Abstract
The interferon-inducible transmembrane protein BST-2 (CD317, tetherin) restricts the release of several enveloped viruses from infected cells. BST-2 is broadly active against retroviruses, including HIV-1 and HIV-2. To counteract this host defense, HIV-1 uses the accessory protein Vpu, whereas HIV-2 uses its envelope glycoprotein (Env). In both cases, viral antagonism is associated with decreased expression of BST-2 at the cell surface. Here, we provide evidence supporting a role for the clathrin-mediated endocytic pathway in the downregulation of BST-2 from the cell surface and the counteraction of restricted virion release. A catalytically inactive, dominant negative version of the vesicle “pinch-ase” dynamin 2 (dyn2K44A) inhibited the downregulation of BST-2 by Vpu, and it inhibited the release of wild-type (Vpu-expressing) HIV-1 virions. Similarly, dyn2K44A inhibited the downregulation of BST-2 by HIV-2 Env, and it inhibited the release of vpu-negative HIV-1 virions when HIV-2 Env was provided in trans. dyn2K44A inhibited Env more robustly than Vpu, suggesting that dynamin 2, while a cofactor for both Env and Vpu, might support just one of several pathways though which Vpu counteracts BST-2. In support of a role for clathrin in these effects, the C-terminal domain of the clathrin assembly protein AP180 also inhibited the downregulation of BST-2 by either Vpu or HIV-2 Env. Consistent with modulation of the postendocytic itinerary of BST-2, Vpu enhanced the accumulation of cell surface-derived BST-2 in transferrin-containing endosomes. Vpu also inhibited the transport of BST-2 from a brefeldin A-insensitive compartment to the cell surface, consistent with a block to endosomal recycling. We propose that HIV-1 Vpu, and probably HIV-2 Env, traps BST-2 in an endosomal compartment following endocytosis, reducing its level at the cell surface to counteract restricted viral release.
INTRODUCTION
The lentiviruses that infect humans encode proteins that antagonize the interferon-inducible restriction factor BST-2—Vpu in the case of HIV-1 and Env in the case of HIV-2 (10, 15, 22, 35). Both of these viral gene products remove BST-2 from the cell surface, the site at which BST-2 restricts the release and spread of virions by preventing their detachment from the cell after membrane scission during budding (6, 24).
The mechanism(s) by which Vpu and Env modulate the expression of BST-2 remains incompletely understood. Both viral proteins interact with BST-2, but how they affect the intracellular itinerary of BST-2 is poorly defined (15, 30). Vpu has been proposed to stimulate the degradation of BST-2 via proteasomes or lysosomes (4, 8, 12), to retain BST-2 within endosomes by blocking its recycling after endocytosis (19), and/or to trap BST-2 in biosynthetic membranes such as the trans-Golgi network (TGN) (5). HIV-2 Env also appears to trap BST-2 within intracellular membranes but without stimulating its degradation (10, 15). Recently, we and others have provided evidence that the downregulation of BST-2 by Vpu occurs in a post-endoplasmic reticulum (ER) membrane compartment, presumably in endosomes (2, 33).
Both Vpu and Env are transmembrane proteins that are in large part endosomal. HIV Env contain a classical Yxxφ clathrin-adaptor protein (AP) complex binding motif (23), and this is required for the HIV-2 Env-mediated removal of BST-2 from the cell surface and the enhancement of virion release (10, 15). In contrast, AP-binding motifs that are of functional importance within Vpu are less clearly defined (31).
Here, we tested the role of the endocytic pathway in the counteraction of BST-2 by human lentiviruses. We find that a dominant negative version of dynamin 2 (dyn2), a GTPase involved in membrane scission during the formation of both clathrin-coated and non-clathrin-coated vesicles (11, 37), inhibits the activities of both HIV-1 Vpu and HIV-2 Env. Moreover, the C-terminal fragment of the clathrin assembly protein AP180 similarly inhibits activity. Since neither Vpu nor Env affect the endocytic rate of BST-2, and Vpu inhibits the delivery of BST-2 to the cell surface, we propose that a block to recycling is one mechanism by which these viral proteins antagonize endogenously expressed BST-2. This possibility is supported by the observation that Vpu directs BST-2 from the cell surface to endosomes that contain transferrin (Tf), a prototypical marker of a recycling pathway.
MATERIALS AND METHODS
Plasmids, reagents, and antibodies.
pcDNA3.1 (Invitrogen), pCA2 (a pcDNA-based plasmid expressing HLA-A2, provided by Olivier Schwartz), pCIneo (Promega), and pCDM8 were used as empty vector controls or as controls expressing an irrelevant protein. pcDNA-BST2 (34) was mutated to encode BST-2 in which tyrosines 6 and 8 in the cytoplasmic domain were replaced with alanines (BST-2–Y6,8A) using a QuikChange kit (Stratagene) and confirmed by nucleotide sequencing. Dynamin 2-encoding plasmids, DN(K44A)-dyn2 and WT-dyn2 (His/Myc- and green fluorescent protein [GFP]-tagged versions), were obtained from Mark McNiven. pVphu, encoding a codon-optimized Vpu independent of Rev, and a Vpu-defective version of the proviral plasmid pNL4-3 (vpudel-1; “Δvpu”) were provided by Klaus Strebel. Mutations were introduced in pVphu using a QuikChange kit as described above. A plasmid expressing HIV-2 Rod-10 Env was provided by Paula Cannon (1). A plasmid expressing HIV-1 Rev was previously cloned in our laboratory. A plasmid expressing GFP (pcgGFP) was provided by Jacek Skowronski. A plasmid expressing Tac (IL-2 receptor α chain)-DKQTLL was provided by Juan Bonifacino (17). A plasmid expressing the C-terminal fragment of the clathrin assembly cofactor AP180 (AP180-C) fused to GFP was provided by Massimo Pizzato (25). Fluorophore-labeled transferrin and epidermal growth factor (EGF) were obtained from Molecular Probes/Invitrogen. Rabbit antibody to HIV-2ST gp120 was obtained from the AIDS Research and Reference Reagent Program and contributed by Raymond Sweet, SmithKline Beecham Pharmaceuticals. The murine monoclonal antibody against BST-2/HM1.24 used for most cell surface staining was a gift from Chugai Pharmaceutical Co. (Kanagawa, Japan). For the surface deposition assays, unconjugated and phycoerythrin (PE)-conjugated anti-BST-2 clone RS38 was obtained from BioLegend, San Diego, CA.
Cells and transfections.
HeLa cells were the clone P4R5, a stable line expressing CD4 and CCR5, and were obtained from Nathaniel Landau and maintained in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), penicillin-streptomycin, and puromycin. HEK293T cells were also obtained from Nathaniel Landau and maintained in Eagle minimal essential medium (EMEM) supplemented with 10% FBS and penicillin-streptomycin. For most experiments, cells were plated in wells of 12-well plates and transfected with 1.6 μg of total DNA using Lipofectamine 2000 (Invitrogen) and manufacturer-recommended procedures. For immunofluorescence, cells were plated in 24-well plates and transfected with 0.8 μg of total DNA. Where indicated, cells were plated in 6-well plates and transfected with 2.5 μg of total DNA. The amounts of specific plasmids used are indicated in the figure legends. Assays were performed approximately 16 to 24 h posttransfection, unless otherwise indicated.
Western blotting.
Cells were detached from plates using 0.5 mM EDTA in phosphate-buffered saline (PBS), pelleted, and resuspended in Western loading buffer containing SDS and dithiothreitol (DTT). Lysates were boiled and loaded onto 12% polyacrylamide gel for SDS-PAGE, blotted onto polyvinylidene difluoride (PVDF) membranes, and analyzed as follows. His-Myc-tagged dynamin 2, p55 Gag, Vpu, HIV-2 Env, and actin were probed using horseradish peroxidase (HRP)-conjugated mouse anti-His (Sigma), mouse anti-p24 (clone 31-90-25; ATCC), rabbit anti-Vpu (from the AIDS Research and Reference Reagent Program and contributed by Klaus Strebel), rabbit anti-Env (described above), and anti-actin (Sigma). p55, Vpu, HIV-2 Env, and actin were detected with HRP-conjugated secondary antibodies goat anti-mouse IgG and goat anti-rabbit IgG (both from Bio-Rad). The blot was treated with chemiluminescent HRP substrate (GE Amersham) and visualized using autoradiographic film (Danville Scientific).
Flow cytometry.
Cells were detached from plates with 0.25% trypsin-EDTA. Incubations with antibodies were done at 4°C. An indirect method was used to stain the cell surface for BST-2. Cells were treated with a primary mouse anti-BST2 (Chugai) at 10 μg/ml, followed by an allophycocyanin (APC)-conjugated secondary goat anti-mouse IgG (BioLegend). For Tac-DKQTLL, a direct stain was done using phycoerythrin (PE)-conjugated antibody to CD25 (Becton Dickinson). For surface deposition assays, cells were treated with anti-BST2 clone RS38 at 4°C to block antigenic sites and then incubated at 37°C for various times before restaining with PE-conjugated RS38. The cells were washed using PBS containing 2% FBS and sodium azide. After being stained, all cells were fixed in 1% formaldehyde-PBS and analyzed using two-color flow cytometry. Flow cytometric gates were set by using cells stained with mouse isotype-matched IgG antibodies or, in the case of GFP, by using cells not expressing GFP. Composite data profiles were created using the FlowJo application package (Tree Star Inc.).
p24-release assay.
Culture supernates were first clarified by centrifugation at 300 × g. In the case of virions produced from HEK293T cells, the virions were purified by centrifugation through a 20% sucrose cushion. The concentration of p24 antigen was measured by enzyme-linked immunosorbent assay (ELISA; PerkinElmer). Western blots of the cell lysates were probed for p55 to confirm equivalent expression of the viral Gag polyprotein.
Immunofluorescence microscopy.
Cells were fixed in 3% formaldehyde-PBS, followed by permeabilization in 0.1% NP-40–PBS as previously described (36). The cells were then stained for Vpu or Env and BST-2 using the antibodies described above. Images were obtained using a wide-field fluorescence microscope fitted with a 100× objective (Olympus). For each field, a set of images was taken along the Z-axis and then processed with a “nearest-neighbor” deconvolution algorithm (SlideBook; Imaging Innovations Inc.). Single-image planes are shown. Composite multichannel images were assembled using Photoshop (Adobe Inc.).
RESULTS
Dominant negative dynamin 2 inhibits the antagonism of BST-2 by HIV-1 Vpu.
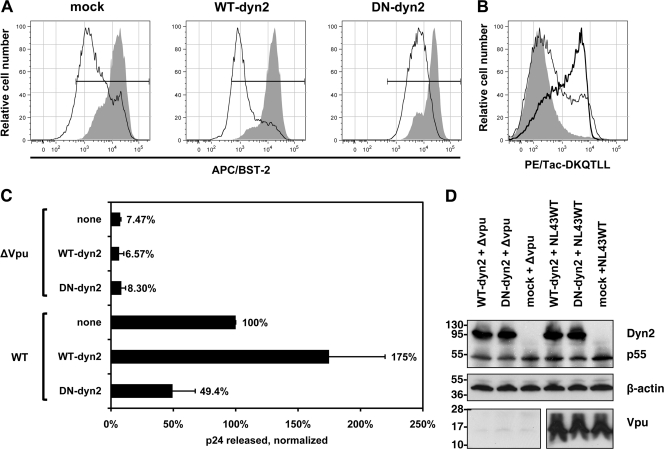
We tested whether a dominant negative version of the GTPase dynamin 2 (dyn2) inhibited the ability of Vpu to downregulate BST-2 from the cell surface and to enhance virion release (Fig. 1). Dynamin 2 is required for membrane scission during the formation of transport vesicles involved in both clathrin-mediated and non-clathrin-mediated endocytosis (11, 37). The catalytically inactive K44A mutant of dynamin 2 has dominant negative activity and can inhibit these processes. As previously reported (12), dyn2K44A inhibited the downregulation of BST-2 from the cell surface by HIV-1 Vpu (Fig. 1A). Here, Vpu was expressed from a codon-optimized expression plasmid (pVphu) in HeLa cells (clone P4.R5), along with either a plasmid expressing wild-type dynamin 2 (WT-dyn2), dyn2K44A (dominant negative dyn2 [DN-dyn2]), or a plasmid expressing an irrelevant protein (the major histocompatibility complex class I [MHC-I] A2 α-chain; mock). The cells, which express BST-2 constitutively, were analyzed by flow cytometry for their expression of BST-2 the next day. Vpu downregulated the surface expression of BST-2, with the peak fluorescence of the cells reduced by about 20-fold. This effect was not inhibited by the overexpression of wild-type dynamin 2. In contract, the expression of dyn2K44A inhibited the downregulation of BST-2 by Vpu, which reduced peak fluorescence by only about 3-fold under these conditions. In the absence of Vpu, dyn2K44A upregulated surface BST-2 very slightly, by about 1.3-fold (30%). In a parallel experiment, we validated the activity of dyn2K44A by coexpressing the endocytic indicator protein Tac-DKQTLL (Fig. 1B). Tac-DKQTLL is normally expressed poorly at the cell surface due to its E/DxxxLφ clathrin adaptor protein (AP)-binding motif (17), but it was markedly upregulated by the expression of dyn2K44A. These data indicated that dyn2K44A is acting as an inhibitor of clathrin-mediated endocytosis and that it inhibits the downregulation of BST-2 from the cell surface by Vpu.
Fig. 1.
Dominant negative dynamin 2 inhibits the Vpu-mediated downregulation of BST-2 and the enhancement of virion release. (A) Inhibition of Vpu-mediated downregulation of cell surface BST-2 by dyn2K44A. Cells (HeLa, which express BST-2 constitutively) were transfected to express dynamin 2 (WT-dyn2; 1.2 μg of plasmid), the dominant negative mutant dyn2K44A (DN-dyn2; 1.2 μg of plasmid), or an irrelevant protein (HLA-A2 [“mock”]; 1.2 μg of plasmid), together with GFP as a transfection marker (0.2 μg of plasmid) and either with or without Vpu (0.2 μg of plasmid expressing Vpu or pCIneo as an empty vector control). The next day, the cells were stained and analyzed by flow cytometry for GFP expression and surface BST-2. Histograms show the relative number of cells versus BST-2 (APC) fluorescence intensity for the GFP-positive cells. Shaded histograms represent cells not expressing Vpu; open histograms represent cells expressing Vpu. Horizontal bars indicate the BST-2-positive gate set using an isotype-matched control antibody. (B) Upregulation of the endocytic indicator protein Tac-DKQTLL by dyn2K44A. Cells (HeLa) were transfected to express Tac-DKQTLL (0.2 μg of plasmid) and GFP (0.2 μg of plasmid) along with either dynamin 2 (thin line) or the dominant negative mutant dyn2K44A (thick line), as described above, or transfected to express GFP and Tac-DKQTLL only (shaded) and then stained the next day for surface Tac antigen and analyzed by flow cytometry. Histograms show the relative number of cells versus Tac (PE) fluorescence intensity for the GFP-positive cells. (C) Inhibition of Vpu-mediated enhancement of virion release by dyn2K44A. Cells (HeLa) were transfected to express the indicated complete viral genomes (0.4 μg of plasmid) along with either dynamin 2 (WT-dyn2; 1.2 μg of plasmid), dyn2K44A (DN-dyn2; 1.2 μg of plasmid), or an irrelevant protein (HLA-A2 [“none”]; 1.2 μg of plasmid). The next day, culture supernates were collected, and the concentration of p24 capsid antigen was measured by ELISA. The data were normalized by setting wild-type virus in the absence of either dynamin 2 overexpression or the dominant negative dyn2 expression (“none”) to 100%. Data were obtained from duplicate transfections. (D) Verification of the expression of dynamin 2 (WT-dyn2), dyn2K44A (DN-dyn2), HIV-1 Gag precursor (p55), and Vpu during the virion release experiments by immunoblotting; the dynamin 2 proteins were detected using an antibody recognizing their polyhistidine tags.
To test whether dyn2K44A inhibited the enhancement of virion release by Vpu, we cotransfected HeLa cells with plasmids expressing either wild-type HIV-1NL43 (NL43WT) or an isogenic vpu mutant (ΔVpu), along with plasmids expressing either dynamin 2 (WT-dyn2), dyn2K44A (DN-dyn2), or an unrelated protein (mock). The culture supernates were collected the next day, and the concentration of p24 capsid antigen was measured by ELISA (Fig. 1C). The cell lysates were analyzed by Western blotting to confirm equal expression of the dynamin proteins, as well as of p55 Gag precursor and Vpu (Fig. 1D). The release of virions, as measured by secreted p24, was unaffected by the dynamin constructs in the absence of Vpu. In contrast, Vpu enhanced the release of virions by 27-fold when dynamin 2 was overexpressed but by only 6-fold when dyn2K44A was coexpressed. Vpu enhanced the release of virions by 13-fold when an unrelated protein (the MHC-I A2 α-chain) was coexpressed (mock). The amount of wild-type, vpu-positive virions released was 3.5-fold greater when wild-type dynamin 2 was coexpressed compared to when dyn2K44A was coexpressed. These data indicated that dyn2K44A inhibits the enhancement of virion release by Vpu. The data also suggested that the overexpression of dynamin 2 slightly enhances Vpu activity.
Dominant negative dynamin 2 inhibits the antagonism of BST-2 by HIV-2 Env.
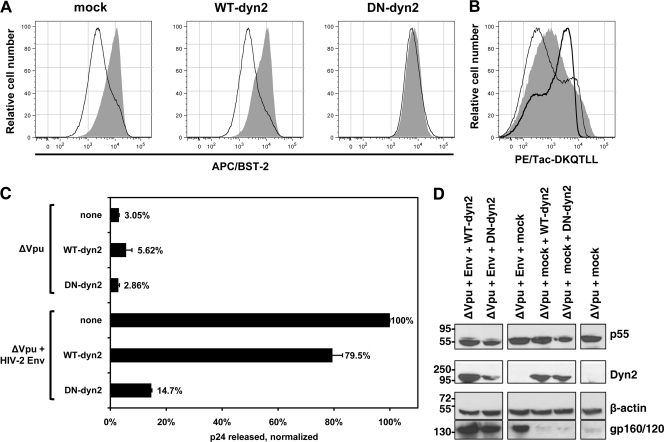
Next, we tested whether dyn2K44A inhibits the antagonism of BST-2 by HIV-2 Env. Unlike Vpu, the activity of HIV-2 Env as a BST-2 antagonist is mechanistically linked to clathrin-dependent protein trafficking within the endosomal system, because it requires a classical Yxxφ clathrin-AP-binding motif in the cytoplasmic domain of gp41. Consistent with this model of clathrin-dependent trafficking, we observed that coexpression of dyn2K44A effectively inhibited the downregulation of BST-2 from the cell surface by HIV-2 EnvROD10, whereas the overexpression of wild-type dynamin 2 did not (Fig. 2 A). The inhibition of Env activity by dyn2K44A in this assay was nearly complete. As described above, we verified that dyn2K44A was acting as an inhibitor of clathrin-mediated endocytosis in these experiments using Tac-DKQTLL as an indicator protein (Fig. 2B).
Fig. 2.
Dominant negative dynamin 2 inhibits the HIV-2 Env-mediated downregulation of BST-2 and the enhancement of virion release. (A) Inhibition of Env-mediated downregulation of cell surface BST-2 by dyn2K44A. The experiment was performed as described in the legend to Fig. 1A, except that instead of Vpu, HIV-2 Env was expressed along with HIV-1 Rev (0.2 μg of each plasmid). Histograms show the relative number of cells versus BST-2 (APC) fluorescence intensity for the GFP-positive cells. Shaded histograms represent cells not expressing Env; open histograms represent cells expressing Env. (B) Upregulation of the endocytic indicator protein Tac-DKQTLL by dyn2K44A. The experiment was performed as described in the legend to Fig. 1B. (C) Inhibition of Env-mediated enhancement of virion release by dyn2K44A. The experiment was performed as described in the legend to Fig. 1C, except that instead of a wild-type HIV-1 genome expressing Vpu, cells expressed a genome lacking vpu (after transfection with 0.4 μg of plasmid), with HIV-2 Env provided in trans (0.2 μg of plasmid) along with the dynamins (1.0 μg of plasmids). (D) Verification of the expression of dynamin 2 (WT-dyn2), dyn2K44A (DN-dyn2), HIV-1 Gag precursor (p55), and HIV-2 Env during the virion release experiments by immunoblotting.
To test whether dyn2K44A inhibited the enhancement of virion release by HIV-2 Env, we cotransfected HeLa cells with plasmids expressing the vpu-negative mutant of HIV-1 (ΔVpu) with or without a plasmid expressing HIV-2 Env, along with plasmids expressing either dynamin 2 (WT-dyn2), dyn2K44A (DN-dyn2), or an unrelated protein (mock). The culture supernates were collected the next day, and the concentration of p24 capsid antigen was measured by ELISA (Fig. 2C). The cell lysates were analyzed by Western blotting to confirm equal expression of the dynamin proteins as well as of p55 Gag precursor and Env (Fig. 2D). The release of virions, as measured by secreted p24, was unaffected by the dynamin constructs in the absence of both vpu and HIV-2 env. Env enhanced the release of virions by 14-fold when dynamin 2 was overexpressed but by only 6-fold when dyn2K44A was coexpressed. Env enhanced the release of virions by 32-fold when an unrelated protein (the MHC-I A2 α-chain) was coexpressed (mock). The amount of vpu-negative HIV-1 virions released in the presence of HIV-2 Env was 5.4-fold greater when wild-type dynamin 2 was coexpressed compared to when dyn2K44A was coexpressed. These data indicated that dyn2K44A inhibits the enhancement of virion release by Env.
Dominant negative dynamin 2 does not appreciably affect the subcellular distribution of Vpu or Env.
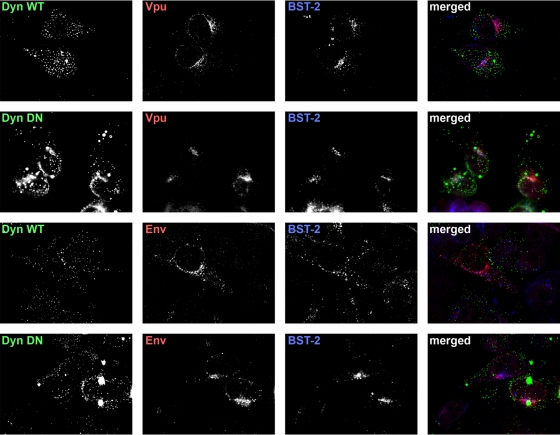
We considered that dynamin 2 might behave as a cofactor for both Vpu and HIV-2 Env because of a key role in enabling these proteins to follow their proper itinerary within the endosomal system. To test this, we transfected HeLa cells with plasmids expressing either Vpu or HIV-2 Env (together with HIV-1 Rev), along with plasmids expressing either wild-type GFP-dyn2 or GFP-dyn2K44A, stained the cells the following day for Vpu or HIV-2 Env along with BST-2, and examined them by immunofluorescence microscopy (Fig. 3). Both wild-type dynamin 2 and dyn2K44A were distributed in fine puncta, many of which were along the surface of the cells opposed to the cover glass, although dyn2K44A also formed large aggregates. Vpu was found throughout the cytoplasm in punctate, endosomal structures that were often relatively concentrated in a juxtanuclear region near the cell center, a region rich in TGNs and perinuclear recycling endosomes, as previously shown (36, 38). This distribution of Vpu was unchanged by the coexpression of dyn2K44A. In contrast to Vpu, HIV-2 EnvROD10 was found not only in an endosomal pattern but also in a ring around the nucleus together with a feathery cytoplasmic pattern, consistent with residence in the endoplasmic reticulum (Fig. 3; see Fig. 5). This distribution of Env was unchanged by the coexpression of dyn2K44A (Fig. 3). The apparent distribution of BST-2 was also unchanged by the coexpression of dyn2K44A; it partially coincided with Vpu and to a lesser extent with Env regardless of the expression of the dynamin constructs. These data weighed against the notion that dyn2K44A prevented Vpu or Env from reaching their proper subcellular destinations, including BST-2-positive compartments, at steady state.
Fig. 3.
Dominant negative dynamin 2 does not appreciably affect the subcellular distributions of Vpu or HIV-2 Env. Cells (HeLa) were transfected to express either wild-type dynamin 2 (Dyn WT; 0.6 μg of plasmid) or dyn2K44A (Dyn DN; 0.6 μg of plasmid), both as GFP fusions, along with either Vpu (0.1 μg of plasmid) or HIV-2 Env with HIV-1 Rev (0.1 μg of each plasmid). The next day, the cells were fixed, permeabilized, and stained for Vpu or Env, together with BST-2, and imaged using wide-field fluorescence microscopy. A Z series of images was obtained, and these were processed by a deconvolution algorithm before export of the single-plane images shown. In the merged images, dynamin-GFP fusion proteins are shown in green, Vpu or Env is red, and BST-2 is blue. Overlap between the viral proteins and BST-2 appears purple.
Fig. 5.
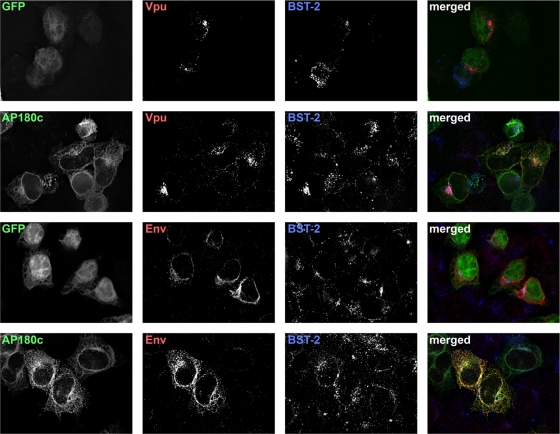
The C-terminal fragment of the clathrin assembly cofactor AP180 does not affect the subcellular distribution of Vpu or Env. Cells (HeLa) were transfected to express either the AP180 C terminus fused to GFP (AP180-C; 0.35 μg of plasmid) or GFP (0.03 μg of plasmid), along with either Vpu (0.1 μg of plasmid) or HIV-2 Env with HIV-1 Rev (0.1 μg of each plasmid); total plasmid was made up to 0.8 μg in each case with the empty vector pCDM8. The next day, the cells were fixed, permeabilized, and stained for Vpu or Env, together with BST-2, and imaged using wide-field fluorescence microscopy. A Z series of images was obtained, and these were processed by a deconvolution algorithm before export of the single-plane images shown. In the “merged” images, GFP proteins are shown in green, Vpu or Env is red, and BST-2 is blue. Overlap between the viral proteins and BST-2 appears purple, whereas overall between the viral proteins and AP180-C or GFP appears yellow.
AP180-C inhibits the downregulation of cell surface BST-2 by Vpu and HIV-2 Env.
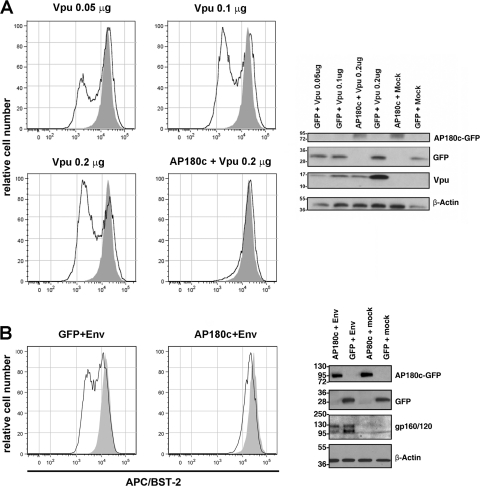
Dynamin 2 plays key roles in endocytosis mediated by both clathrin-dependent and clathrin-independent mechanisms. To specifically implicate clathrin-dependent pathways in the activities of Vpu and HIV-2 Env, we expressed a C-terminal fragment of the clathrin assembly protein AP180 (as a GFP fusion; GFP–AP180-C), which inhibits clathrin-mediated endocytosis (7). The expression of AP180-C inhibited the downregulation of BST-2 from the cell surface by both Vpu and Env (Fig. 4 A and B). In the absence of either Vpu or Env, AP180-C increased the surface expression of BST-2 by about 2-fold, consistent with the reported role of clathrin in the physiologic trafficking of BST-2 (29) (4B, shaded histograms). Unexpectedly, AP180-C decreased the expression of both Vpu and Env. We mitigated this, however, with an experimental design in which the cells were transfected with relatively reduced amounts of the plasmid encoding AP180-C (along with the plasmids encoding Vpu and Env) and allowed expression for only 8 h. Under these conditions, AP180-C had less of an effect on the expression of either Vpu or Env (Fig. 4), yet it inhibited their ability to downregulate BST-2. To support the notion that the inhibition of Vpu activity was not the consequence of reduced expression, we used a dose-response format to document that the levels of Vpu protein detected in the AP180-C experiments were sufficient to downregulate BST-2 (Fig. 4A). These data suggested that the assembly of clathrin coats is involved in the downregulation of BST-2 from the cell surface by both Vpu and HIV-2 Env. The data also confirmed that this downregulation occurs very rapidly, within as little as 8 h from the onset of gene expression.
Fig. 4.
The C-terminal fragment of the clathrin assembly cofactor AP180 inhibits the downregulation of cell surface BST-2 by Vpu and HIV-2 Env. (A) Inhibition of Vpu-mediated downregulation of cell surface BST-2 by the C-terminal fragment of the clathrin assembly cofactor AP180 (GFP–AP180-C). Cells (HeLa) were transfected to express the AP180 C-terminal fragment fused to GFP (AP180-C; 0.7 μg of plasmid) with Vpu (0.2 μg of plasmid) or GFP alone (0.06 μg of plasmid) with Vpu (0.05, 0.1, or 0.2 μg of plasmid). Total plasmid DNA was made (up to 1.6 μg using pcDM8). Eight hours later, the cells were stained for surface BST-2 and analyzed by two-color flow cytometry. GFP-positive cells are shown as open histograms in the plots of the relative number of cells versus fluorescence intensity of APC/BST-2. Shaded histograms are derived from cells expressing neither Vpu nor GFP–AP180-C. The cells were also analyzed for the expression on GFP–AP180-C, GFP, Vpu, and actin by immunoblotting. (B) Inhibition of Env-mediated downregulation of cell surface BST-2 by AP180-C. Cells were transfected as described above, except that instead of Vpu, HIV-2 Env (0.4 μg of plasmid) was expressed along with HIV-1 Rev (0.4 μg of plasmid). Shaded histograms represent cells not expressing Env but expressing GFP (left) or GFP–AP180-C (right); open histograms represent cells expressing Env and GFP (left) or Env and GFP–AP180-C (right). The cells were also analyzed for the expression on AP180-C, GFP, Env (gp160/120), and actin by immunoblotting.
AP180-C does not affect the subcellular distribution of Vpu or Env.
As was the case with dynamin 2, we wondered whether AP180 might behave as a cofactor for both Vpu and HIV-2 Env by enabling these proteins to follow their proper itinerary within the endosomal system. To test this, we transfected HeLa cells with plasmids expressing either Vpu or HIV-2 Env (together with HIV-1 Rev), along with plasmids expressing either GFP or GFP–AP180-C, stained the cells the following day for Vpu or HIV-2 Env along with BST-2, and examined them by immunofluorescence microscopy (Fig. 5). AP180-C was distributed throughout the cytoplasm; in many cells this included a ring around the nucleus and a feathery cytoplasmic distribution consistent with association with the ER, as well as cytoplasmic puncta consistent with endosomes. The distribution of AP180-C coincided substantially with Env and to a much lesser extent with Vpu. However, as was the case with dyn2K44A, AP180-C did not markedly affect the distribution of either Env or Vpu. The distribution of BST-2 partially coincided with Vpu and to a lesser extent with Env, regardless of the expression of AP180-C. These data weighed against the notion that AP180-C prevented Vpu or Env from reaching their proper subcellular destinations, including BST-2-positive compartments, at steady state.
Dominant negative dynamin 2 and AP180-C each inhibit the endocytic rate of BST-2.
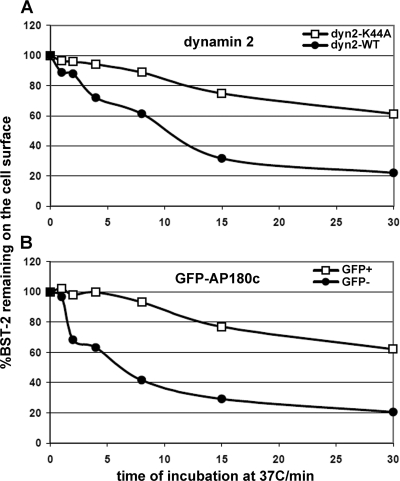
We considered that decreasing the constitutive rate of endocytosis of BST-2 could block the activity of Vpu and Env, especially if a major effect of these proteins is to inhibit the return of endocytosed BST-2 to the cell surface. To test this, we transfected HeLa cells with plasmids expressing either dyn2K44A or AP180-C. We then used flow cytometry to measure the rate of disappearance of antibody-labeled BST-2 from the cell surface, and we calculated the fractional internalization rate of BST-2. (Fig. 6). As described previously, the constitutive rate of endocytosis of BST-2 in HeLa cells is rapid (5, 19). Moreover, the rate of endocytosis is inhibited by expression of dyn2K44A or AP180-C. These results, in particular the effect of AP180-C, are consistent with the notion that BST-2 is internalized via a clathrin-dependent process (29).
Fig. 6.
Dominant negative dynamin 2 or AP180-C inhibits the endocytic rate of BST-2. (A) Dynamin2 K44A. Cells (HeLa) were transfected to express either GFP-dyn2K44A (0.9 μg of plasmid) or GFP-dyn2 wild type (WT). The next day, a flow cytometric assay was used to measure the rate of internalization of BST-2. After labeling of the cell surface at 4°C with antibody to BST-2, the cells were incubated for various times at 37°C before staining with a fluorophore-conjugated secondary antibody and analysis by two-color flow cytometry. The fraction of BST-2 remaining on the cell surface versus the time of incubation at 37°C is graphed, using the mean BST-2/APC fluorescence intensity of the GFP-positive cells and normalizing to the amount of BST-2 present at time zero in each case. (B) AP180-C. Cells (HeLa) were transfected to express GFP–AP180-C (0.7 μg of plasmid). The next day, the flow cytometric assay described above was used to measure the rate of internalization of BST-2. The GFP− curve is derived from the GFP-negative cell population (not expressing AP180-C), whereas the GFP+ curve is derived from the GFP-positive cell population (expressing AP180-C).
Role of potential clathrin adaptor protein binding motifs Y29RKI and EVSAL63V in the cytoplasmic domain of Vpu.
We considered the possibility that Vpu, like Env, might contain a signal in its cytoplasmic domain that would direct it, and BST-2, to clathrin-coated endosomes. We noticed two potential sequences in HIV-1 VpuNL43 that conformed to those of tyrosine- or leucine-based AP-binding motifs, Y29RKI and EVSAL63V (3). Y29 is relatively well conserved among HIV-1 group M Vpu proteins, whereas L63V is poorly conserved, with the valine 64 residue more often a serine or alanine. We used plasmids encoding codon-optimized versions of Vpu-Y29A (encoding an alanine in place of tyrosine 29 in Vpu) and Vpu-LV63,64AA (encoding alanines in place of leucine 63 and valine 64 in Vpu) to test the roles of these residues. These mutated Vpu proteins downregulated BST-2 from the cell surface as effectively as the wild-type protein (Fig. 7 A), they were reasonably well expressed, as assessed by Western blotting (Fig. 7B), and their subcellular distributions were indistinguishable from that of wild-type Vpu (Fig. 7C). These data indicated that neither of these potential AP-binding sequences in the cytoplasmic domain of Vpu is required for the downregulation of BST-2.
Fig. 7.
Role of potential clathrin adaptor protein binding motifs Y29RKI and EVSAL63V in the cytoplasmic domain of Vpu. (A) Vpu-mediated downregulation of cell surface BST-2. Cells (HeLa) were transfected to express GFP as a transfection marker (0.2 μg of plasmid) either with or without Vpu (0.4 μg of plasmid, wild type [WT] or the indicated mutants). The next day, the cells were stained and analyzed by flow cytometry for GFP expression and surface BST-2. Histograms show the relative number of cells versus BST-2 (APC) fluorescence intensity for the GFP-positive cells. Shaded histograms represent cells not expressing Vpu; open histograms represent cells expressing Vpu or the indicated mutated proteins. (B) Cells analyzed by flow cytometry as shown in panel A were analyzed by immunoblotting for the expression of Vpu, GFP, and actin. (C) Subcellular distributions of Vpu-Y29A and Vpu-LV63,64AA by immunofluorescence microscopy. Cells (HeLa) were transfected to express Vpu or the indicated mutated Vpu protein. The next day, the cells were fixed, permeabilized, and stained for Vpu, together with BST-2, and imaged using wide-field fluorescence microscopy. A Z-series of images was obtained, and these were processed by a deconvolution algorithm before export of the single-plane images shown. In the “merged” images, Vpu is shown in red, and BST-2 is blue. Overlap between Vpu and BST-2 appears purple.
Effect of HIV-2 Env on the endocytic rate of BST-2.
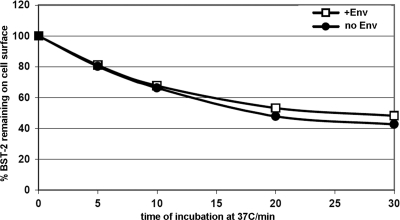
The simplest model for the downregulation of BST-2 and the relief of restricted virion release is that HIV-1 Vpu and HIV-2 Env directly remove the protein from the cell surface. We and others have reported, however, that Vpu does not affect the rate of endocytosis of BST-2 (5, 19). Here, we used flow cytometry to measure the rate of disappearance of antibody-labeled BST-2 from the cell surface, and we calculated the fractional internalization rate for BST-2 either with or without the coexpression of HIV-2 Env (Fig. 8). Like Vpu, Env had no apparent effect on the rate of endocytosis of BST-2, despite the clear role of the Yxxφ clathrin-AP-binding motif in the cytoplasmic domain of gp41 in the downregulation of BST-2 and the counteraction of restriction (1, 15). We confirmed that the endocytic rate of BST-2 is not stimulated in these experiments by antibody-mediated cross-linking: the same internalization rate for BST-2 is obtained using a monovalent Fab1 fragment (data not shown). These data suggested that the action of HIV-2 Env, like that of Vpu, is either pre- or postendocytic, potentially involving entrapment of BST-2 within the endosomal system.
Fig. 8.
Effect of HIV-2 Env on the endocytic rate of BST-2. A flow cytometric assay was used to measure the effect of HIV-2 Env on the rate of internalization of BST-2. The assay was performed as described previously for Vpu (19), except that the cells (HeLa) were transfected to express GFP (0.06 μg of plasmid) either with or without HIV-2 Env and HIV-1 Rev [0.8 μg of each plasmid or 1.6 μg of pcDM8 control (no Env)]. After labeling of the cell surface at 4°C with antibody to BST-2, the cells were incubated for various times at 37°C before staining with a fluorophore-conjugated secondary antibody and analysis by two-color flow cytometry. The fraction of BST-2 remaining on the cell surface versus the time of incubation at 37°C is graphed in the presence and absence of HIV-2 Env, using the mean BST-2/APC fluorescence intensity of the GFP-positive cells and normalizing to the amount of BST-2 present at time zero in each case.
Vpu directs cell surface-derived BST-2 to transferrin-positive endosomes.
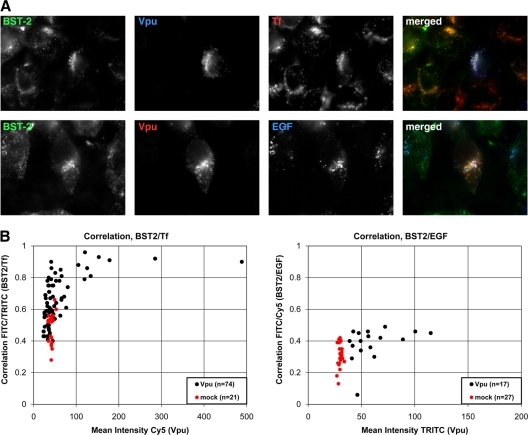
To determine whether Vpu affects the postendocytic fate of BST-2, we tracked surface BST-2 by allowing cells to internalize the antibody-labeled protein along with fluorophore-labeled transferrin (Tf) and fluorophore-labeled epidermal growth factor (EGF). Tf identifies a prototypical recycling compartment, which includes perinuclear endosomes, whereas EGF identifies a degradation compartment, which includes late endosomes and multivesicular bodies. Due to the low levels of BST-2 on the surfaces of cells expressing Vpu, we exposed the cells while alive to antibody to BST-2 together with labeled Tf and EGF for 1 h at 37°C. This allowed for BST-2 that was newly delivered to the plasma membrane to be labeled and tracked (Fig. 9). The cells were also transfected with less Vpu expression plasmid than usual. In the absence of Vpu, internalized BST-2 tracked with both Tf and EGF, but it colocalized more closely with Tf, a result consistent with the observed colocalization of BST-2 at steady state with transferrin receptor (12, 34). Moreover, the delivery of BST-2 from the cell surface to transferrin-containing endosomes was markedly enhanced by Vpu, whereas the delivery of BST-2 to EGF-containing endosomes was less enhanced (Fig. 9). In some cells, internalized BST-2, internalized Tf, and Vpu colocalized almost completely. These data indicated that Vpu primarily directs BST-2 from the cell surface to transferrin-positive endosomes, a conclusion consistent with the reported redistribution of BST-2 to endosomes containing transferrin receptor in cells expressing vpu-positive HIV-1 (9).
Fig. 9.
Effect of Vpu on the fate of BST-2 after internalization from the cell surface. Cells (HeLa) were transfected to express either Vpu (0.05 μg of plasmid) or no Vpu (mock; 0.05 μg of pcDNA3.1). The next day, the cells were incubated while alive at 37°C with antibody to BST-2 and either fluorophore-labeled transferrin (Tf; 10 μg/ml) or fluorophore-labeled epidermal growth factor (EGF; 10 μg/ml) for 1 h. The cells were then fixed, permeabilized, and stained with a fluorophore-conjugated secondary antibody to detect internalized BST-2 as well as with primary and secondary antibodies to detect Vpu. (A) Cells transfected to express Vpu were treated as just described and imaged using wide-field fluorescence microscopy. Z series of images were obtained, and these were processed by a deconvolution algorithm before exporting the single-plane images shown. In the “merged” image in the top row, overlap between BST-2 and Tf appears yellow, overlap between Vpu and Tf appears purple, and overlap between BST-2, Tf, and Vpu appears white. In the “merged” image in the bottom row, overlap between BST-2 and Vpu appears yellow, and overlap between BST-2, Vpu, and EGF appears white. (B) The overlap between internalized BST-2 and Tf (left) and between internalized BST-2 and EGF (right) was quantified as a function of the level of Vpu expression. The Pearson correlation coefficient, in which zero indicates no relationship between the two signals and 1.0 indicates a complete positive correlation, was calculated using SlideBook software. Each dot represents an individual cell. Vpu-expressing cells were chosen to cover a range of expression levels. FITC, fluorescein isothiocyanate; TRITC, tetramethyl rhodamine isothiocyanate.
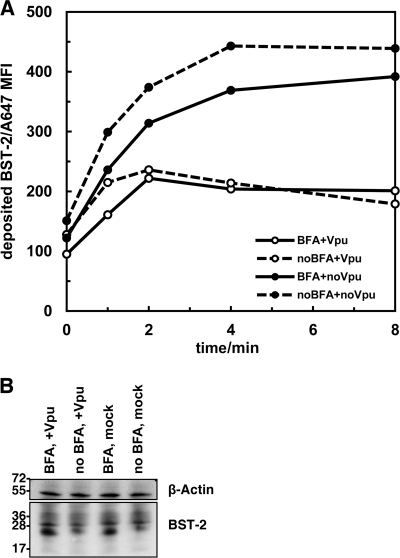
Vpu inhibits the deposition of BST-2 on the cell surface.
We suspected, based on the above-described data, that BST-2 might be retained in recycling endosomes as part of a clathrin-dependent downregulation mechanism. To test this, we used a flow cytometric approach similar to that recently used to support a model in which Vpu blocks the endosomal recycling of CD1d (20). Here, HeLa cells (either transfected or not to express Vpu) were treated with saturating amounts of anti-BST-2 monoclonal antibody at 4°C to block antigenic sites on the cell surface. The cells were then incubated for various times at 37°C, before being stained with the same antibody, but this time conjugated directly to a fluorophore. In this way, BST-2 that was newly deposited at the cell surface was measurable over time (Fig. 10 A). We also preincubated some cells with brefeldin A (BFA) for 30 min before the initial staining and during the 37°C incubation, reasoning that this would block egress of BST-2 from the ER and Golgi complex, since BFA causes dissociation of COP (coatomer) coats and the collapse of the Golgi complex into the ER (18). In the absence of Vpu and BFA, BST-2 was deposited on the cell surface and reached a maximal plateau level in 8 to 12 min. Vpu inhibited this deposition, without causing a detectable decrease in total cellular levels, measured by Western blotting (Fig. 10). BFA only modestly inhibited the deposition of surface BST-2 (although its effects on BST-2 maturation are apparent in the immunoblot shown in Fig. 10B, depicting accumulation of low-molecular-weight, incompletely glycosylated forms), indicating that most of the signal in this assay likely derives from a post-ER compartment, presumably recycling endosomes, which are insensitive to BFA (18). The effects of Vpu and BFA were not additive, consistent with the notion that Vpu not only inhibits the endosomal recycling of BST-2, but also might inhibit the anterograde transport of BST-2 through biosynthetic membranes to the plasma membrane (39).
Fig. 10.
Vpu inhibits the deposition of BST-2 at the cell surface. (A) Cells (HeLa in wells of a 6-well plate) were transfected to express GFP (1.0 μg of plasmid) either with or without Vpu (1.0 μg of plasmid). The next day, the cells were removed from the plates and either treated or not with brefeldin A (BFA; 10 μM) for 30 min at 37°C before blocking surface BST-2 antigen by incubation with unlabeled monoclonal antibody RS38 (30 μg/ml) for 1 h at 4°C. Cells were then incubated for the indicated times at 37°C (either with or without BFA) before staining at 4°C with phycoerythrin (PE)-conjugated RS38 (10 μg/ml) to detect BST-2 that had been deposited at the cell surface during the 37°C incubation. The mean fluorescence intensity (MFI) of the GFP-positive cells (less the MFI of cells stained with a PE-conjugated antibody isotype control) is graphed. (B) The cells analyzed as shown in panel A were also analyzed by immunoblotting for BST-2 and actin.
The YxY sequence in the cytoplasmic domain of BST-2 is not obligatory for constitutive endocytosis.
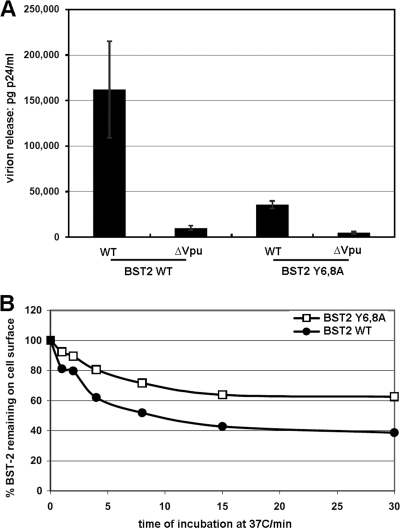
The preceding data support a block-to-recycling model in which Vpu induces removal of surface BST-2 by preventing BST-2 that has been constitutively endocytosed from returning to the cell surface. We challenged this model by evaluating a BST-2 mutant in which tyrosines 6 and 8 were replaced with alanines (BST-2–Y6,8A). This YxY sequence that reportedly binds both AP-1 and AP-2 is required for maximally efficient endocytosis of BST-2 (29). We reasoned that if BST-2–Y6,8A were not endocytosed but remained responsive to Vpu, then a block-to-recycling model could not account for the downregulation and antagonism of BST-2. Here, we confirmed that the YxY sequence is not required for Vpu responsiveness (Fig. 11 A). BST-2–Y6,8A appeared to restrict the release of vpu-negative virus more efficiently than wild-type BST-2, and virus encoding Vpu was released less efficiently when BST-2–Y6,8A was expressed in comparison to wild-type BST-2. Nevertheless, Vpu was able to antagonize BST-2–Y6,8A, as previously reported (12). We then compared the rate of constitutive endocytosis of this mutant to that of wild-type BST-2 (Fig. 11B). The steady-state surface level of BST-2–Y6,8A was about 50% greater than that of the wild type (data not shown), and BST-2–Y6,8A was endocytosed less rapidly than the wild-type protein. Nevertheless, BST-2–Y6,8A was robustly endocytosed, with nearly 40% of the protein internalized within 15 min. These data indicated that the YxY sequence in the cytoplasmic domain of BST-2 stimulates but is not required for the constitutive endocytosis of BST-2. Consequently, the ability of Vpu to antagonize restriction by this mutated BST-2 protein is consistent with the block-to-recycling model.
Fig. 11.
The YxY sequence in the cytoplasmic domain of BST-2 facilitates but is not essential for endocytosis. (A) Virion release. Cells (HEK 293T in wells of a 6-well plate) were transfected to express either wild-type (WT) BST-2 (75 ng of plasmid) or BST-2 in which tyrosines 6 and 8 in the cytoplasmic domain were replaced with alanines (Y6,8A) along with either wild-type or vpu-negative (ΔVpu) proviral plasmids (3.75 μg). The next day, the culture supernatants were removed and clarified by centrifugation at 300 × g. Virions were purified by centrifugation through a 20% sucrose cushion at 23,500 × g, suspended in the original supernatant volume, then analyzed in duplicate by p24 capsid antigen ELISA. (B) Constitutive endocytosis rate. Cells (HEK 293T in wells of a 12-well plate) were transfected to express either wild-type BST-2 (60 ng of plasmid) or BST-2 in which the tyrosines 6 and 8 in the cytoplasmic domain were replaced with alanines (Y6,8A), along with GFP (0.1 μg of plasmid) as a transfection marker. The next day, the fractional rate of endocytosis of BST-2 was measured, as shown in Fig. 6 and 8. Data are normalized to 100% at time zero in each case.
DISCUSSION
We have investigated the role of the endocytic pathway in the counteraction of BST-2 by the human lentiviral proteins HIV-1 Vpu and HIV-2 Env. Our investigation of an endocytic mechanism was prompted by a number of lines of previously published evidence. First, BST-2 acts at the cell surface to restrict virion release (6, 24), and most virally encoded antagonists remove BST-2 from the cell surface (14–16, 35). The most rapid way to accomplish this seems likely to be via an endocytic process. Second, the activity of HIV-2 Env in removing BST-2 from the cell surface depends on a Yxxφ motif in the cytoplasmic domain of gp41 that binds to clathrin adaptor protein (AP) complexes, including AP-2, the complex associated specifically with endocytosis (10, 15). Third, although HIV-1 Vpu contains no clearly defined AP-binding motif, small interfering RNA (siRNA)-mediated suppression of AP-2 expression inhibits Vpu's ability to remove BST-2 from the cell surface (19). Nevertheless, a number of alternative mechanisms of downregulation of BST-2 by Vpu have been reported, including degradation via proteasomes or lysosomes and retention within juxtanuclear membrane systems, either along the biosynthetic (TGN) or endosomal recycling pathways (4, 5, 8, 10, 12, 19). Furthermore, a role for the endocytic pathway in the virologic effect of Vpu, that is, in its ability to enhance virion release, has not been shown directly. Here we document that the virologic activity of Vpu, as well as HIV-2 Env, is at least partly dependent on an endocytic process.
An enzymatically defective, dominant negative version of the GTPase dynamin 2 (dyn2K44A) interfered with the abilities of Vpu and HIV-2 Env to decrease the levels of BST-2 at the cell surface and to enhance virion release. Dynamin 2 is required for the pinching off of several types of vesicles that mediate membrane transport, including those that are clathrin coated and those that are non-clathrin coated (11, 37). While the expression of dyn2K44A was previously shown to inhibit the Vpu-mediated decrease in the levels of BST-2 at the cell surface (12), data herein show that dynamin 2 is a cofactor for the Vpu-mediated enhancement of virion release. dyn2K44A was previously examined for its potential to rescue the release of vpu-negative virions, based on the notion that a blockade to endocytosis might allow such virions to escape the cell surface independently of Vpu (21). dyn2K44A had no such activity, because the tethering activity of BST-2 restricts the release of nascent virions whether or not they are subsequently endocytosed. Instead of promoting the release of vpu-negative virions, we show here that dyn2K44A inhibits the release of wild-type virions, presumably because dynamin 2 is itself a cofactor for Vpu. Given the obligatory role of the AP-binding motif in HIV-2 Env for the antagonism of BST-2 (1, 10, 15), the ability of dyn2K44A to inhibit the Env-mediated decrease in the levels of BST-2 at the cell surface and the enhancement of virion release were expected. This inhibition was more effective in the case of Env than in the case of Vpu, suggesting that dynamin 2, while a cofactor for both Env and Vpu, might support just one of several pathways through which Vpu counteracts BST-2. Pathways of Vpu activity independent of dynamin 2 might include degradation (which HIV-2 Env does not induce) and entrapment within biosynthetic membranes, as discussed below.
We used the cellular protein AP180 to further validate and dissect the role of the endocytic pathway in the activities of Vpu and HIV-2 Env. AP180 is a clathrin assembly protein whose domain structure includes an N-terminal phosphatidylinositol 4,5-biphosphate (PIP2) plasma membrane-binding domain and a C-terminal clathrin-binding domain (7). The C- terminal fragment of the clathrin assembly cofactor AP180 (AP180-C) prevents the assembly of clathrin coats at the plasma membrane. Consequently, the inhibition of both the Vpu- and HIV-2 Env-mediated downregulation of BST-2 by AP180-C suggested a specific role for clathrin-mediated endocytosis in these processes.
How is clathrin-mediated endocytic trafficking involved in the activities of Vpu and HIV-2 Env as antagonists of BST-2? Clathrin-mediated endocytic pathways might support the trafficking of BST-2 and/or the viral proteins themselves. This might be expected based on the presence of a YxY sequence in the cytoplasmic domain of BST-2 that reportedly directs clathrin-mediated endocytosis (29) and based on the presence of the Yxxφ clathrin-AP-binding motif in Env. However, neither dyn2K44A nor AP180-C displaced Vpu or Env from internal endosomes to the plasma membrane, as detected by fluorescence microscopy; this might have been expected if these viral proteins relied on clathrin-mediated endocytosis to reach their proper endosomal compartments at steady state. Moreover, sequences in Vpu with the potential to bind clathrin adaptor protein complexes had no apparent role in Vpu activity with respect to the downregulation of BST-2. Finally, the rate of endocytosis of BST-2 was not increased by HIV-2 Env, just as it is not increased by Vpu (5, 19).
Both dyn2K44A and AP180-C, however, inhibited the constitutive rate of endocytosis of BST-2. These mutated cellular proteins thus could indirectly inhibit the activity of Vpu and Env by inhibiting the delivery of surface-derived BST-2 to endosomes, where the viral proteins act. This model was not refuted by testing a putative endocytosis mutant of BST-2 (29), BST-2–Y6,8A, because this mutant, though responsive to Vpu, was only modestly impaired in its rate of constitutive endocytosis.
These considerations led us to consider that the activity of Vpu and Env as BST-2 antagonists is manifest at a postendocytic step. For example, Vpu could downregulate cell surface BST-2 by specifically blocking the return of endocytosed BST-2 to the plasma membrane. We suggested previously that such a block to the recycling of BST-2 could be a consequence of ubiquitination, which is promoted by the Vpu-dependent recruitment of a β-TrCP-containing SCF E3 ubiquitin ligase complex (4, 19, 34). Notably, the ESCRT-0 protein Hrs is a monomeric clathrin adaptor that binds ubiquitin and could recognize ubiquitinated BST-2, and Hrs has recently been reported as a cofactor in the downregulation and degradation of BST-2 by Vpu (13, 27). Endosomes containing Hrs are partly coated with flat clathrin lattices, and these membrane domains participate in the routing of endocytosed proteins toward lysosomes at the expense of recycling to the plasma membrane (26, 28). The role of dynamin 2 and clathrin assembly in the activities of Vpu (and Env) as BST-2 antagonists could involve the function of these specialized endosomal domains.
However, we observed here that in the presence of Vpu, the predominant route followed by endocytosed BST-2 is shared by transferrin, a prototypical marker of recycling endosomes, rather than by EGF, a prototypical marker of the ESCRT pathway. Presumably, BST-2 becomes trapped in the transferrin-positive compartment by Vpu. In further support of this model, Vpu inhibited the deposition of BST-2 on the plasma membrane. Newly deposited BST-2 came predominantly from a BFA-insensitive compartment, consistent with recycling endosomes (18). Whether this apparent block to recycling by Vpu is linked to ubiquitination or is solely the consequence of the interaction between Vpu and BST-2 remains to be determined.
Can the various models of counteraction of BST-2 by Vpu be reconciled? One possibility is that Vpu acts in a multifaceted manner but from a common subcellular location—juxtanuclear endosomes that include both recycling endosomes and the TGN. This location, at which Vpu is concentrated (5, 10, 36, 38), is a crossroads of biosynthetic and endocytic trafficking at which both newly synthesized and endocytosed BST-2 can be intercepted. From there, Vpu's activities might include the endocytic/block-to-recycling mechanism supported here, sequestration of BST-2 along the biosynthetic pathway, and enhanced degradation of BST-2 by ubiquitin-mediated lysosomal targeting. Such a multifaceted mechanism was very recently supported by “recycling” experiments similar to the surface deposition experiments reported here, as well as by microinjection experiments that document the potential for Vpu to trap newly synthesized BST-2 in juxtanuclear membranes, including the TGN (32). Nevertheless, an endocytic mechanism seems particularly well suited to address the virus's problem of how to eliminate BST-2 already resident on the plasma membrane when it begins to express Vpu. In this regard, we think it is useful to consider that when HIV-1 propagates in an infected individual, it likely encounters target cells that are in the antiviral state induced by interferon and are already expressing BST-2. Moreover, we have reported previously that inhibition of the biosynthesis or biosynthetic transport of BST-2 seems insufficient on a kinetic basis to account for the rapid downregulation induced by Vpu (33).
In summary, we have documented a role for the clathrin-mediated endocytic pathway in the downregulation of BST-2 from the cell surface and in the antagonism of restricted virion release by the human lentiviral proteins HIV-1 Vpu and HIV-2 Env. We favor the hypothesis that these viral proteins modulate the postendocytic itinerary of BST-2 by trapping BST-2 in recycling endosomes. This, together with inhibition of biosynthetic transport and ubiquitin-mediated degradation, enables downregulation of cell surface BST-2 and the counteraction of restriction.
ACKNOWLEDGMENTS
Several antisera, as noted in Materials and Methods, were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH. We thank Chugai Pharmaceutical Co., Kanagawa, Japan, for providing the antibody to HM1.24 (BST-2); Klaus Strebel, Paula Cannon, Jacek Skowronski, Juan Bonifacino, Olivier Schwartz, Massimo Pizzato, and Mark McNiven for plasmids; the USCD CFAR for the p24 ELISAs and flow cytometry support; and Andrey Tokarev for discussions.
This work was supported by grant R01AI081668 from the NIAID, National Institutes of Health.
Footnotes
Published ahead of print on 3 August 2011.
REFERENCES
- 1. Abada P., Noble B., Cannon P. M. 2005. Functional domains within the human immunodeficiency virus type 2 envelope protein required to enhance virus production. J. Virol. 79:3627–3638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Andrew A. J., Miyagi E., Strebel K. 2011. Differential effects of human immunodeficiency virus type 1 Vpu on the stability of BST-2/tetherin. J. Virol. 85:2611–2619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bonifacino J. S., Traub L. M. 2003. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 72:395–447 [DOI] [PubMed] [Google Scholar]
- 4. Douglas J. L., et al. 2009. Vpu directs the degradation of the human immunodeficiency virus restriction factor BST-2/Tetherin via a {beta}TrCP-dependent mechanism. J. Virol. 83:7931–7947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dube M., et al. 2010. Antagonism of tetherin restriction of HIV-1 release by Vpu involves binding and sequestration of the restriction factor in a perinuclear compartment. PLoS Pathog. 6:e1000856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fitzpatrick K., et al. 2010. Direct restriction of virus release and incorporation of the interferon-induced protein BST-2 into HIV-1 particles. PLoS Pathog. 6:e1000701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ford M. G., et al. 2001. Simultaneous binding of PtdIns(4,5)P2 and clathrin by AP180 in the nucleation of clathrin lattices on membranes. Science 291:1051–1055 [DOI] [PubMed] [Google Scholar]
- 8. Goffinet C., et al. 2009. HIV-1 antagonism of CD317 is species specific and involves Vpu-mediated proteasomal degradation of the restriction factor. Cell Host Microbe 5:285–297 [DOI] [PubMed] [Google Scholar]
- 9. Habermann A., et al. 2010. CD317/tetherin is enriched in the HIV-1 envelope and downregulated from the plasma membrane upon virus infection. J. Virol. 84:4646–4658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hauser H., et al. 2010. HIV-1 Vpu and HIV-2 Env counteract BST-2/tetherin by sequestration in a perinuclear compartment. Retrovirology 7:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Henley J. R., Krueger E. W., Oswald B. J., McNiven M. A. 1998. Dynamin-mediated internalization of caveolae. J. Cell Biol. 141:85–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Iwabu Y., et al. 2009. HIV-1 accessory protein Vpu internalizes cell-surface BST-2/tetherin through transmembrane interactions leading to lysosomes. J. Biol. Chem. 284:35060–35072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Janvier K., et al. 2011. The ESCRT-0 component HRS is required for HIV-1 Vpu-mediated BST-2/tetherin down-regulation. PLoS Pathog. 7:e1001265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jia B., et al. 2009. Species-specific activity of SIV Nef and HIV-1 Vpu in overcoming restriction by tetherin/BST2. PLoS Pathog. 5:e1000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Le Tortorec A., Neil S. J. 2009. Antagonism and intracellular sequestration of human tetherin by the HIV-2 envelope glycoprotein. J. Virol. 83:11966–11978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lopez L. A., et al. 2010. Ebola virus glycoprotein counteracts BST-2/Tetherin restriction in a sequence-independent manner that does not require tetherin surface removal. J. Virol. 84:7243–7255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marks M. S., Woodruff L., Ohno H., Bonifacino J. S. 1996. Protein targeting by tyrosine- and di-leucine-based signals: evidence for distinct saturable components. J. Cell Biol. 135:341–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miller S. G., Carnell L., Moore H. H. 1992. Post-Golgi membrane traffic: brefeldin A inhibits export from distal Golgi compartments to the cell surface but not recycling. J. Cell Biol. 118:267–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mitchell R. S., et al. 2009. Vpu antagonizes BST-2-mediated restriction of HIV-1 release via beta-TrCP and endo-lysosomal trafficking. PLoS Pathog. 5:e1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moll M., Andersson S. K., Smed-Sorensen A., Sandberg J. K. 2010. Inhibition of lipid antigen presentation in dendritic cells by HIV-1 Vpu interference with CD1d recycling from endosomal compartments. Blood 116:1876–1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Neil S. J., Eastman S. W., Jouvenet N., Bieniasz P. D. 2006. HIV-1 Vpu promotes release and prevents endocytosis of nascent retrovirus particles from the plasma membrane. PLoS Pathog. 2:e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Neil S. J., Zang T., Bieniasz P. D. 2008. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 451:425–430 [DOI] [PubMed] [Google Scholar]
- 23. Ohno H., et al. 1997. Interaction of endocytic signals from the HIV-1 envelope glycoprotein complex with members of the adaptor medium chain family. Virology 238:305–315 [DOI] [PubMed] [Google Scholar]
- 24. Perez-Caballero D., et al. 2009. Tetherin inhibits HIV-1 release by directly tethering virions to cells. Cell 139:499–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pizzato M., et al. 2007. Dynamin 2 is required for the enhancement of HIV-1 infectivity by Nef. Proc. Natl. Acad. Sci. U. S. A. 104:6812–6817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Raiborg C., et al. 2002. Hrs sorts ubiquitinated proteins into clathrin-coated microdomains of early endosomes. Nat. Cell Biol. 4:394–398 [DOI] [PubMed] [Google Scholar]
- 27. Raiborg C., Bache K. G., Mehlum A., Stang E., Stenmark H. 2001. Hrs recruits clathrin to early endosomes. EMBO J. 20:5008–5021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Raiborg C., Wesche J., Malerod L., Stenmark H. 2006. Flat clathrin coats on endosomes mediate degradative protein sorting by scaffolding Hrs in dynamic microdomains. J. Cell Sci. 119:2414–2424 [DOI] [PubMed] [Google Scholar]
- 29. Rollason R., Korolchuk V., Hamilton C., Schu P., Banting G. 2007. Clathrin-mediated endocytosis of a lipid-raft-associated protein is mediated through a dual tyrosine motif. J. Cell Sci. 120:3850–3858 [DOI] [PubMed] [Google Scholar]
- 30. Rong L., et al. 2009. The transmembrane domain of BST-2 determines its sensitivity to down-modulation by human immunodeficiency virus type 1 Vpu. J. Virol. 83:7536–7546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ruiz A., Hill M. S., Schmitt K., Guatelli J., Stephens E. B. 2008. Requirements of the membrane proximal tyrosine and dileucine-based sorting signals for efficient transport of the subtype C Vpu protein to the plasma membrane and in virus release. Virology 378:58–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schmidt S., Fritz J. V., Bitzegeio J., Fackler O. T., Keppler O. T. 2011. HIV-1 Vpu blocks recycling and biosynthetic transport of the intrinsic immunity factor CD317/tetherin to overcome the virion release restriction. mBio 2(3):e000036–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Skasko M., et al. 2011. BST-2 is rapidly down-regulated from the cell surface by the HIV-1 protein Vpu: evidence for a post-ER mechanism of Vpu-action. Virology 411:65–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tokarev A. A., Munguia J., Guatelli J. C. 2010. Serine-threonine ubiquitination mediates downregulation of BST-2/tetherin and relief of restricted virion release by HIV-1 Vpu. J. Virol. 85:551–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Van Damme N., et al. 2008. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe 3:245–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Van Damme N., Guatelli J. 2008. HIV-1 Vpu inhibits accumulation of the envelope glycoprotein within clathrin-coated, Gag-containing endosomes. Cell. Microbiol. 10:1040–1057 [DOI] [PubMed] [Google Scholar]
- 37. van der Bliek A. M., et al. 1993. Mutations in human dynamin block an intermediate stage in coated vesicle formation. J. Cell Biol. 122:553–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Varthakavi V., et al. 2006. The pericentriolar recycling endosome plays a key role in Vpu-mediated enhancement of HIV-1 particle release. Traffic 7:298–307 [DOI] [PubMed] [Google Scholar]
- 39. Vincent M. J., Abdul Jabbar M. 1995. The human immunodeficiency virus type 1 Vpu protein: a potential regulator of proteolysis and protein transport in the mammalian secretory pathway. Virology 213:639–649 [DOI] [PubMed] [Google Scholar]