Abstract
γδ T cells are essential constituents of antimicrobial and antitumor defenses. We have recently reported that phosphoantigen isopentenyl pyrophosphate (IPP)-expanded human Vγ9Vδ2 T cells participated in anti-influenza virus immunity by efficiently killing both human and avian influenza virus-infected monocyte-derived macrophages (MDMs) in vitro. However, little is known about the noncytolytic responses and trafficking program of γδ T cells to influenza virus. In this study, we found that Vγ9Vδ2 T cells expressed both type 1 cytokines and chemokine receptors during influenza virus infection, and IPP-expanded cells had a higher capacity to produce gamma interferon (IFN-γ). Besides their potent cytolytic activity against pandemic H1N1 virus-infected cells, IPP-activated γδ T cells also had noncytolytic inhibitory effects on seasonal and pandemic H1N1 viruses via IFN-γ but had no such effects on avian H5N1 or H9N2 virus. Avian H5N1 and H9N2 viruses induced significantly higher CCL3, CCL4, and CCL5 production in Vγ9Vδ2 T cells than human seasonal H1N1 virus. CCR5 mediated the migration of Vγ9Vδ2 T cells toward influenza virus-infected cells. Our findings suggest a novel therapeutic strategy of using phosphoantigens to boost the antiviral activities of human Vγ9Vδ2 T cells against influenza virus infection.
INTRODUCTION
Influenza A (FluA) virus is a major causative pathogen of acute respiratory diseases worldwide and accounts for substantial morbidity and mortality annually (8, 20). The newly emerged strains produced by natural reassortment (e.g., avian or swine-origin pandemic influenza viruses) have posed many challenges for our community (12, 27, 28). Innate immunity plays a critical role in the host defense by limiting viral replication and helping initiate adaptive immune responses during the early phase of viral infection (21, 27, 35). Therefore, enhancing innate immunity has obvious benefit as an early therapeutic intervention for FluA virus infection.
Natural killer (NK) cells are key effector cells in innate immunity in that they destroy virus-infected cells directly without the need for prior antigen stimulation during acute viral infections (9, 21). However, recently we along with others found that FluA virus can evade NK cell immunity by directly infecting NK cells and inhibiting NK cell cytotoxicity (16, 23, 24). γδ T cells, as the innate-like T cells, have recently been demonstrated to have abilities to kill microbially infected cells and initiate adaptive immune responses (4–6, 29, 34). Although γδ T cells constitute only a small proportion (2% to 10%) of T cells in the peripheral blood of most adult mammals and humans (5, 6), they are essential constituents of antimicrobial and antitumor defenses (4). More intriguingly, unlike NK cells, γδ T cells have been proven not to be susceptible to FluA virus infection (24, 26).
Both natural and synthetic phosphoantigens can selectively expand Vγ9Vδ2 T cells in vitro or in vivo (37). Human peripheral Vγ9Vδ2 T cells or phosphoantigen-activated ones have been proven to have a broad antiviral activity through their cytolytic and noncytolytic mechanisms (29). Recently, we have found that phosphoantigen isopentenyl pyrophosphate (IPP)-expanded human Vγ9Vδ2 T cells participate in anti-influenza virus immunity by efficiently killing both human and avian influenza virus-infected monocyte-derived macrophages (MDMs) in vitro (32). However, little is known about their noncytolytic antiviral activity against FluA viruses. Moreover, it remains unclear whether the chemokine receptors expressed in Vγ9Vδ2 T cells could facilitate their trafficking to the sites of FluA virus infection for subsequent immune responses.
In the present study, we examined the cytokine and chemokine production and chemokine receptor expression profile in human Vγ9Vδ2 T cells during human and avian FluA virus infection. Using a transwell culture system, we further determined the noncytolytic antiviral activity of the soluble factors released from these cells against human and avian FluA viruses and their underlying mechanisms.
MATERIALS AND METHODS
Cells.
Peripheral blood mononuclear cells (PBMCs) were isolated from buffy coats of healthy donors (from the Hong Kong Red Cross) by Ficoll-Hypaque (Pharmacia) gradient centrifugation. The research protocol was approved by the Institutional Review Board of the University of Hong Kong. The peripheral resting γδ T cells were purified by negative selection with a T-cell receptor (TCR) γ/δ+ T cell isolation kit according to the manufacturer's instruction (Miltenyi Biotec). The IPP-expanded Vγ9Vδ2 T cells were generated as we described before (32). Briefly, PBMCs were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS). IPP (Sigma) was added at day 0 and day 3 to a final concentration of 6 μg/ml. Recombinant human interleukin-2 (IL-2; Invitrogen) was added to a final concentration of 500 IU/ml every 3 days from day 3. Following 14 days of culture, the cells were purified by negative selection with a TCR γ/δ+ T cell isolation kit according to the manufacturer's instruction (Miltenyi Biotec). The purity of γδ T cells, as determined by flow cytometry with anti-CD3 and anti-Vδ2 monoclonal antibodies (MAbs), was consistently >98%.
Human MDMs were generated from PBMCs as we described before (39). The purity of monocytes, as determined by flow cytometry with anti-CD14 MAb, was consistently >90%. A549 immortalized human alveolar type II epithelial cells were grown in Dulbecco's modified Eagle's medium (DMEM) plus 10% FBS.
Influenza viruses.
As we described in a previous study (39), human seasonal influenza virus H1N1 (A/Hong Kong/54/98) and avian H9N2 (A/Quail/HK/G1/97) and H5N1 (A/HK/483/97) were cultured in Madin-Darby canine kidney (MDCK) cells (ATCC). Pandemic H1N1 (A/California/04/2009; pdmH1N1) was propagated in embryonated chicken eggs. The viruses were concentrated and purified over a sucrose step gradient as described elsewhere (2). The virus titer was determined by daily observation of cytopathic effect (CPE), and the 50% tissue culture infective dose (TCID50) was calculated according to the Reed-Muench formula.
Flow cytometry.
Cells were stained for surface markers with the following monoclonal antibodies: anti-CD3 (HIT3a), anti-Vδ2 (B6), anti-CD69 (FN50), anti-CCR5 (HEK/1/85a) (Biolegend), and anti-CXCR5 (51505) (R&D Systems). Intracellular staining was performed after cell fixation and permeabilization as we described before (43), and the following MAbs were used: anti-gamma interferon ([IFN-γ] 25723.11) and anti-IL-4 (3010.211) antibodies (BD Biosciences). A total of 2 × 105 to 5 × 105 events for each sample were acquired by gating on small lymphocytes (using forward versus side scatter) on a BD FACSAria (BD Biosciences) and analyzed by Flowjo software (Tree Star, Inc.).
Profiling of chemokine receptor expression.
PBMCs were infected with mock, H1N1, or H9N2 virus at a multiplicity of infection (MOI) of 2. At 18 h postinfection (p.i.), CD3+ Vδ2+ cells in mock-treated PBMCs and CD3+ Vδ2+ CD69+ and CD3+ Vδ2+ CD69− cells in virus-infected PBMCs were sorted by FACSAria. The purity of sorted cells was routinely >97%. Total RNA from sorted cells was isolated and reverse transcribed to cDNA as we described before (32). The gene expression levels of CCR1 to CCR7, CCR9 and CCR10, and CXCR1 to CXCR6 were assessed with an ABI Prism 7900 sequence detection system (Applied Biosystems). The housekeeping gene β-actin was used to normalize chemokine receptor expression. Primers used for PCR are shown in Table 1. Relative quantification of chemokine receptors was performed using the comparative threshold cycle (CT) method (ΔΔCT method).
Table 1.
Primer sequences used in relative quantification real-time PCR assays
| Gene | Sequence (5′→3′)a | Amplification product length (bp) |
|---|---|---|
| CCR1 | F, ACCATAGGAGGCCAACCCAAAATA | 103 |
| R, TCCATGCTGTGCCAAGAGTCA | ||
| CCR2 | F, CTACCTTCCAGTTCCTCATTTTT | 100 |
| R, ACATTTACAAGTTGCAGTTTTCAGC | ||
| CCR3 | F, TTTGTCATCATGGCGGTGTTTTTC | 169 |
| R, GGTTCATGCAGCAGTGGGAGTAG | ||
| CCR4 | F, GAGAAGAAGAACAAGGCGGTGAAGA | 200 |
| R, GGATTAAGGCAGCAGTGAACAAAAG | ||
| CCR5 | F, CAACCACAGGCAGCATTTAGCAC | 147 |
| R, GGCAGGCAGCATCTTAGTTTTTCAG | ||
| CCR6 | F, CTGCCTGAACCCTGTGCTCTACG | 171 |
| R, TTATCTGCGGTCTCACTGGTCTGC | ||
| CCR7 | F, GCCGAGACCACCACCACCTT | 105 |
| R, AGTCATTGCATCTGCTCCCTATCC | ||
| CCR10 | F, GGGCTGGAGTCTGGGAAGTGC | 183 |
| R, ACGATGACGGAGACCAAGTGTGC | ||
| CCR11 | F, TCCTCCCTGTATTCCTCACAATAG | 310 |
| R, CTGGGGACTTTAGTTACTGCCAC | ||
| CXCR1 | F, CTGAGCCCCAAGTGGAACGAGACA | 152 |
| R, GCACGGAACAGAAGCTTTATTAGGA | ||
| CXCR2 | F, CAATGAATGAATGAATGGCTAAG | 118 |
| R, AAAGTTTTCAAGGTTCGTCCGTGTT | ||
| CXCR3 | F, CCCGCAACTGGTGCCGAGAAAG | 148 |
| R, AGGCGCAAGAGCAGCATCCACAT | ||
| CXCR4 | F, ATCCCTGCCCTCCTGCTGACTATTC | 231 |
| R, GAGGGCCTTGCGCTTCTGGTG | ||
| CXCR5 | F, TCCCCTCCTCACTCCCTTCCCATAA | 224 |
| R, CCTGCGGTTCCATCTGAGTGACATC | ||
| CXCR6 | F, TTGTTTATAGCTTGCGCATTCTCAT | 189 |
| R, ATCCCCCTTGGTTTCAGCATTCTT | ||
| β-Actin | F, GGATGCAGAAGGAGATCACTG | 90 |
| R, CAAGTACTCCGTGTGGATCG |
F, forward; R, reverse.
Chemotaxis.
MDMs were infected by H1N1 or H9N2 virus at an MOI of 2. Supernatant was collected at 48 h p.i. The in vitro migration of purified peripheral γδ T cells in response to virus-infected MDM (vMDM) supernatants was assessed in a transwell system (24-well; pore size, 5.0 μm; polycarbonate membranes; Corning-Costar) as we described before (18). Briefly, supernatants from H1N1- or H9N2-infected MDMs (H1-sup or H9-sup) were loaded in the lower compartment. A total of 100 μl of autologous γδ T cells (105) in serum-free RPMI 1640 medium was added to the upper compartment of the chamber. After 4 h, the cells that had migrated through the membrane to the lower compartment were collected and counted microscopically with crystal violet. The data are expressed as a percentage of the migrated cells in total cells. In blocking experiments, γδ T cells were preincubated for 30 min with either anti-CCR5 MAb (1 to 20 μg/ml; clone 45531; R&D Systems) or isotype control mouse IgG2b ([mIgG2b] 1 to 20 μg/ml; R&D Systems) MAb.
Profiling of chemokines.
MDMs were infected with H1N1, H9N2, or H5N1 virus for 1 h for antigen processing. Then, vMDMs were exposed to 0.1% paraformaldehyde (PFA) briefly for silencing of cytokine production (42). After PFA was washed away, the vMDMs were cocultured with purified peripheral γδ T cells for the time indicated in the legend to Fig. 2. Then the cell-free supernatants were collected and used for quantification of the chemokines secreted by γδ T cells with a bead-based Flow Cytomix assay according to the manufacturer's instructions (BenderMed System). The concentrations of the chemokines were calculated by extrapolating the mean fluorescence intensities (MFI) on the respective standard curves and are expressed as pg/ml, using Flow Cytomix Pro software (version 2.3).
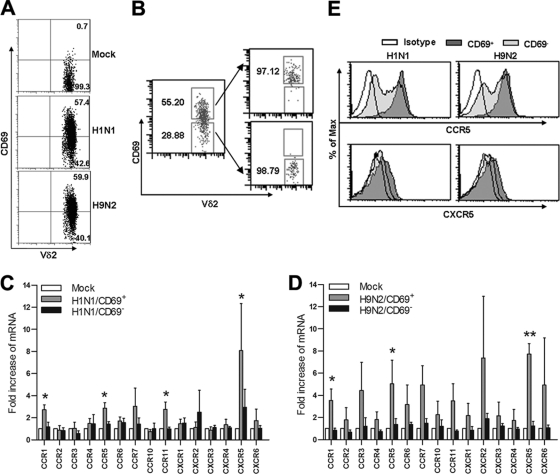
Fig. 2.
FluA virus-activated Vγ9Vδ2 T cells express type 1 chemokine receptors. (A and B) PBMCs were infected with mock, H1N1, or H9N2 virus at an MOI of 2. At 18 h p.i., the cells were stained for CD3, Vδ2, and CD69 and then analyzed by flow cytometry. CD69 expression in Vγ9Vδ2 T cells is shown after virus stimulation. Data shown are representative of five separate experiments. CD3+ Vδ2+ cells were sorted from mock-infected PBMCs, while CD3+ Vδ2+ CD69+ and CD3+ Vδ2+ CD69− cells were sorted from virus-infected PBMCs by FACSAria (B). (C and D) Total RNA was extracted from sorted cells, and gene expression levels of chemokine receptors were determined by relative quantitative real-time RT-PCR. The fold increase of chemokine receptor mRNA relative to the level in mock-treated γδ T cells (mean ± SEM) was calculated from CT values using the formula 2−ΔCT. A Newman-Keuls one-way ANOVA test was used to compare chemokine receptor expression levels in mock-treated cells and in virus-reactive CD69+ and CD69− subsets (n = 4). (E) PBMCs were infected by mock, H1N1, or H9N2 virus for 24 h. The cells were stained for CD3, Vδ2, CD69, CCR5, and CXCR5. Histogram plots of CCR5 and CXCR5 expression in CD69+ and CD69− Vδ2 T subsets are representative for four separate experiments. *, P < 0.05; **, P < 0.01. Max, maximum.
Quantification of FluA virus viral copies by RT-PCR.
MDMs (1 × 105) were infected with H1N1, pdmH1N1, or H9N2 at an MOI of 2. One hour later, unadsorbed virus was washed away carefully, and the vMDMs were cultured alone or with 1 × 106 γδ T cells for 48 h. Viral matrix (M1) gene copies in the cells and supernatant were quantified by SYBR green real-time reverse-transcription PCR (RT-PCR) as we described before (32). Results are expressed as the number of target gene copies per 105 MDMs.
Human lung epithelial A549 cells (2 × 105) in the bottom wells were infected by H1N1, pdmH1N1, or H9N2 at an MOI of 2. Purified γδ T cells (1 × 106) were added into transwell inserts (24 wells; pore size, 0.4 mm; Millipore), with or without the IPP (6 μg/ml), and cocultured for 4 days. For blocking experiments, anti-IFN-γ (10 μg/ml; R&D Systems) or isotype goat IgG (gIgG) were added in H1N1 and pdmH1N1 infection experiments. The A549 cells were collected. Viral copies were quantified by real-time PCR and normalized by β-actin.
Cytotoxicity assay.
The cytotoxicity of IPP-expanded γδ T cells (effector) against pdmH1N1 virus-infected MDMs (target) was assessed by flow cytometry as we described before (32).
Statistical analysis.
Data are expressed as means ± standard errors of the means (SEM). Statistical significance was determined by a Student t test or one-way analysis of variance (ANOVA), using GraphPad Prism, version 5, software. A P value of <0.05 was considered to be significant.
RESULTS
Human Vγ9Vδ2 T cells express type 1 cytokines in response to FluA viruses.
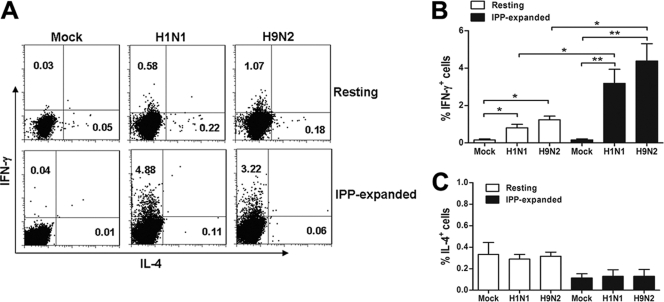
We first examined whether influenza virus can trigger a type 1 or type 2 response in the resting and IPP-expanded Vγ9Vδ2 T cells. As shown in Fig. 1A and B, both H1N1 and H9N2 viruses induced significantly higher expression levels of IFN-γ in resting Vγ9Vδ2 T cells than mock-treated cells although the frequencies of IFN-γ+ cells were relatively low after virus stimulation. H1N1 and H9N2 viruses also induced IFN-γ expression in IPP-expanded Vγ9Vδ2 T cells significantly, and the frequencies of IFN-γ+ cells in IPP-expanded Vγ9Vδ2 T cells were three times greater than in resting Vγ9Vδ2 T cells after virus stimulation (Fig. 1A and B). In contrast, there was no change in IL-4 expression in resting or IPP-expanded Vγ9Vδ2 T cells upon FluA virus stimulation (Fig. 1A and C). These results indicated that FluA virus can trigger the type 1 response of Vγ9Vδ2 T cells and that IPP-expanded cells have a higher capacity to produce IFN-γ upon influenza virus infection.
Fig. 1.
Human Vγ9Vδ2 T cells express type 1 cytokines in response to FluA viruses. (A) PBMCs were infected by FluA H1N1 or H9N2 virus at an MOI of 2 for 12 h (upper row). MDMs were infected by H1N1 or H9N2 virus (target) at an MOI of 2 for 1 h and then cocultured with the purified IPP-expanded Vγ9Vδ2 T cells (effector) at an effector-to-target ratio of 1:1 for 12 h (lower row). Cells were stained for CD3, TCR γδ, IFN-γ, and IL-4. The intracellular contents of IFN-γ (y axis) and IL-4 (x axis) in resting γδ T cells and IPP-expanded γδ T cells are representative of six separate experiments. (B) The percentages of IFN-γ+ cells in resting and IPP-expanded γδ T cell populations (means ± SEM) are shown (n = 6). (C) The percentages of IL-4+ cells (means ± SEM) in resting and IPP-expanded γδ T cell populations are shown (n = 6). *, P < 0.05; **, P < 0.01.
FluA virus-activated Vγ9Vδ2 T cells express type 1 chemokine receptors.
As FluA viruses activated Vγ9Vδ2 T cells, evidenced by increased expression of the activation marker CD69 in these cells upon FluA virus stimulation (Fig. 2A), the CD69+ cells were referred to as FluA virus-activated Vγ9Vδ2 T cells. We sorted CD69+ and CD69− Vγ9Vδ2 T cell subsets from virus-infected PBMCs (Fig. 2B) and determined their chemokine receptor expression levels. As shown in Fig. 2C and D, both H1N1 and H9N2 virus-activated Vγ9Vδ2 T cells had higher expression levels of certain CC receptors (CCR1 and CCR5) and CXC receptor (CXCR5) than mock-treated Vγ9Vδ2 T cells or virus-treated CD69− Vγ9Vδ2 T cells. Meanwhile, the differences in gene expression of other chemokine receptors, such as CCR2, CCR3, CCR4, CCR7, and CXCR3, among Vγ9Vδ2 T cell subsets were not statistically significant. There were no significant differences in any of the above chemokine receptor expression levels between H1N1 and H9N2 virus-activated Vγ9Vδ2 T cells. Furthermore, the Th1 signature marker, CCR5 protein, was strongly expressed on virus-activated CD69+ Vγ9Vδ2 T cells compared with the CD69− counterparts (Fig. 2C and D), as confirmed by flow cytometry (Fig. 2E). The surface expression levels of CXCR5 were quite low in both subsets (Fig. 2E). These data demonstrated that FluA virus-activated Vγ9Vδ2 T cells preferentially express type 1 chemokine receptors.
CCR5 mediates Vγ9Vδ2 T cell migration to virus-infected cells.
Using a transwell chemotaxis assay, we found that the supernatants from H1N1 and H9N2 virus-infected MDMs had significantly higher chemotactic abilities for Vγ9Vδ2 T cells than supernatants from mock-treated MDMs. This effect was not caused by chemokinesis as the migration of Vγ9Vδ2 T cells was not observed when the supernatant from virus-infected MDMs was added to both upper and lower wells (Fig. 3A). Importantly, the migration induced by supernatant from either H1N1- or H9N2-infected MDMs was abrogated by CCR5 neutralizing MAb (Fig. 3B), and the inhibition of Vγ9Vδ2 T cell migration by CCR5 neutralizing MAb was dose dependent (Fig. 3C). These results suggested that the migration of Vγ9Vδ2 T cells to virus-infected sites may mainly be mediated by CCR5.
Fig. 3.
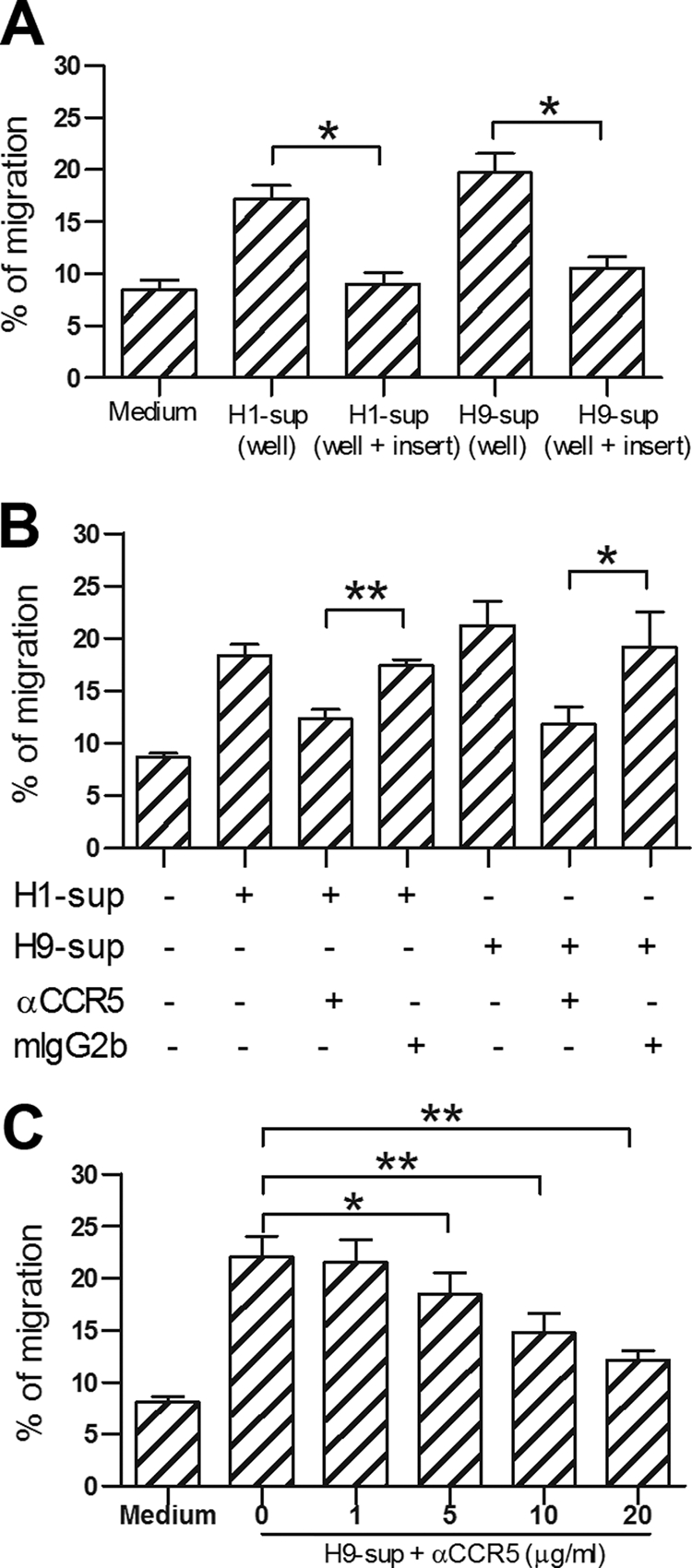
CCR5 mediates Vγ9Vδ2 T cell migration to virus-infected cells. (A) Purified peripheral Vγ9Vδ2 T cells were assayed for their chemotactic response to the supernatants from FluA virus-infected MDMs (Materials and Methods). Background migration was measured with medium in the lower well. Supernatants were also added to both upper and lower wells as controls. (B) Vγ9Vδ2 T cells were preincubated with anti-CCR5 MAb (20 μg/ml) or isotype control mouse IgG2b (mIgG2b; 20 μg/ml) for 30 min and placed in the upper well. H1N1-infected MDM supernatant (H1-sup) or H9N2-infected MDM supernatant (H9-sup) was added (+) into the lower well. (C) Titration of anti-CCR5 MAb (1 to 20 μg/ml) effects on the migration of Vγ9Vδ2 T cells. The percentages of cells (means ± SEM) that have migrated from the upper well are shown (n = 4). *, P < 0.05; **, P < 0.01. α, anti.
Vγ9Vδ2 T cells produce differential chemokine expression upon human versus avian FluA virus stimulation.
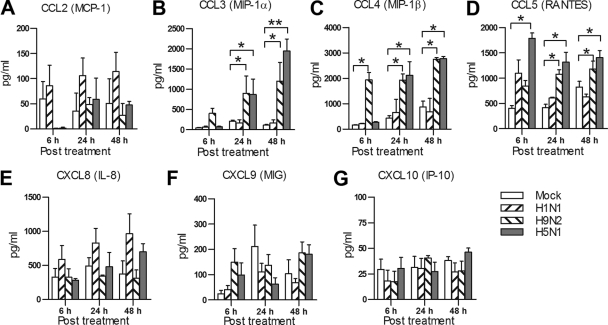
To determine whether there are differences in chemokine secretions from Vγ9Vδ2 T cells following human seasonal FluA or avian FluA virus stimulation, we examined the chemokine secretion from Vγ9Vδ2 T cells. As observed in Fig. 4, avian FluA H9N2 and H5N1 viruses induced significantly higher production levels of CCL3 (MIP-1α), CCL4 (MIP-1β), and CCL5 (RANTES) at nearly all time points (6, 24, and 48 h) postinfection than human seasonal H1N1 virus. There was no significant difference in the production levels of other chemokines, including CCL2 (MCP-1), CXCL8 (IL-8), CXCL9 (monokine induced by γ-IFN [MIG]), and CXCL10 (IP-10), between human and avian FluA viruses. No significant differences were observed in the production levels of any of the above chemokines in Vγ9Vδ2 T cells infected with either H5N1 or H9N2. These data indicated that avian FluA viruses can induce stronger chemokine production in Vγ9Vδ2 T cells.
Fig. 4.
Vγ9Vδ2 T cells produce differential chemokines upon human versus avian FluA virus stimulation. MDMs (105) were infected with H1N1, H9N2, or H5N1 virus at an MOI of 2 for 1 h. Then, virus-infected MDMs (vMDMs; target) were fixed with 0.1% PFA for 10 min. Purified Vγ9Vδ2 T cells were cocultured with the fixed vMDMs at an effector/target ratio of 1:1 for the indicated time. The amounts of chemokines secreted by Vγ9Vδ2 T cells were quantified by a bead-based Flow Cytomix assay (BenderMed System). Data are means ± SEM for four independent experiments. *, P < 0.05; **, P < 0.01.
IFN-γ released from Vγ9Vδ2 T cells inhibits human H1N1 virus replication but not avian FluA viruses.
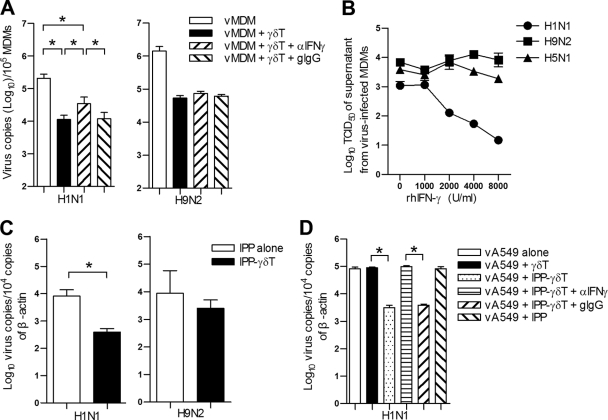
To investigate whether IFN-γ secreted by Vγ9Vδ2 T cells is involved in the inhibition of viral replication, blocking antibody against IFN-γ was applied when Vγ9Vδ2 T cells were cocultured with virus-infected MDMs. The inhibition of H1N1 virus replication was significantly but partially abrogated by IFN-γ neutralizing MAb. In contrast, this neutralizing MAb did not affect avian H9N2 viral replication (Fig. 5A). To confirm this, we further examined the antiviral activity of recombinant human IFN-γ in human and avian FluA virus-infected MDMs. As shown in Fig. 5B, recombinant human IFN-γ significantly inhibited human seasonal H1N1 virus replication in a dose-dependent manner, but it did not affect avian H9N2 or H5N1 virus replication. Using a transwell culture system, we found that the soluble factors released from IPP-activated Vγ9Vδ2 T cells significantly inhibited the replication of human seasonal H1N1 virus but not avian H9N2 virus in human lung epithelial A549 cells (Fig. 5C). With the addition of IFN-γ neutralizing MAb, the inhibition of H1N1 viral replication was almost abrogated completely (Fig. 5D). These results indicated that the noncytolytic antiviral response of IPP-activated Vγ9Vδ2 T cells against H1N1 virus mainly relies on IFN-γ, but avian FluA viruses are resistant to this noncytolytic antiviral activity.
Fig. 5.
IFN-γ released from Vγ9Vδ2 T cells inhibits human seasonal H1N1 virus replication but not avian FluA viruses. (A) MDMs were infected with H1N1 or H9N2 virus at an MOI of 2 for 1 h. Then vMDMs were cultured alone or with purified IPP-expanded Vγ9Vδ2 T cells at an effector/target ratio of 10:1 in the presence of anti-IFN-γ MAb (αIFN-γ; 10 μg/ml) or its isotype control, goat IgG (gIgG; 10 μg/ml), for 48 h. Total RNA was extracted from both cells and supernatants, and viral M1 gene copies were quantified by real-time RT-PCR. Data (means ± SEM) for M1 gene copies per 105 MDMs from four separate experiments are shown. (B) MDMs were pretreated with human recombinant IFN-γ at the indicated doses for 24 h and then infected with H1N1, H9N2, or H5N1 virus at an MOI of 2 for an additional 48 h. The virus titers of supernatants were determined by TCID50 assay on MDCK cells. Data are means ± SEM of TCID50 titers of four separate experiments. (C) A549 cells (target) were infected by human seasonal H1N1 or avian H9N2 virus at an MOI of 2 for 1 h. Purified Vγ9Vδ2 T cells (effector) were added into transwell inserts at an effector-to-target ratio of 5:1 in the presence of IPP (6 μg/ml) and cocultured for 4 days. In the control group (IPP alone), IPP was added to infected A549 cells in the absence of Vγ9Vδ2 T cells. (D) A549 cells in the bottom wells were infected by H1N1 virus at an MOI of 2. Purified Vγ9Vδ2 T cells were added into transwell inserts. IPP (6 μg/ml) was added alone or together with anti-IFN-γ MAb (10 μg/ml) or goat IgG (10 μg/ml); The A549 cells were collected at day 4 postinfection. Viral copies were quantified by real-time PCR. Data are means ± SEM for viral M1 gene copies per 104 copies of β-actin from four separate experiments. *, P < 0.05; **, P < 0.01.
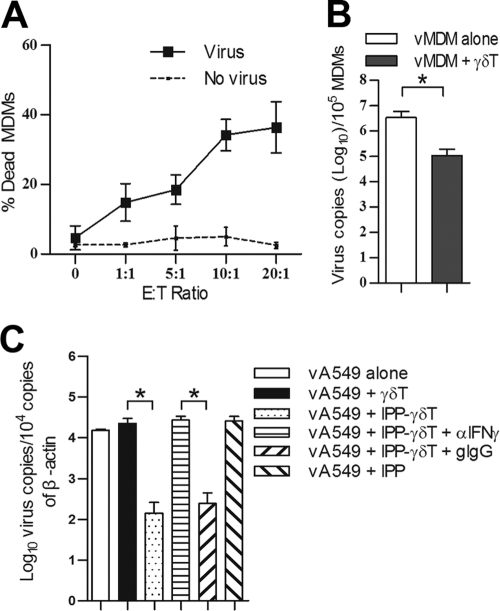
Vγ9Vδ2 T cells inhibit pandemic H1N1 virus replication in both a cytotoxic and noncytotoxic manner.
Previously, we reported that IPP-expanded Vγ9Vδ2 T cells can kill human seasonal H1N1 and avian H5N1 and H9N2 virus-infected cells (32), but it remains unknown whether Vγ9Vδ2 T cells have similar effects on pandemic H1N1 (pdmH1N1) virus-infected cells. Using the coculture of IPP-expanded Vγ9Vδ2 T cells and pdmH1N1-infected MDMs and then flow cytometry to identify the dead target cells as CD3− ethidium homodimer 2-positive (EthD-2+) population, we found that IPP-expanded Vγ9Vδ2 T cells displayed potent cytotoxicity against pdmH1N1 virus-infected MDMs in a dose-dependent manner (Fig. 6A). Moreover, virus replication was significantly reduced in pdmH1N1 virus-infected MDMs after treatment with IPP-expanded Vγ9Vδ2 T cells (Fig. 6B). Using a transwell culture system, we further found that the soluble factors released from IPP-expanded Vγ9Vδ2 T cells significantly inhibited the replication of pdmH1N1 virus in human lung epithelial A549 cells, and this inhibition could be reversed by IFN-γ neutralizing MAb (Fig. 6C). The results indicated that IPP-expanded Vγ9Vδ2 T cells have both cytotoxic and noncytolytic antiviral effects against pdmH1N1 virus.
Fig. 6.
IPP-expanded Vγ9Vδ2 T cells inhibit pandemic H1N1 virus replication in both a cytotoxic and noncytolytic manner. (A) MDMs (target) were infected by mock or pdmH1N1 virus at an MOI of 2 for 1 h and then cocultured with purified IPP-expanded Vγ9Vδ2 T cells (effector) at indicated effector/target (E/T) ratios (range, 0:1 to 20:1) for 6 h. The percentages (means ± SEM) of dead MDMs among whole target cells (CD3− population) identified as CD3− EthD-2+ from four separate experiments are shown. (B) MDMs were infected with pdmH1N1 virus at an MOI of 2 and cultured alone or with Vγ9Vδ2 T cells at an E/T ratio of 10:1 for 48 h. Total RNA was extracted from both cells and supernatant, and viral matrix gene copies were quantified by real-time RT-PCR, as described in Materials and Methods. Data are means ± SEM for viral M1 gene copies per 105 MDMs from four separate experiments. (C) A549 cells (target) in the bottom wells were infected with pdmH1N1 virus at an MOI of 2 for 1 h. Then, purified Vγ9Vδ2 T cells (effector) were added into transwell inserts at an E/T ratio of 5:1. IPP (6 μg/ml) was added alone or together with anti-IFN-γ MAb (αIFN-γ; 10 μg/ml) or goat IgG (gIgG; 10 μg/ml). The A549 cells were collected at day 4 postinfection. Viral copies were quantified by real-time PCR. Data are shown as means ± standard error for viral M1 gene copies per 104 copies of β-actin from four separate experiments. *, P < 0.05.
DISCUSSION
γδ T cells, like αβ T cells, can differentiate into type 1 or type 2 cells in different contexts (10, 41). The balance of type 1 and type 2 responses is thought to be one key determinant of the outcome of immune responses in many pathological conditions, including microbial and virus infections (36). Our earlier studies suggested that virus-specific Th1 responses for cytomegalovirus (CMV) are critical for the control of viral replication (38, 40). In this study, we demonstrated for the first time that human Vγ9Vδ2 T cells, especially IPP-expanded cells, exhibit a type 1 response upon FluA virus infection, and soluble factors such as IFN-γ released from Vγ9Vδ2 T cells contribute to their noncytolytic antiviral activity against human and pandemic H1N1 FluA viruses. IPP-expanded Vγ9Vδ2 T cells have more capacity to produce IFN-γ upon FluA virus infection, indicating that phosphoantigen could be used as an alternative therapeutic option to treat FluA virus infections through boosting the noncytolytic antiviral activity of Vγ9Vδ2 T cells.
The antiviral mechanisms of Vγ9Vδ2 T cells among different viral infections are diverse. For instance, human Vγ9Vδ2 T cells can kill herpes simplex virus (HSV)-, Epstein-Barr virus (EBV)-, or CMV-infected target cells in an HLA-unrestricted manner in vitro (7, 13, 17). IPP-activated Vγ9Vδ2 T cells not only kill human immunodeficiency virus (HIV)-infected target cells but also inhibit viral replication by releasing certain CCR5 ligand chemokines to block the HIV entry coreceptor CCR5 (30, 31). Moreover, phosphoantigen-activated Vγ9Vδ2 T cells can induce noncytolytic inhibition of hepatitis C virus (HCV) replication through the secretion of IFN-γ (1). Previously, we have demonstrated that IPP-expanded Vγ9Vδ2 T cells can efficiently kill both human seasonal and avian influenza virus-infected MDMs and consequently inhibit viral replication (32). Here, we further found that these cells can kill pandemic H1N1 virus-infected MDMs. With exogenous IFN-γ and blocking assays, we further showed that IPP-expanded Vγ9Vδ2 T cells inhibit human and pandemic H1N1 virus replication through the secretion of IFN-γ. Our results indicate that both cytotoxicity and soluble factors are involved in their antiviral activity against FluA viruses.
Interestingly, compared to the effect on human seasonal and pandemic H1N1 viruses, the soluble factors and IFN-γ released from IPP-expanded Vγ9Vδ2 T cells had no such inhibitory effects on avian H5N1 (A/HK/483/97) and H9N2 (A/Quail/HK/G1/97) viruses. In supporting our findings, Seo et al. also reported that avian H5N1 (A/HK/156/97) virus is resistant to the antiviral effects of IFNs even at very high concentrations. They also reported that an Asp92Glu amino acid substitution in the NS1 gene may be related to this resistance (33). The Asp92Glu amino acid substitution in the NS1 gene was also found in H5N1 (A/HK/483/97) and H9N2 (A/Quail/HK/G1/97) viruses (15, 25), suggesting that this amino acid substitution in the NS1 genes of the H5N1 (A/HK/483/97) and H9N2 (A/Quail/HK/G1/97) viruses might also contribute to their IFN resistance. Therefore, it is particularly important to boost the cytotoxic activity of Vγ9Vδ2 T cells for controlling avian FluA virus infections. In fact, phosphoantigen IPP has been found to enhance the cytotoxic activity of Vγ9Vδ2 T cells and lead the clearance of avian H5N1 and H9N2 viruses in vitro (32).
Different expression profiles of chemokine receptors associate with functionally distinct T lymphocyte subsets. CCR5, CXCR3, and CCR1 have been found preferentially on Th1 cells, while Th2 cells frequently express CCR3 and CCR4 (3). Here, we found that FluA virus-activated Vγ9Vδ2 T cells expressed upregulated type 1 chemokine receptors, such as CCR1, CCR5, and CXCR5. Human and avian FluA virus-infected cells can produce chemokines CCL2, CCL3, and CCL5 (44), and CCR5 can bind CCL3 and CCL5 (14), suggesting that FluA virus-activated Vγ9Vδ2 T cells can migrate to infected sites. Indeed, our data here demonstrated that CCR5 expressed on FluA virus-activated Vγ9Vδ2 T cells mediates their migration to virus-infected cells.
Chemokines act as important mediators for immune cell activation and recruitment and contribute to the inflammatory response (22). Via CCR5, chemokines such as CCL3, CCL4, and CCL5 potentially contribute to lung pathology by inducing the activation and migration of leukocytes to the site of influenza virus infection (22). Here, we also found that Vγ9Vδ2 T cells produced significantly higher levels of CCL3, CCL4, and CCL5 in response to avian H5N1 and H9N2 viruses than in response to human seasonal H1N1 virus. In support of this, one of our earlier studies also found higher expression levels of CCL2, CCL3, CCL5, and CXCL10 in avian FluA virus-infected cells than in human seasonal FluA virus-infected cells (44), suggesting that these higher production levels of chemokines may account for the severity of H5N1 disease.
Altogether, our study demonstrated that human Vγ9Vδ2 T cells exhibited a type 1 response in terms of cytokine and chemokine receptor expression during FluA virus infection. Besides their cytotoxic activity against human seasonal and pandemic FluA viruses, IPP-activated Vγ9Vδ2 T cells can inhibit both human seasonal and pandemic H1N1 viral replication in a noncytolytic manner, mainly through IFN-γ. In contrast, avian H5N1 (A/HK/483/97) and H9N2 (A/Quail/HK/G1/97) viruses are resistant to the IFN-γ-mediated antiviral activity of Vγ9Vδ2 T cells, but they are sensitive to the cytotoxicity of these cells (32). Vγ9Vδ2 T cells have the potential to migrate to FluA virus-infected sites through a CCR5-dependent mechanism. In conclusion, our findings suggest a novel therapeutic strategy of using phosphoantigens to boost the cytotoxic and noncytolytic antiviral activities of human Vγ9Vδ2 T cells for FluA virus infection.
ACKNOWLEDGMENTS
This work was supported in part by the Area of Excellence program on influenza supported by the University Grants Committee of the Hong Kong SAR, China (project number AoE/M-12/06; to J.S.M.P., Y.-L.L., and W.T.); the General Research Fund, Research Grants Council of Hong Kong (HKU 777108 M, HKU777407, and HKU768108; to W.T. and Y.-L.L.); NSFC, China (30973235; to H.L. and W.T.); and the Research Fund for the Control of Infectious Diseases, Hong Kong government (HK-09-03-05).
We declare that we have no potential conflicts of interests.
Footnotes
Published ahead of print on 13 July 2011.
REFERENCES
- 1. Agrati C., et al. 2006. Activation of Vγ9Vδ2 T cells by non-peptidic antigens induces the inhibition of subgenomic HCV replication. Int. Immunol. 18:11–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arora D. J., Tremblay P., Bourgault R., Boileau S. 1985. Concentration and purification of influenza virus from allantoic fluid. Anal. Biochem. 144:189–192 [DOI] [PubMed] [Google Scholar]
- 3. Bonecchi R., et al. 1998. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J. Exp. Med. 187:129–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bonneville M., Scotet E. 2006. Human Vγ9Vδ2 T cells: promising new leads for immunotherapy of infections and tumors. Curr. Opin. Immunol. 18:539–546 [DOI] [PubMed] [Google Scholar]
- 5. Born W. K., Reardon C. L., O'Brien R. L. 2006. The function of γδ T cells in innate immunity. Curr. Opin. Immunol. 18:31–38 [DOI] [PubMed] [Google Scholar]
- 6. Brandes M., Willimann K., Moser B. 2005. Professional antigen-presentation function by human γδ T cells. Science 309:264–268 [DOI] [PubMed] [Google Scholar]
- 7. Bukowski J. F., Morita C. T., Brenner M. B. 1994. Recognition and destruction of virus-infected cells by human gamma delta CTL. J. Immunol. 153:5133–5140 [PubMed] [Google Scholar]
- 8. Chiu S. S., et al. 2009. Virologically confirmed population-based burden of hospitalization caused by influenza A and B among children in Hong Kong. Clin. Infect. Dis. 49:1016–1021 [DOI] [PubMed] [Google Scholar]
- 9. Cooper M. A., Fehniger T. A., Caligiuri M. A. 2001. The biology of human natural killer-cell subsets. Trends Immunol. 22:633–640 [DOI] [PubMed] [Google Scholar]
- 10. Dagna L., et al. 2002. Skewing of cytotoxic activity and chemokine production, but not of chemokine receptor expression, in human type-1/-2 gamma delta T lymphocytes. Eur. J. Immunol. 32:2934–2943 [DOI] [PubMed] [Google Scholar]
- 11. Reference deleted.
- 12. Dawood F. S., et al. 2009. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N. Engl. J. Med. 360:2605–2615 [DOI] [PubMed] [Google Scholar]
- 13. Fujishima N., et al. 2007. Skewed T cell receptor repertoire of Vδ1+ γδ T lymphocytes after human allogeneic haematopoietic stem cell transplantation and the potential role for Epstein-Barr virus-infected B cells in clonal restriction. Clin. Exp. Immunol. 149:70–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Glatzel A., et al. 2002. Patterns of chemokine receptor expression on peripheral blood γδ T lymphocytes: strong expression of CCR5 is a selective feature of Vδ2/Vγ9 gamma delta T cells. J. Immunol. 168:4920–4929 [DOI] [PubMed] [Google Scholar]
- 15. Guan Y., Shortridge K. F., Krauss S., Webster R. G. 1999. Molecular characterization of H9N2 influenza viruses: were they the donors of the “internal” genes of H5N1 viruses in Hong Kong? Proc. Natl. Acad. Sci. U. S. A. 96:9363–9367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guo H., et al. 2009. The functional impairment of natural killer cells during influenza virus infection. Immunol. Cell Biol. 87:579–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Halary F., et al. 2005. Shared reactivity of Vδ2− γδ T cells against cytomegalovirus-infected cells and tumor intestinal epithelial cells. J. Exp. Med. 201:1567–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ip W. K., Lau Y. L. 2004. Distinct maturation of, but not migration between, human monocyte-derived dendritic cells upon ingestion of apoptotic cells of early or late phases. J. Immunol. 173:189–196 [DOI] [PubMed] [Google Scholar]
- 19. Reference deleted.
- 20. Lewis D. B. 2006. Avian flu to human influenza. Annu. Rev. Med. 57:139–154 [DOI] [PubMed] [Google Scholar]
- 21. Lodoen M. B., Lanier L. L. 2006. Natural killer cells as an initial defense against pathogens. Curr. Opin. Immunol. 18:391–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mahalingam S., Karupiah G. 1999. Chemokines and chemokine receptors in infectious diseases. Immunol. Cell Biol. 77:469–475 [DOI] [PubMed] [Google Scholar]
- 23. Mao H., et al. 2010. Inhibition of human natural killer cell activity by influenza virions and hemagglutinin. J. Virol. 84:4148–4157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mao H., et al. 2009. Influenza virus directly infects human natural killer cells and induces cell apoptosis. J. Virol. 83:9215–9222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Matrosovich M. N., Matrosovich T. Y., Gray T., Roberts N. A., Klenk H. D. 2004. Human and avian influenza viruses target different cell types in cultures of human airway epithelium. Proc. Natl. Acad. Sci. U. S. A. 101:4620–4624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Meuter S., Eberl M., Moser B. 2010. Prolonged antigen survival and cytosolic export in cross-presenting human γδ T cells. Proc. Natl. Acad. Sci. U. S. A. 107:8730–8735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Peiris J. S., Tu W. W., Yen H. L. 2009. A novel H1N1 virus causes the first pandemic of the 21st century. Eur. J. Immunol. 39:2946–2954 [DOI] [PubMed] [Google Scholar]
- 28. Peiris J. S., et al. 2004. Re-emergence of fatal human influenza A subtype H5N1 disease. Lancet 363:617–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Poccia F., et al. 2005. Antiviral reactivities of γδ T cells. Microbes Infect. 7:518–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Poccia F., et al. 1999. Phosphoantigen-reactive Vγ9Vδ2 T lymphocytes suppress in vitro human immunodeficiency virus type 1 replication by cell-released antiviral factors including CC chemokines. J. Infect. Dis. 180:858–861 [DOI] [PubMed] [Google Scholar]
- 31. Poccia F., et al. 1997. CD94/NKG2 inhibitory receptor complex modulates both anti-viral and anti-tumoral responses of polyclonal phosphoantigen-reactive Vγ9Vδ2 T lymphocytes. J. Immunol. 159:6009–6017 [PubMed] [Google Scholar]
- 32. Qin G., et al. 2009. Phosphoantigen-expanded human γδ T cells display potent cytotoxicity against monocyte-derived macrophages infected with human and avian influenza viruses. J. Infect. Dis. 200:858–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Seo S. H., Hoffmann E., Webster R. G. 2002. Lethal H5N1 influenza viruses escape host anti-viral cytokine responses. Nat. Med. 8:950–954 [DOI] [PubMed] [Google Scholar]
- 34. Shen Y., et al. 2002. Adaptive immune response of Vγ2Vδ2+ T cells during mycobacterial infections. Science 295:2255–2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shinya K., et al. 2006. Avian flu: influenza virus receptors in the human airway. Nature 440:435–436 [DOI] [PubMed] [Google Scholar]
- 36. Spellberg B., Edwards J. E., Jr 2001. Type 1/type 2 immunity in infectious diseases. Clin. Infect. Dis. 32:76–102 [DOI] [PubMed] [Google Scholar]
- 37. Tanaka Y., Morita C. T., Nieves E., Brenner M. B., Bloom B. R. 1995. Natural and synthetic non-peptide antigens recognized by human gamma delta T cells. Nature 375:155–158 [DOI] [PubMed] [Google Scholar]
- 38. Tu W., et al. 2004. Persistent and selective deficiency of CD4+ T cell immunity to cytomegalovirus in immunocompetent young children. J. Immunol. 172:3260–3267 [DOI] [PubMed] [Google Scholar]
- 39. Tu W., et al. 2010. Cytotoxic T lymphocytes established by seasonal human influenza cross-react against 2009 pandemic H1N1 influenza virus. J. Virol. 84:6527–6535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tu W., et al. 2006. T-cell immunity to subclinical cytomegalovirus infection reduces cardiac allograft disease. Circulation 114:1608–1615 [DOI] [PubMed] [Google Scholar]
- 41. Wen L., et al. 1998. Primary γδ cell clones can be defined phenotypically and functionally as Th1/Th2 cells and illustrate the association of CD4 with Th2 differentiation. J. Immunol. 160:1965–1974 [PubMed] [Google Scholar]
- 42. Zenhaeusern G., et al. 2007. Investigation of alloreactive NK cells in mixed lymphocyte reactions using paraformaldehyde-silenced target cells. J. Immunol. Methods 321:196–199 [DOI] [PubMed] [Google Scholar]
- 43. Zheng J., et al. 2009. Efficient induction and expansion of human alloantigen-specific CD8 regulatory T cells from naive precursors by CD40-activated B cells. J. Immunol. 183:3742–3750 [DOI] [PubMed] [Google Scholar]
- 44. Zhou J., et al. 2006. Differential expression of chemokines and their receptors in adult and neonatal macrophages infected with human or avian influenza viruses. J. Infect. Dis. 194:61–70 [DOI] [PMC free article] [PubMed] [Google Scholar]