Abstract
California sea lions are one of the major marine mammal species along the Pacific coast of North America. Sea lions are susceptible to a wide variety of viruses, some of which can be transmitted to or from terrestrial mammals. Using an unbiased viral metagenomic approach, we surveyed the fecal virome in California sea lions of different ages and health statuses. Averages of 1.6 and 2.5 distinct mammalian viral species were shed by pups and juvenile sea lions, respectively. Previously undescribed mammalian viruses from four RNA virus families (Astroviridae, Picornaviridae, Caliciviridae, and Reoviridae) and one DNA virus family (Parvoviridae) were characterized. The first complete or partial genomes of sapeloviruses, sapoviruses, noroviruses, and bocavirus in marine mammals are reported. Astroviruses and bocaviruses showed the highest prevalence and abundance in California sea lion feces. The diversity of bacteriophages was higher in unweaned sea lion pups than in juveniles and animals in rehabilitation, where the phage community consisted largely of phages related to the family Microviridae. This study increases our understanding of the viral diversity in marine mammals, highlights the high rate of enteric viral infections in these highly social carnivores, and may be used as a baseline viral survey for comparison with samples from California sea lions during unexplained disease outbreaks.
INTRODUCTION
California sea lions (Zalophus californianus) have a population of approximately 240,000 and along with seals and walruses are members of the subgroup Pinnipedia in the suborder Caniformia in the order Carnivora. They inhabit mainland shorelines and coastal islands along the west coast of North America and migrate along the coast during the nonbreeding season. California sea lions are strict carnivores, eating a variety of marine prey, including more than 50 species of fishes and cephalopods. Sea lion pups start eating fish at about 5 months of age, in addition to their mother's milk; are weaned at 10 to 12 months old; and can live up to 15 to 25 years. California sea lions are gregarious animals, forming large rookeries at breeding sites, and aggregate at high densities on haul-out sites (7).
California sea lions share beaches and coastal waters with humans, often resting on human-made structures such as docks and boats, and are affected by pathogens and chemicals that enter coastal waters through runoff and sewage outfalls (5). Features of California sea lions, including their large population, wide geographic distribution and migration, gregarious nature, long life span, and shared environment with humans, may favor the transmission of viruses among themselves and to and/or from humans and other mammals.
A commonly reported sea lion virus is San Miguel sea lion virus (SMSV), a calicivirus in the genus Vesivirus. SMSV was first isolated from California sea lions from San Miguel Island in 1972 (53). SMSV causes vesicular lesions of the skin and mucosa, abortion, pneumonia, and encephalitis in sea lions and is transmissible to swine, generating a disease identical to that caused by vesicular exanthema of swine virus (VESV), a very closely related calicivirus (43, 65). The epidemics of VESV in North America from the 1930s to 1950s were shown by classical virological investigations to be serotypes of marine origin (56, 57, 65). SMSV was also found in vesicular lesions in humans, and antibodies were detected in blood donors (54, 55).
Canine distemper virus (CDV), a paramyxovirus of the genus Morbillivirus, was first described in 1905 (48). CDV most commonly affects dogs and causes gastrointestinal and respiratory symptoms as well as neurological symptoms. It also infects other domestic and wild carnivores, including ferrets, mink, foxes, and raccoons (38). CDV infection is not confined to terrestrial host species and has caused significant problems in marine mammals in the past 2 decades. It was identified as the cause of death of several thousand Baikal seals (Phoca sibirica) in 1988 (22) and 10,000 Caspian seals (Phoca caspica) in 2000 (30). CDV was also detected in the brain tissue of one captive California sea lion that died of unknown causes in 1995 in Europe (4).
Besides SMSV and CDV, viruses detected in California sea lions also include astrovirus (AstV) (50), polyomavirus (15), anellovirus (45), gammaherpesvirus (10, 32, 36), parapoxvirus (46, 47), retrovirus (31), and adenovirus (18). Otarine herpesvirus 1 is considered a possible contributing factor to the unusually high occurrence of tumors in California sea lions (10, 32, 44). A parapoxvirus was isolated from cutaneous nodular lesions, and the prevalence of antiparapoxviral antibodies in 761 free-ranging California sea lions was 91% (46, 47).
In this study, we used next-generation sequencing to get a comprehensive view of the fecal viral populations from wild and temporarily captive California sea lions. We report previously uncharacterized California sea lion viruses, including astroviruses, picornaviruses, bocaviruses, sapoviruses, and other viruses. These results provide a baseline for the current enteric viral burden in this marine mammal species that can be compared to later virome surveys to detect alterations associated with changes in their health or population size.
MATERIALS AND METHODS
Animal specimen collection.
Fecal specimens from three groups of California sea lions were collected by The Marine Mammal Center (TMMC) during July to October 2010. A total of 47 fecal specimens were analyzed. One group consisted of feces from 14 pups (3 months old) on San Miguel Island, CA. A second group consisted of feces from 19 juveniles (2 to 3 years old) also on San Miguel Island, CA. A third group consisted of samples from 14 California sea lions (>1 year old) being rehabilitated at TMMC for reasons including malnutrition, domoic acid toxicosis, leptospirosis, cancer, pneumonia, entanglement, and trauma (23). Fecal specimens were stored in small plastic bags and frozen at −80°C. Sample collection was performed under Marine Mammal Protection Act (MMPA) permit no. 932-1905-00/MA-009526 while animals were handled for veterinary examination.
Sample preparation and viral nucleic acid extraction.
Fecal samples were processed as previously described (62). Briefly, fecal samples were resuspended by vigorous vortexing in Hanks' buffered saline solution (Gibco BRL) at a concentration of ∼0.5 g/ml. The stool suspension was then centrifuged at 10,000 × g for 3 min, and the supernatant was filtered through a 0.45-μm filter (Millipore) to remove bacteria or cellular debris. The viral-particle containing filtrates were digested with a mixture of DNases and RNases to remove unprotected (not in viral capsids) nucleic acids (1). Viral RNA and DNA were then extracted by using the QIAamp viral RNA Minikit (Qiagen). Extracted viral nucleic acids were protected from degradation by the addition of 40 U of RNase inhibitor (Fermentas) and stored at −80°C for future use.
Library construction and pyrosequencing.
Combined viral RNA and DNA libraries were constructed by random PCR amplification using reverse transcription (RT) and PCR primers with degenerate 3′ ends as previously described (62). Random PCR products were pooled and separated on a 2% agarose gel, and DNA fragments from 500 bp to 1,000 bp were excised and extracted. The resulting purified product was prepared for sequencing by use of a GS FLX Titanium general library preparation kit (454 Life Science, Roche), and the library of single-stranded DNA fragments was sequenced on a single pyrosequencing gasket by use of a Genome Sequencer FLX instrument (454 Life Science, Roche), generating approximately 616,000 high-quality nucleotide sequence reads with an average length of 260 bases.
Bioinformatics.
The pyrosequencing reads were sorted into their fecal samples of origin according to their unique sequence tag (20 fixed bases of the random PCR primer). Primer sequences plus the adjacent 8 nucleotides were then trimmed from each read. Trimmed reads from each sample were assembled de novo by using the Mira assembly program (13), with a criterion of 95% identity or greater over ≥35 bp. The sequences greater than 100 bp were compared to the GenBank nonredundant nucleotide and protein databases using BLASTn and BLASTx, respectively. Sequences were classified into eukaryotic viruses, phages, bacteria, and eukaryotes based on the taxonomic origin of the best-hit sequence. An E value of 0.001 was used as the cutoff value of significant hits.
Phylogenetic analysis.
Reference viral sequences from different viral families were obtained from GenBank. Amino acid sequence alignments were generated by using ClustalW, implemented in MEGA 4 with the default settings (33). Aligned sequences were trimmed to match the genomic regions of the viral sequences obtained in this study, and phylogenetic trees were generated by MEGA4 using the neighbor-joining method with amino acid p-distances and 1,000 bootstrap replicates. The GenBank accession numbers of the viral sequences used in the phylogenetic analyses are shown in the trees.
Nucleotide sequence accession number.
High-quality sequences and contigs of veterinary sample metagenomes have been deposited in the short-read archive of GenBank under accession no. SRA044033.
RESULTS
Virome overview.
Viral nucleic acids enriched from 47 fecal samples from California sea lions were randomly amplified and pyrosequenced, generating >600,000 sequence reads. For each sample, sequence contigs were assembled and, together with singlets longer than 100 bases, were taxonomically classified based on the best BLAST scores (E < 0.001). Approximately 25% of the total reads showed detectable similarity to eukaryotic viral sequences, and 21% matched bacteriophage sequences.
The majority of the eukaryotic viruses detected belonged to DNA and RNA viral species not previously reported for marine mammals (Table 1). The most abundant eukaryotic viruses were bocaviruses, from the family Parvoviridae (39% of the total eukaryotic virus reads), followed by astroviruses, from the Astroviridae (30%); densoviruses, from the Parvoviridae (10%); dependoviruses, from the Parvoviridae (9%); caliciviruses, from the Caliciviridae (7%); picornaviruses, from the Picornaviridae (2%); and rotaviruses, from the Reoviridae (2%). The most prevalent viruses were astrovirus, bocavirus, and rotavirus, detected in 51%, 38%, and 28%, respectively, of the animals tested. Averages of 1.6, 2.5, and 2.1 distinct mammalian viruses were identified in the feces of individual pups, juveniles, and animals in rehabilitation, respectively.
Table 1.
Summary of mammalian viruses found in California sea lion feces
| Sample | Age of California sea lion | Total no. of reads | Eukaryotic virus(es) (no. of reads) |
|---|---|---|---|
| 1203 | Pup | 15,293 | Bocavirus (3) |
| 1207 | Pup | 5,258 | Astrovirus (879), rotavirus (1) |
| 1209 | Pup | 3,118 | Bocavirus (1) |
| 1211 | Pup | 10,315 | Bocavirus (505), anellovirus (4) |
| 1214 | Pup | 5,751 | None |
| 1215 | Pup | 7,981 | Astrovirus (76), bocavirus (17), sapelovirus (45), picobirnavirus (12) |
| 1218 | Pup | 10,829 | Bocavirus (6,743), dependovirus (1), sapovirus (16) |
| 1219 | Pup | 13,525 | None |
| 1222 | Pup | 6,312 | Astrovirus (47), cardiovirus (17) |
| 1223 | Pup | 15,554 | None |
| 1230 | Pup | 8,780 | Astrovirus (1), dependovirus (8) |
| 1234 | Pup | 9,895 | Astrovirus (6,091), bocavirus (35) |
| 1244 | Pup | 7,353 | Rotavirus (723), anellovirus (1) |
| 1249 | Pup | 2,150 | Bocavirus (1) |
| 1125 | Juvenile | 19,677 | Astrovirus (341) |
| 1136 | Juvenile | 17,100 | Astrovirus (2,500), bocavirus (5,917), dependovirus (5,709), sapovirus (79) |
| 1137 | Juvenile | 10,551 | Norovirus (6), rotavirus (916), anellovirus (7) |
| 1140 | Juvenile | 10,463 | Astrovirus (313), rotavirus (257), anellovirus (2) |
| 1141 | Juvenile | 8,430 | Astrovirus (133), bocavirus (1), rotavirus (7), anellovirus (3) |
| 1148 | Juvenile | 9,247 | Astrovirus (7,701), rotavirus (4), sapelovirus (2) |
| 1153 | Juvenile | 14,878 | Astrovirus (4,332), bocavirus (6,589), parvovirus (2), rotavirus (68), anellovirus (2), enterovirus/sapelovirus (1,716) |
| 1157 | Juvenile | 8,034 | Bocavirus (1), rotavirus (7) |
| 1162 | Juvenile | 15,735 | Bocavirus (12), enterovirus/sapelovirus (1,318) |
| 1166 | Juvenile | 5,151 | Sapovirus (1), anellovirus (2), sapelovirus (4) |
| 1169 | Juvenile | 9,403 | Astrovirus (1,987), dependovirus (1) |
| 1170 | Juvenile | 4,376 | Astrovirus (212), norovirus (49) |
| 1174 | Juvenile | 9,174 | Astrovirus (32), parvovirus (2), rotavirus (37), sapelovirus (1) |
| 1181 | Juvenile | 5,111 | Astrovirus (109), norovirus (1) |
| 1182 | Juvenile | 16,827 | None |
| 1185 | Juvenile | 6,319 | Astrovirus (115), bocavirus (1), norovirus (43), rotavirus (5) |
| 1187 | Juvenile | 20,882 | Bocavirus (7,779), dependovirus (7,292) |
| 1194 | Juvenile | 19,289 | Astrovirus (3) |
| 1199 | Juvenile | 17,383 | None |
| 9715 | Rehab. juvenile | 17,882 | Astrovirus (6,450), bocavirus (1), densovirus (612), picobirnavirus (1) |
| 9775 | Rehab. yearling | 16,798 | Bocavirus (1), sapovirus (9,672), cardiovirus (28) |
| 9795 | Rehab. juvenile | 9,993 | Astrovirus (1,564), enterovirus (5) |
| 9801 | Rehab. adult | 17,382 | Astrovirus (103) |
| 9805 | Rehab. subadult | 13,132 | Astrovirus (1), bocavirus (7,889), picornavirus (79), picobirnavirus (1), hepevirus (1) |
| 9806 | Rehab. adult | 13,747 | Calicivirus (199), picornavirus (8) |
| 9807 | Rehab. adult | 7,519 | None |
| 9810 | Rehab. yearling | 7,133 | Rotavirus (3) |
| 9813 | Rehab. adult | 11,386 | Astrovirus (65), vesivirus (3) |
| 9814 | Rehab. adult | 14,920 | None |
| 9816 | Rehab. juvenile | 14,631 | Astrovirus (1), |
| 9822 | Rehab. juvenile | 29,857 | Astrovirus (8,615), bocavirus (19,140), dependovirus (2), sapovirus (1), rotavirus (4), picobirnavirus (61) |
| 9828 | Rehab. yearling | 18,610 | Parvovirus (253), papillomavirus (1) |
| 9830 | Rehab. juvenile | 3,497 | Rotavirus (76), picobirnavirus (1), asfarvirus (5) |
Comparison of viruses in different sea lion groups.
The fecal viral communities of 14 unweaned pups (3 months old), 19 juveniles (2 to 3 years old), and 14 Californian sea lions in rehabilitation for various symptoms (>1 year old) were then compared (Fig. 1). The percentages of eukaryotic virus reads compared to the total number of sequences read were 12%, 24%, and 35% for pups, juveniles, and animals in rehabilitation, respectively. The percentage of eukaryotic virus reads compared to total virus reads (i.e., including prokaryotic viruses) was lowest for the pups (22%), intermediate for wild juveniles (47%), and highest for the animals in rehabilitation (88%) (Fig. 1A). The most abundant bacteriophage-like sequences found were those with similarities to the major double-stranded DNA podoviruses, siphoviruses, and myoviruses and the single-stranded DNA microviruses. The phage community in the pups had the most complex composition, with siphoviruses, podoviruses, myoviruses, and microviruses at 42%, 20%, 5%, and 26% of the total phage reads, respectively, while the phages in juveniles and animals in rehabilitation were less diverse, with 89% and 73% of their phage reads being related to microviruses (Fig. 1B). Using ≥80% of the total viral reads as a criterion, phages dominated in 11/14 (79%) pups, 12/19 (63%) juveniles, and 5/14 (36%) animals in rehabilitation, while eukaryotic viruses dominated in 2/14 (14%) pups, 5/19 (26%) juveniles, and 6/14 (49%) animals in rehabilitation.
Fig. 1.
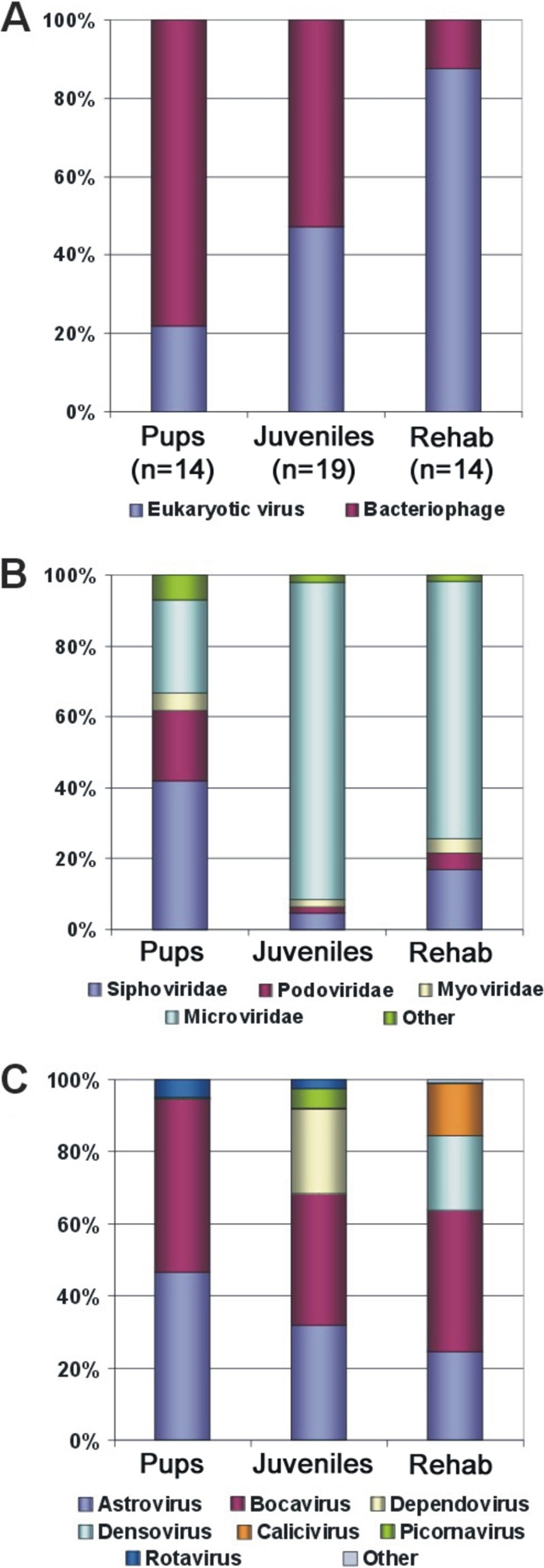
Virome comparisons for three California sea lion groups based on BLASTx comparison to the GenBank nonredundant database (E value of <0.001). (A) Percentage of virus-like sequence reads with similarity to bacteriophages and eukaryotic viruses. (B) Percentage of phage-related sequences in different viral families. (C) Percentage of eukaryotic virus-related sequences in different viral groups.
For all three animal groups, astroviruses and bocaviruses constituted the majority of the eukaryotic virus community (Fig. 1C). Among eukaryotic virus reads, the percentages for astroviruses and bocaviruses combined were 95%, 68%, and 64% for pups, juveniles, and animals in rehabilitation, respectively. Compared with the other two groups, the juvenile group had higher percentages of dependovirus (23% of the total eukaryotic virus reads) and picornavirus (6%) reads, while the group in rehabilitation had higher percentages of calicivirus (14%) and densovirus (21%) reads (Fig. 1C).
Astrovirus.
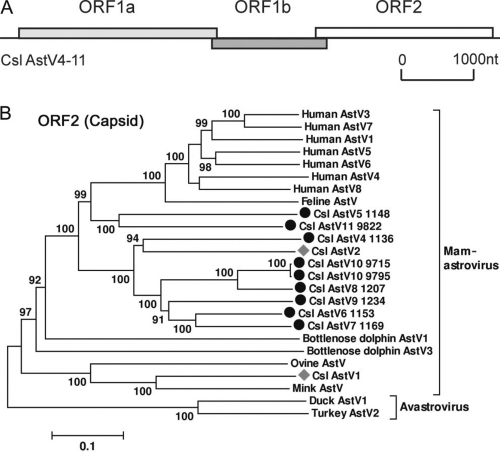
Astroviruses are positive single-stranded RNA viruses with a genome of 6.4 to 7.3 kb and have been identified in a wide variety of terrestrial mammals and birds (42). Human astrovirus is a significant cause of acute pediatric gastroenteritis (24). Recently, astroviruses have also been detected in marine mammals, including California sea lion, Steller sea lion (50), bottlenose dolphin, killer whale, and minke whale (64). In this study, astroviruses were present in 24 of the 47 California sea lion samples and had high titers in 9 samples (>500 sequence reads). Sequence assembly within each samples generated 9 near-complete genome sequences, covering 80% to 99% of genome length (GenBank accession no. JN420351 to JN420358). As the astroviruses in samples 9715 and 9795 shared 99% nucleotide similarity with each other, a total of 8 astrovirus species were identified and temporarily named California sea lion astrovirus 4 (Csl AstV4) to Csl AstV11.
As typical mamastroviruses, Csl AstV4 to Csl AstV11 had three putative open reading frames (ORFs) encoding nonstructural proteins with ORF1a and ORF1b and a structural protein with ORF2 (Fig. 2A and Table S1 in the supplemental material). The conserved protease motifs, RNA-dependent RNA polymerase (RdRp) motifs, and a ribosomal frameshift signal (AAAAAAC) in the ORF1a/1b overlap region were found in all Csl AstVs.
Fig. 2.
(A) Genome organization of California sea lion astroviruses (Csl AstVs). (B) Phylogenetic analysis of California sea lion astroviruses. Trees are based on complete capsid (ORF2) proteins. The novel Csl AstV4 and Cs2 AstV11 are marked by black circles, and the previously reported Csl AstV1 and Csl AstV2 are marked by gray diamonds. nt, nucleotides.
To determine the divergence in sequence among Csl AstV species and those of other AstVs, sequence alignments of ORF2 (encoding capsid) and ORF1b (encoding RdRp) were performed, and neighbor-joining trees were generated. The tree for the capsid protein confirmed that Csl AstV4 to Csl AstV11 were novel AstV species, having less than 60% amino acid similarity with the recently characterized Csl AstV1 to Csl AstV3 (50) and other AstVs, showing a high level of diversity among AstVs from a single host species (Fig. 2B). The tree for the RdRp region revealed that California sea lion astroviruses formed three genetic clusters (see Fig. S1 in the supplemental material). The use of available RdRp sequences revealed that Csl AstVs 3, 5, and 11 were the mamastroviruses most closely related to the clade comprised of human AstV1 to AstV8, sharing as high as 92% amino acid similarity in the RdRp region.
Picornavirus.
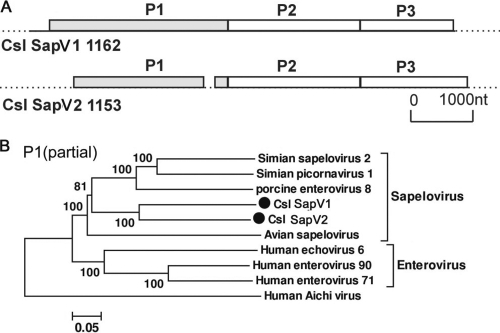
Picornaviruses are small, nonenveloped, positive-sense, single-stranded RNA viruses with a genome size of 7.1 to 8.9 kb, encoding a single polyprotein (58). Here, we found picornavirus sequences in 11 California sea lion samples that were abundant (>1,000 reads) in 2 juvenile samples (samples 1153 and 1162) from San Miguel Island. Sample 1162 contained two distinct picornaviruses, one having 99% nucleotide similarity to the strain in sample 1153. Assembly of the viral reads in samples 1153 and 1162 generated long contigs of 2 distinct picornaviruses, each covering more than 70% of the genome (GenBank accession no. JN420367 and JN420368). In sample 1162, a large 6.5-kb sequence spanned a partial 5′ untranslated region (5′UTR); the complete leader protein L, P1, and P2 regions; and a partial P3 region, while in sample 1153, two fragments of 2 kb and 3.9 kb yielded a partial P1 region, the complete P2 region, and a partial P3 region (Fig. 3A).
Fig. 3.
(A) Genome organization of California sea lion sapeloviruses (Csl SaVs). (B) Phylogenetic analysis of the partial P1 region of sapeloviruses, including representative enteroviruses.
According to the International Committee on Taxonomy of Viruses (ICTV) (http://www.picornastudygroup.com/definitions/genus_definition.htm), the members of a picornavirus genus should share >40%, >40%, and >50% amino acid similarity in their P1, P2, and P3 regions, respectively. As the P1, P2, and partial P3 regions of the picornavirus in sample 1162 shared 46%, 39%, and 52% amino acid similarities with its closest relative, simian sapelovirus 2, the virus was considered a novel species in the genus Sapelovirus. The P1, P2, and P3 regions of the sapelovirus in sample 1153 shared 60%, 70%, and 75% amino acid similarity with its closest relative, the sapelovirus in sample 1162. Therefore, we temporarily named these two novel picornaviruses California sea lion sapelovirus 1 (Csl SapV1) and Csl SapV2. Known hosts for sapeloviruses therefore include pigs, monkeys, birds, and now Californian sea lions.
The genome organization of Csl SapV1 is typical of a picornavirus, with a single large ORF encoding a near-complete polyprotein of 2,054 amino acids (aa). The polyprotein comprised a putative leader protein; the capsid proteins VP4, VP2, VP3, and VP1; and nonstructural proteins 2A to 2C and 3A to 3D (partially sequenced). The L protein was 94 aa and did not have significant similarity to any other protein. Phylogenetic analyses were performed on the partial P1 region and confirmed that Csl SapVs fell into the sapelovirus genus and were located close to the basal nodes of this clade, with a preferred association with mammalian sapeloviruses (Fig. 3B). Phylogenetic analyses based on the partial 3D region yielded a similar topology (data not shown).
Calicivirus.
Caliciviruses are single-stranded, positive-sense, nonenveloped RNA viruses with a genome size of 7.3 to 8.3 kb. The family Caliciviridae includes multiple genera, including Sapovirus, Vesivirus, Norovirus, and Lagovirus (20). Among caliciviruses, only vesiviruses have been reported in marine mammals (41, 53). We detected calicivirus sequences in 10 California sea lion samples. The vesivirus identified in one TMMC rehabilitation sample was San Miguel sea lion virus (SMSV), with approximately 90% nucleotide similarity to a previously sequenced isolate. The other caliciviruses identified here were novel sapoviruses (4 samples) and noroviruses (4 samples) and an unclassified calicivirus (1 sample).
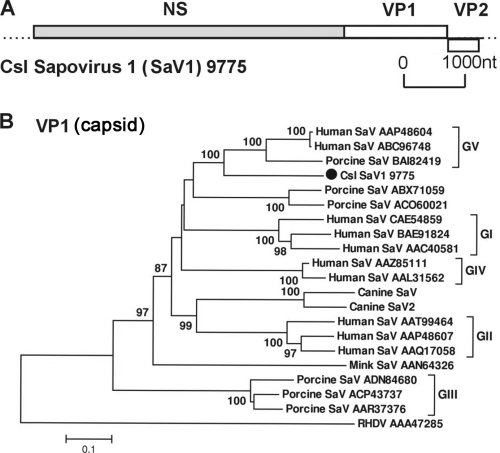
Sapoviruses (SaVs), previously known as Sapporo-like viruses, are important enteric pathogens that cause diarrhea in humans, pigs, dogs, and mink (14, 39, 61). Sapovirus sequences were found at a high abundance of 9,672 reads in a California sea lion with severe osteomyelitis and nephrolithiasis in rehabilitation at TMMC (sample 9775). One near-complete (7.4-kb) genome from sample 9775 and one partial genome (2.2 kb) from sample 1136 could be assembled (GenBank accession no. JN420369 to JN420370). These sapoviruses were temporarily named California sea lion sapovirus 1 (Csl SaV1) and Csl SaV2.
Csl SaV1 has the typical SaV genome organization, with two major ORFs (Fig. 4A). The near-complete ORF1 encodes a polyprotein of 2,259 aa that can be theoretically cleaved into the nonstructural protein NTPase, a 3C-like protease, an RdRp, and a major capsid protein (VP1; 563 aa). ORF2 encoded a minor structural protein (VP2; 167 aa). Csl SaV1 shared as high as 57% amino acid similarity in the polyprotein region with human SaVs, while the VP2 protein showed an amino acid similarity of 65% with human and swine SaVs. The complete VP1 region showed the highest similarity (65% amino acid similarity) with human SaVs. The phylogenetic analysis of the complete VP1 protein of representative SaVs in the GenBank database confirmed that Csl SaV1 belonged to that genus and was most closely related to SaV genogroup V (Fig. 4B). The partial VP1 region of 204 aa from Csl SaV2 shared the highest similarity (72% amino acid similarity) and phylogenetically grouped with human SaVs in genogroup II (see Fig. S2 in the supplemental material).
Fig. 4.
(A) Genome organization of California sea lion sapovirus 1 (Csl SaV1). (B) Phylogenetic analysis of the VP1 regions of Csl SaV1 and sapoviruses from different genogroups. GenBank accession numbers are shown at the end of the branches. GV, genogroup V; RHDV, rabbit hemorrhagic disease virus.
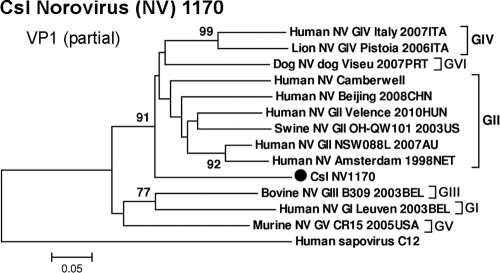
Low titers of noroviruses (<50 sequence reads) were detected in 4 fecal samples from California sea lions. Norovirus is one of the leading causes of human viral gastroenteritis and a major contributor to cases of food-borne illness worldwide. The contamination of water ecosystems (25, 51, 63) and seafood (17, 60) by norovirus has been widely reported. The norovirus genome has three overlapping ORFs. ORF1 encodes 6 nonstructural proteins, including the viral polymerase RdRp, while ORF2 encodes the capsid protein VP1, and ORF3 encodes a minor structural protein, VP2 (20). The norovirus sequences detected here were small fragments of different genome regions. For sample 1170, phylogenetic analyses based on the amino acid sequences of a 399-bp RdRp region (GenBank accession no. JN420373) (see Fig. S3 in the supplemental material) and a 319-bp VP1 region (GenBank accession no. JN420374) (Fig. 5) showed that the newly discovered sea lion norovirus (Csl NV1170) was most related to genogroup II noroviruses, sharing >70% amino acid similarity.
Fig. 5.
Phylogenetic analysis of California sea lion norovirus (Csl NV1170) and representatives from different genogroups based on the amino acid sequence of the partial VP1 region.
Rotavirus.
Rotaviruses are nonenveloped, double-stranded RNA viruses consisting of 11 genomic segments (0.6 to 3.3 kb) with a total genome size of approximately 18 kb. The genus Rotavirus belongs to the family Reoviridae and contains 7 serologically distinct species (A to G). Rotaviruses have a triple-layered capsid structure made of 6 proteins (VP1 to VP4, VP6, and VP7). Rotaviruses are the most common cause of acute gastroenteritis in infants and young children and are common in a wide variety of terrestrial mammals and birds (49). A recent study reported that rotavirus antibodies were detected in approximately 20% of the serum samples from Galapagos sea lion pups (n = 125) and Galapagos fur seal pups (n = 22). Rotavirus RNA was detected in 1 out of 18 fecal sample from Galapagos sea lion pups (16).
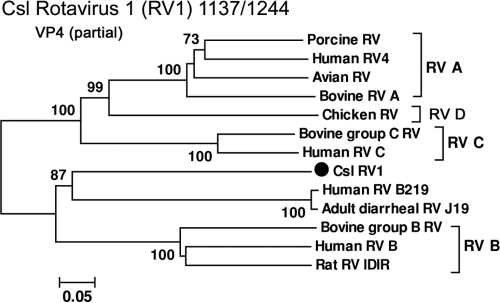
In this study, rotavirus sequences were identified in 2 out of 14 samples from 3-month-old pups and 11 out of 27 samples from yearling/juvenile sea lions. Sequence assemblies produced fragments of different genomic regions, most of them too short to be phylogenetically informative. The rotavirus sequences from two samples (samples 1137 and 1244, each yielding >500 reads) shared 99% nucleotide similarity with each other. This rotavirus was temporally labeled California sea lion rotavirus 1 (Csl RV1). Phylogenetic analyses were performed on the amino acid sequences of a partial VP4 sequence (∼900 bp) and a partial VP2 sequence (∼500 bp) from sample 1137/1244 (GenBank accession no. JN420375 to JN420378). The outer capsid protein, VP4, and inner capsid protein, VP2, have both been used to define the rotavirus group/species genetically (16). Csl RV1 VP4 was closest to adult diarrheal rotavirus J19/B219, with a preferred association with group B lineages (Fig. 6). A similar topology was seen with the partial VP2 region (see Fig. S4 in the supplemental material).
Fig. 6.
Phylogenetic analysis of California sea lion rotavirus 1 (Csl RV1) and representative rotaviruses from other species based on the amino acid sequence of the partial VP4 region.
Bocavirus.
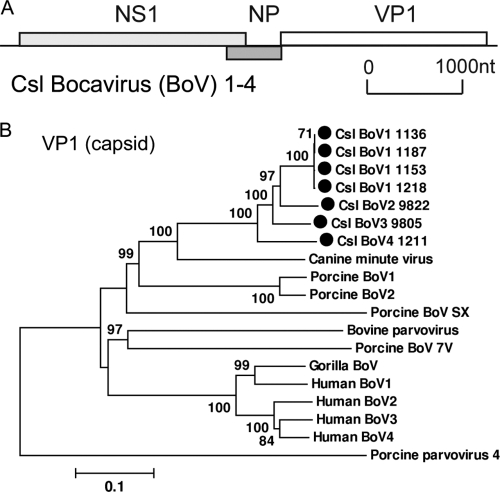
Members of the genus Bocavirus from the family Parvoviridae are small, nonenveloped, autonomously replicating, single-stranded DNA viruses with a genome length of about 5.4 kb (59). Bocaviruses were initially discovered in the 1960s with two species, bovine parvovirus and canine minute virus (37). Human bocaviruses are newly recognized human parvoviruses first reported in 2005 (2) and have been associated with respiratory tract disease and, possibly, gastroenteritis. Related species of human bocaviruses in feces have since been reported and associated with gastroenteritis (3, 26, 29). Recently, bocavirus species were also identified in swine (6, 12), gorilla (27), and chimpanzee (52). Here, bocaviruses were identified in 18 of the 47 California sea lion samples and were abundant in 7 samples (>500 reads). The assembly of each sample generated 6 near-complete genome sequences (5.1 to 5.4 kb) and 1 partial genome (2 kb) (GenBank accession no. JN420360 to JN420366). As the bocaviruses in four samples (samples 1136, 1153, 1187, and 1218) were nearly identical (>99% nucleotide similarity), a total of 4 bocavirus species were identified and temporarily named California sea lion bocavirus 1 (Csl BoV1) to Csl BoV4.
The genome organization of the Csl BoVs was similar to that of the other known bocaviruses (Fig. 7A). It is predicted to contain three major ORFs, encoding the nonstructural proteins NS1 and NP1 and the structural protein VP1. The NS1 protein was 793 aa for Csl BoV1, Csl BoV2, and Csl BoV4 and 802 aa for Csl BoV3. Conserved motifs associated with rolling-circle replication, helicase, and ATPase were present in NS1. The NP1 protein encoded by the middle ORF, a unique feature of bocaviruses, was 193 aa in all 4 Csl BoVs. The VP1 protein was 719 aa for Csl BoV1, Csl BoV3, and Csl BoV4 and 718 aa for Csl BoV2. Csl BoVs were most closely related to canine minute virus, showing 54%, 60 to 64%, and 64 to 67% amino acid similarities in the NS1, NP, and VP1 regions, respectively. A phylogenetic analysis of the entire VP1 protein was performed to determine the relationship between Csl BoV1 to Csl BoV4 and other bocaviruses. All Csl BoVs clustered together and were closest to canine minute virus (Fig. 7B).
Fig. 7.
(A) Genome organization of California sea lion bocaviruses (Csl BoVs). (B) Phylogenetic analysis of Csl BoVs and representative bocaviruses based on the complete VP1 protein.
Dependovirus.
The genus Dependovirus belongs to the family Parvoviridae and contains small, nonenveloped, single-stranded DNA viruses with a genome length of about 4.7 kb (59). Dependoviruses are mostly replication-defective adeno-associated viruses (AAVs), but some autonomous avian parvoviruses have also been classified into this genus (66). Dependoviruses were found in human and several other mammalian species, avian species, and amphibian species and are considered commensal viruses (21). Here, AAVs were identified in 6 California sea lion samples and were very abundant (>5,000 reads) in 2 juvenile samples from San Miguel Island. The assembly of each sample generated 2 near-complete genome sequences (∼4.4 kb) (GenBank accession no. JN420371 and JN420372), sharing 99% nucleotide similarity with each other. We temporarily named the novel dependovirus California sea lion adeno-associated virus 1 (Csl-AAV1).
The genome organization of Csl-AAV1 was similar to those of other known AAVs, with two large ORFs (see Fig. S5A in the supplemental material) encoding the putative nonstructural (Rep) and capsid (VP) proteins, respectively. Multiple Rep and VP proteins may be produced by alternative initiation or mRNA splicing. The left ORF encoded a putative Rep1 protein of 600 aa, which showed 64% amino acid similarity to bovine and caprine AAVs. The putative VP1 protein consisted of 718 aa, showing the highest similarity (63% amino acid similarity) with AAV11 from cynomolgus monkey. Phylogenetic analyses of the VP1 protein revealed that Csl-AAV1 fell inside the mammalian AAV clade and was most closely related to the bovine AAV cluster (see Fig. S5B in the supplemental material).
DISCUSSION
This study describes the composition of the viral communities in the feces of three groups of California sea lions. Pups showed the smallest overall number of eukaryotic virus reads and the smallest ratio of eukaryotic virus/bacteriophage sequences, possibly reflecting protection by maternal antibodies in these approximately 3-month-old animals. Diseased animals in rehabilitation showed the largest overall number of eukaryotic virus reads and the largest ratio of eukaryotic virus/phage sequences, an observation that may reflect increased host susceptibility to enteric infections in these weakened animals.
Sixty-seven percent of phage-like reads in unweaned pups were related to siphoviruses, podoviruses, and myoviruses, similar to the phage compositions of human and equine feces reported by previous fecal viral metagenomic studies (8, 9, 11), showing that the majority of the phages (∼70%) were related to the tailed bacteriophages of the order Caudovirales. In contrast, the majority of the phage-like reads in juveniles (89%) and in diseased animals in rehabilitation (73%) were related to the single-stranded DNA microviruses. The less diverse phage composition in juveniles and animals in rehabilitation may reflect a similar change in their gut bacterial population.
All three animal groups showed a high level of coinfections, with averages of 1.6 distinct mammalian viruses for pups, 2.5 for juveniles, and 2.1 for animals in rehabilitation. This high level of coinfections with mammalian viruses may be an underestimate, and even deeper sequencing of the viral nucleic acids may have revealed an even greater diversity of viruses shed at lower levels. Whether the high number of viruses detected per animal is the result of frequent but rapidly resolving infections or of less frequent infections with long-term viral shedding, as seen, for example, with human bocavirus (40) will require analyses of longitudinally collected samples. Differences in viral load as reflected by the numbers of sequence reads might also reflect differences in the immune and health statuses of the hosts. The unweaned pups may have been partially protected from infections by maternal antibodies, whereas the animals in rehabilitation may have had reduced immune responses due to their concurrent diseases.
The presence of some eukaryotic viruses may also reflect the host diet. For example, feces from insectivorous bats contain a significant fraction of insect viruses and plant viruses (19, 35), and porcine circovirus and plant viruses can be detected in human feces (34, 67). Densoviruses, known to infect insects and crustaceans, were found exclusively in 6/14 sea lions in rehabilitation, which may be attributable to differences in the diet of captive versus wild sea lions, with the former being fed frozen herring (at times contaminated with nematode larvae and insects) rather than a variety of prey. A few other virus-like sequences of possible dietary origin were also detected in two animals in rehabilitation, namely, 2 nodavirus-related sequences (nodaviruses are known to infect insects and fishes) and 243 sequences which assembled into a >3-kb contig. This contig showed 23 and 34% amino acid identities with the capsid and nonstructural genes, respectively, of calhevirus, an unclassified member of the order Picornavirales thought to infect insects (28). The relative paucity of eukaryotic viruses from expected food sources may be due in part to the low number of genetically characterized fish and cephalopod viruses relative to insect or plant viruses, making their detection by sequence similarity more difficult. The still-nursing pups are also expected to be less exposed to viruses from solid foods.
We therefore detected viruses of mammalian origin belonging to multiple RNA and DNA virus families in California sea lion feces. Except for SMSV, none of these viruses had close homologues among previously described viruses. The sapelovirus, sapovirus, norovirus, bocavirus, and dependovirus sequences are the first ones from a marine mammal host to be reported.
Astroviruses had the highest prevalence and were found in 51% of the California sea lion fecal samples. A greater level of astrovirus genetic diversity than that previously reported (Csl AstV1 to Csl AstV3) (50) was noted here (Csl AstV4 to Csl AstV11) in this small population sampling. Bocaviruses showed the second highest prevalence, with 38% of animals being infected with diverse variants. Overall, astroviruses and bocaviruses were the most abundant eukaryotic viruses detected, consisting of approximately 70% of the total eukaryotic virus reads. Whether these common viral infections are always commensal or can at times be pathogenic may depend on the overall health and immune status of the host and the presence of coinfections.
Our study provides an overview of the fecal virome of the California sea lion and significantly increases the diversity of viruses known to infect marine mammals, which now includes sapeloviruses, sapoviruses, noroviruses, bocavirus, and dependovirus. Characterization of the current baseline fecal virome of the California sea lion will help monitor future changes in its composition, which may be associated with disease outbreaks or population declines (22, 30, 57, 65).
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge NHLBI grants R01HL083254 and R01HL105770, the BSRI for sustained support to E.D., and NSF award CNS-0619926 to the Bio-X2 cluster at Stanford University for computer resources.
We thank Jennifer Soper and Denise Creig from TMMC for sample collection and Robert DeLong for logistic support on San Miguel Island.
Footnotes
Supplemental material for this article may be found at http://jvi.asm.org/.
Published ahead of print on 27 July 2011.
REFERENCES
- 1. Allander T., Emerson S. U., Engle R. E., Purcell R. H., Bukh J. 2001. A virus discovery method incorporating DNase treatment and its application to the identification of two bovine parvovirus species. Proc. Natl. Acad. Sci. U. S. A. 98:11609–11614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allander T., et al. 2005. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc. Natl. Acad. Sci. U. S. A. 102:12891–12896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arthur J. L., Higgins G. D., Davidson G. P., Givney R. C., Ratcliff R. M. 2009. A novel bocavirus associated with acute gastroenteritis in Australian children. PLoS Pathog. 5:e1000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barrett T., Wohlsein P., Bidewell C. A., Rowell S. F. 2004. Canine distemper virus in a Californian sea lion (Zalophus californianus). Vet. Rec. 154:334–336 [DOI] [PubMed] [Google Scholar]
- 5. Blasius M. E., Goodmanlowe G. D. 2008. Contaminants still high in top-level carnivores in the Southern California Bight: levels of DDT and PCBs in resident and transient pinnipeds. Mar. Pollut. Bull. 56:1973–1982 [DOI] [PubMed] [Google Scholar]
- 6. Blomstrom A. L., et al. 2009. Detection of a novel porcine boca-like virus in the background of porcine circovirus type 2 induced postweaning multisystemic wasting syndrome. Virus Res. 146:125–129 [DOI] [PubMed] [Google Scholar]
- 7. Bonner W. 2004. Seals and sea lions of the world. Facts on File, New York, NY [Google Scholar]
- 8. Breitbart M., et al. 2008. Viral diversity and dynamics in an infant gut. Res. Microbiol. 159:367–373 [DOI] [PubMed] [Google Scholar]
- 9. Breitbart M., et al. 2003. Metagenomic analyses of an uncultured viral community from human feces. J. Bacteriol. 185:6220–6223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Buckles E. L., et al. 2006. Otarine herpesvirus-1, not papillomavirus, is associated with endemic tumours in California sea lions (Zalophus californianus). J. Comp. Pathol. 135:183–189 [DOI] [PubMed] [Google Scholar]
- 11. Cann A. J., Fandrich S. E., Heaphy S. 2005. Analysis of the virus population present in equine faeces indicates the presence of hundreds of uncharacterized virus genomes. Virus Genes 30:151–156 [DOI] [PubMed] [Google Scholar]
- 12. Cheng W. X., et al. 2010. Identification and nearly full-length genome characterization of novel porcine bocaviruses. PLoS One 5:e13583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chevreux B. 2005. MIRA: an automated genome and EST assembler. Ruprecht-Karls University, Heidelberg, Germany [Google Scholar]
- 14. Chiba S., et al. 1979. An outbreak of gastroenteritis associated with calicivirus in an infant home. J. Med. Virol. 4:249–254 [DOI] [PubMed] [Google Scholar]
- 15. Colegrove K. M., et al. 2010. Polyomavirus infection in a free-ranging California sea lion (Zalophus californianus) with intestinal T-cell lymphoma. J. Vet. Diagn. Invest. 22:628–632 [DOI] [PubMed] [Google Scholar]
- 16. Coria-Galindo E., et al. 2009. Rotavirus infections in Galapagos sea lions. J. Wildl. Dis. 45:722–728 [DOI] [PubMed] [Google Scholar]
- 17. David S. T., et al. 2007. An outbreak of norovirus caused by consumption of oysters from geographically dispersed harvest sites, British Columbia, Canada, 2004. Foodborne Pathog. Dis. 4:349–358 [DOI] [PubMed] [Google Scholar]
- 18. Dierauf L. A., Lowenstine L. J., Jerome C. 1981. Viral hepatitis (adenovirus) in a California sea lion. J. Am. Vet. Med. Assoc. 179:1194–1197 [PubMed] [Google Scholar]
- 19. Donaldson E. F., et al. 2010. Metagenomic analysis of the viromes of three North American bat species: viral diversity among different bat species that share a common habitat. J. Virol. 84:13004–13018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Emerson S. U., et al. 2005. Caliciviridae, p. 353–369 In Fauquet C., Mayo M., Maniloff J., Desselberger U., Ball L. (ed.), Virus taxonomy: eighth report of the International Committee on Taxonomy of Viruses. Academic Press, San Diego, CA [Google Scholar]
- 21. Flotte T. R., Berns K. I. 2005. Adeno-associated virus: a ubiquitous commensal of mammals. Hum. Gene Ther. 16:401–407 [DOI] [PubMed] [Google Scholar]
- 22. Grachev M. A., et al. 1989. Distemper virus in Baikal seals. Nature 338:209–210 [DOI] [PubMed] [Google Scholar]
- 23. Greig D. J., Gulland F. M. D., Kreuder C. 2005. A decade of live California sea lion (Zalophus californianus) strandings along the central California coast: causes and trends, 1991–2000. Aquat. Mammals 31:40–51 [Google Scholar]
- 24. Guix S., Bosch A., Pinto R. M. 2005. Human astrovirus diagnosis and typing: current and future prospects. Lett. Appl. Microbiol. 41:103–105 [DOI] [PubMed] [Google Scholar]
- 25. Hernandez-Morga J., Leon-Felix J., Peraza-Garay F., Gil-Salas B. G., Chaidez C. 2009. Detection and characterization of hepatitis A virus and norovirus in estuarine water samples using ultrafiltration-RT-PCR integrated methods. J. Appl. Microbiol. 106:1579–1590 [DOI] [PubMed] [Google Scholar]
- 26. Kahn J. 2008. Human bocavirus: clinical significance and implications. Curr. Opin. Pediatr. 20:62–66 [DOI] [PubMed] [Google Scholar]
- 27. Kapoor A., et al. 2010. Identification and characterization of a new bocavirus species in gorillas. PLoS One 5:e11948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kapoor A., Simmonds P., Lipkin W. I., Zaidi S., Delwart E. Use of nucleotide composition analysis to infer hosts for three novel picorna-like viruses. J. Virol. 84:10322–10328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kapoor A., et al. 2009. A newly identified bocavirus species in human stool. J. Infect. Dis. 199:196–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kennedy S., et al. 2000. Mass die-off of Caspian seals caused by canine distemper virus. Emerg. Infect. Dis. 6:637–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kennedy-Stoskopf S., Stoskopf M. K., Eckhaus M. A., Strandberg J. D. 1986. Isolation of a retrovirus and a herpesvirus from a captive California sea lion. J. Wildl. Dis. 22:156–164 [DOI] [PubMed] [Google Scholar]
- 32. King D. P., et al. 2002. Otarine herpesvirus-1: a novel gammaherpesvirus associated with urogenital carcinoma in California sea lions (Zalophus californianus). Vet. Microbiol. 86:131–137 [DOI] [PubMed] [Google Scholar]
- 33. Kumar S., Nei M., Dudley J., Tamura K. 2008. MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief. Bioinform. 9:299–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li L., et al. 2010. Multiple diverse circoviruses infect farm animals and are commonly found in human and chimpanzee feces. J. Virol. 84:1674–1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li L., et al. 2010. Bat guano virome: predominance of dietary viruses from insects and plants plus novel mammalian viruses. J. Virol. 84:6955–6965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lipscomb T. P., et al. 2000. Common metastatic carcinoma of California sea lions (Zalophus californianus): evidence of genital origin and association with novel gammaherpesvirus. Vet. Pathol. 37:609–617 [DOI] [PubMed] [Google Scholar]
- 37. Manteufel J., Truyen U. 2008. Animal bocaviruses: a brief review. Intervirology 51:328–334 [DOI] [PubMed] [Google Scholar]
- 38. Martella V., Elia G., Buonavoglia C. 2008. Canine distemper virus. Vet. Clin. North Am. Small Anim. Pract. 38:787–797 [DOI] [PubMed] [Google Scholar]
- 39. Martella V., et al. 2008. Identification of a porcine calicivirus related genetically to human sapoviruses. J. Clin. Microbiol. 46:1907–1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Martin E. T. Frequent and prolonged shedding of bocavirus in young children attending daycare. J. Infect. Dis. 201:1625–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McClenahan S. D., et al. 2008. Genomic characterization of novel marine vesiviruses from Steller sea lions (Eumetopias jubatus) from Alaska. Virus Res. 138:26–35 [DOI] [PubMed] [Google Scholar]
- 42. Monroe S., Carter M., Hermann J., Mitchell D., Sanchez-Fauquier A. 2005. Astroviridae, p. 859–864 In Fauquet C., Mayo M., Maniloff J., Desselberger U., Ball L. (ed.), Virus taxonomy: eighth report of the International Committee on Taxonomy of Viruses. Academic Press, San Diego, CA [Google Scholar]
- 43. Neill J. D., Meyer R. F., Seal B. S. 1995. Genetic relatedness of the caliciviruses: San Miguel sea lion and vesicular exanthema of swine viruses constitute a single genotype within the Caliciviridae. J. Virol. 69:4484–4488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Newman S. J., Smith S. A. 2006. Marine mammal neoplasia: a review. Vet. Pathol. 43:865–880 [DOI] [PubMed] [Google Scholar]
- 45. Ng T. F., Suedmeyer W. K., Wheeler E., Gulland F., Breitbart M. 2009. Novel anellovirus discovered from a mortality event of captive California sea lions. J. Gen. Virol. 90:1256–1261 [DOI] [PubMed] [Google Scholar]
- 46. Nollens H. H., et al. 2006. Seroepidemiology of parapoxvirus infections in captive and free-ranging California sea lions Zalophus californianus. Dis. Aquat. Organ. 69:153–161 [DOI] [PubMed] [Google Scholar]
- 47. Nollens H. H., et al. 2006. Pathology and preliminary characterization of a parapoxvirus isolated from a California sea lion (Zalophus californianus). J. Wildl. Dis. 42:23–32 [DOI] [PubMed] [Google Scholar]
- 48. Pomeroy L. W., Bjornstad O. N., Holmes E. C. 2008. The evolutionary and epidemiological dynamics of the paramyxoviridae. J. Mol. Evol. 66:98–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ramig R. F., Ciarlet M., Mertens P. P. C., Dermody T. S. 2005. Rotavirus, p. 859–864 In Fauquet C., Mayo M., Maniloff J., Desselberger U., Ball L. (ed.), Virus taxonomy: eighth report of the International Committee on Taxonomy of Viruses. Academic Press, San Diego, CA [Google Scholar]
- 50. Rivera R., Nollens H. H., Venn-Watson S., Gulland F. M., Wellehan J. F., Jr 2010. Characterization of phylogenetically diverse astroviruses of marine mammals. J. Gen. Virol. 91:166–173 [DOI] [PubMed] [Google Scholar]
- 51. Saitoh M., Kimura H., Kozawa K., Nishio O., Shoji A. 2007. Detection and phylogenetic analysis of norovirus in Corbicula fluminea in a freshwater river in Japan. Microbiol. Immunol. 51:815–822 [DOI] [PubMed] [Google Scholar]
- 52. Sharp C. P., et al. 2010. Widespread infection with homologues of human parvoviruses B19, PARV4, and human bocavirus of chimpanzees and gorillas in the wild. J. Virol. 84:10289–10296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Smith A. W., Akers T. G., Madin S. H., Vedros N. A. 1973. San Miguel sea lion virus isolation, preliminary characterization and relationship to vesicular exanthema of swine virus. Nature 244:108–110 [DOI] [PubMed] [Google Scholar]
- 54. Smith A. W., et al. 1998. In vitro isolation and characterization of a calicivirus causing a vesicular disease of the hands and feet. Clin. Infect. Dis. 26:434–439 [DOI] [PubMed] [Google Scholar]
- 55. Smith A. W., et al. 2006. Vesivirus viremia and seroprevalence in humans. J. Med. Virol. 78:693–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Smith A. W., Skilling D. E., Dardiri A. H., Latham A. B. 1980. Calicivirus pathogenic for swine: a new serotype isolated from opaleye Girella nigricans, an ocean fish. Science 209:940–941 [DOI] [PubMed] [Google Scholar]
- 57. Smith A. W., Vedros N. A., Akers T. G., Gilmartin W. G. 1978. Hazards of disease transfer from marine mammals to land mammals: review and recent findings. J. Am. Vet. Med. Assoc. 173:1131–1133 [PubMed] [Google Scholar]
- 58. Stanway G., et al. 2005. Picornaviridae, p. 859–864 In Fauquet C., Mayo M., Maniloff J., Desselberger U., Ball L. (ed.), Virus taxonomy: eighth report of the International Committee on Taxonomy of Viruses. Elsevier Academic Press, San Diego, CA [Google Scholar]
- 59. Tattersall P., et al. 2005. Parvoviridae, p. 353–369 In Fauquet C., Mayo M., Maniloff J., Desselberger U., Ball L. (ed.), Virus taxonomy: eighth report of the International Committee on Taxonomy of Viruses. Academic Press, San Diego, CA [Google Scholar]
- 60. Terio V., et al. 2010. Norovirus in retail shellfish. Food Microbiol. 27:29–32 [DOI] [PubMed] [Google Scholar]
- 61. Usuku S., Kumazaki M., Kitamura K., Tochikubo O., Noguchi Y. 2008. An outbreak of food-borne gastroenteritis due to sapovirus among junior high school students.. Jpn. J. Infect. Dis. 61:438–441 [PubMed] [Google Scholar]
- 62. Victoria J. G., et al. 2009. Metagenomic analyses of viruses in stool samples from children with acute flaccid paralysis. J. Virol. 83:4642–4651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Victoria M., et al. 2010. Assessment of norovirus contamination in environmental samples from Florianopolis City, Southern Brazil. J. Appl. Microbiol. 109:231–238 [DOI] [PubMed] [Google Scholar]
- 64. Wellehan J. J. 2010. Discovery, phylogenetic analysis, diagnostic test development, and surveillance of the astroviruses of marine mammals. Ph.D. thesis. University of Florida, Gainesville, FL [Google Scholar]
- 65. Wilder F. W., Dardiri A. H. 1978. San Miguel sea lion virus fed to mink and pigs. Can. J. Comp. Med. 42:200–204 [PMC free article] [PubMed] [Google Scholar]
- 66. Zadori Z., Stefancsik R., Rauch T., Kisary J. 1995. Analysis of the complete nucleotide sequences of goose and muscovy duck parvoviruses indicates common ancestral origin with adeno-associated virus 2. Virology 212:562–573 [DOI] [PubMed] [Google Scholar]
- 67. Zhang T., et al. 2006. RNA viral community in human feces: prevalence of plant pathogenic viruses. PLoS Biol. 4:e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.