Abstract
Enterovirus 71 (EV71) infections continue to remain an important public health problem around the world, especially in the Asia-Pacific region. There is a significant mortality rate following such infections, and there is neither any proven therapy nor a vaccine for EV71. This has spurred much fundamental research into the replication of the virus. In this review, we discuss recent work identifying host cell factors which regulate the synthesis of EV71 RNA and proteins. Three of these proteins, heterogeneous nuclear ribonucleoprotein A1 (hnRNP A1), far-upstream element-binding protein 2 (FBP2), and FBP1 are nuclear proteins which in EV71-infected cells are relocalized to the cytoplasm, and they influence EV71 internal ribosome entry site (IRES) activity. hnRNP A1 stimulates IRES activity but can be replaced by hnRNP A2. FBP2 is a negative regulatory factor with respect to EV71 IRES activity, whereas FBP1 has the opposite effect. Two other proteins, hnRNP K and reticulon 3, are required for the efficient synthesis of viral RNA. The cleavage stimulation factor 64K subunit (CstF-64) is a host protein that is involved in the 3′ polyadenylation of cellular pre-mRNAs, and recent work suggests that in EV71-infected cells, it may be cleaved by the EV71 3C protease. Such a cleavage would impair the processing of pre-mRNA to mature mRNAs. Host cell proteins play an important role in the replication of EV71, but much work remains to be done in order to understand how they act.
INTRODUCTION
Enterovirus 71 (EV71) is an important neurotropic enterovirus for which there is currently no effective therapy and no vaccine. Outbreaks of infection with this virus have occurred around the world (1, 2, 34, 43, 60, 72, 78, 98). EV71 manifests most frequently as a childhood exanthem known as hand, foot, and mouth disease (HFMD). However, acute EV71 infection can also be associated with severe neurological disease and significant mortality. Children under 5 years old are particularly susceptible to severe forms of EV71-associated neurological complications, including aseptic meningitis, brainstem and/or cerebellar encephalitis, and acute flaccid paralysis, as well as myocarditis and rapid fatal pulmonary edema with hemorrhage (9, 40, 57, 79).
EV71, first described by Schmidt et al. in 1974 (74), belongs to human enterovirus species A of the genus Enterovirus, family Picornaviridae, a large diverse group of small RNA viruses, for which poliovirus is the prototype. EV71 contains a positive-strand RNA genome of approximately 7,400 nucleotides (nt). Although not all the details of EV71 replication are understood, all enteroviruses do share a similar viral genome structure and strategy of replication. The most intensively studied enterovirus is poliovirus, which can be taken as a general model for EV71 (23).
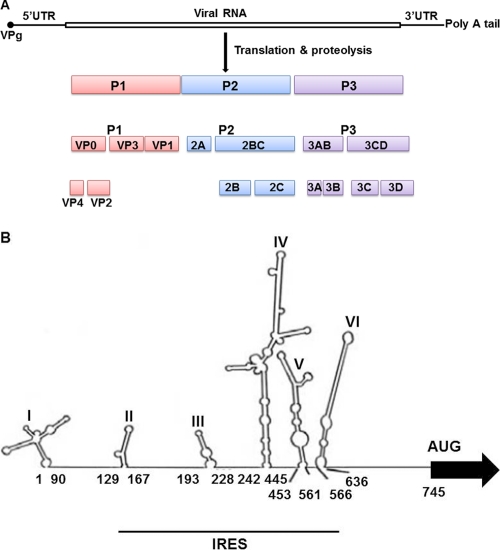
Enteroviruses are nonenveloped viruses about 30 nm in diameter. They encode four structural proteins and have an icosahedral capsid of 60 identical subunits, each of which is made up of one copy of VP1, VP2, and VP3. VP4 is an internal protein. The viral RNA has a small protein called VPg, the 3B protein, covalently attached to its 5′ end and is polyadenylated at its 3′ terminus. EV71 enters into cells via specific receptors: human P-selectin glycoprotein ligand 1 and scavenger receptor B2 (62, 97). After infection of the host cells, the genome, which lacks a 5′cap but has an internal ribosome entry site (IRES) in its 5′ untranslated region (5′UTR), is translated in a cap-independent manner into a single polyprotein, which is subsequently processed by virus-encoded proteases 2Apro and 3Cpro into the structural capsid proteins and the nonstructural proteins; the latter are involved mainly in the replication of the viral RNA. Possibly related to the limited coding capacity of picornavirus genomes, precursor polyproteins as well as mature cleavage products actively participate in viral replication (Fig. 1A) (5, 50, 56, 71, 95, 104).
Fig. 1.
(A) Structure of the EV71 genome. The single open reading frame (ORF) is flanked by highly structured 5′UTR and 3′UTR followed by a poly(A) tail. The 5′ end of the viral genome is covalently bound to the viral VPg protein. The ORF is divided into three regions. P1 encodes four structural proteins, VP1 to -4. P2 and P3 encode seven nonstructural proteins, 2A to 2C and 3A to 3D, respectively. (B) Schematic representation of EV71 5′UTR. The first and the last nucleotides in each stem-loop and the position of the functional initiator AUGs are indicated. The body of the IRES within the 5′UTR is underlined. Reprinted from reference 53 with permission.
There have been several recent review articles describing the clinical features, epidemiology, diagnosis, and pathogenesis of EV71 infection, as well as possible anti-EV71 therapy and vaccine development (45, 64, 83, 93, 96). This review will focus on recent work describing the role of host factors involved in EV71 replication, specifically on viral RNA synthesis and IRES-dependent translation.
HOST FACTORS INVOLVED IN EV71 REPLICATION. (i) IRES-DEPENDENT TRANSLATION INITIATION OF EV71 RNA
Translation initiation of EV71 RNA is dependent on the IRES element which, in concerted action with the 3′UTR, controls the viral replication cycle (65, 85). An IRES is a cis-acting element that forms secondary and tertiary RNA structures that with the assistance of cellular and viral proteins recruits the cellular translation machinery to an internal position in the viral RNA. Accordingly, the translation initiation of picornaviruses is cap independent, and the recruitment of the 40S subunit does not require the 4E subunit of eukaryotic initiation factor (eIF4E) (6, 55, 65). During infection by poliovirus, human rhinovirus, or coxsackievirus, the viral proteases, 3Cpro and 2Apro, cleave several cellular proteins, including the translation initiation factor eIF4G and poly(A)-binding protein (PABP) (39, 44); these cleavages lead to a rapid shutoff of host translation; it should be noted, however, that the shutoff is not synchronous with protein cleavage. Thus, the IRES-mediated initiation of translation allows translation of viral RNA while at the same time host cell translation is shut off.
Since their discovery nearly 2 decades ago in encephalomyocarditis virus (EMCV) and poliovirus (PV) RNA (38, 68), IRESs have been identified for all picornaviruses as well as for an increasing number of other viral and cellular mRNAs. The picornavirus IRES elements have been classified into four distinct groups on the basis of their primary sequences, their secondary structures, and their requirements for optimal activity in vitro (7, 8, 61, 73). EV71 RNA contains a type I IRES (Fig. 1B). The IRES region of EV71 spans about 500 nt, a little more than 100 nt upstream of the functional translation start codon of the polyprotein (85). Type I IRES elements (those of the enteroviruses and rhinoviruses; e.g., PV and human rhinovirus [HRV]) are inefficient in driving translation initiation in the absence of certain cellular proteins. The generation of a proper secondary and ternary structure, including several stem-loops, is crucial for internal translation initiation driven by the picornavirus IRESs (6, 28, 55, 73).
(ii) CANONICAL TRANSLATION FACTORS
IRES-mediated translation initiation of picornaviruses depends on the recognition of the IRES by some of the canonical translation initiation factors. Using purified components, several groups have demonstrated with reconstitution assays that types I and II IRESs of picornaviral RNA require a portion of, but not the entire, eIF4G, eIF4A, eIF2, eIF3, and ATP, but not eIF4E, eIF1, or eIF1A, in order to assemble 48S initiation complexes (3, 22, 42). These requirements of the picornavirus IRESs contrast with those of the IRESs of hepatitis C virus (HCV) and cricket paralysis virus, which require few or no canonical factors (33). The fact that the EV71 IRES is a type I IRES implies that it also requires eIF4A, eIF2, eIF3, and ATP in order to assemble the 48S complex for translation initiation. It has also been reported that a fragment of eIF4G stimulates EV71 IRES activity (86).
(iii) ITAFs
IRES-dependent translation requires a number of trans-acting protein factors, collectively known as IRES-specific trans-acting factors (ITAFs), to recruit the ribosome and initiate translation. These proteins may serve as IRES chaperones, binding to RNA across multiple domains and stabilizing the entire IRES in a structure that is suitable for binding canonical translation factors, and ribosomal subunits (70, 100). Several reports indicate that the activity of these ITAFs is dependent on their subcellular localization (24, 46, 47, 49). While the requirement by EV71 for the eIFs has been well studied, only a few studies have been carried out with the EV71 IRES and the ITAFs with which it interacts. It is likely that there are ITAFs relevant to EV71 replication that remain to be identified and that there is much to be learned about the interactions of ITAFs with the EV71 IRES.
Using streptavidin beads to capture cellular proteins which bound to a biotinylated EV71 5′UTR, and matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) analysis, Lin et al. (51) identified 12 cellular proteins which interact with the 5′UTR of EV71. Among these proteins, polypyrimidine tract-binding protein (PTB), poly(rC)-binding protein 1 (PCBP1; also known as heterogeneous nuclear ribonucleoprotein E [hnRNP E] or hnRNP αCP), PCBP2, the autoantigen La, and upstream N-ras protein (Unr) had previously been shown to interact with the 5′UTR of various picornaviruses and to regulate virus replication. However, there have been no reports pointing to a role for any of these proteins in EV71 replication.
Four other proteins, hnRNP K, hnRNP A1, far-upstream element-binding protein 1 (FBP1), and FBP2, until recently had not been reported to bind to the 5′UTR of any picornavirus or to be involved in the replication of EV71. Below, we discuss the interaction of the EV71 IRES with hnRNP A1, FBP1, and FBP2. In addition, the interaction of EV71 with hnRNP K, which was found to regulate viral RNA replication, will be discussed. It is notable that all four of these nuclear proteins were found to relocate from the nucleus to the cytoplasm after EV71 infection (P.-N. Huang, J.-Y. Lin, N. Locker, Y.-A. Kung, J.-Y. Lin, C.-T. Hung, H.-I. Huang, M.-L. Li, and S.-R. Shih, submitted for publication; 51–53).
hnRNP A1.
Heterogeneous nuclear ribonucleoproteins (hnRNPs) are a family of proteins (named hnRNP A1 to hnRNP U) which have RNA-binding and protein-binding motifs. Several hnRNPs, such as A1, C1/C2, E1/E2, I (PTB), and L, are involved in the translational control of cellular or viral mRNAs which contain IRESs (12, 31, 47, 84, 100). As noted previously, hnRNP A1 and hnRNP K were found to be associated with the EV71 5′UTR and to play essential roles in EV71 replication (51, 53).
hnRNP A1 is a nucleocytoplasmic shuttling protein that functions in many aspects of mRNA metabolism. It is involved in the regulation of alternative splicing, and it antagonizes the activity of serine-arginine rich (SR) family proteins. It influences constitutive splicing by modulating the conformation of mammalian pre-mRNAs, and it is involved in the biogenesis of telomerase and microRNA (13, 15, 58, 86). The shuttling of hnRNP A1 between the nucleus and cytoplasm has been linked to its posttranscription regulatory roles, such as IRES-mediated translation and its effect on mRNA stability (25, 31, 53, 92). The hnRNP A1 protein is composed of 320 amino acids and has two highly conserved RNA recognition motifs (RRMs) at the N terminus and a glycine-rich domain at the C terminus; the latter is involved in protein-protein interactions. The C-terminal 38 amino acids, termed M9, are the signal that mediates shuttling between the nucleus and cytoplasm (81, 91).
hnRNP A1 has been shown to be involved in the replication of many viruses, such as murine hepatitis virus, hepatitis C virus, dengue virus, vesicular stomatitis virus, Sindbis virus, human papillomavirus 16, and human cytomegalovirus (17, 31, 41, 53, 66, 69, 75, 90, 103). For instance, hnRNP A1 interacts with both the genomic and subgenomic promoters of Sindbis virus RNA and actively participates in the synthesis of genomic and subgenomic RNA. Knockdown of hnRNP A1 resulted in a drastic decrease in the synthesis of genomic and subgenomic RNA, both in infected cells and in vitro (31, 53). hnRNP A1 was also shown to be involved in HCV replication. Kim et al. (41) reported that hnRNP A1 and septin 6 proteins were coimmunoprecipitated with HCV NS5B protein. Septin 6 and hnRNP A1 also interact with each other. In addition, hnRNP A1 interacts with the 5′UTR and the 3′UTR of HCV RNA. Knockdown of either hnRNP A1 or septin 6 reduced HCV replication. These results indicate that the host proteins hnRNP A1 and septin 6 play critical roles in the replication of HCV through RNA-protein and protein-protein interactions.
Making use of an electrophoretic mobility shift assay (EMSA), Lin et al. (53) demonstrated that hnRNP A1 bound to a 32P-labeled EV71 5′UTR, but not to a unrelated 31-mer RNA oligonucleotide. To identify the RNA sequences in the EV71 5′UTR which bind to hnRNP A1, various EV71 IRES sequences were reacted with hnRNP A1. hnRNP A1 slowed the migration of RNA probes containing nt 91 to 167, nt 167 to 636, nt 167 to 745, nt 561 to 636, and nt 561 to 745. These results indicate that hnRNP A1 protein likely associated with stem-loops II and VI of the EV71 IRES but not with the cloverleaf structure (nt 1 to 90) of the 5′UTR.
To examine the effect of hnRNP A1 on EV71 IRES activity, a bicistronic luciferase reporter system, cytomegalovirus (CMV)-RLuc-EV715′UTR-FLuc RNA, was transfected into cells. Knocking down the expression of endogenous hnRNP A1 by small interfering RNA (siRNA) had very little effect on the EV71 IRES activity in the cells, nor did the knockdown of hnRNP A2. However, when both hnRNP A1 and hnRNP A2 were knocked down by siRNAs, there was a dramatic reduction in EV71 IRES activity and virus yield, suggesting that either hnRNP A1 or hnRNP A2 is required for the activity of the EV71 IRES, but not both (53).
By treating a nuclear extract from HeLa cells with 3Cpro and making use of two-dimensional (2D) electrophoresis and MALDI-TOF analysis, Weng et al. (94) identified hnRNP A1 as one of several nuclear proteins that could be cleaved by EV71 3Cpro. Whether 3Cpro cleaves hnRNP A1 in the cytoplasm of infected cells is not known; such a cleavage would not be expected to impair EV71 IRES activity if hnRNP A2 can substitute for hnRNP A1 as shown above in vitro. As hnRNP A1 is also an ITAF for the IRESs of several cellular mRNAs (12), the cleavage of hnRNP A1 by 3Cpro might shut off the translation of these mRNAs unless it could be replaced by hnRNP A2.
hnRNP A1 has been reported to downregulate the IRES activity of apoptotic peptidase activating factor 1 (Apaf-1) mRNA. Apaf-1 is known to interact with cytochrome c to activate caspase-3 and lead to rapid and irreversible apoptosis (105). Some years ago, Li et al. (48) reported that 3Cpro protein triggers apoptosis in EV71-infected human glioblastoma cells by the activation of caspase-3. In a work by Cammas et al. (12), hnRNP A1 was found to inhibit UV-induced apoptosis in 293T cells. Following UV irradiation of these cells, hnRNP A1 relocated to the cytoplasm, bound to the IRES of the human Apaf-1 mRNA, and blocked its translation. If Apaf-1 is involved in apoptosis of EV71-infected cells, perhaps the cleavage of hnRNP A1 by 3Cpro would render it unable to bind to the IRES of Apaf-1 mRNA and would thus relieve hnRNP A1's inhibitory effect on apoptosis. Further study to determine the mechanism involved in 3Cpro-induced apoptosis will make clearer the complex interplay between hnRNP A1, the EV71 IRES, 3Cpro, and Apaf-1.
FBP1 and FBP2.
The family of FUSE-binding proteins (FBPs) was named for its interaction with the far-upstream element (FUSE) upstream of the c-myc gene (21, 26, 32). Three variants are known in humans (far-upstream element-binding protein 1 [FBP1], FBP2, and FBP3), and they display strong primary sequence and predicted secondary structure homology. Each of these proteins has three distinct functional domains. The N terminus region represses transcription of c-myc both in cis and in trans. The C terminus region activates transcription of c-myc in trans through multiple repeats of a tyrosine-rich activation motif. The central domain of these proteins binds single-stranded nucleic acids of specific sequences and is composed of four distinct K homology (KH) motifs, each followed by an amphipathic helix (27, 88).
As noted above (52), FBP2 binds to the EV71 5′UTR. This was shown by an RNA protein pulldown assay using biotinylated EV71 5′UTR and lysates of human glioblastoma cells (SF268). Addition of nonbiotinylated EV71 5′UTR, but not yeast tRNA, reduced the pull down of FBP2, suggesting that this interaction is specific. The interaction of FBP2 with biotinylated EV71 5′UTR was observed not only with lysates of SF268 cells but also with lysates of human embryonal rhabdomyosarcoma (RD) cells. To determine the FBP2-binding site(s) on the EV71 5′UTR, RNA pulldown assays using various EV71 5′UTR sequences and SF268 cell lysates were carried out. The biotinylated probes containing nt 1 to 167, 91 to 228, 91 to 636, 91 to 745, 453 to 636, 453 to 745, or 566 to 745 pulled down FBP2 from SF268 cell lysates. The results suggest that FBP2 interacts with the stem-loop I-II region, the stem-loop II-III region, and the stem-loop V-VI and linker regions of EV71 5′UTR. Various truncated forms of FBP2 were tested in a similar assay to map the 5′UTR-binding site(s) on FBP2. The results indicated that the KH2 and KH4 motifs are essential for FBP2 to bind to the EV71 5′UTR (52).
Viral protein synthesis in infected cells was increased when cells were depleted of FBP2 by siRNA targeting FBP2 but decreased in cells in which FBP2 was overexpressed. The IRES activity of EV71, as indicated by the luciferase reporter gene assay, was also increased when FBP2 was knocked down. FBP2 out competed PTB, one of the positive-acting ITAFs of picornaviral IRESs, for binding to the EV71 IRES. Thus, FBP2 appears to be a negative regulator of EV71 IRES activity (52).
FBP1, another member of the FBP family which shares a highly similar primary sequence and structure with FBP2, was reported to interact with both the 3′UTR and with NS5A of HCV and to be essential for HCV replication (102). A recent report by Chien et al. (18) indicates that FBP1 binds to the 5′ and 3′UTRs of Japanese encephalitis virus RNA and functions as a host anti-JEV defense molecule by repressing viral protein expression.
In contrast to FBP2, FBP1 enhanced the IRES activity of EV71 RNA (Huang et al., submitted). As indicated by RNA pulldown assays with lysates of either neural or nonneural cells. FBP1, like FBP2, interacted with the EV71 5′UTR. As shown by both an RNA pulldown assay and EMSA, FBP1 interacts with only the linker region (nt 637 to 745) of the EV71 5′UTR. The EV71 5′UTR-binding site on FBP1 was mapped to the KH3 and KH4 motifs.
Studies using fluorescence confocal microscopy and specific antibodies showed that both FBP2 and FBP1 relocalized from the nucleus to the cytoplasm in EV71-infected cells. FBP1 contains three nuclear localization signals (NLS), a classical bipartite NLS in the N-terminal domain, a typical alpha 4 NLS in the central domain, and a tyrosine-rich motif (YM) in the C-terminal domain of FBP1, which also functions as an NLS. Plasmids coding the different NLS coding regions of FBP1 fused to a coding sequence for triple green fluorescent proteins (GFPs) were transfected into cells, after which the localization of GFP was monitored by fluorescence confocal microscopy. The results suggested that any of the three NLS of FBP1 can lead to the relocalization of FBP1. The IRES activity of EV71, as indicated by the luciferase reporter gene assay, and viral protein synthesis were decreased when FBP1 was knocked down, indicating that in contrast to FBP2, FBP1 acts as a positive-regulating ITAF of EV71 IRES-dependent translation. Both FBP1 and FBP2 bind to the sequence of the 5′UTR containing the linker region (nt 637 to 745), and the results of competition binding assays suggested that FBP1 and FBP2 compete with each other for binding to this region.
FBPs interact with certain mRNAs and participate at various steps in transcription, in RNA processing, RNA transport, or RNA catabolism in the nucleus or in the cytoplasm (30, 37, 59). However, the involvement of FBPs in translation, especially viral translation, has remained largely unexplored. The mechanism underlying the regulation of EV71 IRES activity by FBP1 and FBP2 is unclear. How is it that these two very similar proteins compete with each other for binding to the linker region of the EV71 5′UTR (nt 637 to 745) but have opposite effects on EV71 IRES activity and presumably viral replication? Structural probing, such as RNA footprinting of FBP1- and FBP2-binding sites on the EV71 5′UTR and mapping the IRES interaction sites in the KH domains of FBP1 and FBP2 should help explain the involvement of FBP1 and FBP2 in EV71 translation.
(iv) HOST FACTORS INVOLVED IN VIRAL RNA REPLICATION
hnRNP K.
hnRNP K is an RNA-binding protein originally identified as a component of the heterogeneous nuclear ribonucleoprotein (hnRNP) complex. It has three KH domains (KH1 to -3) and a proline-rich domain flanked by the KH2 and KH3 domains. The KH domain is one of the most common RNA-binding domains that directly contacts single-stranded RNA. The proline-rich domain is important for protein-protein interactions (82, 87).
hnRNP K is involved in the replication of several DNA viruses. Zhang et al. (101) reported that overexpression of hnRNP K stimulated the replication of hepatitis B virus (HBV), whereas knockdown of endogenous hnRNP K resulted in a significant reduction in the HBV virus yield. hnRNP K also interacts with human herpesvirus 6 immediate-early protein 2 (77) and with herpes simplex virus 1 IE63 protein (10) and is involved in the replication of several RNA viruses. It interacts with Sindbis virus nonstructural proteins and viral subgenomic RNA. Knockdown of hnRNP K resulted in decreased expression of GFP driven by the Sindbis virus subgenomic promoter (11). In addition, hnRNP K interacts with the core proteins of both dengue virus (14) and the hepatitis C virus (35).
As noted above, Lin et al. (51) identified hnRNP K as one of the proteins that bound to the biotinylated EV71 5′UTR. In a competition assay, this interaction was outcompeted by nonbiotinylated EV71 5′UTR but not by yeast tRNA.
To map the hnRNP K-binding site(s) in the 5′UTR, various deleted forms of biotinylated EV71 5′UTR were reacted with RD cell lysate and subjected to an RNA pulldown assay. RNA molecules containing nt 1 to 167, 91 to 445, 91 to 561, 91 to 636, 91 to 745, or 242 to 445 were able to pull down hnRNP K, suggesting that hnRNP K interacts with the cloverleaf structure which is critical for the viral RNA synthesis, or with stem-loop II of the 5′UTR, and with stem-loop IV of the IRES (51).
To determine the functional domains of hnRNP K that interact with the EV71 5′UTR, various deleted forms of hnRNP K were constructed, expressed, and tested for their interaction with the EV71 5′UTR. The data suggested that the EV71 5′UTR interacts with the KH2 domain, the proline-rich domain, and one neighboring KH domain (KH1 or KH3) of hnRNP K. Depletion of endogenous hnRNP K in infected cells by siRNA knockdown decreased the yield of EV71 and delayed the synthesis of both positive and negative strands of viral RNA, indicating that hnRNP K is essential for EV71 replication (51).
Studies with poliovirus had demonstrated that the cloverleaf structure of the 5′UTR forms a ternary complex with the cellular poly(rC)-binding protein (PCBP) and the viral polymerase precursor, 3CD. This complex functions in both viral RNA synthesis and translation. The binding of PCBP to the cloverleaf structure promotes viral translation, while binding of 3CD to the cloverleaf structure represses viral translation and promotes negative-strand RNA synthesis (29, 89). As hnRNP K also interacts with the cloverleaf structure of EV71 and enhances EV71 RNA synthesis (51), it will be important to determine how these proteins interact with each other to regulate viral RNA synthesis and translation. Since hnRNP K also interacts with stem-loop IV of the IRES, the effect of this interaction on IRES-dependent translation needs to be defined as well.
RTN 3.
The reticulon (RTN) family of proteins contains four members. Each contains the reticulon homology domain (RHD), a conserved region at the C terminus consisting of two putative transmembrane regions separated by a hydrophilic loop (99). Proteins in this family are involved in membrane trafficking, structural stabilization of the endoplasmic reticulum (ER) network, proapoptotic mechanisms, and protein secretion. RTN 3 is expressed in all human cells and is highly expressed in the brain. It is localized predominately in the ER and is often activated under ER stress (63). Recently Chen et al. (16) reported that RTN 3 enhances the binding of Bcl-2 to Beclin 1, thereby inhibiting Beclin 1-dependent autophagic clearance of cytosolic prion aggregates.
The 2C protein is one of the most highly conserved proteins among the picornaviruses (4). The amino acid sequence of EV71 2C shares 65% similarity with that of poliovirus. Like the 2C protein of PV, EV71 2C plays an important role in forming the viral RNA replication complex by binding to and rearranging mammalian cytoplasmic membranes (84). Using a yeast two-hybrid system to screen a human fetal brain cDNA library, Tang et al. (84) demonstrated that RTN 3 interacts with the N-terminal domain of EV71 2C. This interaction was further confirmed by an in vitro binding assay using recombinant RTN 3 and EV71 2C and by coimmunoprecipitation of 2C with RTN 3 in HEK-293T cells. In addition to RTN3, RTN1C was identified by the yeast two-hybrid system as another protein possibly interacting with EV71 2C. As each member of the reticulon family shares a conserved C-terminal RHD, the binding of EV71 2C to the RHDs of all RTNs was tested. The RHDs of all four RTNs, from a human fetal brain cDNA library, were subcloned in frame with a His tag at its C terminus into a mammalian expression vector and individually transfected into HEK-293T cells. The cells were cotransfected with plasmid expressing EV71 2C protein, and lysates were prepared for coimmunoprecipitation using a monoclonal antibody to the His tag. The EV71 2C protein was coimmunoprecipitated with the RHDs of all four RTNs, indicating a specific interaction between EV71 2C and the RHDs of all four reticulon proteins.
Immunofluorescent staining of EV71-infected cells with anti-RTN3 and anti-2C antibodies indicated that endogenous RTN3 colocalized not only with EV71 2C but also with double-stranded RNA (dsRNA). Thus, it appears that RTN3 is associated with the replication complex probably through a direct and specific interaction with the EV71 2C protein. Site-directed mutagenesis of the N-terminal conserved motif of the EV71 2C protein showed that isoleucine 25 is critical for the interaction of EV71 2C with the RHD of RTN3. The I25K mutation in the PV 2C protein is known to inhibit P2/P3 cleavage and thus leads to defective viral RNA replication (67). When an I25K mutation was introduced into the EV71, the cytopathic effect was reduced compared to that seen after infection with wild-type virus. These findings are consistent with the findings of Paul et al. (67) and imply that RTN3 plays a role in modulating the viral replication of EV71 and possibly other enteroviruses. The synthesis of viral dsRNA and viral proteins in EV71-infected cells was inhibited when RTN3 was knocked down by siRNA. Transfection of a plasmid encoding RTN3 to overexpress RTN3 in siRTN3 knockdown cells negated the effect of the knockdown on viral RNA and protein synthesis. As the 2C sequence is highly conserved among enteroviruses, the interactions of RTN3 with the 2C proteins of poliovirus and coxsackievirus A16 were also tested. Immunoprecipitation studies showed that RTN3 could bind to the 2C of PV, CA16, and EV71, suggesting that RTN 3 plays a role in the replication of many enteroviruses (84).
(v) 3Cpro CLEAVAGE OF CstF-64
Although the replication of picornaviruses occurs in the cytoplasm, some viral 3C protease has been found, as described below, to enter the nucleus. While its role in the nucleus has usually been thought to prevent host transcription, recent work has indicated that it can also act at the posttranscriptional level.
Polyadenylation of eukaryotic cells is a stepwise process. The cleavage/polyadenylation specificity factor (CPSF) binds to the polyadenylation signal, the AAUAAA motif. The cleavage stimulation factor (CstF), which can interact with CPSF, then binds through its 64K subunit (CstF-64) to an appropriately positioned U-rich sequence downstream of the cleavage site on the RNA substrate. Thus, both CPSF and CstF interact with the pre-mRNA, and they interact with each other, spanning the polyadenylation cleavage site. CstF-64 binding to the U-rich regions located downstream of the cleavage site is essential for efficient pre-mRNA cleavage (20, 54).
By treating nuclear extracts of HeLa cells with EV71 3Cpro and then analyzing the proteins using 2D electrophoresis and MALDI-TOF, Weng et al. (94) found that the 3Cpro of EV71 was able to cleave CstF-64. The cleavage was confirmed by (i) the observation that the amount of CstF-64 was reduced in nuclear extracts incubated with wild-type EV71 3Cpro but not with C147S mutant 3Cpro which lacked proteolytic activity and (ii) the in vitro cleavage by recombinant wild-type 3Cpro of 35S-labeled CstF-64, which had been generated by in vitro transcription and translation (TNT). In another experiment, cells were infected by EV71, and the lysate was subjected to Western blot analysis using anti-CstF-64 antibody. The results showed that the amount of 3Cpro was increased from 6 to 10 h postinfection (p.i.) and that the amount of host CstF-64 protein was reduced. That CstF-64 remained in the nucleus of the infected cells from 6 to 10 h p.i. was shown by confocal microscopy using anti-CstF-64 antibody, and the presence of 3Cpro in the nucleus as well as in the cytoplasm was shown by Western blot analysis using anti-3Cpro antibody.
The effect of the cleavage of CstF-64 on the pre-mRNA processing of interleukin 10 receptor beta (IL-10RB) was also examined by Weng et al. (95). The relative amounts of IL-10RB pre-mRNA and poly(A) mRNA in EV71-infected cells were monitored by real-time reverse transcription-PCR (RT-PCR). By 8 h after infection, the IL-10RB mRNA in EV71-infected cells was decreased to 63% of that in mock-infected cells. This is consistent with the cDNA microarray data reported by Shih et al. (76). In contrast, the relative amount of IL-10RB pre-mRNA increased to 156% of that in mock-infected cells. These results confirm the idea that the processing of pre-mRNA was impaired in EV71-infected cells. In another experiment, HeLa cell nuclear extract (as the source of the polyadenylation machinery) was incubated with a capped pre-mRNA, which contained an simian virus 40 (SV40) late gene polyadenylational cleavage site and treated with EV71 3Cpro. The cleavage of pre-mRNA and polyadenylation proceeded efficiently when nuclear extract was treated with C147S mutant 3Cpro or was left untreated. In contrast, the pre-mRNA processing was impaired when the nuclear extract was treated with wild-type 3Cpr; however, this impairment was overcome by adding purified recombinant CstF-64. These results further support the idea that 3Cpro cleavage of CstF-64 inhibits the 3′ end pre-mRNA processing and polyadenylation. As a result, less polyadenylated host mRNA is synthesized and more cellular resources, such as the various translation factors, are available for the translation of viral RNA. As a specific example, the fact that the poly(A) tail of poliovirus is required for viral replication suggests that one or more host poly(A)-associated factors may also be required (80). If so, interference with polyadenylation of host cellular mRNA may be advantageous for virus replication.
CONCLUSIONS
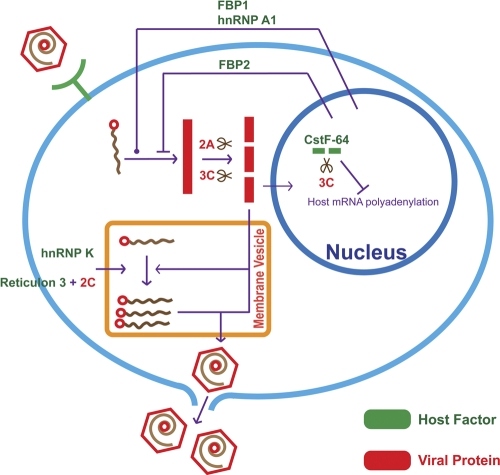
Figure 2 summarizes the roles of host factors described above for EV71 replication. As described in this review, the replication of EV71 depends on multiple host factors. It is likely that more such host factors remain to be identified. Furthermore, of those identified, much remains to be learned about how and exactly where in the cell they act. For instance, the ITAFs hnRNP A1, FBP2, and FBP1 discussed above, which associate with the EV71 IRES, were found to redistribute from the nucleus to the cytoplasm following infection with EV71. This is consistent with previous reports that the activity of proteins controlling IRES-dependent translation initiation is dependent on their subcellular localization (46). The fact that cellular proteins, which function as ITAFs, normally shuttle back and forth between nucleus and cytoplasm indicates that the redistribution of ITAFs is an absolute requirement for IRES-dependent translation. Thus, it will be of interest to determine whether EV71 infection affects the nuclear import/export signaling pathways that control the subcellular redistribution of ITAFs. Along these lines, Weng et al. (94) made use of two-dimensional electrophoresis and MALDI-TOF analysis to identify karyopherin β2, the nuclear import receptor (19), as one of the likely targets for EV71 3Cpro cleavage. A logical next step would be to study how the cleavage of karyopherin β2 by EV71 3Cpro in infected cells affects the nuclear import and export of ITAFs. In addition to ITAFs, host factors associated with the EV71 3′UTR deserve more attention, since the 3′UTR, like the 5′UTR, plays a critical role in viral replication. The family Picornaviridae contains viruses that cause diverse and severe diseases in humans and animals, yet picornaviruses all employ a similar strategy of replication. Further study of the host cell proteins required for replication of viruses in this family should reveal which of these proteins are unique to the replication of EV71 and to what extent they are responsible for the pathogenesis of EV71 disease.
Fig. 2.
Overview of the interplay between host factors and EV71 replication. Virus binds to a cellular receptor, and the genome is released into the cytoplasm where the viral replication takes place. Genomic RNA is first translated to produce the viral polyprotein. The polyprotein is co- and posttranslationally processed to produce the various precursors and processed proteins that are needed for EV71 replication (Fig. 1A). RNA synthesis occurs on membrane vesicles. Replicated RNA enters either the translation-replication cycle or the viral particle assembly step. For virus assembly, RNA encapsidation and virus particle maturation must occur. Newly synthesized viral particles are released from the cell by lysis. hnRNP A1 and FBP1 exert a positive effect on EV71 IRES activity and thus on the synthesis of EV71 proteins; FBP2 has the opposite effect. hnRNP K and reticulon 3 are required for the efficient synthesis of viral RNA. EV71 3Cpro cleaves CstF-64 and thus impairs the processing of pre-mRNA to mature mRNAs.
ACKNOWLEDGEMENTS
Our work in this area was supported by U.S. Public Health Service grant AI-70668 from the National Institutes of Health to M.-L.L. and NSC-99-3112-B-182-007 from the National Science Council in Taiwan to S.-R.S.
We are grateful to Kuo-Feng Weng for the preparation of Fig. 2.
Biographies

Shin-Ru Shih, Ph.D., got her bachelor's degree in Medical Biotechnology and masters degree in Biochemistry from National Taiwan University and her Ph.D. in Biochemistry and Molecular Biology from Rutgers University, NJ. In 1996, she established a molecular virology laboratory in Chang Gung University, and in 2008, she established the Research Center for Emerging Viral Infections. Her team has been studying many aspects of emerging RNA viruses, including identification of unknown viruses, mechanisms of pathogenesis, and development of antiviral compounds. Influenza virus and enterovirus 71 (EV71) are their major focuses. Their study of EV71 began in 1998, when a large EV71 outbreak occurred in Taiwan. Their participation contributed significantly to the laboratory diagnosis of EV71. They subsequently focused on virus-host interactions, developing molecular targets for drug discovery and a series of anti-EV71 compounds. Dr. Shih was awarded the National Medal for Outstanding Youth in 2004 for contributing to EV71 research in Taiwan.

Victor Stollar, M.D., is Professor of Molecular Genetics and Microbiology at Robert Wood Johnson Medical School (UMDNJ) in Piscataway, NJ. For most of his career, his research focused on the replication and genetics of alphaviruses and flaviviruses; much of his work concerned the replication of these viruses in mosquito cells. In the last few years, he has also become involved in the study of enterovirus 71.

Mei-Ling Li, Ph.D., is an assistant professor of Molecular Genetics, Microbiology & Immunology at Robert Wood Johnson Medical School-UMDNJ, Piscataway, NJ. She received her Ph.D. in Molecular Genetics & Microbiology from Rutgers University. She then did her postdoctoral training with Dr. Robert Krug. Her research at present focuses on the replication and genetics of Sindbis virus and the interaction of enterovirus 71 with host factors.
Footnotes
Published ahead of print on 29 June 2011.
REFERENCES
- 1. AbuBakar S., et al. 2009. Enterovirus 71 outbreak, Brunei. Emerg. Infect. Dis. 15:79–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alexander J. P., Jr., Baden L., Pallansch M. A., Anderson L. J. 1994. Enterovirus 71 infections and neurologic disease—United States, 1977-1991. J. Infect. Dis. 169:905–908 [DOI] [PubMed] [Google Scholar]
- 3. Andreev D. E., et al. 2007. Differential factor requirement to assemble translation initiation complexes at the alternative start codons of foot-and-mouth disease virus RNA. RNA 13:1366–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Argos P., Kamer G., Nicklin M. J., Wimmer E. 1984. Similarity in gene organization and homology between proteins of animal picornaviruses and a plant comovirus suggest common ancestry of these virus families. Nucleic Acids Res. 12:7251–7267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bedard K. M., Semler B. L. 2004. Regulation of picornavirus gene expression. Microbes Infect. 6:702–713 [DOI] [PubMed] [Google Scholar]
- 6. Belsham G. J. 2009. Divergent picornavirus IRES elements. Virus Res. 139:183–192 [DOI] [PubMed] [Google Scholar]
- 7. Borman A. M., Bailly J. L., Girard M., Kean K. M. 1995. Picornavirus internal ribosome entry segments: comparison of translation efficiency and the requirements for optimal internal initiation of translation in vitro. Nucleic Acids Res. 23:3656–3663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Borman A. M., Michel Y. M., Kean K. M. 2001. Detailed analysis of the requirements of hepatitis A virus internal ribosome entry segment for the eukaryotic initiation factor complex eIF4F. J. Virol. 75:7864–7871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brown B. A., Oberste M. S., Alexander J. P., Jr., Kennett M. L., Pallansch M. A. 1999. Molecular epidemiology and evolution of enterovirus 71 strains isolated from 1970 to 1998. J. Virol. 73:9969–9975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bryant H. E., et al. 2000. Interaction between herpes simplex virus type 1 IE63 protein and cellular protein p32. J. Virol. 74:11322–11328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Burnham A. J., Gong L., Hardy R. W. 2007. Heterogeneous nuclear ribonuclear protein K interacts with Sindbis virus nonstructural proteins and viral subgenomic mRNA. Virology 367:212–221 [DOI] [PubMed] [Google Scholar]
- 12. Cammas A., et al. 2007. Cytoplasmic relocalization of heterogeneous nuclear ribonucleoprotein A1 controls translation initiation of specific mRNAs. Mol. Biol. Cell 18:5048–5059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cartegni L., Chew S. L., Krainer A. R. 2002. Listening to silence and understanding nonsense: exonic mutations that affect splicing. Nat. Rev. Genet. 3:285–298 [DOI] [PubMed] [Google Scholar]
- 14. Chang C. J., et al. 2001. The heterogeneous nuclear ribonucleoprotein K (hnRNP K) interacts with dengue virus core protein. DNA Cell Biol. 20:569–577 [DOI] [PubMed] [Google Scholar]
- 15. Chen M., Manley J. L. 2009. Mechanisms of alternative splicing regulation: insights from molecular and genomics approaches. Nat. Rev. Mol. Cell Biol. 10:741–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen R., et al. 2011. Reticulon 3 attenuates the clearance of cytosolic prion aggregates via inhibiting autophagy. Autophagy 7:205–216 [DOI] [PubMed] [Google Scholar]
- 17. Cheunim T., Zhang J., Milligan S. G., McPhillips M. G., Graham S. V. 2008. The alternative splicing factor hnRNP A1 is up-regulated during virus-infected epithelial cell differentiation and binds the human papillomavirus type 16 late regulatory element. Virus Res. 131:189–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chien H. L., Liao C. L., Lin Y. L. 2011. The FUSE binding protein 1 interacts with untranslated regions of Japanese encephalitis virus RNA and negatively regulates viral replication. J. Virol. 85:4698–4706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chook Y. M., Blobel G. 1999. Structure of the nuclear transport complex karyopherin-β2-Ran·GppNHp. Nature 399:230–237 [DOI] [PubMed] [Google Scholar]
- 20. Colgan D. F., Manley J. L. 1997. Mechanism and regulation of mRNA polyadenylation. Genes Dev. 11:2755–2766 [DOI] [PubMed] [Google Scholar]
- 21. Davis-Smyth T., Duncan R. C., Zheng T., Michelotti G., Levens D. 1996. The far upstream element-binding proteins comprise an ancient family of single-strand DNA-binding transactivators. J. Biol. Chem. 271:31679–31687 [DOI] [PubMed] [Google Scholar]
- 22. de Breyne S., Yu Y., Unbehaun A., Pestova T. V., Hellen C. U. 2009. Direct functional interaction of initiation factor eIF4G with type 1 internal ribosomal entry sites. Proc. Natl. Acad. Sci. U. S. A. 106:9197–9202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. De Jesus N. H. 2007. Epidemics to eradication: the modern history of poliomyelitis. Virol. J. 4:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dobbyn H. C., et al. 2008. Regulation of BAG-1 IRES-mediated translation following chemotoxic stress. Oncogene 27:1167–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dreyfuss G., Matunis M. J., Pinol-Roma S., Burd C. G. 1993. hnRNP proteins and the biogenesis of mRNA. Annu. Rev. Biochem. 62:289–321 [DOI] [PubMed] [Google Scholar]
- 26. Duncan R., et al. 1994. A sequence-specific, single-strand binding protein activates the far upstream element of c-myc and defines a new DNA-binding motif. Genes Dev. 8:465–480 [DOI] [PubMed] [Google Scholar]
- 27. Engidawork E., Afjehi-Sadat L., Yang J. W., Slavc I., Lubec G. 2006. Protein chemical identification and characterization of the human variants of far upstream element binding protein in medulloblastoma DAOY cell line. Int. J. Oncol. 29:721–736 [PubMed] [Google Scholar]
- 28. Fitzgerald K. D., Semler B. L. 2009. Bridging IRES elements in mRNAs to the eukaryotic translation apparatus. Biochim. Biophys. Acta 1789:518–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gamarnik A. V., Andino R. 1998. Switch from translation to RNA replication in a positive-stranded RNA virus. Genes Dev. 12:2293–2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Grossman J. S., et al. 1998. The use of antibodies to the polypyrimidine tract binding protein (PTB) to analyze the protein components that assemble on alternatively spliced pre-mRNAs that use distant branch points. RNA 4:613–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gui H., Lu C. W., Adams S., Stollar V., Li M. L. 2010. hnRNP A1 interacts with the genomic and subgenomic RNA promoters of Sindbis virus and is required for the synthesis of G and SG RNA. J. Biomed. Sci. 17:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. He L., Weber A., Levens D. 2000. Nuclear targeting determinants of the far upstream element binding protein, a c-myc transcription factor. Nucleic Acids Res. 28:4558–4565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hellen C. U. 2009. IRES-induced conformational changes in the ribosome and the mechanism of translation initiation by internal ribosomal entry. Biochim. Biophys. Acta 1789:558–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ho M., et al. 1999. An epidemic of enterovirus 71 infection in Taiwan. N. Engl. J. Med. 341:929–935 [DOI] [PubMed] [Google Scholar]
- 35. Hsieh T. Y., et al. 1998. Hepatitis C virus core protein interacts with heterogeneous nuclear ribonucleoprotein K. J. Biol. Chem. 273:17651–17659 [DOI] [PubMed] [Google Scholar]
- 36. Reference deleted.
- 37. Irwin N., Baekelandt V., Goritchenko L., Benowitz L. I. 1997. Identification of two proteins that bind to a pyrimidine-rich sequence in the 3′-untranslated region of GAP-43 mRNA. Nucleic Acid Res. 25:1281–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jang S. K., et al. 1988. A segment of the 5′ nontranslated region of encephalomyocarditis virus RNA directs internal entry of ribosomes during in vitro translation. J. Virol. 62:2636–2643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Joachims M., Van Breugel P. C., Lloyd R. E. 1999. Cleavage of poly(A)-binding protein by enterovirus proteases concurrent with inhibition of translation in vitro. J. Virol. 73:718–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kehle J., Roth B., Metzger C., Pfitzner A., Enders G. 2003. Molecular characterization of an enterovirus 71 causing neurological disease in Germany. J. Neurovirol. 9:126–128 [DOI] [PubMed] [Google Scholar]
- 41. Kim C. S., Seol S. K., Song O. K., Park J. H., Jang S. K. 2007. An RNA-binding protein, hnRNP A1, and a scaffold protein, septin 6, facilitate hepatitis C virus replication. J. Virol. 81:3852–3865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kolupaeva V. G., de Breyne S., Pestova T. V., Hellen C. U. 2007. In vitro reconstitution and biochemical characterization of translation initiation by internal ribosomal entry. Methods Enzymol. 430:409–439 [DOI] [PubMed] [Google Scholar]
- 43. Koroleva G. A., Gracheva L. A., Voroshilova M. K. 1978. Isolation of type 71 enterovirus from patients with a poliomyelitis-like disease during an outbreak in Bulgaria. Vopr. Virusol. 5:611–618 (In Russian) [PubMed] [Google Scholar]
- 44. Krausslich H. G., Nicklin M. J., Toyoda H., Etchison D., Wimmer E. 1987. Poliovirus proteinase 2A induces cleavage of eucaryotic initiation factor 4F polypeptide p220. J. Virol. 61:2711–2718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lee B. Y., et al. 2010. Forecasting the economic value of an enterovirus 71 (EV71) vaccine. Vaccine 28:7731–7736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lewis S. M., Holcik M. 2008. For IRES trans-acting factors, it is all about location. Oncogene 27:1033–1035 [DOI] [PubMed] [Google Scholar]
- 47. Lewis S. M., et al. 2007. Subcellular relocalization of a trans-acting factor regulates XIAP IRES-dependent translation. Mol. Biol. Cell 18:1302–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li M. L., et al. 2002. The 3C protease activity of enterovirus 71 induces human neural cell apoptosis. Virology 293:386–395 [DOI] [PubMed] [Google Scholar]
- 49. Lin J. C., Hsu M., Tarn W. Y. 2007. Cell stress modulates the function of splicing regulatory protein RBM4 in translation control. Proc. Natl. Acad. Sci. U. S. A. 104:2235–2240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lin J. Y., et al. 2009. Viral and host proteins involved in picornavirus life cycle. J. Biomed. Sci. 16:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lin J. Y., et al. 2008. Heterogeneous nuclear ribonuclear protein K interacts with the enterovirus 71 5′ untranslated region and participates in virus replication. J. Gen. Virol. 89:2540–2549 [DOI] [PubMed] [Google Scholar]
- 52. Lin J. Y., Li M. L., Shih S. R. 2009. Far upstream element binding protein 2 interacts with enterovirus 71 internal ribosomal entry site and negatively regulates viral translation. Nucleic Acids Res. 37:47–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lin J. Y., et al. 2009. hnRNP A1 interacts with the 5′ untranslated regions of enterovirus 71 and Sindbis virus RNA and is required for viral replication. J. Virol. 83:6106–6114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. MacDonald C. C., Wilusz J., Shenk T. 1994. The 64-kilodalton subunit of the CstF polyadenylation factor binds to pre-mRNAs downstream of the cleavage site and influences cleavage site location. Mol. Cell. Biol. 14:6647–6654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Martinez-Salas E. 2008. The impact of RNA structure on picornavirus IRES activity. Trends Microbiol. 16:230–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. McMinn P. C. 2002. An overview of the evolution of enterovirus 71 and its clinical and public health significance. FEMS Microbiol. Rev. 26:91–107 [DOI] [PubMed] [Google Scholar]
- 57. Melnick J. L. 1984. Enterovirus type 71 infections: a varied clinical pattern sometimes mimicking paralytic poliomyelitis. Rev. Infect. Dis. 6(Suppl. 2):S387–S390 [DOI] [PubMed] [Google Scholar]
- 58. Michlewski G., Caceres J. F. 2010. Antagonistic role of hnRNP A1 and KSRP in the regulation of let-7a biogenesis. Nat. Struct. Mol. Biol. 17:1011–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Min H., Turck C. W., Nikolic J. M., Black D. L. 1997. A new regulatory protein, KSRP, mediates exon inclusion through an intronic splicing enhancer. Genes Dev. 11:1023–1036 [DOI] [PubMed] [Google Scholar]
- 60. Nagy G., Takatsy S., Kukan E., Mihaly I., Domok I. 1982. Virological diagnosis of enterovirus type 71 infections: experiences gained during an epidemic of acute CNS diseases in Hungary in 1978. Arch. Virol. 71:217–227 [DOI] [PubMed] [Google Scholar]
- 61. Niepmann M. 2009. Internal translation initiation of picornaviruses and hepatitis C virus. Biochim. Biophys. Acta 1789:529–541 [DOI] [PubMed] [Google Scholar]
- 62. Nishimura Y., et al. 2009. Human P-selectin glycoprotein ligand-1 is a functional receptor for enterovirus 71. Nat. Med. 15:794–797 [DOI] [PubMed] [Google Scholar]
- 63. Oertle T., Schwab M. E. 2003. Nogo and its paRTNers. Trends Cell Biol. 13:187–194 [DOI] [PubMed] [Google Scholar]
- 64. Ooi M. H., Wong S. C., Lewthwaite P., Cardosa M. J., Solomon T. 2010. Clinical features, diagnosis, and management of enterovirus 71. Lancet Neurol. 9:1097–1105 [DOI] [PubMed] [Google Scholar]
- 65. Pacheco A., Martinez-Salas E. 2010. Insights into the biology of IRES elements through riboproteomic approaches. J. Biomed. Biotechnol. 2010:458927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Paranjape S. M., Harris E. 2007. Y box-binding protein-1 binds to the dengue virus 3′-untranslated region and mediates antiviral effects. J. Biol. Chem. 282:30497–30508 [DOI] [PubMed] [Google Scholar]
- 67. Paul A. V., Molla A., Wimmer E. 1994. Studies of a putative amphipathic helix in the N-terminus of poliovirus protein 2C. Virology 199:188–199 [DOI] [PubMed] [Google Scholar]
- 68. Pelletier J., Sonenberg N. 1988. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature 334:320–325 [DOI] [PubMed] [Google Scholar]
- 69. Pettit Kneller E. L., Connor J. H., Lyles D. S. 2009. hnRNPs relocalize to the cytoplasm following infection with vesicular stomatitis virus. J. Virol. 83:770–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pilipenko E. V., Viktorova E. G., Guest S. T., Agol V. I., Roos R. P. 2001. Cell-specific proteins regulate viral RNA translation and virus-induced disease. EMBO J. 20:6899–6908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Racaniello V. R. 2007. Picornaviridae: the viruses and their replication, p. 796–838 In Knipe D. M., et al. (ed.), Fields virology, 5th ed Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 72. Samuda G. M., Chang W. K., Yeung C. Y., Tang P. S. 1987. Monoplegia caused by enterovirus 71: an outbreak in Hong Kong. Pediatr. Infect. Dis. J. 6:206–208 [DOI] [PubMed] [Google Scholar]
- 73. Sanz M. A., Welnowska E., Redondo N., Carrasco L. 2010. Translation driven by picornavirus IRES is hampered from Sindbis virus replicons: rescue by poliovirus 2A protease. J. Mol. Biol. 402:101–117 [DOI] [PubMed] [Google Scholar]
- 74. Schmidt N. J., Lennette E. H., Ho H. H. 1974. An apparently new enterovirus isolated from patients with disease of the central nervous system. J. Infect. Dis. 129:304–309 [DOI] [PubMed] [Google Scholar]
- 75. Shi S. T., Huang P., Li H. P., Lai M. M. 2000. Heterogeneous nuclear ribonucleoprotein A1 regulates RNA synthesis of a cytoplasmic virus. EMBO J. 19:4701–4711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Shih S. R., et al. 2004. Identification of genes involved in the host response to enterovirus 71 infection. J. Neurovirol. 10:293–304 [DOI] [PubMed] [Google Scholar]
- 77. Shimada K., Kondo K., Yamanishi K. 2004. Human herpesvirus 6 immediate-early 2 protein interacts with heterogeneous ribonucleoprotein K and casein kinase 2. Microbiol. Immunol. 48:205–210 [DOI] [PubMed] [Google Scholar]
- 78. Shimizu H., et al. 1999. Enterovirus 71 from fatal and nonfatal cases of hand, foot and mouth disease epidemics in Malaysia, Japan and Taiwan in 1997-1998. Jpn. J. Infect. Dis. 52:12–15 [PubMed] [Google Scholar]
- 79. Shindarov L. M., et al. 1979. Epidemiological, clinical, and pathomorphological characteristics of epidemic poliomyelitis-like disease caused by enterovirus 71. J. Hyg. Epidemiol. Microbiol. Immunol. 23:284–295 [PubMed] [Google Scholar]
- 80. Silvestri L. S., Parilla J. M., Morasco B. J., Ogram S. A., Flanegan J. B. 2006. Relationship between poliovirus negative-strand RNA synthesis and the length of the 3′ poly(A) tail. Virology 345:509–519 [DOI] [PubMed] [Google Scholar]
- 81. Siomi H., Dreyfuss G. 1995. A nuclear localization domain in the hnRNP A1 protein. J. Cell Biol. 129:551–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Siomi H., Matunis M. J., Michael W. M., Dreyfuss G. 1993. The pre-mRNA binding K protein contains a novel evolutionarily conserved motif. Nucleic Acids Res. 21:1193–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Solomon T., et al. 2010. Virology, epidemiology, pathogenesis, and control of enterovirus 71. Lancet Infect. Dis. 10:778–790 [DOI] [PubMed] [Google Scholar]
- 84. Tang W. F., et al. 2007. Reticulon 3 binds the 2C protein of enterovirus 71 and is required for viral replication. J. Biol. Chem. 282:5888–5898 [DOI] [PubMed] [Google Scholar]
- 85. Thompson S. R., Sarnow P. 2003. Enterovirus 71 contains a type I IRES element that functions when eukaryotic initiation factor eIF4G is cleaved. Virology 315:259–266 [DOI] [PubMed] [Google Scholar]
- 86. Ting N. S., Pohorelic B., Yu Y., Lees-Miller S. P., Beattie T. L. 2009. The human telomerase RNA component, hTR, activates the DNA-dependent protein kinase to phosphorylate heterogeneous nuclear ribonucleoprotein A1. Nucleic Acids Res. 37:6105–6115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Tomonaga T., Levens D. 1995. Heterogeneous nuclear ribonucleoprotein K is a DNA-binding transactivator. J. Biol. Chem. 270:4875–4881 [DOI] [PubMed] [Google Scholar]
- 88. Vindigni A., Ochem A., Triolo G., Falaschi A. 2001. Identification of human DNA helicase V with the far upstream element-binding protein. Nucleic Acids Res. 29:1061–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Vogt D. A., Andino R. 2010. An RNA element at the 5′-end of the poliovirus genome functions as a general promoter for RNA synthesis. PLoS Pathog. 6:e1000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wang Y. F., Chen S. C., Wu F. Y., Wu C. W. 1997. The interaction between human cytomegalovirus immediate-early gene 2 (IE2) protein and heterogeneous ribonucleoprotein A1. Biochem. Biophys. Res. Commun. 232:590–594 [DOI] [PubMed] [Google Scholar]
- 91. Weighardt F., Biamonti G., Riva S. 1995. Nucleo-cytoplasmic distribution of human hnRNP proteins: a search for the targeting domains in hnRNP A1. J. Cell Sci. 108:545–555 [DOI] [PubMed] [Google Scholar]
- 92. Weighardt F., Biamonti G., Riva S. 1996. The roles of heterogeneous nuclear ribonucleoproteins (hnRNP) in RNA metabolism. Bioessays 18:747–756 [DOI] [PubMed] [Google Scholar]
- 93. Weng K. F., Chen L. L., Huang P. N., Shih S. R. 2010. Neural pathogenesis of enterovirus 71 infection. Microbes. Infect. 12:505–510 [DOI] [PubMed] [Google Scholar]
- 94. Weng K. F., Li M. L., Hung C. T., Shih S. R. 2009. Enterovirus 71 3C protease cleaves a novel target CstF-64 and inhibits cellular polyadenylation. PLoS Pathog. 5:e1000593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wimmer E., Nomoto A. 1993. Molecular biology and cell-free synthesis of poliovirus. Biologicals 21:349–356 [DOI] [PubMed] [Google Scholar]
- 96. Wu K. X., Ng M. M., Chu J. J. 2010. Developments towards antiviral therapies against enterovirus 71. Drug Discov. Today 15:1041–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Yamayoshi S., et al. 2009. Scavenger receptor B2 is a cellular receptor for enterovirus 71. Nat. Med. 15:798–801 [DOI] [PubMed] [Google Scholar]
- 98. Yang F., et al. 2009. Enterovirus 71 outbreak in the People's Republic of China in 2008. J. Clin. Microbiol. 47:2351–2352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Yang Y. S., Strittmatter S. M. 2007. The reticulons: a family of proteins with diverse functions. Genome Biol. 8:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Yu Y., Abaeva I. S., Marintchev A., Pestova T. V., Hellen C. U. 2011. Common conformational changes induced in type 2 picornavirus IRESs by cognate trans-acting factors. Nucleic Acids Res. 39:4851–4865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Zhang W., et al. 2008. Cytidine deaminase APOBEC3B interacts with heterogeneous nuclear ribonucleoprotein K and suppresses hepatitis B virus expression. Cell Microbiol. 10:112–121 [DOI] [PubMed] [Google Scholar]
- 102. Zhang Z., Harris D., Pandey V. N. 2008. The FUSE binding protein is a cellular factor required for efficient replication of hepatitis C virus. J. Virol. 82:5761–5773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Zhao X., Rush M., Schwartz S. 2004. Identification of an hnRNP A1-dependent splicing silencer in the human papillomavirus type 16 L1 coding region that prevents premature expression of the late L1 gene. J. Virol. 78:10888–10905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Zoll J., Heus H. A., van Kuppeveld F. J., Melchers W. J. 2009. The structure-function relationship of the enterovirus 3′-UTR. Virus Res. 139:209–216 [DOI] [PubMed] [Google Scholar]
- 105. Zou H., Henzel W. J., Liu X., Lutschg A., Wang X. 1997. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell 90:405–413 [DOI] [PubMed] [Google Scholar]