Abstract
The dengue viruses (DENVs) exist as numerous genetic strains that are grouped into four antigenically distinct serotypes. DENV strains from each serotype can cause severe disease and threaten public health in tropical and subtropical regions worldwide. No licensed antiviral agent to treat DENV infections is currently available, and there is an acute need for the development of novel therapeutics. We found that a synthetic small interfering RNA (siRNA) (DC-3) targeting the highly conserved 5′ cyclization sequence (5′CS) region of the DENV genome reduced, by more than 100-fold, the titers of representative strains from each DENV serotype in vitro. To determine if DC-3 siRNA could inhibit DENV in vivo, an “in vivo-ready” version of DC-3 was synthesized and tested against DENV-2 by using a mouse model of antibody-dependent enhancement of infection (ADE)-induced disease. Compared with the rapid weight loss and 5-day average survival time of the control groups, mice receiving the DC-3 siRNA had an average survival time of 15 days and showed little weight loss for approximately 12 days. DC-3-treated mice also contained significantly less virus than control groups in several tissues at various time points postinfection. These results suggest that exogenously introduced siRNA combined with the endogenous RNA interference processing machinery has the capacity to prevent severe dengue disease. Overall, the data indicate that DC-3 siRNA represents a useful research reagent and has potential as a novel approach to therapeutic intervention against the genetically diverse dengue viruses.
INTRODUCTION
A variety of genetically distinct virus strains within four antigenically distinguishable serotypes (dengue virus type 1 [DENV-1], DENV-2, DENV-3, and DENV-4) comprise the DENV species, a member of the genus Flavivirus of the family Flaviviridae (7, 55, 66). Dengue viruses are transmitted to humans primarily by Aedes aegypti mosquitoes and represent a considerable threat to public health in tropical and subtropical regions worldwide. Infection can be asymptomatic or can cause a spectrum of clinical syndromes ranging from self-limiting febrile illness to life-threatening severe dengue disease. Annually, hundreds of thousands of cases of clinical dengue disease are reported by clinicians to the WHO, with a case-fatality rate of <0.5 to 5.0% (23, 24, 27, 67). DENV genomic sequences can vary up to approximately 19% between strains in a single serotype and up to approximately 34% between strains of different serotypes (24, 66). Infection by various strains from each serotype can cause severe disease in humans, and all four serotypes now circulate globally (24, 27).
The DENV genome is a ∼10.7-kb positive-sense single-stranded RNA consisting of a single open reading frame (ORF) flanked by a 5′ untranslated region (5′UTR) and a 3′UTR. The ORF is translated into a single polyprotein that is co- and posttranslationally cleaved to produce three structural (C, prM/M, and E) and seven nonstructural (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) proteins. The viral genome features a 5′ type 1 cap structure but lacks a 3′ poly(A) tail and serves both as mRNA and as a template for replication via the production of a minus-strand intermediate (40). During viral replication, a number of long-range RNA-RNA cis interactions are necessary for efficient minus-strand synthesis (reviewed in references 32 and 65). The 3′UTR of all mosquito-vectored flaviviruses contains a region of at least 8 nucleotides (nt), termed conserved sequence 1 (CS1), which is both highly conserved and complementary to the 5′ cyclization sequence (5′CS) located in the N-terminal region of the capsid gene (C) coding sequence (9, 26). A base-pairing interaction between the CS1 and 5′CS sequences of DENV and other flaviviruses was shown to promote the cyclization of the genome and contribute to efficient minus-strand synthesis (1, 2, 20, 65, 75, 76, 78).
Although many advances have been made recently in the development of vaccines and antiviral drugs to address DENV (reviewed in references 17, 23, 47, and 63), to date there is no antiviral intervention formally approved to prevent or treat DENV infections. To warrant commercial development, a prospective antiviral drug against DENV would likely require demonstrated efficacy against infections by representative strains from each of the four DENV serotypes. The targeting of flaviviral RNA with nucleic acid-based agents has provided insight into virus biology and transmission and may provide novel interventional approaches (57, 61). RNA interference (RNAi) is a cellular process induced by double-stranded RNA (dsRNA) capable of producing the RNase-mediated degradation of specific mRNA. The activation of RNAi machinery via the introduction of a small dsRNA targeted against viral RNA has been used with experimental success to inhibit various RNA virus infections (18, 30, 44). Recently, several studies have described the antiviral efficacy of chemical-, plasmid-, or virus-vectored small dsRNAs in both cell cultures and mouse models infected with various flaviviruses such as West Nile virus (WNV) (4, 5, 37, 48, 71), Japanese encephalitis virus (JEV) (37, 41, 46, 53), and yellow fever virus (YFV) (49). DENV infection and transmission could be suppressed in mosquitoes transgenically engineered to produce DENV-targeted small interfering RNA (siRNA) (21). A synthetic siRNA targeting prM coding sequence inhibited the infection of DENV-1 in mosquito cell cultures (69). The peptide-mediated delivery of siRNA targeting a highly conserved sequence in the DENV envelope (E) gene decreased DENV-2 replication in human monocyte-derived dendritic cells and macrophages in culture (62). Finally, adeno-associated virus (AAV) vectors containing short hairpin RNA (shRNA) targeted to the CS1 region inhibited DENV-2 infections in mammalian cell cultures (79). While RNAi has been shown to have efficacy against DENV infections in vitro, there have not been any reports of antiviral efficacy in in vivo models.
Chemically synthesized siRNAs are typically 19 to 21 bp in length with 2-nt 3′ overhangs and can be delivered into cell cultures with lipid-based transfection reagents. To design a synthetic siRNA suitable for use against one or more serotypes of DENV, we employed two approaches. In one approach we used genomic sequences from specific strains of DENV to program a commercial siRNA design algorithm. In a second approach we used a comparative genome analysis to identify siRNA target sequences that are highly conserved across the four serotypes of DENV. The siRNAs were then tested for activity against DENV infections in cell cultures and in mice.
The AG129 mouse model has been used to investigate the biology of DENV disease and to evaluate vaccine and antiviral drug candidates (reviewed in references 68 and 72). A recent version of this in vivo model system used DENV-2 strain S221 in the presence of an anti-prM antibody to mimic the antibody-dependent enhancement (ADE)-mediated disease thought to occur for a significant portion of cases of severe dengue disease in humans (77). AG129 mice infected in this model exhibit some of the same pathological features observed for humans with severe dengue disease, including vascular leakage, intestinal hemorrhage, elevated cytokine levels, low platelet counts, and elevated hematocrit levels. We found that an siRNA targeting a highly conserved sequence in the 5′CS region of the DENV capsid gene was a powerful inhibitor of representative strains of all four DENV serotypes in Huh7 cell cultures and DENV-2 in this mouse model.
MATERIALS AND METHODS
Cells and viruses.
Huh7 cells (a human hepatoma-derived cell line) and Vero cells were obtained from F. Chisari (Scripps Research Institute) and the ATCC, respectively. All cells were propagated in complete growth medium consisting of Dulbecco's modification of Eagle's medium (DMEM; Cellgro) supplemented with 10% heat-inactivated fetal bovine serum (FBS) and antibiotics (100 units/ml penicillin and 100 g/ml streptomycin). All cell culture incubations were carried out at 37°C in a humidified atmosphere containing 5% CO2. DENV-1 (strain West-Pac; GenBank accession number DVU88535) was obtained from Robert Putnak (Walter Reed Army Research Institute). DENV-2 strain New Guinea C (NGC) (GenBank accession number AF038403), DENV-3 (strain H87; GenBank accession number M93130), and DENV-4 (strain H241; GenBank accession number AY947539) were obtained from the ATCC. The WNV strain used (strain 385-99; GenBank accession number AY842931) was previously described (70). Viral stocks were prepared and titers were determined as previously described (59). WHO primary seed lot 213/77 yellow fever virus (YFV) 17D passage 237 (GenBank accession number NC_002031) was propagated on Vero cells. For in vivo experiments, DENV-2 strain S221, a triple-plaque-purified clone isolated from mouse-passaged DENV-2 strain D2S10, was prepared and quantified as previously described (58).
siRNA design and bioinformatics.
To design siRNAs optimized to target the individual genomes of each of the four DENV strains used in this study, we used the Dharmacon algorithm (Dharmacon siRNA Design Center) (6, 54). This analysis generated a list of sequences scored hierarchically with respect to their likelihood to represent effective siRNA targets (data not shown). Two nonoverlapping high-scoring sequences per serotype were selected from these lists (algorithm siRNA; sequences D1-1 through D4-2) (Table 1).
Table 1.
siRNAs and their sequences and target locations
| siRNA | siRNA target sequence | Virus(es) targeted | Positions of siRNA target location in viral genome (genome region)a |
|---|---|---|---|
| NC | ACGUGACGUUCGGAGAAUU | NA | NA (negative control) |
| D1-1 | GGGCAAUGGUUGUGGGCUA | DENV–1 | 1237–1255 (E) |
| D1-2 | GGAUGGAGCUUGAGAGAAA | DENV–1 | 9803–9821 (NS5) |
| D2-1 | CGGGAAAGACGAAGAGAUA | DENV–2 | 5111–5129 (NS3) |
| D2-2 | CCAAAGAGGUAGUGGACAA | DENV–2 | 9360–9378 (NS5) |
| D3-1 | GAGGAAUGCUUGUGAGAAA | DENV–3 | 8096–8114 (NS5) |
| D3-2 | GGAUGGAGCCUUAGAGAAA | DENV–3 | 9727–9745 (NS5) |
| D4-1 | CCAAAGAGGUAGUGGACAA | DENV–4 | 9353–9371 (NS5) |
| D4-2 | GGAUGGAGCUUAAGAGAAA | DENV–4 | 9798–9816 (NS5) |
| DC-1 | AGUUGUUAGUCUACGUGGAC | DENV1–DENV4 | 1–20 (5′ UTR) |
| DC-2 | AUUAGAGAGCAGAUCUCUG | DENV1–DENV4 | 78–96 (5′UTR) |
| DC-3 | UGCUGAAACGCGAGAGAAA | DENV1–DENV4 | 140–158 (C) |
| DC-4 | GGUUAGAGGAGACCCCUCC | DENV1–DENV4 | 10501–10519 (3′UTR) |
| DC-5 | GGACUAGAGGUUAGAGGAG | DENV1–DENV4 | 10580–10598 (3′UTR) |
| DC-6 | AACAGCAUAUUGACGCUGG | DENV1–DENV4 | 10616–10634 (3′UTR) |
| DC-7 | CCAGAGAUCCUGCUGUCUC | DENV1–DENV4 | 10641–10659 (3′UTR) |
| WNV-1 | GUCAAUAUGCUAAAACGCG | WNV | 136–154 (C) |
| YFV-1 | CCCUGGGCGUCAAUAUGGU | YFV | 147–165 (C) |
Based on the following GenBank accessions numbers: NC_001477 for DENV-1, NC_001474 for DENV-2, NC_001475 for DENV-3, NC_002640 for DENV-4, DQ211652 for WNV, and NC_002031 for YFV. The genome region containing the siRNA target is shown in parentheses. For siRNAs targeting multiple DENV serotypes, the target nucleotide positions correspond to DENV-2 (accession number NC_001474). NA, not applicable.
To design an siRNA targeted against a sequence that is highly conserved across the DENV virome (conserved siRNA), full-length genomic sequences of the NCBI reference strains of the four serotypes of DENV were aligned (Table 1) by using TCoffee (http://www.tcoffee.org). This analysis revealed six regions (sequences DC-2 through DC-7) (Table 1) of 19 contiguous nucleotides containing a 0- or 1-base difference between the four reference strains. A 20-nt region having perfect conservation between the GenBank reference sequences for DENV-1 to DENV-3 but with 2 mismatches to DENV-4 was also selected for targeting (DC-1) (Table 1). Eight sequences (DC-1 through DC-7 and D2-2) were aligned with a library of 2,754 full-length DENV genomes from GenBank (GI numbers are available upon request). These genomes have representatives from each of the four serotypes: 1,223 genomes of serotype 1, 852 genomes of serotype 2, 587 genomes of serotype 3, and 92 genomes of serotype 4. NCBI BLAST was used to align each of the candidate sequences against all of the genomes in the library by using the blastn program, setting the number of results reported to 3,000, with a gap-opening penalty of 2, a gap extension penalty of 1, a nucleotide mismatch penalty of −1, and a nucleotide match reward of +1, with an E value cutoff of 10.0. Multiple alignments for any query/genome pair were eliminated, and only the top alignment was considered. Exact matches were defined as BLAST alignments of the full length of the query (20 for DC-1 and 19 for all others) with 100% identities. Single mismatches were defined as BLAST alignments of a length of query of −1 (19 for DC-1 and 18 for all others) with 100% identities or full-length alignments with 1 mismatch.
Considerations for the design of siRNAs targeting WNV and YFV (sequences WNV-1 and YFV-1, respectively) (Table 1) are described below in Results. The WNV and YFV sequences were compared to libraries of WNV and YFV from the NCBI nucleotide database having 284 and 18 complete genomes, respectively. The same run-time parameters as those for the DENV calculations were used to calculate blastn alignments between the WNV-1 and YFV-1 siRNA queries and the corresponding libraries.
A random sequence negative-control siRNA (sequence NC) (Table 1) was also prepared to control for off-target effects of the siRNA chemistry. To reduce the chances of unintentional hybridization events, all siRNAs were further analyzed with blastn against all human transcript sequences and relevant siRNAs against mouse transcript sequences.
siRNA transfections of cell cultures.
For cell culture experiments, reverse transfection in 48-well plates was employed. For each sample, the appropriate amount of a 1 or 10 μM stock of siRNA (Dharmacon Inc., Lafayette, CO) and 1 μl of RNAiMAX (Invitrogen) were mixed into 100 μl of Opti-MEM for 30 min at room temperature, after which 300 μl of DMEM (with 2% FBS and no antibiotics) containing 3 × 104 Huh7 cells was added and mixed gently. Two days later, the transfection-reagent-containing medium was removed, and the cells were infected with virus (multiplicity of infection [MOI] of 0.2 or 0.5, as indicated) in 200 μl of DMEM (containing 2% FBS and antibiotics) for 2 h with gentle rocking, after which the virus-containing medium was removed. The cells were washed twice and replenished with 250 μl DMEM (containing 2% FBS and antibiotics). When an MOI of 0.5 was used, supernatants were collected at 24 h (WNV), 48 h (DENV), or 72 h (YFV) postinfection (p.i.) and stored at −80°C for subsequent plaque assay analysis. In addition, the cells were lysed with 200 μl of 1× SDS buffer (50 mM Tris [pH 8], 2% SDS, 10% glycerol) for 30 min and then collected for immunoblotting. For growth curve experiments the same protocol as that described above was employed, except that an MOI of 0.2 and a p.i. volume of 400 μl DMEM (containing 2% FBS and antibiotics) were used, with 50 μl of the supernatant collected daily for 3 or 5 days, as indicated starting at 1 day p.i., for subsequent analysis by plaque assays.
Immunoblots.
Lysed cell samples were heated to 95°C for 5 min and subjected to 10% SDS-PAGE before transfer onto a polyvinylidene difluoride (PVDF) membrane (Millipore). Membranes were blocked for 1 h in PBST (1% phosphate-buffered saline [PBS] with 0.1% Tween 20) and 10% instant nonfat dry milk, after which two monoclonal antibodies were added, one to detect the envelope (E) protein of DENV and WNV (mouse monoclonal antibody 4G2-4-15; ATCC) at 1 μg/ml and one to detect glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (used at a 1:7,500 dilution; Santa Cruz BioTechnology), for 2 h of incubation. After 2 rinses in PBST, secondary antibody consisting of horseradish peroxidase-conjugated goat anti-mouse IgG (Santa Cruz Biotechnology) at a 1:7,500 dilution was added in PBST and 10% milk as described above for 1 h before PBST rinsing and detection with SuperSignal West Pico chemiluminescent substrate (Thermo Scientific).
Titer determination.
Viral titers for DENV and WNV were determined on Vero cells by the immunostaining of foci (plaque assay). Briefly, serial dilutions of virus in DMEM (with 2% FBS and antibiotics) were used to infect confluent monolayers of Vero cells for 90 min. The inoculation solution was then replaced with DMEM containing 2% FBS, antibiotics, and 0.5% carboxymethylcellulose sodium salt (CMC) (Sigma). The culture medium was removed 2 days (for WNV) or 4 days (for DENV) later, and the cells fixed with 4% (wt/vol) paraformaldehyde (PFA) (in 1× PBS) for 15 min before immunostaining, as follows. After the removal of PFA the cells were washed twice with 1× PBS and then blocked for 1 h in 1× PBS containing 2% (vol/vol) normal goat serum (NGS) and 0.4% (vol/vol) Triton X-100 (TX-100). Mouse monoclonal anti-E antibody, suspended in 1× PBS with 2% NGS, was added to each well of the assay plates at 4 μg/ml for 1 h, followed by two washes with 1× PBS. As a secondary antibody, horseradish peroxidase-conjugated goat anti-mouse IgG (Santa Cruz Biotechnology) at a 1:5,000 dilution in 1× PBS with 2% NGS was added, and the mixture was incubated for 1 h, followed by two rinses with 1× PBS. Immunoreactive foci were stained with a Vector VIP peroxidase substrate kit (Vector Labs), and the numbers of PFU/ml were calculated. For YFV, viral titers were determined on Vero cells by a plaque assay. Briefly, serial dilutions of virus in DMEM (with 10% FBS) were used to infect confluent monolayers of Vero cells for 60 min. Following infection, the monolayers were overlaid 1:1 with 1% agar in double-distilled water (ddH2O)-2× Eagle's minimal essential medium (with 5% FBS and antibiotics). At 3 days postinfection, an additional overlay with 0.015% (wt/vol) neutral red in 1% agar was added. At 4 days postinfection, viral plaques were counted and recorded.
Cell viability assay.
Uninfected Huh7 cells were subjected to the indicated treatment using the same reverse transfection and cell culture conditions as those used for the antiviral assays described above (for siRNA transfections of cell cultures), except that 96-well plates and appropriately scaled reagent volumes, with 104 cells per well, were used. Cell viability was measured at 48 h after plating by using the CellTiter Glo (Promega) cell proliferation assay kit according to the manufacturer's instructions and a Turner Biosystems (Sunnyvale, CA) plate reader with Veritas (Mountain View, CA) software. The absorbance values of siRNA-treated cells were converted to percentages by comparison to mock-treated samples containing no transfection reagent or siRNA, set at 100%.
Construction of Huh7-ISRE-luc cells and assay for siRNA induction of interferon-stimulated gene expression.
To investigate possible interferon (IFN)-stimulated gene expression, Huh7 cells were transduced with two recombinant lentiviral vectors, one containing the interferon-stimulated response element (ISRE) fused with the coding sequence for firefly luciferase (catalog number CLS-008L-1; SABiosciences) and the other containing the coding sequence for Renilla luciferase driven by the cytomegalovirus (CMV) promoter/enhancer (catalog number CLS-RCL-1; SABiosciences), resulting in a cell line stable for the inducible and constitutive expression of firefly and Renilla luciferases, respectively. These Huh7-ISRE-luc cells were subjected to the indicated treatments using reverse transfection and cell culture conditions similar to those used for the antiviral assays described above (for siRNA and cell culture transfections), in a 96-well plate. The respective luciferase levels were measured at 8 or 48 h after plating by use of the Dual-Glo luciferase assay system (Promega) according to the manufacturer's instructions, using the same equipment as that used for the cell viability assays described above. As a positive control for this experiment, 500 U IFN-β (PBL InterferonSource, NJ) was used.
Evaluation of siRNA antiviral efficacy in AG129 mice.
129/Sv mice doubly deficient in IFN-α/β and -γ receptors (AG129 mice) were bred and housed at the La Jolla Institute for Allergy & Immunology (LIAI) (La Jolla, CA). The mice were 5 to 6 weeks old at the beginning of the experiments. Mice were allowed food and water ad libitum throughout the studies, and all experimental procedures were preapproved and performed according to the guidelines set by the LIAI Animal Care and Use Committee. For in vivo studies, mice were infected intravenously (tail vein) with 109 genomic equivalents (GE) (equivalent to 20,000 PFU, as measured by a plaque assay on BHK-21 cells [58]) of S221 and administered 5 μg monoclonal antibody 2H2 by intraperitoneal injection on day 0. In addition, mice received a retro-orbital intravenous administration of either PBS or a mixture of Silencer In Vivo Ready siRNA (10 mg/kg of body weight/dose; Ambion) and Invivofectamine 2.0 reagent (Invitrogen), combined as recommended by the manufacturers, at 24 h before and 24 h and 72 h after DENV infection. For survival studies, all mice were weighed daily and euthanized when moribund or at the first signs of paralysis. Animals whose weight fell below 80% of the weight at the start of the experiment were considered moribund. For tissue analysis, mice were euthanized by isoflurane inhalation at 24 and 72 h postinfection, and blood and tissues were harvested as described previously (51). Quantitative reverse transcription (RT)-PCR was performed to detect DENV genomes and cellular 18S RNA as previously described (52). Viral loads are expressed as GE per ml in serum or GE normalized to copies of 18S RNA in tissues.
TNF measurements.
Serum from infected animals was analyzed by use of the tumor necrosis factor alpha (TNF-α) enzyme-linked immunosorbent assay (ELISA) Ready-Set-Go kit (eBioscience) according to the manufacturer's instructions.
Statistical analysis.
Cell culture data were analyzed with the Student t test, with the exception of growth curves, where a repeated-measures analysis of variance (ANOVA) (SAS, version 9.2) followed by contrast tests was used for day-by-day comparisons of virus growth under the two treatments. For in vivo data, Kaplan-Meier survival curves were analyzed by a log rank test. The viral load in mouse tissues and TNF levels determined by ELISA were analyzed with the Student t test. Results with error bars are expressed as the standard errors of the means (SEM). Unless noted otherwise, statistical analyses were performed with Prism 5 (GraphPad Software).
RESULTS
Design and antiviral activity of algorithm and conserved siRNAs against four DENV serotypes.
All siRNAs used in this study are defined in Table 1. To design siRNAs against DENV genomic sequences, we used two different strategies. In one strategy, we applied a predictive algorithm and selected two high-scoring siRNA target sequences for each strain of DENV that we planned to use in our experiments (one strain from each of the four serotypes [for strain designations, see Materials and Methods]) (sequences D1-1 through D4-2; algorithm siRNA). In a second strategy, we identified seven regions that were highly conserved across the GenBank reference sequences (listed in Table 1) for the four serotypes of DENV (DC-1 through DC-7; conserved siRNA) and encompassed at least 19 nt, as required for effective siRNA targeting. The sequences of the algorithm siRNA were not well conserved across serotypes (data not shown), with the exception of D2-2, which showed a moderately high level of conservation compared with the reference sequences (Table 2). None of the conserved-siRNA targets were present in the list of potential siRNA targets determined with the predictive algorithm, and we reasoned that conserved siRNAs could therefore benefit from empirical comparisons to the algorithm siRNAs for the ability to inhibit different strains of DENV.
Table 2.
Sequence agreement between conserved siRNA and DENV strains
| Virus (strain)b | No. of base differences |
|||||||
|---|---|---|---|---|---|---|---|---|
| DC-1 | DC-2 | DC-3 | DC-4 | DC-5 | DC-6 | DC-7 | D2-2 | |
| DENV-1 (West-Paca) | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 3 |
| DENV-2 (NGCa) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| DENV-2 (S210a) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| DENV-3 (H87a) | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 2 |
| DENV-4 (H241a) | NAc | 2 | 0 | 0 | 0 | 0 | 0 | 1 |
| DENV-1 (Ref Seq) | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 3 |
| DENV-2 (Ref Seq) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| DENV-3 (Ref Seq) | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 |
| DENV-4 (Ref Seq) | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
Strain of DENV used in this study (see Materials and Methods).
Ref Seq, GenBank reference sequence (see Table 1 for accession numbers).
NA, not applicable. The 5′-terminal nucleotides of this GenBank entry may be somewhat inaccurate (see Results).
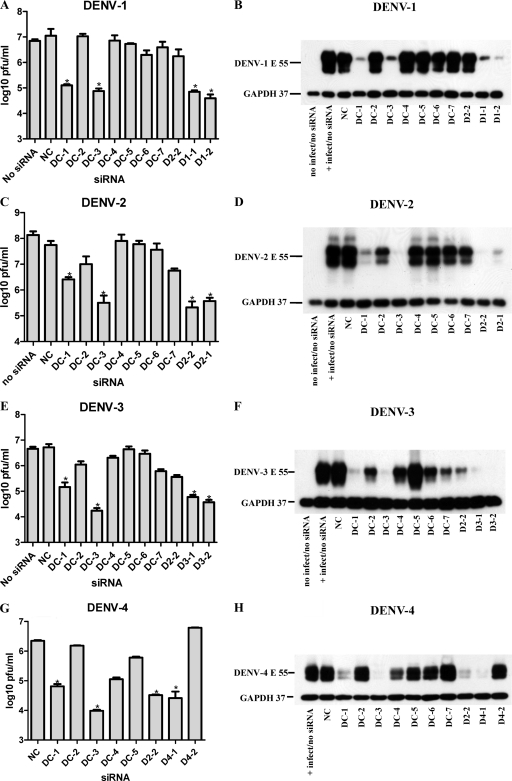
To determine the antiviral efficacy of the algorithm and conserved siRNAs, we used Huh7 cells, as they are both easily infected by DENV and easily transfected with siRNA and are derived from human liver, an organ that has been shown to be infected by DENV in vivo (33, 50). The growth of four DENV strains, each one representing a different serotype, was monitored in Huh7 cells after transfection with the conserved siRNA and the algorithm siRNA D2-2 as well as with the appropriate strain-specific algorithm siRNA. A negative-control siRNA (NC) and a no-treatment sample (containing no siRNA or transfection reagent) were included in each experiment. Cells were reverse transfected with 100 nM siRNA 2 days before infection with 0.5 MOIs of DENV. To assess the impact of each siRNA on viral protein production, we used Western blotting to detect viral envelope glycoprotein (anti-E antibody) in lysates collected at 2 days after infection. Cellular GAPDH was detected simultaneously as a loading control and cell viability indicator. A noninfected control is present in three of the four Western blots shown in Fig. 1, demonstrating the specificity of the two antibodies used. The reduction of infectious viral particle release was monitored with an immunostain-based plaque assay of the cell culture supernatants at 2 days postinfection. The viruses used in this study varied to some extent in growth characteristics, with DENV-2 titers reaching approximately an order of magnitude higher in peak titer than those of the other three viruses. The results of the Western blots were generally consistent with those of the plaque assays. A 10- to 100-fold reduction of infectious virus corresponded to a marked reduction in viral protein levels (Fig. 1), with viral protein being detectable only upon the prolonged exposure of Western blots (not shown). With the exception of D4-2, all the algorithm siRNAs markedly suppressed virus protein production and the amount of infectious virus produced (Fig. 1). This result is consistent with the predictive power of the design methodology, and we therefore used the activity of select algorithm siRNAs as benchmarks to evaluate the efficacy of conserved siRNA. The DC-5 and DC-6 sequences were generally ineffective, despite a perfect agreement between siRNAs and target sequences for all four strains (with the exception of a single-base mismatch in the DC-5 target for DENV-3) (Table 2). Of note, the DC-6 siRNA contains the DENV CS1 sequence (CAGCAUAUUG), a functional and highly conserved motif in mosquito-borne flaviviruses (26, 35, 45, 65, 76). DC-4 showed some activity against DENV-3 and DENV-4, whereas DC-7 had some activity against DENV-2 and DENV-3. The algorithm siRNA D2-2 was active not only against DENV-2 but also against DENV-3 and DENV-4. D2-2 has 0, 1, 2, and 3 mismatches with the DENV-2, -4, -3, and -1 strains used in this study, respectively (Table 2). The most consistent results against all strains were obtained with DC-1 and DC-3. The DC-1 sequence has no disagreement with the strains of DENV-1 to -3 used in this study but uncertain agreement with strain H241 of DENV-4 used in this study, due to uncertainty regarding the accuracy of the GenBank sequence annotation at the H241 5′-terminal region (M. Schreiber, Novartis Institute for Tropical Diseases, personal communication). DC-3 suppressed the titers of all four strains robustly, by more than 98% compared to control siRNA (Fig. 1). DC-3 has perfect agreement with the DENV-2 and -4 strains and 1 mismatched base with the DENV-1 and -3 strains used here (and similarly with the GenBank reference sequences) (Table 2). These data show that at least some of the siRNAs (DC-1 and DC-3) designed solely on the basis of their conservation across GenBank reference sequences were active against representative strains from each of the four serotypes.
Fig. 1.
DENV-specific siRNAs reduce the production of viral protein and infectious virus in Huh7 cells. Cell cultures received a single transfection with 100 nM the indicated siRNA at 48 h before infection with the respective strains from each of the serotypes of DENV (for strain designations see Materials and Methods) at an MOI of 0.5. After a 2-h adsorption period the virus was removed and replaced with medium containing no siRNA. At 48 h postinfection, supernatants were collected for focus-forming (plaque) assays (A, C, E, and G), and cell lysates were prepared for Western blotting (B, D, F, and H). Western blots were detected with two monoclonal antibodies, one against the DENV Env protein and one against GAPDH, simultaneously. A sample of an uninfected-cell lysate was included in the Western blots for DENV-1 to -3. A few of the treatments that did not appear to reduce virus protein levels by Western blotting were omitted from the plaque assays for DENV-4. Each experiment was repeated at least twice, with similar results. A representative Western blot is shown, and histograms represent mean values of triplicate plaque assays ± SEM. Asterisks indicate a significant difference relative to the negative-control siRNA-treated group (*, P < 0.05, Student t test).
To rule out that the cytotoxicity of siRNA reagents had contributed to the inhibition of DENV recovery observed in Fig. 1, we measured the viability of uninfected Huh7 cells subjected to siRNA under the conditions used in the antiviral assays. Huh7 cells were transfected with 100 nM NC, DC-1, DC-3, D2-2, WNV-1, and YFV-1 siRNAs under the same conditions as those used in the experiments described above for Fig. 1 and incubated for 48 h. When ATP utilization was measured as an indicator of cellular metabolism, we did not observe a significant effect on cell viability for any of the siRNAs combined with the amount of RNAiMAX used for the transfections in this study (1 μl per transfection in a total volume of 400 μl). However, a higher concentration of RNAiMAX (5 μl) did cause a ∼60% diminishment of cell viability (data not shown). These results indicate that the siRNA and transfection conditions used in this study did not impact cell viability.
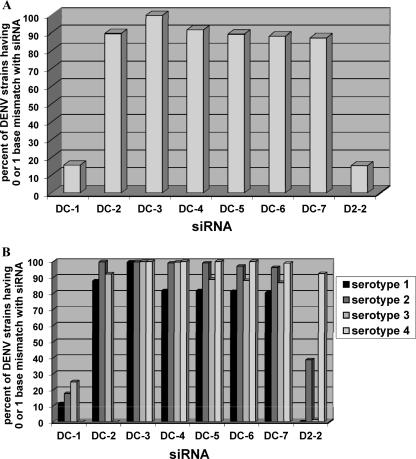
Next, we sought to determine the level of conservation of the conserved siRNAs against all full-length DENV sequences present in the GenBank database. We aligned full-length sequences of 2,754 strains of DENV from the various serotypes and determined the percentages of sequences that had a 0- or 1-base difference compared to the sequence of DC-1 to -7 and D2-2. The sequences of DC-2, -4, -5, -6, and -7 were conserved at between 86 and 92% (Fig. 2A). However, the target sequences for DC-1 and D2-2 were each conserved in only 15 to 16% of the sequences analyzed (Fig. 2A). Importantly, the target sequence for DC-3 was found to be highly conserved in more than 99% of all DENV sequences analyzed (Fig. 2A). A similar disposition was observed when these full-length sequences were subdivided into individual serotypes. Whereas most of the conserved-siRNA targets showed lower conservation across strains of at least one of the four DENV serotypes, the DC-3 target sequence was highly conserved in all four serotypes (Fig. 2B). Thus, our empirical approach yielded at least one panspecific siRNA (DC-3) that was highly active in cell cultures against representative strains from all four serotypes and conserved in almost all DENV strains annotated to date.
Fig. 2.
Conservation of sequence between different strains of DENV at 8 siRNA target sites. Shown are the fractions of DENV strains with a 0- or 1-base mismatch to various siRNAs. (A) Total numbers of best alignments with an exact match or 1 mismatch as defined in Materials and Methods for each query/target pair identified by blast divided by 2,754, the total number of DENV strains in the library. (B) Total number of best alignments having an exact match or 1 mismatch in each DENV serotype divided by the number of strains in each serotype (defined in Materials and Methods).
Panspecific siRNA DC-3 inhibits multiple rounds of DENV infection.
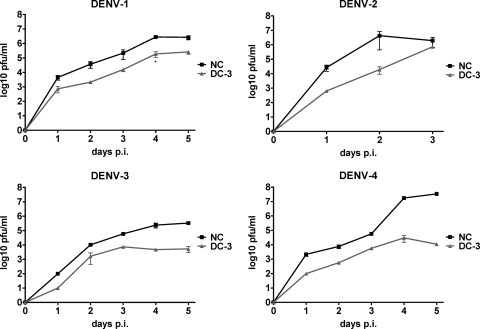
To further characterize the activity of DC-3, we evaluated its activity over several rounds of viral replication, in multistep growth curves. Huh7 cells were transfected with siRNA at 2 days before infection, and supernatants were collected daily for evaluations of virus production. At an input MOI of 0.2, the level of recovery of DENV-1, -3, and -4 steadily increased over a period of 5 days, consistent with several rounds of infection (Fig. 3). At each of these time points, DC-3 reduced the recovery of infectious virus for each of these strains by 90 to 99%. DC-3 thus delayed the accumulation of extracellular virus by 1 to 2 days for up to 5 days postinfection, a full week after the single transfection. DENV-2 strain NGC grew more quickly and to a higher titer, resulting in noticeable cell death beginning at day 3 postinfection. Taken together, these data suggest that DC-3 was active over several days, reducing virus production during sequential rounds of infection.
Fig. 3.
Multistep growth curves of 4 DENV serotypes after DC-3 siRNA treatment. Huh7 cell cultures received a single transfection with 100 nM DC-3 or NC siRNA at 48 h before infection with a strain from each serotype of DENV as indicated, at an MOI of 0.2. After a 2-h adsorption period the virus was removed and replaced with medium containing no siRNA. Supernatants were collected daily for 3 to 5 days after infection. Error bars indicate the SEM of data from triplicate plaque assays. The growth of each virus was significantly different with the two treatments at each day postinfection (P < 0.05, contrast test), except for DENV-2 at day 3.
Comparison of the inhibitory concentrations of an algorithm siRNA and those of a conserved siRNA against DENV-2 infection.
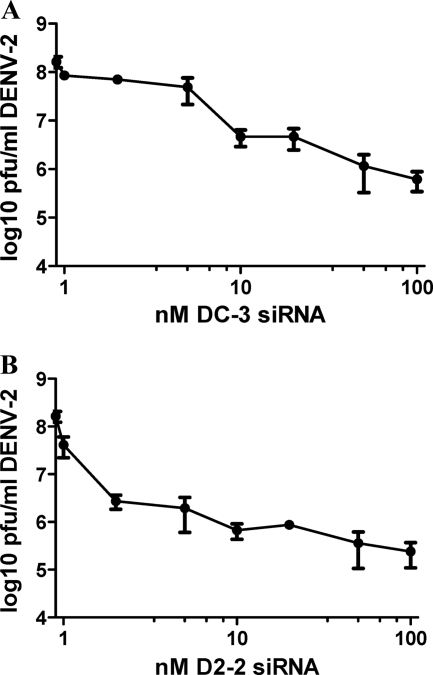
The conserved siRNA DC-3 and the algorithm siRNA D2-2 each reduced DENV-2 production by more than 2 logs at a concentration of 100 nM (Fig. 1C). To further compare the activities of these two siRNAs, we tested them against DENV-2, the most robustly growing virus, in a dose-response format. Cells were transfected with different concentrations of siRNA at 2 days before infection, and virus titers in the supernatant were measured at 2 days postinfection. As shown in Fig. 4, viral release was suppressed by more than 10-fold when D2-2 was present at under 2 nM, generating an 90% inhibitory concentration (IC90) (the concentration of drug producing a 90% reduction in the amount of virus compared to mock treatment) of 1.4 nM. DC-3 was less potent, with an IC90 of 9.8 nM. Thus, at a concentration of approximately 10 nM, D2-2 reduced DENV-2 production 7-fold more than did DC-3. However, both siRNAs reduced viral titers by more than 100-fold at 50 nM.
Fig. 4.
DENV-specific siRNAs reduce the production of infectious virus in Huh7 cells in a dose-responsive manner. Cell cultures received a single transfection with the indicated concentration of DC-3 (A) or D2-2 (B) siRNA at 48 h before infection with DENV-2 at an MOI of 0.5. After a 2-h adsorption period the virus was removed and replaced with medium containing no siRNA. At 48 h postinfection, supernatants were collected. Each experiment was carried out at least twice, with similar results, and the mean values from triplicate plaque assays ± SEM are shown.
siRNA targeting of the 5′CS inhibits WNV moderately and YFV potently.
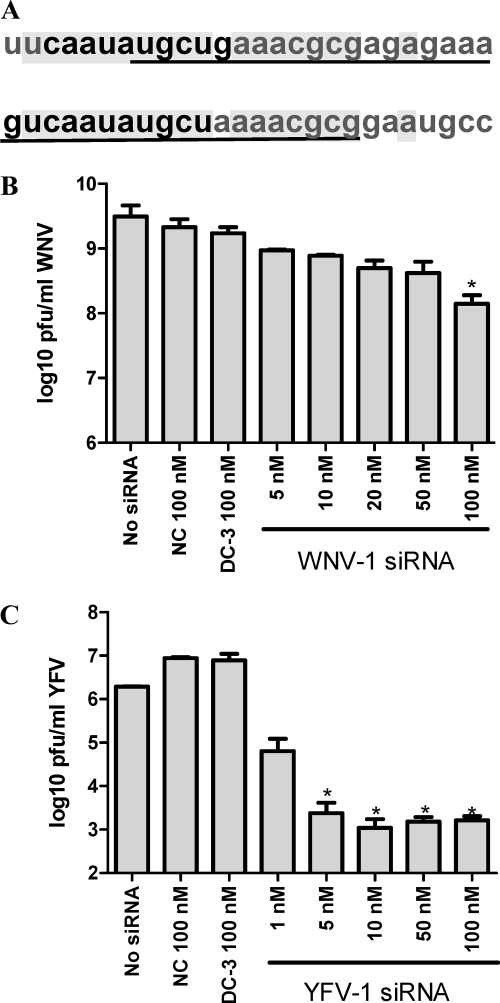
Since the DC-3 target includes 5 of the 10 nt of the DENV 5′CS (Fig. 5A), we tested whether 5′CS-directed siRNA would also be effective against other mosquito-borne flaviviruses. Although the 5′CS regions of all mosquito-borne flaviviruses share 8 nt of sequence, the adjacent sequences vary between species. We therefore designed species-specific siRNAs to target the 5′CS region of WNV and YFV. For WNV, we designed an siRNA (WNV-1) to target the 11-nt 5′CS and the adjacent 8 nt in the 3′ direction, as the sequence located 5′ to the 5′CS is thought to be part of a highly ordered region and, therefore, perhaps less accessible to siRNA (16, 65, 78). WNV-1 was 100% conserved across all 284 complete genomes against which it was tested by comparative alignment. Interestingly, the sequences targeted by DC-3 and WNV-1 are similar, with 12 bases targeted by DC-3 being present in WNV and 17 of the 19 bases targeted by WNV-1 being present in DENV-2 (Fig. 5A). Up to 18 nt in a sequence of 19 contiguous nucleotides are predicted to comprise the 5′CS region for YFV (65), and we therefore chose that entire region for siRNA targeting. All 18 YFV genomes analyzed had 100% conservation with the YFV-1 sequence.
Fig. 5.
Comparison of genomic sequences and siRNA targets in DENV-2 and WNV at the 5′CS region and dose-response efficacy of siRNAs targeting the 5′CS of WNV or YFV in Huh7 cell cultures. (A) Alignment of the 5′CS regions of DENV-2 (GenBank accession number NC_001474) bases 131 to 158 (top) and WNV (GenBank accession number DQ211652) bases 136 to 161 (bottom). The 5′CS (10 nt for DENV-2 and 11 nt for WNV [66]) of each virus is shown in boldface type, and the targets of DC-3 (in DENV-2) and WNV-1 (in WNV) are underlined. The aligned sequence in common between DENV-2 and WNV is shaded. (B and C) Cells received a single transfection with the indicated concentration of the indicated siRNAs at 48 h before infection with WNV (B) or YFV (C) at an MOI of 0.5. After a 2-h adsorption period the virus was removed and replaced with medium containing no siRNA. At 24 h (WNV) or 72 h (YFV) postinfection, supernatants were collected for plaque assays. Each experiment was carried out at least twice, with similar results, and the histograms show the mean values of triplicate plaque assays ± SEM. Asterisks indicate a significant difference relative to that of the negative-control siRNA-treated group (*, P < 0.05, Student t test).
The antiviral activities of the 5′CS-targeting siRNAs against WNV and YFV were examined in a dose-response manner. The WNV 5′CS-directed siRNA (WNV-1) reduced the production of WNV by only about 5- to 20-fold when present at 50 and 100 nM, respectively (Fig. 5B). We note that WNV grows quickly and to high titers (more than 109 PFU/ml under the conditions used here) in Huh7 cells, and for that reason we measured the infectious virus produced at 24 h p.i. In contrast, the YFV 5′CS-directed siRNA (YFV-1) reduced viral release by several logs when used at low-nanomolar concentrations (Fig. 5C).
As a further control for the sequence specificity of the siRNA in this study, Huh7 cells were transfected with 100 nM NC, WNV-1, and YFV-1 siRNAs and then infected with 0.5 MOI of DENV-2 under the same conditions as those used for the antiviral experiments described in the legend of Fig. 1. WNV-1 produced a 3- to 4-fold, and YFV-1 or NC produced a negligible, reduction in the titer of DENV-2 (data not shown). Likewise, the DC-3 siRNA, which was potently inhibitory against DENV (Fig. 1 and 4), was not significantly active against WNV or YFV (Fig. 5B and C).
These results suggest that although 5′CS-targeting siRNA must be designed species specifically, the 5′CS region in general represents a sensitive site for the targeting of mosquito-borne flaviviruses with exogenous siRNA.
Antiflavivirus siRNAs do not induce interferon-stimulated gene expression.
It was reported previously that siRNA can produce innate immune stimulation in a sequence-specific manner (34, 56). To rule out the possibility that the stimulation of interferon production had contributed to the antiviral activity observed in this study, we tested select siRNAs at 100 nM on uninfected Huh7-ISRE-luc cells, which contain a stably integrated interferon-stimulated response element (ISRE) fused to the luciferase coding sequence. Under the conditions used in the antiviral experiments described above, none of the siRNAs generated a significant stimulation of interferon, measured at either 8 h (optimal time for a firefly luciferase readout with these cells) or 48 h (matching the time point at which cells were infected in our antiviral assays), whereas the beta interferon positive control induced a high level of reporter signal at both time points (data not shown).
DC-3 inhibits DENV-2 infection and disease in AG129 mice.
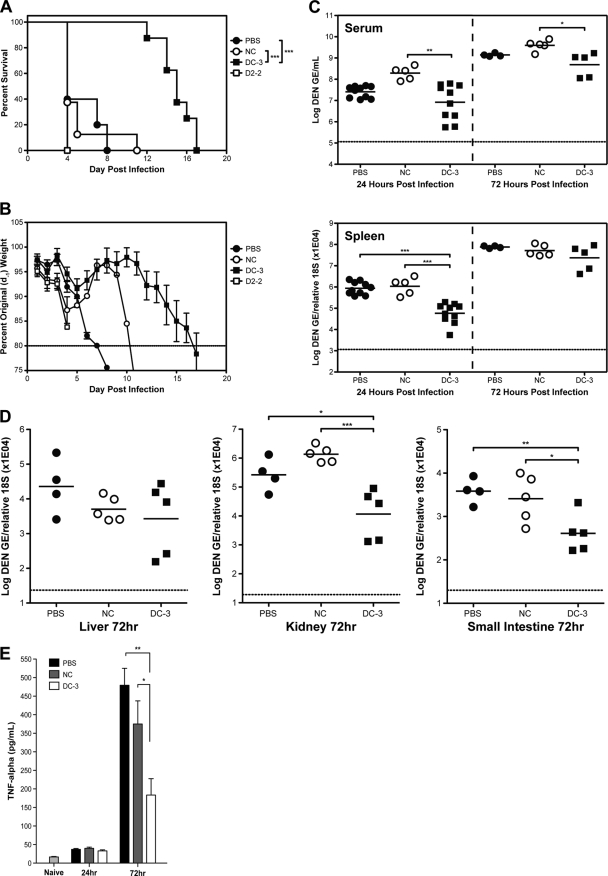
To investigate if siRNA sequences could interfere with DENV infection in vivo, we utilized a model of ADE-mediated disease in AG129 mice. In this model, AG129 mice were infected with DENV-2 strain S221 in the presence of DENV-1- to -4-reactive antibody (anti-prM, clone 2H2), which mediates severe pathology and death between days 4 and 8 (77). We chose to test three siRNAs, a negative control (NC); DC-3, as it possessed high levels of activity against all 4 representative serotypes; and D2-2, as it consistently produced higher antiviral activity against DENV-2 than any other siRNA (Fig. 1 and 4). Mice received the selected siRNAs via retro-orbital intravenous injection at −24 h, +24 h, and +72 h in relation to infection. Although the same siRNA sequences were used, in vivo application required the use of a specialized siRNA (Silencer In Vivo Ready siRNA; Ambion) and transfection agent (Invivofectamine 2.0; Invitrogen). One group was treated with PBS only, and the other three groups received the transfection reagent mixed with NC, DC-3, or D2-2 siRNA. The time of survival of animals treated with DC-3 was significantly extended (median survival time of 14 days) compared to that of PBS- or NC-treated animals (median survival time of 5 days) (Fig. 6A). In contrast to the efficacy observed in cultured cells, D2-2 failed to increase the survival times of the infected mice compared to NC- or PBS-treated mice. Seventeen out of 18 animals in the PBS-, NC-, and D2-2-treated groups succumbed to early death, a phenotype indicated by hunched posture, ruffled fur, and severe weight loss, within the first 8 days following infection. In contrast, the eight DC-3-treated mice maintained normal appearance and behavior until at least day 11 and were euthanized between days 12 and 17 following signs of hind-limb paralysis. Body weight measurements mirrored the survival pattern and reflected disease severity, with DC-3-treated mice retaining on average around 95% of their preinfection body weight for at least 11 days after infection (Fig. 6B). The siRNA dose of 10 mg/kg was chosen based on recommendations from the manufacturer, and the normal health and body weight of DC-3-treated animals after infection indicate that the dosing was both subtoxic and efficacious. However, it may be noteworthy that treatment with siRNA that did not produce an antiviral effect in vivo (NC and D2-2) appeared to be associated with accelerated weight loss in infected animals (Fig. 6B).
Fig. 6.
DC-3 siRNA limits the viral load and TNF response and protects mice from lethal ADE-DENV infection. AG129 mice were treated with 10 mg/kg of the indicated siRNAs via retro-orbital intravenous injection at 24 h before and 24 h and 72 h after the tail vein administration of 109 genomic equivalents of DENV-2 (S221) and the intraperitoneal injection of 5 μg of antibody 2H2 (anti-prM/M, DENV-1 to -4 reactive). (A) Survival time of AG129 mice (PBS, n = 5; NC, n = 8; DC-3, n = 8; D2-2, n = 5). The curve shows the combined results from two survival experiments carried out under similar conditions. Log rank analysis indicates that the difference between the DC-3-treated groups and the other groups is statistically significant at a P value of ≤0.0001. (B) Body weight measured daily and represented as a percentage of the animal weight 24 h prior to infection. (C and D) Viral loads in serum and spleen at 24 and 72 h p.i. (for 24 h p.i., n = 10 for PBS, n = 5 for NC, and n = 10 for DC-3; for 72 h p.i., n = 5 per group) (C) and in the liver, kidney, and small intestine at 72 h p.i. (n = 5 per group) (D). Viral RNA was quantified by RT-PCR as described in Materials and Methods (***, P < 0.0005; **, P < 0.005; *, P < 0.05 [Student t test]). (E) Levels of TNF in serum collected from naïve and infected mice at 24 h and 72 h p.i. (n = 5 per group), detected by ELISA as described in Materials and Methods (**, P < 0.005; *, P < 0.05 [Student t test]).
To examine the effects of the DC-3 siRNA treatment on viral replication directly, the viral load was assessed in several tissues by RT-PCR at 24 and 72 h postinfection. In the ADE model of infection used here, initial viral replication occurs in the spleen, followed by increasing viral loads in other tissues during the several days following infection. By 24 h p.i., mice treated with DC-3 possessed an 18-fold-lower viral load in the spleen than either control group of animals (PBS- or NC-treated group) (Fig. 6C). DC-3 treatment also generated a statistically significant 20-fold reduction in viremia compared to NC treatment; however, the 7-fold reduction of the viremia in the DC-3-treated group compared to the PBS-treated group was not significant. By 72 h postinfection, the viral loads in the serum and spleen had increased in all sample groups regardless of treatment, and the differences were less pronounced. The level of viremia in DC-3-treated mice was 8-fold lower than that in the NC-treated group and remained consistently 3-fold lower than that in the PBS-treated group. The greatest effect from DC-3 treatment at 72 h postinfection was observed for the kidney, where the viral load was 23-fold and 117-fold lower than those of the PBS-treated and NC-treated groups, respectively (Fig. 6D). DC-3 treatment also significantly suppressed levels of viral RNA in the small intestine at 72 h compared to those of the PBS-treated or NC-treated group (Fig. 6D). It is noteworthy that in this model death from S221 is typically preceded by intestinal hemorrhage (77) and that gastrointestinal bleeding is the most common type of severe hemorrhage in patients with severe dengue disease (13). Together, these data indicate that treatment with DC-3 siRNA suppressed the accumulation of viral RNA in selected tissues, thereby promoting prolonged survival.
Tumor necrosis factor (TNF) is a key mediator of severe DENV disease in this mouse model (77). Measurements taken at 72 h postinfection showed that PBS- and NC-treated mice had significantly elevated serum TNF levels compared to those of mice treated with DC-3 (Fig. 6E). This result indicates that DC-3 treatment was associated with reduced levels of a cytokine known to mediate the early-death phenotype in this animal model.
DISCUSSION
In this study we demonstrate that a synthetic siRNA, DC-3, targeting the highly conserved 5′CS region, inhibits representative strains from each of the four DENV serotypes in cell cultures. Furthermore, we show that an analog of DC-3, chemically modified for in vivo applications, inhibited pathogenic progression in a murine model of severe dengue disease. DC-3 was identified by the systematic testing of the antiviral activities of a panel of siRNAs designed against sequences that are highly conserved in the genomes of genetically diverse dengue viruses. The majority of siRNAs designed solely on the basis of the targeting of highly conserved sequences were not efficient at inhibiting the growth of various strains of DENV in cell cultures, presumably because other parameters were not considered in their design. In contrast, most of the siRNAs designed against individual strains of DENV by a commercial algorithm, which factors in such considerations as G/C content and secondary structure, were effective against their targeted strains in cell cultures. The target specificity of DC-3 was confirmed in part by challenge against heterologous viruses. Cell viability, TNF, and interferon assays further ruled out that the antiviral activity of DC-3 was due to off-target effects. We conclude therefore that DC-3 acted via RNA interference targeting the 5′CS region.
During the course of this study, two other reports documented the antiflaviviral efficacy of nucleic acid-based agents targeting the 5′CS. DENV-2 replication in mosquito cell cultures was suppressed by group 1 splicing introns targeted to the 5′CS region (8), and WNV and St. Louis encephalitis virus infections were inhibited in vitro and WNV infection was inhibited in a mouse model by a 5′CS-targeting siRNA conjugated to a 41-mer peptide (73). We observed a potent inhibition of YFV and a modest inhibition of WNV by the targeting of their respective 5′CS regions. Taken together, these studies show that the flavivirus 5′CS region is susceptible to RNAi-mediated interventional strategies and that it warrants further exploration as a target for the development of nucleic acid-based inhibitors of flavivirus infections.
The 10-nt DENV 5′CS is highly conserved in dengue viruses and complementary to the CS1 sequence (corresponding to nt 135 to 144 and 10618 to 10627, respectively, in DENV-2 [GenBank accession number NC_001474]). The base-pairing interaction between the 5′CS and CS1 has been shown to be important for the efficient replication of DENV (20, 35, 64, 76), WNV (42, 78), and YFV (14). The importance of the interaction between the 5′CS and CS1 regions may render these sites particularly vulnerable to RNAi, especially when the flavivirus genome is present in a noncyclized form prior to minus-strand synthesis. Alvarez et al. showed previously that while the disruption of base pairing between the 5′CS and CS1 reduced DENV RNA synthesis, the replacement of base pairs with different but complementary nucleotide sequences rescued viral RNA production. This result suggested that complementarity rather than the specific nucleotide sequence of the CS interaction was essential for viral replication (1). Thus, DENV mutants with simultaneous replacements of complementary bases at the 5′CS and CS1 sequences could conceivably escape DC-3-mediated inhibition. However, the requirement for multiple mutations in disparate regions of the genome lowers the likelihood of such a scenario. The fact that the 5′CS and the CS1 regions are highly conserved across the dengue viruses suggests that mutations in either region would likely result in viruses with lower fitness in vivo.
The DENV CS1 region was previously found to be a productive target site for sequence-specific antiviral inhibition by peptide-conjugated morpholino oligomers (PPMO) (29, 36, 60) and shRNA (79). However, the DC-6 siRNA, designed against the CS1 region, was consistently inactive. While a possible explanation for the discrepancy between results obtained with the PPMO and those obtained with siRNAs could be their differing mechanisms of action (steric blockade versus the RNase-mediated cleavage of the target, respectively), the difference in results with AAV-vectored shRNA compared to those with lipid-transfected siRNA remains unexplained. Interestingly, an siRNA designed against the first 20 nt of the DENV genome (DC-1) was highly active against a DENV strain from each of the four serotypes. The DC-1 target site is of functional significance for various aspects of the DENV life cycle, including translation (12, 28), RNA synthesis (19, 20, 31, 43), and RNA 5′ cap methylation (15), and was also an effective target site for PPMO (29, 36, 60). The efficacy of DC-1 was somewhat unexpected considering that its target is part of the stable hairpin structure formed by the first 70 nt of the DENV 5′UTR (22, 45) and suggests that highly ordered RNA secondary structures in flaviviral genomes can be susceptible to siRNA targeting. Although the DC-1 target sequence is not as highly conserved across the DENV virome as the other conserved siRNAs (Fig. 2), the 5′-terminal region of the genome also appears to warrant further investigation as a target for nucleic acid-based inhibitors.
In addition to producing multiple-log reductions of viral titers in cell cultures, the DC-3 siRNA proved to be highly effective against a lethal DENV infection in vivo. Compared to two control groups, AG129 mice receiving DC-3 showed reduced TNF levels and suppressed viral RNA levels in the spleen at 24 h p.i. and in kidney and small intestine at 72 h p.i. DC-3-treated mice were protected from lethality for at least 2 weeks, in contrast to the other groups, in which most of the mice died by day 5. Furthermore, DC-3-treated mice appeared to eventually succumb to neurologic symptoms rather than to the vascular pathology known to be a hallmark of the demise of untreated AG129 mice infected with DENV strain S221 (77), indicating robust antiviral activity early in the infection process. The lack of antiviral efficacy of two other in vivo-ready siRNAs (NC and D2-2) along with the lack of interferon stimulation by DC-3 in vitro (data not shown) suggest that the antiviral activity of DC-3 in vivo is most likely a result of sequence-specific inhibition and not an off-target effect. The ADE mouse model used in this study constitutes a severely pathogenic DENV challenge, and the efficacy exhibited by the DC-3 siRNA treatment suggests that the activation of endogenous mammalian RNAi machinery by exogenous siRNA may be sufficient to thwart a natural DENV infection.
In contrast to DC-3, D2-2 was unable to prevent DENV-2-mediated disease in vivo despite the fact that D2-2 inhibited DENV-2 more potently than DC-3 in vitro. The reason for D2-2's lack of efficacy in vivo is currently unknown. Although different strains of DENV-2 were used in the in vitro and in vivo arms of the study, both strains have identical sequences in the D2-2 target region. It is possible that during replication in vivo, viral mutants arose that were resistant to D2-2, which targets a sequence which is less conserved and perhaps less constrained than the 5′CS targeted by DC-3. Alternatively, it is also possible that the stabilities of the two compounds differed or that the siRNA sequence had some other effect on the respective antiviral efficacy of each compound in vivo, although neither of these possibilities has been addressed experimentally. As the DC-3 treatment regimen employed here was unable to fully eradicate the virus, future studies will include in vivo dose responsiveness, an extended time of treatment, and postinfection-only dosing and will explore the potential generation and characterization of escape mutants.
To date, few compounds have been documented to extend the time of survival of AG129 mice challenged with a lethal DENV infection. A small-molecule adenosine nucleotide analog was reported previously to completely protect S221-infected mice for up to 11 days, while untreated mice succumbed to primary infection by day 4 (11, 74), in a mortality pattern similar to that reported here. In two other studies, one using a PPMO targeting the 5′-terminal region of the genome and/or the CS1 region (roughly equivalent in targeting to the DC-1 and DC-6 siRNAs here) (60) and the other using a heparin sulfate mimetic (39), the average survival time of AG129 mice receiving a lethal challenge of mouse brain-adapted DENV-2 strain New Guinea C was extended for about a week. Together, the PPMO-, self-splicing intron-, and RNAi-based studies suggest that the targeting of regions of highly conserved DENV RNA with antisense-based methodologies may represent a productive strategy for the development of antiflaviviral compounds.
The study here represents a further advancement in the development of siRNA inhibitors of DENV infections. The targeting of pathogenically relevant RNA is valuable for mechanistic analyses and is being developed clinically against various human diseases (3, 10, 25, 38, 44). Further study will be necessary to investigate whether this technology could be developed as the basis for a therapeutic system to address DENV infections in humans.
ACKNOWLEDGMENTS
This work was supported by the NIAID Pacific Northwest Regional Center of Excellence for Biodefense and Emerging Infectious Diseases Research (grant U54 AI 081680), National Center for Research Resources support for the Oregon National Primate Research Center (grant RR00163), and National Institutes of Health grants UO1 AI082196 and R44 AI079898 (M.K.S.). M.A.F. and J.L.S. were supported by OHSU training grants T32 A1074494 and T32 AI07472.
Footnotes
Published ahead of print on 27 July 2011.
REFERENCES
- 1. Alvarez D. E., De Lella Ezcurra A. L., Fucito S., Gamarnik A. V. 2005. Role of RNA structures present at the 3′UTR of dengue virus on translation, RNA synthesis, and viral replication. Virology. 339:200–212 [DOI] [PubMed] [Google Scholar]
- 2. Alvarez D. E., Lodeiro M. F., Luduena S. J., Pietrasanta L. I., Gamarnik A. V. 2005. Long-range RNA-RNA interactions circularize the dengue virus genome. J. Virol. 79:6631–6643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alvarez-Salas L. M. 2008. Nucleic acids as therapeutic agents. Curr. Top. Med. Chem. 8:1379–1404 [DOI] [PubMed] [Google Scholar]
- 4. Anthony K. G., Bai F., Krishnan M. N., Fikrig E., Koski R. A. 2009. Effective siRNA targeting of the 3′ untranslated region of the West Nile virus genome. Antiviral Res. 82:166–168 [DOI] [PubMed] [Google Scholar]
- 5. Bai F., et al. 2005. Use of RNA interference to prevent lethal murine West Nile virus infection. J. Infect. Dis. 191:1148–1154 [DOI] [PubMed] [Google Scholar]
- 6. Birmingham A., et al. 2007. A protocol for designing siRNAs with high functionality and specificity. Nat. Protoc. 2:2068–2078 [DOI] [PubMed] [Google Scholar]
- 7. Calisher C. H., et al. 1989. Antigenic relationships between flaviviruses as determined by cross-neutralization tests with polyclonal antisera. J. Gen. Virol. 70(Pt. 1):37–43 [DOI] [PubMed] [Google Scholar]
- 8. Carter J. R., Keith J. H., Barde P. V., Fraser T. S., Fraser M. J., Jr 2010. Targeting of highly conserved dengue virus sequences with anti-dengue virus trans-splicing group I introns. BMC Mol. Biol. 11:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chambers T. J., Hahn C. S., Galler R., Rice C. M. 1990. Flavivirus genome organization, expression, and replication. Annu. Rev. Microbiol. 44:649–688 [DOI] [PubMed] [Google Scholar]
- 10. Chen S. H., Zhaori G. 2011. Potential clinical applications of siRNA technique: benefits and limitations. Eur. J. Clin. Invest. 41:221–232 [DOI] [PubMed] [Google Scholar]
- 11. Chen Y. L., et al. 2010. Inhibition of dengue virus RNA synthesis by an adenosine nucleoside. Antimicrob. Agents Chemother. 54:2932–2939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chiu W. W., Kinney R. M., Dreher T. W. 2005. Control of translation by the 5′- and 3′-terminal regions of the dengue virus genome. J. Virol. 79:8303–8315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chiu Y. C., et al. 2005. Endoscopic findings and management of dengue patients with upper gastrointestinal bleeding. Am. J. Trop. Med. Hyg. 73:441–444 [PubMed] [Google Scholar]
- 14. Corver J., et al. 2003. Fine mapping of a cis-acting sequence element in yellow fever virus RNA that is required for RNA replication and cyclization. J. Virol. 77:2265–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dong H., et al. 2010. Biochemical and genetic characterization of dengue virus methyltransferase. Virology 405:568–578 [DOI] [PubMed] [Google Scholar]
- 16. Dong H., Zhang B., Shi P. Y. 2008. Terminal structures of West Nile virus genomic RNA and their interactions with viral NS5 protein. Virology 381:123–135 [DOI] [PubMed] [Google Scholar]
- 17. Durbin A. P., Whitehead S. S. 2010. Dengue vaccine candidates in development. Curr. Top. Microbiol. Immunol. 338:129–143 [DOI] [PubMed] [Google Scholar]
- 18. Dykxhoorn D. M., Lieberman J. 2006. Silencing viral infection. PLoS Med. 3:e242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Filomatori C. V., Iglesias N. G., Villordo S. M., Alvarez D. E., Gamarnik A. V. 2011. RNA sequences and structures required for the recruitment and activity of the dengue virus polymerase. J. Biol. Chem. 286:6929–6939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Filomatori C. V., et al. 2006. A 5′ RNA element promotes dengue virus RNA synthesis on a circular genome. Genes Dev. 20:2238–2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Franz A. W., et al. 2006. Engineering RNA interference-based resistance to dengue virus type 2 in genetically modified Aedes aegypti. Proc. Natl. Acad. Sci. U. S. A. 103:4198–4203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gritsun T. S., Gould E. A. 2007. Origin and evolution of flavivirus 5′UTRs and panhandles: trans-terminal duplications? Virology 366:8–15 [DOI] [PubMed] [Google Scholar]
- 23. Guy B., Almond J. W. 2008. Towards a dengue vaccine: progress to date and remaining challenges. Comp. Immunol. Microbiol. Infect. Dis. 31:239–252 [DOI] [PubMed] [Google Scholar]
- 24. Guzman M. G., et al. 2010. Dengue: a continuing global threat. Nat. Rev. Microbiol. 8:S7–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Haasnoot J., Berkhout B. 2009. Nucleic acids-based therapeutics in the battle against pathogenic viruses. Handb. Exp. Pharmacol. 2009:243–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hahn C. S., et al. 1987. Conserved elements in the 3′ untranslated region of flavivirus RNAs and potential cyclization sequences. J. Mol. Biol. 198:33–41 [DOI] [PubMed] [Google Scholar]
- 27. Halstead S. B. 2007. Dengue. Lancet 370:1644–1652 [DOI] [PubMed] [Google Scholar]
- 28. Holden K. L., Harris E. 2004. Enhancement of dengue virus translation: role of the 3′ untranslated region and the terminal 3′ stem-loop domain. Virology 329:119–133 [DOI] [PubMed] [Google Scholar]
- 29. Holden K. L., et al. 2006. Inhibition of dengue virus translation and RNA synthesis by a morpholino oligomer targeted to the top of the terminal 3′ stem-loop structure. Virology 344:439–452 [DOI] [PubMed] [Google Scholar]
- 30. Huang D. D. 2008. The potential of RNA interference-based therapies for viral infections. Curr. HIV/AIDS Rep. 5:33–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Iglesias N. G., Filomatori C. V., Gamarnik A. V. 2011. The F1 motif of dengue virus polymerase NS5 is involved in promoter-dependent RNA synthesis. J. Virol. 85:5745–5756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Iglesias N. G., Gamarnik A. V. 2011. Dynamic RNA structures in the dengue virus genome. RNA Biol. 8:249–257 [DOI] [PubMed] [Google Scholar]
- 33. Jessie K., Fong M. Y., Devi S., Lam S. K., Wong K. T. 2004. Localization of dengue virus in naturally infected human tissues, by immunohistochemistry and in situ hybridization. J. Infect. Dis. 189:1411–1418 [DOI] [PubMed] [Google Scholar]
- 34. Judge A. D., et al. 2005. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat. Biotechnol. 23:457–462 [DOI] [PubMed] [Google Scholar]
- 35. Khromykh A. A., Meka H., Guyatt K. J., Westaway E. G. 2001. Essential role of cyclization sequences in flavivirus RNA replication. J. Virol. 75:6719–6728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kinney R. M., et al. 2005. Inhibition of dengue virus serotypes 1 to 4 in Vero cell cultures with morpholino oligomers. J. Virol. 79:5116–5128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kumar P., Lee S. K., Shankar P., Manjunath N. 2006. A single siRNA suppresses fatal encephalitis induced by two different flaviviruses. PLoS Med. 3:e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lares M. R., Rossi J. J., Ouellet D. L. 2010. RNAi and small interfering RNAs in human disease therapeutic applications. Trends Biotechnol. 28:570–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee E., Pavy M., Young N., Freeman C., Lobigs M. 2006. Antiviral effect of the heparan sulfate mimetic, PI-88, against dengue and encephalitic flaviviruses. Antiviral Res. 69:31–38 [DOI] [PubMed] [Google Scholar]
- 40. Lindenbach B. D., Theil H. J., Rice C. M. 2007. Flaviviridae: the viruses and their replication, p. 1102–1152In Knipe D. M., et al. (ed.), Fields virology, 5th ed., vol. 1 Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 41. Liu X., et al. 2006. Inhibition of Japanese encephalitis virus NS1 protein expression in cell by small interfering RNAs. Virus Genes 33:69–75 [DOI] [PubMed] [Google Scholar]
- 42. Lo M. K., Tilgner M., Bernard K. A., Shi P. Y. 2003. Functional analysis of mosquito-borne flavivirus conserved sequence elements within 3′ untranslated region of West Nile virus by use of a reporting replicon that differentiates between viral translation and RNA replication. J. Virol. 77:10004–10014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lodeiro M. F., Filomatori C. V., Gamarnik A. V. 2009. Structural and functional studies of the promoter element for dengue virus RNA replication. J. Virol. 83:993–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lopez-Fraga M., Wright N., Jimenez A. 2008. RNA interference-based therapeutics: new strategies to fight infectious disease. Infect. Disord. Drug Targets 8:262–273 [DOI] [PubMed] [Google Scholar]
- 45. Markoff L. 2003. 5′- and 3′-noncoding regions in flavivirus RNA. Adv. Virus Res. 59:177–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Murakami M., Ota T., Nukuzuma S., Takegami T. 2005. Inhibitory effect of RNAi on Japanese encephalitis virus replication in vitro and in vivo. Microbiol. Immunol. 49:1047–1056 [DOI] [PubMed] [Google Scholar]
- 47. Noble C. G., et al. 2010. Strategies for development of dengue virus inhibitors. Antiviral Res. 85:450–462 [DOI] [PubMed] [Google Scholar]
- 48. Ong S. P., Chu J. J., Ng M. L. 2008. Inhibition of West Nile virus replication in cells stably transfected with vector-based shRNA expression system. Virus Res. 135:292–297 [DOI] [PubMed] [Google Scholar]
- 49. Pacca C. C., et al. 2009. RNA interference inhibits yellow fever virus replication in vitro and in vivo. Virus Genes 38:224–231 [DOI] [PubMed] [Google Scholar]
- 50. Paes M. V., et al. 2005. Liver injury and viremia in mice infected with dengue-2 virus. Virology 338:236–246 [DOI] [PubMed] [Google Scholar]
- 51. Perry S. T., Buck M. D., Lada S. M., Schindler C., Shresta S. 2011. STAT2 mediates innate immunity to dengue virus in the absence of STAT1 via the type I interferon receptor. PLoS Pathog. 7:e1001297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Prestwood T. R., Prigozhin D. M., Sharar K. L., Zellweger R. M., Shresta S. 2008. A mouse-passaged dengue virus strain with reduced affinity for heparan sulfate causes severe disease in mice by establishing increased systemic viral loads. J. Virol. 82:8411–8421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Qi W. B., et al. 2008. Effective inhibition of Japanese encephalitis virus replication by small interfering RNAs targeting the NS5 gene. Virus Res. 132:145–151 [DOI] [PubMed] [Google Scholar]
- 54. Reynolds A., et al. 2004. Rational siRNA design for RNA interference. Nat. Biotechnol. 22:326–330 [DOI] [PubMed] [Google Scholar]
- 55. Rico-Hesse R. 1990. Molecular evolution and distribution of dengue viruses type 1 and 2 in nature. Virology 174:479–493 [DOI] [PubMed] [Google Scholar]
- 56. Robbins M., Judge A., MacLachlan I. 2009. siRNA and innate immunity. Oligonucleotides. 19:89–102 [DOI] [PubMed] [Google Scholar]
- 57. Sanchez-Vargas I., et al. 2004. RNA interference, arthropod-borne viruses, and mosquitoes. Virus Res. 102:65–74 [DOI] [PubMed] [Google Scholar]
- 58. Shresta S., Sharar K. L., Prigozhin D. M., Beatty P. R., Harris E. 2006. Murine model for dengue virus-induced lethal disease with increased vascular permeability. J. Virol. 80:10208–10217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shum D., et al. High-content assay to identify inhibitors of dengue virus infection. Assay Drug Dev. Technol. 8:553–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Stein D. A., et al. 2008. Treatment of AG129 mice with antisense morpholino oligomers increases survival time following challenge with dengue 2 virus. J. Antimicrob. Chemother. 62:555–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Stein D. A., Shi P. Y. 2008. Nucleic acid-based inhibition of flavivirus infections. Front. Biosci. 13:1385–1395 [DOI] [PubMed] [Google Scholar]
- 62. Subramanya S., et al. 2010. Targeted delivery of small interfering RNA to human dendritic cells to suppress dengue virus infection and associated proinflammatory cytokine production. J. Virol. 84:2490–2501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Swaminathan S., Khanna N. 2009. Dengue: recent advances in biology and current status of translational research. Curr. Mol. Med. 9:152–173 [DOI] [PubMed] [Google Scholar]
- 64. Villordo S. M., Alvarez D. E., Gamarnik A. V. 2011. A balance between circular and linear forms of the dengue virus genome is crucial for viral replication. RNA 16:2325–2335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Villordo S. M., Gamarnik A. V. 2009. Genome cyclization as strategy for flavivirus RNA replication. Virus Res. 139:230–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Weaver S. C., Vasilakis N. 2009. Molecular evolution of dengue viruses: contributions of phylogenetics to understanding the history and epidemiology of the preeminent arboviral disease. Infect. Genet. Evol. 9:523–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wilder-Smith A., Ooi E. E., Vasudevan S. G., Gubler D. J. 2011. Update on dengue: epidemiology, virus evolution, antiviral drugs, and vaccine development. Curr. Infect. Dis. Rep. 12:157–164 [DOI] [PubMed] [Google Scholar]
- 68. Williams K. L., Zompi S., Beatty P. R., Harris E. 2009. A mouse model for studying dengue virus pathogenesis and immune response. Ann. N. Y. Acad. Sci. 1171(Suppl. 1):E12–E23 [DOI] [PubMed] [Google Scholar]
- 69. Wu X., et al. 2010. Inhibitory effect of small interfering RNA on dengue virus replication in mosquito cells. Virol. J. 7:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Xiao S. Y., Guzman H., Zhang H., Travassos da Rosa A. P., Tesh R. B. 2001. West Nile virus infection in the golden hamster (Mesocricetus auratus): a model for West Nile encephalitis. Emerg. Infect. Dis. 7:714–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yang Y., et al. 2008. Inhibition of West Nile virus replication by retrovirus-delivered small interfering RNA in human neuroblastoma cells. J. Med. Virol. 80:930–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Yauch L. E., Shresta S. 2008. Mouse models of dengue virus infection and disease. Antiviral Res. 80:87–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ye C., Abraham S., Wu H., Shankar P., Manjunath N. 2011. Silencing early viral replication in macrophages and dendritic cells effectively suppresses flavivirus encephalitis. PLoS One 6:e17889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yin Z., et al. 2009. An adenosine nucleoside inhibitor of dengue virus. Proc. Natl. Acad. Sci. U. S. A. 106:20435–20439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. You S., Falgout B., Markoff L., Padmanabhan R. 2001. In vitro RNA synthesis from exogenous dengue viral RNA templates requires long range interactions between 5′- and 3′-terminal regions that influence RNA structure. J. Biol. Chem. 276:15581–15591 [DOI] [PubMed] [Google Scholar]
- 76. You S., Padmanabhan R. 1999. A novel in vitro replication system for dengue virus. Initiation of RNA synthesis at the 3′-end of exogenous viral RNA templates requires 5′- and 3′-terminal complementary sequence motifs of the viral RNA. J. Biol. Chem. 274:33714–33722 [DOI] [PubMed] [Google Scholar]
- 77. Zellweger R. M., Prestwood T. R., Shresta S. 2010. Enhanced infection of liver sinusoidal endothelial cells in a mouse model of antibody-induced severe dengue disease. Cell Host Microbe 7:128–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zhang B., Dong H., Stein D. A., Iversen P. L., Shi P. Y. 2008. West Nile virus genome cyclization and RNA replication require two pairs of long-distance RNA interactions. Virology 373:1–13 [DOI] [PubMed] [Google Scholar]
- 79. Zhang W., et al. 2004. Attenuation of dengue virus infection by adeno-associated virus-mediated siRNA delivery. Genet. Vaccines Ther. 2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]