Abstract
The human papillomavirus (HPV) type 16 E1^E4 (16E1^E4) protein is expressed in the middle to upper layers of infected epithelium and has several roles within the virus life cycle. It is apparent that within the epithelium there are multiple species of 16E1^E4 that differ in length and/or degree of phosphorylation and that some or all of these can associate with the cellular keratin networks, leading to network disruption. We show here that the cellular cysteine protease calpain cleaves the 16E1^E4 protein after amino acid 17 to generate species that lack the N terminus. These C-terminal fragments are able to multimerize and form amyloid-like fibers. This can lead to accumulation of 16E1^E4 and disruption of the normal dynamics of the keratin networks. The cleavage of E1^E4 proteins by calpain may be a common strategy used by α-group viruses, since we show that cleavage of type 18 E1^E4 in raft culture is also dependent on calpain. Interestingly, the cleavage of 16E1^E4 by calpain appears to be highly regulated as differentiation of HPV genome-containing cells by methylcellulose is insufficient to induce cleavage. We hypothesize that this is important since it ensures that the formation of the amyloid fibers is not prematurely triggered in the lower layers and is restricted to the upper layers, where calpain is active and where disruption of the keratin networks may aid virus release.
INTRODUCTION
Papillomaviruses are small DNA viruses that infect epithelial tissue and can give rise to hyperproliferative lesions (16). The majority of these lesions are benign, but a small number of papillomaviruses, so called high-risk types, cause lesions that may become malignant. Human papillomavirus type 16 (HPV16) is such a virus and is responsible for the majority of cases of cervical cancer (35). As with all PV, the life cycle of HPV16 is tightly linked to the differentiation of the host epithelium. Initial infection occurs in the basal cells, and as these cells divide and move toward the surface of the epithelium, differentiating as they ascend, the expression of different viral proteins is triggered (reviewed in reference 9). In the upper layers of the epithelium, the viral DNA is vastly amplified for packaging into new virions. Coincident with this is the high-level expression of the viral E1^E4 protein, the product of a spliced transcript of the E1 and E4 open reading frames (ORFs) (15). However, while viral DNA replication occurs in the nucleus, 16E1^E4 appears to be cytoplasmic, and its exact function in the virus life cycle is not fully understood.
The HPV16 E1^E4 protein appears to have multiple activities that may contribute to different aspects of the virus life cycle. HPV16 mutants with disrupted E4 ORFs show altered abilities to replicate viral DNA (24). In particular, consistent with other PV types, these mutants have less viral DNA amplification in the late stages of the virus life cycle. Possibly related to this, it has been shown that 16E1^E4 has the ability to bind to Cdk/cyclin complexes and arrest the cell cycle prior to mitosis (7, 8). It may be that by preventing further cellular DNA replication, viral DNA replication is favored. Alternatively, the interaction of 16E1^E4 with the viral E2 replication protein may explain the ability of 16E1^E4 to affect viral DNA replication (6). 16E1^E4 has a well-characterized interaction with keratin that leads to disruption of the cytokeratin networks (13, 37); an effect hypothesized to promote viral egress. The leucine cluster, LLKLL, of 16E1^E4 is required for binding to keratin (27), and this motif also facilitates the interaction of 16E1^E4 with mitochondria (25). This latter association is thought to mediate 16E1^E4-induced apoptosis. The C terminus of 16E1^E4 binds to a cellular DEAD-box RNA helicase, but the role of this interaction is unknown (11). Multimerization of the C terminus of 16E1^E4 is thought to be involved in producing amyloid-like fibers, which are hypothesized to cross-link and disrupt the cytokeratin network (23, 28, 37).
It is becoming increasingly apparent, both from work on 16E1^E4 and from studies of related proteins from other PV types, that the E1^E4 protein is subject to various posttranslational modifications. As well as the aforementioned multimerization, 16E1^E4 can also be phosphorylated by extracellular signal-regulated kinase (ERK), cyclin-dependent kinase 1 (Cdk1), Cdk2, protein kinase A (PKA), and PKC (38). E1^E4 proteins from other PV types have been shown to be phosphorylated by SRPK1 (type 1 E1^E4) (1) and by PKA and mitogen-activated protein kinase protein (type 11 E1^E4) (3) and to bind zinc ions (29). In multiple HPV types, E1^E4 species smaller than full-length protein have been observed (type 1 E1^E4) (10). In particular, HPV16-containing differentiating epithelium contains at least two different species of 16E1^E4 that can be distinguished by their antibody-binding capabilities (15, 23). Failure of one of these forms to bind to an antibody to the N terminus suggests that at least some in vivo species lacks this end of the protein. As is the case with E1^E4 proteins from other PV types (2), it is not clear whether these truncated species arise from differential splicing or from posttranslational modifications.
In the present study, we confirm proteolytic cleavage as the mechanism of generating truncated 16E1^E4 species, identify calpain as the protease responsible for cleavage in differentiating epithelium, determine the peptide bond between amino acids 17 and 18 as the site of cleavage, and demonstrate the role of cleavage in the formation of amyloid-like fibers, the accumulation of 16E1^E4, and the reorganization of the 16E1^E4 and keratin networks.
MATERIALS AND METHODS
Mutagenesis.
A QuikChange site-directed mutagenesis kit (Stratagene) was used to mutate pMV11.16E1^E4, and the W12 HPV16 genome contained within an Escherichia coli vector (pSPW12). pMV11.16E1^E4 was mutated to pMV11.L15S16E1^E4, pMV11.L16S16E1^E4, pMV11.Gly17_Ser18insPro, and pMV11.GS17,18DN using the forward primers ACGAAGTATCCTCTCCTGAAATCATTAGGCAGCACTTGG, ACGAAGTATCCTCTCCTGAAATTATCAGGCAGCACTTGG, ACGAAGTATCCTCTCCTGAAATTATTAGGCCCCAGCACTTGG, ACGAAGTATCCTCTCCTGAAATTATTAGACAACACTTGG, respectively, and their complementary reverse primers. Mutations were confirmed by sequencing, and the complete HPV genomes of all mutants were sequenced.
Cos7 culture, transfection, and lysis.
Cos7 cells were maintained in Dulbecco modified Eagle Medium (DMEM) supplemented with 10% fetal calf serum. For transfections, pMV11, pMV11.16E1^E4 wild-type (8), and mutants were prepared by using an EndoFree plasmid maxi kit (Qiagen) and transfected into Cos7 cells by using Effectene (Qiagen). When required, the following inhibitors (Calbiochem) were included in the DMEM for 18 h prior to harvest: N-[N-(N-acetyl-l-leucyl)-l-leucyl]-l-norleucine (ALLN; 100 μM), calpain inhibitor III (100 μM), calpeptin (100 μM), (2S,3S)-trans-epoxysuccinyl-l-leucylamido-3-methylbutane ethyl ester (EST; 100 μM), and 3-(4-lodophenyl)-2-mercapto-(Z)-2-propenoic acid (PD 150606; 100 μM). At these concentrations (recommended by the manufacturers), there was no apparent toxicity to the cells. Equivalent volumes of control solvents were applied to the controls. For Western blotting, Cos7 cells were treated with Benzonase (25 U of cells) and then lysed in 1× Red Loading Buffer (New England Biolabs) containing 100 mM dithiothreitol (DTT). The protein concentrations were checked by using a BCA protein assay kit (Bio-Rad).
NIKS culture, transfection, differentiation, and lysis.
NIKS, a spontaneously immortalized, HPV-negative human keratinocyte cell line, was maintained at subconfluent levels on gamma-irradiated J2 3T3 feeder cells in F medium with all supplements as previously described (21). Linearized HPV16 DNA was obtained from plasmid vectors by digestion with BamHI and was twice recircularized and recovered as previously described (21). The two independent recircularized DNAs for each of wild-type and mutant HPV16 genomes were cotransfected twice with a blasticidin resistance plasmid (pCDN6; Invitrogen) in triplicate into NIKS cells using Effectene (Qiagen), and the NIKS cells were selected in blasticidin for 4 days. Cultures were amplified for a further 3 weeks before the cells were used in experiments. Total DNA was extracted from 106 cells and digested with HindIII and DpnI restriction enzymes. The presence and levels of episomal HPV16 DNA was assessed by Southern blot (compared to known copy numbers of BamHI-linearized recombinant plasmid HPV16 DNA) and quantitative PCR (using forward [5′-GACTATCCAGCGACCAAGATCAG] and reverse [5′-CTGAGTCTCTGTGCAACAACTTAGTG] primers to detect the viral genomes and forward [CCTCCCGCTTCGCTGTGT] and reverse [CTGGCGACGCAAAAGAAGA] primers to detect cellular GAPDH). Differentiation of the NIKS cells in organotypic raft culture was performed essentially as described previously (38), and raft cultures were grown from two independently derived HPV16-positive cell lines and one HPV18-positive NIKS cell line between passages 6 and 8. The rafts were cultured for 15 days at the air-liquid interface and, when required, calpain inhibitor III (75 μM) was added to raft culture medium every other day from lifting to harvest. At this concentration, there was no apparent toxicity to the rafts. An equivalent volume of dimethyl sulfoxide (DMSO) solvent was applied to control rafts. For Western blot analysis, the tissue and cells were treated with Benzonase (50 U per raft epithelium or per 106 methylcellulose/calcium-differentiated cells) on ice for 20 min and then lysed at 95°C for 40 min in 6% sodium dodecyl sulfate (SDS), 1.5 M urea, and 0.1 M DTT. The lysate was centrifuged at 10,000 × g for 5 min to yield the supernatant.
In vitro cleavage reactions.
To prepare Cos7 cell lysate, cells from a confluent T25-sized flask were treated with Benzonase (300 U) and then lysed in 500 μl of lysis buffer (1% NP-40, 50 mM Na2HPO4, 50 mM NaH2PO4, 100 mM NaCl, 0.2 mM DTT [pH 7]). The lysate was centrifuged at 10,000 × g for 5 min at 4°C to yield the supernatant. C-terminally His-tagged 16E1^E4 (16E1^E4-His) was prepared as previously described (23), and 400 ng of protein was incubated at 37°C for 30 min with either Cos7 cell lysate supernatant or μ-calpain (Calbiochem) in lysis buffer containing 1 mM CaCl2 in a total volume of 200 μl. When required, the following inhibitors were included in the reactions: AEBSF [4-(2-aminoethyl)-benzenesulfonyl fluoride], (1 mM), 6-aminohexanoic acid (5 mg ml−1), antipain (100 μM), aprotinin (800 nM), benzamidine HCl (4 mM), bestatin (40 μM), chymostatin (100 μM), E64 (10 μM), EDTA (10 mM), N-ethylmaleimide (1 mM), leupeptin (100 μM), pepstatin (1 μg ml−1), and phosphoramidon (10 μM) from Sigma and ALLN (100 μM), calpain inhibitor III (100 μM), calpeptin (100 μM), EST (100 μM), and PD 150606 (100 μM) from Calbiochem. Reactions were stopped by addition of 2× Red Loading Buffer containing 100 mM DTT (New England Biolabs).
Electrospray ionization-mass spectrometry (ESI-MS) and N-terminal sequencing.
Calpain-cleaved 16E1^E4-His was eluted from a desalting column in 60% acetonitrile, 0.1% acetic acid, and injected into a microTOFQ electrospray mass spectrometer (Bruker Daltonics, United Kingdom). Protein was infused into the mass spectrometer at 3 μl/min using an electrospray voltage of 4.5 kV. Mass spectra were deconvoluted using maximum entropy software (Bruker Daltonics). Alternatively, calpain-cleaved 16E1^E4 was separated by SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membrane. The membranes were stained with Coomassie brilliant blue, and protein bands were excised and commercially analyzed by N-terminal sequencing.
Antibody generation.
A peptide with the sequence PETPATPLSC was synthesized by using the FastMoc solid-phase peptide synthesis method, purified by reversed-phase high-pressure liquid chromatography, and then freeze-dried. The peptide was then conjugated to carrier protein by using an Imject maleimide-activated mariculture keyhole limpet hemocyanin kit, according to the manufacturer's instructions (Pierce, United Kingdom). The polyclonal antibody (anti-PETPAT) was raised in rabbits at Harlan Sera-Lab (Loughborough, United Kingdom).
Western blotting.
Samples were separated either on 15% polyacrylamide-SDS-Tris-glycine or 16% polyacrylamide-SDS-Tris-Tricine denaturing gels (Invitrogen). Proteins were transferred to an immunoblot PVDF membrane (Bio-Rad). 16E4 was detected with TVG402 (it binds to an epitope between proline 33 and serine 44), (12), anti-N-term (it binds to an epitope between methionine 1 and leucine 12), (15), or anti-PETPAT. 18E4, GAPDH (glyceraldehyde-3-phosphate dehydrogenase), Hsp70, and profilaggrin were detected with anti-E4 R705 (H. Griffin, Z. Wu, R. Marname, V. Dewar, A. Molijn, W. Quint, C. Van Hoof, F. Strauyf, B. Colau, J. Doorbar, and D. Jenkins, unpublished data), anti-GAPDH clone 374 (Chemicon), anti-Hsp70 clone W27 (Santa Cruz), and anti-profilaggrin clone 15C10 (Vector Laboratories), respectively, and then with anti-rabbit (NA9340)- or anti-mouse (NA931)-horseradish peroxidase conjugates (GE Healthcare), followed by detection using the ECL or ECL Plus kits (GE Healthcare). The images were adjusted for brightness and contrast by using Adobe Photoshop software.
Amyloid-binding thioflavin fluorescence assay.
N-terminally His-tagged 16E1^E4 (His-16E1^E4) was expressed, purified, and refolded as previously described (23). His-16E1^E4 (23 μM) was incubated at room temperature in a total volume of 500 μl in the presence of 1 mM CaCl2 with or without 1 μl of human μ-calpain (Sigma) or with 1 μl of human caspase-14 (Biomol). After 30 min, the reaction was stopped by the addition of the cysteine protease inhibitor calpeptin and transferred to 4°C. Thioflavin-T (20 μM; Sigma) was added, and the fluorescent emission spectra were measured between 480 and 600 nm with an excitation wavelength of 446 nm by using a Safire 2 plate reader (Tecan).
Microscopy.
Raft sectioning, dewaxing, rehydration, and epitope exposure and blocking were carried out as described previously (37). Sections were stained in a humidified chamber at 4°C overnight, with primary antibody followed by a 2-h incubation with secondary antibody. Profilaggrin, keratin 10, and MCM2 were detected with anti-profilaggrin clone 15C10 (Vector Laboratories), anti-K10 clone DE-K10 (Neomarkers), and anti-MCM2 Ab53136 (Abcam) primary antibodies, followed by Alexa Fluor 594-conjugated anti-mouse IgG (Invitrogen). 16E4 and 18E4 were detected with clone TVG405 Fab (15) directly conjugated to Alexa Fluor 488, applied at the same time as 1 μg of DAPI (4′,6′-diamidino-2-phenylindole; Sigma) ml−1. Dewaxed sections were also stained with hematoxylin and eosin; the slides were stained with Harris hematoxylin (Surgipath) for 2 min, washed in water, differentiated in 1% HCl in 70% ethanol for 5 s, washed in water, and stained with eosine (Surgipath) for 8 min before dehydration through graded alcohols and xylene and mounting under DPX (Sigma). Cos7 cells were fixed in 5% formaldehyde in phosphate-buffered saline for 5 min and blocked in 2% bovine serum albumin (BSA) for 30 min. Keratin 18 was detected with anti-K18 clone CY90 monoclonal antibody (Sigma), followed by Alexa Fluor 594-conjugated anti-mouse IgG antibody (Invitrogen). 16E4 was detected with clone TVG405 Fab (which binds to an epitope between proline 29 and arginine 40) (15) directly conjugated to Alexa Fluor 488. Antibodies were diluted in 2% BSA and incubated for 1 h on cells. Nuclei were stained with 1 μg of DAPI ml−1 for 1 h. Sections and cells were examined by using a bright-field fluorescence microscope, and digitally captured whole images were adjusted for brightness, contrast, and color levels by using Adobe Photoshop software. For electron microscopy, protein samples were applied to carbon-coated grids and negatively stained with 1% sodium silico-tungstate (pH 7). The grids were viewed under minimum-dose, accurate defocus conditions with a JEOL 1200EX instrument operated at 100 kV.
RESULTS
The truncated species of 16E1^E4 result from proteolytic cleavage by a cysteine protease.
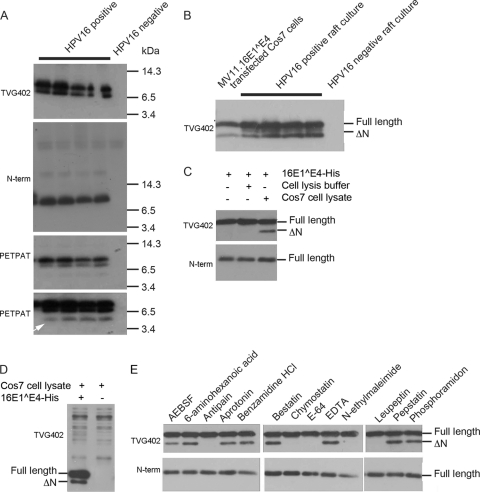
Previous analysis of HPV16-positive raft cultures has identified the presence of E1^E4 species that lack the N terminus (23). To further characterize N-terminal truncation of 16E1^E4, the nature of these species and how they are generated was investigated. First, the 16E1^E4 species present in the differentiating epithelium of a raft culture were analyzed using Tricine gels and a range of anti-16E4 antibodies (Fig. 1A). Tricine gels often display superior abilities to separate low-molecular-weight species than the more commonly used glycine gels. On these gels the TVG402 antibody, which binds to amino acids 37 to 41, detects two closely spaced species that correspond to the full-length and the previously identified N-terminally truncated forms of 16E1^E4. As expected, the lower band is not detected when an antibody to the N terminus is used. To determine whether further N-terminally truncated species were present that were not recognized by the TVG402 antibody, a polyclonal antibody, anti-PETPAT, was raised in rabbit against a peptide corresponding to amino acids 52 to 61 of 16E1^E4. The anti-PETPAT antibody detects the full-length and N-terminally truncated species that TVG402 reacts with but also detects an additional, albeit minor, smaller species. This suggests that this minor species lacks amino acid 37 and maybe even more of 16E1^E4. Differentiating raft cultures are not the easiest system within which to study molecular changes in proteins. It was already known that on expression of 16E1^E4 in Cos7 cells, the protein was also N-terminally truncated and the equivalence in size of this cleaved form with that present in rafts was confirmed (Fig. 1B). This suggests that whatever the mechanism that is generating the truncated form of 16E1^E4 in differentiating epithelial cells, it may also be present in Cos7 cells. We have been unable to detect in Cos7 cell extracts the minor smaller sized 16E1^E4 band that is present in raft extracts, so the findings presented here will focus on the N-terminally truncated band that is detected by the TVG402 antibody.
Fig. 1.
Proteolytic processing of full-length 16E1^E4 by a cysteine protease generates an N-terminally truncated species. (A) Total cell extracts from one negative and four positive HPV16 raft cultures were separated on Tricine gels and analyzed by Western blotting with anti-16E4 antibodies: TVG402 (top panel, the gel has split such that lane 4 appears in two sections), anti-N-term (second panel), and anti-PETPAT (low exposure, third panel; high exposure, bottom panel). An arrow indicates a truncated species that is only apparent with an antibody that binds to an epitope of 16E1^E4 within amino acids 52 to 61. (B) Total cell extracts from HPV16-positive and HPV16-negative raft cultures were compared to an extract from Cos7 cells transfected with a plasmid that expresses 16E1^E4, using glycine gels and Western blotting with the anti-16E4 antibody TVG402. (C) 16E1^E4-His expressed and purified from E. coli (lane 1) was incubated with Cos7 cell lysate (lane 3) or, as a control, the lysis buffer used to break open the Cos7 cells (lane 2). The reactions were analyzed by Western blotting with the anti-16E4 antibodies TVG402 (upper panel) and anti-N-term (lower panel). (D) Cos7 cell lysate was incubated with or without 16E1^E4-His and analyzed by Western blotting with the anti-16E4 antibody TVG402. (E) 16E1^E4-His expressed and purified from E. coli was incubated with Cos7 cell lysate containing a range of protease inhibitors. The reactions were analyzed by Western blotting with the anti-16E4 antibodies TVG402 (upper panels) and anti-N-term (lower panels).
The truncated 16E1^E4 species might arise by either pre- or posttranscriptional processes, such as mRNA processing or proteolytic cleavage, respectively. It was hypothesized that the latter was true and, if proteolysis was indeed the mechanism for generating the shorter form of 16E1^E4, then Cos7 cells would be expected to express whatever protease was responsible for cleavage. We reasoned therefore that if Cos7 cell extract was incubated with full-length 16E1^E4, it might be possible to convert 16E1^E4 to the truncated form. Full-length 16E1^E4 was expressed in E. coli and purified by means of a C-terminal hexahistidine tag (16E1^E4-His). This was then incubated with Cos7 cell extract, leading to the production of the truncated form of 16E1^E4 (Fig. 1C). That this form again lacks the N terminus was confirmed by its lack of reactivity with the anti-N-term antibody. This band was not apparent in Cos7 cell extract in the absence of 16E1^E4 (Fig. 1D). The observation that in vitro Cos7 cell extract was able to cleave 16E1^E4 gave us a convenient system within which to further investigate 16E1^E4 cleavage.
Depending on the mechanism that proteases use to hydrolyze the peptide bond, they can be classified into groups, the most common of which are the serine, cysteine, aspartate, and metalloproteases. To determine what type of protease is responsible for 16E1^E4 cleavage, the in vitro cleavage experiment was repeated with the inclusion of a range of protease inhibitors (Fig. 1E). The cleavage of 16E1^E4 is unaffected by AEBSF, aprotinin, or benzamidine HCl (serine protease inhibitors); 6-aminohexanoic acid (a lysine analogue that can inhibit binding of enzymes to substrate lysines); bestatin, EDTA, or phosphoramidon (metalloprotease inhibitors); and pepstatin (aspartyl protease inhibitor). However, cleavage is inhibited in the presence of antipain, chymostatin, and leupeptin, which all inhibit cysteine and serine proteases, and in the presence of E-64 and N-ethylmaleimide, which specifically target cysteine proteases, suggesting that 16E1^E4 is proteolytically cleaved by a cysteine protease present in Cos7 cell lysate.
The protease responsible for 16E1^E4 cleavage is calpain.
Having established that it is a member of the cysteine family of proteases that is responsible for cleaving 16E1^E4, the identity of the protease was sought. The activity of cysteine proteases within epithelial tissue has not been extensively characterized. However, one cysteine protease that is known to be active in epithelial tissue is calpain. Calpains are a family of structurally related, calcium-activated cysteine proteases (reviewed in reference 4). Unlike degradative proteases, calpains often cleave their substrates specifically (33) in such a way as to generate products with altered activities. Calpain substrates include protein components of the cytoskeleton, transcription factors, receptors, and enzymes, and it is thought that calpain activity is important in a range of physiological and pathological processes. Two calpains, μ- and m-, are found ubiquitously in vertebrates and are so called because of their dependence on μM and mM concentrations of Ca2+ respectively. Interestingly, HPV16 E7 was shown to reduce the calcium requirement of μ-calpain, suggesting that HPV infection might lead to calpain activation (5). We decided to test whether calpain is capable of cleaving off the N terminus of 16E1^E4.
The in vitro cleavage experiment was repeated, this time using purified calpain, obtained from a commercial source (Fig. 2A). That calpain is able to cleave 16E1^E4 is clearly seen with increasing concentrations of enzyme, and the band appears of a similar size to that obtained when 16E1^E4 is cleaved by Cos7 cell extract. Again, the lack of N terminus after calpain treatment is confirmed by the inability of the lower band to react with the anti-N-term antibody, and this time, that the C terminus remains intact was confirmed by the anti-His antibody (the 16E1^E4 is tagged with six histidines on its C terminus).
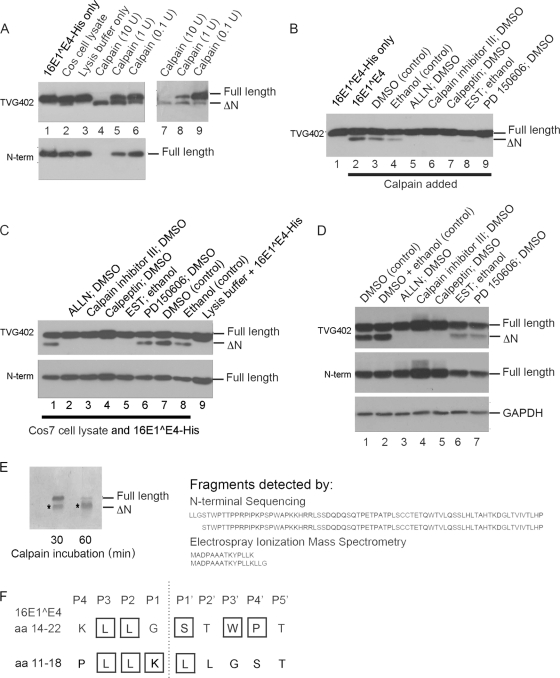
Fig. 2.
Cleavage of 16E1^E4 by calpain results in a predominant fragment consisting of amino acids 18 to 92. (A) 16E1^E4-His expressed and purified from E. coli (lane 1) was incubated with Cos7 cell lysate (lane 2) or with lysis buffer only (lane 3) or with 10, 1, and 0.1 U of human μ-calpain (lanes 4 to 6 and lanes 7 to 9). The reactions were analyzed by Western blotting with the anti-16E4 antibodies TVG402 (upper left panel) or anti-N-term (lower left panel) or with anti-His-tag (right panel). (B) 16E1^E4-His expressed and purified from E. coli (lane 1) was incubated with calpain (lane 2) or calpain treated with an inhibitor of calpain (lanes 5 to 9) or, as controls, calpain treated with the solvents used to dissolve the inhibitors (lanes 3 and 4). The reactions were analyzed by Western blotting with the anti-16E4 antibody TVG402. (C) 16E1^E4-His expressed and purified from E. coli was incubated with Cos7 cell lysate (lane 1) or Cos7 cell lysate treated with calpain inhibitors (lanes 2 to 6) or, as controls, Cos7 cell lysate treated with the solvents used to dissolve the inhibitors (lanes 7 and 8) or with only lysis buffer (lane 9). The reactions were analyzed by Western blotting with the anti-16E4 antibodies TVG402 (upper panel) and anti-N-term (lower panel). (D) Calpain inhibitors (lanes 3 to 7) or, as controls, the solvents used to dissolve the inhibitors (lanes 1 and 2) were added to Cos7 cells 6 h after the cells were transfected with pMV11.16E1^E4. At 24 h posttransfection, the cells were harvested and analyzed by Western blotting with the anti-16E4 antibodies TVG402 (upper panel) and anti-N-term (middle panel) and with anti-GAPDH (lower panel). (E) 16E1^E4-His expressed and purified from E. coli was incubated with calpain for 30 or 60 min before being separated by SDS-PAGE and transferred to nitrocellulose membrane. The membrane was stained with Coomassie blue, and the lower 16E1^E4 bands were excised and subjected to N-terminal sequencing analysis. Peptides beginning at serine 18 or leucine 15 were identified with the serine 18 peptides predominating with the longer exposure to calpain. SI-MS of calpain-digested 16E1^E4-His identified two predominant peaks corresponding to N-terminal peptides ending either in lysine 14 or glycine 17. (F) Analysis of the amino acids close to the cleavage sites (dotted line) in 16E1^E4 shows some (in boxes) to be residues conserved in a number of calpain substrates.
To test whether in Cos7 cells calpain was indeed cleaving the 16E1^E4, chemical inhibitors of calpain were used to try to abrogate the reaction. Due to the somewhat variable specificity of protease inhibitors, we tested five different known calpain inhibitors for their ability to inhibit calpain-mediated 16E1^E4 truncation in our in vitro cleavage experiment (Fig. 2B). Since the inhibitors were dissolved in organic solvents, controls were included in which only DMSO or ethanol were added. Although these controls showed some inhibition of cleavage, the results with the calpain inhibitors, ALLN, calpain inhibitor III, calpeptin, PD 150606 and, to a lesser extent EST, were more dramatic. Having confirmed that these inhibitors were indeed active against calpain in our in vitro assay, the experiment was repeated using Cos7 cell lysate instead of calpain (Fig. 2C). Again, the inhibitors showed an ability to prevent 16E1^E4 cleavage compared to controls. This suggested that calpain was the enzyme in Cos7 cell extract that was cleaving 16E1^E4. The PD 150606 inhibitor did not seem to work in this experiment and, interestingly, PD 150606 has a different mode of action from the other calpain inhibitors used here (36). This may manifest as a lack of suitability in using this inhibitor in the context of a complex mix of proteins. One final test to show that calpain was responsible for cleaving 16E1^E4 in Cos7 cells was to use the inhibitors on cells transfected with pMV11.16E1^E4 (Fig. 2D). In this case ALLN, calpain inhibitor III, and calpeptin all showed an impressive ability to inhibit 16E1^E4 cleavage. EST and PD 150606 were less able to do this, and this may reflect problems in them accessing the cells (perhaps as a result of failure to be internalized or lack of stability in the medium), or being ineffectual in the environment of the cell. In summary, 16E1^E4 can be cleaved by calpain in Cos7 cells to yield a C-terminal fragment that is of equivalent size to that found in differentiating epithelium.
Calpain can cleave 16E1^E4 between amino acids 14 and 15 and between amino acids 17 and 18.
Having identified the enzyme responsible for 16E1^E4 cleavage, the exact site at which calpain cleaves 16E1^E4 was investigated. Unlike many other proteases, calpain does not have a strict consensus sequence that it cleaves; instead, it is thought that a loose consensus sequence coupled with elements of secondary structure are involved in recognition of its substrates (4). To determine the peptide bond that calpain was cleaving, two techniques were used: N-terminal sequencing and ESI-MS. 16E1^E4-His expressed in E. coli and purified on a nickel column was incubated with calpain for 30 or 60 min and then separated by SDS-PAGE. The proteins were transferred to nitrocellulose membrane and stained with Coomassie blue (Fig. 2E). The lower bands were excised and analyzed by N-terminal sequencing to identify the amino acids present at the N terminus of the fragment. After 30 min of incubation with calpain, an approximately 50:50 mix of N-terminal ends beginning either with leucine 15 or serine 18 was obtained. After a 60 min of incubation with calpain, the majority of the N termini began with serine 18.
Calpain-cleaved 16E1^E4-His was then analyzed by electrospray ionization mass spectrometry. The predominant peaks obtained by ESI-MS corresponded to peptides 1-14 and 1-17 (Fig. 2E). The C-terminal fragments did not appear as peaks, and subsequent analysis suggested that these were unable to be detected because they formed aggregates that were incompatible with the MS equipment (data not shown). However, the presence of the particular N-terminal fragments supports the data obtained by N-terminal sequence analysis in suggesting that calpain is able to cleave at two sites within 16E1^E4—between amino acids 14 and 15 and between amino acids 17 and 18.
Although these two cleavage sites were detected in the context of treating 16E1^E4 with purified calpain, it was possible that the cellular milieu would alter the specificity of calpain for its substrates. To determine whether calpain cleaved 16E1^E4 at the same site in the context of a cell extract, 16E1^E4-His was incubated with Cos7 cell extract and analyzed by N-terminal sequencing. In contrast to the two N-terminally truncated forms identified in the presence of calpain, only a single form starting with serine 18 was identified. Although this does not rule out that the species beginning leucine 15 was initially present and was then rapidly converted by further cleavage to serine 18, the C-terminal fragment beginning with serine 18 appears to be the predominant truncated species in cells.
How do the observed sites of cleavage of 16E1^E4 fit with what is known about calpain? Despite calpain not having a strict consensus for identifying cleavage sites, a number of residues are often found within calpain substrates (34). These include leucine positions P3 and P2, lysine position P1, and leucine position P1′ relative to the cleavage site between amino acids 14 and 15 and leucine positions P3 and P2, serine position P1′, tryptophan position P3′, and proline position P4′ relative to the cleavage site between amino acids 17 and 18 (Fig. 2F). In fact, all six of the amino acids found surrounding the two 16E1^E4 scissile bonds are also present in the same position in one or more other substrates of calpain (34). It is also of note that the C-terminal decapeptide after the scissile bond lacks secondary structure in ca. 90% of calpain substrates, and this is predicted to also be the case with cleavage site between 16E1^E4 amino acids 17 and 18 (23).
16E1^E4 mutants that are not cleaved by calpain show additional phenotypes that are unrelated to their susceptibility to truncation.
To investigate the function of 16E1^E4 cleavage within the virus life cycle, it would be ideal to have an 16E1^E4 mutant in the context of the HPV16 genome that was not susceptible to cleavage, and we set out to make mutants insensitive to calpain. The choices of mutants were also affected by the fact that the E4 and E2 ORFs of HPV16 overlap, and any changes were designed to minimize disturbance of E2 when the mutations were introduced into the HPV16 genome.
Given the strong preference shown by calpain for serine at position P1′ (34), this residue was mutated to asparagine, for which calpain shows low preference, while the glycine at position P1 was mutated to aspartic acid, which again is not favored by calpain. Neither mutation would alter the sequence of E2. To test whether this double mutant was cleaved by calpain, the mutation was first introduced into the pMV11.16E1^E4 plasmid and transfected into Cos7 cells (see Fig. S1A in the supplemental material). Although some degree of inhibition of cleavage was observed with this GS17,18DN mutant, this was far from complete. Previous studies have shown that proline is rarely found at the P1′, position and mutation of this residue to proline can result in inhibition of cleavage at this site (34). For this reason, a mutant having proline at this site (G17_S18insP) was expressed in Cos7 cells (see Fig. S1A in the supplemental material). Although cleavage was again reduced, it was not completely ablated, and this mutation would have been extremely difficult to engineer into the HPV genome. Two further mutants (L16S and L15S) were analyzed in which the leucine in position P3 or position P2 that seemed to be strongly favored by calpain (34) was mutated to serine (an amino acid not favored by calpain at these positions), without affecting the potential E2 ORF. In contrast to the other mutants, these mutants appeared to be completely insensitive to calpain (see Fig. S1B in the supplemental material).
To characterize the L15S and L16S mutants further, they were each expressed in Cos7 cells from plasmid vectors for immunostaining and in NIKS cells in the context of the HPV16 genome for copy number analysis. It became apparent that these mutants are phenotypically different from wild-type 16E1^E4 in respect to their immunostaining patterns (see Fig. S1C in the supplemental material). Unlike the wild type, in which typical staining patterns include filamentous (in association with keratin networks), partially rearranged networks, perinuclear bundles, and mitochondrion association, this was not the case with the L15S and L16S mutants. Both mutants failed to show characteristic filamentous keratin colocalization or mitochondrial colocalization staining patterns, and this is perhaps not surprising given that these mutants have a disrupted leucine cluster. In addition, the mutants also had a strong tendency to adopt an unusual phenotype in which the 16E1^E4 appeared as smooth aggregates, often strung out in lines across the cell. This phenotype was observed in 40 to 50% of cells expressing mutant 16E1^E4 but was only rarely seen in wild-type 16E1^E4-expressing cells. We considered whether the lack of filamentous or mitochondrial staining and the alternative unusual staining could be a result of the inhibition of cleavage but neither of these characteristics was significantly observed when wild-type 16E1^E4 was treated with calpain inhibitors (see below). This led us to conclude that these mutants are not useful for analyzing the effect of 16E1^E4 cleavage because they show disruptions in known activities of 16E1^E4 that are unrelated to their inability to be cleaved. At the same time, we analyzed the mutations in the context of the HPV genome transfected into NIKS cells. Upon culturing the cells, it became apparent that the growth kinetics of the cells containing the mutant genomes were different from the wild-type genomes. To test whether this was due to differences in copy number of the viral genomes, DNA from the populations was analyzed by Southern blotting and quantitative PCR (see Fig. S1D and E in the supplemental material). These analyses demonstrated that the copy number of the mutant genomes was significantly reduced compared to the wild type, although it was still maintained as an episome. The L15S mutation affected copy number in monolayer cells less dramatically than L16S mutant, and the highest copy number seen with this mutant did on one occasion come close to the lowest copy number seen with the wild-type genome. Although we carried out five independent transfection and selection experiments into NIKS cells, we were unable to produce cell lines in which the HPV16 L15S mutant genome was maintained at levels typical of those seen with the wild-type genome. It seems unlikely that this is due to the lack of calpain cleavage since these are undifferentiated cells and do not express detectable 16E1^E4 (and we also have been unable to detect calpain activity in undifferentiated NIKS cells [data not shown]). The L16S gene was much more dramatically compromised in genome maintenance than the L15S mutant and, interestingly, we have seen a similar effect previously with another 16E1^E4 mutation at this position (L16STOP), in which the leucine 16 codon was replaced with a termination linker, and in this case both NIKS (five repeats) and primary cells (three repeats) were used. Importantly, the L15STOP mutant was not affected in genome maintenance levels, whereas the L15STOP/L16STOP double mutant was severely compromised (24; data not shown). These data suggest that nucleotides around codon position 16 may affect maintenance genome copy number levels through routes other than through changes in the 16E1^E4 protein. This might result indirectly from changes to the E2 mRNA or directly as a result of changes to the DNA. In any case, the inability of our these 16E1^E4 mutants to be maintained at wild-type levels in monolayer keratinocytes (NIKS and primary keratinocytes), coupled with their unusual staining patterns, precluded their use in further analyses of 16E1^E4 cleavage. Since it appeared that mutation of the 16E1^E4 sequence in the region of the cleavage site had both the potential to disrupt important functions of 16E1^E4 and aspects of HPV16 biology unrelated to 16E1^E4 expression, we decided to avoid using mutants and to focus on the use of calpain inhibitors on wild-type 16E1^E4 for further studies.
Inhibition of calpain prevents N-terminal cleavage of high risk HPV E1^E4 proteins in raft culture.
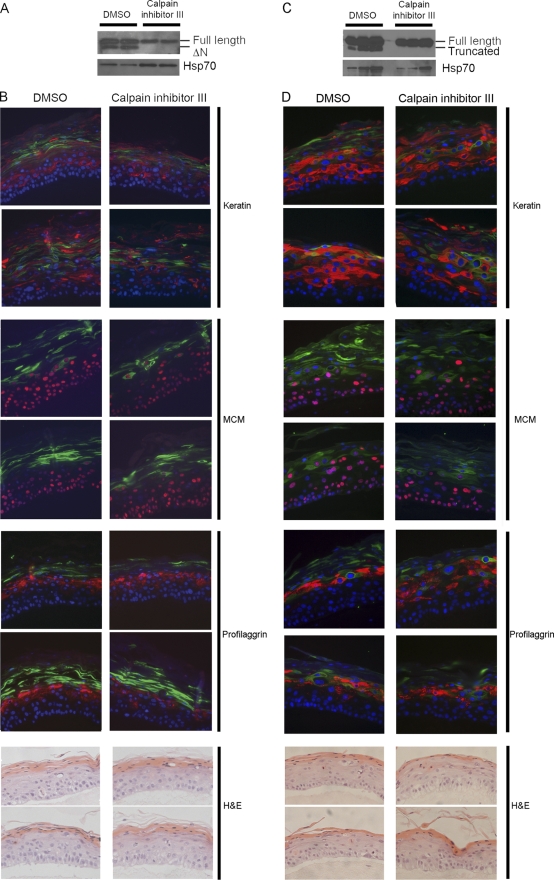
To substantiate the role of calpain cleavage of 16E1^E4 in differentiating epithelium, the calpain inhibitor “calpain inhibitor III” was added to the wild-type HPV16 organotypic raft cultures. Total cell extracts from rafts were analyzed by Western blotting (Fig. 3A) and compared to extracts from control rafts treated with DMSO. As expected, the calpain inhibitor III prevented the appearance of the lower band in the raft extracts, confirming the importance of calpain activity for cleavage of 16E1^E4 in differentiating epithelium. Interestingly, the overall levels of E1^E4 protein are lower in the calpain inhibitor III-treated rafts than in those treated with DMSO. This is consistent with the previous hypothesis that disruption to the N terminus of 16E1^E4 frees the C terminus to multimerize, leading to stable structures that accumulate in the cell (23). By inhibiting cleavage we are preventing the N-terminal disruption that would facilitate multimerization. To confirm that the lack of cleavage was not an indirect effect of calpain inhibitor III on some other aspect of the raft culture, we analyzed three inhibitor-treated rafts for MCM2, keratin 10, and profilaggrin, As far as we could tell, there was no apparent delay in the appearance of the keratin 10 or profilaggrin or the disappearance of MCM2 associated with calpain inhibitor III treatment (Fig. 3B). Neither were there any obvious differences in raft morphology when we compared inhibitor-treated and control rafts using hematoxylin and eosin staining (Fig. 3B).
Fig. 3.
Calpain activity in differentiating epithelium is responsible for cleavage of HPV16 and 18 E1^E4 proteins in the context of the virus life cycle. Organotypic raft cultures of NIKS cells containing HPV16 (A and B) or HPV18 (C and D) genomes were treated with calpain inhibitor III or control DMSO for 15 days from lifting until harvest. Total cell extracts from HPV16 and HPV18 organotypic raft cultures were analyzed by Western blotting with the anti-16E4 antibody TVG402 (A) or the anti-E4 antibody R705 (C), and loading levels are indicated by the Hsp70 blots. Sections of the organotypic raft cultures were immunostained for keratin 10, MCM2, or profilaggrin (red) and 16E1^E4 (B) or 18E1^E4 (D) (green) with a DAPI nuclear stain (blue) or were stained with hematoxylin and eosin (H&E). The images are representative of data obtained from triplicate rafts.
In addition to using stratified differentiating organotypic raft cultures, we also attempted to analyze cleavage of the 16E1^E4 protein using differentiation of monolayers cells (see Fig. S2 in the supplemental material). NIKS cells containing HPV16 genomes were treated with either methylcellulose for 3 days or calcium for up to 12 days. Western blots showed that despite the appearance of differentiation (as determined by the appearance of profilaggrin, transglutaminase, and 16E1^E4), both methods resulted in a single species of 16E1^E4 being detected, which corresponded in size to full-length 16E1^E4 expressed in Cos7 cells. Previously, we had observed that expression of 16E1^E4 from transient vectors in NIKS cells did not result in cleavage of 16E1^E4 (38), and we hypothesized that calpain was not active in these cells. We had anticipated that differentiation of the cells would be sufficient to induce calpain activation, as was the case with differentiation in organotypic raft culture. However, this was not the case with either methylcellulose or calcium treatment. One possible explanation is that a greater degree of differentiation than that obtained with these methods is required to activate calpain in these cells. This might be expected, since our previous observations of antibody pairs that differentiate full-length and N-terminally cleaved proteins show differential immunostaining in the upper, i.e., most differentiated layers of the epithelium (23).
Given the high degree of sequence homology observed between alpha group E1^E4 proteins, we sought to determine whether calpain was responsible for generating similarly truncated HPV18 E1^E4 species in raft cultures. Total cell extracts from three HPV18 organotypic raft cultures treated with calpain inhibitor III were compared to extracts from three control rafts treated with DMSO (Fig. 3C). Again, it was found that treatment with calpain inhibitor III prevented the appearance of the lower E1^E4 band, suggesting that calpain is also able to cleave the type 18 E1^E4 protein. Again, that this was not due to disruption of the normal differentiation pathways of the raft culture was determined using immunostaining of keratin 10, MCM2, and profilaggrin and staining with hematoxylin and eosin (Fig. 3D).
Inhibition of calpain results in disruption of the reorganization of the 16E1^E4 and keratin filament networks.
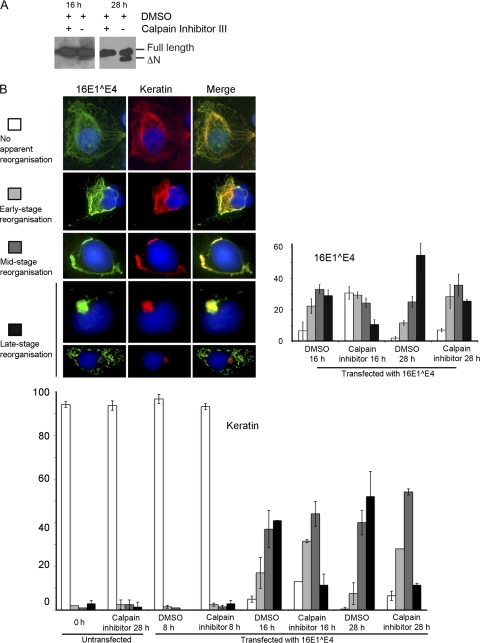
It is already known that when expressed in epithelial cells, 16E1^E4 forms networks associated with the cytokeratin networks (13). With time, the 16E1^E4 multimerizes through its C terminus, and this causes the networks of 16E1^E4 and keratin to reorganize (28, 37). In cells expressing keratins 8 and 18, this manifests as a change from a fine filamentous pattern (images labeled as “no apparent reorganization” in Fig. 4B) through thickening of the filaments (images labeled as “early and mid-stage reorganization” in Fig. 4B) to reorganization of the networks into a perinuclear bundle (13, 25) (images labeled as “late-stage reorganization” in Fig. 4B). Subsequent to this, 16E1^E4 may then associate with mitochondria, giving it a punctate appearance in cells (25) (see the lower panels in Fig. 4B). Thus, the rate of appearance of these different patters serves as an indicator of how effectively 16E1^E4 can disrupt or reorganize the cellular keratin filaments. Disruptions to the keratin networks in differentiating epithelium are hypothesized to be involved in the release of new virus particles, but further work is needed to confirm this. To determine what role cleavage has on the network reorganization, Cos7 cells expressing 16E1^E4 were treated with calpain inhibitor III (or control DMSO) for 16 or 28 h prior to fixation (Fig. 4). Total cell extracts were analyzed by Western blotting to confirm the activity of the inhibitor and that it did not significantly alter the expression levels of 16E1^E4 (Fig. 4A). The proportion of cleaved compared to uncleaved 16E1^E4 protein is much smaller at 16 h than at 28 h, but since 16E1^E4 is so abundantly expressed (both from the transient-transfection cytomegalovirus promoter used here and from the HPV promoter), the actual amount of cleaved 16E1^E4 is potentially quite high. Furthermore, it has been suggested that the cleaved form of 16E1^E4 may function as a template for the multimerization of full-length 16E1^E4 and, as such, may exert a functional effect even at low concentrations relative to the full-length protein. To investigate this, the 16- and 28-h time point cells were immunostained for 16E1^E4 and keratin 18, and the degree of reorganization of the networks was assessed by fluorescence microscopy (Fig. 4B). Analysis of both 16E1^E4 and keratin 18 patterns showed that treatment with calpain inhibitor III significantly reduced the degree of reorganization at both time points. Compared to previous reports of 16E1^E4-associated reorganization in cell culture (13, 22), reorganization occurring at relatively early time points in our system, which we think is partly a function of the cell line that we are using but mostly because the necessary control addition of DMSO, which has been found to greatly increase the expression levels of 16E1^E4 in transient-transfection experiments (data not shown). At 16 h, in cells treated with DMSO, the majority of the 16E1^E4 networks are in the middle or late stages of reorganization. In contrast, in cells treated with calpain inhibitor III, at this time point the majority of the cells display either no or little reorganization of the 16E1^E4 network. In fact, by 28 h, the calpain inhibitor III-treated cells are only showing the same degree of reorganization that was apparent at 16 h with the DMSO treatment. The effect is even more dramatic when we observe the keratin networks. Even after 28 h, the calpain inhibitor III-treated cells have still not reached the same level of reorganization that was observed at 16 h with the DMSO treatment. That the keratin and 16E1^E4 networks do not exactly mimic each other is not surprising since they have been shown to be dynamically distinct (22). The cells were categorized according to the pattern adopted by the majority of each network within a cell, and in many cells some of the keratin appeared to be associated with 16E1^E4, whereas some was not and vice versa. However, the overall slowing and reduction in the extent of network reorganization in the presence of calpain inhibitor III suggests the cleaved form of 16E1^E4, which lacks the keratin-binding domain, plays an important contribution to 16E1^E4-mediated keratin reorganization. Exactly how this occurs has not been fully elucidated, but our data suggest that the cleaved form of 16E4 can contribute to reorganization even when present at low levels relative to the full-length protein. Given that both N-terminally cleaved 16E1^E4 and keratin-bound 16E1^E4 multimerize through their C termini (37), it is reasonable to propose a working model in which the multimeric forms act to associate with and cross-link the keratin. This would explain the observed role of N-terminally cleaved 16E1^E4 in keratin reorganization. Therefore, by preventing calpain cleavage of 16E1^E4, this process can be disrupted. We would not expect the inhibition of cleavage to completely prevent reorganization since some degree of reorganization would also be expected as a result of monomeric 16E1^E4 binding, similar to the way that intracellular anti-keratin antibodies can act (17).
Fig. 4.
Calpain activity is important for reorganization of the 16E1^E4 and keratin networks. Cos7 cells were transfected with pMV11.16E1^E4 (or left untransfected). Six hours later, 75 μM calpain inhibitor III or an equivalent control volume of DMSO was added to the cells. (A) At 16 or 28 h posttransfection, the cells were Western blotted for 16E1^E4 with TVG402 anti-16E4 antibody (16E1^E4 is not detectable at 8 h posttransfection). (B) Cells immunostained for 16E1^E4 (green) and keratin 18 (red). A DAPI nuclear stain was included (blue). For each treatment, 3 × 102 cells were analyzed for their degree of reorganization of the 16E1^E4 and keratin 18 networks according to the following categories: no apparent reorganization or early-stage, mid-stage, or late-stage reorganization. Late-stage reorganization also included a mitochondrial pattern since 16E1^E4 relocates to these structures once the keratin network has been depleted. Examples are shown of each category (from DMSO-treated cells). The bar charts show the mean number of cells in each category ± the standard deviation for keratin at 0, 8, 16, and 28 h posttransfection and for 16E1^E4 at 16 and 28 h posttransfection.
Calpain cleavage of 16E1^E4 results in the formation of amyloid fibers.
Previous work has shown that the multimeric form of the protein that results from disruption of the N terminus is amyloid fiber-like in nature (23). However, that study utilized 16E1^E4 mutants that lack amino acids 2 to 5 or amino acids 12 to 16. These species are not thought to occur naturally during infections; however, the N-terminally truncated form of 16E1^E4 described here also lacks these residues and might be expected to behave in a similar way. To test whether calpain cleaved 16E1^E4 forms fibers, 16E1^E4-His was treated with calpain for 30 min to generate a ratio of full-length to cleaved protein similar to that observed in cells (Fig. 5A). The reaction was stopped by addition of a calpain inhibitor (calpeptin), and the ability of the protein to form fibers was assessed using an electron microscope (Fig. 5B). After 30 min, single fibers, comparable in diameter to those of the Δ2-5 and Δ12-16 mutant proteins (23), were observed. This was not observed in samples lacking 16E1^E4 (data not shown). The cleaved protein was then left at 4°C for a further 3 weeks, after which analysis on an electron microscope revealed the association of individual fibrils into twisted fiber bundles.
Fig. 5.
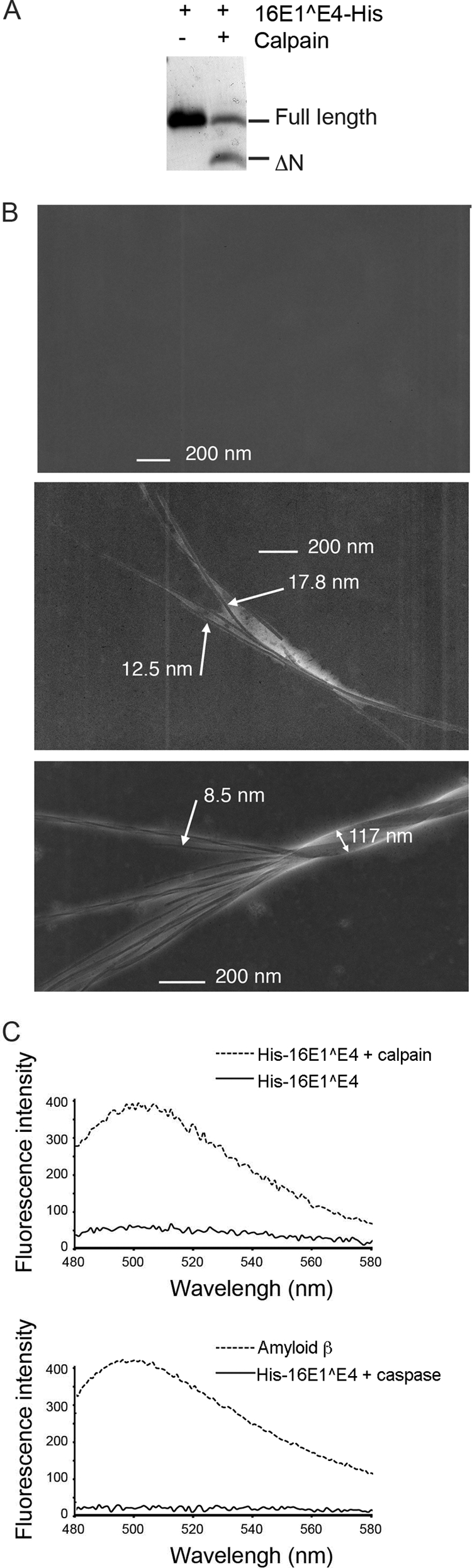
Calpain cleavage of 16E1^E4 results in the assembly of amyloid fibers. 16E1^E4-His was incubated with calpain or a buffer control for 30 min before addition of the calpain inhibitor calpeptin. (A) Proteins were separated by SDS-PAGE and stained with Coomassie blue. (B) Electron micrographs of individual amyloid-like E4 fibrils formed from 16E1^E4-His treated with calpain (middle panel) or buffer control (top panel). 16E1^E4-His treated with calpain was left for a further 3 weeks at 4°C, whereupon individual fibrils were observed to assemble into twisted bundles (bottom panel). (C) His-16E1^E4 treated or not treated with calpain were mixed with the amyloid stain thioflavin-T, and the fluorescence emission spectrum measured at between 480 and 600 nm. Thioflavin-T mixed with amyloid-β or caspase 14-treated His-16E1^E4 were used as positive and negative controls, respectively. The fluorescence peak observed at 485 nm is typically observed upon association of thioflavin-T with amyloid-like fibrils.
To determine whether the fibers that formed are amyloid in nature, the calpain-cleaved His-16E1^E4 was incubated with the dye, thioflavin-T, which gives a strong fluorescence emission at around 485 nm when bound to amyloid (Fig. 5C). No fluorescent signal was observed when 16E1^E4 was not present (data not shown), but the calpain-treated His-16E1^E4 gave a significant signal compared to non-calpain-treated His-16E1^E4, showing that fibers observed by electron microscopy are indeed amyloid-like. The appearance of the amyloid signal was not detected when His-16E1^E4 was treated with another cysteine protease, caspase 14.
These data confirm that the N-terminally truncated from of 16E1^E4 that exists in differentiating epithelium does indeed form amyloid-like fibrils and that calpain cleavage is crucial to the accumulation of 16E1^E4. Many amyloidogenic proteins have been shown to act as a template for inclusion of monomeric protein into fibrils (20, 39). By this mechanism, the inclusion of full-length 16E1^E4 into amyloid-like fibrils might explain why, as observed above, the presence of cleaved protein accelerates reorganization of the keratin network. Indeed, if this is the case the lateral packing of fibers observed here may also be crucial to the reorganization process.
DISCUSSION
It has been clear for some time that there are multiple species of 16E1^E4 protein found during infections and that some of these lack the N terminus. The consequences of some E1^E4 proteins lacking the N terminus has been investigated (19, 31), but how these species arise has not be proven. Our results show that these N-terminally truncated species can arise in differentiating epithelium as a result of proteolytic processing by calpain. Calpain cleavage of 16E1^E4 generates a C-terminal fragment of amino acids 18 to 92 that is amyloidogenic and assembles into fibers. In the absence of calpain cleavage, the reorganization of the 16E1^E4 and cytokeratin networks that is normally observed is inhibited, and levels of 16E1^E4 do not accumulate to the extent seen in cells in which cleavage is not inhibited.
We hypothesize that the generation of cleaved 16E1^E4 and its associated assembly into amyloid-like fibers has a role in the later stages of the virus life cycle, in which disruption of the keratin networks might aid in virus release. In addition to this, cleavage may have other roles in the virus life cycle. In the case of HPV1, N-terminally truncated species of E4 have been found to have altered localizations compared to full-length (30) and to have novel effects on the cell cycle (18). It will also be interesting to determine whether the even smaller species of 16E1^E4 (detected in Fig. 1A) have a functional role. It is already known that the HPV1 E4 protein comes in several truncated forms, and it may be that it is similarly the case with HPV16 E1^E4. Theoretically, calpain cleavage should also generate an N-terminal fragment consisting of amino acids 1 to 17. Although we have been unable to detect this fragment, this may be because of its small size rather than its absence. Previous work that expressed N-terminal peptides in the context of the HPV16 genome suggested that these might be active in viral DNA replication (24).
When analyzing the function of proteins, it is often desirable to have mutant proteins that lack these functions. However, the data are often difficult to interpret when mutations have been made in small, multi-activity proteins, particularly those whose ORFs overlap other ORFs or potential nucleic acid control regions. Nakahara et al. have analyzed the effects of making a stop codon mutation in the DNA encoding the leucine cluster region and concluded that disruption of the leucine cluster itself inhibits maintenance of viral DNA (24). These authors showed that an HPV genome having a stop codon TTA instead of the codon TAA encoding the leucine 15 of 16E1^E4 was maintained, while mutation of the leucine cluster from LLKLL to LPKSS inhibited viral genome maintenance. These researchers also found that the 16E1^E4 was expressed in nondifferentiating NIKS cells and concluded that the leucine cluster was somehow important for maintenance. One possibility that arises from our results is that the leucine cluster is important because of its role in determining the site of cleavage, since our L15S and L16S mutant genomes also showed decreased maintenance. It may be that maintenance requires the presence of an N-terminal peptide of 16E1^E4 corresponding to approximately amino acids 1 to 17. This would be produced in wild-type 16E1^E4 by the cleavage of full-length protein and would be perhaps mimicked in the L15 stop codon mutant of Nakahara et al. by the expression of amino acids 1 to 14 of 16E1^E4. Mutation of the leucine cluster to LPKSS, we predict, would abolish cleavage and therefore the N-terminal peptide would not be produced. However, since our two leucine cluster genome mutants, L15S and L16S, which both failed to cleave, show different levels of maintenance, this may indicate that disruption to the cleavage recognition site is not the issue. Another potential explanation is that it is the nucleic acid sequence found encoding the leucine cluster region that is important for maintenance. Indeed, this is further suggested by our finding that a genomic mutant with a stop codon at 16E1^E4 amino acid position 15 is maintained at wild-type levels, whereas a double mutant that introduces a stop codon at positions 15 and 16 (but which introduces only silent mutations in E2) is defective. Either the DNA sequence present at these sites or the corresponding sequence of the mRNA message may be sensitive to mutation; perhaps changing the secondary structure and hence the access of binding proteins. In fact sequences in this region have already been shown to affect HPV16 splicing (32), demonstrating the care that must be taken when making and interpreting mutant HPV genomes. We experienced additional problems while trying to characterize our noncleavable mutant 16E1^E4 proteins expressed from transient transfection cDNAs. Although the L15S and L16S mutations were successful in abrogating cleavage, these mutants had other undesirable phenotypes, i.e., the lack of mitochondrial binding and/or unusual immunostaining patterns, that made them of limited use in determining the role of calpain cleavage in the virus life cycle. For this reason, we decided to use chemical inhibitors of calpain activity.
While care must also be taken in the interpretation of the data obtained using chemical inhibition of calpain, the results thus far are consistent with a model in which calpain activity within the differentiating epithelium gives rise to species that lack the N terminus of 16E1^E4. These are able to form amyloid-like fibers and promote disruption of the 16E1^E4/cytokeratin networks. Unfortunately and despite considerable effort, our attempts to inhibit calpain activity in Cos7 cells using small interfering RNA (siRNA) has not yet been successful, which may reflect the fact that calpains are a complex multigene family whose regulation is not yet fully understood. This alternative approach is further complicated by the fact that Cos7 cells are derived from the African green monkey rather than from humans and are likely to have as-yet-unidentified polymorphisms that compromise the use of the commercially available siRNA sequences. The more relevant human keratinocyte cell line (NIKS) does not support 16E1^E4 cleavage unless induced to differentiate in organotypic raft culture, which makes good sense given the timing of 16E1^E4 cleavage and its eventual accumulation in the upper layers of infected mucosal epithelium in vivo. Ensuring the correctly timed expression of siRNA against all calpain isoforms in this system is technically challenging and has thus far proved unfeasible. Unfortunately, the methylcellulose system does not appear to support differentiation as extensively as in the raft and, although it is easier to use, it does not provide an alternative for the evaluation of E1^E4 processing. Our finding that purified calpain cleaves 16E1^E4 at the same position as occurs in cells in culture and in rafts, as well as stimulating the formation of 16E1^E4 amyloid-like fibers, lends weight to the hypothesis that calpain is likely to be an important (and possibly the key) mediator of 16E1^E4 function in terminally differentiating keratinocytes.
The extent of cleavage required to stimulate filament formation is not yet clear; however, we have observed here a significant acceleration in keratin network reorganization in the presence of a small amount of calpain-cleaved 16E1^E4. This species readily forms amyloid-like fibrils, and it will be interesting to see whether, once formed, the 16E1^E4 amyloid can associate with the C termini of full-length 16E1^E4, thus disrupting the structure of the protein and seeding the inclusion of full-length 16E1^E4 into amyloid fibers. If this aspect of the model proves to be true, the inclusion of 16E1^E4, with an intact keratin-binding domain, into fibrils presents a potent cytokeratin cross-linking structure, particularly in light of the observation of twisted multifibril 16E1^E4 structures.
Filament reorganization is seen in vivo as well as in vitro, although in vivo, where cells are not dividing in the upper epithelial layers, bundling is generally toward the cell periphery rather than the cell nucleus. These cells, uniquely modified by the deposition of insoluble 16E1^E4 amyloid and reorganization of the cytokeratin network, are also laden with virus. Indeed, 16E1^E4 deposits are undoubtedly exposed to assembled virus on nuclear degeneration. We can speculate that virus transmission may be facilitated by some degree of association with this modified squame.
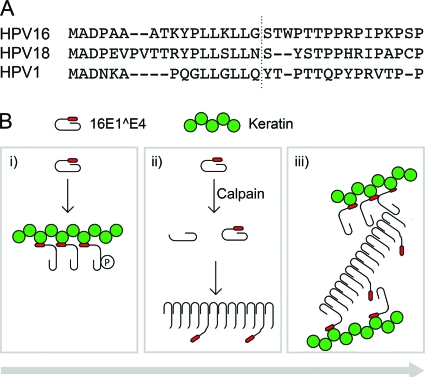
The amino acid sequence of E1^E4 proteins from different HPV types is not well conserved, but if cleavage is an important event in the virus life cycle, then it might be expected that the cleavage site would be present in all types. In the present study we have shown that in addition to the HPV16 E1^E4 protein, the HPV18 E1^E4 protein is also cleaved by calpain and might, given the relative position of the leucine cluster, be expected to be cleaved between amino acids asparagine 19 and serine 20 (Fig. 6A). This would explain why the expression of 18E1^E4 in Cos7 cells results in the observation of amyloid-like staining (23). Interestingly, although the HPV1 E1^E4 sequence is somewhat different from that of 16E1^E4, the site of cleavage appears (relative to the leucine cluster) to be the same (27). However, we have not observed cleavage of 1E1^E4 when we have expressed it in Cos7 cells (data not shown). This may be because 1E1^E4 is not correctly folded in Cos7 cells and thus is not recognized by calpain. Alternatively, it may suggest that 1E1^E4 has evolved to be cleaved by a different protease (that is not active in Cos7 cells), albeit at roughly the same site.
Fig. 6.
Conservation of E1^E4 cleavage sites and a model for the role of cleavage in the life cycle. (A) Alignment of the N termini of HPV type 16, 18, and 1 E1^E4 amino acid sequences according to the CLUSTAL W method. Vertical dotted line shows the position at which the N-terminally truncated protein begins in types 16 and 1 and the predicted position in type 18. (B) Model showing the proposed role of 16E1^E4 cleavage in reorganization of the keratin networks. (i) Monomeric E1^E4 binds to cytokeratin filaments, which may inhibit E1^E4 cleavage (38). At this stage, the cells are still in cycle, and some of the E1^E4 is phosphorylated by ERK. Keratin binding is facilitated by transient ERK phosphorylation of E1^E4. Keratin binding displaces the C terminus of E1^E4 from its N terminus. (ii) As E1^E4/keratin binding increases, unbound E1^E4 becomes available for cleavage. As the E1^E4-expresing cells exit the cell cycle and begin to differentiate, calpain is activated and cleaves the free E1^E4. Truncated E4 acts as a template to seed the formation of E4 amyloid fibers, which may incorporate the full-length protein in both its free and keratin-bound forms. (iii) These heterogeneous E1^E4 fibers associate with and cross-link the keratin network, preventing normal keratin network dynamics, which may facilitate the release of viral particles.
Why has 16E1^E4 evolved to be cleaved by calpain rather than some other protease? Unlike lysosomal and proteosomal enzymes, calpains tend to be involved in the processing of proteins to further active forms, rather than in their total degradation. Previous data from the HPV type 1 E1^E4 protein suggest that the full-length protein is processed into shorter forms as the cells move toward the surface of the epithelium (2, 14). If E1^E4 proteins are to form amyloid structures, it is likely that the virus needs this to happen in a very controlled way, since the premature formation of large fibrous structures within the cell could be very detrimental to the virus life cycle. While it may be that in cells some other modification of 16E1^E4 promotes proteolysis by calpain, our in vitro results suggest that this may not be the case, since calpain is readily able to cleave 16E1^E4 purified from E. coli. Instead, we hypothesize that 16E1^E4 is protected from cleavage in the lower layers by two mechanisms. First, 16E1^E4 molecules bound to keratins may be protected from cleavage since the keratin binding site is very close to the cleavage site. Therefore, free 16E1^E4 molecules would only become available for cleavage once all of the keratin was saturated with 16E1^E4. In support of this, we have previously observed that in a pair of isogenic cell lines that either do or do not express keratins the extent of 16E1^E4 cleavage is significantly greater in the cell line that lacks keratins (38). Also, in a time course study of 16E1^E4 expression in Cos7 cells, the degree of cleavage appeared to increase disproportionately to the amount of protein (23), which again may be indicative of some kind of saturation of the keratin occurring. A further means by which premature amyloid formation is prevented in a stratified epithelium may involve the complex regulatory mechanisms that govern the activity of calpain. Calpains can be regulated by the availability of calcium ions, their endogenous inhibitor (calpastatin), binding of other proteins, intracellular location, and autolysis. In the differentiating epithelium, calpain is thought to be activated in the upper layers, where it is involved in processing profilaggrin to filaggrin (26), a finding consistent with our previous observations of truncated species of 16E1^E4 being observed in this region (23). The requirement for reaching a certain stratified layer before calpain cleavage can occur is supported by our observation that methylcellulose- or calcium-induced differentiation of HPV16-containing NIKS cells was insufficient to induce the cleavage of 16E1^E4. However, once cleaved in the stratifying epithelium, the accumulation of 16E1^E4 and the effect on the keratin networks are promoted. We propose the model shown in Fig. 6B to explain the regulation of cleavage of 16E1^E4 and its role in keratin network reorganization. It will be interesting to determine how HPV exploits the complex mechanisms of calpain regulation to control its own life cycle.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Steve Howell for assistance with mass spectrometry, Radma Mahmood for histology support, Rina Sorathia for producing the polyclonal anti-PETPAT antibody, and Ken Raj, Jonathan Stoye, Mike Blackman, and Alison McBride for many useful discussions.
This study was funded by the United Kingdom Medical Research Council grant U117584278 to J.D.
Footnotes
Supplemental material for this article may be found at http://jvi.asm.org/.
Published ahead of print on 13 July 2011.
REFERENCES
- 1. Bell I., Martin A., Roberts S. 2007. The E1^E4 protein of human papillomavirus interacts with the serine-arginine-specific protein kinase SRPK1. J. Virol. 81:5437–5448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bretiburd F., Croissant O., Orth G. 1987. Expression of human papillomavirus type-1 E4 gene products in warts, p. 115–122 In Steinberg B., et al. (ed.), Papillomaviruses, vol. 5 Cold Spring Harbor Laboratory Press, New York, NY [Google Scholar]
- 3. Bryan J. T., Han A., Fife K. H., Brown D. R. 2000. The human papillomavirus type 11 E1E4 protein is phosphorylated in genital epithelium. Virology 268:430–439 [DOI] [PubMed] [Google Scholar]
- 4. Croall D. E., Ersfeld K. 2007. The calpains: modular designs and functional diversity. Genome Biol. 8:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Darnell G. A., et al. 2007. Human papillomavirus E7 requires the protease calpain to degrade the retinoblastoma protein. J. Biol. Chem. 282:37492–37500 [DOI] [PubMed] [Google Scholar]
- 6. Davy C., et al. 2009. A novel interaction between the human papillomavirus type 16 E2 and E1^E4 proteins leads to stabilization of E2. Virology 394:266–275 [DOI] [PubMed] [Google Scholar]
- 7. Davy C. E., et al. 2005. Human papillomavirus type 16 E1 E4-induced G2 arrest is associated with cytoplasmic retention of active Cdk1/cyclin B1 complexes. J. Virol. 79:3998–4011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davy C. E., et al. 2002. Identification of a G2 arrest domain in the E1^E4 protein of human papillomavirus type 16. J. Virol. 76:9806–9818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Doorbar J. 2006. Molecular biology of human papillomavirus infection and cervical cancer. Clin. Sci. (London) 110:525–541 [DOI] [PubMed] [Google Scholar]
- 10. Doorbar J., Campbell D., Grand R. J., Gallimore P. H. 1986. Identification of the human papillomavirus-1a E4 gene products. EMBO J. 5:355–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Doorbar J., et al. 2000. The E1E4 protein of human papillomavirus type 16 associates with a putative RNA helicase through sequences in its C terminus. J. Virol. 74:10081–10095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Doorbar J., et al. 1992. Epitope-mapped monoclonal antibodies against the HPV16E1^E4 protein. Virology 187:353–359 [DOI] [PubMed] [Google Scholar]
- 13. Doorbar J., Ely S., Sterling J., McLean C., Crawford L. 1991. Specific interaction between HPV-16 E1^E4 and cytokeratins results in collapse of the epithelial cell intermediate filament network. Nature 352:824–827 [DOI] [PubMed] [Google Scholar]
- 14. Doorbar J., Evans H. S., Coneron I., Crawford L. V., Gallimore P. H. 1988. Analysis of HPV-1 E4 gene expression using epitope-defined antibodies. EMBO J. 7:825–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Doorbar J., et al. 1997. Characterization of events during the late stages of HPV16 infection in vivo using high-affinity synthetic Fabs to E4. Virology 238:40–52 [DOI] [PubMed] [Google Scholar]
- 16. Howley P. M., Lowy D. R. 2006. Papillomaviruses, p. 2299–2354 In Fields B. N., et al. (ed.), Virology, vol. 2 Lippincott/The Williams & Wilkins Co., Philadelphia, PA [Google Scholar]
- 17. Klymkowsky M. W., Miller R. H., Lane E. B. 1983. Morphology, behavior, and interaction of cultured epithelial cells after the antibody-induced disruption of keratin filament organization. J. Cell Biol. 96:494–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Knight G. L., Grainger J. R., Gallimore P. H., Roberts S. 2004. Cooperation between different forms of the human papillomavirus type 1 E4 protein to block cell cycle progression and cellular DNA synthesis. J. Virol. 78:13920–13933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Knight G. L., Turnell A. S., Roberts S. 2006. Role for Wee1 in inhibition of G2-to-M transition through the cooperation of distinct human papillomavirus type 1 E4 proteins. J. Virol. 80:7416–7426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Krebs M. R. H., Morozova-Roche L. A., Daniel K., Robinson C. V., Dobson C. M. 2004. Observation of sequence specificity in the seeding of protein amyloid fibrils. Protein Sci. 13:1933–1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lambert P. F., et al. 2005. Using an immortalized cell line to study the HPV life cycle in organotypic “raft” cultures. Methods Mol. Med. 119:141–155 [DOI] [PubMed] [Google Scholar]
- 22. McIntosh P. B., et al. 2010. E1^E4-mediated keratin phosphorylation and ubiquitylation: a mechanism for keratin depletion in HPV16-infected epithelium. J. Cell Sci. 123:2810–2822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McIntosh P. B., et al. 2008. Structural analysis reveals an amyloid form of the human papillomavirus type 16 E1^E4 protein and provides a molecular basis for its accumulation. J. Virol. 82:8196–8203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nakahara T., Peh W. L., Doorbar J., Lee D., Lambert P. F. 2005. Human papillomavirus type 16 E1^E4 contributes to multiple facets of the papillomavirus life cycle. J. Virol. 79:13150–13165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Raj K., Berguerand S., Southern S., Doorbar J., Beard P. 2004. E1 empty set E4 protein of human papillomavirus type 16 associates with mitochondria. J. Virol. 78:7199–7207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Resing K. A., al-Alawi N., Blomquist C., Fleckman P., Dale B. A. 1993. Independent regulation of two cytoplasmic processing stages of the intermediate filament-associated protein filaggrin and role of Ca2+ in the second stage. J. Biol. Chem. 268:25139–25145 [PubMed] [Google Scholar]
- 27. Roberts S., et al. 1994. Mutational analysis of human papillomavirus E4 proteins: identification of structural features important in the formation of cytoplasmic E4/cytokeratin networks in epithelial cells. J. Virol. 68:6432–6445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Roberts S., Ashmole I., Rookes S. M., Gallimore P. H. 1997. Mutational analysis of the human papillomavirus type 16 E1^E4 protein shows that the C terminus is dispensable for keratin cytoskeleton association but is involved in inducing disruption of the keratin filaments. J. Virol. 71:3554–3562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roberts S., Ashmole I., Sheehan T. M., Davies A. H., Gallimore P. H. 1994. Human papillomavirus type 1 E4 protein is a zinc-binding protein. Virology 202:865–874 [DOI] [PubMed] [Google Scholar]
- 30. Rogel-Gaillard C., Breitburd F., Orth G. 1992. Human papillomavirus type 1 E4 proteins differing by their N-terminal ends have distinct cellular localizations when transiently expressed in vitro. J. Virol. 66:816–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rogel-Gaillard C., Pehau-Arnaudet G., Breitburd F., Orth G. 1993. Cytopathic effect in human papillomavirus type 1-induced inclusion warts: in vitro analysis of the contribution of two forms of the viral E4 protein. J. Invest. Dermatol. 101:843–851 [DOI] [PubMed] [Google Scholar]
- 32. Rush M., Zhao X., Schwartz S. 2005. A splicing enhancer in the E4 coding region of human papillomavirus type 16 is required for early mRNA splicing and polyadenylation as well as inhibition of premature late gene expression. J. Virol. 79:12002–12015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Takahashi K. 1990. Calpain substrate specificity, p. 55–74 In Mellgren R., et al. (ed.), Intracellular calcium-dependent proteolysis. CRC Press, Inc., Boca Raton, FL [Google Scholar]
- 34. Tompa P., et al. 2004. On the sequential determinants of calpain cleavage. J. Biol. Chem. 279:20775–20785 [DOI] [PubMed] [Google Scholar]
- 35. Walboomers J., et al. 1999. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 189:12–19 [DOI] [PubMed] [Google Scholar]
- 36. Wang K. K., et al. 1996. An alpha-mercaptoacrylic acid derivative is a selective nonpeptide cell-permeable calpain inhibitor and is neuroprotective. Proc. Natl. Acad. Sci. U. S. A. 93:6687–6692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang Q., et al. 2004. Functional analysis of the human papillomavirus type 16 E1^E4 protein provides a mechanism for in vivo and in vitro keratin filament reorganization. J. Virol. 78:821–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang Q., et al. 2009. Phosphorylation of the human papillomavirus type 16 E1^E4 protein at T57 by ERK triggers a structural change that enhances keratin binding and protein stability. J. Virol. 83:3668–3683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Westermark P. 2005. Aspects on human amyloid forms and their fibril polypeptides. FEBS J. 272:5942–5949 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.