Abstract
Innate immune response is important for viral clearance during influenza virus infection. Galectin-1, which belongs to S-type lectins, contains a conserved carbohydrate recognition domain that recognizes galactose-containing oligosaccharides. Since the envelope proteins of influenza virus are highly glycosylated, we studied the role of galectin-1 in influenza virus infection in vitro and in mice. We found that galectin-1 was upregulated in the lungs of mice during influenza virus infection. There was a positive correlation between galectin-1 levels and viral loads during the acute phase of viral infection. Cells treated with recombinant human galectin-1 generated lower viral yields after influenza virus infection. Galectin-1 could directly bind to the envelope glycoproteins of influenza A/WSN/33 virus and inhibit its hemagglutination activity and infectivity. It also bound to different subtypes of influenza A virus with micromolar dissociation constant (Kd) values and protected cells against influenza virus-induced cell death. We used nanoparticle, surface plasmon resonance analysis and transmission electron microscopy to further demonstrate the direct binding of galectin-1 to influenza virus. More importantly, we show for the first time that intranasal treatment of galectin-1 could enhance survival of mice against lethal challenge with influenza virus by reducing viral load, inflammation, and apoptosis in the lung. Furthermore, galectin-1 knockout mice were more susceptible to influenza virus infection than wild-type mice. Collectively, our results indicate that galectin-1 has anti-influenza virus activity by binding to viral surface and inhibiting its infectivity. Thus, galectin-1 may be further explored as a novel therapeutic agent for influenza.
INTRODUCTION
Influenza is an important disease in humans and animals. Influenza epidemics may result in significant morbidity, mortality, and economic burden. The emergence of novel influenza viruses poses pandemic threats to human health. Both vaccines and antiviral drugs are vital to combat influenza viruses. In light of the current pandemic threat and the emergence of drug resistance, development of novel antiviral strategies is important. Considerable efforts are being made to discover novel therapeutic agents against influenza virus.
Hemagglutinin and neuraminidase are two determinants in the pathogenicity of influenza viruses. Hemagglutinin plays a dual role in the initiation of infection by binding the virus to sialic acid-containing glycans on the host cell surface and by promoting penetration of the viral genome through membrane fusion. Neuraminidase cleaves the sialic acid on viral glycoproteins and thereby prevents clumping and promotes virus release. The degree or pattern of glycosylation of hemagglutinin and neuraminidase of influenza virus involves receptor binding, infectivity, virus release, and neurovirulence (25, 46, 48). In particular, carbohydrates are important for the interaction of hemagglutinin and neuraminidase, in which a balance is required between receptor-binding activity and virus release (27). These two viral surface glycoproteins are also important targets of host innate and adaptive immune responses.
A number of proteins can recognize influenza virus and inhibit its infectivity (11–14, 22). Collectins, a family of collagenous C-type lectins present in the mucosal secretions and serum, play roles in protecting the host from the invasion of pathogens and enhancing their clearance (44). They can bind via their carbohydrate recognition domains (CRDs) to distinctive patterns of carbohydrates on the surfaces of a wide variety of microorganisms. The collectins appear to play roles in the innate immune response to influenza virus infection by restricting viral infection and preventing overwhelming inflammation (53). Notably, surfactant proteins D and A (SP-D and SP-A), which are collectins expressed in the airway and alveolar epithelium, contribute to the innate response and enhance phagocytosis after recognition and binding to influenza virus (11–14, 22).
Galectins, which belong to S-type lectins, are a family of animal lectins containing conserved CRDs recognizing galactose-containing oligosaccharides with diverse biological activities (32). To date, 15 galectins have been identified in mammals (32). Galectins bind not only to glycan structures expressed by host cells but also to glycans on the surface of some microorganisms, suggesting their roles as soluble pattern recognition receptors in pathogen recognition (33). Galectin-1 is an important contributor in the regulation of immune homeostasis (4, 33). During infection, galectin-1 exhibits anti-inflammatory activity by inhibiting leukocyte infiltration, migration, and recruitment, whereas galectin-3 displays proinflammatory activity by enhancing macrophage survival and recruitment (40). It was shown that recombinant human galectin-1 inhibits cell fusion and syncytial formation mediated by the fusion (F) envelope glycoprotein of Nipah virus through binding to specific N-glycans in the F glycoprotein (23). Such effects were also demonstrated recently in endothelial and neural cells, the targets of Nipah virus infection (7). However, galectin-1 was also reported to increase HIV-1 infectivity through stabilization of virus attachment to host cells (26, 30). Galectin-1 has high affinity with lactose, glycoproteins with terminal β-linked galactosyl residue or extracellular matrix, such as N-acetyllactosamine (LacNAc) disaccharides and poly-N-acetyl-lactosamines, including laminin and fibronectin (56). Recently, a lectin microarray study suggested that galectin-1 binding to HIV-1 may occur through interactions with the clustered high-mannose ligands present on the viral surface, in addition to its known interactions with LacNAc residues (20).
Since hemagglutinin and neuraminidase of influenza virus carry N-linked oligosaccharide side chains (19) and galectin-1 is a β-galactoside-binding protein, in the present study we investigated the role of galectin-1 in influenza virus infection in vitro and in mice. We show for the first time that galectin-1 can directly bind to the surface of influenza viruses and inhibit viral infection. Moreover, intranasal treatment with galectin-1 enhances the survival of influenza virus-infected mice by reducing viral load and attenuating lung inflammation and apoptosis. Thus, our results suggest that galectin-1 may be further explored for amelioration of influenza virus pathogenesis.
MATERIALS AND METHODS
Cells, viruses, and mice.
MDCK cells were routinely maintained in Dulbecco modified Eagle medium supplemented with 10% cosmic calf serum (HyClone, Logan, UT), 2 mM l-glutamine, and 50 μg of gentamicin/ml. Influenza A/WSN/33 (H1N1), influenza A/Philippine/2/82 (H3N2), and influenza A/England/12/64 (H2N2) viruses were obtained from K. Y. Huang, which were originally from National Institute of Allergy and Infectious Diseases, Bethesda, MD. Influenza A/Taiwan/N39/06 (H1N1) and influenza A/Taiwan/N2723/06 (H3N2) viruses were isolated from National Cheng Kung University (NCKU) Hospital. All human influenza viruses were propagated in MDCK cells (9). Influenza A/chicken/Taiwan/2838V/00 (H6N1) and influenza A/duck/Yunlin/04 (H5N2) viruses, which are two low-pathogenicity avian influenza viruses isolated from Taiwan (3, 49), were propagated in embryonic chicken eggs and inactivated with 1% (vol/vol) of 0.1 M 2-bromoethylamine hydrobromide (BEI; Sigma, St. Louis, MO) dissolved in 0.2 N NaOH by constant shaking overnight at 37°C (1). Influenza A/WSN/33 (H1N1) virus was used in all of the experiments, unless stated otherwise. Adenovirus type 5 was propagated in 293 cells. All in vitro work on influenza virus was carried out in biosafety level 2 laboratories. Female C57BL/6 mice were purchased from the Laboratory Animal Center of NCKU or the National Laboratory Animal Center (Taipei, Taiwan). Galectin-1 knockout mice with C57BL/6 background were originally deposited to the Mutant Mouse Regional Resource Center (MMRRC) by the Consortium for Functional Glycomics (31) and were maintained in the Laboratory Animal Center of NCKU. All work with animals was carried out in animal biosafety level 2 facilities at NCKU. The experimental protocols adhered to the rules of the Animal Protection Act of Taiwan and were approved by the Animal Care and Use Committee of the NCKU.
Construction of galectin-1 expression vectors and production of recombinant galectin-1 protein.
Histidine-tagged galectin-1 protein was produced by inserting the cDNA encoding human galectin-1 in-frame into the prokaryotic expression vector pRSET (Invitrogen, Carlsbad, CA) (17). The fusion protein was expressed in Escherichia coli strain BL21(DE3) LysS transformed with the recombinant plasmid. After induction by isopropyl-β-d-thiogalactopyranoside (IPTG), recombinant galectin-1 protein was expressed, purified by the Talon metal affinity resin (Clontech, Palo Alto, CA) under denaturing conditions, and loaded onto a 1-ml HighTrap Q FPLC column (Amersham Biosciences, Piscataway, NJ). Fractions were collected in the NaCl buffer (0.7 M NaCl, 5 mM 2-mercaptoethanol [pH 7.0]) and concentrated with Amicon Ultra-15 device centrifugal filters (Millipore, Boston, MA). The purified galectin-1 protein was identified by SDS-PAGE and immunoblot analysis.
To facilitate the secretion of galectin-1 expressed from the eukaryotic expression vector pCEP4 (Invitrogen), the coding sequence encoding the signal peptide of CD5 (1 to 23 amino acids) was obtained by PCR amplification of a plasmid containing the CD5 signal peptide with the sense primer 5′-TTTAAATCTAGAATGCCCATGGGGTCTCTGCAA-3′ and the antisense primer 5′-GATATCAGATCTGTACTCACCCTCGGGATCCGC-3′, in which an XbaI site (underlined) and a BglII site (underlined) were introduced onto the 5′ and 3′ ends, respectively. The resulting PCR product was digested with XbaI and BglII and fused in frame and upstream of the coding region of human galectin-1 in the yT-hGal-1 plasmid at the XbaI/BglII sites, yielding the yT-cd5-Gal-1 plasmid. The whole sequence encompassing the CD5 secretory signal sequence and the galectin-1 cDNA was further recovered from yT-cd5-Gal-1 plasmid by SalI and SmaI digestion and ligated into the XhoI/NaeI sites of pCEP4, resulting in pCEP4-cd5-Gal-1.
Hemagglutination and plaque assays.
For determining the virus titers, serial 2-fold dilutions of influenza virus in phosphate-buffered saline (PBS) were mixed with 0.8% human O-type erythrocyte suspension in U-shaped 96-well plates at room temperature for 1 h. The reciprocal of the highest dilution of the test sample capable of causing partial or complete hemagglutination was expressed as the hemagglutinating unit (HAU). MDCK cells that had been transfected with 0.8 μg of pCEP4-cd5-Gal-1 or pCEP4 plasmid DNA using Lipofectamine 2000 (Invitrogen) were infected with influenza virus (0.1 HAU) for 48 h. Moreover, MDCK cells were infected with various doses of influenza virus in the presence or absence of recombinant galectin-1 (10 μg/ml). Titers of influenza virus produced from these cells at the indicated time points were determined with a standard plaque assay in MDCK cells and expressed as PFU/ml. On the basis of the titers of PFU and HAU, we estimated that 200 PFU of our influenza A/WSN/33 virus stocks equaled ∼1 HAU.
A hemagglutination test was also used to verify the binding of influenza virus to galectin-1. Influenza viruses (2 HAU) were incubated with various concentrations of galectin-1 with or without the concomitant addition of different concentrations of lactose for 1 h at room temperature, followed by addition of 0.8% human O-type erythrocyte suspension. After an additional 60-min incubation at room temperature, hemagglutination was recorded.
Colorimetric binding assay.
An enzyme-linked immunosorbent assay (ELISA)-based method was used to demonstrate direct binding between galectin-1 and influenza virus. Washing was performed three times between all steps with PBS containing 0.1% Tween 20 (PBST), unless stated otherwise. Galectin-1 (10 μg/ml, 50 μl) diluted in 0.1 M carbonate/bicarbonate buffer (pH 9.6) was used to coat 96-well plates (Nunc-Immuno plate, MaxiSorp surface; Nunc, Roskilde, Denmark) overnight at room temperature. The free binding site was blocked with 1% (wt/vol) bovine serum albumin (BSA) at room temperature for 2 h. Control wells were coated with BSA only. A total of 50 μl of influenza virus ranging from 0 to 64 HAU diluted in 1% BSA/PBS was added and incubated overnight at 4°C. Goat anti-influenza A virus (H1N1) antiserum (1:200; ViroStat, Portland, ME) was then added, followed by incubation at room temperature for 2 h. Subsequently, horseradish peroxidase (HRP)-conjugated donkey anti-goat IgG diluted 1:200 in BSA diluent/blocking solution (KPL, Gaithersburg, MD) was added to each well and incubated at room temperature for 2 h, and the plates were developed with 3,3′,5,5′-tetramethyl benzidine (KPL). The enzyme reaction was stopped with 2 N H2SO4 after incubation for 5 to 10 min at room temperature, and the absorption was measured at 450 nm.
To test the carbohydrate specificity of the binding, a similar binding assay was done except that influenza virus (2 HAU/well), rather than galectin-1, served as the capture reagent. Subsequently, galectin-1 (0.5 μg/well) in the presence of different concentrations of lactose or mannose was added to the wells, followed by incubation at 4°C overnight. Biotinylated goat anti-galectin-1 antibody (10 ng; R&D, Minneapolis, MN) was added, followed by incubation at room temperature for 2 h and then the addition of HRP-conjugated streptavidin (1:200; R&D) for an additional 20 min at room temperature. The remaining steps for enzymatic color development were carried out as described above. A similar experiment was performed to examine the binding between galectin-1 and different subtypes of influenza A viruses, except that a wide range of galectin-1 concentrations was used to apply to the wells coated with 4 HAU of different influenza A viruses/well. The dissociation constant (Kd) was calculated as 1/2 Vmax by nonlinear regression analysis, based on the fitting of the dissociation data to one-phase exponential decay, using the Prism 5.0 software package (GraphPad Software, San Diego, CA).
Preparation and characterization of galectin-1 conjugated to AuNP.
The gold nanoparticle (AuNP) was prepared as previously described, with minor modifications (10). Briefly, 108 ml of HAuCl4 (0.6 mM) was reduced with sodium citrate (195 mM, 2 ml) by boiling with vigorous stirring for 10 min. After the resulting wine-red suspension was fully cooled, it was sterile filtered and stored in glass bottles at room temperature or 4°C. The AuNP was spherical and well dispersed, with an approximate diameter of 13 ± 1.2 nm, as confirmed by transmission electron microscopy (TEM). The particle concentration was ∼9.2 nM (235 μg/ml), as quantified by an absorption at 450 nm or calculation from initial Au3+ ion concentration. For fabrication of galectin-1-conjugated AuNP (Au/Gal-1), 1 ml of AuNP was incubated with 20 μg of galectin-1, and this mixture was gently shaken, centrifuged, and finally resuspended in sodium phosphate buffer (10 nM, pH 7). Because gold binds strongly to the thiols, we prepared Au/PEG, a thiolated polyethylene glycol (PEG)-coated AuNP, as a control particle as previously described (43).
To study the interaction of Au/Gal-1 with influenza virus, Au/Gal-1 (20 μg/ml) or Au/PEG was incubated with 5.12 × 104 PFU of influenza virus or adenovirus for 1 h at room temperature, followed by centrifugation to concentrate the nanocomplexes. They were fixed and negatively stained with 1% (wt/vol) phosphotungstic acid (pH 6.5) for 3 min. The samples were examined by TEM with an acceleration voltage of 100 kV. Surface plasmon resonance (SPR) was performed to analyze the binding of influenza virus with Au/Gal-1 using an UV-visible spectrophotometer (TECAN Infinite 200, Salzburg, Austria). Briefly, different samples (100 μl), including Au/Gal-1 plus virus, Au/Gal-1, Au/BSA plus virus, and Au/BSA, were applied to the wells of a 96-well plate, and the UV-visible absorption spectra were recorded from wavelength 100 to 800 nm with a 1-nm interval at room temperature.
Animal studies.
Groups of female C57BL/6 mice at 5 to 6 weeks of age and galectin-1 knockout mice at 4 to 5 weeks of age were intranasally inoculated with 512 HAU of influenza virus that corresponded to 1.5 × the 50% lethal dose at day 0 and treated with recombinant galectin-1 (50 μg) or NaCl buffer at days 2, 4, and 5 via the nasal route. The mice were monitored daily for illness, weight loss, and death for 15 days after viral infection.
Histochemical, immunohistochemical, and apoptotic analyses.
Influenza virus-infected mice that had received different treatments were killed at day 6 postinfection (p.i.). The lungs were removed, formalin fixed, and paraffin embedded for hematoxylin and eosin (H&E) staining according to standard methods. Inflammatory changes on the basis of numbers of inflammatory cells and tissue damage in the lungs were determined by histology from H&E-stained longitudinal cross-sections and graded as no change (score = 0), mild (score = 1), moderate (score = 2), or severe (score = 3) as previously described (2). Sections were reviewed and scored in a blind fashion by a pathologist (C.-C.L.). For immunochemical staining, tissue sections were deparaffinized, antigen retrieved using protease K (100 μg/ml; Life Technologies, Carlsbad, CA) digestion for 10 min at room temperature, and incubated with monoclonal antibody specific for nucleoprotein of all influenza A virus strains (1:200; Virostat). After sequential incubation with appropriate HRP-labeled secondary antibody at room temperature and 3-amino-9-ethyl carbazole (AEC) as a substrate chromogen, the slides were counterstained with hematoxylin. Paraffin-embedded lung tissue sections were also subjected to TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling) assay according to the manufacturer's instructions (Promega, Madison, WI).
ELISA.
Bronchoalveolar lavage (BAL) was performed by injection of 1 ml of PBS to the alveolar space of influenza virus-infected mice at different time points through the trachea three times, and BAL fluid was then collected by gentle suction. The BAL fluid was centrifuged, and cell-free supernatant was collected. The levels of galectin-1 in the BAL fluid were quantified using mouse galectin-1 DuoSet ELISA kit (R&D). Levels of human galectin-1 in the medium of MDCK cells transfected with pCEP4-cd5-Gal-1 encoding secretory human galectin-1 or pCEP4 were determined with a sandwich ELISA. Briefly, tested media (50 μl) were added to each well of 96-well plates coated with polyclonal goat anti-human galectin-1 antibody (R&D) using the same method for preparing galectin-1-coated plates and incubated at 4°C overnight. After washing, biotinylated goat anti-human galectin-1 antibody (10 ng; R&D) was added, followed by incubation for 2 h at room temperature. The remaining steps for enzymatic color development were carried out as described above.
Immunoblot analysis.
Lung tissues collected at different time points from influenza virus-infected mice were homogenized, and their lysates were subjected to immunoblotting to detect galectin-1. Lysates from MDCK cells that had been transfected with pCEP4-cd5-Gal-1 or pCEP4 plasmid DNA, followed by infection with influenza virus as described above, were examined for influenza virus protein expression at 48 h p.i. Equal protein amounts (20 μg) of tissue or cell lysates were fractionated on SDS-10% PAGE, transferred onto nitrocellulose membrane, and immunoblotted with polyclonal goat anti-human galectin-1 antibody (R&D) or goat anti-influenza A virus (H1N1) antiserum (1:1,000; ViroStat). HRP-conjugated Affinipure donkey anti-goat IgG (Jackson, West Grove, PA) was used as the secondary antibody, and protein-antibody complexes were visualized by the enhanced chemiluminescence system (Amersham, Uppsala, Sweden). The blots reprobed with HRP-conjugated anti-mouse β-actin antibody (1:10,000; Sigma) served as the loading control.
Statistical analysis.
Data are expressed as means ± the standard deviations (SD). Differences in body weights between two groups were compared by repeated-measures analysis of variance (ANOVA) using SAS software version 9.1 (GLM program; SAS Institute, Cary, NC). Differences in the remaining data between two groups were compared by using a Student t test. Pearson correlation analysis was used to evaluate the correlation between galectin-1 levels and viral loads. The survival analysis was performed using the Kaplan-Meier survival curve and log-rank test. The differences were considered significant if P values were <0.05.
RESULTS
Galectin-1 expression is upregulated in mice infected with influenza virus.
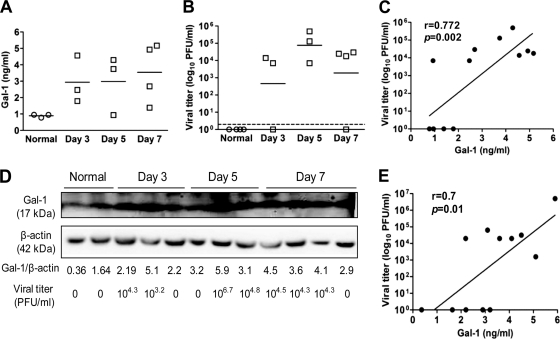
We first determined the levels of galectin-1 and viral loads in mice intranasally infected with 512 HAU of influenza A/WSN/33 virus. In the BAL fluid, galectin-1 was upregulated (Fig. 1A) with a concomitant increase in viral loads (Fig. 1B). On the basis of the best-fit linear trend line, a positive correlation (r = 0.772, P = 0.002) between galectin-1 level and viral load in the BAL fluid during the acute phase of viral infection was found (Fig. 1C). In the lung, galectin-1 was also upregulated after influenza virus infection (Fig. 1D). Viruses were detectable in most of the infected mice (Fig. 1D). A positive correlation (r = 0.7, P = 0.01) obtained from a best-fit linear trend line between galectin-1 level and viral load was also noted (Fig. 1E).
Fig. 1.
Upregulation of galectin-1 in mice after infection with influenza virus. Mice were intranasally inoculated with influenza virus (512 HAU), and the BAL fluid and lung tissue were collected at different time points. (A) Levels of galectin-1 in the BAL fluid determined by ELISA. (B) Viral loads in the BAL fluid determined by the plaque assay. The horizontal bars shown in panels A and B denote the mean value for each group. The threshold of virus detection is indicated by the horizontal dashed line. (C) Pearson correlation analysis for the correlation of galectin-1 levels and virus titers in the BAL fluid. The best-fit linear trend line is shown. (D) Levels of galectin-1 in the lung lysate (20 μg) determined by immunoblot analysis. Expression of β-actin served as the loading control. Ratios between the intensity of the bands corresponding to galectin-1 and those corresponding to β-actin analyzed by densitometry were calculated. The levels of virus titers in the whole lung lysate suspended in 1 ml of saline were quantified with the plaque assay. (E) Pearson correlation analysis for the correlation of galectin-1 ratios (between the intensity of the bands corresponding to galectin-1 and those corresponding to β-actin) and virus titers in the lungs. The best-fit linear trend line is shown.
Galectin-1 reduces influenza virus production in MDCK cells.
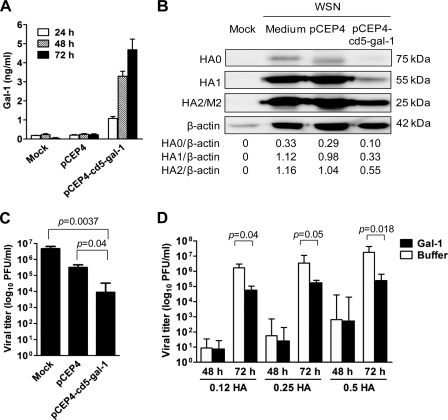
Given that galectin-1 was upregulated during influenza virus infection (Fig. 1) and it was shown to bind to specific N-glycans in the F glycoprotein of Nipah virus (23), we tested to see whether galectin-1 affected influenza virus infection in vitro. We constructed a secretory galectin-1 expression vector, pCEP4-cd5-Gal-1 by incorporating the signal peptide of CD5. MDCK cells transfected with pCEP4-cd5-Gal-1, allowing secretion of galectin-1 to the culture medium (Fig. 2A), produced substantially less HA0, HA1, HA2, and M2 proteins (Fig. 2B) and yielded lower virus titers (Fig. 2C) than those transfected with the control plasmid pCEP4. To further verify that galectin-1 secreted from the transfected cells contributed to the anti-influenza virus effect, we treated cells with various doses of influenza virus in the presence or absence of recombinant galectin-1 (10 μg/ml) produced from E. coli, and quantified virus titers at 24, 48, and 72 h p.i. As shown in Fig. 2D, galectin-1-treated cells generated lower viral yields than buffer-treated cells at 72 h p.i. in all of the viral doses tested. There was no difference in virus titers between the treated and control cells at 48 h p.i. The viral levels were all below the limit of detection at 24 h p.i. The anti-influenza virus effect of galectin-1 was not restricted to influenza A/WSN/33 virus, as galectin-1 also protected cells from infection with different subtypes of influenza A virus (data not shown).
Fig. 2.
Reduction of influenza virus production in MDCK cells by galectin-1. (A) Levels of galectin-1 in the conditioned medium of cells transfected with 0.8 μg of pCEP4-cd5-gal-1 plasmid DNA encoding secretory galectin-1 or the control vector pCEP4 at different time points after transfection, as determined by ELISA. The bars shown represent means ± the SD (n = 4). (B) Immunoblot analysis of viral protein expression in the lysates of pCEP4-cd5-gal-1-transfected, pCEP4-transfected, or untransfected cells after infection with 0.1 HAU of influenza virus (WSN) for 48 h. Goat anti-influenza A virus (H1N1) antiserum and HRP-conjugated donkey anti-goat IgG were used as primary and secondary antibodies, respectively. Mock-infected cells served as negative controls. Expression of β-actin served as the loading control. Values shown at the bottom are the ratios between the intensity of the bands corresponding to the indicated proteins, as determined by densitometric analysis. (C) Quantification of influenza virus produced from pCEP4-cd5-gal-1-transfected, pCEP4-transfected, or untransfected cells after infection with influenza virus (0.1 HAU) for 48 h by the plaque assay. The bars shown represent means ± the SD (n = 4). (D) Quantification of influenza virus produced from cells infected with indicated doses of influenza virus in the presence or absence of galectin-1 (10 μg/ml) at 24, 48, and 72 h p.i. Note that viral levels were below the limit of detection at 24 h p.i. The bars shown represent means ± the SD (n = 4 to 6).
Intranasal treatment with galectin-1 enhances survival of mice against lethal challenge with influenza virus.
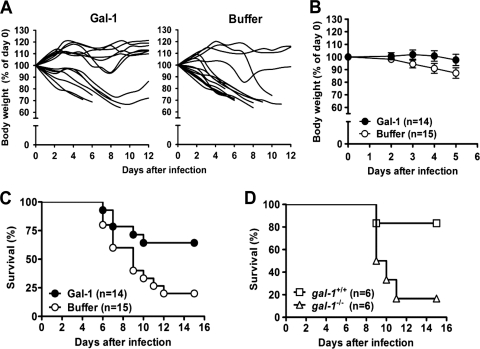
Since galectin-1 could reduce influenza virus production in MDCK cells, we next investigated whether galectin-1 treatment would protect mice against lethal influenza virus infection. Mice that had been infected with influenza A/WSN/33 virus, known to be neurovirulent to mice (37, 41), were treated with recombinant galectin-1 or the vehicle (NaCl buffer) and monitored daily for morbidity as measured by weight loss and mortality. More buffer-treated mice continued to lose weight over time, compared to galectin-1-treated mice (Fig. 3A). Analysis of the entire body weight curves from day 0 through day 5, while all mice were still alive reveals that mice treated with galectin-1 significantly lost less weight than those treated with buffer (P = 0.0127) (Fig. 3B). All galectin-1-treated mice that survived the infection were recovered at day 10 p.i. Importantly, galectin-1 treatment significantly enhanced the survival of the infected mice compared to buffer treatment (P = 0.023) (Fig. 3C). To verify the importance of endogenous galectin-1 in host defense against influenza virus infection, we compared the survival curves between galectin-1 knockout mice and wild-type C57BL/6 mice after influenza virus infection. Figure 3D shows that galectin-1 knockout mice indeed had lower survival compared to wild-type mice (P = 0.03), indicating that galectin-1 knockout mice were more susceptible to influenza virus infection than wild-type mice.
Fig. 3.
Protection of mice against lethal influenza virus challenge by galectin-1 treatment. (A and B) Groups of C57BL/6 mice were intranasally inoculated with influenza virus (512 HAU) at day 0 and treated with recombinant galectin-1 (50 μg) or NaCl buffer at days 2, 4, and 5 p.i. via the nasal route. (A) Changes in body weights in individual mice from two groups. Body weights were recorded and are expressed as a percentage of preinfection (day 0) body weight. (B) Mean changes in body weights from day 0 through day 5 while all mice were still alive (P = 0.0127 by repeated-measures ANOVA). (C) Kaplan-Meier survival curves (P = 0.023 by the log-rank test). (D) C57BL/6 (gal-1+/+) and galectin-1 knockout (gal-1−/−) mice were infected with influenza virus (512 HAU), and their survival time was recorded. Kaplan-Meier survival curves were shown (P = 0.03 by the log-rank test).
Galectin-1 reduces viral loads in influenza virus-infected mice.
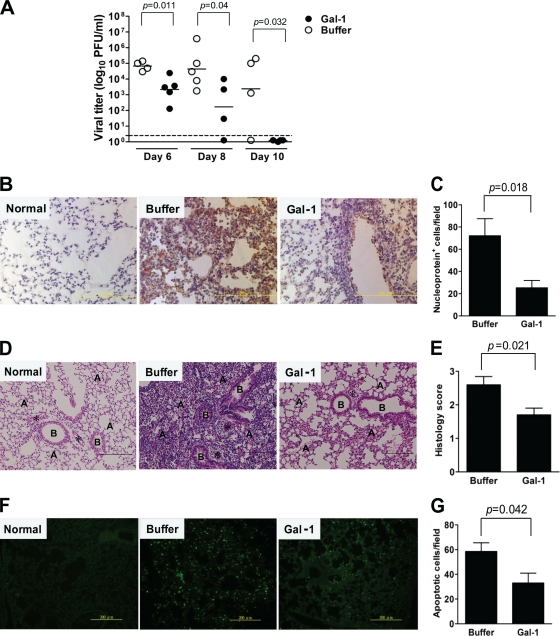
Determination of influenza virus titers in the BAL fluid revealed that viral loads were significantly lower in galectin-1-treated mice than in buffer-treated mice at each time point (Fig. 4A). Of note, at day 10 p.i., viral levels were below the limit of detection in galectin-1-treated mice, whereas three of four buffer-treated mice still harbored significant amounts of viruses. Immunohistochemical examination at day 6 p.i. reveals that expression of nucleoprotein was less abundant in the lungs of galectin-1-treated mice than in their control counterparts (Fig. 4B), indicating lower degree of viral replication in the lungs of galectin-1-treated mice. Quantification of cells expressing nucleoprotein also confirms this observation (Fig. 4C).
Fig. 4.
Reduction of viral load, lung inflammation, and apoptosis in the lung of influenza virus-infected mice by galectin-1 treatment. Groups of C57BL/6 mice were inoculated with influenza virus (384 HAU) at day 0 and treated with 50 μg of recombinant galectin-1 or NaCl buffer at days 2, 4, and 5. (A) Virus titers in the BAL fluid collected at days 6, 8, and 10 p.i., as determined by the plaque assay. Horizontal bars shown denote the mean value for each group (n = 4 to 5). The threshold of virus detection is indicated by the horizontal dashed line. (B) Immunohistochemical staining of the lungs collected at day 6 p.i. for viral nucleoprotein using monoclonal antibody specific for nucleoprotein of all influenza virus A strains (original magnification, ×400; scale bar, 200 μm). Lung sections from normal mice served as controls for background staining levels. (C) Numbers of nucleoprotein-positive cells indicative of viral infection were calculated from six randomly selected fields in each section. The bars shown represent means ± the SD (n = 6). (D) H&E-stained lung sections collected at day 6 p.i. (original magnification, ×200; scale bar, 200 μm). Lungs from uninfected mice served as the normal control. B, bronchiole; A, alveolus; *, microvessel. (E) Lung histology scores. H&E-stained longitudinal cross-sections were graded as no changes (score = 0), mild (score = 1), moderate (score = 2), or severe (score = 3) based on the severity of inflammation and tissue damage. The bars shown represent means ± the SD (n = 5). (F) Detection of apoptotic cells in the lungs collected at day 6 p.i. by the TUNEL assay (original magnification, ×200; scale bar, 200 μm). (G) Numbers of TUNEL-positive cells in the lungs were determined from five randomly selected fields in each section. The bars shown represent means ± the SD (n = 5).
Galectin-1 attenuates lung inflammation and apoptosis in influenza virus-infected mice.
Histological examination of the lungs collected at day 6 p.i. demonstrates that influenza virus-infected mice treated with galectin-1 had relatively fewer inflammatory cells and less damaged tissue than those treated with buffer (Fig. 4D). Furthermore, lung histology scores were lower in galectin-1-treated mice than in control mice (Fig. 4E). Since apoptotic cell death induced by influenza virus may contribute to its pathogenesis (29, 50), we detected apoptotic cells in the lung using TUNEL staining. TUNEL-positive cells were abundantly detected in the buffer control group, whereas fewer TUNEL-positive cells were detected in galectin-1-treated group (Fig. 4F). Galectin-1 reduced the percentage of cells undergoing virus-induced apoptosis, suppressing apoptosis by approximately half compared to buffer treatment (Fig. 4G).
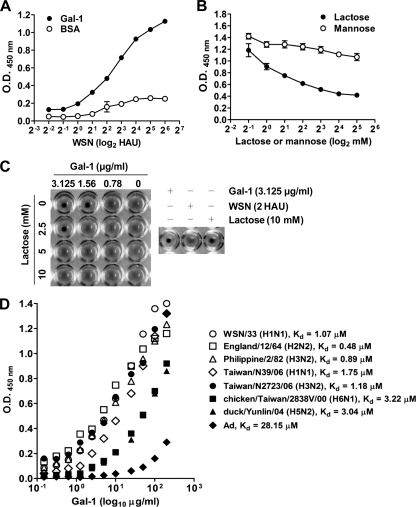
Galectin-1 binds to influenza virus and inhibits viral hemagglutination activity.
Since galectin-1 had anti-influenza virus activity in vitro and in vivo, we then examined whether galectin-1 directly bound to influenza virus and thereby reduced viral infectivity. As shown in Fig. 5A, influenza virus bound to galectin-1 coated on a solid phase in a dose-dependent manner, whereas the virus failed to bind to BSA that served as the negative control. Furthermore, such binding was dose dependently abolished by the addition of lactose that is expected to compete for the CRD of galectin-1 and act as its antagonist, but not by mannose, which does not bind galectin-1 (Fig. 5B). We further examined the loss of influenza virus hemagglutination activity after binding with galectin-1 and whether the addition of lactose could reverse such an effect. As shown in Fig. 5C, galectin-1 at concentrations ≥1.56 μg/ml could block the hemagglutination activity of influenza virus (2 HAU). The concomitant addition of lactose abrogated the hemagglutination inhibition activity of galectin-1. In fact, high concentrations of galectin-1 per se may induce hemagglutination by binding to erythrocytes and obscure its hemagglutination inhibition activity. In this assay, galectin-1 alone up to 3.125 μg/ml had no hemagglutination activity. Therefore, we could observe the effect of galectin-1 on the inhibition of influenza virus-induced hemagglutination in such assay conditions. This result agrees with previous reports demonstrating that lectins can inhibit the activities of hemagglutinin of a variety of viruses (18, 35, 36).
Fig. 5.
Binding of galectin-1 on the surface of influenza virus and inhibition of viral hemagglutination activity. (A) Binding of galectin-1 to influenza virus in a dose-dependent manner. Various doses of influenza virus (WSN) were applied to 96-well plates coated with 10 μg of recombinant galectin-1/ml, and the bound virus particles were detected by ELISA with goat anti-influenza A virus (H1N1) antiserum. BSA-coated wells served as the negative control. Each value represents a mean ± the SD (n = 4). (B) Abrogation of galectin-1 binding by lactose. Galectin-1 (0.5 μg/well) admixed with various concentrations of lactose or mannose was applied to 96-well plates coated with influenza virus (2 HAU/well). The bound galectin-1 was detected by ELISA with anti-galectin-1 antibody. Each value represents a mean ± the SD (n = 4). (C) Hemagglutination inhibition activity of galectin-1. Influenza viruses (2 HAU) were incubated with various concentrations of galectin-1 with or without the concomitant addition of different concentrations of lactose for 1 h, followed by the addition of erythrocyte suspension. Hemagglutination was recorded after 1 h. (D) Binding of galectin-1 to different subtypes of influenza A virus. Serial 2-fold dilutions of galectin-1, ranging from 200 to 0.195 μg/well, were applied to 96-well plates coated with various subtypes of influenza A virus (4 HAU/well equivalent to 800 PFU/well) or adenovirus (Ad, 800 PFU/well). The bound galectin-1 was detected by ELISA with anti-galectin-1 antibody. The dissociation constant (Kd) was calculated as 1/2 Vmax by nonlinear regression analysis, based on the fitting of the dissociation data to one-phase exponential decay. Note that galectin-1 bound to adenovirus much weaker than to influenza virus.
Galectin-1 binds to different subtypes of influenza A virus with micromolar Kd values.
To further demonstrate that the binding of galectin-1 to the surface of influenza A virus is not restricted to influenza A/WSN/33 virus, different subtypes of influenza A viruses were used for the binding assay. Figure 5D shows that galectin-1 could bind to H1N1, H2N2, H3N2, H5N2, and H6N1 subtypes of influenza A virus with Kd values ranging between 0.48 and 3.22 μM. In contrast, the binding of galectin-1 to adenovirus was much weaker than to influenza virus, with a Kd value of 28.15 μM. These data indicate that galectin-1 can bind to influenza A viruses prevalent in humans and avian influenza A viruses.
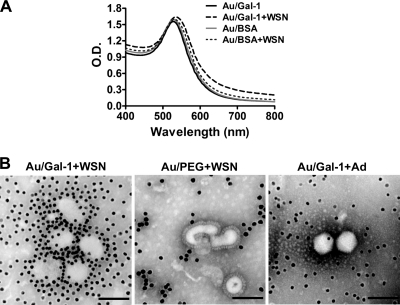
Au/Gal-1 nanocomplex binds to the surface of influenza virus detected by SPR and TEM analysis.
To further demonstrate the binding of galectin-1 to influenza virus, galectin-1 was conjugated onto AuNP. A broadening shift of the surface plasmon band from the UV-visible absorption spectrum was noted in the mixture of Au/Gal-1 and influenza virus but not in the mixture of Au/BSA and influenza virus, indicating that the interaction between galectin-1 and influenza virus indeed existed (Fig. 6A). Notably, TEM clearly demonstrates that Au/Gal-1 particles directly bind to the surface of influenza virus and aggregate the virus (Fig. 6B, left panel). To exclude the possibility of nonspecific binding of AuNP to influenza virus, AuNP coated with PEG on the surface (Au/PEG) was examined for its interaction with influenza virus. As expected, Au/PEG did not bind to the surface of influenza virus (Fig. 6B, middle panel). In accordance with the data shown in Fig. 4D, Au/Gal-1 also failed to bind to adenovirus (Fig. 6B, right panel). Therefore, these results provide evidence that the binding between Au/Gal-1 and influenza virus was attributable to the interaction of galectin-1 molecule, rather than AuNP, with glycans on the surface of influenza virus.
Fig. 6.
Detection of the binding between Au/Gal-1 nanocomplex and influenza virus by SPR and TEM analysis. (A) Galectin-1- or BSA-conjugated AuNPs were incubated with or without influenza virus (5 HAU) for 1 h, followed by centrifugation to concentrate the nanocomplexes. The sample solution (100 μl) was applied to wells of a 96-well plate. The binding between influenza viruses and nanocomplexes was analyzed by SPR. The UV-visible absorption spectra were recorded from wavelength 100 to 800 nm with a 1-nm interval. (B) Galectin-1-conjugated Au nanoparticles (Au/Gal-1) or PEG-coated Au nanoparticles (Au/PEG) were incubated with influenza virus (WSN) or adenovirus (Ad) for 1 h, followed by centrifugation to concentrate the nanocomplexes. They were fixed and negatively stained for examination by TEM (original magnification, ×300,000; scale bar, 100 nm).
DISCUSSION
Viral infections have been reported to modulate expression and function of galectins in vitro, suggesting that galectins may participate in antiviral defense from the initial recognition and blocking of surface glycoproteins to the activation and amplification of innate and adaptive immune responses (45). Galectins, such as galectin-1 and galectin-3, are upregulated after pathogen invasion and may serve as a regulator in innate defense during inflammation (6, 8, 28, 47). In the present study, we show in vivo that galectin-1 was upregulated in mice after influenza virus infection, and its levels positively correlated with viral loads. Galectin-1, which is ubiquitously expressed in a variety of cells, is present intracellularly in the cytoplasm and the nucleus. It can be secreted extracellularly despite the lack of a signal peptide. During infection or inflammation, galectin-1 may be produced and released by various cells, such as infected epithelium, activated macrophages, and endothelial cells. In our infection model, although galectin-1 was upregulated in the respiratory tract during influenza virus infection, it may be too late to produce enough galectin-1 to inhibit viral infection and attenuate inflammation. Our viral challenge experiment supports this notion. We demonstrate that treatment with galectin-1 in mice after influenza virus infection significantly reduced the morbidity and mortality, as well as enhanced the survival time. Moreover, galectin-1 knockout mice were more susceptible to influenza virus infection than wild-type mice, resulting in death in five of six mice, whereas only one of six wild-type mice died after viral infection. These results suggest that galectin-1 might play a part in the host defense against influenza virus infection. We used influenza A/WSN/33 virus for the present study, which causes neurovirulence and death in mice (37, 41). However, the lung, rather than the brain, is the major target organ for influenza virus pathogenesis in humans. Therefore, we also infected mice with lower doses of influenza virus and examined inflammatory responses and viral loads in the lung and respiratory tract, which may mimic influenza virus infection in humans more closely. Our results show that galectin-1 treatment reduced the viral load and attenuated inflammation and apoptosis in the lungs. Alleviation of inflammation might be attributed to the reduced levels of viral load. However, the contribution of the anti-inflammatory activity of galectin-1 could not be excluded. Our results suggest that the regulatory role of galectin-1 on immune responses may be orchestrated with its direct antiviral effect in influenza virus infection. Our findings provide evidence to show for the first time that galectin-1 has anti-influenza virus activity in vivo.
In our in vitro studies, we show that MDCK cells transfected with an expression vector encoding a secretory form of galectin-1 produced lower levels of viral proteins and viral particles after influenza virus infection compared to those transfected with a control vector. Exogenous addition of recombinant human galectin-1 also verified its anti-influenza virus activity. On the viral surface, hemagglutinin and neuraminidase are glycosylated to various degrees in different subtypes and strains of influenza virus. The oligosaccharides attached to individual glycosylation sites are either complex or oligomannosidic. We show that galectin-1 binds to influenza virus via the interaction of its CRD and viral glycans, as lactose but not mannose abrogated such binding. This interaction may have resulted in the inhibition of influenza virus infection by preventing hemagglutinin binding to sialylated receptors, which may be attributed to the multivalent binding and cross-linking activities of galectin-1. The influenza virion contains about 350 to 400 hemagglutinin trimers and 50 neuraminidase tetramers on its surface (24). All hemagglutinin molecules are glycosylated to various degrees, with glycosylation sites ranging between 5 and 11 (38, 39, 54). The neuraminidase molecule is also a glycoprotein with three to five potential glycosylation sites (5, 15). Our results show that galectin-1 can bind to different subtypes of influenza A virus with micromolar Kd values, which may explain its broad activities against influenza virus. Galectin-1 is expected to bind to hemagglutinin and possibly neuraminidase and thereby inhibit influenza virus infection. In contrast, we found that galectin-1 has little, if any, binding activity to nonenveloped adenovirus compared to its binding to influenza virus.
In addition to the binding assays in solid phase, by using 13-nm Au/Gal-1 nanoconjugates to increase multivalent binding, we also demonstrate the direct binding of Au/Gal-1 with influenza virus particles by detection of a shift of the SPR band. It has been known that AuNP has a prominent SPR band at the visible wavelength region (at 520 nm for 13 nm AuNP). In our SPR analysis, the peak of the SPR absorption was shifted from 520 to 530 nm after addition of galectin-1 or BSA for adsorption. This 10-nm red shift was related to a change in the local dielectric constant around the AuNP as a result of protein adsorption. A more significant red shift for the SPR band at the long-wavelength region was observed when Au/Gal-1 was mixed with influenza virus compared to Au/BSA admixed with the virus. This red shift reflected that a significant increase in the aggregate size occurred from Au/Gal-l linked structures during the mixing process with influenza virus and that aggregate size significantly influenced the optical properties of the AuNP network materials due to the increase in the electron mean-free path of AuNP. Therefore, the SPR analysis clearly demonstrates that Au/Gal-1 binds to influenza virus via the galectin-1 molecule. More importantly, multivalent interactions of Au/Gal-1 with carbohydrate moieties on the surface glycoproteins of influenza virus allow detection of the binding between galectin-1 and influenza virus. Our TEM analysis demonstrates for the first time that Au/Gal-1 directly binds to the surface of influenza virus via the galectin-1 molecule.
Galectin-1 is conjugated to the surface of AuNP through the formation of the Au-S covalent bond. The functionalized AuNP can cause aggregation of influenza virus via the formation of a sandwich complexation, virus-Au/Gal-1-virus (42). The induced dipole-dipole interaction from this sandwich complexation is also reflected in the red shift of SPR in UV-visible absorption spectroscopy. The binding affinities of mammalian lectins for carbohydrate ligands can be dramatically increased by a concomitant clustering of lectin binding sites and CRDs. The affinity enhancement due to multivalent interaction has been known as the “glycoside cluster effect” (21). Galectins can form multivalent complexes with cell surface glycoconjugates and deliver intercellular signals to modulate cell activation, differentiation, and survival (34). It was shown that galectin-1 inhibits cell fusion and syncytial formation of Nipah virus through binding to specific N-glycans on viral glycoproteins and thereby inducing its oligomerization (23). Nevertheless, galectin-1 was also reported to increase HIV-1 infectivity through stabilizing virus attachment to host cells and cross-linking viral glycoproteins with the target cells (30). In the present study, similar to the sandwich complexation of influenza virus-Au/Gal-1-influenza virus, the multivalent interaction of galectin-1-influenza virus is expected to occur.
Several lectin-like molecules are involved in influenza virus infection. C-type lectin SP-D plays a key role in the defense against influenza virus infection through the recognition of mannose residue on the viral hemagglutinin with CRDs and thereby inhibits early stages of viral infection (52). SP-D also binds to neuraminidase and inhibits its activity (51). Pentraxin PTX3, another C-type lectin, preserves the pathogen recognition ability by offering sialylated ligands similar to cellular receptors for influenza virus infection, as well as blocks the binding between the receptor and hemagglutinin (35). Serum amyloid P component (SAP), which is a pentraxin protein, can inhibit hemagglutinin cleavage of influenza virus (16). Furthermore, the extract of Japanese plum contains lectin-like molecules capable of inhibiting viral infection by reducing hemagglutination activity of influenza virus (55). In the present study, we used the whole virion, rather than viral glycoproteins used in other studies (7, 23, 26), to demonstrate that galectin-1 inhibits influenza virus infection by direct binding to galactose-containing glycans on the viral envelope, inhibiting viral hemagglutination activity, and blocking viral entry. Moreover, compared to previous in vitro studies on the effects of galectin-1 on viral infection (23, 30), our study is the first to show in vivo that galectin-1 can ameliorate influenza virus pathogenesis through direct anti-influenza virus activity.
In conclusion, we show here that galectin-1 can bind to the envelope glycoproteins of influenza virus and inhibit viral infectivity, which may be attributed to the multivalent binding and cross-linking activities of galectin-1. Reduction in the viral load after galectin-1 treatment may also lead to the attenuation of lung inflammation and injury. Alternatively, galectin-1, known to possess anti-inflammatory activity, may also be involved in alleviating lung inflammation during influenza virus infection. Since galectin-1 knockout mice are more susceptible to influenza virus infection, galectin-1 might also play a part in the host defense against influenza virus. Therefore, galectin-1 may have promise as a novel therapeutic agent for influenza. Furthermore, galectin-1 may also be explored for targeting other viruses with glycoproteins on their surface.
ACKNOWLEDGMENTS
We acknowledge the National Institutes of Health-sponsored MMRRC National System as the source of galectin-1 knockout mice for use in this study. The mice were produced and deposited with the MMRRC by the Consortium for Functional Glycomics supported by the National Institute of General Medical Sciences (GM62116). This study was supported by grants from the National Science Council, Taiwan (NSC 99-2120-M-006-007 and NSC 99-2321-B-006-009) and the Center for Frontier Materials and Micro/Nano Science and Technology, National Cheng Kung University, Taiwan.
Footnotes
Published ahead of print on 27 July 2011.
REFERENCES
- 1. Bahnemann H. G. 1990. Inactivation of viral antigens for vaccine preparation with particular reference to the application of binary ethylenimine. Vaccine 8:299–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Buchweitz J. P., Karmaus P. W., Harkema J. R., Williams K. J., Kaminski N. E. 2007. Modulation of airway responses to influenza A/PR/8/34 by Δ9-tetrahydrocannabinol in C57BL/6 mice. J. Pharmacol. Exp. Ther. 323:675–683 [DOI] [PubMed] [Google Scholar]
- 3. Chen Y.-C., Chen C.-H., Wang C.-H. 2008. H5 antibody detection by blocking enzyme-linked immunosorbent assay using a monoclonal antibody. Avian Dis. 52:124–129 [DOI] [PubMed] [Google Scholar]
- 4. Dhirapong A., Lleo A., Leung P., Gershwin M. E., Liu F.-T. 2009. The immunological potential of galectin-1 and -3. Autoimmun. Rev. 8:360–363 [DOI] [PubMed] [Google Scholar]
- 5. Fields S., Winter G., Brownlee G. G. 1981. Structure of the neuraminidase gene in human influenza virus A/PR/8/34. Nature 290:213–217 [DOI] [PubMed] [Google Scholar]
- 6. Fowler M., Thomas R. J., Atherton J., Roberts I. S., High N. J. 2006. Galectin-3 binds to Helicobacter pylori O-antigen: it is upregulated and rapidly secreted by gastric epithelial cells in response to H. pylori adhesion. Cell. Microbiol. 8:44–54 [DOI] [PubMed] [Google Scholar]
- 7. Garner O. B., et al. 2010. Endothelial galectin-1 binds to specific glycans on Nipah virus fusion protein and inhibits maturation, mobility, and function to block syncytium formation. PLoS Pathog. 6:e1000993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gauthier S., et al. 2008. Induction of galectin-1 expression by HTLV-1 Tax and its impact on HTLV-1 infectivity. Retrovirology 5:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Govorkova E. A., Fang H.-B., Tan M., Webster R. G. 2004. Neuraminidase inhibitor-rimantadine combinations exert additive and synergistic anti-influenza virus effects in MDCK Cells. Antimicrob. Agents Chemother. 48:4855–4863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grabar K. C., Freeman R. G., Hommer M. B., Natan M. J. 2002. Preparation and characterization of Au colloid monolayers. Anal. Chem. 67:735–743 [Google Scholar]
- 11. Hartshorn K. L., et al. 1997. Mechanisms of anti-influenza activity of surfactant proteins A and D: comparison with serum collectins. Am. J. Physiol. 273:L1156–1166 [DOI] [PubMed] [Google Scholar]
- 12. Hartshorn K. L., White M. R., Tecle T., Holmskov U., Crouch E. C. 2006. Innate defense against influenza A virus: activity of human neutrophil defensins and interactions of defensins with surfactant protein D. J. Immunol. 176:6962–6972 [DOI] [PubMed] [Google Scholar]
- 13. Hartshorn K. L., et al. 2000. Mechanism of binding of surfactant protein D to influenza A viruses: importance of binding to haemagglutinin to antiviral activity. Biochem. J. 351(Pt. 2):449–458 [PMC free article] [PubMed] [Google Scholar]
- 14. Hawgood S., et al. 2004. Pulmonary collectins modulate strain-specific influenza A virus infection and host responses. J. Virol. 78:8565–8572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hiti A. L., Nayak D. P. 1982. Complete nucleotide sequence of the neuraminidase gene of human influenza virus A/WSN/33. J. Virol. 41:730–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Horváth A., et al. 2001. Serum amyloid P component inhibits influenza A virus infections: in vitro and in vivo studies. Antivir. Res. 52:43–53 [DOI] [PubMed] [Google Scholar]
- 17. Hsieh S. H., et al. 2008. Galectin-1, a novel ligand of neuropilin-1, activates VEGFR-2 signaling and modulates the migration of vascular endothelial cells. Oncogene 27:3746–3753 [DOI] [PubMed] [Google Scholar]
- 18. Jia W., Li H., He Y. W. 2008. Pattern recognition molecule mindin promotes intranasal clearance of influenza viruses. J. Immunol. 180:6255–6261 [DOI] [PubMed] [Google Scholar]
- 19. Keil W., et al. 1985. Carbohydrates of influenza virus. Structural elucidation of the individual glycans of the FPV hemagglutinin by two-dimensional 1H NMR and methylation analysis. EMBO J. 4:2711–2720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Krishnamoorthy L., Bess J. W., Preston A. B., Nagashima K., Mahal L. K. 2009. HIV-1 and microvesicles from T cells share a common glycome, arguing for a common origin. Nat. Chem. Biol. 5:244–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee R. T., Lee Y. C. 2000. Affinity enhancement by multivalent lectin-carbohydrate interaction. Glycoconj. J. 7:543–551 [DOI] [PubMed] [Google Scholar]
- 22. LeVine A. M., Whitsett J. A., Hartshorn K. L., Crouch E. C., Korfhagen T. R. 2001. Surfactant protein D enhances clearance of influenza A virus from the lung in vivo. J. Immunol. 167:5868–5873 [DOI] [PubMed] [Google Scholar]
- 23. Levroney E. L., et al. 2005. Novel innate immune functions for galectin-1: galectin-1 inhibits cell fusion by Nipah virus envelope glycoproteins and augments dendritic cell secretion of proinflammatory cytokines. J. Immunol. 175:413–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Matrosovich M., Klenk H.-D. 2003. Natural and synthetic sialic acid-containing inhibitors of influenza virus receptor binding. Rev. Med. Virol. 13:85–97 [DOI] [PubMed] [Google Scholar]
- 25. Matsuoka Y., et al. 2009. Neuraminidase stalk length and additional glycosylation of the hemagglutinin influence the virulence of influenza H5N1 viruses for mice. J. Virol. 83:4704–4708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mercier S., et al. 2008. Galectin-1 promotes HIV-1 infectivity in macrophages through stabilization of viral adsorption. Virology 371:121–129 [DOI] [PubMed] [Google Scholar]
- 27. Mitnaul L. J., et al. 2000. Balanced hemagglutinin and neuraminidase activities are critical for efficient replication of influenza A virus. J. Virol. 74:6015–6020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nieminen J., St-Pierre C., Bhaumik P., Poirier F., Sato S. 2008. Role of galectin-3 in leukocyte recruitment in a murine model of lung infection by Streptococcus pneumoniae. J. Immunol. 180:2466–2473 [DOI] [PubMed] [Google Scholar]
- 29. Nunoi H., et al. 2005. Apoptosis under hypercytokinemia is a possible pathogenesis in influenza-associated encephalopathy. Pediatr. Int. 47:175–179 [DOI] [PubMed] [Google Scholar]
- 30. Ouellet M., et al. 2005. Galectin-1 acts as a soluble host factor that promotes HIV-1 infectivity through stabilization of virus attachment to host cells. J. Immunol. 174:4120–4126 [DOI] [PubMed] [Google Scholar]
- 31. Poirier F., Robertson E. J. 1993. Normal development of mice carrying a null mutation in the gene encoding the L14 S-type lectin. Development 119:1229–1236 [DOI] [PubMed] [Google Scholar]
- 32. Rabinovich G. A., Liu F. T., Hirashima M., Anderson A. 2007. An emerging role for galectins in tuning the immune response: lessons from experimental models of inflammatory disease, autoimmunity and cancer. Scand. J. Immunol. 66:143–158 [DOI] [PubMed] [Google Scholar]
- 33. Rabinovich G. A., Toscano M. A. 2009. Turning “sweet” on immunity: galectin-glycan interactions in immune tolerance and inflammation. Nat. Rev. Immunol. 9:338–352 [DOI] [PubMed] [Google Scholar]
- 34. Rabinovich G. A., Toscano M. A., Jackson S. S., Vasta G. R. 2007. Functions of cell surface galectin-glycoprotein lattices. Curr. Opin. Struct. Biol. 17:513–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Reading P. C., et al. 2008. Antiviral activity of the long chain pentraxin PTX3 against influenza viruses. J. Immunol. 180:3391–3398 [DOI] [PubMed] [Google Scholar]
- 36. Reading P. C., Morey L. S., Crouch E. C., Anders E. M. 1997. Collectin-mediated antiviral host defense of the lung: evidence from influenza virus infection of mice. J. Virol. 71:8204–8212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schulman J. L., Palese P. 1977. Virulence factors of influenza A viruses: WSN virus neuraminidase required for plaque production in MDBK cells. J. Virol. 24:170–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Skehel J. J., et al. 1984. A carbohydrate side chain on hemagglutinins of Hong Kong influenza viruses inhibits recognition by a monoclonal antibody. Proc. Natl. Acad. Sci. U. S. A. 81:1779–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Skehel J. J., Wiley D. C. 2000. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu. Rev. Biochem. 69:531–569 [DOI] [PubMed] [Google Scholar]
- 40. Stowell S. R., et al. 2008. Differential roles of galectin-1 and galectin-3 in regulating leukocyte viability and cytokine secretion. J. Immunol. 180:3091–3102 [DOI] [PubMed] [Google Scholar]
- 41. Sugiura A., Ueda M. 1980. Neurovirulence of influenza virus in mice. I. Neurovirulence of recombinants between virulent and avirulent virus strains. Virology 101:440–449 [DOI] [PubMed] [Google Scholar]
- 42. Thanh N. T., Rosenzweig Z. 2002. Development of an aggregation-based immunoassay for anti-protein A using gold nanoparticles. Anal. Chem. 74:1624–1628 [DOI] [PubMed] [Google Scholar]
- 43. Tsai C. Y., et al. 2007. Amelioration of collagen-induced arthritis in rats by nanogold. Arthritis Rheum. 56:544–554 [DOI] [PubMed] [Google Scholar]
- 44. van Kooyk Y., Rabinovich G. A. 2008. Protein-glycan interactions in the control of innate and adaptive immune responses. Nat. Immunol. 9:593–601 [DOI] [PubMed] [Google Scholar]
- 45. Vasta G. R. 2009. Roles of galectins in infection. Nat. Rev. Microbiol. 7:424–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vigerust D. J., et al. 2007. N-linked glycosylation attenuates H3N2 influenza viruses. J. Virol. 81:8593–8600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vray B., et al. 2004. Up-regulation of galectin-3 and its ligands by Trypanosoma cruzi infection with modulation of adhesion and migration of murine dendritic cells. Glycobiology 14:647–657 [DOI] [PubMed] [Google Scholar]
- 48. Wagner R., Wolff T., Herwig A., Pleschka S., Klenk H.-D. 2000. Interdependence of hemagglutinin glycosylation and neuraminidase as regulators of influenza virus growth: a study by reverse genetics. J. Virol. 74:6316–6323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang C.-W., Wang C.-H. 2003. Experimental selection of virus derivatives with variations in virulence from a single low-pathogenicity H6N1 avian influenza virus field isolate. Avian Dis. 47:1416–1422 [DOI] [PubMed] [Google Scholar]
- 50. Welliver T. P., et al. 2007. Severe human lower respiratory tract illness caused by respiratory syncytial virus and influenza virus is characterized by the absence of pulmonary cytotoxic lymphocyte responses. J. Infect. Dis. 195:1126–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. White M., et al. 2008. Multimerization of surfactant protein D, but not its collagen domain, is required for antiviral and opsonic activities related to influenza virus. J. Immunol. 181:7936–7943 [DOI] [PubMed] [Google Scholar]
- 52. White M. R., Crouch E., Chang D., Hartshorn K. L. 2001. Increased antiviral and opsonic activity of a highly multimerized collectin chimera. Biochem. Biophys. Res. Commun. 286:206–213 [DOI] [PubMed] [Google Scholar]
- 53. White M. R., Doss M., Boland P., Tecle T., Hartshorn K. L. 2008. Innate immunity to influenza virus: implications for future therapy. Expert Rev. Clin. Immunol. 4:497–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wilson I. A., Skehel J. J., Wiley D. C. 1981. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 Å resolution. Nature 289:366–373 [DOI] [PubMed] [Google Scholar]
- 55. Yingsakmongkon S., et al. 2008. In vitro inhibition of human influenza A virus infection by fruit-juice concentrate of Japanese plum (Prunus mume SIEB. et ZUCC). Biol. Pharm. Bull. 31:511–515 [DOI] [PubMed] [Google Scholar]
- 56. Zhou Q., Cummings R. D. 1993. L-14 lectin recognition of laminin and its promotion of in vitro cell adhesion. Arch. Biochem. Biophys. 300:6–17 [DOI] [PubMed] [Google Scholar]