Abstract
Karposi's sarcoma-associated herpesvirus (KSHV) is found predominantly in a latent state in most cell types, impeding investigations of the lytic replication cycle. Here, we engineered the cloned KSHV genome, bacterial artificial chromosome 36 (BAC36), to enforce constitutive expression of the main lytic switch regulator, the replication and transcription activator (RTA) (open reading frame 50 [ORF50]). The resulting virus, KSHV-lyt, activated by default the lytic cycle and replicated to high titers in various cells. Using KSHV-lyt, we showed that ORF33 (encoding a tegument protein) is essential for lytic KSHV replication in cell culture, but ORF73 (encoding the latent nuclear antigen [LANA]) is not. Thus, KSHV-lyt should be highly useful to study viral gene function during lytic replication.
TEXT
Kaposi's sarcoma-associated herpesvirus (KSHV; human herpesvirus 8) is the etiologic agent of Kaposi's sarcoma and the B-cell-derived malignancies multicentric Castleman's disease (MCD) and primary effusion lymphoma (PEL) (3, 5). KSHV infects a variety of cell types in vivo but predominantly establishes a latent state of infection (1). During latency, the viral genome is maintained in the nucleus as an episome, and only a few viral genes are expressed. Although latent infection seems to be sufficient to promote cellular transformation, lytic replication is also required for KS development (17) and for virus dissemination. The switch from latency to lytic replication is initiated by the open reading frame 50 (ORF50)-encoded replication and transcription activator (RTA) protein. The ORF50 transcript consists of two exons separated by an intron, which encoded ORF49 on the complementary strand (Fig. 1A). Splicing of ORF50 mRNA allows the expression of RTA, which functions as an immediate-early transcription factor capable of inducing many viral and cellular genes (9, 15, 20). RTA expression is both necessary and sufficient to induce the complete progression of KSHV through the lytic cycle (9).
Fig. 1.
Construction of KSHV-lyt. (A) Schematic illustration of KSHV BAC36 mutagenesis. ORF50 exon 1 and ORF49 were first replaced by a zeo cassette. ORF49 was then reinserted in reverse orientation together with a PGK promoter and ORF50 exon 1. The kan cassette flanked by FRT sites (black ovals) was subsequently removed with FLP recombinase. Nucleotide positions refer to GenBank accession no. HQ404500. (B) XbaI restriction pattern of the parental and mutant BAC genomes in an ethidium bromide-stained agarose gel. For better visibility, images of the upper and lower parts of the gel were taken separately. Fragments affected by mutagenesis as predicted in panel A are indicated by arrowheads.
In vitro, KSHV infection is either abortive or results in latency in most cell types (1). Lytic replication was found to some extent after infection of primary human endothelial cells but was not self-sustaining over longer periods of time (4, 7). In latently infected cells, KSHV reactivation can be triggered by chemical inducers, such as 12-O-tetradecanoylphorbol-13- acetate (TPA) or sodium butyrate (13). However, these chemicals are quite toxic and can cause unwanted side effects (6). Alternatively, enforced RTA expression in trans by a plasmid or viral vector can be used to drive KSHV lytic replication (1). However, these systems are not self-sustaining, and replication stops when the inducing stimulus is withdrawn. Here, we describe the construction and characterization of a recombinant KSHV carrying an intronless ORF50 gene under the control of a constitutively active promoter. The recombinant virus enters the lytic replication cycle by default in different cell types without the need for further induction. Infected cells display a cytopathic effect (CPE), produce expanding foci, express all kinetic classes of viral genes, and release considerable amounts of virus progeny into the supernatant. We further show that this lytically replicating KSHV can be used to analyze viral gene function during lytic replication.
A KSHV genome derived from the PEL cell line BCBL1 has been cloned as a bacterial artificial chromosome (termed BAC36) in Escherichia coli, making it amenable to bacterial mutagenesis techniques (26). We used BAC36 to construct a lytically replicating KSHV clone. First, we replaced ORF50 exon 1 and ORF49 (nucleotides [nt] 71110 to 72093 in BAC36, GenBank accession number HQ404500) by homologous recombination using a zeocin resistance cassette PCR amplified with primers 5′-CAACCTTACTCCGCAAGGGGTAGTCTGTTGTGAGAATACTGTCCAGGCAGTCAAGTCCTGCTCCTCCTCGGCCA-3′ and 5′-CCGAGAGGCCGACGAAGCTTTCCACACAGGACCGCCGAAGCTTCTTACCCTTGTTGACAATTAATCATCGGCA-3′ essentially as described previously (21). The resulting BAC was termed KSHVΔ49 (Fig. 1A). For the second recombination step, a helper plasmid was constructed. It contained ORF49, a kanamycin resistance (kan) marker, a cellular phosphoglycerate kinase (PGK) promoter, ORF50 exon 1, and 50-nt flanking sequences for homologous recombination. Briefly, two oligonucleotides (5′-GATCTCCACCATGGCGCAAGATGACAAGGGTAAGAAGCTTCGGCGGTCCTGTGTGGAAAGCTTCGTCGGCCTCTCGGGCC-3′ and 5′-GGCCGCAACCTTACTCCGCAAGGGGTAGTCTGTTGTGAGAATACTGTCCAGG CAG-3′) were inserted into pReplacer (11) between the BglII and ApaI sites and the NotI and EcoRI sites, respectively. ORF49 was then PCR amplified and inserted between the BamHI and EcoRI sites. A kan marker flanked by FLP recombination target (FRT) sites was introduced at the EcoRI site. The recombination cassette was excised with NotI and ApaI from the helper plasmid and used to modify KSHVΔ49 (Fig. 1A). Finally, the kan marker was removed with FLP recombinase yielding KSHV-lyt, in which an intronless ORF50 gene is driven by a PGK promoter. All constructs were analyzed by restriction digest and gel electrophoresis (Fig. 1B), by PCR, and by sequencing the relevant regions (data not shown).
Transfection of BAC DNA into telomerase-immortalized retinal pigment epithelial cells (hTERT-RPE1, ATCC CRL-4000) was monitored by expression of green fluorescent protein (GFP), which is encoded adjacent to the BAC cassette (26). Transfection of KSHV-lyt resulted in the development of morphologically distinct foci of GFP-expressing cells (Fig. 2A), suggesting that KSHV-lyt-derived virus was able to cause CPE and spread to neighboring cells without any further exogenous stimulus. Foci increased in size and number over time until the entire monolayer was infected. In contrast, the parental BAC36 did not spread and did not form foci. After transfer of the supernatant from infected cells to fresh RPE1 cells, new foci appeared, even if the supernatant was passed through a 0.45-μm filter.
Fig. 2.
Analysis of KSHV-lyt replication. (A) Virus reconstitution following BAC transfection into RPE1 cells. GFP expression and focus formation was observed by fluorescence microscopy over time. Images were taken 1 and 8 days posttransfection (dpt). (B) Focus formation 8 days after transferring infected cell supernatant onto fresh RPE1 cells. (C) Comparison of KSHV-lyt BAC and virion DNA. Equal amounts of DNA were digested with PmeI or XhoI and analyzed by gel electrophoresis. Fragments representing the BAC cassette are indicated by arrowheads. Asterisks indicate additional differences. (D) The PmeI and XhoI restriction maps with the expected fragments in the BAC cassette region are depicted. (E) Replication of KSHV-lyt on different cells after low-MOI infection. Titers (focus-forming units) were determined on RPE1 cells using the TCID50 method. DL, detection limit.
By the same three-step procedure outlined in Fig. 1A, we also constructed KSHV-lyt[−49]. ORF49 was not reinserted in the second recombination step, but otherwise KSHV-lyt[−49] was constructed analogously to KSHV-lyt. KSHV-lyt[−49] formed foci and replicated in RPE1 like KSHV-lyt (data not shown), indicating that ORF49 is not required for KSHV lytic replication. However, since a previous study has shown that ORF49 cooperates with RTA to activate several KSHV lytic promoters (8), we decided to continue further work only with KSHV-lyt.
Surprisingly, serial passaging of KSHV-lyt virus resulted in a loss of GFP expression within the first 2 to 3 passages (Fig. 2B). KSHV virion DNA was recovered from supernatants of infected RPE1 cells and analyzed by restriction digest. Compared to KSHV-lyt BAC DNA, the virion DNA lacked the region containing the BAC cassette (Fig. 2C and D), suggesting that the BAC replicon and the adjacent GFP expression cassette were lost during virus reconstitution and lytic replication. In an attempt to avoid the loss of GFP expression, we constructed a modified version of KSHV-lyt, in which the BAC cassette contained a Cre recombinase gene and was flanked with loxP sites to make it self-excising. Such a procedure has been used previously for other herpesvirus BACs (19, 24). The GFP gene was kept outside the loxP-flanked region in order to preserve GFP expression after excision of the BAC replicon. Much to our chagrin, this modification did not prevent loss of GFP expression during virus reconstitution (data not shown). When we further investigated the underlying reason for this phenomenon, we found out that the region in which the BAC cassette was inserted was duplicated in the parental BAC36. The second copy of the region, ranging approximately from ORF K5 to ORF19, is located within the terminal repeats (TRs) and contains the BAC cassette (data not shown). While this work was in progress, an analysis of the complete BAC36 sequence was published by others (23). The paper identified and documented the same duplication that we had observed. Hence, we did not further investigate this property of BAC36. However, the presence of the BAC cassette within the duplicated region within the TR region offers a rational explanation for the rapid loss of the BAC cassette and the GFP gene. In fact, a recent publication suggested that the TRs are a suitable location for insertion of the BAC cassette, because it will be automatically excised upon virus reconstitution by terminal repeat-mediated homologous recombination (25).
RPE1 cells were used routinely for virus reconstitution from BAC DNA and for titration of infectious KSHV. They have an intact contact inhibition response and tolerate the prolonged culturing required to recover replicating virus after BAC transfection. RPE1 cells were also the preferred cell type for titration, because they developed the clearest CPE and showed the most reliable and reproducible results. Viral titers (focus-forming units) were determined using the 50% tissue culture infective dose (TCID50) method (16). Larger quantities of infectious virus were grown on RPE1 cells and harvested from the supernatants of infected cells. High-titer KSHV-lyt stocks were obtained by pelleting the virus (180 min at 27,000 × g) and resuspending it in a smaller volume of complete medium. To determine the replication capability of KSHV-lyt, growth kinetics were determined on different cells. RPE1 cells, Vero cells (ATCC CRL-1587D), and primary human umbilical vein endothelial cells (HUVEC) (Lonza, Switzerland) were infected at a multiplicity of infection (MOI) of 0.02 using 5 μg/ml Polybrene and centrifugal enhancement of infection (30 min at 900 × g). On all cell lines used here, KSHV-lyt infection led to cell rounding and swelling, release of infectious virions into the supernatant, and finally cell demise several days after infection. After low-MOI infection, the virus replicated to maximum titers of about 105 TCID50/ml in RPE1 cells.
Next, we tested whether KSHV-lyt can be used to determine the importance of viral genes for lytic replication. First, we replaced ORF33, encoding a tegument protein (27), with a galK-kan cassette with methods described previously (18). In a second step, galK-kan was removed by homologous recombination using a synthetic oligonucleotide (5′-GCTATAGGGCGTCGAAGGAGGATCTGGTGTTCATTCGAGGCCGCTATGGCTAGCAGCATGTTGCGCACATCAGCGAGCTGGACCGTCCTCCGGGTCGCGT-3′), generating the seamless deletion mutant KSHV-lytΔ33. The revertant virus, Rev33, was obtained by replacing galK-kan with a PCR-amplified ORF33 sequence (Fig. 3A). Mutant BACs were checked by restriction digest (Fig. 3B) and transfected into RPE1 cells (Fig. 3C). As expected, parental and revertant BACs yielded infectious virus that replicated to comparable titers (Fig. 3D). The Δ33 deletion mutants (with or without galK-kan) repeatedly failed to reconstitute infectious virus, indicating that ORF33 is essential for lytic replication. This result matches similar findings for the related gammaherpesvirus murid herpesvirus 68 (MHV-68), in which ORF33 is also essential for replication (10).
Fig. 3.
ORF33 is required for KSHV-lyt lytic replication. (A) Genomic organization of KSHV-lyt ORF33 mutants. Nucleotide positions refer to GenBank accession no. HQ404500. (B) Acc65I (isoschizomer of KpnI) restriction pattern of the parental and mutant BAC genomes in an ethidium bromide-stained agarose gel. The relevant fragments (see panel A) are indicated by arrowheads. (C) RPE1 cells were transfected with recombinant BAC genomes as indicated, and GFP fluorescence was observed 10 days posttransfection. (D) RPE1 cells were infected at an MOI of 0.02 TCID50/cell of the indicated viruses. Infectious virus (focus-forming units) released into the supernatants was titrated on RPE1 cells. DL, detection limit.
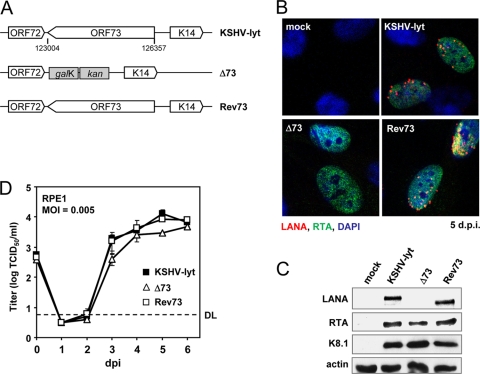
The LANA-encoding ORF73 was deleted by a similar approach (Fig. 4A). ORF73 was first replaced with galK-kan, yielding KSHV-lytΔ73. A cloned KSHV fragment containing ORF73 and approximately 150 nt of flanking KSHV sequence (kindly provided by Rolf Renne) was then used to generate the revertant Rev73. LANA expression from the recombinant KSHVs was checked by immunofluorescence (Fig. 4B) and immunoblotting (Fig. 4C). As the cloned ORF73 sequence used for Rev73 was derived from a different KSHV clone and contained fewer internal repeats, Rev73-expressed LANA was slightly smaller in size than that of the parental virus. All three recombinant viruses expressed RTA and K8.1 proteins in infected RPE1 cells (Fig. 4C), demonstrating that LANA expression is not essential for KSHV lytic replication. However, the Δ73 mutant grew to slightly lower titers than the parental and revertant viruses, indicating that LANA might contribute directly or indirectly to lytic virus production (Fig. 4D). In contrast, LANA-deficient KSHV BAC36 and rhesus rhadinovirus showed increased virus production in previous studies, which was attributed to the loss of LANA-mediated repression of the RTA promoter (14, 22). This repression is probably absent in KSHV-lyt, as RTA expression is driven by a heterologous promoter.
Fig. 4.
ORF73 is dispensable for KSHV-lyt lytic replication. (A) Genomic organization of KSHV-lyt ORF73 mutants. Nucleotide positions refer to GenBank accession no. HQ404500. (B) RPE1 cells were infected as indicated and analyzed by immunofluorescence. RTA was stained with a rabbit antiserum (kindly provided by Gary Hayward) and Alexa Fluor 488-coupled anti-rabbit IgG (Invitrogen, Germany). LANA was visualized with mouse anti-LANA (Acris, Germany) and Alexa Fluor 568-coupled anti-mouse IgG. Nuclei were counterstained with DAPI (4′,6-diamidino-2-phenylindole). (C) Viral protein expression in infected RPE1 cells was analyzed by immunoblotting with the same primary antibodies as those described above or anti-K8.1 (Santa Cruz, CA) and peroxidase-coupled (Dako, Germany) secondary antibodies. (D) RPE1 cells were infected at an MOI of 0.005 TCID50/cell, and viral titers (focus-forming units) in the supernatant were determined. DL, detection limit.
In summary, we demonstrated that a molecular KSHV clone modified to express RTA constitutively replicates to substantial titers in cultured cells. The lytically replicating KSHV is well suited to study viral gene function during lytic replication. Another promising application for lytically replicating KSHV is the field of antiviral drug testing. Most of the available antiherpesviral drugs target the lytic replication phase (12, 17) and are therefore difficult to test with previously available systems in which KSHV is predominantly latent (2). Of course, PEL cell lines and unmodified BAC36 remain the systems of choice for investigating latency and reactivation.
Acknowledgments
We thank Shou-Jiang Gao for BAC36, Gary Hayward and Keji Ueda for antibodies, Thomas Schulz and Cornelia Henke-Gendo for sharing the BAC36 sequence before publication, and Antonio Gallo and Adam Grundhoff for a critical reading of the manuscript.
Footnotes
Published ahead of print on 27 July 2011.
REFERENCES
- 1. Bechtel J. T., Liang Y., Hvidding J., Ganem D. 2003. Host range of Kaposi's sarcoma-associated herpesvirus in cultured cells. J. Virol. 77:6474–6481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Casper C., Wald A. 2006. The use of antiviral drugs in the prevention and treatment of Kaposi sarcoma, multicentric Castleman disease and primary effusion lymphoma, p. 289–307. In Compans R. W., et al. (ed.), Current topics in microbiology and immunology, vol. 312. Springer Verlag, Berlin, Germany. [DOI] [PubMed] [Google Scholar]
- 3. Chang Y., et al. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865–1869 [DOI] [PubMed] [Google Scholar]
- 4. Ciufo D. M., et al. 2001. Spindle cell conversion by Kaposi's sarcoma-associated herpesvirus: formation of colonies and plaques with mixed lytic and latent gene expression in infected primary dermal microvascular endothelial cell cultures. J. Virol. 75:5614–5626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Damania B. 2004. Oncogenic gamma-herpesviruses: comparison of viral proteins involved in tumorigenesis. Nat. Rev. Microbiol. 2:656–668 [DOI] [PubMed] [Google Scholar]
- 6. Filippovich I., Sorokina N., Khanna K. K., Lavin M. F. 1994. Butyrate induced apoptosis in lymphoid cells preceded by transient over-expression of HSP70 mRNA. Biochem. Biophys. Res. Commun. 198:257–265 [DOI] [PubMed] [Google Scholar]
- 7. Gao S. J., Deng J. H., Zhou F. C. 2003. Productive lytic replication of a recombinant Kaposi's sarcoma-associated herpesvirus in efficient primary infection of primary human endothelial cells. J. Virol. 77:9738–9749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gonzalez C. M., et al. 2006. Identification and characterization of the Orf49 protein of Kaposi's sarcoma-associated herpesvirus. J. Virol. 80:3062–3070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gradoville L., et al. 2000. Kaposi's sarcoma-associated herpesvirus open reading frame 50/Rta protein activates the entire viral lytic cycle in the HH-B2 primary effusion lymphoma cell line. J. Virol. 74:6207–6212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guo H., Wang L., Peng L., Zhou Z. H., Deng H. 2009. Open reading frame 33 of a gammaherpesvirus encodes a tegument protein essential for virion morphogenesis and egress. J. Virol. 83:10582–10595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jurak I., Brune W. 2006. Induction of apoptosis limits cytomegalovirus cross-species infection. EMBO J. 25:2634–2642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kedes D. H., Ganem D. 1997. Sensitivity of Kaposi's sarcoma-associated herpesvirus replication to antiviral drugs. Implications for potential therapy. J. Clin. Invest. 99:2082–2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lagunoff M., et al. 2002. De novo infection and serial transmission of Kaposi's sarcoma-associated herpesvirus in cultured endothelial cells. J. Virol. 76:2440–2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li Q., Zhou F., Ye F., Gao S. J. 2008. Genetic disruption of KSHV major latent nuclear antigen LANA enhances viral lytic transcriptional program. Virology 379:234–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lukac D. M., Kirshner J. R., Ganem D. 1999. Transcriptional activation by the product of open reading frame 50 of Kaposi's sarcoma-associated herpesvirus is required for lytic viral reactivation in B cells. J. Virol. 73:9348–9361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mahy B. W. J., Kangro H. O. 1996. Virology methods manual. Academic Press, San Diego, CA. [Google Scholar]
- 17. Martin D. F., et al. 1999. Oral ganciclovir for patients with cytomegalovirus retinitis treated with a ganciclovir implant. N. Engl. J. Med. 340:1063–1070 [DOI] [PubMed] [Google Scholar]
- 18. Qian Z., Xuan B., Hong T. T., Yu D. 2008. The full-length protein encoded by human cytomegalovirus gene UL117 is required for the proper maturation of viral replication compartments. J. Virol. 82:3452–3465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Smith G. A., Enquist L. W. 2000. A self-recombining bacterial artificial chromosome and its application for analysis of herpesvirus pathogenesis. Proc. Natl. Acad. Sci. U. S. A. 97:4873–4878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sun R., et al. 1998. A viral gene that activates lytic cycle expression of Kaposi's sarcoma-associated herpesvirus. Proc. Natl. Acad. Sci. U. S. A. 95:10866–10871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Valchanova R. S., Picard-Maureau M., Budt M., Brune W. 2006. Murine cytomegalovirus m142 and m143 are both required to block protein kinase R-mediated shutdown of protein synthesis. J. Virol. 80:10181–10190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wen K. W., Dittmer D. P., Damania B. 2009. Disruption of LANA in rhesus rhadinovirus generates a highly lytic recombinant virus. J. Virol. 83:9786–9802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yakushko Y., et al. 2011. Kaposi's sarcoma-associated herpesvirus bacterial artificial chromosome contains a duplication of a long unique-region fragment within the terminal repeat region. J. Virol. 85:4612–4617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yu D., Smith G. A., Enquist L. W., Shenk T. 2002. Construction of a self-excisable bacterial artificial chromosome containing the human cytomegalovirus genome and mutagenesis of the diploid TRL/IRL13 gene. J. Virol. 76:2316–2328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhou F., Li Q., Wong S. W., Gao S. J. 2010. Autoexcision of bacterial artificial chromosome facilitated by terminal repeat-mediated homologous recombination: a novel approach for generating traceless genetic mutants of herpesviruses. J. Virol. 84:2871–2880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhou F. C., et al. 2002. Efficient infection by a recombinant Kaposi's sarcoma-associated herpesvirus cloned in a bacterial artificial chromosome: application for genetic analysis. J. Virol. 76:6185–6196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhu F. X., Chong J. M., Wu L., Yuan Y. 2005. Virion proteins of Kaposi's sarcoma-associated herpesvirus. J. Virol. 79:800–811 [DOI] [PMC free article] [PubMed] [Google Scholar]