Abstract
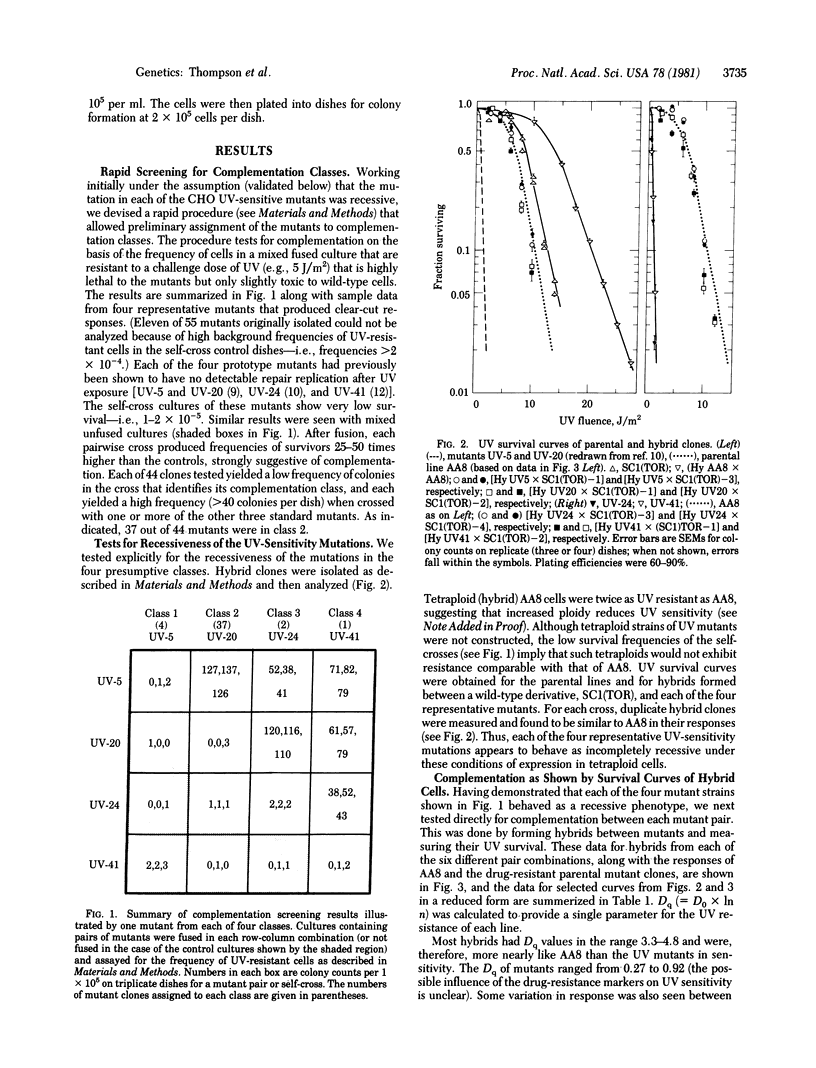
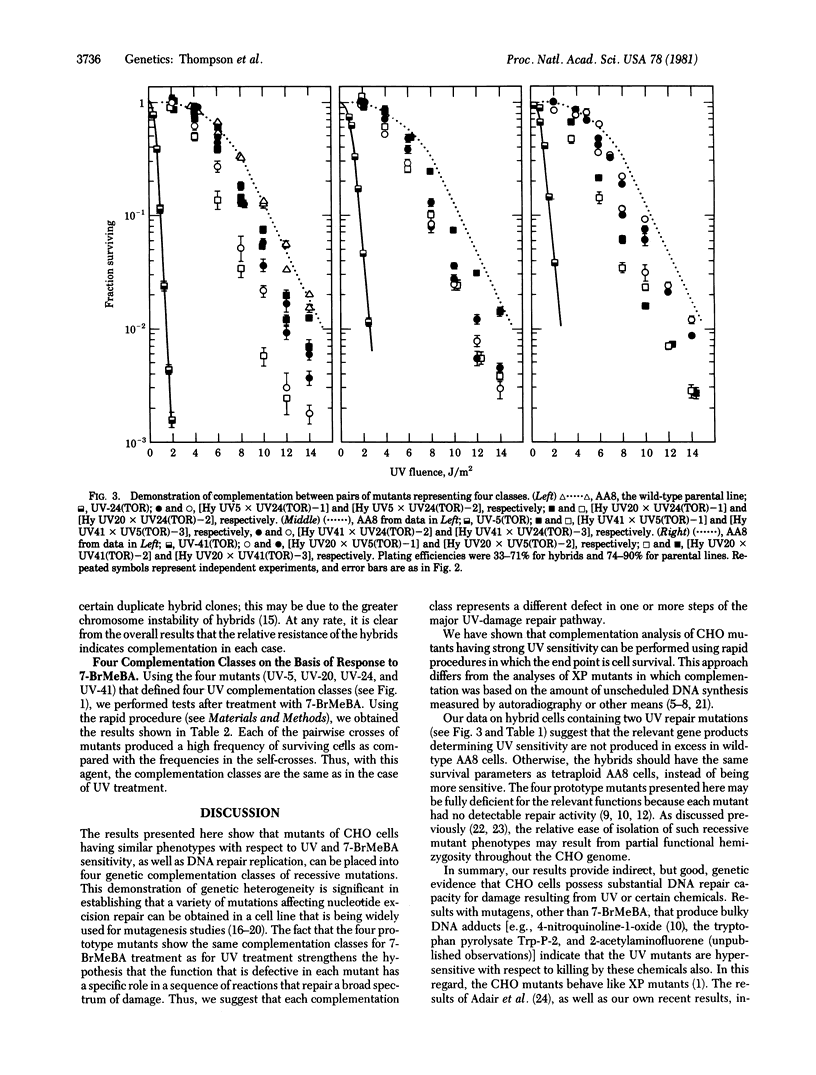
Mutant lines of Chinese hamster ovary cells that show hypersensitivity to killing and mutagenesis by UV light were analyzed by genetic complementation analysis to determine whether defects in different gene loci might underlie a common cellular phenotype,. To facilitate rapid screening of mutant clones, a procedure was devised that allowed presumptive complementation to be assessed on the basis of the frequency of UV-resistant cells after fusion by polyethylene glycol. Four classes were identified among 44 clones tested. By using drug-resistance markers for selection of hybrid cells in crosses between UV mutant and wild type, a mutant from each of the four classes was shown to behave as phenotypically recessive. Hybrids were also isolated from crosses between each of the pair combinations of the four mutants. All such hybrids were relatively resistant to UV killing, providing confirmation of the complementation classes. When mutants representing the four UV-complementation classes were tested with the polyaromatic hydrocarbon 7-bromomethylbenz(a)anthracene, complementation was again seen for all pair combinations. These results suggest that each class of mutants represents a biochemical defect that plays a common role in the repair of both UV-induced and chemically induced lesions in the DNA.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adair G. M., Carver J. H., Wandres D. L. Mutagenicity testing in mammalian cells. I. Derivation of a Chinese hamster ovary cell line heterozygous for the adenine phosphoribosyltransferase and thymidine kinase loci. Mutat Res. 1980 Sep;72(2):187–205. doi: 10.1016/0027-5107(80)90035-4. [DOI] [PubMed] [Google Scholar]
- Busch D. B., Cleaver J. E., Glaser D. A. Large-scale isolation of UV-sensitive clones of CHO cells. Somatic Cell Genet. 1980 May;6(3):407–418. doi: 10.1007/BF01542792. [DOI] [PubMed] [Google Scholar]
- Carver J. H., Adair G. M., Wandres D. L. Mutagenicity testing in mammalian cells. II. Validation of multiple drug-resistance markers having practical application for screening potential mutagens. Mutat Res. 1980 Sep;72(2):207–230. doi: 10.1016/0027-5107(80)90036-6. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E., Bootsma D. Xeroderma pigmentosum: biochemical and genetic characteristics. Annu Rev Genet. 1975;9:19–38. doi: 10.1146/annurev.ge.09.120175.000315. [DOI] [PubMed] [Google Scholar]
- Keijzer W., Jaspers N. G., Abrahams P. J., Taylor A. M., Arlett C. F., Zelle B., Takebe H., Kinmont P. D., Bootsma D. A seventh complementation group in excision-deficient xeroderma pigmentosum. Mutat Res. 1979 Aug;62(1):183–190. doi: 10.1016/0027-5107(79)90231-8. [DOI] [PubMed] [Google Scholar]
- Kraemer K. H., Coon H. G., Petinga R. A., Barrett S. F., Rahe A. E., Robbins J. H. National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20014, USA. Proc Natl Acad Sci U S A. 1975 Jan;72(1):59–63. doi: 10.1073/pnas.72.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer K. H., De Weerd-Kastelein E. A., Robbins J. H., Keijzer W., Barrett S. F., Petinga R. A., Bootsma D. Five complementation groups in xeroderma pigmentosum. Mutat Res. 1975 Dec;33(2-3):327–340. doi: 10.1016/0027-5107(75)90208-0. [DOI] [PubMed] [Google Scholar]
- Lehmann A. R., Stevens S. A rapid procedure for measurement of DNA repair in human fibroblasts and for complementation analysis of xeroderma pigmentosum cells. Mutat Res. 1980 Jan;69(1):177–190. doi: 10.1016/0027-5107(80)90187-6. [DOI] [PubMed] [Google Scholar]
- Mortelmans K., Friedberg E. C., Slor H., Thomas G., Cleaver J. E. Defective thymine dimer excision by cell-free extracts of xeroderma pigmentosum cells. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2757–2761. doi: 10.1073/pnas.73.8.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill J. P., Hsie A. W. Chemical mutagenesis of mammalian cells can be quantified. Nature. 1977 Oct 27;269(5631):815–817. doi: 10.1038/269815a0. [DOI] [PubMed] [Google Scholar]
- Siminovitch L., Thompson L. H. The nature of conditionally lethal temperature-sensitive mutations in somatic cells. J Cell Physiol. 1978 Jun;95(3):361–364. doi: 10.1002/jcp.1040950314. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Fong S., Brookman K. Validation of conditions for efficient detection of HPRT and APRT mutations in suspension-cultured Chinese hamster ovary cells. Mutat Res. 1980 Feb;74(1):21–36. doi: 10.1016/0165-1161(80)90188-0. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Rubin J. S., Cleaver J. E., Whitmore G. F., Brookman K. A screening method for isolating DNA repair-deficient mutants of CHO cells. Somatic Cell Genet. 1980 May;6(3):391–405. doi: 10.1007/BF01542791. [DOI] [PubMed] [Google Scholar]



