Abstract
The P protein of parainfluenza virus 5 (PIV5) is an essential cofactor of the viral RNA-dependent RNA polymerase. Phosphorylation of the P protein can positively or negatively regulate viral gene expression, depending on the precise phosphorylation sites. Sumoylation, a process of adding small ubiquitin-like modifier (SUMO) to proteins posttranslationally, plays an important role in regulating protein function. In this study, we have found that the P protein of PIV5 was sumoylated with SUMO1 in both transfected and infected cells. The K254 residue of the P protein is within a consensus sumoylation motif. Mutation of the P protein at K254 to arginine (P-K254R) reduced PIV5 minigenome activity, as well as the sumoylation level of the P protein. Incorporation of K254R into a recombinant PIV5 (rPIV5-P-K254R) resulted in a virus that grew to a lower titer and had lower levels of viral RNA synthesis and protein expression than wild-type PIV5, suggesting that sumoylation of the P protein at K254 is important for PIV5 growth. Biochemical studies did not reveal any defect of P-K254R in its interactions with viral proteins NP and L or formation of homotetramers. We propose that sumoylation of the P protein at K254 regulates PIV5 gene expression through a host protein.
INTRODUCTION
Small ubiquitin-related modifier, or small ubiquitin-like modifier (SUMO), was first identified in 1996 as a protein that is covalently linked to a target protein posttranslationally (24). Many proteins, including RanGap1, Sp3, ERK, P53, IκBα, PIAS, STAT1, MDA5, and RIG-I, have been found to be covalently linked to SUMO through a process called sumoylation (7, 22, 25, 28–30, 35, 43). Sumoylated proteins are involved in transcriptional regulation, nuclear-cytosolic transport, protein stability, stress response, signal transduction, DNA repair, and the cell cycle (1, 2, 9, 15, 36, 38). The reversible process of sumoylation is carried out similarly to ubiquitination through activation, conjugation, and ligation (9, 15). Immature SUMO is cleaved by SUMO-specific isopeptidases (sentrin-specific proteases [SENP]) to expose its C-terminal Gly-Gly motif to become mature SUMO. Activation of mature SUMO protein, carried out by the SUMO-specific E1-activating enzyme, forms a SUMO-adenylate conjugate as an intermediate. SUMO is then transferred to an E2-conjugating enzyme, such as Ubc9 for SUMO1. Finally, the E2-conjugating enzyme transfers SUMO to a substrate, a reaction which E3 ligase may facilitate and for which it may determine the specificity (9). Unlike in ubiquitination, a poly-SUMO1 chain does not occur at a single site because there is no lysine residue within SUMO1 protein to serve as an acceptor for additional SUMO. Furthermore, sumoylation is not known to mediate protein degradation.
SUMO proteins are approximately 100 amino acid residues in length and 12 kDa in size. There are four SUMO isoforms encoded by the human genome, SUMO1, SUMO2, SUMO3, and SUMO4. SUMO1, SUMO2, and SUMO3 are ubiquitously expressed; however, SUMO4 is not. It is unclear whether SUMO4 can be processed to its mature form in vivo (9). The mature forms of SUMO2 and SUMO3 share 97% identity, but they have only 50% identity with SUMO1 (9). As a result, SUMO1 and SUMO2/3 are conjugated to different target proteins and serve different functions. While SUMO1 is conjugated to target protein as a monomer, SUMO2/3 can be conjugated as a monomer or a polymer (33).
Among the hundreds of sumoylated proteins, most are nuclear proteins (4, 15). Viral proteins that have been found to be sumoylated are mostly from DNA viruses; examples include IE2p86 and IE1p72 of human cytomegalovirus (HCMV) (3, 14, 18) and E1, E2, and L2 capsid protein of human papillomavirus (HPV) (23, 26, 39). For RNA viruses, sumoylation of viral proteins has also been detected. Sumoylation of Gag protein of human immunodeficiency virus (HIV) may affect HIV infectivity (12). Sumoylation of CA protein of Moloney murine leukemia virus (MMLV) has been found to be important in early events during virus infection (44). The NS1 proteins of most influenza virus strains are sumoylated, which enhances NS1 stability and promotes rapid growth of virus (41). It has been reported that viral proteins can promote or reduce sumoylation of host proteins (4, 16, 37). For example, the VP35 protein of Ebola Zaire virus has been found to block type I interferon (IFN) production by increasing sumoylation of PIAS1 (protein inhibitor of activated STAT1) (6). It was reported that the L3 endoprotease of adenovirus, I7 protein of poxvirus, and S273R protein of African swine fever virus (ASFV) have sequence and functional similarity to SUMO-specific protease Ulp1 (the yeast homolog of SENP, which can process the SUMO1 precursor and remove SUMO from substrates), indicating that these viral proteases may process sumoylation and desumoylation of viral or host proteins (19, 37).,
Parainfluenza virus 5 (PIV5) is a prototypic member of the paramyxovirus family, which contains many important human and animal pathogens, including mumps virus (MuV), measles virus (MeV), Sendai virus (SeV), and the emerging Hendra virus (HeV) and Nipah virus (NiV) (17). The negative-stranded RNA genome of PIV5 is composed of seven genes and yet encodes eight known proteins (17). The viral RNA-dependent RNA polymerase consists of large (L) protein and phosphoprotein (P). The L protein has enzymatic activities capable of initiation, elongation, and termination of viral RNA synthesis, as well as addition of the 5′ cap structure and 3′ poly(A) sequence to the viral mRNA. The P protein is the cofactor for the polymerase, playing an essential role in regulating viral RNA synthesis (17). The P protein is heavily phosphorylated, and its phosphorylation status regulates the function of the P protein in viral RNA synthesis (8, 31, 31a, 34). In this study, we have investigated the role of P protein sumoylation in regulating viral gene expression.
MATERIALS AND METHODS
Plasmids, viruses and cells.
Human SUMO1, SUMO2, and SUMO3 genes were purchased (Open Biosystems) and cloned into pCAGGS vector. Plasmids encoding the wild-type P protein (P-WT), P-K254R, His-P, His-P-K254R, and the full-length rPIV5-P-K254R were made similarly to what has been previously described (13, 31, 34). rPIV5-P-K254R virus was recovered from the plasmid as previously described (31). The whole genome of the mutant virus rPIV5-P-K254R was confirmed by sequencing. All cell lines used in this study were grown at 37°C with 5% CO2. MDBK and HeLa cells were grown in Dulbecco's modified Eagle medium (DMEM) (Invitrogen) containing 10% fetal bovine serum (FBS) and 100 IU/ml penicillin–100 μg/ml streptomycin. BHK cells were grown in the same medium as for MDBK cells plus 10% tryptose phosphate broth (TPB). The medium for BSR-T7 cells contains an additional 400 μg/ml G418 (5). The growth medium for infected cells contains only 2% FBS.
Immunoblotting (IB).
To detect sumoylation of the P protein, SUMO1, SUMO2, or SUMO3 was cotransfected with P into BSR-T7 cells. At 20 to 24 h posttransfection, the cells were lysed with whole-cell extraction buffer (WCEB) (50 mM Tris-HCl [pH 8], 280 mM NaCl, 0.5% NP-40, 0.2 mM EDTA, 2 mM EGTA, and 10% glycerol) (34). The lysates were centrifuged, and the supernatants were mixed with the same volume of 2× SDS loading buffer (100 mM Tris-HCl [pH 6.8], 20% glycerol, 4% SDS, 200 mM dithiothreitol [DTT], and 0.1% bromophenol blue) (34), heated at 95°C for 5 min, and resolved by 10% SDS-PAGE. The proteins were transferred onto a methanol-soaked polyvinylidene difluoride (PVDF) (Millipore) membrane. The membrane was incubated with mouse anti-P (Pk) antibody, followed by incubation with anti-mouse secondary antibody labeled with horseradish peroxidase (HRP). After washing, the PVDF membrane was incubated with ECL Advance Substrate (GE Healthcare) and scanned using a Kodak Image Station 440. To detect viral protein expression, MDBK cells were infected with PIV5 or rPIV5-P-K254R at a multiplicity of infection (MOI) of 3. The cells were lysed with WCEB at different time points. The proteins were resolved by SDS-PAGE, followed by transfer as described above. Mouse anti-NP antibody and Pk antibody (which recognize both P and V protein) were used to detect viral proteins NP, P, and V. β-Actin was used as a protein loading control.
IP-IB.
To further confirm sumoylation of the P protein, SUMO1 and P were transfected separately or together into BSR-T7 cells. The cells were lysed with WCEB, and the supernatants were incubated with mouse anti-P and protein G-Sepharose beads at 4°C for 2 to 3 h. The immunoprecipitation (IP) products were washed three times with WCEB and mixed with SDS loading buffer, followed by SDS-PAGE. Proteins were detected using immunoblotting as described above. Three individual experiments were performed, and the relative level of P protein sumoylation was normalized and quantified. To detect sumoylation of the P protein in infected cells, HeLa cells were mock infected or infected with PIV5 or rPIV5-P-K254R at an MOI of 5. At 24 h postinfection (hpi), the cells were lysed with WCEB, and IP-IB experiments similar to those described above were performed. To compare the interaction between L and P/P-K254R, 3 μg Flag-L was cotransfected with 1 μg P or P-K254R. Anti-Pk antibody recognizing P was used for immunoprecipitation, followed by immunoblotting with anti-Flag antibody. An aliquot of the cell lysate was used for immunoblotting to show the input amount of Flag-L and P/P-K254R.
PIV5 minigenome system and dual luciferase assay.
The PIV5 minigenome system used in this study had been previously described (31). Briefly, increasing amounts (0.01 to 0.16 μg/well, 24-well plate, 4 replicates for each condition) of P-WT or P-K254R were transfected together with other plasmids (0.2 μg pSMG-Rluc, 0.2 μg NP, 0.3 μg L, and 1 ng FF-Luc) into BSR-T7 cells. After 20 to 22 h, the cells were lysed with passive lysis buffer and 1/10 of the lysate from each well were used for dual-luciferase assay (Promega). Relative luciferase activity was normalized as the ratio of Renilla luciferase (R-Luc) activity to firefly luciferase (FF-Luc) activity. An aliquot of the cell lysate from transfected cells was used for immunoblotting to detect the input amount of NP and P-WT/P-K254R. For the PIV5 transcription-only minigenome system, a mutant minigenome plasmid, pSMG-m-Rluc, carrying a deletion in the region important for PIV5 replication was used (34). The experimental process was similar to that for the PIV5 minigenome system; however, the dual-luciferase assay was performed at 2 days posttransfection.
Protein purification and CD.
Circular dichroism (CD) was performed using His-P-WT and His-P-K254R purified from bacteria. Briefly, P-WT or P-K254R with 8 histidines (His) at the N terminus in a pET15b vector was transformed into BL21(DE3)PolyS competent cells. A single colony was selected and grown in lysogeny broth (LB) with ampicillin (50 ng/ml) and chloramphenicol (34 ng/ml). When the optical density at 600 nm (OD600) of the bacteria was within the range of 0.5 to 0.6, isopropyl β-d-1-thiogalactopyranoside (IPTG) was added to the bacterial culture at a 1 mM final concentration to induce protein expression for 4 h at 37°C. Proteins were purified using nickel (Ni)-charged resin (Novagen) and examined by SDS-PAGE and Coomassie blue staining (50% methanol, 10% acetic acid, 0.25% Coomassie brilliant blue R250). The purified proteins were desalted and resuspended in a buffer containing 10 mM potassium phosphate (KH2PO4) and 10 mM potassium chloride (KCl) (pH 7.0). Three hundred microliters of 10 μM His-P-WT and His-P-K254R was analyzed on a Jasco-J715 spectropolarmeter using a 0.1-cm-path-length cuvette (10). Three measurements were taken for each sample, and the average of millidegrees is shown.
Growth curve and plaque assay.
MDBK cells in 6-well plates were infected with PIV5 or rPIV5-P-K254R at an MOI of 0.01. The supernatants were collected at 0, 1, 2, 3, 4, and 5 days postinfection and centrifuged to remove cell debris. For high-MOI infection, MDBK cells in 6-well plates were infected with PIV5 or rPIV5-P-K254R at an MOI of 3, and the supernatants were collected at 0, 12, 24, 36, and 48 h postinfection. BHK cells in 6-well plates were infected with the virus stocks in serial dilution (1:10 to 1:107). After 2 h, the inoculating mixture was removed and replaced with 5 ml DMEM containing 2% FBS, 10% TPB, 100 IU/ml penicillin, 100 μg/ml streptomycin, and 1% low-melting-point agarose. The plaques were counted at 4 to 5 days postinfection. Three replicates for each time point were collected for statistical analysis.
Flow cytometry.
To further compare viral protein expression levels in PIV5- and rPIV5-P-K254R-infected cells, flow cytometry was performed as previously described (20, 31, 32). MDBK cells were mock infected or infected with PIV5 or rPIV5-P-K254R at an MOI of 3. The cells were collected at different time points, fixed with 0.5% formaldehyde, and resuspended in 0.5 ml DMEM-FBS (1:1), and 1.5 ml 70% ethanol was added to permeabilize the cells. After washing, the cells were incubated with Pk antibody (anti-P/V) and secondary antibody against mouse labeled with fluorescein isothiocyanate (FITC). The mean fluorescence intensity was measured using an LSRII flow cytometer (BD). Similar flow cytometry was performed in MDBK, HeLa, and BSR-T7 cells at 10 h postinfection (hpi) with an MOI of 1.
RT and real-time RT-PCR.
MDBK cells in 6-well plates were infected with PIV5 or rPIV5-P-K254R at an MOI of 3 or 0.5. Total RNAs from the infected cells were extracted using the RNeasy minikit (Qiagen) at different time points. One-tenth of the total RNA from each sample was used for reverse transcription (RT) using Superscript III reverse transcriptase (Invitrogen). Oligo(dT)15 was used in RT to detect viral mRNA level; BH191 annealing to the M gene of the genomic RNA was used in RT to measure the viral genome level. One percent of the cDNAs from RT were used for real-time PCR on the Step One Plus real-time PCR system as described before (34). Relative levels of viral mRNA and viral genome were determined by calculating ΔCT and normalized with the level of the input genome, defined as the viral genome level at 2 hpi. Three replicates for each sample were used for statistical analysis.
Immunoprecipitation and DSP cross-linking.
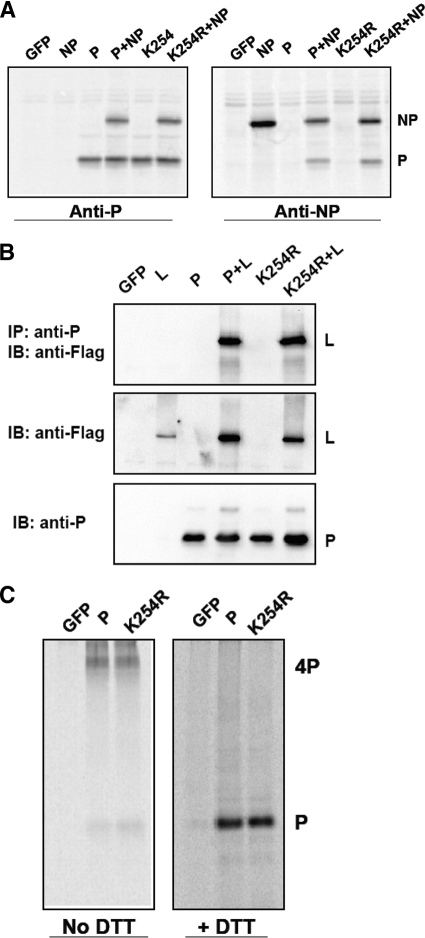
To compare interactions between NP and P-WT/P-K254R, BSR-T7 cells in 6-cm plates were transfected with 1 μg NP together with 1 μg P-WT or P-K254R. After 18 to 20 h, the cells were starved and metabolically labeled with [35S]Met-Cys for 3 h. The labeled cells were lysed with WCEB and immunoprecipitated with either anti-P or anti-NP antibody. The IP products were washed and resolved by SDS-PAGE. The gel was dried, and the proteins were visualized using a Typhoon 9700 phosphorimager (GE Healthcare) (see Fig. 8). To detect tetramer formation of P-WT or P-K254R, the transfected BSR-T7 cells were starved and labeled with [35S]Cys-Met for 3 h at 37°C. The labeled cells were incubated with 1 mM disuccinimidyltartarate (DSP) (Pierce, Rockford, IL) in phosphate-buffered saline (PBS)–0.5% NP-40 to cross-link the disulfide bond as previously described (34). After cross-linking, the cells were lysed with WCEB and immunoprecipitation was performed as described above using anti-P antibody. After washing, half of the IP products were mixed with SDS loading buffer without DTT to detect P tetramer, and half of the IP products were mixed with SDS loading buffer with 200 mM DTT to show the P band after removing the cross-linking. The mixture was resolved in a 10% SDS gel, and the proteins were visualized using a Typhoon 9700 phosphorimager (GE Healthcare).
Fig. 8.
Interactions of P with NP, L, and itself. (A) Interaction between NP and P-WT/P-K254R. BSR-T7 cells were transfected with NP, along with P-WT or P-K254R, and metabolically labeled with [35S]Met-Cys. Lysates of the labeled cells were used for immunoprecipitation with anti-P antibody or anti-NP antibody. (B) Interaction between L and P-WT/P-K254R. BSR-T7 cells were transfected with Flag-L along with P-WT or P-K254R. The transfected cells were lysed and used for IP with anti-P antibody and IB using anti-Flag antibody. Aliquots of the cells lysates were used for immunoblotting to show the input amounts of L and P-WT/P-K254R. (C) Tetramer formation of P-WT/P-K254R. The transfected cells were labeled with [35S]Met-Cys, and DSP was used for cross-linking followed by immunoprecipitation using anti-P antibody. Half of the IP products were mixed with SDS loading buffer without DTT, and the other half were mixed with SDS loading buffer with DTT.
RESULTS
The P protein is sumoylated by SUMO1 but not by SUMO2 or SUMO3.
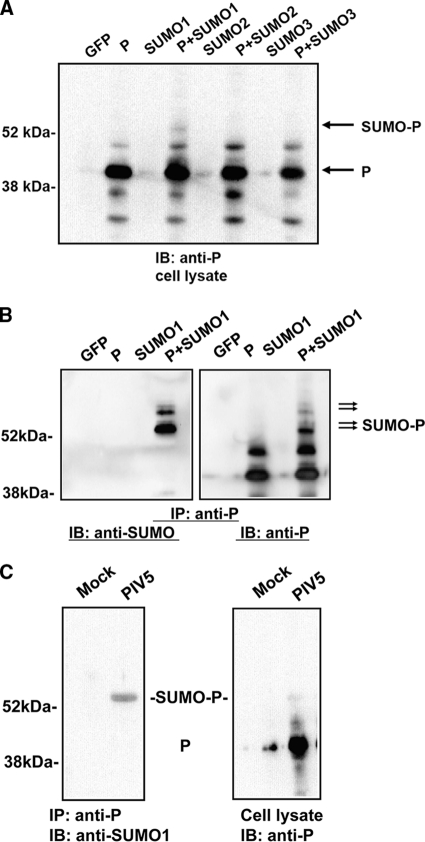
To investigate whether the P protein is sumoylated, P and SUMO1, SUMO2, or SUMO3 were cotransfected into cells and modification of the P protein was examined using immunoblotting with Pk antibody (anti-P). A slower-moving band, consistent with the size of the P protein plus 12 kDa, was detected in the cells transfected with both P and SUMO1, indicating that the P protein might be sumoylated by SUMO1 (Fig. 1A). Interestingly, there was no corresponding band in P- and SUMO2-transfected cells or in P- and SUMO3-transfected cells, suggesting that P may not be sumoylated by SUMO2 or SUMO3. Similar results were observed using Flag-tagged SUMO1, -2, and -3 (data not shown). To further confirm that the slower-moving P band was indeed sumoylated P protein, an IP-IB experiment was performed using mouse anti-P antibody for IP and rabbit anti-SUMO1 (Santa Cruz Biotechnology) for IB. The slower-moving band was recognized by both anti-P antibody and anti-SUMO1 antibody; the difference in size between the P protein band and the slower-moving band is about 12 kDa, indicating that the P protein was sumoylated by SUMO1. Additional sumoylation products were also detected in the lane containing P plus SUMO1. Since a poly-SUMO1 chain cannot form at the same site, there are likely multiple sumoylation sites within the P protein. The same results were obtained using different anti-P antibodies (data not shown), indicating that the P protein is sumoylated. Sumoylation of the P protein was also detected in PIV5-infected cells using a similar IP-IB approach (Fig. 1C). Interestingly, even though the antibody used (Pk) recognizes both V and P, which precipitate with NP, no sumoylated NP or V was detected.
Fig. 1.
Sumoylation of the P protein by SUMO1. (A) Immunoblotting. P was cotransfected with SUMO1, SUMO2, or SUMO3 into BSR-T7 cells. After 24 h, the cells were lysed and an aliquot of the supernatant was resolved by SDS-PAGE. Anti-P antibody (Pk) was used for immunoblotting. The green fluorescent protein (GFP) gene was used as a control. (B) IP-IB of transfected cells. P was cotransfected with SUMO1 in BSR-T7 cells. At 20 to 24 h posttransfection, the cells were lysed. Anti-P antibody (Pk, from mouse) was used for IP. Anti-SUMO1 antibody (from rabbit) and a different anti-P antibody (from rabbit) were used for IB. (C) IP-IB of infected cells. HeLa cells were mock infected or infected with PIV5. At 24 h postinfection, the cells were lysed and a similar IP-IB was performed.
Identification of one consensus sumoylation site within the P protein.
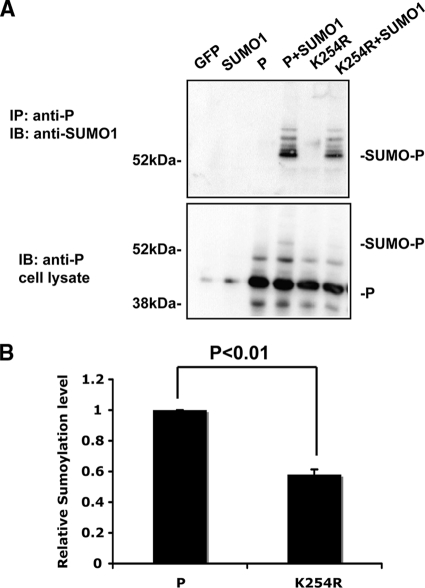
Most sumoylated proteins share a consensus motif, ΨKXD/E, with a hydrophobic amino acid residue Ψ at the −1 location and an acidic amino acid residue at +2; +1 can be any amino acid residue (15, 27). We have identified several potential sumoylation sites within the P protein. K254 of the P-WT protein fits the consensus motif the best. To investigate whether K254 of the P-WT protein is indeed a sumoylation site, a K254R mutation of the P protein was generated and analyzed. The K254R mutation reduced the sumoylation level of the P protein by approximately 40% in transfected cells (Fig. 2A and B), indicating that K254 is a sumoylation site of the P protein. The statistically significant reduction of sumoylation, but not a complete lack of sumoylation, of P-K254R indicates that there are other sumoylation sites within the P protein, consistent with the observation in Fig. 1.
Fig. 2.
K254 of the P protein is one sumoylation site. (A) Sumoylation of P-K254R. K254 was mutated to arginine (R), and this P-K254R was used for IP-IB to compare the sumoylation level to that of wild-type P. (B) Quantification of the sumoylation level. Data from three individual experiments from panel A were used for quantification and statistical analysis. The ratio of the singly sumoylated P protein to total P protein was set to 1 for wild-type P protein, and the relative sumoylation level of P-K254R was normalized to that of wild-type P. The P value was calculated using a t test.
The K254R mutation reduces PIV5 minigenome activity.
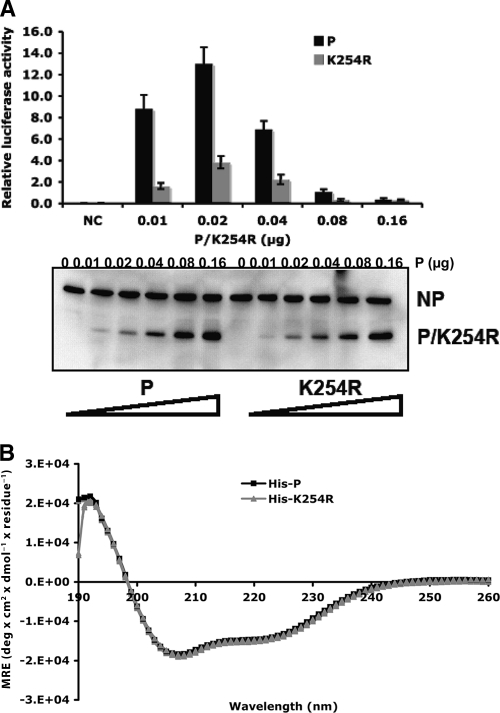
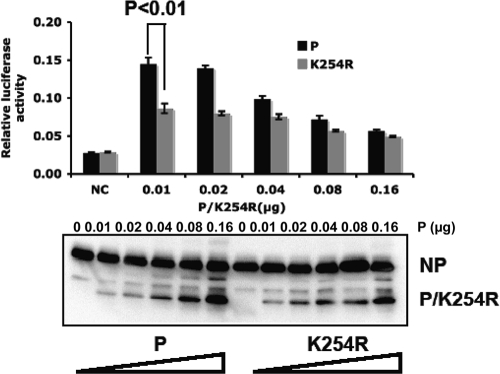
To examine whether sumoylation of the P-WT protein plays a role in regulating PIV5 gene expression, the effect of a K254R mutation in the P protein was studied using a PIV5 minigenome system. It is known that the ratio of P to NP affects the level of viral RNA synthesis and that too high a level of expression of P inhibits viral gene expression. To ensure that the maximal level of viral gene expression was detected in the minigenome system, a range of concentrations of P was used and expression levels of P and NP were examined. P-K254R significantly reduced minigenome activity (Fig. 3A), suggesting that sumoylation of the P-WT protein at K254 affects PIV5 gene expression. To investigate the possibility that the negative impact of the P-K254R mutation on viral gene expression may be due to a change of protein secondary structure, circular dichroism was performed to compare the secondary structures of P-K254R and the P protein. These two proteins showed identical absorption from wavelength 190 to 260 nm (Fig. 3B), suggesting that the K-to-R mutation at amino acid residue 254 did not affect the secondary structure of the P protein.
Fig. 3.
P-K254R has lower activity in PIV5 minigenomes. (A) Minigenome activity of P-K254R. Increasing amounts of P-K24R or the P protein were used in the minigenome system. Immunoblotting was performed to show the input amounts of NP, P, or P-K254R. (B) CD analysis of His-P-WT and His-P-K254R purified from bacteria.
K254 of the P-WT protein is a sumoylation site in PIV5-infected cells.
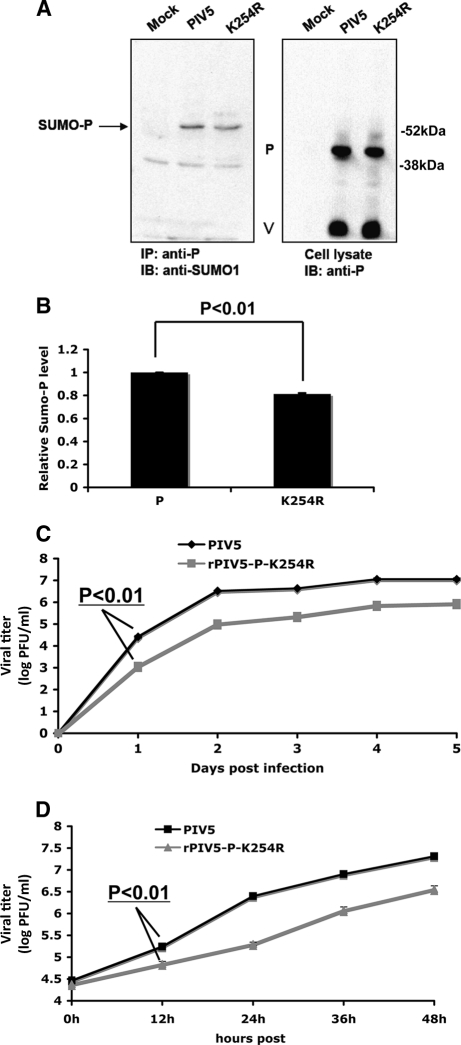
To examine the role of sumoylation of the P protein in virus infection, the K254R mutation was incorporated into PIV5 to generate a recombinant virus (rPIV5-P-K254R). The genome of the mutant virus rPIV5-P-K254R was sequenced, and no other amino acid residue change was detected. The sumoylation level of P-K254R in rPIV5-P-K254R-infected cells was compared with that of the P protein in PIV5-infected cells. There was a lower level of P-K254R sumoylation in rPIV5-P-K254R virus-infected cells, indicating that K254 is a sumoylation site in PIV5-infected cells (Fig. 4A and B). Interestingly, the reduction of sumoylation is modest, indicating that there is an additional sumoylation site(s) or an alternate sumoylation site is sumoylated. As in Fig. 1C, only sumoylated P was detected and no sumoylated NP or V was detected in infected cells, suggesting that NP and V are not sumoylated. Growth of rPIV5-P-K254R virus was characterized with low-MOI (0.01) (Fig. 4C) and high-MOI (3) (Fig. 4D) infection in MDBK cells. rPIV5-P-K254R grew slower and to a lower titer than PIV5, suggesting that mutation of the P protein at K254 caused a defect in growth of PIV5.
Fig. 4.
Sumoylation of K254R in virus infection. (A) Sumoylation of P-K254R in infected cells. HeLa cells were mock infected or infected with PIV5 or rPIV5-P-K254R at an MOI of 5. At 24 hpi, the cells were lysed and the supernatants used for IP-IB. (B) Quantification of the sumoylation levels. Three individual experiments from panel B were performed for quantification and statistical analysis. (C) Growth rate of rPIV5-P-K254R at an MOI of 0.01. MDBK cells were infected with PIV5 or rPIV5-P-K254R, and the supernatants were collected at different time points for plaque assay. (D) Growth rate of rPIV5-P-K254R at an MOI of 3 in MDBK cells.
The K254R mutation reduces viral protein expression.
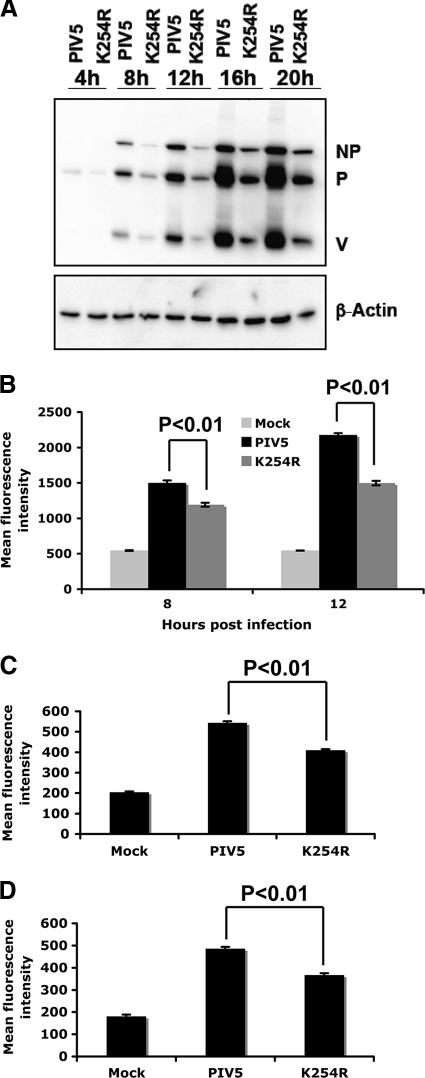
To study whether the defect in rPIV5-P-K254R growth is due to the defect in viral gene expression, levels of viral protein expression in rPIV5-P-K254R-infected cells were compared to those in PIV5-infected cells. At an MOI of 3, a lower level of viral protein expression in rPIV5-P-K254R-infected cells was found at 8 hpi, as well as at later time points, by immunoblotting and flow cytometry (Fig. 5A and B). With low-MOI infection (MOI = 1), rPIV5-P-K254R also showed a significant defect in viral protein expression at 10 hpi (data not shown). In addition, the defect of rPIV5-P-K254R in viral protein expression was also detected in HeLa and BSR-T7 cells, indicating that the phenotype is not cell line specific (Fig. 5C and D).
Fig. 5.
Viral protein expression levels in rPIV5-P-K254R-infected cells. (A) Immunoblotting. MDBK cells were infected with PIV5 or rPIV5-P-K254R at an MOI of 3. The cells were collected at different time points and used for immunoblotting using anti-NP and Pk (anti-P/V) antibodies. β-Actin was used as a protein loading control. K254R indicates rPIV5-P-K254R virus. (B) Flow cytometry. MDBK cells were mock infected or infected with PIV5 or rPIV5-P-K254R at an MOI of 3. Flow cytometry was performed to compare viral protein expression at different time points using Pk antibody. (C and D) Flow cytometry in HeLa (C) and BSR-T7 (D) cells. Similar experiments were performed at an MOI of 1 in HeLa and BSR-T7 cells at 10 hpi.
rPIV5-P-K254R-infected cells have lower levels of viral genome RNA and viral mRNA.
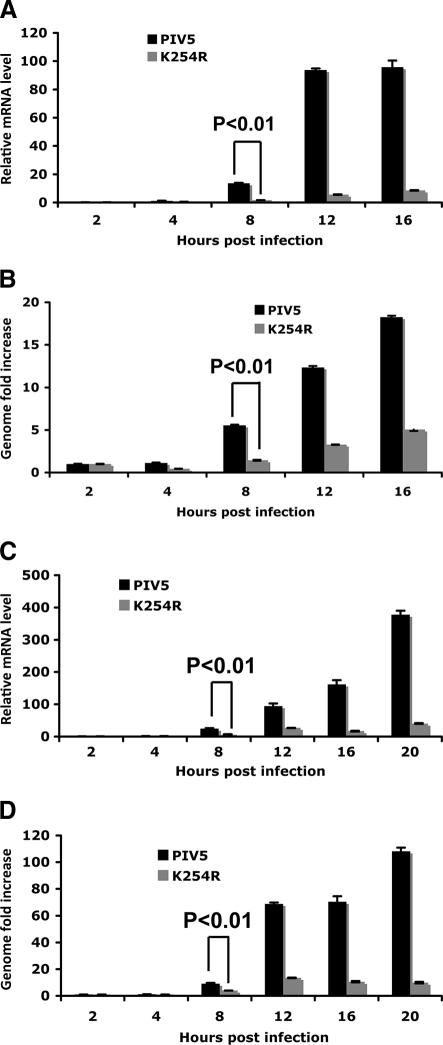
To further examine the mechanism of the defect in rPIV5-P-K254R growth, quantitative real-time RT-PCR (qRT-PCR) was used to compare the levels of viral mRNA and viral genome RNA in MDBK cells. With high-MOI infection (MOI = 3), rPIV5-P-K254R had a lower level of viral mRNA at 8 hpi, as well as at later time points (Fig. 6A). The genome RNA level was also reduced in rPIV5-P-K254R-infected cells at 8 hpi and at later time points (Fig. 6B). Similar results were observed with low-MOI infection (MOI = 0.5). (Fig. 6C and D), suggesting that the K254R mutation reduced viral RNA transcription and replication.
Fig. 6.
Viral RNA levels in rPIV5-P-K254R-infected cells. (A) Viral mRNA levels after high-MOI infection. MDBK cells were infected with PIV5 or rPIV5-P-K254R at an MOI of 3. The cells were collected at different time points for RNA extraction and real-time RT-PCR. Oligo(dT) was used for RT. The relative mRNA levels were normalized with the input genome defined as the viral genome at 2 hpi. (B) Viral genome levels after high-MOI infection. The same RNA from panel A was used for RT using BH191 annealing to the M gene of the viral genome. The cDNA was used for real-time PCR analysis. The genome level at 2 hpi was used as the baseline for normalization. (C) Viral mRNA levels after low-MOI infection. MDBK cells were infected with PIV5 or rPIV5-P-K254R at an MOI of 0.5. Total RNA was extracted from the cells at different time points for RT and real-time PCR analysis. (D) Viral genome levels after low-MOI infection. The same RNA from panel C was used for RT using BH191 to measure viral genome levels.
The K254R mutation affects viral RNA transcription.
To determine which step in viral RNA synthesis was affected by the K254R mutation of the P protein, a transcription-only minigenome, which is functional only in viral transcription and not in replication, was used. P-K254R reduced the activity of the transcription-only minigenome (P < 0.01 by analysis of variance [ANOVA]) (Fig. 7), indicating that P-K254R was defective in viral mRNA transcription.
Fig. 7.
P-K254R affects viral RNA transcription. P-K254R was compared to P-WT using a transcription-only minigenome system. The plasmids were transfected similarly to the normal PIV5 minigenome system. A dual-luciferase assay was carried out at 2 days posttransfection. Aliquots of the cell lysates were used for immunoblotting with anti-NP and anti-P antibodies.
P-K254R does not affect NP-P or P-L interaction or P tetramer formation.
The interactions of NP and P and of P and L, as well as P tetramer formation, are essential for PIV5 RNA synthesis. To study the mechanism of the defect of P-K254R, the NP and P/K254R interaction, and the L and P-WT/P-K254R interaction, were examined using coimmunoprecipitation or IP-IB in transfected cells (Fig. 8A and B). P-K254R can bind to NP and L protein, similarly to P-WT. In addition, P-K254R formed tetramers as does P-WT (Fig. 8C), indicating that there is no defect in P-NP or P-L interactions or tetramer formation.
DISCUSSION
Sumoylation is an important protein posttranslational modification and is involved in many essential cellular processes. In this study, we have found that PIV5 P protein, a viral protein from a nonsegmented, negative-strand RNA virus, was sumoylated by SUMO1 but not by SUMO2 or SUMO3. The mechanism of preference of SUMO1 over SUMO2/3 is not clear. Interestingly, no sumoylation of NP, L, or V was detected (Fig. 1B and 4A). Based on a consensus sumoylation motif, we have identified K254 of the P-WT protein as a sumoylation site. A K254R mutation reduced, but did not eliminate, the sumoylation level of the P protein, suggesting that there are other sumoylation sites within the P protein. Further studies need to identify these extra sumoylation sites and their function. We speculate that the other sumoylation sites are likely in the C terminus of the P protein, since the V protein, which has an N terminus of 164 amino acid residues identical to that of P, was not sumoylated. Sumoylation of the P protein at K254 plays an important role in PIV5 minigenome activity, as well as PIV5 growth, indicating that sumoylation of the P protein may regulate PIV5 gene expression. We also found that sumoylation of the P protein at K254 affected viral RNA transcription, which may lead to a reduced level of viral replication in infected cells. We propose that sumoylation of the P protein regulates viral gene expression through regulating viral RNA transcription.
Among the effects of sumoylation, transcriptional regulation is an important one. In eukaryotes, sumoylation usually has an inhibitory effect on gene transcription. Mutations at the sumoylation sites of transcription factors Elk, Sp-3, STAT-1, and P300 lead to transcriptional activation (15). Although the steady-state sumoylation level is less than 5% of the given protein, most transcription factors can be significantly activated when sumoylation sites are mutated (9). SUMO can also have positive effects on transcription, for example, through activating β-catenin-activated factor Tcf-4 (42). For viruses, most studies suggest that sumoylation plays a positive role in viral gene expression. Bovine papillomavirus (BPV) E1 protein can be sumoylated at K154, and a K154R mutation results in sequestration of E1 in the cytoplasm and therefore loss of replication capacity (26). Sumoylation of human papillomavirus (HPV) E2 proteins affects its activity in both transcriptional activation and repression (40). Sumoylation of HCMV IE2p86 protein is important for IE2-mediated transactivation (14). Despite the different roles of sumoylation of host and viral proteins in transcription, the underlying mechanisms may be similar.
Sumoylation may function through modulating protein-protein interaction or altering substrate conformation (15). SUMO-dependent transcriptional repression is likely due to SUMO-dependent recruitment of downstream effector proteins. For example, only sumoylated p300 can recruit HDAC6, a transcriptional repressor (11). Interestingly, HDAC6 can also interact with PIAS proteins, which also bind directly to SUMO and sumoylated proteins (15, 21). Therefore, sumoylation may be important in the complex formation of transcription factors. Similarly, one possible mechanism for the effect of viral protein sumoylation on viral transcription could be the recruitment of downstream effector proteins, of either the virus or host cells. Our study has shown that P-K254R had no defect in interacting with NP and L proteins or in homotetramer formation by the P protein. We propose that sumoylation of the P protein at K254 regulates PIV5 gene expression through interaction with an as-yet-unidentified host protein(s). However, we cannot exclude the possibility that sumoylation of the P protein at K254 also directly affects viral RNA replication. It is possible that the small portion of sumoylated P behaves differently, such as by having different affinity for its interacting partners.
ACKNOWLEDGMENTS
We appreciate helpful discussion and technical assistance from all the members of Biao He's laboratory. We are grateful to Kaori Sakamoto for carefully reading the manuscript prior to submission.
This work was supported by grants from the National Institute of Allergy and Infectious Disease (R01AI070847, K02AI65795, and R56AI081816) to B.H.
Footnotes
Published ahead of print on 27 July 2011.
REFERENCES
- 1. Andreou A. M., Tavernarakis N. 2009. SUMOylation and cell signalling. Biotechnol. J. 4:1740–1752 [DOI] [PubMed] [Google Scholar]
- 2. Bergink S., Jentsch S. 2009. Principles of ubiquitin and SUMO modifications in DNA repair. Nature 458:461–467 [DOI] [PubMed] [Google Scholar]
- 3. Berndt A., Hofmann-Winkler H., Tavalai N., Hahn G., Stamminger T. 2009. Importance of covalent and noncovalent SUMO interactions with the major human cytomegalovirus transactivator IE2p86 for viral infection. J. Virol. 83:12881–12894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boggio R., Chiocca S. 2006. Viruses and sumoylation: recent highlights. Curr. Opin. Microbiol. 9:430–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Buchholz U. J., Finke S., Conzelmann K. K. 1999. Generation of bovine respiratory syncytial virus (BRSV) from cDNA: BRSV NS2 is not essential for virus replication in tissue culture, and the human RSV leader region acts as a functional BRSV genome promoter. J. Virol. 73:251–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chang T. H., et al. 2009. Ebola Zaire virus blocks type I interferon production by exploiting the host SUMO modification machinery. PLoS Pathog. 5:e1000493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fu J., Xiong Y., Xu Y., Cheng G., Tang H. 2011. MDA5 is SUMOylated by PIAS2beta in the upregulation of type I interferon signaling. Mol. Immunol. 48:415–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fuentes S. M., Sun D., Schmitt A. P., He B. 2010. Phosphorylation of paramyxovirus phosphoprotein and its role in viral gene expression. Future Microbiol. 5:9–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Geiss-Friedlander R., Melchior F. 2007. Concepts in sumoylation: a decade on. Nat. Rev. Mol. Cell Biol. 8:947–956 [DOI] [PubMed] [Google Scholar]
- 10. Gilmore J. M., et al. 2010. Determinants of affinity and activity of the anti-sigma factor AsiA. Biochemistry 49:6143–6154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Girdwood D., et al. 2003. P300 transcriptional repression is mediated by SUMO modification. Mol. Cell 11:1043–1054 [DOI] [PubMed] [Google Scholar]
- 12. Gurer C., Berthoux L., Luban J. 2005. Covalent modification of human immunodeficiency virus type 1 p6 by SUMO-1. J. Virol. 79:910–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. He B., et al. 2002. Recovery of paramyxovirus simian virus 5 with a V protein lacking the conserved cysteine-rich domain: the multifunctional V protein blocks both interferon-beta induction and interferon signaling. Virology 303:15–32 [DOI] [PubMed] [Google Scholar]
- 14. Hofmann H., Floss S., Stamminger T. 2000. Covalent modification of the transactivator protein IE2-p86 of human cytomegalovirus by conjugation to the ubiquitin-homologous proteins SUMO-1 and hSMT3b. J. Virol. 74:2510–2524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Johnson E. S. 2004. Protein modification by SUMO. Annu. Rev. Biochem. 73:355–382 [DOI] [PubMed] [Google Scholar]
- 16. Kubota T., et al. 2008. Virus infection triggers SUMOylation of IRF3 and IRF7, leading to the negative regulation of type I interferon gene expression. J. Biol. Chem. 283:25660–25670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lamb R. A., Parks G. D. 2007. Paramyxovirudae: the viruses and their replication, p. 1450–1497.In Knipe D. M., Howley P. M. (ed.), Fields virology, 5th ed. Lippincott, Williams and Wilkins, Philadelphia, PA. [Google Scholar]
- 18. Lee H. R., Ahn J. H. 2004. Sumoylation of the major immediate-early IE2 protein of human cytomegalovirus Towne strain is not required for virus growth in cultured human fibroblasts. J. Gen. Virol. 85:2149–2154 [DOI] [PubMed] [Google Scholar]
- 19. Li S. J., Hochstrasser M. 1999. A new protease required for cell-cycle progression in yeast. Nature 398:246–251 [DOI] [PubMed] [Google Scholar]
- 20. Lin Y., Bright A. C., Rothermel T. A., He B. 2003. Induction of apoptosis by paramyxovirus simian virus 5 lacking a small hydrophobic gene. J. Virol. 77:3371–3383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Long J., et al. 2003. Repression of Smad transcriptional activity by PIASy, an inhibitor of activated STAT. Proc. Natl. Acad. Sci. U. S. A. 100:9791–9796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mahajan R., Delphin C., Guan T., Gerace L., Melchior F. 1997. A small ubiquitin-related polypeptide involved in targeting RanGAP1 to nuclear pore complex protein RanBP2. Cell 88:97–107 [DOI] [PubMed] [Google Scholar]
- 23. Marusic M. B., Mencin N., Licen M., Banks L., Grm H. S. 2010. Modification of human papillomavirus minor capsid protein L2 by sumoylation. J. Virol. 84:11585–11589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Matunis M. J., Coutavas E., Blobel G. 1996. A novel ubiquitin-like modification modulates the partitioning of the Ran-GTPase-activating protein RanGAP1 between the cytosol and the nuclear pore complex. J. Cell Biol. 135:1457–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mi Z., Fu J., Xiong Y., Tang H. 2010. SUMOylation of RIG-I positively regulates the type I interferon signaling. Protein Cell 1:275–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rangasamy D., Woytek K., Khan S. A., Wilson V. G. 2000. SUMO-1 modification of bovine papillomavirus E1 protein is required for intranuclear accumulation. J. Biol. Chem. 275:37999–38004 [DOI] [PubMed] [Google Scholar]
- 27. Ren J., et al. 2009. Systematic study of protein sumoylation: development of a site-specific predictor of SUMOsp 2.0. Proteomics 9:3409–3412 [DOI] [PubMed] [Google Scholar]
- 28. Ross S., Best J. L., Zon L. I., Gill G. 2002. SUMO-1 modification represses Sp3 transcriptional activation and modulates its subnuclear localization. Mol. Cell 10:831–842 [DOI] [PubMed] [Google Scholar]
- 29. Sapetschnig A., et al. 2002. Transcription factor Sp3 is silenced through SUMO modification by PIAS1. EMBO J. 21:5206–5215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Song L., Bhattacharya S., Yunus A. A., Lima C. D., Schindler C. 2006. Stat1 and SUMO modification. Blood 108:3237–3244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sun D., Luthra P., Li Z., He B. 2009. PLK1 down-regulates parainfluenza virus 5 gene expression. PLoS Pathog. 5:e1000525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31a. Sun D., Luthra P., Xu P., Yoon H., He B. 2011. Identification of a phosphorylation site within the P protein important for mRNA transcription and growth of parainfluenza virus. 5. J. Virol. 85:8376–8385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sun M., et al. 2004. Conserved cysteine-rich domain of paramyxovirus simian virus 5 V protein plays an important role in blocking apoptosis. J. Virol. 78:5068–5078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tatham M. H., et al. 2001. Polymeric chains of SUMO-2 and SUMO-3 are conjugated to protein substrates by SAE1/SAE2 and Ubc9. J. Biol. Chem. 276:35368–35374 [DOI] [PubMed] [Google Scholar]
- 34. Timani K. A., et al. 2008. A single amino acid residue change in the P protein of parainfluenza virus 5 elevates viral gene expression. J. Virol. 82:9123–9133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ungureanu D., et al. 2003. PIAS proteins promote SUMO-1 conjugation to STAT1. Blood 102:3311–3313 [DOI] [PubMed] [Google Scholar]
- 36. Wilkinson K. A., Henley J. M. 2010. Mechanisms, regulation and consequences of protein SUMOylation. Biochem. J. 428:133–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wilson V. G., Rangasamy D. 2001. Viral interaction with the host cell sumoylation system. Virus Res. 81:17–27 [DOI] [PubMed] [Google Scholar]
- 38. Wu S. Y., Chiang C. M. 2009. Crosstalk between sumoylation and acetylation regulates p53-dependent chromatin transcription and DNA binding. EMBO J. 28:1246–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wu Y. C., Deyrieux A. F., Wilson V. G. 2007. Papillomaviruses and the host SUMOylation system. Biochem. Soc. Trans. 35:1433–1435 [DOI] [PubMed] [Google Scholar]
- 40. Wu Y. C., Roark A. A., Bian X. L., Wilson V. G. 2008. Modification of papillomavirus E2 proteins by the small ubiquitin-like modifier family members (SUMOs). Virology 378:329–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xu K., et al. 2011. Modification of nonstructural protein 1 of influenza A virus by SUMO1. J. Virol. 85:1086–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yamamoto H., Ihara M., Matsuura Y., Kikuchi A. 2003. Sumoylation is involved in beta-catenin-dependent activation of Tcf-4. EMBO J. 22:2047–2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yang S. H., Jaffray E., Hay R. T., Sharrocks A. D. 2003. Dynamic interplay of the SUMO and ERK pathways in regulating Elk-1 transcriptional activity. Mol. Cell 12:63–74 [DOI] [PubMed] [Google Scholar]
- 44. Yueh A., et al. 2006. Interaction of Moloney murine leukemia virus capsid with Ubc9 and PIASy mediates SUMO-1 addition required early in infection. J. Virol. 80:342–352 [DOI] [PMC free article] [PubMed] [Google Scholar]