Abstract
CD11c is expressed on the surface of dendritic cells (DCs) and is one of the main markers for identification of DCs. DCs are the effectors of central innate immune responses, but they also affect acquired immune responses to infection. However, how DCs influence the efficacy of adaptive immunity is poorly understood. Here, we show that CD11c+ DCs negatively orchestrate both adaptive and innate immunity against herpes simplex virus type 1 (HSV-1) ocular infection. The effectiveness and quantity of virus-specific CD8+ T cell responses are increased in CD11c-deficient animals. In addition, the levels of CD83, CD11b, alpha interferon (IFN-α), and IFN-β, but not IFN-γ, were significantly increased in CD11c-deficient animals. Higher levels of IFN-α, IFN-β, and CD8+ T cells in the CD11c-deficient mice may have contributed to lower virus replication in the eye and trigeminal ganglia (TG) during the early period of infection than in wild-type mice. However, the absence of CD11c did not influence survival, severity of eye disease, or latency. Our studies provide for the first time evidence that CD11c expression may abrogate the ability to reduce primary virus replication in the eye and TG via higher activities of type 1 interferon and CD8+ T cell responses.
INTRODUCTION
Following primary ocular herpes simplex virus type 1 (HSV-1) infection, the virus replicates in the eye (27). The higher that the viral load is, the longer that it takes for the immune system to clear the virus from the eye, which leads to more extensive and protracted ocular disease (19). In addition, a hallmark of infection with HSV-1 is the establishment of latency in ganglia of the infected individual (13, 57). During the lifetime of an infected individual, the latent virus can occasionally reactivate, travel back to the eye, and cause recurrent eye disease. Indeed, a major cause of corneal scarring (CS) is the scarring induced by the immune response to HSV-1 following reactivation from latency (3, 27). Although recurrent cold sores in the mouth are more common than ocular HSV-1 disease, recurrent ocular infection is the leading cause of corneal blindness due to an infectious agent in developed countries. In the United States, approximately 450,000 people have a history of recurrent ocular herpes that requires doctor visits, medication, and, in severe cases, corneal transplants (10, 11, 44, 47). HSV-1 also causes pharyngitis, genital lesions, and encephalitis (2, 34, 52, 55). Because of the preexisting immune response, CS is more likely to occur following recurrent than primary infection (6, 12). One way to decrease latency and thus subsequent recurrent infections and loss of vision is to reduce the ocular viral load by accelerating ocular viral clearance.
Viruses have evolved several mechanisms for immune evasion and immune counterattack. In the context of HSV-1, infection of immature dendritic cells (DCs) causes downregulation of costimulatory molecules, adhesion molecules, and major histocompatibility complex (MHC) class I molecules (25, 35). The result is a reduction in antigen presentation, as CD8+ T cells are seemingly unable to recognize affected DCs (66). The inhibition continues until a late stage of infection, when HSV-1 induces apoptosis of immature DCs in a caspase 8-dependent manner (5, 41). In addition, it has been shown that HSV-1 infection induces downregulation of chemokine receptors needed for migration in mature DCs, further slowing DC migration and antigen presentation (49).
DCs are powerful innate sentinels which serve to link innate and adaptive immune responses (9, 51). DCs are rapidly differentiating cells able to capture/process antigen and migrate to lymphoid sites to present antigen to T cells, thereby inducing adaptive immunity (56). CD11c is a major marker for identification of DCs (9, 51). CD11c is abundantly expressed on DCs, while low levels of expression have been detected on NK cells, cytotoxic T lymphocytes (CTLs), and macrophages (16, 50). However, CD11c is the most widely accepted DC marker, and targeting of DCs through CD11c and its receptor has shown promise in boosting adaptive immunity in vaccine studies (31, 63).
Mice genetically deficient in CD11c were previously characterized and have been shown to have reduced development of atherosclerosis (65). We have shown earlier that the increase of HSV-1 latency is correlated with the presence of lymphoid DCs and that depletion of DCs caused reduced latency in two strains of mice (39). We furthered this observation with a vaccine study, whereby DC depletion via diphtheria toxin combined with the 5gp DNA vaccine reduced latency by 5-fold in BALB/c mice (36). Taken together, these results suggest that DCs serve more as viral allies than enemies. Thus, we sought to determine if, similar to a previous study (65), the negative impact of DCs is associated with expression of CD11c. Here we investigated what role, if any, CD11c plays in control of ocular HSV-1 infection and latency by comparing the generation, maintenance, and in vivo effectiveness of antiviral responses to HSV-1 in CD11c−/− (knockout) versus CD11c+/+ (wild-type [wt] parental) mice. We report that at an early time after ocular infection, CD11c-deficient mice had decreased HSV-1 replication in both the eye and the trigeminal ganglia (TG). The decrease in virus replication is correlated with increased expression of CD83- and CD11b-positive cells in the spleen, increased alpha interferon (IFN-α) and IFN-β production in the eye, and increased HSV-1 glycoprotein B (gB)-specific CD8+ T cells in the TG. Taken together, the results of this study provide evidence that the mechanism behind HSV-1 exploitation of DCs may involve CD11c and its reduction can overcome the inhibitory effects of the virus on DC maturation and production of type 1 interferon, without obvious negative side effects in this murine model.
MATERIALS AND METHODS
Virus, cells, and mice.
Plaque-purified HSV-1 strain McKrae was grown in rabbit skin (RS) cell monolayers in minimal essential medium (MEM) containing 5% fetal calf serum (FCS), as described previously (46, 48).
Wild-type C57BL/6 mice were purchased from Jackson Laboratories. C57BL/6 CD11c−/− mice have been reported previously (65) and were bred in-house. All animal procedures adhered to the Association for Research in Vision and Ophthalmology (ARVO) statement for the Use of Animals in Ophthalmic and Vision Research and according to Cedars-Sinai Medical Center animal care and use guidelines. In all experiments, both knockout and wild-type mice were randomly allocated to groups.
Ocular infection.
Mice were infected ocularly with 2 × 105 PFU of strain McKrae suspended in 2 μl of tissue culture medium and administered as an eye drop.
Titration of virus in tears.
Tear films were collected from both eyes of 20 mice per group as described previously (17). Each swab was placed in 1 ml tissue culture medium, and the amount of virus in the medium was determined by a standard plaque assay on RS cells.
Titration of virus in trigeminal ganglia.
CD11c−/− and wt C57BL/6 mice were infected as described above. On days 3 and 5 postinfection (p.i.), 5 mice per group were euthanized and individual TG were isolated for a total of 10 TG per group. Individual TG were homogenized by centrifugation at 10,000 rpm for 30 s on ice. Any cellular debris was removed by centrifugation at 3,000 rpm for 10 min. The viral titer in the supernatant was then measured by standard plaque assay on RS cells as described previously (18, 21).
Monitoring of eye disease.
The severity of CS in 40 eyes from 4 separate experiments was scored in a masked fashion on day 30 p.i. Disease was scored on a scale of from 0 to 4 (0 = no disease, 1 = 25% involvement, 2 = 50% involvement, 3 = 75% involvement, and 4 = 100% involvement), as we described previously (17).
Cell surface staining of cells isolated from cornea, TG, and spleen.
Tissues from 5 mice per group were harvested and pooled into 2 samples per group on days 5 and 10 p.i. Cornea and TG were digested in a phosphate-buffered saline solution containing collagenase type I (3 mg/ml; Sigma-Aldrich, St. Louis, MO) and incubated for 2 h at 37°C with trituration approximately every 30 min as we described previously (39). The recovered cells were washed and stained with the following monoclonal antibodies (MAbs) and subjected to standard multicolor fluorescence-activated cell sorter (FACS) analyses to assess surface expression of various markers. We used peridinin chlorophyll protein CD3, PacBlue CD11b, allophycocyanin CD83, and a phycoerythrin HSV-1 CD8+ T cell pentamer specific for a peptide derived from residues 498 to 505 of gB (gB+498-505 pentamer) (all antibodies were from eBioscience and BD Biosciences, San Diego, CA, while HSV-1 gB+498-505 pentamer was from ProImmune Inc., Bradenton, FL). For cell surface staining, cells were incubated with CD3/CD11b/CD83/gB+498-505 pentamer MAbs following the manufacturer's protocol. Stained cells were washed twice with FACS buffer and fixed with BD Cytofix/Cytoperm solution for 20 min at 4°C. Following fixation, the cells were washed twice in BD Perm/Wash buffer, resuspended in 4% paraformaldehyde, and analyzed using a multicolor five-laser LSR II instrument (Applied Biosystems, Foster City, CA).
DNA extraction and PCR analysis for HSV-1 gB DNA.
DNA was isolated on days 3 and 5 p.i. from homogenized individual corneas and TG from 5 mice per group using a DNeasy blood and tissue kit (Qiagen, Stanford, CA) according to the manufacturer's instructions. PCR analyses were done using gB-specific primers (forward primer, 5′-AACGCGACGCACATCAAG-3′; reverse primer, 5′-CTGGTACGCGATCAGAAAGC-3′; probe, 5′-FAM-CAGCCGCAGTACTACC-3′, where FAM is 6-carboxyfluorescein). The amplicon length for this primer set is 72 bp. Relative copy numbers for gB were calculated using standard curves generated from the plasmid pAc-gB1. In all experiments, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used for normalization of transcripts.
RNA extraction, cDNA synthesis, and TaqMan reverse transcription-PCR.
The corneas, TG, and spleen from individual mice that survived ocular infection were collected on days 2, 4, and 6 p.i., immersed in RNAlater RNA stabilization reagent, and stored at −80°C until processing. There were a total of 4 mice per group per time point (n = 8 for cornea and TG and n = 4 for spleen). Tissue processing of spleen, corneas, and TG, total RNA extraction, and RNA yield determination were carried out as we have described previously (37, 38). Following RNA extraction, 1,000 ng of total RNA was reverse transcribed using random hexamer primers and murine leukemia virus (MuLV) reverse transcriptase from a high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA), in accordance with the manufacturer's recommendations.
The differences in the expression levels of IFN-α, IFN-β, and IFN-γ mRNA were evaluated using the custom-made TaqMan gene expression primers described below. In all experiments, GAPDH was used for normalization of transcripts. The expression levels of the various transcripts were evaluated using commercially available TaqMan gene expression assays (Applied Biosystems, Foster City, CA) with optimized primer and probe concentrations. Primer probe sets consisted of two unlabeled PCR primers and the FAM dye-labeled TaqMan minor groove-binding probe formulated into a single mixture. Additionally, all cellular amplicons included an intron-exon junction to eliminate signal from genomic DNA contamination. The assays used in this study were as follows: (i) for IFN-α, ABI assay Mm00833961_s1 and an amplicon length of 158 bp; (ii) for IFN-β, ABI assay Mm00439552_s1 and an amplicon length of 69; and (iii) for IFN-γ, ABI assay Mm00801778_m1 and an amplicon length of 101 bp.
Quantitative real-time PCR (qRT-PCR) was performed using an ABI Prism 7900HT sequence detection system (Applied Biosystems, Foster City, CA) in 384-well plates as we described previously (37, 38). Real-time PCR was performed in triplicate for each tissue sample. The threshold cycle (CT) values, which represent the PCR cycles at which there is a noticeable increase in the reporter fluorescence above baseline, were determined using SDS (version 2.2) software.
Immunohistochemistry (IHC).
Corneas and spleens were collected from four individual mice per group that survived ocular infection on days 5 and 10 p.i. The tissues were embedded in optimal cutting temperature compound (OCT; Tissue-Tek, Sakura) for cryosectioning and stored at −80°C. Cryostat sections 10 μm thick were cut, air dried overnight, and then fixed in 4% paraformaldehyde for 1 h at 4°C Single immunostaining was performed using anti-CD8 MAb (clone 53-6.7) according to the manufacturer's protocol at 4°C overnight. Alexa Fluor 488 (Invitrogen) antirat secondary antibody was incubated on tissue for 1 h at room temperature. Washed sections were air dried and mounted with 4′,6-diamidino-2-phenylindole (DAPI) prolong Gold (Invitrogen). The fluorophores were imaged in separate channels with a Zeiss ApoTome-equipped Axio Imager Z1 (Carl Zeiss Microimaging). Images were then analyzed using Image J software, release 1.40g. The number of cells immunopositive for CD8 was counted in a double-blind fashion from the entire tissue section. To reduce possible staining or sampling variability, three consecutive sections from each tissue sample were examined on each slide. One section was used to quantitate the number and distribution of cells. The second and third sections were used to verify these results, thereby confirming accuracy and ensuring a lack of artifacts due to staining or tissue manipulation. Spleen tissue was used as a positive histological control for CD8 staining.
Statistical analysis.
Student's t test and analysis of variance were performed using the computer program Instat (GraphPad, San Diego, CA). Results were considered statistically significant when the P value was less than 0.05.
RESULTS
Effect of CD11c deficiency on survival and virus replication following ocular HSV-1 infection.
Four independent experiments were done using a total of 33 CD11c−/− mice and 20 C57BL/6 wt mice. CD11c−/− mice and wt mice were infected ocularly with strain McKrae, a neurovirulent, stromal disease-causing HSV-1 strain, as described in Materials and Methods. Thirty of 33 (91%) CD11c−/− mice survived ocular infection to day 30 p.i. This was not significant compared to the rate of survival for parental C57BL/6 mice in this experiment (20 of 20 survived; P = 0.28, the Fisher exact test).
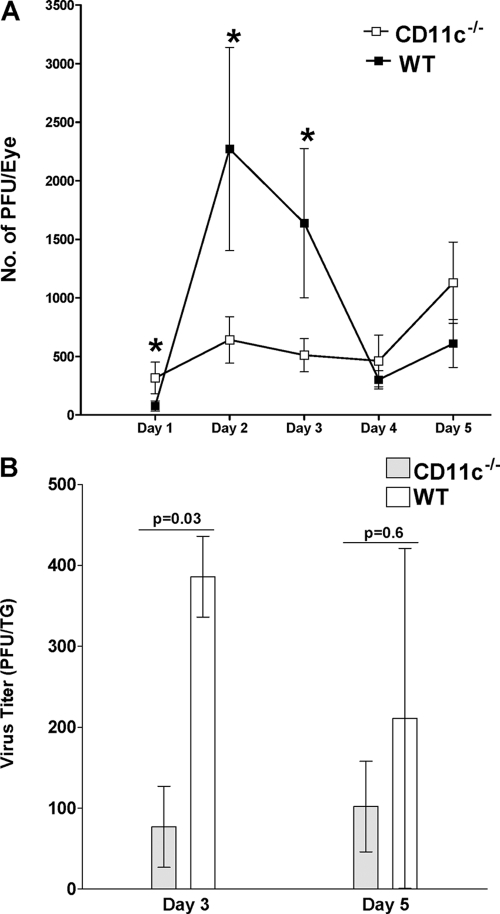
To determine the effect of CD11c deficiency on virus replication in the eye, tear films from the infected mice described above were collected (40 eyes/group) on days 1, 2, 3, 4, and 5 p.i. The collected tear films were titrated for the presence of infectious virus on RS cells as described in Materials and Methods. More infectious virus was detected in tear films of CD11c−/− mice than wt mice on day 1 p.i., while on days 2 and 3 p.i., the reverse was found (Fig. 1A; P < 0.05, Student's t test). Thus, the absence of CD11c appeared to reduce virus titers in the eyes of infected mice on days 2 and 3 p.i.
Fig. 1.
Virus titers in eyes and TG following ocular infection of mice. (A) Ocular viral titers. CD11c−/− and wt mice were infected ocularly, and the amount of infectious HSV-1 in tear films was determined daily by standard plaque assays as described in Materials and Methods. For each time point, the virus titer (y axis) represents the average of the titers from 40 eyes ± SEM. (B) TG viral titers. CD11c−/− and wt mice were infected ocularly, and the amount of infectious HSV-1 in TG was determined on days 3 and 5 p.i. as described in Materials and Methods. For each time point, the virus titer (y axis) represents the average of the titers from 10 TG per time point ± SEM.
To determine the effect of CD11c deficiency on viral titers in the TG, 10 mice per group were infected ocularly as described above. On days 3 and 5 p.i., TG were harvested and individually homogenized, and standard plaque assays were performed as described in Materials and Methods. On day 3 p.i., the TG from CD11c−/− mice had less infectious virus than those from wt mice (Fig. 1B; P = 0.03, Student's t test). However, by day 5 p.i. the differences were not statistically significant (Fig. 1B; P = 0.6). Thus, in the CD11c−/− mice, early in infection less infectious HSV-1 was detected in both the eyes and TG, suggesting that the same mechanism may be involved.
CD11c affects CD8+ T cell numbers in the cornea.
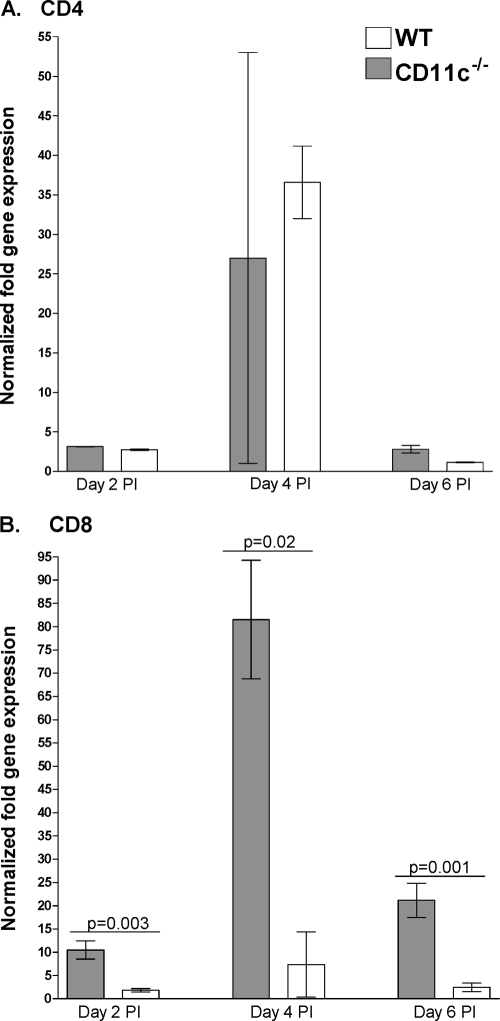
One explanation of the above results is that CD11c may decrease the protective immune response to HSV-1 in the eye and TG early after infection. To begin to assess the effect of a lack of CD11c on CD4 and CD8 T cell numbers, we infected CD11c−/− and wt mice as described above. The relative amounts of CD4 and CD8 mRNAs in the cornea on days 2, 4, and 6 p.i. were determined by qRT-PCR as described in Materials and Methods. We did not detect differences in CD4 mRNA levels between the CD11c−/− and wt groups (Fig. 2A; P > 0.05). However, we detected a significant increase in the levels of CD8 mRNA in the corneas of CD11c−/− mice on days 2 p.i. (Fig. 2B; P = 0.003, Student's t test), 4 p.i. (Fig. 2B; P = 0.02, Student's t test), and 6 p.i. (Fig. 2B; P = 0.001, Student's t test) compared with those in the corneas of wt mice.
Fig. 2.
Elevated CD8, but not CD4, mRNA levels in corneas of CD11c-deficient mice. CD11c−/− and wt mice were infected ocularly as described in the legend to Fig. 1. Individual corneas from infected mice were isolated on days 2, 4, and 6 p.i. Total RNA was extracted, and cDNA was synthesized. Transcript expression in naive mice was used to estimate the relative expression of each transcript in corneas of infected mice. GAPDH expression was used to normalize the relative expression of each transcript in corneas of infected mice. Each point represents the mean ± SEM from 8 corneas per time point. (A) CD4; (B) CD8.
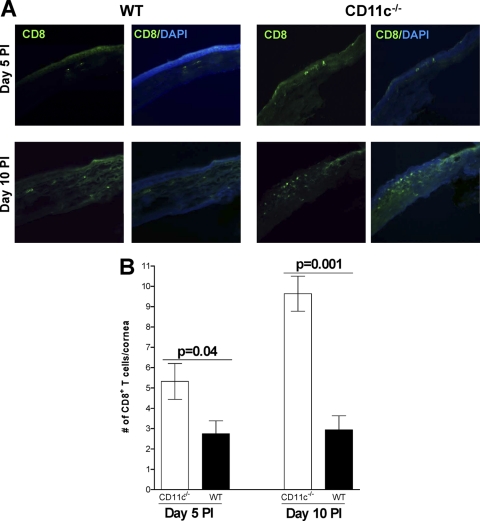
To determine if the increased CD8 mRNA levels described above (Fig. 2B) correlated with increased numbers of CD8+ T cells, CD11c−/− and wt mice were infected as described above and the number of CD8+ T cell infiltrates in the corneas of infected mice were determined on days 5 and 10 p.i. by IHC as described in Materials and Methods. We found an increase in CD8+ T cells in the corneas of CD11c−/− mice on both day 5 p.i. (Fig. 3 A, upper right) and day 10 p.i. (Fig. 3A, lower right) relative to wt mice. These differences were quantified, and the increased number of CD8+ T cells in the corneas of CD11c−/− mice on both day 5 p.i. (Fig. 3B; P = 0.04, Student's t test) and day 10 p.i. (Fig. 3B; P = 0.001, Student's t test) was significantly higher than that in wt mice. These results suggest that CD11c normally acts to decrease the number of CD8+ T cells in the cornea during primary ocular infection.
Fig. 3.
Effect of CD11c on CD8-positive T cells in corneas of ocularly infected mice. CD11c−/− and wt mice were infected ocularly as described in the legend to Fig. 1. (A) Qualitative analysis. Eyes from infected mice were isolated on days 5 and 10 p.i., fixed in OCT, sectioned, and stained for the presence of CD8+ T cells (green) as described in Materials and Methods. Cells were costained with DAPI (blue) to identify nuclei. Each individual panel shows CD8 staining alone or merge of CD8 and DAPI staining. Representative results are shown. (B) Quantitative analysis. The number of CD8+ T cells from the entire corneal section was counted in a double-blind fashion and plotted. Numbers shown represent the mean ± SEM from 18 CD11c-deficient corneal sections and 20 wt corneal sections from five mice per group.
CD11c affects CD8+ T cell numbers in TG.
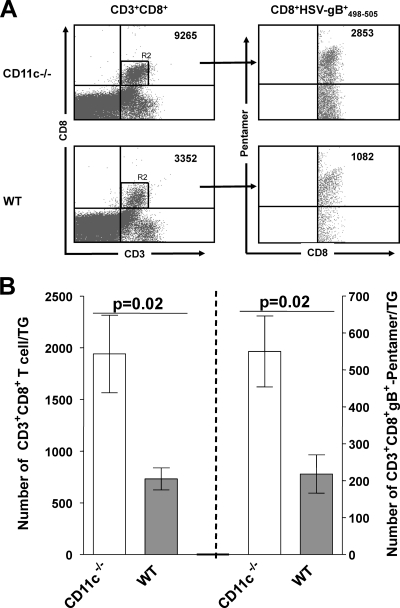
To assess if, similar to the cornea, the levels of CD8+ T cells are also elevated in the TG of CD11c−/− mice, additional mice were infected ocularly as described above, and the presence of CD8+ T cells from pooled TG and their specificity to HSV-1 were determined by FACS analyses on day 10 p.i., as described in Materials and Methods. We detected a significant increase in CD3+ CD8+ T cells (Fig. 4 A, upper left) and HSV gB-specific CD3+ CD8+ gB+498-505 pentamer T cells (Fig. 4A, upper right) in the TG of CD11c−/− mice compared to wt mice. Quantification showed a significant increase in the number of CD8+ T cells (Fig. 4B, left; P = 0.02, Student's t test) and CD8+ gB+498-505 pentamer HSV-specific T cells (Fig. 4B, right; P = 0.02, Student's t test). Taken together, these results from the cornea and TG suggested that the presence of CD11c decreases the number of total CD8+ T cells and HSV-1-specific CD8 T cells in the TG during primary HSV-1 infection.
Fig. 4.
Effect of CD11c on T cells in TG of CD11c-deficient mice. CD11c-deficient and wt mice were infected as described in the legend to Fig. 1. On day 10 p.i., TG from 4 mice per group were homogenized individually and stained with anti-CD3, anti-CD8, and anti-HSV gB+498-505 pentamer antibodies as described in Materials and Methods. (A) Representative dot plots. Dot plots of T cells that are CD3+ CD8+ (left) and CD3+ CD8+ gB+498-505 pentamer (right) are shown. The number of positive cells in each quadrant is indicated at the upper right. (B) Quantification of CD8+ T cells. The mean numbers of CD3+ CD8+ (left) and CD3+ CD8+ gB+ (right) T cells per individual TG in CD11c-deficient and wt mice are shown. Each point represents the mean of 4 FACS analyses ± SEM.
CD11c affects innate cell populations in the spleen.
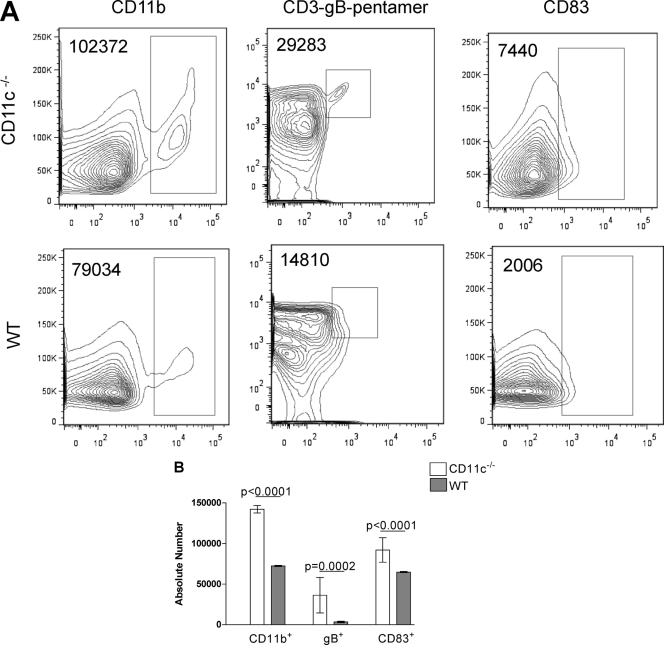
Our results with virus replication in the eye and TG suggested that the absence of CD11c reduced virus replication in the eye, while our IHC and FACS analysis results demonstrated an increase in CD8+ T cells in the eye and an increase in HSV-1-specific CD8+ T cells in the TG. To further characterize the infiltrates in CD11c−/− mice versus wt mice, we determined the number of cells that were CD11b+, CD83+, or gB+498-505 pentamer (HSV-1 specific) in extracts from the corneas, spleens, and TG on days 5 and 10 p.i. by FACS analysis as described in Materials and Methods. On day 5 p.i., we found an increase in the individual populations of CD11b+, gB+498-505 pentamer, and CD83+ cells in the spleens of CD11c−/− mice compared to wt mice (Fig. 5 A, representative dot plots). These differences were statistically significant for CD11b+ (Fig. 5B; P < 0.0001 Student's t test), gB+498-505 pentamer (Fig. 5B; P = 0.0002, Student's t test), and CD83+ (Fig. 5B; P < 0.0001, Student's t test). Increased numbers of CD11b+, gB+498-505 pentamer, and CD83+ cells were also found in the corneas and TG of CD11c−/− mice compared to wt mice, but the differences did not reach statistical significance (data not shown). These results suggested that the lack of CD11c in the CD11c−/− mice might increase the number of innate immune cells and HSV-1-specific CD8+ T cells, which in turn may account for the reduced amount of HSV-1 found in the eyes and TG during primary infection.
Fig. 5.
Effect of CD11c on various responses in spleens of CD11c-deficient mice. CD11c-deficient and wt mice were infected as described in the legend to Fig. 1. On day 5 p.i., spleens from individual mice were homogenized and stained with antibodies specific for CD11b, HSV gB+498-505 pentamer, and CD83. (A) Representative dot plots. Dot plots from CD11b-positive (left), gB+498-505 pentamer-positive (center), and CD83-positive (right) cells. The number of positive cells is indicated in the upper left corner of each panel. (B) Quantification of the mean number of CD83+ (right), gB+498-505 pentamer (gB+; center), and CD11b+ (left) cells per individual spleen in CD11c-deficient and wt mice. The experiment was repeated 2 times for a total of 4 spleens ± SEM.
Interferon mRNA levels during primary HSV-1 infection in CD11c-deficient mice.
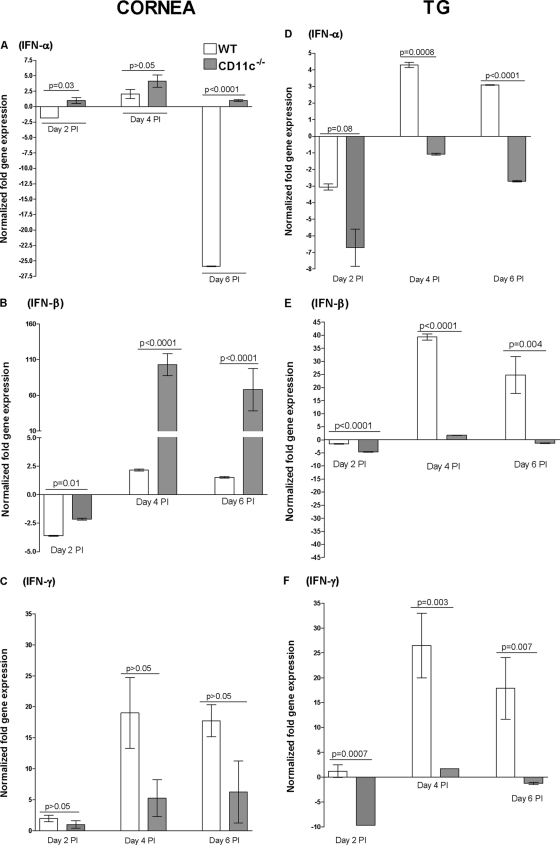
Faster clearance of HSV-1 from the eyes and TG of CD11c−/− mice may suggest that these mice have higher antiviral activity (normally associated with innate immune responses). Thus, we investigated potential differences in expression levels for IFN-α and IFN-β mRNAs, two type I interferons that are mainly produced by innate immune cells, and the type II interferon IFN-γ. CD11c−/− and wt mice were infected as described above, and the corneas and TG were harvested on days 2, 4, and 6 p.i. Spleen cells from the same mice were used as marker controls. qRT-PCR was performed on total RNA from individual corneas, TG, (Fig. 6), and spleens (see Fig. S1 in the supplemental material). We found a significant increase in expression of IFN-α mRNA (Fig. 6A; P < 0.03, Student's t test) and IFN-β mRNA (Fig. 6B; P < 0.01, Student's t test) in the corneas of CD11c−/− mice from days 2 to 6 compared with wt mice, except on day 4, when the difference in IFN-α did not reach statistical significance. However, this trend was reversed in the TG, where we found decreased expression of IFN-α mRNA (Fig. 6D; P < 0.008, Student's t test) and IFN-β mRNA (Fig. 6E; P < 0.004, Student's t test) in CD11c−/− mice compared to wt mice. The increase in IFN-α and IFN-β mRNAs in the eye may explain the initial decrease in primary virus replication in CD11c-deficient mice compared with wt mice. In contrast to IFN-α and IFN-β mRNAs, in wt mice the IFN-γ mRNA level appeared to be higher in the TG (Fig. 6F). The differences did not reach significance in the cornea (Fig. 6C). The levels of IFN-α, IFN-β, and IFN-γ mRNAs in the spleens of CD11c−/− and wt control mice on days 2, 4, and 6 are shown in Fig. S1 in the supplemental material. On day 2 p.i., the level of IFN-α expression was the same between CD11c−/− and wt mice, while we found a significant decrease in the IFN-α transcript on days 4 and 6 p.i. in the CD11c−/− group (see Fig. S1A in the supplemental material). In contrast, no significant differences were found for IFN-β expression between CD11c−/− and wt mice at any time point examined (see Fig. S1B in the supplemental material). Finally, from days 2 to 6 we found a decrease in IFN-γ expression in CD11c−/− mice compared with wt mice (see Fig. S1C in the supplemental material). These results suggest that in the cornea, CD11c negatively regulates IFN-α and IFN-β, while it may have a positive effect on IFN-γ. In contrast, in the TG, the absence of CD11c had a negative effect on the levels of IFN-α and IFN-β mRNAs but not the level of IFN-γ mRNA. These differences between cornea and TG with regard to IFN-α and IFN-β mRNA levels could be due to higher innate immune responses due to the presence of more resident fibroblasts and leukocytes in the cornea and/or more infiltrates after ocular infection with HSV-1.
Fig. 6.
Effect of CD11c on interferon mRNAs in corneas and TG of CD11c-deficient mice. Total RNA was isolated from corneas and TG of infected mice on days 2, 4, and 6 p.i. and used for quantitative reverse transcription-PCR. IFN-α, IFN-β, and IFN-γ mRNA levels in naive mice were used to estimate the relative expression of each transcript in treated mice. GAPDH mRNA levels were used to normalize the relative expression of each transcript in corneas and TG of infected mice. Each point represents the mean ± SEM from 8 corneas or TG per time point.
CS and latency in CD11c−/− mice.
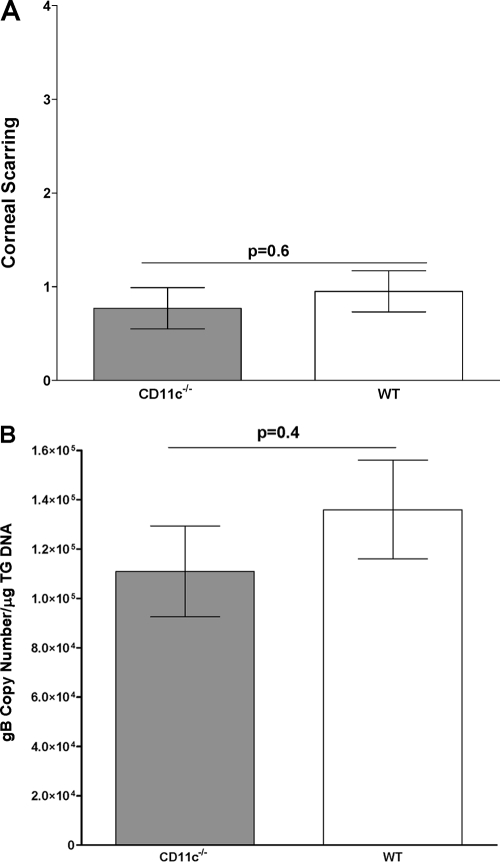
To determine if the absence of CD11c would affect the level of CS, eyes from infected mice were scored in a masked fashion as described in Materials and Methods. The average CSs in CD11c−/− mice and wt C57BL/6 control mice were similar (Fig. 7A; P = 0.6, Student's t test). Thus, the lack of CD11c expression appeared to have little effect on HSV-1-induced CS.
Fig. 7.
Degree of CS and level of latency in infected mice. (A) CS. CS in surviving mice was determined 30 days after ocular infection. For each bar, CS (y axis) represents the average of 40 eyes from 4 separate experiments. (B) Latency. TG from infected mice used for measuring CS were removed individually at autopsy on day 30 p.i. TaqMan PCR of total DNA isolated from TG from each mouse was performed as described in Materials and Methods using gB primers. GAPDH DNA was used to normalize the relative expression of gB DNA in TG. In each experiment, an estimated relative copy number of gB was calculated using standard curves generated from pGem-gB1. Briefly, DNA template was serially diluted 10-fold, such that 5 μl contained from 103 to 1011 copies of gB, and then subjected to TaqMan PCR with the same set of primers. By comparing the normalized threshold cycle of each sample to the threshold cycle of the standard, the copy number for each reaction was determined. Each bar represents the mean ± SEM of 40 TG.
The surviving wt and CD11c−/− mice described above (Fig. 6A) were euthanized on day 30 p.i., and qPCR was performed to quantitate HSV-1 genomic DNA levels in TG of latently infected mice. No significant differences were detected (Fig. 7B; P = 0.4, Student's t test). This suggests that despite the reduction in the amount of infectious virus detected in the eyes and TG of CD11c−/− mice on days 2 and 3 and on day 3, respectively, the amount of latent virus was not altered compared to that in wt mice.
DISCUSSION
HSV has developed several strategies to evade immune cells and to counteract their attacks (61). One such method is the mechanism by which HSV inhibits DC maturation (43). Mature DCs are highly resistant to HSV infection; however, immature DCs can be readily infected, which leads to morphological changes and the downregulation of costimulatory molecules needed for antigen presentation, T cell activation, and IFN-γ production (26, 40). HSV also induces the decreased display of the CD83 activation marker on the surface of DCs via lysosomal degradation (30, 69). Mature DCs are necessary to prime CD4+ and CD8+ T cells, which in turn expand the primary populations of cytotoxic T lymphocytes and generate memory T cells (4). Thus, DC maturation is critical to generating effective immunity against HSV. The ability of HSV to infect immature DCs and decrease DC maturation appears to help HSV survive within its host (43).
In this study, we found an increase in CD83, a marker of DC activation (maturation), in CD11c-deficient mice infected with HSV-1 compared to wt mice infected with HSV-1. This is consistent with CD11c being involved in HSV blockage of DC maturation (43). If so, removal of CD11c should prevent this blockage and prevent HSV-1 from blocking DC maturation. Consistent with this, our results also suggested that CD11c deficiency resulted in increased innate and CD8+ T cell responses following ocular HSV-1 infection. We and others have previously reported that within the first 5 days p.i. the CD8+ T cell infiltrates are very low (<2.2/cornea) (37) or undetectable (6, 12) in corneas of HSV-1-infected mice. In this study we found an increase in the levels of both total CD8+ cells in eyes and HSV-1-specific CD8+ T cells in TG in HSV-1-infected CD11c-deficient mice. DCs serve to link innate and adaptive immunity, as such more mature (CD83) DCs would be expected to increase the T cell response, as seen with CD11c-deficient mice in this study. However, increased numbers of HSV-1-specific CD8+ T cells in the CD11c-deficient mice did not have a major impact on latency, pathology, or survival. However, as mentioned in Results, the death of 3 CD11c-deficient mice following ocular HSV-1 infection was unique compared to the 100% survival of hundreds of similarly infected wt mice. Thus, even though viral replication appeared to be slightly reduced at some early times p.i., neurovirulence (death from encephalitis) may have been slightly increased. The neurovirulence would then likely not be due to increased amounts of virus in the brain but, rather, due to an increased im- mune response in the brain that led to pathology.
In this study we found a significant increase in the levels of IFN-α and IFN-β in the corneas but not in the TG of CD11c-deficient mice. Many cell types secrete IFN-α and IFN-β, with their secretion eliciting antiviral responses; these cell types include lymphocytes (NK cells, B cells, and T cells), macrophages, fibroblasts, endothelial cells, and osteoblasts (53). We also found an increase in CD11b, whose presence in combination with CD11c-negative DCs could explain the increase of IFN-α and IFN-β in the corneas of CD11c-deficient mice. The seemingly rare CD11b (CD11c-negative DCs) has previously been shown to produce large amounts of IFN-α (8, 54), and our result is consistent with these observations. It was previously shown that injection of mice with either IFN-α or IFN-β DNA greatly improves resistance to HSV-1 infection (24, 42). Taken together, our results suggest that the enhancement of early IFN-α and IFN-β production by leukocytes at the site of viral infection may be the major cause of reduced replication in the eyes of CD11c-deficient mice.
The results presented in this study suggest that decreased virus replication in the eyes and TG of CD11c-deficient mice correlated with IFNα/β but not IFN-γ. This is consistent with the findings of previous studies showing a significant role for IFN-α/β in control of early acute replication of HSV-1 and only a relatively minor role of IFN-γ (20, 33, 58). In addition, lower IFN-γ responses in the CD11c-deficient mice, despite the presence of more CD8 T cells, may suggest CD8 T cell exhaustion, as we reported previously (1).
In addition to high expression of CD11c on DCs, CD11c is expressed at low levels on NK cells, CTLs, and macrophages (16, 50). Thus, the absence of CD11c in CD11c-deficient mice might theoretically alter the function of NK cells, CTLs, or macrophages. However, in the CD11c-knockout mice used in these studies, macrophage, NK cell, and T cell responses appear to be normal (7).
The effect of CD11c deficiency on reducing infection is not universal and appears to be dependent on the triggering antigen. CD11c deficiency did not alter the staphylococcal enterotoxin response in mice (65). The lack of CD11c decreased development of atherosclerosis in the apoE−/− mouse model of hypercholesterolemia (64). Similar to this study, CD11c−/− was shown to exacerbate Lyme disease pathology in mice (22). In the context of HSV-1 infection, it is likely that the decreased primary replication of HSV-1 in CD11c-deficient mice was caused by the robust generation of innate responses due to increased maturation of DCs and production of type I interferon. As such, blocking the interactions between CD11c and HSV, potentially by pushing expansion of CD11c-negative populations, such as CD11b+ CD11c− CD83+, may be a useful approach for prophylactic vaccine development. The possibility of such immune modulation is unknown; however, the differential stimulation requirements of CD11c-positive and CD11c-negative DCs have already been elucidated (28). Modulation of CD83 may also be a potential target, as increased CD83 has been correlated with an increased antibody response in B cells (29) and increased expression of MHC class II and CD86 costimulatory molecules on DCs (60). Similar to our results, the engagement of the CD83 ligand (CD83L) preferentially enriches and significantly amplifies the number of antigen-specific CD8+ T cells (23). In addition, CD83-deficient mice demonstrated a specific block in CD4+ CD8− thymocyte development without increased CD4− CD8− or CD4− CD8+ thymocytes (15). CD83 is highly expressed on mature DCs, and its function might be associated with either T cell expansion or T cell survival (14, 67, 68). The therapeutic potential of CD83 has been demonstrated by increased allograft tolerance in mice treated with CD83 (32) and in adenoviral transfection of CD83+ DCs (59).
Due to the problems associated with recurrent ocular infection, prevention and/or reduction of virus replication in the eye should be a major goal of any prophylactic immunization against ocular HSV-1. Thus, shifting the immune response as we have shown here, in conjunction with immunization with HSV-1 subunit vaccines that we have described previously (45), should further improve vaccination efficacy against virus replication in the eye and establishment of latency in the TG. In this study, in the absence of antiviral antibody or virus-specific CTL responses during the early stage of infection, nonsensitized innate immune responses reduced viral replication in the eye, but they could not control latency. Adaptive immune responses require sensitization by exposure to the foreign antigens. Innate immune responses occur more rapidly after infection and do not require prior sensitization (62). Thus, in terms of vaccine design, a decrease in CD11c could generate higher virus-specific CTL and higher type I interferon responses, in addition to antiviral antibody. These combinations of higher innate and adaptive immune responses may significantly reduce virus replication in the eye and reduce latency.
Using a model of ocular viral infection, we have defined a novel link between components of the innate and adaptive immune systems, whereby CD11c in conjunction with HSV-1 limits the expansion of CD8+ T cells and innate cell responses without any deleterious effect in mice ocularly infected with HSV-1. Therefore, the absence of CD11c provides a unique situation whereby activation of CD8+ T cells and innate antiviral responses in order to generate long-lived antigen-specific CTL responses and higher IFN-α and IFN-β levels could be used for more effective control of infection.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants EY13615 and EY15557 from the National Eye Institute to H.G. S.L.W. was supported by Public Health Service grant EY013191 and a Research to Prevent Blindness Challenge grant. S.J.A. was partially supported by NIH training grant T32-AI-89553.
Footnotes
Supplemental material for this article may be found at http://jvi.asm.org/.
Published ahead of print on 20 July 2011.
REFERENCES
- 1. Allen S. J., et al. 2011. The role of LAT in increased CD8+ T cell exhaustion in trigeminal ganglia of mice latently infected with herpes simplex virus type 1. J. Virol. 85:4184–4197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Auslander B. A., Biro F. M., Rosenthal S. L. 2005. Genital herpes in adolescents. Semin. Pediatr. Infect. Dis. 16:24–30 [DOI] [PubMed] [Google Scholar]
- 3. Barron B. A., et al. 1994. Herpetic Eye Disease Study. A controlled trial of oral acyclovir for herpes simplex stromal keratitis. Ophthalmology 101:1871–1882 [DOI] [PubMed] [Google Scholar]
- 4. Behrens G., et al. 2004. Helper T cells, dendritic cells and CTL immunity. Immunol. Cell Biol. 82:84–90 [DOI] [PubMed] [Google Scholar]
- 5. Bosnjak L., et al. 2005. Herpes simplex virus infection of human dendritic cells induces apoptosis and allows cross-presentation via uninfected dendritic cells. J. Immunol. 174:2220–2227 [DOI] [PubMed] [Google Scholar]
- 6. Brown D. C., Nesburn A. B., Nauheim J. S., Pavan-Langston D., Kaufman H. E. 1968. Recurrent herpes simplex conjunctivitis. Arch. Ophthalmol. 79:733–735 [DOI] [PubMed] [Google Scholar]
- 7. Bullard D. C., Hu X., Adams J. E., Schoeb T. R., Barnum S. R. 2007. p150/95 (CD11c/CD18) expression is required for the development of experimental autoimmune encephalomyelitis. Am. J. Pathol. 170:2001–2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cella M., et al. 1999. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat. Med. 5:919–923 [DOI] [PubMed] [Google Scholar]
- 9. Colonna M., Pulendran B., Iwasaki A. 2006. Dendritic cells at the host-pathogen interface. Nat. Immunol. 7:117–120 [DOI] [PubMed] [Google Scholar]
- 10. Corey L. 1994. The current trend in genital herpes. Progress in prevention. Sex. Transm. Dis. 21:S38–S44 [PubMed] [Google Scholar]
- 11. Dawson C. R., Togni B. 1976. Herpes simplex eye infections: clinical manifestations, pathogenesis and management. Surv. Ophthalmol. 21:121–135 [DOI] [PubMed] [Google Scholar]
- 12. Dix R. D. 1987. Prospects for a vaccine against herpes simplex virus types 1 and 2. Prog. Med. Virol. 34:89–128 [PubMed] [Google Scholar]
- 13. Fraser N. W., Valyi-Nagy T. 1993. Viral, neuronal and immune factors which may influence herpes simplex virus (HSV) latency and reactivation. Microb. Pathog. 15:83–91 [DOI] [PubMed] [Google Scholar]
- 14. Fujimoto Y., Tedder T. F. 2006. CD83: a regulatory molecule of the immune system with great potential for therapeutic application. J. Med. Dent. Sci. 53:85–91 [PubMed] [Google Scholar]
- 15. Fujimoto Y., et al. 2002. CD83 expression influences CD4+ T cell development in the thymus. Cell 108:755–767 [DOI] [PubMed] [Google Scholar]
- 16. Geissmann F., Jung S., Littman D. R. 2003. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19:71–82 [DOI] [PubMed] [Google Scholar]
- 17. Ghiasi H., Bahri S., Nesburn A. B., Wechsler S. L. 1995. Protection against herpes simplex virus-induced eye disease after vaccination with seven individually expressed herpes simplex virus 1 glycoproteins. Invest. Ophthalmol. Vis. Sci. 36:1352–1360 [PubMed] [Google Scholar]
- 18. Ghiasi H., Cai S., Nesburn A. B., Wechsler S. L. 1996. Vaccination with herpes simplex virus type 1 glycoprotein K impairs clearance of virus from the trigeminal ganglia resulting in chronic infection. Virology 224:330–333 [DOI] [PubMed] [Google Scholar]
- 19. Ghiasi H., Kaiwar R., Slanina S., Nesburn A. B., Wechsler S. L. 1994. Expression and characterization of baculovirus expressed herpes simplex virus type 1 glycoprotein L. Arch. Virol. 138:199–212 [DOI] [PubMed] [Google Scholar]
- 20. Ghiasi H., et al. 2002. Infection of BALB/c mice with a herpes simplex virus type 1 recombinant virus expressing IFN-g driven by the LAT promoter. Virology 302:144–154 [DOI] [PubMed] [Google Scholar]
- 21. Ghiasi H., Perng G., Nesburn A. B., Wechsler S. L. 1999. Either a CD4(+) or CD8(+) T cell function is sufficient for clearance of infectious virus from trigeminal ganglia and establishment of herpes simplex virus type 1 latency in mice. Microb. Pathog. 27:387–394 [DOI] [PubMed] [Google Scholar]
- 22. Guerau-de-Arellano M., Alroy J., Bullard D., Huber B. T. 2005. Aggravated Lyme carditis in CD11a−/− and CD11c−/− mice. Infect. Immun. 73:7637–7643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hirano N., et al. 2006. Engagement of CD83 ligand induces prolonged expansion of CD8+ T cells and preferential enrichment for antigen specificity. Blood 107:1528–1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. John-Philip B., Carr D. J. 2005. Transfection of Muller cells with type I interferon transgenes: resistance to HSV-1 infection. Methods Mol. Med. 116:221–232 [DOI] [PubMed] [Google Scholar]
- 25. Jugovic P., Hill A. M., Tomazin R., Ploegh H., Johnson D. C. 1998. Inhibition of major histocompatibility complex class I antigen presentation in pig and primate cells by herpes simplex virus type 1 and 2 ICP47. J. Virol. 72:5076–5084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kobelt D., Lechmann M., Steinkasserer A. 2003. The interaction between dendritic cells and herpes simplex virus-1. Curr. Top. Microbiol. Immunol. 276:145–161 [DOI] [PubMed] [Google Scholar]
- 27. Koelle D. M., Ghiasi H. 2005. Prospects for developing an effective vaccine against ocular herpes simplex virus infection. Curr. Eye Res. 30:929–942 [DOI] [PubMed] [Google Scholar]
- 28. Kohrgruber N., et al. 1999. Survival, maturation, and function of CD11c− and CD11c+ peripheral blood dendritic cells are differentially regulated by cytokines. J. Immunol. 163:3250–3259 [PubMed] [Google Scholar]
- 29. Kretschmer B., Luthje K., Schneider S., Fleischer B., Breloer M. 2009. Engagement of CD83 on B cells modulates B cell function in vivo. J. Immunol. 182:2827–2834 [DOI] [PubMed] [Google Scholar]
- 30. Kurt-Jones E. A., et al. 2004. Herpes simplex virus 1 interaction with Toll-like receptor 2 contributes to lethal encephalitis. Proc. Natl. Acad. Sci. U. S. A. 101:1315–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kurts C. 2008. CD11c: not merely a murine DC marker, but also a useful vaccination target. Eur. J. Immunol. 38:2072–2075 [DOI] [PubMed] [Google Scholar]
- 32. Lan Z., et al. 2010. Prevention of chronic renal allograft rejection by soluble CD83. Transplantation 90:1278–1285 [DOI] [PubMed] [Google Scholar]
- 33. Leib D. A., et al. 1999. Interferons regulate the phenotype of wild-type and mutant herpes simplex viruses in vivo. J. Exp. Med. 189:663–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liesegang T. J. 2001. Herpes simplex virus epidemiology and ocular importance. Cornea 20:1–13 [DOI] [PubMed] [Google Scholar]
- 35. Mikloska Z., Bosnjak L., Cunningham A. L. 2001. Immature monocyte-derived dendritic cells are productively infected with herpes simplex virus type 1. J. Virol. 75:5958–5964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mott K. R., Ghiasi H. 2008. Role of dendritic cells in enhancement of herpes simplex virus type 1 latency and reactivation in vaccinated mice. Clin. Vaccine Immunol. 15:1859–1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mott K. R., et al. 2007. The corneas of naive mice contain both CD4+ and CD8+ T cells. Mol. Vis. 13:1802–1812 [PubMed] [Google Scholar]
- 38. Mott K. R., Perng G. C., Osorio Y., Kousoulas K. G., Ghiasi H. 2007. A recombinant herpes simplex virus type 1 expressing two additional copies of gK is more pathogenic than wild-type virus in two different strains of mice. J. Virol. 81:12962–12972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mott K. R., UnderHill D., Wechsler S. L., Ghiasi H. 2008. Lymphoid-related CD11c+ CD8a+ dendritic cells are involved in enhancing HSV-1 latency. J. Virol. 82:9870–9879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mott K. R., Underhill D., Wechsler S. L., Town T., Ghiasi H. 2009. A role for the JAK-STAT1 pathway in blocking replication of HSV-1 in dendritic cells and macrophages. Virol. J. 6:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Muller D. B., Raftery M. J., Kather A., Giese T., Schonrich G. 2004. Frontline: induction of apoptosis and modulation of c-FLIPL and p53 in immature dendritic cells infected with herpes simplex virus. Eur. J. Immunol. 34:941–951 [DOI] [PubMed] [Google Scholar]
- 42. Noisakran S., Carr D. J. 2001. Type I interferons and herpes simplex virus infection: a naked DNA approach as a therapeutic option? Immunol. Res. 24:1–11 [DOI] [PubMed] [Google Scholar]
- 43. Novak N., Peng W. M. 2005. Dancing with the enemy: the interplay of herpes simplex virus with dendritic cells. Clin. Exp. Immunol. 142:405–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Oh J. O., Kimura S. J., Ostler H. B., Dawson C. R., Smolin G. 1976. Oculogenital transmission of type 2 herpes simplex virus in adults. Surv. Ophthalmol. 21:106–109 [DOI] [PubMed] [Google Scholar]
- 45. Osorio Y., Cohen J., Ghiasi H. 2004. Improved protection from primary ocular HSV-1 infection and establishment of latency using multigenic DNA vaccines. Invest. Ophthalmol. Vis. Sci. 45:506–514 [DOI] [PubMed] [Google Scholar]
- 46. Osorio Y., Ghiasi H. 2003. Comparison of adjuvant efficacy of herpes simplex virus type 1 recombinant viruses expressing TH1 and TH2 cytokine genes. J. Virol. 77:5774–5783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pavan-Langston D. 1983. Ocular viral infections. Med. Clin. North Am. 67:973–990 [DOI] [PubMed] [Google Scholar]
- 48. Perng G. C., et al. 1994. The latency-associated transcript gene of herpes simplex virus type 1 (HSV-1) is required for efficient in vivo spontaneous reactivation of HSV-1 from latency. J. Virol. 68:8045–8055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Prechtel A. T., et al. 2005. Infection of mature dendritic cells with herpes simplex virus type 1 dramatically reduces lymphoid chemokine-mediated migration. J. Gen. Virol. 86:1645–1657 [DOI] [PubMed] [Google Scholar]
- 50. Probst H. C., et al. 2005. Histological analysis of CD11c-DTR/GFP mice after in vivo depletion of dendritic cells. Clin. Exp. Immunol. 141:398–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pulendran B., Tang H., Denning T. L. 2007. Division of labor, plasticity, and crosstalk between dendritic cell subsets. Curr. Opin. Immunol. 12:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Roberts C. M., Pfister J. R., Spear S. J. 2003. Increasing proportion of herpes simplex virus type 1 as a cause of genital herpes infection in college students. Sex. Transm. Dis. 30:797–800 [DOI] [PubMed] [Google Scholar]
- 53. Schultz U., Kaspers B., Staeheli P. 2004. The interferon system of non-mammalian vertebrates. Dev. Comp. Immunol. 28:499–508 [DOI] [PubMed] [Google Scholar]
- 54. Siegal F. P., et al. 1999. The nature of the principal type 1 interferon-producing cells in human blood. Science 284:1835–1837 [DOI] [PubMed] [Google Scholar]
- 55. Singh A. E., et al. 2005. Herpes simplex virus seroprevalence and risk factors in 2 Canadian sexually transmitted disease clinics. Sex. Transm. Dis. 32:95–100 [DOI] [PubMed] [Google Scholar]
- 56. Steinman R. M., Hemmi H. 2006. Dendritic cells: translating innate to adaptive immunity. Curr. Top. Microbiol. Immunol. 311:17–58 [DOI] [PubMed] [Google Scholar]
- 57. Stevens J. G. 1989. Human herpesviruses: a consideration of the latent state. Microbiol. Rev. 53:318–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Su Y. H., Oakes J. E., Lausch R. N. 1990. Ocular avirulence of a herpes simplex virus type 1 strain is associated with heightened sensitivity to alpha/beta interferon. J. Virol. 64:2187–2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tuting T., et al. 1999. Dendritic cell-based genetic immunization in mice with a recombinant adenovirus encoding murine TRP2 induces effective anti-melanoma immunity. J. Gene Med. 1:400–406 [DOI] [PubMed] [Google Scholar]
- 60. Tze L. E., et al. 2011. CD83 increases MHC II and CD86 on dendritic cells by opposing IL-10-driven MARCH1-mediated ubiquitination and degradation. J. Exp. Med. 208:149–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Vandevenne P., Sadzot-Delvaux C., Piette J. 2010. Innate immune response and viral interference strategies developed by human herpesviruses. Biochem. Pharmacol. 80:1955–1972 [DOI] [PubMed] [Google Scholar]
- 62. Welsh R. M., Jr 1978. Mouse natural killer cells: induction specificity, and function. J. Immunol. 121:1631–1635 [PubMed] [Google Scholar]
- 63. White A. L., et al. 2010. Ligation of CD11c during vaccination promotes germinal centre induction and robust humoral responses without adjuvant. Immunology 131:141–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wu H., et al. 2009. Functional role of CD11c+ monocytes in atherogenesis associated with hypercholesterolemia. Circulation 119:2708–2717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wu H., et al. 2004. Deficiency of CD11b or CD11d results in reduced staphylococcal enterotoxin-induced T cell response and T cell phenotypic changes. J. Immunol. 173:297–306 [DOI] [PubMed] [Google Scholar]
- 66. York I. A., et al. 1994. A cytosolic herpes simplex virus protein inhibits antigen presentation to CD8+ T lymphocytes. Cell 77:525–535 [DOI] [PubMed] [Google Scholar]
- 67. Zhou L. J., Schwarting R., Smith H. M., Tedder T. F. 1992. A novel cell-surface molecule expressed by human interdigitating reticulum cells, Langerhans cells, and activated lymphocytes is a new member of the Ig superfamily. J. Immunol. 149:735–742 [PubMed] [Google Scholar]
- 68. Zhou L. J., Tedder T. F. 1995. Human blood dendritic cells selectively express CD83, a member of the immunoglobulin superfamily. J. Immunol. 154:3821–3835 [PubMed] [Google Scholar]
- 69. Zinser E., Turza N., Steinkasserer A. 2004. CNI-1493 mediated suppression of dendritic cell activation in vitro and in vivo. Immunobiology 209:89–97 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.