Abstract
In the present study, we have investigated the anatomic distribution in blood and gut mucosal tissues of memory poxvirus-specific CD4 and CD8 T cells in subjects vaccinated with smallpox and compared it with vector (NYVAC)-specific and HIV insert-specific T-cell responses induced by an experimental DNA-C/ NYVAC-C vaccine regimen. Smallpox-specific CD4 T-cell responses were present in the blood of 52% of the subjects studied, while smallpox-specific CD8 T cells were rarely detected (12%). With one exception, smallpox-specific T cells were not measurable in gut tissues. Interestingly, NYVAC vector-specific and HIV-specific CD4 and CD8 T-cell responses were detected in almost 100% of the subjects immunized with DNA-C/NYVAC-C in blood and gut tissues. The large majority (83%) of NYVAC-specific CD4 T cells expressed α4β7 integrins and the HIV coreceptor CCR5. These results demonstrate that the experimental DNA-C/NYVAC-C HIV vaccine regimen induces the homing of potentially protective HIV-specific CD4 and CD8 T cells in the gut, the port of entry of HIV and one of the major sites for HIV spreading and the depletion of CD4 T cells.
INTRODUCTION
Replication-defective adenovirus (Ad) and poxvirus-derived vectors are among the most studied T-cell-based vaccine platforms against human immunodeficiency virus (HIV) (21, 36).
Recently, two HIV vaccine trials evaluating the efficacy of these two vectors generated different clinical outcomes. The phase IIb test-of-concept efficacy study called STEP, which evaluated a trivalent Ad5-Gag/Pol/Nef vaccine candidate, was prematurely terminated due to a lack of efficacy of the vaccine (6, 20). Furthermore, unexpectedly, the vaccine recipients with higher titers of preexisting neutralizing antibodies (Abs) (NAbs) to Ad5 (Ad5-NAbs) (Ad5 titers of >18) and who were uncircumcised showed increased susceptibility to HIV infection compared to vaccine recipients with lower titers (<18) of Ad5-NAbs and/or subjects who were circumcised and placebo recipients (6). The increased acquisition of HIV infection in the group of vaccine recipients with higher titers of Ad5-NAbs and who were uncircumcised has been further confirmed by long-term follow-up analyses (A. Duerr, H. Y. Z. Moodie, D. Lawrence, M. Robertson, and S. Buchbinder, presented at the AIDS Vaccine Meeting 2010, Atlanta, GA, 2010). These analyses have indicated that the increased susceptibility to HIV infection was concentrated during the 6 months post-vaccine administration, suggesting a causal link between Ad5 vaccination, the circumcision status, and increased HIV acquisition.
Several hypotheses were proposed to explain the increased acquisition of HIV infection among vaccine recipients in the STEP trial. These include (i) the Ad5 vaccine-mediated activation of Ad5-specific CD4 T cells that in turn may become the ideal targets for HIV infection and support virus replication and spreading; (ii) the generation of “enhancing antibodies” that facilitate HIV infection; (iii) the activation of dendritic cells (DC) through immune complexes composed of Ad5 particles and Ad5-NAbs, which may facilitate HIV infection of DC and spreading to CD4 T cells at the site of virus entry, i.e., mucosal surfaces; and (iv) the unique microenvironment of the mucosal compartment, where the mechanisms for the increased acquisition of HIV infection likely operate (8, 31, 35). Despite major efforts, the mechanism(s) responsible for the increased susceptibility to HIV infection in vaccine recipients with high Ad5-NAb titers remains elusive.
The RV-144 trial evaluated the efficacy of the poxvirus ALVAC-HIV (vCP1521) in combination with a recombinant gp120 subunit vaccine (AIDSVAX B/E) (32). The results indicated that this vaccine combination was effective in preventing infection, i.e., 31.2% efficacy, while it showed no effect on the levels of viremia and/or CD4 T-cell counts in vaccinated subjects for whom HIV-1 infection was subsequently diagnosed (32). These results, although showing a modest efficacy, demonstrate for the first time that an HIV vaccine is capable of preventing HIV infection.
HIV vaccine candidates based on rare adenovirus serotypes, such as Ad26 and Ad35 vectors, and poxvirus vectors are important components of promising vaccine candidates for advanced clinical development.
For these reasons, it is important to comprehensively characterize the preexisting immunity against the virus vector-based vaccines and vaccine-induced immune responses in different anatomical compartments and particularly at mucosal sites, which represent the primary port of entry and replication for HIV (4). In the present study, we have characterized the distribution of memory poxvirus- and Ad-specific T-cell responses in blood and gut mucosal tissues (rectum and ileum) induced by smallpox vaccination and by adenovirus natural infection in order to evaluate preexisting immunity against the poxvirus and Ad vector HIV vaccine candidate. Furthermore, poxvirus vector-specific and HIV insert-specific T-cell responses induced by a DNA-C/NYVAC-C (HIV clade C) vaccine regimen were also studied. This vaccine regimen was previously shown (EuroVacc 02 phase I/II clinical trial with HIV-uninfected individuals) to be safe, to be highly immunogenic (90% of responders), and to induce vigorous, polyfunctional, broad, and durable T-cell responses (12).
MATERIALS AND METHODS
Study groups.
Blood samples and gut biopsy specimens were obtained from 30 HIV-uninfected volunteers and 6 individuals enrolled in a previous HIV vaccine trial, i.e., the EV03 trial (Y. E. Levy et al., presented at the 17th Conference of Retroviruses and Opportunistic Infections, 2010), who agreed to colonoscopy and gut biopsy. The average age of the smallpox-vaccinated group was 52 years, and since smallpox vaccination in Switzerland (Europe) was stopped in 1974, all the subjects in this group received smallpox vaccination. The average age of the subjects in the six DNA/NYVAC vaccine groups who were enrolled in the present study was 30 years, and only 1 out of 6 subjects (42 years old) was smallpox vaccinated. The individuals enrolled in the EV03 trial were immunized with 3 DNA-C injections (4 mg at weeks 0, 4, and 8) plus 1 NYVAC-C injection (107 PFU at week 24). The six individuals were studied about 2 years after receiving the last immunization. This study was approved by the Institutional Review Board of the Centre Hospitalier Universitaire Vaudois, and informed consent was obtained from all volunteers.
Vectors.
Two E1/E3-deleted Ad5 vectors (Adβgal and AdGFP) were used in this study and were previously described (30). Briefly, Adβgal harbors a lacZ expression cassette and was used to stimulate Ad-specific T cells and for in vitro HIV infection (6 × 109 physical particles [pp]/ml). AdGFP harbors a green fluorescent protein (GFP) expression cassette and was used for the assessment of NAb titers. Ad5 vector titers were measured in virus physical particles (pp/cell) as described previously by Mittereder et al. (23). Parental NYVAC and a recombinant vector expressing Gag, Pol, and Nef of HIV-1 from clade C (referred to as NYVAC-C) were previously described (9, 10).
Ad5-NAb titers.
The neutralizing activity of the plasma of each volunteer enrolled was determined by transduction inhibition assays using the 911 cell line (28).
Flow cytometry analyses.
The following antibodies were used: CD4-allophycocyanin (APC), CD8-peridinin chlorophyll protein (PerCP)-Cy5.5, CD3-electron-coupled dye (ECD), CCR5-AF700, β7 integrin-phycoerythrin (PE), β4 integrin-PE-CY5, cutaneous lymphocyte antigen (CLA)-biotin, and streptavidin-PE-CY7. CD4-APC, CD8-PerCP-Cy5.5, and β7 integrin-PE were purchased from BD Biosciences; CD3-ECD was purchased from Beckman Coulter; and CLA-biotin, CCR5-AF700, and α4 integrin-PE-CY5 were purchased from Biolegend. Dead cells were excluded by use of the violet Live/Dead stain kit (Invitrogen).
Ex vivo proliferation assays.
Mononuclear cells were isolated either from peripheral blood using Ficoll-Histopaque separation (29) or following collagenase digestion of gut biopsy specimens. Five tissue samples were collected from both the terminal ileum and the rectum. Gut samples were taken during routine colonoscopy in patients with normal colonoscopic findings. Colonoscopies were performed under conscious sedation with midazolam and pethidine in moderate doses with a Pentax type EC 3890 Fi colonoscope (Pentax, Tokyo, Japan). Tissue samples were collected with Radial Jaw 4 forceps (Boston Scientific Corporate, Natick, MA). Biopsy specimens were then incubated at 37°C for 90 min with RPMI medium containing 0.5 mg/ml type II-S collagenase (Sigma). Mononuclear cells from each anatomic region were resuspended at 106 cells/ml in phosphate-buffered saline (PBS) and incubated for 7 min at 37°C with 0.25 μM 5,6-carboxyfluorescein succinimidyl ester (CFSE; Molecular Probes). The reaction was quenched with 1 volume of fetal bovine serum (FBS). Subsequently, cells were washed, cultured in 4% human AB serum (Institut Jacques Boy, France) and RPMI medium, and stimulated with ΔE1 Adβgal (109 pp/ml), empty NYVAC (106 PFU/ml), or HIV peptide pools encompassing the Gag, Pol, Nef, and Env regions (1 μg/ml) for 6 days (1). Cells were also stimulated with Staphylococcus enterotoxin B (SEB) (100 ng/ml) (positive control). At the end of the incubation period, cells were washed and stained with Abs (see “Flow cytometry analyses”) to CD3, CD4, CD8, CCR5, α4 and β7 integrins, and the cell adhesion molecule CLA. Data were acquired on an LSRII four-laser instrument (408, 488, 633, and 405 nm) and analyzed by using FlowJo. Between 2 × 105 and 2 × 106 events were acquired in the flow cytometry experiments. The percentage of proliferating CD4+ and CD8+ T cells, i.e., CFSE-low cells, was determined from the CD3+ cell population. The criteria for scoring of the proliferating cell cultures as positive included (i) a percentage of CFSE-low cells of >1% after subtracting background (percentage of CFSE-low cells in unstimulated cell cultures) and (ii) a stimulation index (SI) of >3. The SI was calculated by the fold increase between stimulated and unstimulated cell cultures. The expressions of integrins, chemokine receptors, and cell adhesion molecules on antigen-specific CD4 and CD8 T cells were established on CFSE-low proliferating CD4 or CD8 T cells.
HIV infection in vitro.
Mononuclear cells isolated either from peripheral blood or from gut tissues (rectum plus ileum) (n = 5) were stimulated with Adβgal (6 × 109 pp/ml) or SEB (positive control) or remained unstimulated. After 3 days of exposure, cell cultures were washed and exposed to 30 picograms of HIV-1BAL for 3 h. At the end of the incubation, cells were washed and replated, and HIV replication was assessed by measuring p24 levels in the culture supernatant at days 0, 4, and 7 postexposure. HIV infection was also determined intracellularly by flow cytometry. Following 7 days of HIV exposure, mononuclear cells were permeabilized and stained with CD3-ECD, CD4-APC, CD8-PerCP-Cy5.5, and P24-RD-1 (KC57; Beckman Coulter) monoclonal antibodies (MAbs). Cell culture supernatants were also assayed for P24 content at days 0, 4, and 7 by using an enzyme-linked immunosorbent assay (ELISA) (Innogenetics).
Statistical analyses.
P values were derived from either chi-square analysis, for comparison of positive proportions; one-way analysis of variance (ANOVA) (Kruskal-Wallis test) followed by a Student t test, in the context of multiple comparisons; or a Spearman rank test for correlations.
RESULTS
Ad-specific but not poxvirus-specific CD4 or CD8 T cells reside in gut mucosal tissues.
Cellular immunity (T cells) to different Ad serotypes is highly cross-reactive (29, 37). For these reasons, we have used the terminology of “Ad-specific T cells” and not “Ad5-induced T cells” to define T-cell responses specific to adenovirus. We have also used the terminology of “smallpox-specific T cells” to define memory T-cell responses observed for subjects vaccinated with smallpox and NYVAC-specific T-cell responses to define memory T-cell responses detected in subjects who received the DNA-C/NYVAC-C vaccine regimen.
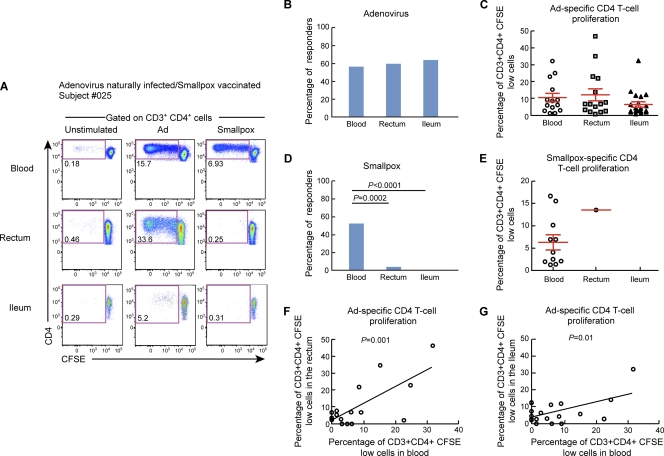
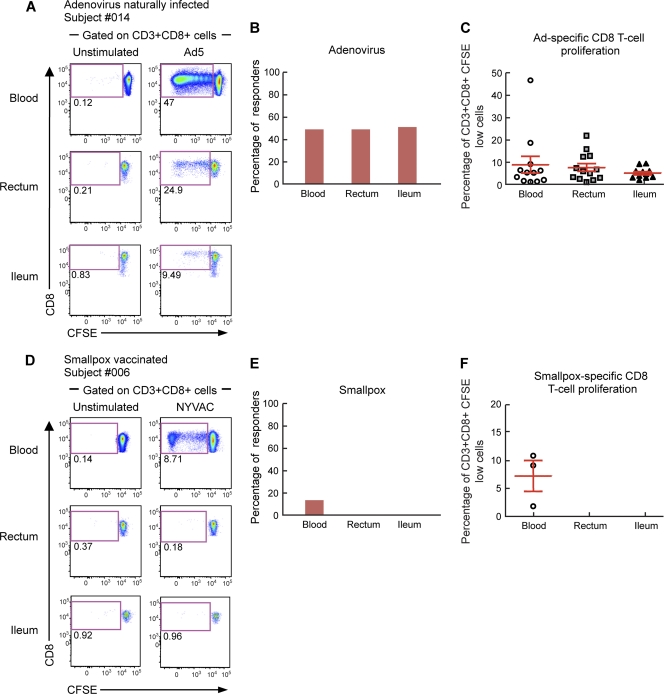
To evaluate the anatomic distribution of Ad-specific versus smallpox-specific CD4 and CD8 T cells, we analyzed the presence of Ad-specific and smallpox-specific CD4 and CD8 T cells in mononuclear cell populations isolated from blood and gut mucosal tissues (ileum and rectum). To assess virus-specific T-cell responses, blood mononuclear cells isolated from both blood and gut mucosal tissues (ileum and rectum) from HIV-uninfected individuals were labeled with CFSE and stimulated for 6 days with Ad5 or empty NYVAC vectors. At the end of the stimulation period, cells were stained with CD3, CD4, and CD8 MAbs. As shown by representative flow cytometry profiles of subjects 025 and 014 (Fig. 1A and 2A) and by the cumulative data from the 25 subjects studied (Fig. 1B and C and 2B and C), Ad-specific CD4 and CD8 T cells were detected in both blood and gut mucosal tissues. The proportions of individuals having detectable Ad-specific CD4 and CD8 T cells (referred to as responders) were similar in the two compartments, i.e., 56% in blood versus 58.3% in the rectum and 62.5% in the ileum for CD4 T cells and 48% in blood versus 48% in the rectum and 50% in the ileum for CD8 T cells (Fig. 1B and 2B). The levels of T-cell proliferation (percentage of CFSE-low cells among total CD4 or CD8 T cells) were similar among the two compartments for both Ad-specific CD4 and CD8 T cells (Fig. 1C and 2C). These results demonstrated that preexisting Ad-specific CD4 and CD8 T cells also resided in gut mucosal tissues. Interestingly, the frequencies of CD4 T cells proliferating in response to Ad5 stimulation correlated between blood and gut tissues (rectum, P = 0.001; ileum, P = 0.01), indicating that the evaluation of immunological measures of Ad-specific CD4 T-cell responses in blood might reflect those of Ad-specific CD4 T cells resident in gut mucosal tissues.
Fig. 1.
Adenovirus-specific but not smallpox-specific CD4 T cells are detected in gut mucosal tissues. Mononuclear cells were isolated from blood and gut mucosal tissues (rectum and ileum), stained with CFSE, and stimulated with Ad5 or NYVAC vectors. Following 6 days of incubation, cells were stained with CD3, CD4, and CD8 MAbs. (A) Flow cytometric profiles of proliferating Ad-specific and smallpox-specific CD4 T cells from mononuclear cells isolated from blood and mucosal tissues (rectum and ileum). Data from one representative subject (subject 025) are shown. The flow cytometric profile of unstimulated cells (negative control) is also shown. (B and C) Percentages of Ad-specific (B) and smallpox-specific (C) CD4 T-cell responders (volunteers with CD4 T-cell responses). (D and E) Frequencies of proliferating Ad-specific (D) and smallpox-specific (E) CD4 T cells. (F and G) Direct correlation between Ad-specific CD4 T-cell proliferation detected in blood and the rectum (F) or the ileum (G). Statistical analyses were performed by using chi-square methods for comparisons of positive proportions, a Student t test for multiple comparisons, or a Spearman rank test for correlations.
Fig. 2.
Adenovirus-specific but not smallpox-specific CD8 T cells are detected in gut mucosal tissues. Mononuclear cells were isolated from blood and gut mucosal tissues (rectum and ileum), stained with CFSE, and stimulated with Ad5 or NYVAC vectors. Following 6 days of incubation, cells were stained with CD3, CD4, and CD8 MAbs. (A and D) Flow cytometric profiles of proliferating Ad-specific (A) and smallpox-specific (D) CD8 T cells from mononuclear cells isolated from blood and mucosal tissues (rectum and ileum). Data from two representative subjects (subject 014 and 006) are shown. The flow cytometric profiles of unstimulated cells (negative control) are also shown. (B and C) Percentages of Ad-specific (B) and smallpox-specific (C) CD8 T-cell responders (volunteers with CD8 T-cell responses). (E and F) Frequencies of proliferating Ad-specific (E) and smallpox-specific (F) CD8 T cells. Statistical analyses were performed by using chi-square methods for comparisons of positive proportions and a Student t test for multiple comparisons.
As shown for one representative individual (Fig. 1A and 2D) and by the cumulative data (Fig. 1D and E and 2E and F), smallpox-specific CD4 and CD8 T cells were detected mainly in blood and very rarely (1 out of 12) in gut mucosal tissues. The proportions of smallpox-specific T-cell responders were dramatically reduced (4% and 0%) for both CD4 T-cell responses in gut mucosal tissues (rectum and ileum, respectively) compared to blood (52%; P = 0.0002 and P < 0.0001, respectively) and CD8 T cells (12% in blood versus 0% in gut tissues; P > 0.05) (Fig. 1D and 2D). Therefore, memory smallpox-specific T-cell responses studied more than 30 years after vaccination were rarely detected in gut mucosal tissues.
Frequencies of Ad-specific CD4 T cells in blood and gut mucosal tissues do not correlate with Ad5-specific neutralizing antibody titers.
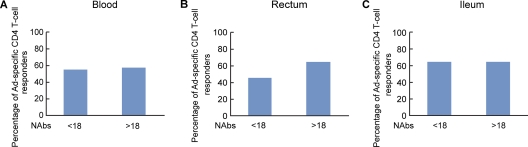
Recently, two studies have found no correlation between Ad5-NAb titers and levels of proliferating Ad-specific CD4 T cells isolated from blood before or after Ad5 immunization (15, 25). It was then relevant to determine whether Ad5-NAb titers correlated with the presence and/or the proliferative capacity of Ad-specific CD4 T cells isolated from gut mucosal tissues in naturally adenovirus-infected volunteers. To address this issue, Ad5-NAb titers were determined for each volunteer as previously described (28). The volunteers were stratified into two groups based on their Ad5 serostatus: (i) Ad5 NAb low (titer, <18) and (ii) Ad5 NAb high (>18) (6, 20). The proportions of Ad-specific responders (individuals with CD4 T-cell proliferation) were similar between volunteers with low and those with high titers of Ad5 NAbs regardless of the anatomic compartment investigated (Fig. 3). In addition, the percentage of Ad-specific CD4 T cells (percentage of CD3+ CD4+ CFSE-low cells) did not correlate with Ad5 NAb titers in cells isolated from both blood and gut (see Fig. S1 in the supplemental material).
Fig. 3.
Frequencies of Ad-specific CD4 T cells in blood and in mucosal tissues do not correlate with Ad5-specific neutralizing antibody titers. Ad5-NAb titers were determined for each volunteer as described in Materials and Methods. The volunteers were arbitrarily stratified by their Ad5 serostatus into two groups: Ad5-NAb low (Ad5-NAb titers of <18; n = 12) and Ad5-NAb high (Ad5-NAb titers of >18; n = 13). The percentages of Ad-specific CD4 T-cell responders (volunteers with Ad-specific CD4 T-cell proliferation) were compared in volunteers with low and high Ad5-NAb titers for each compartment: peripheral blood (A), rectum (B), and ileum (C). Statistical analyses were performed by using chi-square methods for comparisons of positive proportions.
Anatomic distribution of NYVAC- and HIV-specific CD4 and CD8 T-cell populations.
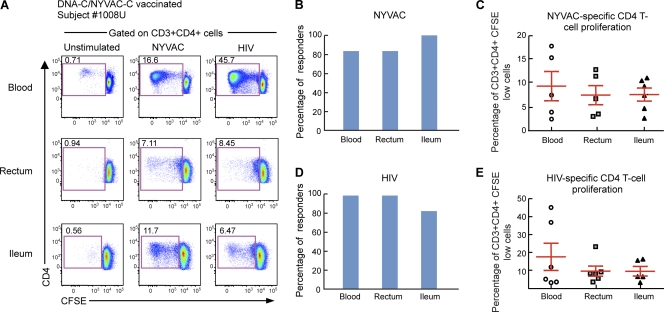
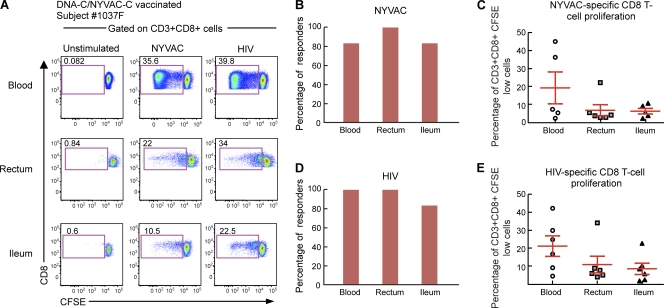
Six individuals enrolled in a previous HIV vaccine trial, i.e., the EV03 trial (Levy et al., presented at the 17th Conference of Retroviruses and Opportunistic Infections, 2010), who agreed to colonoscopy and gut biopsy, were investigated for the presence of vector (NYVAC)-specific and insert (HIV)-specific T-cell responses in both blood and gut after vaccination. The individuals enrolled in the EV03 trial were immunized with 3 DNA-C injections (4 mg at weeks 0, 4, and 8) plus 1 NYVAC-C injection (107 PFU at week 24). The DNA and NYVAC vaccines expressed Env, Gag, Pol, and Nef from HIV-1 clade C isolate CN54 (9). The six individuals were studied about 2 years after receiving the last immunization. As for the Ad- and smallpox-specific T cells (Fig. 1 and 2), NYVAC- and HIV-specific T-cell responses were measured by antigen-specific proliferation in mononuclear cells isolated from both blood and gut mucosal tissues (ileum and rectum) of the same subjects. Cells were labeled with CFSE and stimulated for 6 days with the empty (not expressing HIV antigens) NYVAC vector or with HIV peptide pools. At the end of the incubation period, cells were stained with CD3, CD4, and CD8 MAbs. As shown by a representative flow cytometry profile of subjects 1008U and 1037F (Fig. 4A and 5A) and by the cumulative data (Fig. 4B and C and 5B and C), NYVAC-specific CD4 and CD8 T cells were detected in both blood and gut mucosal tissues (ileum and rectum). The proportions of individuals having detectable proliferating NYVAC-specific CD4 and CD8 T cells were similar for blood (83.3%) and gut tissues (rectum, 83.8%; ileum, 100%) (Fig. 4B and 5B). The magnitudes of the T-cell proliferation were similar between the two anatomic compartments for NYVAC-specific CD4 and CD8 T cells (Fig. 4C and 5C).
Fig. 4.
NYVAC-specific and HIV-specific CD4 T cells home to gut mucosal tissues. Mononuclear cells were isolated from blood and gut mucosal tissues (rectum and ileum) of subjects (n = 6) vaccinated with the DNA-C/NYVAC-C regimen in the EV03 study, stained with CFSE, and stimulated with NYVAC empty vectors or HIV peptide pools encompassing the Env, Gag, Pol, and Nef regions. Following 6 days of incubation, cells were stained with CD3, CD4, and CD8 MAbs. (A) Flow cytometric profiles of proliferating NYVAC-specific and HIV-specific CD4 T cells in mononuclear cells isolated from blood and mucosal tissues (rectum and ileum). Data from one representative subject (subject 1008U) are shown. The flow cytometric profiles of unstimulated cells (negative control) are also shown. (B and C) Percentage of NYVAC-specific (B) or HIV-specific (C) responders (volunteers with CD4 T-cell responses). (D and E) Frequencies of proliferating NYVAC-specific (D) or HIV-specific (E) CD4 T cells. Statistical analyses were performed by using chi-square methods for comparisons of positive proportions or by a Student t test in the case of multiple comparisons.
Fig. 5.
NYVAC-specific and HIV-specific CD8 T cells home to gut mucosal tissues. Mononuclear cells were isolated from blood and gut mucosal tissues (rectum and ileum) of subjects (n = 6) vaccinated with the DNA-C/NYVAC-C regimen in the EV03 study, stained with CFSE, and stimulated with NYVAC empty vectors or HIV peptide pools encompassing the Gag, Pol, Nef, and Env regions. Following 6 days of incubation, cells were stained with CD3, CD4, and CD8 MAbs. (A) Flow cytometric profiles of proliferating NYVAC-specific and HIV-specific CD8 T cells in mononuclear cells isolated from blood and mucosal tissues (rectum and ileum). Data from one representative subject (subject 1037F) are shown. The flow cytometric profiles of unstimulated cells (negative control) are also shown. (B and C) Percentage of NYVAC-specific (B) or HIV-specific (C) responders (volunteers with CD8 T-cell responses). (D and E) Frequencies of proliferating NYVAC-specific (D) or HIV-specific (E) CD8 T cells. Statistical analyses were performed by using chi-square methods for comparisons of positive proportions or by a Student t test in the case of multiple comparisons.
These results demonstrate that recent (about 2 years) intramuscular immunization with DNA-C plus NYVAC-C led to the generation of NYVAC-specific CD4 and CD8 T cells in blood and in gut tissues.
Along the same line, the generation of HIV-specific CD4 and CD8 T in both blood and gut tissues cells was investigated. Interestingly, HIV-specific CD4 and CD8 T cells were detected in both blood and gut mucosal tissues (ileum and rectum). Data for representative subjects (subjects 1008U and 1037F) are shown in Fig. 4A and 5A. Cumulative data generated from the analysis of the 6 subjects are also shown (Fig. 4D and E and 5D and E). All subjects studied showed HIV-specific CD4 and CD8 T-cell responses in both blood and rectum and 83.3% in the ileum (Fig. 4D and 5D), indicating that the DNA/NYVAC vaccine regimen induced the generation of HIV-specific CD4 and CD8 T-cell responses in blood and gut mucosal tissues. The frequencies of HIV-specific CD4 and CD8 T-cell responses tended to be higher for blood than for gut tissues (Fig. 4E and 5E). However, these differences were not significant (P > 0.05). The percentage of HIV-specific CD4 T cells was 17.6% in blood versus 9.7% in the rectum and 9.6% in the ileum, and the percentage of CD8 T cells was 21.09% in blood, 10.8% in the rectum, and 7.75% in the ileum (Fig. 4E and 5E).
Proliferating Ad-specific and NYVAC-specific CD4 T cells express higher levels of α4β7 integrins than smallpox-specific CD4 T cells.
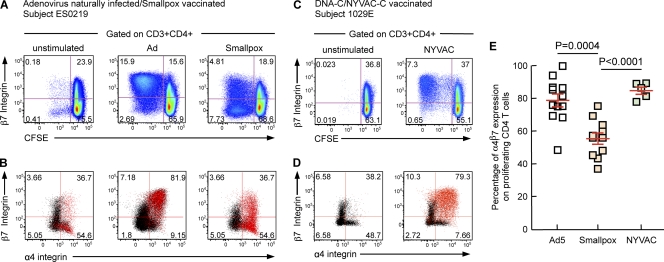
The tissue migration capacity of lymphocytes is orchestrated by the combined expression of specific integrins and chemokine receptors. The classical T-cell migration paradigm opposes mucosal tropism to skin tropism (11, 16, 19, 22). In order to migrate to the small intestine, T lymphocytes have to express the chemokine receptor CCR9 (41), which recognizes CCL25, constitutively released in the small intestine (17, 40) but not in the colon (17, 27, 41), and to express α4β7 integrins, which bind MAdCAM-1 on endothelial cells (3, 39). In order to home to the skin, T lymphocytes have to express both CCR4 and cutaneous lymphocyte antigen (CLA) (7, 33). Of note, whereas CCR9 expression is required for small intestine migration, the chemokine receptor(s) involved in migration to other regions of the gastrointestinal tract remains to be identified (24). We therefore examined the expressions of CCR4, CCR9, α4β7 integrins, and CLA in proliferating Ad-specific, smallpox-, and NYVAC-specific CD4 T cells isolated from the blood of naturally adenovirus-infected (n = 12), smallpox-vaccinated (n = 10), and NYVAC-vaccinated (n = 5) individuals. To perform this analysis, mononuclear cells were isolated from blood, labeled with CFSE, and stimulated for 6 days with Ad5 or NYVAC vectors. The chemokine receptor and cell adhesion molecule profiles of proliferating T cells were determined by multiparametric flow cytometry. As shown by data from the representative individuals ES0219 and 1029E (Fig. 6A and D) and by the cumulative data (Fig. 6E), proliferating (CFSE-low cells) Ad-specific and NYVAC-specific CD4 T cells isolated form peripheral blood expressed higher levels of α4β7 integrins than did smallpox-specific CD4 T cells, i.e., Ad-specific levels of 78.6% versus smallpox-specific levels of 55.27% versus NYVAC-specific levels of 84.62% (P = 0.0002 and P < 0.0001, respectively). In addition, Ad-specific, smallpox-specific, and NYVAC-specific CD4 T cells expressed intermediate levels of CLA, low levels of CCR9, and high levels of CCR4 (data not shown). These results suggested that proliferating Ad-specific, smallpox-specific, and NYVAC-specific CD4 T cells isolated from blood have the potential to home to gut mucosal tissues. The reduced α4β7 integrin expression levels in smallpox-specific CD4 T cells (versus Ad-specific and NYVAC-specific CD4 T cells) might potentially explain the distinct migratory capacity of these antigen-specific CD4 T cells. However, we cannot exclude that the differences between the migratory capacities of NYVAC-, Ad-, and smallpox-specific T cells and specific immune responses might be attributed to the increased time that had passed between antigen exposure and the measurement of antigen-specific immune responses. Furthermore, the route of smallpox vaccination (scarification) differed from those of natural Ad infections (usually mucosal routes [34]) and NYVAC vaccination (intramuscular).
Fig. 6.
Adenovirus-specific and NYVAC-specific CD4 T cells express higher levels of α4β7 integrins than do smallpox-specific CD4 T cells. Mononuclear cells were isolated from blood, stained with CFSE, and stimulated with Ad5 or NYVAC vectors. Following 6 days of incubation, cells were stained with CD3, CD4, CD8, and α4/β7 integrin MAbs. (A to D) Flow cytometric profiles of proliferating Ad-specific (A and B), smallpox-specific (A and B), and NYVAC-specific (C and D) CD4 T cells (CFSE-low cells) expressing β7 integrin or coexpressing α4 and β7 integrins. Black dots correspond to CD3+ CD4+ CFSE-high cells, and red dots correspond to CD3+ CD4+ CFSE-low cells. (E) Cumulative data for proliferating Ad-specific, smallpox-specific, or NYVAC-specific CD4 T cells (CD4 CFSE-low cells) expressing α4β7 integrins.
Ad-specific, smallpox-specific, and NYVAC-specific CD4 T cells isolated from peripheral blood and gut mucosal tissues express the HIV coreceptor CCR5.
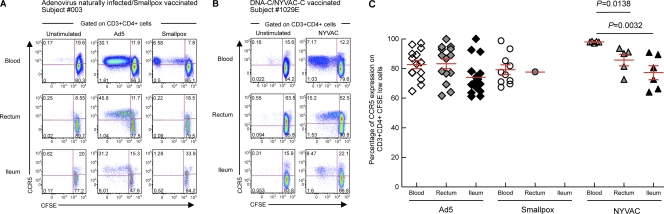
To determine whether proliferating vector-specific CD4 T cells isolated from blood and mucosal tissues were susceptible to HIV infection, the levels of expression of the HIV coreceptor CCR5 were evaluated by multiparametric flow cytometry. Mononuclear cells isolated from blood and gut mucosal tissues (rectum and ileum) of naturally Ad-infected individuals (n = 12), smallpox vaccine recipients (n = 10), and NYVAC vaccine recipients (n = 5) were labeled with CFSE and stimulated for 6 days with Ad5 or NYVAC vectors. At the end of the stimulation period, cells were stained with CD3, CD4, CD8, and CCR5 MAbs. As shown by data from representative subjects 003 and 1029E (Fig. 7A and B) and by the cumulative data (Fig. 7C), proliferating Ad-specific, smallpox-specific, and NYVAC-specific CD4 T cells expressed high levels of CCR5 in both the blood and gut compartments.
Fig. 7.
Proliferating Ad-specific, smallpox-specific, and NYVAC-specific CD4 T cells express high levels of HIV coreceptor CCR5. Mononuclear cells were isolated from peripheral blood and gut mucosal tissues (rectum and ileum), stained with CFSE, and stimulated with Ad5 or NYVAC vectors. Following 6 days of incubation, cells were stained with CD3, CD4, CD8, and CCR5 MAbs. (A and B) Flow cytometric profiles of proliferating (CFSE-low cells) Ad-specific (A), smallpox-specific (A), and NYVAC-specific (B) CD4 T cells in mononuclear cells isolated from blood and mucosal tissues (rectum and ileum) expressing CCR5. Two representative subjects (subjects 003 and 1029E) are shown. The flow cytometric profiles of unstimulated cells (negative control) are also shown. (C) Percentage of Ad-specific, smallpox-specific, and NYVAC-specific CD4 T cells (CD3+ CD4+ CFSE-low cells) expressing CCR5. P values were determined by using a Student t test.
As expected, the proliferating virus-specific CD4 T cells from both blood and gut were able to support efficient HIV infection in vitro (see Fig. S2 in the supplemental material).
DISCUSSION
Several replication-defective adenovirus and poxvirus vectors are among the most frequent strategies explored for the development of an HIV vaccine (21, 26, 36). There is a large consensus within the scientific community on the importance of inducing vaccine responses both systemically (blood) and at mucosal sites, i.e., at the port of entry of HIV (4). In addition, the gut has also been shown to represent a major site for HIV replication and the depletion of CD4 T cells (5). For these reasons, it is important to characterize the vaccine-induced immune responses in different anatomic compartments, including in blood and mucosal tissues such as the gut.
The efficacy of a vaccine strategy can be substantially influenced by the presence of preexisting immunity in the host (13). This issue is particularly important for vectors derived from adenovirus and poxvirus. There are 53 different Ad serotypes that infect a large proportion of the population, depending on the geographic region, and have tropisms for different anatomic compartments (34, 38). While Ad-NAbs are serotype specific, Ad-specific T-cell responses are largely cross-reactive (14, 28, 29, 37). The issue of preexisting immunity to poxvirus concerns only certain age groups, since smallpox vaccination was stopped in the mid-1970s.
In the present study, we have investigated (i) preexisting Ad- and smallpox-specific immunity in both blood and gut tissues, (ii) poxvirus vector (NYVAC)- and HIV-specific T-cell responses in both blood and gut tissues following vaccination with a DNA-C/NYVAC-C regimen in healthy volunteers, and (iii) the potential tropism of Ad-, smallpox-, and NYVAC-specific CD4 and CD8 T cells.
Memory Ad-specific CD4 and CD8 T-cell responses were consistently found in both blood and gut tissues (rectum and ileum) from a large proportion of subjects studied, while memory smallpox T-cell responses were found in blood (predominantly CD4) and were almost absent in gut tissues. The lack of detection of smallpox-specific T cells in the gut might be due either to the low frequency of memory T cells after at least 30 years from the time of smallpox vaccination or to the route of immunization (see below).
Therefore, on the basis of these results, Ad vector-based vaccines may induce cross-reactive T-cell responses both in blood and in gut tissues, while smallpox vaccines may induce cross-reactive T-cell responses predominantly in blood. The data on Ad vectors are consistent with previous studies performed with blood from humans and with both in blood and gut tissues from nonhuman primates (NHPs) (2, 18).
The lack of detection of smallpox-specific T cells in gut tissues raised the question of whether poxvirus vector-induced T-cell responses, both vector specific and HIV transgene specific, were also induced at mucosal sites. Healthy volunteers immunized with the DNA-C/NYVAC-C vaccine regimen were simultaneously investigated for the presence of vector- and HIV transgene-specific CD4 and CD8 T cells in blood and gut (rectum and ileum). The results consistently indicated that this vaccine regimen was able to induce vector- and HIV-specific CD4 and CD8 T-cell responses in both blood and gut tissues. An explanation for the differences between NYVAC and smallpox vaccinations in the induction of T-cell responses at mucosal sites remains unclear but could be related to the degree of virus replication in human tissues, as the smallpox vaccine represents a replication-competent virus, while NYVAC is a nonreplicating virus. In addition to what is mentioned above, it is worth mentioning that NYVAC was administered intramuscularly and that an NYVAC boost was preceded by three DNA administrations. In this regard, DNA priming substantially increases the immunogenicity of NYVAC, resulting in a larger proportion of responders and in a T-cell response several orders of magnitude greater than that with the administration of NYVAC alone (12).
Benlahrech et al. recently showed that proliferating Ad-specific CD4 T cells isolated from the blood of volunteers naturally infected with adenovirus expressed α4β7 integrins and CCR9, thus indicating that Ad5-specific CD4 T cells may migrate to the small intestine (2). In this regard, Masek-Hammerman et al. have clearly shown with NHPs that Ad5-specific CD4 T cells migrate to the small intestine and show no differences in the mucosal tropism of Ad5-induced CD4 T cells in monkeys in the presence or in the absence of preexisting immunity to Ad5 (18). In the present study, we have shown that poxvirus-specific T cells induced by smallpox and NYVAC vaccination also expressed high levels of α4β7 integrins. However, blood smallpox-specific CD4 T cells expressed significantly lower levels of α4β7 integrins than NYVAC-specific or Ad-specific CD4 T cells. The lower levels of expression of α4β7 integrins may partially explain the lack of detection of smallpox-specific T cells in the gut tissues. However, it is possible that the differences observed for the migratory capacities of NYVAC-, Ad-, and smallpox-specific T cells might result from the increased time which had passed between antigen exposure and the measurement of antigen-specific immune responses. Both Ad- and poxvirus-specific CD4 T cells expressed elevated and comparable levels of CCR5, and virus-specific CD4 T cells from both blood and gut tissues showed comparable susceptibility to HIV infection and supported viral replication in vitro.
In conclusion, the present study clearly shows that immunization with the DNA/NYVAC vaccine regimen is capable of inducing HIV-specific T-cell immunity both systemically and at mucosal sites such as the gut, which may serve as the port of entry for HIV and as a major anatomic site for virus replication and the depletion of CD4 T cells (4), and in turn, the immune response triggered might play an important role in the control of HIV infection.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to L. Poupon for technical help and all nurses and resident medical doctors involved in the collection of gut biopsy specimens.
This work was funded by the Swiss National Science Foundation. We declare no conflicting financial interests.
Footnotes
Supplemental material for this article may be found at http://jvi.asm.org/.
Published ahead of print on 20 July 2011.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1. Bart P. A., et al. 2008. EV01: a phase I trial in healthy HIV negative volunteers to evaluate a clade C HIV vaccine, NYVAC-C undertaken by the EuroVacc Consortium. Vaccine 26:3153–3161 [DOI] [PubMed] [Google Scholar]
- 2. Benlahrech A., et al. 2009. Adenovirus vector vaccination induces expansion of memory CD4 T cells with a mucosal homing phenotype that are readily susceptible to HIV-1. Proc. Natl. Acad. Sci. U. S. A. 106:19940–19945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Berlin C., et al. 1993. Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell 74:185–195 [DOI] [PubMed] [Google Scholar]
- 4. Brenchley J. M., Douek D. C. 2008. HIV infection and the gastrointestinal immune system. Mucosal Immunol. 1:23–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brenchley J. M., et al. 2004. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200:749–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buchbinder S. P., et al. 2008. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 372:1881–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Campbell J. J., et al. 1999. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature 400:776–780 [DOI] [PubMed] [Google Scholar]
- 8. Corey L., McElrath M. J., Kublin J. G. 2009. Post-step modifications for research on HIV vaccines. AIDS 23:3–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gomez C. E., et al. 2007. Generation and immunogenicity of novel HIV/AIDS vaccine candidates targeting HIV-1 Env/Gag-Pol-Nef antigens of clade C. Vaccine 25:1969–1992 [DOI] [PubMed] [Google Scholar]
- 10. Guerra S., et al. 2007. Distinct gene expression profiling after infection of immature human monocyte-derived dendritic cells by the attenuated poxvirus vectors MVA and NYVAC. J. Virol. 81:8707–8721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guy-Grand D., Griscelli C., Vassalli P. 1978. The mouse gut T lymphocyte, a novel type of T cell. Nature, origin, and traffic in mice in normal and graft-versus-host conditions. J. Exp. Med. 148:1661–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harari A., et al. 2008. An HIV-1 clade C DNA prime, NYVAC boost vaccine regimen induces reliable, polyfunctional, and long-lasting T cell responses. J. Exp. Med. 205:63–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harro C. D., et al. 2009. Safety and immunogenicity of adenovirus-vectored near-consensus HIV type 1 clade B gag vaccines in healthy adults. AIDS Res. Hum. Retroviruses 25:103–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Horwitz M. 1996. Adenoviruses, p. 2149–2171 In Fields B. N., et al. (ed.), Fields virology, 3rd ed., vol. 2 Raven Press, Philadelphia, PA [Google Scholar]
- 15. Hutnick N. A., et al. 2009. Baseline Ad5 serostatus does not predict Ad5 HIV vaccine-induced expansion of adenovirus-specific CD4+ T cells. Nat. Med. 15:876–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kantele A., Zivny J., Hakkinen M., Elson C. O., Mestecky J. 1999. Differential homing commitments of antigen-specific T cells after oral or parenteral immunization in humans. J. Immunol. 162:5173–5177 [PubMed] [Google Scholar]
- 17. Kunkel E. J., et al. 2000. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J. Exp. Med. 192:761–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Masek-Hammerman K., et al. 2010. Mucosal trafficking of vector-specific CD4+ T lymphocytes following vaccination of rhesus monkeys with adenovirus serotype 5. J. Virol. 84:9810–9816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McDermott M. R., Bienenstock J. 1979. Evidence for a common mucosal immunologic system. I. Migration of B immunoblasts into intestinal, respiratory, and genital tissues. J. Immunol. 122:1892–1898 [PubMed] [Google Scholar]
- 20. McElrath M. J., et al. 2008. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet 372:1894–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McMichael A. J. 2006. HIV vaccines. Annu. Rev. Immunol. 24:227–255 [DOI] [PubMed] [Google Scholar]
- 22. McWilliams M., Phillips-Quagliata J. M., Lamm M. E. 1977. Mesenteric lymph node B lymphoblasts which home to the small intestine are precommitted to IgA synthesis. J. Exp. Med. 145:866–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mittereder N., March K. L., Trapnell B. C. 1996. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J. Virol. 70:7498–7509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mora J. R. 2008. Homing imprinting and immunomodulation in the gut: role of dendritic cells and retinoids. Inflamm. Bowel Dis. 14:275–289 [DOI] [PubMed] [Google Scholar]
- 25. O'Brien K. L., et al. 2009. Adenovirus-specific immunity after immunization with an Ad5 HIV-1 vaccine candidate in humans. Nat. Med. 15:873–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pantaleo G., Esteban M., Jacobs B., Tartaglia J. 2010. Poxvirus vector-based HIV vaccines. Curr. Opin. HIV AIDS 5:391–396 [DOI] [PubMed] [Google Scholar]
- 27. Papadakis K. A., et al. 2000. The role of thymus-expressed chemokine and its receptor CCR9 on lymphocytes in the regional specialization of the mucosal immune system. J. Immunol. 165:5069–5076 [DOI] [PubMed] [Google Scholar]
- 28. Perreau M., Guerin M. C., Drouet C., Kremer E. J. 2007. Interactions between human plasma components and a xenogenic adenovirus vector: reduced immunogenicity during gene transfer. Mol. Ther. 15:1998–2007 [DOI] [PubMed] [Google Scholar]
- 29. Perreau M., Kremer E. J. 2005. Frequency, proliferation, and activation of human memory T cells induced by a nonhuman adenovirus. J. Virol. 79:14595–14605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Perreau M., et al. 2007. Contrasting effects of human, canine, and hybrid adenovirus vectors on the phenotypical and functional maturation of human dendritic cells: implications for clinical efficacy. J. Virol. 81:3272–3284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Perreau M., Pantaleo G., Kremer E. J. 2008. Activation of a dendritic cell-T cell axis by Ad5 immune complexes creates an improved environment for replication of HIV in T cells. J. Exp. Med. 205:2717–2725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rerks-Ngarm S., et al. 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 361:2209–2220 [DOI] [PubMed] [Google Scholar]
- 33. Sallusto F., Geginat J., Lanzavecchia A. 2004. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 22:745–763 [DOI] [PubMed] [Google Scholar]
- 34. Schmitz H., Wigand R., Heinrich W. 1983. Worldwide epidemiology of human adenovirus infections. Am. J. Epidemiol. 117:455–466 [DOI] [PubMed] [Google Scholar]
- 35. Sekaly R. P. 2008. The failed HIV Merck vaccine study: a step back or a launching point for future vaccine development? J. Exp. Med. 205:7–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shiver J. W., Emini E. A. 2004. Recent advances in the development of HIV-1 vaccines using replication-incompetent adenovirus vectors. Annu. Rev. Med. 55:355–372 [DOI] [PubMed] [Google Scholar]
- 37. Smith C. A., Woodruff L. S., Rooney C., Kitchingman G. R. 1998. Extensive cross-reactivity of adenovirus-specific cytotoxic T cells. Hum. Gene Ther. 9:1419–1427 [DOI] [PubMed] [Google Scholar]
- 38. Smith J. G., Wiethoff C. M., Stewart P. L., Nemerow G. R. 2010. Adenovirus. Curr. Top. Microbiol. Immunol. 343:195–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wagner N., et al. 1996. Critical role for beta7 integrins in formation of the gut-associated lymphoid tissue. Nature 382:366–370 [DOI] [PubMed] [Google Scholar]
- 40. Wurbel M. A., et al. 2000. The chemokine TECK is expressed by thymic and intestinal epithelial cells and attracts double- and single-positive thymocytes expressing the TECK receptor CCR9. Eur. J. Immunol. 30:262–271 [DOI] [PubMed] [Google Scholar]
- 41. Zabel B. A., et al. 1999. Human G protein-coupled receptor GPR-9-6/CC chemokine receptor 9 is selectively expressed on intestinal homing T lymphocytes, mucosal lymphocytes, and thymocytes and is required for thymus-expressed chemokine-mediated chemotaxis. J. Exp. Med. 190:1241–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.