Abstract
Herpes simplex type 1 (HSV-1) is a neurotropic virus which establishes lifelong latency in human trigeminal ganglia (TG). Currently, two nonexclusive control mechanisms of HSV-1 latency are discussed: antiviral CD8+ T cells and viral microRNAs (miRNAs) encoded by the latency associated transcript (LAT). We investigate here to what extent these mechanisms may contribute to the maintenance of HSV-1 latency. We show that only a small proportion of LAT+ neurons is surrounded by T cells in human TG. This indicates that viral latency in human TG might be controlled by other mechanisms such as viral miRNAs. Therefore, we assessed TG sections for the presence of HSV-1 miRNA, DNA, and mRNA by combining LAT in situ hybridization, T-cell immunohistochemistry, and single cell analysis of laser-microdissected sensory neurons. Quantitative reverse transcription-PCR (RT-PCR) revealed that LAT+ neurons with or without surrounding T cells were always positive for HSV-1 miRNAs and DNA. Furthermore, ICP0 mRNA could rarely be detected only in LAT+ neurons, as analyzed by single-cell RT-PCR. In contrast, in LAT− neurons that were surrounded by T cells, neither miRNAs nor the DNA of HSV-1, HSV-2, or varicella-zoster virus could be detected. These data indicate that the majority of LAT+ neurons is not directly controlled by T cells. However, miRNA expression in every latently infected neuron would provide an additional checkpoint before viral replication is initiated.
INTRODUCTION
Herpes simplex virus type 1 (HSV-1) is a human herpesvirus with a specific tropism for sensory neurons. After a primary infection of the oral cavity, the virus migrates along the axons of the trigeminal nerve to reach the cell bodies of sensory neurons within the trigeminal ganglion (TG), where it establishes life-long latency (1, 20). This latent state is characterized by the expression of only one prominent viral transcript, called latency-associated transcript (LAT). It accumulates in the nuclei of the infected neurons, where it can easily be visualized by applying in situ hybridization (21). In addition, there is evidence from animal models (3, 4, 7, 13, 17) and also from human postmortem studies (6) that viral immediate-early genes are expressed at very low levels during viral latency.
Latent HSV-1 infection in human TG is accompanied by immune cell infiltrates that are mainly composed of CD8+ T cells (23). This infiltration has previously been seen in TG of latently infected mice (16), where it has been demonstrated that most of the local CD8+ T cells are specific for HSV-1 and can block the virus from reactivation (11, 15, 19). This protective effect of T cells could be mediated partly by gamma interferon (5, 14). Moreover, granzyme B produced by local CD8+ T cells was shown to degrade the HSV-1 immediate-early protein ICP-4 in vitro (12). Since this viral protein is important for viral replication, lack of ICP-4 might stop reactivation of HSV-1 at a very early stage. Interestingly, the local granzyme B production was not accompanied by neuronal apoptosis. The CD8+ T-cell effector functions were demonstrated in ex vivo cultures of mouse TG (12, 15). It was also found in humans, the natural host of HSV-1, that CD8+ T cells isolated from HSV-1 latently infected human TG proliferate in reaction to protein derived from HSV-1 (29). Furthermore, HSV-1-positive human ganglia show an increased expression of inflammatory cytokines (6, 23). The infiltrating CD8+ T cells also express chemokine receptors and show features of clonal expansion, indicating an antigenic stimulus as the cause of the local T-cell proliferation (6).
LAT is believed to play a role in the establishment and maintenance of latency (3, 25) and reactivation (9, 18), but thus far no LAT-derived protein has been found. Recently, it has been shown that HSV-1 encodes several microRNAs (miRNAs), some of them originating from the LAT region (10, 26, 27). The discovery that HSV-1-encoded miRNAs have a regulatory capacity for viral genes helps to show how the virus itself might control its latency state. Two of these miRNAs were shown to reduce expression of the immediate-early proteins ICP0 and ICP4 in vitro (26), thereby potentially inhibiting viral reactivation from latency since these two immediate-early proteins are known to be major transactivators for viral gene expression. Further, ICP34.5, a neurovirulence factor (2), is a potential target for other viral miRNAs. In this way, LAT-encoded miRNAs may facilitate the establishment and maintenance of latency. The presence of the HSV-1-encoded miRNAs has been described in whole RNA from lytic infected cells and also latently infected mouse and human TG (27).
We hypothesized that by downregulating viral proteins, which may be potential viral candidate antigens for the infiltrating T cells, the viral miRNAs may reduce recruitment of antiviral CD8+ T cells to infected neurons. Therefore, we assessed single laser-microdissected neurons for the presence of viral miRNA in relation to LAT expression and immune cell infiltrates using a combination of in situ hybridization, immunohistochemistry, and quantitative reverse transcription-PCR (qRT-PCR). This concomitant analysis of viral miRNAs, LAT, viral DNA, viral mRNA, and T cells on the single-cell level helps to clarify to which proportion the viral miRNAs and host T cells may contribute to the maintenance of HSV-1 latency in human TG.
MATERIALS AND METHODS
The Ethics Committee of the Medical Faculty of the Ludwig Maximilian University of Munich approved the use of autopsy samples for the present study. TG of both sides were removed from 16 subjects 6 to 24 h after death. None of them had lesions suggestive of an active orolabial herpes infection or a history of cranial nerve disorders. Gender, age, and alphaherpesvirus infection state are listed in Table 1. Ganglia were embedded immediately in Jung tissue freezing medium (Leica Microsystems, Nussloch, Germany) and subsequently stored at −80°C. Frozen sections (10 μm) were cut and mounted on positively charged slides (Superfrost Plus; Menzel, Braunschweig, Germany) for immunohistochemistry or on membrane-covered PET slides (Zeiss, Jena, Germany) for laser catapulting microscopy (LCM). Slides were subsequently stored at −20°C or at −80°C, respectively. Further, 10 30-μm tissue sections were collected for RNA extraction. Paraffin sections (4 μm) on positively charged slides were prepared from several TG after fixation in 4% buffered paraformaldehyde for 24 h and paraffin embedding.
Table 1.
Tissue sample overview used in the present study with alphaherpesvirus infection statea
| Subject | Gender | Age (yr)b | Tissue | Presence (+) or absence (−) of: |
|
|---|---|---|---|---|---|
| HSV-1 latency | VZV latency | ||||
| 1 | F | 52 | TG | + | + |
| 2 | M | 12 | TG | + | − |
| 3 | F | 85 | TG | + | ND |
| 4 | F | 71 | TG | + | + |
| 5 | M | 39 | TG | + | − |
| 6 | 5 wks | FG | − | − | |
| 7 | 5 wks | FG | − | − | |
| 8 | M | 56 | TG | + | + |
| 9 | M | 77 | TG | − | + |
| 10 | F | 80 | TG | + | + |
| 11 | M | 62 | TG | − | + |
| 12 | F | 61 | TG | + | − |
| 13 | M | 41 | TG | + | − |
| 14 | M | 78 | TG | + | + |
| 15 | M | 36 | TG | − | − |
| 16 | F | 32 | TG | − | + |
| 17 | M | 35 | TG | − | + |
| 18 | M | 34 | TG | + | + |
| 19 | F | 17 | TG | + | − |
| 20 | M | 29 | TG | − | − |
F, female; M, male; TG, trigeminal ganglia; FG, facial ganglia; ND, not done.
Expressed in years except as noted.
In situ hybridization and immunohistochemistry.
The 25-bp oligonucleotide probe (Dig-LAT probe [5′-CAT AGA GAG CCA GGC ACA AAA ACA C-3′]) used for the detection of the LAT was synthesized and labeled with digoxigenin (Eurofins MWG Synthesis GmbH, Ebersberg, Germany). An in situ hybridization for LAT, followed by CD3 immunohistochemistry, was carried out on TG sections as described previously (6, 22, 24). The polyclonal rabbit anti-human CD3 antibody (Dako Cytomation, Glostrup, Denmark) was detected by using a biotinylated goat anti-rabbit immunoglobulin antibody (Dako Cytomation), followed by Cy2-labeled streptavidin (Dianova, Hamburg, Germany) or horseradish peroxidase (HRP)-conjugated streptavidin (Dako Cytomation) and incubation in diaminobenzidine (DAB; Dako Cytomation).
The frequency of different T-cell neuron interaction patterns was assessed in paraffin sections and cryosections of 19 TG. These sections were concomitantly stained for LAT and CD3. The frequencies of the different T-cell neuron interaction patterns were analyzed by counting all of the neurons of one TG section under the microscope. To confirm the consistency of the pattern, five consecutive sections were stained for LAT, as well as CD3, and analyzed.
Varicella-zoster virus (VZV) latency was determined by staining TG sections for the VZV protein 62 as described in a previous study (23). In brief, frozen TG sections were incubated with the anti-VZV immediate-early gene 62 antibody (Chemicon [Millipore], Billerica, MA). Subsequently, the antibody was detected with a biotinylated rabbit anti-mouse immunoglobulin antibody (Dako Cytomation), followed by HRP-conjugated streptavidin (Dako Cytomation) and incubation in DAB (Dako Cytomation).
HSV-1 miRNA expression in human TG.
Total RNA was isolated from human ganglia with Qiazol (Qiagen, Hilden, Germany) and subsequently purified using an miRNeasy minikit (Qiagen).
For LCM, the tissue was covered with n-propanol to prevent drying and to inhibit RNase activity, and immediately transferred to a PALM Microbeam-Z microscope. Neurons meeting our criteria (positive or negative in situ signal for LAT; positive or negative for local CD3 T-cell clusters) were marked electronically. After evaporation of the n-propanol, five marked neurons were microdissected and laser pressure catapulted into single reaction tubes, which were immediately stored on dry ice. After microdissection of the neurons, it was visually verified that all five neurons were actually captured.
RNA from five pooled LCM cells per sample was prepared with a combination of the miRNeasy minikit and an RNeasy microkit (Qiagen). miRNAs were transcribed using a TaqMan miRNA reverse transcription kit (Applied Biosystems, Darmstadt, Germany) and miRNA specific stem-looped primers (26). These stem-looped primers bind to the miRNAs and generate a longer cDNA transcript in the RT reaction, which can easily be amplified in a normal qPCR.
qPCR was performed on the ABI 7900 (Applied Biosystems) using the qPCR core kit and uracyl N-glycosylase (both from Eurogentec, Cologne, Germany). Primers and probes for qPCR were designed as described recently (26). The total amount of transcribed RNA equivalent used per PCR was 0.8 ng. The miRNA let7a was used for normalization with the ΔCT method (26).
Viral DNA in single neurons.
DNA was extracted from five pooled microdissected neurons or T-cell infiltrates as described before (31). The copy numbers for HSV-1, HSV-2, or VZV DNA in single neurons were determined on the ABI 7900 cycler (Applied Biosystems) using an Artus HSV-1/2 PCR kit or an Artus VZV PCR kit (both from Qiagen), respectively. The mean efficiencies of the PCRs ranged between 96 and 105%. Titration of viral DNA revealed that 10 copies could be detected with a 100% recovery.
HSV-1 ICP0 mRNA expression in single neurons.
First, cDNA of whole TG was analyzed for the expression of ICP0 by nested PCR as described previously (6). Only positive TG were used for microdissection.
To preserve mRNA, membrane-covered PET slides (Zeiss, Jena, Germany) were stained for 5 min with a Cy3-labeled anti-CD8 antibody (clone LT8 [AbD Serotec, Dusseldorf, Germany] and the Cy3 MAb labeling kit [GE Healthcare, Chalfont St. Giles, United Kingdom]). Neurons surrounded by CD8+ T cells were microdissected and immediately stored on dry ice. To obtain sufficient amounts of mRNA, five neurons were pooled. For each of the pools, a multiplex nested LAT and ICP0 PCR was performed as previously described (6). The first round of amplification was carried out in one reaction tube for both products, whereas in the second round of PCR LAT and ICP0 were amplified separately. cDNA from HSV-1-infected Vero cells was used as a positive control. PCR products were separated by using a 2% agarose gel. The identity of the PCR products was verified by sequencing.
Statistics.
Statistical analysis was performed by using GraphPad Prism 5 and Microsoft Office Excel 2003. When Gaussian distributions of results were given, the two-sided Student t test was used. The results without Gaussian distributions were analyzed by the Mann-Whitney U test. Differences with a P value of 0.05 were considered statistically significant.
RESULTS
Frequency of different T-cell neuron interaction patterns.
Four different T cell neuron interactions can be envisioned: (i) LAT-positive neurons that are surrounded by T cells, (ii) LAT-positive neurons that are not surrounded by T cells, (iii) LAT-negative neurons that are surrounded by T cells, and (iv) LAT-negative neurons that are not surrounded by T cells (Fig. 1A). To quantify the different T-cell neuron interaction patterns, all neurons in one TG section of 19 different ganglia were counted. Only 0.15% ± 0.04% (mean ± the standard error of the mean [SEM]) of neurons showed a LAT hybridization signal and were surrounded by T cells. The majority of the LAT+ neurons, 1.13% ± 0.17% of the total neurons, were not surrounded by T cells. This means that only 13.16% of all LAT+ neurons were surrounded by T cells. Furthermore, 3.88% ± 0.49% of the neurons of one TG section showed surrounding T-cell infiltrates, although they were negative for LAT by in situ hybridization (Fig. 1B). Examination of consecutive sections showed the same frequencies of the different T-cell neuron interaction patterns. It is widely accepted that HSV-1 is the trigger for the infiltration of immune cells into the TG, but VZV is also known to establish latency in the sensory neurons of the human TG. To test the hypothesis that the T cells infiltrate the TG due to HSV-1 and not VZV, the occurrence of neurons surrounded by T cells was compared between latently HSV-1-infected and noninfected TG and latently VZV-infected and noninfected TG. HSV-1-infected TG show significantly higher amounts of neurons surrounded by T cells than HSV-1-negative TG (Student t test: P = 0.001). This is not the case for VZV (Fig. 1C). The percentage of LAT+ neurons overall also correlated with the percentage of neurons surrounded by T cells, meaning that TG with a high percentage of LAT+ neurons usually also exhibited more T-cell infiltrates (P = 0.0045, r2 = 0.4095). In TG negative for latent HSV-1, only scattered T cells were present.
Fig. 1.
(A) LAT in situ hybridization (dark nuclear staining) and anti-CD3 immunohistochemistry (brown surface staining) on a 4-μm paraffin section of human TG. All four different T-cell neuron interactions are depicted in this micrograph. Scale bar, 50 μm. (B) Percentage of neuronal patterns in latently HSV-1 infected human TG with range. The values are given as means ± the SEM of the total neurons of one TG section. All bars exhibit highly significant differences (Student t test: P < 0.005). (C) Percentage of neurons surrounded by T cells in latently HSV-1- or VZV-infected TG versus noninfected human TG. The difference in the percentage of neurons surrounded by T cells in latently HSV-1-infected ganglia versus non-HSV-1-infected ganglia is significant (Student t test, P = 0.001), whereas this is not the case with VZV-infected versus noninfected ganglia (Student t test, P = 0.472).
HSV-1 miRNA expression in human TG.
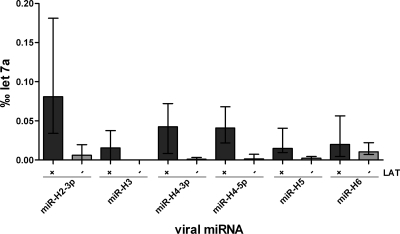
In the present study we examined 10 human TG from five subjects for the expression of HSV-1 miRNAs. Three subjects were latently HSV-1 infected, and two subjects were HSV-1 negative as determined by LAT in situ hybridization. In addition, two facial ganglia (FG) of two different HSV-1-negative cases were also assessed in the present study. The 10 TG and two FG were screened by qPCR for the expression of five HSV-1-encoded miRNAs (H2-3p, H3, H4-3p, H4-5p, H5, and H6). The results were normalized against miRNA let7a levels. All assessed miRNAs were present in the latently infected TG, except for miRNA H3 in one subject (Fig. 2). The two FG from HSV-1-negative cases showed results comparable to those for the HSV-1-negative TG.
Fig. 2.
Expression of HSV-1 miRNAs in latently HSV-1-infected versus noninfected human TG. The expression of the viral miRNAs is shown in ‰ let7a (median with interquartile range), a human housekeeping miRNA. All LAT+ TG show expression of viral miRNAs, whereas hardly any expression is seen in LAT− TG. The difference in the viral miRNA expression of latently HSV-1-infected versus noninfected TG is statistically significant for the miRNAs H2-3p, H4-3p, H4-5p, and H5 (P < 0.05 [Mann-Whitney U test]).
HSV-1 miRNA expression in single neurons.
On the cellular level of the TG it becomes apparent that only a minority of latently infected neurons are surrounded by T cells. Differential expression of viral miRNAs might contribute to this finding. Higher levels of viral miRNAs may lower the amount of viral antigen presented to T cells due to their ability to block transcription of immediate-early genes (26).
Therefore, we analyzed the expression of viral miRNAs in single neurons using a combination of in situ hybridization, immunohistochemistry, and LCM, followed by qPCR. Thus, the expression of viral miRNAs could be analyzed on the single-cell level in relation to LAT expression and T-cell infiltration.
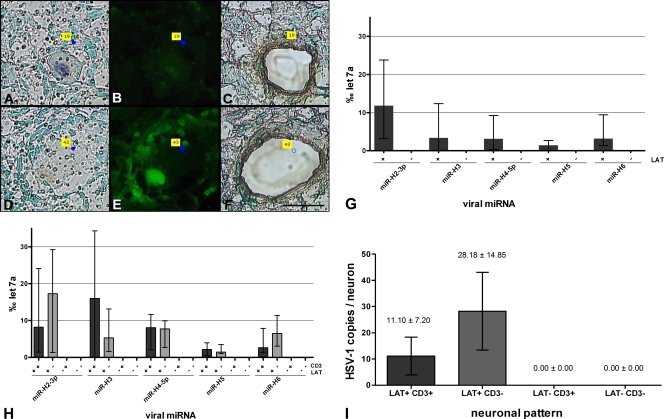
Frozen TG sections were stained for LAT and CD3+ T cells, and neurons were then extracted by laser microdissection (Fig. 3A to F). Five neurons from each group (LAT+ neurons with surrounding T cells, LAT+ neurons without surrounding T cells, LAT− neurons with surrounding T cells, and LAT− neurons without surrounding T cells) were pooled and analyzed by qRT-PCR for HSV-1 miRNAs. HSV-1 miRNAs were only expressed in LAT+ neurons and not in LAT− neurons (Fig. 3G). The expression level of viral miRNAs did not differ in LAT+ neurons surrounded or not surrounded by infiltrating T cells (Fig. 3H). In contrast to whole-tissue RNA (Fig. 2), on the single-cell level miRNA H6 showed an obvious difference in expression in LAT-positive versus LAT-negative neurons (Fig. 3G and H).
Fig. 3.
Analysis of single neurons. (A to F) Representative micrographs of TG sections on membrane-covered PET slides used for LCM. Panels A and D show human TG sections stained for LAT by in situ hybridization. The micrographs show a LAT+ (“19”) and a LAT− (“43”) neuron. Panels B and E show CD3 staining of the same TG sections showing neurons surrounded (“43”) or not surrounded (“19”) by T cells. Panels C and F show neurons that were microdissected. Magnification, ×400. Scale bar, 50 μm. (G and H) HSV-1 miRNA expression in single neurons. The results are given in ‰ let7a (median with interquartile range). In panel G, the expression of viral miRNAs in LAT+ versus LAT− neurons is shown. Viral miRNAs are only present in LAT+ neurons. The difference in the expression of viral miRNAs in LAT+ versus LAT− neurons is highly significant for all miRNAs (P < 0.001 [Mann-Whitney U test]). In panel H, HSV-1 miRNA expression in LAT+ and LAT− neurons with respect to T cells is shown. An abundant expression of viral miRNAs was detected in LAT+ neurons but not in LAT− neurons. There is no obvious difference in miRNA expression with regard to the T-cell infiltration. (I) Viral DNA in single microdissected neurons. The values are given as means ± the SEM of the HSV-1 DNA copies per neuron. HSV-1 DNA was only detected in LAT+ neurons. There is no significant difference between neurons surrounded or not surrounded by T cells. No HSV-2 or VZV DNA was detected in any of the neurons (data not shown).
No viral DNA in single LAT− neurons surrounded by T cells.
Since 95.51% of the neurons that were surrounded by T cells were negative for LAT and did not show any expression of viral miRNAs, these neurons were screened for the presence of viral DNA in order to determine the potential trigger for the immune cell infiltration. LAT+ and LAT− neurons, surrounded or not surrounded by T cells, were microdissected and assessed for the occurrence of HSV-1, HSV-2, and VZV DNA by qPCR. Further T-cell infiltrates that surrounded neurons were microdissected and analyzed. HSV-1 DNA was only detected in LAT+ neurons and not in LAT− neurons (Fig. 3I). All of the 12 LAT+ neuron pools were positive for HSV-1 DNA and all of the 16 LAT− neuron pools were negative for HSV-1 DNA. There was no obvious difference in the HSV-1 DNA copy number of LAT+ neurons surrounded or not surrounded by T cells (P > 0.05 [Mann-Whitney U test]). A broad distribution in the amount of HSV-1 DNA copies per neuron was observed (Fig. 3I). The HSV-1 DNA copy numbers ranged from 1 to 558 copies per neuron pool.
Neither HSV-2 nor VZV DNA was present in any of the neurons (data not shown). Further, no herpesviral DNA could be found in the T-cell infiltrates, which is in accordance with the neurotropism of the Alphaherpesviridae (data not shown).
HSV-1 ICP0 mRNA expression in single neurons.
To identify a potential antigenic trigger for the immune cell infiltration, neurons that were surrounded by CD8+ T cells were analyzed for the presence of the immediate-early gene ICP0 mRNA. ICP0 was chosen because it is a potent activator of gene expression with functions in reactivation, and it has also been reported to be present at low levels in latently infected human TG (6). Therefore, it may be a possible antigenic trigger to infiltrating T cells. Expression of ICP0 was found in whole RNA from two of six analyzed TG. Frozen sections from these two positive TG were used for microdissection of neurons surrounded by CD8+ T cells. A total of 16 pools of five neurons each from two different subjects were assessed for the presence of LAT and ICP0 mRNA. LAT transcripts were amplified from one of the neuron pools. This pool also showed an ICP0 amplification signal. Sequencing confirmed the identity of the transcripts.
DISCUSSION
The present study aimed at deciphering to what extent CD8+ T cells and viral miRNA are involved in the HSV-1 latency process in human sensory neurons. It has been assumed that CD8+ T cells and miRNAs are both implicated in the control of viral latency (15, 26). Different interaction patterns of these two mechanisms can be proposed: (i) latently infected neurons need both control mechanisms to keep the latent state, and (ii) latent infection is controlled in some neurons by miRNA in the others by T cells. To distinguish which interaction pattern occurs during HSV-1 latency in humans, TG were assessed by immunohistochemistry for the presence of T cells and by in situ hybridization for the presence of LAT, followed by laser microdissection of single neurons on the same histological section. Our investigation demonstrated that only a minority of the LAT+ neurons were surrounded by T cells. This is in line with previous findings from the mouse model and human TG (7, 23, 29). It can be assumed that these neurons surrounded by T cells represent foci of viral gene expression beyond the expression of LAT. This is supported by our demonstration of ICP0 and LAT in single neurons that were surrounded by T cells.
Surprisingly, many LAT− neurons were surrounded by T cells. To exclude that this finding is due to an artifact (e.g., neurons were not cut at the nuclei level, where LAT accumulates during latency), T cells and LAT were simultaneously stained on consecutive sections. These findings confirmed that T cells are frequently found around LAT-free neurons (data not shown). Furthermore, no HSV-1, HSV-2, or VZV DNA was present in any of the LAT− neurons, whereas all LAT+ neurons contained HSV-1 DNA. This is in contrast to earlier studies in humans (31) and mice (reviewed in reference 30) wherein LAT could be detected by PCR also in neurons that were found to be negative by in situ hybridization. These contradictory results could be explained by the use of frozen samples in the present study, a different probe for the in situ detection of LAT, and possibly a more specific PCR protocol.
It still remains unresolved why T cells surround LAT− neurons. One explanation could be that these or neighboring neurons were the origin of former herpesviral reactivations. This would be in line with reports demonstrating that memory T cells remain resident in the skin long after a resolved HSV-1 infection (8). The local cytokine milieu produced by antiviral T cells following repeated reactivations of HSV-1 might also keep other T cells, the so-called bystander T cells, in the ganglion (28). Finally, it cannot be definitely excluded that these neurons host other viruses or minuscule amounts of viral antigen which are not detectable with the techniques applied in the present study. However, it was shown in animal studies with LAT-deficient mutants that neurons with low DNA copy numbers are less likely to reactivate (18). Therefore, a prominent immune response around neurons with only little viral DNA would not be expected.
Besides immune cells, HSV-1-encoded miRNAs were considered as an additional control mechanism for viral latency. Using RNA extracted from whole TG, we observed the expression of miRNAs H2-3p, H3, H4-3p, H4-5p, H5, and H6 in latently infected TG. This is in accordance with the results of Umbach et al. (27). On the single-cell level, we could detect HSV-1 miRNA exclusively in LAT+ neurons. However, there was no significant difference in the expression of viral miRNAs in neurons that were or were not surrounded by T cells.
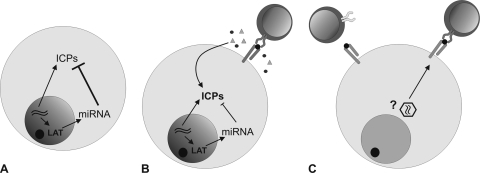
Based on these findings, we propose the following model for the control of HSV-1 latency in humans: in the majority of latently HSV-1-infected neurons, cellular and viral control mechanisms, such as the viral miRNAs, are sufficient to block the virus from reactivation. In a small percentage of latently infected neurons, the virus escapes these control mechanisms, and viral gene expression is initiated. This leads to the presentation of viral antigens on MHC. As a consequence, CD8+ T cells are attracted as a backup to keep the virus in the latent state by a noncytolytic mechanism (Fig. 4).
Fig. 4.
Proposed model of latent HSV-1 infection in human TG. (A) In the majority of latently HSV-1-infected neurons miRNA levels and cellular mechanisms are sufficient to block the virus from reactivation. (B) In a small percentage of latently infected neurons, the equilibrium is shifted toward viral gene production, and immediate-early proteins get translated. The CD8+ T cells infiltrate and keep the virus in the latent state. (C) The T cells surrounding LAT− neurons may be attracted by as-yet-unknown factors (former HSV-1 reactivations, a different virus, or nonspecific by the inflammatory milieu).
ACKNOWLEDGEMENTS
This study was supported by Deutsche Forschungsgemeinschaft grant TH 894 (to D.T., T.D., and K.D.) and Friedrich Baur Stiftung grant 68/10 (to K.H.).
We thank Katie Ogston for carefully copyediting the manuscript.
Footnotes
Published ahead of print on 27 July 2011.
REFERENCES
- 1. Baringer J. R., Swoveland P. 1973. Recovery of herpes simplex virus from human trigeminal ganglions. N. Engl. J. Med. 288:648–650 [DOI] [PubMed] [Google Scholar]
- 2. Brown S. M., MacLean A. R., Aitken J. D., Harland J. 1994. ICP34.5 influences herpes simplex virus type 1 maturation and egress from infected cells in vitro. J. Gen. Virol. 75:3679–3686 [DOI] [PubMed] [Google Scholar]
- 3. Chen S. H., Kramer M. F., Schaffer P. A., Coen D. M. 1997. A viral function represses accumulation of transcripts from productive-cycle genes in mouse ganglia latently infected with herpes simplex virus. J. Virol. 71:5878–5884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen S. H., et al. 2002. Neither LAT nor open reading frame P mutations increase expression of spliced or intron-containing ICP0 transcripts in mouse ganglia latently infected with herpes simplex virus. J. Virol. 76:4764–4772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Decman V., Kinchington P. R., Harvey S. A., Hendricks R. L. 2005. Gamma interferon can block herpes simplex virus type 1 reactivation from latency, even in the presence of late gene expression. J. Virol. 79:10339–10347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Derfuss T., et al. 2007. Presence of HSV-1 immediate-early genes and clonally expanded T cells with a memory effector phenotype in human trigeminal ganglia. Brain Pathol. 17:389–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Feldman L. T., et al. 2002. Spontaneous molecular reactivation of herpes simplex virus type 1 latency in mice. Proc. Natl. Acad. Sci. U. S. A. 99:978–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gebhardt T., et al. 2009. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat. Immunol. 10:524–530 [DOI] [PubMed] [Google Scholar]
- 9. Hill J. M., Sedarati F., Javier R. T., Wagner E. K., Stevens J. G. 1990. Herpes simplex virus latent phase transcription facilitates in vivo reactivation. Virology 174:117–125 [DOI] [PubMed] [Google Scholar]
- 10. Jurak I., et al. 2010. Numerous conserved and divergent microRNAs expressed by herpes simplex viruses 1 and 2. J. Virol. 84:4659–4672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khanna K. M., Bonneau R. H., Kinchington P. R., Hendricks R. L. 2003. Herpes simplex virus-specific memory CD8+ T cells are selectively activated and retained in latently infected sensory ganglia. Immunity 18:593–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Knickelbein J. E., et al. 2008. Noncytotoxic lytic granule-mediated CD8+ T cell inhibition of HSV-1 reactivation from neuronal latency. Science 322:268–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kramer M. F., Coen D. M. 1995. Quantification of transcripts from the ICP4 and thymidine kinase genes in mouse ganglia latently infected with herpes simplex virus. J. Virol. 69:1389–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu T., Khanna K. M., Carriere B. N., Hendricks R. L. 2001. Gamma interferon can prevent herpes simplex virus type 1 reactivation from latency in sensory neurons. J. Virol. 75:11178–11184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu T., Khanna K. M., Chen X., Fink D. J., Hendricks R. L. 2000. CD8+ T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neurons. J. Exp. Med. 191:1459–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu T., Tang Q., Hendricks R. L. 1996. Inflammatory infiltration of the trigeminal ganglion after herpes simplex virus type 1 corneal infection. J. Virol. 70:264–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Margolis T. P., et al. 2007. Spontaneous reactivation of herpes simplex virus type 1 in latently infected murine sensory ganglia. J. Virol. 81:11069–11074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Perng G. C., et al. 1994. The latency-associated transcript gene of herpes simplex virus type 1 (HSV-1) is required for efficient in vivo spontaneous reactivation of HSV-1 from latency. J. Virol. 68:8045–8055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sheridan B. S., Cherpes T. L., Urban J., Kalinski P., Hendricks R. L. 2009. Reevaluating the CD8 T-cell response to herpes simplex virus type 1: involvement of CD8 T cells reactive to subdominant epitopes. J. Virol. 83:2237–2245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stevens J. G., Cook M. L. 1971. Latent herpes simplex virus in spinal ganglia of mice. Science 173:843–845 [DOI] [PubMed] [Google Scholar]
- 21. Stevens J. G., Wagner E. K., vi-Rao G. B., Cook M. L., Feldman L. T. 1987. RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science 235:1056–1059 [DOI] [PubMed] [Google Scholar]
- 22. Theil D., et al. 2001. Prevalence of HSV-1 LAT in human trigeminal, geniculate, and vestibular ganglia and its implication for cranial nerve syndromes. Brain Pathol. 11:408–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Theil D., et al. 2003. Latent herpesvirus infection in human trigeminal ganglia causes chronic immune response. Am. J. Pathol. 163:2179–2184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Theil D., et al. 2003. Dually infected (HSV-1/VZV) single neurons in human trigeminal ganglia. Ann. Neurol. 54:678–682 [DOI] [PubMed] [Google Scholar]
- 25. Thompson R. L., Sawtell N. M. 1997. The herpes simplex virus type 1 latency-associated transcript gene regulates the establishment of latency. J. Virol. 71:5432–5440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Umbach J. L., et al. 2008. MicroRNAs expressed by herpes simplex virus 1 during latent infection regulate viral mRNAs. Nature 454:780–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Umbach J. L., Nagel M. A., Cohrs R. J., Gilden D. H., Cullen B. R. 2009. Analysis of human {alpha}-herpesvirus microRNA expression in latently infected human trigeminal ganglia. J. Virol. 83:10677–10683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van Lint A. L., et al. 2005. Latent infection with herpes simplex virus is associated with ongoing CD8+ T-cell stimulation by parenchymal cells within sensory ganglia. J. Virol. 79:14843–14851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Verjans G. M., et al. 2007. Selective retention of herpes simplex virus-specific T cells in latently infected human trigeminal ganglia. Proc. Natl. Acad. Sci. U. S. A. 104:3496–3501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wagner E. K., Bloom D. C. 1997. Experimental investigation of herpes simplex virus latency. Clin. Microbiol. Rev. 10:419–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang K., Lau T. Y., Morales M., Mont E. K., Straus S. E. 2005. Laser-capture microdissection: refining estimates of the quantity and distribution of latent herpes simplex virus 1 and varicella-zoster virus DNA in human trigeminal ganglia at the single-cell level. J. Virol. 79:14079–14087 [DOI] [PMC free article] [PubMed] [Google Scholar]