.ppat.1000502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackney DE, Scott JC, Sagawa F, Woodward JE, Miller NA, Schilkey FD, et al. C6/36 Aedes albopictus cells have a dysfunctional antiviral RNA interference response. PLoS Negl Trop Dis. 2010;4:e856. doi: 10.1371/journal.pntd.0000856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brault AC, Huang CYH, Langevin SA, Kinney RM, Bowen RA, Ramey WN, et al. A single positively selected West Nile viral mutation confers increased virogenesis in American crows. Nature Genetics. 2007;39:1162–1166. doi: 10.1038/ng2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulet P, Stocklin R. Insect antimicrobial peptides: structures, properties and gene regulation. Protein Pept Lett. 2005;12:3–11. doi: 10.2174/0929866053406011. [DOI] [PubMed] [Google Scholar]
- Byrne SN, Halliday GM, Johnston LJ, King NJ. Interleukin-1beta but not tumor necrosis factor is involved in West Nile virus-induced Langerhans cell migration from the skin in C57BL/6 mice. The Journal of investigative dermatology. 2001;117:702–709. doi: 10.1046/j.0022-202x.2001.01454.x. [DOI] [PubMed] [Google Scholar]
- Campbell CL, Black W.C.t., Hess AM, Foy BD. Comparative genomics of small RNA regulatory pathway components in vector mosquitoes. BMC Genomics. 2008a;9:425. doi: 10.1186/1471-2164-9-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell CL, Keene KM, Brackney DE, Olson KE, Blair CD, Wilusz J, Foy BD. Aedes aegypti uses RNA interference in defense against Sindbis virus infection. BMC Microbiol. 2008b;8:47. doi: 10.1186/1471-2180-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caparros E, Munoz P, Sierra-Filardi E, Serrano-Gomez D, Puig-Kroger A, Rodriguez-Fernandez JL, et al. DC-SIGN ligation on dendritic cells results in ERK and PI3K activation and modulates cytokine production. Blood. 2006;107:3950–3958. doi: 10.1182/blood-2005-03-1252. [DOI] [PubMed] [Google Scholar]
- Cheng G, Cox J, Wang P, Krishnan MN, Dai J, Qian F, et al. A C-type lectin collaborates with a CD45 phosphatase homolog to facilitate West Nile virus infection of mosquitoes. Cell. 2010;142:714–725. doi: 10.1016/j.cell.2010.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chotkowski HL, Ciota AT, Jia Y, Puig-Basagoiti F, Kramer LD, Shi PY, Glaser RL. West Nile virus infection of Drosophila melanogaster induces a protective RNAi response. Virology. 2008;377:197–206. doi: 10.1016/j.virol.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christophides GK, Zdobnov E, Barillas-Mury C, Birney E, Blandin S, Blass C, et al. Immunity-related genes and gene families in Anopheles gambiae. Science (New York, N.Y. 2002;298:159–165. doi: 10.1126/science.1077136. [DOI] [PubMed] [Google Scholar]
- Cirimotich CM, Scott JC, Phillips AT, Geiss BJ, Olson KE. Suppression of RNA interference increases alphavirus replication and virus-associated mortality in Aedes aegypti mosquitoes. BMC Microbiol. 2009;9:49. doi: 10.1186/1471-2180-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A, Jan E, Sarnow P, Schneider D. The Imd pathway is involved in antiviral immune responses in Drosophila. PLoS One. 2009;4:e7436. doi: 10.1371/journal.pone.0007436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daffis S, Samuel MA, Keller BC, Gale M, Jr., Diamond MS. Cell-specific IRF-3 responses protect against West Nile virus infection by interferon-dependent and -independent mechanisms. PLoS pathogens. 2007;3:e106. doi: 10.1371/journal.ppat.0030106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daffis S, Samuel MA, Suthar MS, Gale M, Jr., Diamond MS. Toll-like receptor 3 has a protective role against West Nile virus infection. Journal of virology. 2008;82:10349–10358. doi: 10.1128/JVI.00935-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daffis S, Suthar MS, Gale M, Jr., Diamond MS. Measure and countermeasure: type I IFN (IFN-alpha/beta) antiviral response against West Nile virus. J Innate Immun. 2009;1:435–445. doi: 10.1159/000226248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CT, Ebel GD, Lanciotti RS, Brault AC, Guzman H, Siirin M, et al. Phylogenetic analysis of North American West Nile virus isolates, 2001–2004: evidence for the emergence of a dominant genotype. Virology. 2005;342:252–265. doi: 10.1016/j.virol.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Davis CW, Mattei LM, Nguyen HY, Ansarah-Sobrinho C, Doms RW, Pierson TC. The location of asparagine-linked glycans on West Nile virions controls their interactions with CD209 (dendritic cell-specific ICAM-3 grabbing nonintegrin) The Journal of biological chemistry. 2006a;281:37183–37194. doi: 10.1074/jbc.M605429200. [DOI] [PubMed] [Google Scholar]
- Davis CW, Nguyen HY, Hanna SL, Sanchez MD, Doms RW, Pierson TC. West Nile virus discriminates between DC-SIGN and DC-SIGNR for cellular attachment and infection. Journal of virology. 2006b;80:1290–1301. doi: 10.1128/JVI.80.3.1290-1301.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiasi RL, Tyler KL. West Nile virus meningoencephalitis. Nat Clin Pract Neurol. 2006;2:264–275. doi: 10.1038/ncpneuro0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deddouche S, Matt N, Budd A, Mueller S, Kemp C, Galiana-Arnoux D, et al. The DExD/H-box helicase Dicer-2 mediates the induction of antiviral activity in drosophila. Nature immunology. 2008;9:1425–1432. doi: 10.1038/ni.1664. [DOI] [PubMed] [Google Scholar]
- Diamond MS, Mehlhop E, Oliphant T, Samuel MA. The host immunologic response to West Nile encephalitis virus. Front Biosci. 2009;14:3024–3034. doi: 10.2741/3432. [DOI] [PubMed] [Google Scholar]
- Dostert C, Jouanguy E, Irving P, Troxler L, Galiana-Arnoux D, Hetru C, et al. The Jak-STAT signaling pathway is required but not sufficient for the antiviral response of drosophila. Nature immunology. 2005;6:946–953. doi: 10.1038/ni1237. [DOI] [PubMed] [Google Scholar]
- Ebel GD, Rochlin I, Longacker J, Kramer LD. Culex restuans (Diptera: Culicidae) relative abundance and vector competence for West Nile Virus. Journal of Medical Entomology. 2005;42:838–843. doi: 10.1603/0022-2585(2005)042[0838:CRDCRA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Fang H, Welte T, Zheng X, Chang GJ, Holbrook MR, Soong L, Wang T. gammadelta T cells promote the maturation of dendritic cells during West Nile virus infection. FEMS Immunol Med Microbiol. 2010;59:71–80. doi: 10.1111/j.1574-695X.2010.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragkoudis R, Attarzadeh-Yazdi G, Nash AA, Fazakerley JK, Kohl A. Advances in dissecting mosquito innate immune responses to arbovirus infection. J Gen Virol. 2009;90:2061–2072. doi: 10.1099/vir.0.013201-0. [DOI] [PubMed] [Google Scholar]
- Fredericksen BL, Gale M., Jr. West Nile virus evades activation of interferon regulatory factor 3 through RIG-I-dependent and -independent pathways without antagonizing host defense signaling. Journal of virology. 2006;80:2913–2923. doi: 10.1128/JVI.80.6.2913-2923.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Tapia D, Loiacono CM, Kleiboeker SB. Replication of West Nile virus in equine peripheral blood mononuclear cells. Veterinary immunology and immunopathology. 2006;110:229–244. doi: 10.1016/j.vetimm.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek TB, Van Vliet SJ, Koppel EA, Sanchez-Hernandez M, Vandenbroucke-Grauls CM, Appelmelk B, Van Kooyk Y. Mycobacteria target DC-SIGN to suppress dendritic cell function. The Journal of experimental medicine. 2003;197:7–17. doi: 10.1084/jem.20021229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin L, Barchet W, Gilfillan S, Cella M, Beutler B, Flavell RA, et al. Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:8459–8464. doi: 10.1073/pnas.0603082103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler DJ. The continuing spread of West Nile virus in the western hemisphere. Clin Infect Dis. 2007;45:1039–1046. doi: 10.1086/521911. [DOI] [PubMed] [Google Scholar]
- Hess AM, Prasad AN, Ptitsyn A, Ebel GD, Olson KE, Barbacioru C, et al. Small RNA profiling of Dengue virus-mosquito interactions implicates the PIWI RNA pathway in anti-viral defense. BMC Microbiol. 2011;11:45. doi: 10.1186/1471-2180-11-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt RA, Subramanian GM, Halpern A, Sutton GG, Charlab R, Nusskern DR, et al. The genome sequence of the malaria mosquito Anopheles gambiae. Science (New York, N.Y. 2002;298:129–149. doi: 10.1126/science.1076181. [DOI] [PubMed] [Google Scholar]
- Imler JL, Bulet P. Antimicrobial peptides in Drosophila: structures, activities and gene regulation. Chem Immunol Allergy. 2005;86:1–21. doi: 10.1159/000086648. [DOI] [PubMed] [Google Scholar]
- Johnston LJ, Halliday GM, King NJ. Langerhans cells migrate to local lymph nodes following cutaneous infection with an arbovirus. The Journal of investigative dermatology. 2000;114:560–568. doi: 10.1046/j.1523-1747.2000.00904.x. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. Innate immune recognition of viral infection. Nat Immunol. 2006;7:131–137. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- Keene KM, Foy BD, Sanchez-Vargas I, Beaty BJ, Blair CD, Olson KE. RNA interference acts as a natural antiviral response to O'nyong-nyong virus (Alphavirus; Togaviridae) infection of Anopheles gambiae. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:17240–17245. doi: 10.1073/pnas.0406983101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller BC, Fredericksen BL, Samuel MA, Mock RE, Mason PW, Diamond MS, Gale M., Jr. Resistance to alpha/beta interferon is a determinant of West Nile virus replication fitness and virulence. Journal of virology. 2006;80:9424–9434. doi: 10.1128/JVI.00768-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley TW, Prayson RA, Ruiz AI, Isada CM, Gordon SM. The neuropathology of West Nile virus meningoencephalitis. A report of two cases and review of the literature. American journal of clinical pathology. 2003;119:749–753. doi: 10.1309/PU4R-76JJ-MG1F-81RP. [DOI] [PubMed] [Google Scholar]
- Komar N, Langevin S, Hinten S, Nemeth N, Edwards E, Hettler D, et al. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerging infectious diseases. 2003;9:311–322. doi: 10.3201/eid0903.020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komar N, Panella NA, Burns JE, Dusza SW, Mascarenhas TM, Talbot TO. Serologic evidence for West Nile virus infection in birds in the New York City vicinity during an outbreak in 1999. Emerg Infect Dis. 2001;7:621–625. doi: 10.3201/eid0704.010403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong KF, Delroux K, Wang X, Qian F, Arjona A, Malawista SE, et al. Dysregulation of TLR3 impairs the innate immune response to West Nile virus in the elderly. Journal of virology. 2008a;82:7613–7623. doi: 10.1128/JVI.00618-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong KF, Wang X, Anderson JF, Fikrig E, Montgomery RR. West nile virus attenuates activation of primary human macrophages. Viral Immunol. 2008b;21:78–82. doi: 10.1089/vim.2007.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer LD, Styer LM, Ebel GD. A global perspective on the epidemiology of West Nile virus. Annu Rev Entomol. 2008;53:61–81. doi: 10.1146/annurev.ento.53.103106.093258. [DOI] [PubMed] [Google Scholar]
- Kreil TR, Eibl MM. Nitric oxide and viral infection: NO antiviral activity against a flavivirus in vitro and evidence for contribution to pathogenesis in experimental infection in vivo. Virology. 1996;219:304–306. doi: 10.1006/viro.1996.0252. [DOI] [PubMed] [Google Scholar]
- Kulkarni AB, Mullbacher A, Blanden RV. Functional analysis of macrophages, B cells and splenic dendritic cells as antigen-presenting cells in West Nile virus-specific murine T lymphocyte proliferation. Immunology and cell biology. 1991;69(Pt 2):71–80. doi: 10.1038/icb.1991.12. [DOI] [PubMed] [Google Scholar]
- Lanciotti RS, Roehrig JT, Deubel V, Smith J, Parker M, Steele K, et al. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science (New York, N.Y. 1999;286:2333–2337. doi: 10.1126/science.286.5448.2333. [DOI] [PubMed] [Google Scholar]
- Lim JK, McDermott DH, Lisco A, Foster GA, Krysztof D, Follmann D, et al. CCR5 deficiency is a risk factor for early clinical manifestations of West Nile virus infection but not for viral transmission. J Infect Dis. 2010a;201:178–185. doi: 10.1086/649426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim PY, Louie KL, Styer LM, Shi PY, Bernard KA. Viral pathogenesis in mice is similar for West Nile virus derived from mosquito and mammalian cells. Virology. 2010b;400:93–103. doi: 10.1016/j.virol.2010.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YL, Huang YL, Ma SH, Yeh CT, Chiou SY, Chen LK, Liao CL. Inhibition of Japanese encephalitis virus infection by nitric oxide: antiviral effect of nitric oxide on RNA virus replication. Journal of virology. 1997;71:5227–5235. doi: 10.1128/jvi.71.7.5227-5235.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrey JD, Day CW, Julander JG, Blatt LM, Smee DF, Sidwell RW. Effect of interferon-alpha and interferon-inducers on West Nile virus in mouse and hamster animal models. Antivir Chem Chemother. 2004;15:101–109. doi: 10.1177/095632020401500202. [DOI] [PubMed] [Google Scholar]
- Moudy RM, Meola MA, Morin LL, Ebel GD, Kramer LD. A newly emergent genotype of West Nile virus is transmitted earlier and more efficiently by Culex mosquitoes. Am J Trop Med Hyg. 2007;77:365–370. [PubMed] [Google Scholar]
- Mukhopadhyay S, Kim BS, Chipman PR, Rossmann MG, Kuhn RJ. Structure of West Nile virus. Science (New York, N.Y. 2003;302:248. doi: 10.1126/science.1089316. [DOI] [PubMed] [Google Scholar]
- Myles KM, Wiley MR, Morazzani EM, Adelman ZN. Alphavirus-derived small RNAs modulate pathogenesis in disease vector mosquitoes. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:19938–19943. doi: 10.1073/pnas.0803408105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash D, Mostashari F, Fine A, Miller J, O'Leary D, Murray K, et al. The outbreak of West Nile virus infection in the New York City area in 1999. N Engl J Med. 2001;344:1807–1814. doi: 10.1056/NEJM200106143442401. [DOI] [PubMed] [Google Scholar]
- Nene V, Wortman JR, Lawson D, Haas B, Kodira C, Tu ZJ, et al. Genome sequence of Aedes aegypti, a major arbovirus vector. Science (New York, N.Y. 2007;316:1718–1723. doi: 10.1126/science.1138878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson TC, Diamond MS, Ahmed AA, Valentine LE, Davis CW, Samuel MA, et al. An infectious West Nile virus that expresses a GFP reporter gene. Virology. 2005;334:28–40. doi: 10.1016/j.virol.2005.01.021. [DOI] [PubMed] [Google Scholar]
- Pisarev VB, Shishkina EO, Larichev VF, Grigor'eva NV. Morphofunctional characteristics of antigen-presenting cells in lymph node in mice with experimental West Nile fever. Bulletin of experimental biology and medicine. 2003;135:293–295. doi: 10.1023/a:1024153502265. [DOI] [PubMed] [Google Scholar]
- Qian F, Wang X, Zhang L, Lin A, Zhao H, Fikrig E, Montgomery RR. Impaired Interferon Signaling in Dendritic Cells From Older Donors Infected In Vitro With West Nile Virus. J Infect Dis. 2011 doi: 10.1093/infdis/jir048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JL, Dimopoulos G. The Toll immune signaling pathway control conserved anti-dengue defenses across diverse Ae. aegypti strains and against multiple dengue virus serotypes. Dev Comp Immunol. 34:625–629. doi: 10.1016/j.dci.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawal A, Gavin PJ, Sturgis CD. Cerebrospinal fluid cytology in seasonal epidemic West Nile virus meningo-encephalitis. Diagnostic cytopathology. 2006;34:127–129. doi: 10.1002/dc.20410. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Barker CM, Fang Y, Martinez VM. Does variation in Culex (Diptera: Culicidae) vector competence enable outbreaks of West Nile virus in California? Journal of Medical Entomology. 2008;45:1126–1138. doi: 10.1603/0022-2585(2008)45[1126:dvicdc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Fang Y, Martinez VM. Avian host and mosquito (Diptera: Culicidae) vector competence determine the efficiency of West Nile and St. Louis encephalitis virus transmission. Journal of Medical Entomology. 2005;42:367–375. doi: 10.1093/jmedent/42.3.367. [DOI] [PubMed] [Google Scholar]
- Robinson MJ, Sancho D, Slack EC, LeibundGut-Landmann S, Reis e Sousa C. Myeloid C-type lectins in innate immunity. Nature immunology. 2006;7:1258–1265. doi: 10.1038/ni1417. [DOI] [PubMed] [Google Scholar]
- Sabin LR, Zhou R, Gruber JJ, Lukinova N, Bambina S, Berman A, et al. Ars2 regulates both miRNA- and siRNA- dependent silencing and suppresses RNA virus infection in Drosophila. Cell. 2009;138:340–351. doi: 10.1016/j.cell.2009.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Gale M., Jr. Principles of intracellular viral recognition. Current opinion in immunology. 2007;19:17–23. doi: 10.1016/j.coi.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Samuel CE. Host genetic variability and West Nile virus susceptibility. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:11555–11557. doi: 10.1073/pnas.202448899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel MA, Diamond MS. Alpha/beta interferon protects against lethal West Nile virus infection by restricting cellular tropism and enhancing neuronal survival. Journal of virology. 2005;79:13350–13361. doi: 10.1128/JVI.79.21.13350-13361.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel MA, Diamond MS. Pathogenesis of West Nile Virus infection: a balance between virulence, innate and adaptive immunity and viral evasion. Journal of virology. 2006a;80:9349–9360. doi: 10.1128/JVI.01122-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel MA, Whitby K, Keller BC, Marri A, Barchet W, Williams BR, et al. PKR and RNase L contribute to protection against lethal West Nile Virus infection by controlling early viral spread in the periphery and replication in neurons. Journal of virology. 2006b;80:7009–7019. doi: 10.1128/JVI.00489-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Vargas I, Scott JC, Poole-Smith BK, Franz AW, Barbosa-Solomieu V, Wilusz J, et al. Dengue virus type 2 infections of Aedes aegypti are modulated by the mosquito's RNA interference pathway. PLoS pathogens. 2009;5:e1000299. doi: 10.1371/journal.ppat.1000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Vargas I, Travanty EA, Keene KM, Franz AW, Beaty BJ, Blair CD, Olson KE. RNA interference, arthropod-borne viruses and mosquitoes. Virus research. 2004;102:65–74. doi: 10.1016/j.virusres.2004.01.017. [DOI] [PubMed] [Google Scholar]
- Shao L, Devenport M, Jacobs-Lorena M. The peritrophic matrix of hematophagous insects. Arch Insect Biochem Physiol. 2001;47:119–125. doi: 10.1002/arch.1042. [DOI] [PubMed] [Google Scholar]
- Shirato K, Miyoshi H, Kariwa H, Takashima I. The kinetics of proinflammatory cytokines in murine peritoneal macrophages infected with envelope protein-glycosylated or non-glycosylated West Nile virus. Virus research. 2006;121:11–16. doi: 10.1016/j.virusres.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Shrestha B, Wang T, Samuel MA, Whitby K, Craft J, Fikrig E, Diamond MS. Gamma interferon plays a crucial early antiviral role in protection against West Nile virus infection. Journal of virology. 2006;80:5338–5348. doi: 10.1128/JVI.00274-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MC, Guerrero-Plat A, Gilfoy FD, Garofalo RP, Mason PW. Differential activation of human monocyte-derived and plasmacytoid dendritic cells by West Nile virus generated in different host cells. Journal of virology. 2007 doi: 10.1128/JVI.00857-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu RW, Fragkoudis R, Simmonds P, Donald CL, Chase-Topping ME, Barry G, et al. Antiviral RNA interference responses induced by Semliki Forest virus infection of mosquito cells: characterization, origin and frequency-dependent functions of virus-derived small interfering RNAs. Journal of virology. 2011;85:2907–2917. doi: 10.1128/JVI.02052-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza-Neto JA, Sim S, Dimopoulos G. An evolutionary conserved function of the JAKSTAT pathway in anti-dengue defense. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:17841–17846. doi: 10.1073/pnas.0905006106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthar MS, Ma DY, Thomas S, Lund JM, Zhang N, Daffis S, et al. IPS-1 is essential for the control of West Nile virus infection and immunity. PLoS pathogens. 2010;6:e1000757. doi: 10.1371/journal.ppat.1000757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szretter KJ, Daffis S, Patel J, Suthar MS, Klein RS, Gale M, Jr., Diamond MS. The innate immune adaptor molecule MyD88 restricts West Nile virus replication and spread in neurons of the central nervous system. Journal of virology. 2010;84:12125–12138. doi: 10.1128/JVI.01026-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Ip WE, Michelow IC, Ezekowitz RA. The mannose-binding lectin: a prototypic pattern recognition molecule. Current opinion in immunology. 2006;18:16–23. doi: 10.1016/j.coi.2005.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Town T, Bai F, Wang T, Kaplan AT, Qian F, Montgomery RR, et al. Toll-like receptor 7 mitigates lethal West Nile encephalitis via interleukin 23-dependent immune cell infiltration and homing. Immunity. 2009;30:242–253. doi: 10.1016/j.immuni.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Town T, Jeng D, Alexopoulou L, Tan J, Flavell RA. Microglia recognize double-stranded RNA via TLR3. J Immunol. 2006;176:3804–3812. doi: 10.4049/jimmunol.176.6.3804. [DOI] [PubMed] [Google Scholar]
- Tyler KL, Pape J, Goody RJ, Corkill M, Kleinschmidt-DeMasters BK. CSF findings in 250 patients with serologically confirmed West Nile virus meningitis and encephalitis. Neurology. 2006;66:361–365. doi: 10.1212/01.wnl.0000195890.70898.1f. [DOI] [PubMed] [Google Scholar]
- Tzou P, Ohresser S, Ferrandon D, Capovilla M, Reichhart JM, Lemaitre B, et al. Tissue-specific inducible expression of antimicrobial peptide genes in Drosophila surface epithelia. Immunity. 2000;13:737–748. doi: 10.1016/s1074-7613(00)00072-8. [DOI] [PubMed] [Google Scholar]
- Valanne S, Wang JH, Ramet M. The Drosophila Toll signaling pathway. J Immunol. 2011;186:649–656. doi: 10.4049/jimmunol.1002302. [DOI] [PubMed] [Google Scholar]
- van Marle G, Antony J, Ostermann H, Dunham C, Hunt T, Halliday W, et al. West nile virus-induced neuroinflammation: glial infection and capsid protein-mediated neurovirulence. Journal of virology. 2007;81:10933–10949. doi: 10.1128/JVI.02422-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Dai J, Bai F, Kong KF, Wong SJ, Montgomery RR, et al. Matrix metalloproteinase 9 facilitates West Nile virus entry into the brain. Journal of virology. 2008;82:8978–8985. doi: 10.1128/JVI.00314-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Scully E, Yin Z, Kim JH, Wang S, Yan J, et al. IFN-gamma-producing gamma delta T cells help control murine West Nile virus infection. J Immunol. 2003;171:2524–2531. doi: 10.4049/jimmunol.171.5.2524. [DOI] [PubMed] [Google Scholar]
- Wang T, Town T, Alexopoulou L, Anderson JF, Fikrig E, Flavell RA. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nature medicine. 2004;10:1366–1373. doi: 10.1038/nm1140. [DOI] [PubMed] [Google Scholar]
- Waterhouse RM, Kriventseva EV, Meister S, Xi Z, Alvarez KS, Bartholomay LC, et al. Evolutionary dynamics of immune-related genes and pathways in disease-vector mosquitoes. Science (New York, N.Y. 2007;316:1738–1743. doi: 10.1126/science.1139862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welte T, Reagan K, Fang H, Machain-Williams C, Zheng X, Mendell N, et al. Toll-like receptor 7-induced immune response to cutaneous West Nile virus infection. J Gen Virol. 2009;90:2660–2668. doi: 10.1099/vir.0.011783-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Z, Ramirez JL, Dimopoulos G. The Aedes aegypti toll pathway controls dengue virus infection. PLoS pathogens. 2008;4:e1000098. doi: 10.1371/journal.ppat.1000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassine H, Osta MA. Anopheles gambiae innate immunity. Cell Microbiol. 2010;12:1–9. doi: 10.1111/j.1462-5822.2009.01388.x. [DOI] [PubMed] [Google Scholar]
- Zambon RA, Nandakumar M, Vakharia VN, Wu LP. The Toll pathway is important for an antiviral response in Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:7257–7262. doi: 10.1073/pnas.0409181102. [DOI] [PMC free article] [PubMed] [Google Scholar]