Abstract
Background
Although preclinical and epidemiological data suggest that statins may have antineoplastic properties, the impact of statin use on patient survival after a curative resection of stage III colon cancer is unknown.
Methods
We conducted a prospective observational study of 842 patients with stage III colon cancer enrolled in a randomized adjuvant chemotherapy trial from April 1999 to May 2001 to investigate the relationship between statin use and survival. Disease-free survival (DFS), recurrence-free survival (RFS), and overall survival (OS) were investigated by Kaplan–Meier curves and log-rank tests in the overall study population and in a subset of patients stratified by KRAS mutation status (n = 394), and Cox proportional hazards regression was used to assess the simultaneous impact of confounding variables. All statistical tests were two-sided.
Results
Among 842 patients, 134 (15.9%) reported statin use after completing adjuvant chemotherapy. DFS among statin users and nonusers was similar (hazard ratio [HR] of cancer recurrence or death = 1.04, 95% confidence interval [CI] = 0.73 to 1.49). RFS and OS were also similar between statin users and nonusers (adjusted HR of cancer recurrence = 1.14, 95% CI = 0.77 to 1.69; adjusted HR of death = 1.15, 95% CI = 0.77 to 1.71). Survival outcomes were similar regardless of increasing duration of statin use before cancer diagnosis (Ptrend = .63, .63, and .59 for DFS, RFS, and OS, respectively). The impact of statin use did not differ by tumor KRAS mutation status, with similar DFS, RFS, and OS for statin use among mutant and wild-type subgroups (Pinteraction = .84, .67, and .98 for DFS, RFS, and OS, respectively).
Conclusion
Statin use during and after adjuvant chemotherapy was not associated with improved DFS, RFS, or OS in patients with stage III colon cancer, regardless of KRAS mutation status.
CONTEXT AND CAVEATS
Prior knowledge
Although several studies have investigated the potential chemopreventative activity of statins, and conflicting findings on the relationship between statin use and the risk of colon cancer have been reported, the relationship between statin use and outcome among colon cancer patients has not been studied.
Study design
The relationship between statin use and disease-free, recurrence-free, and overall survival among 842 colon cancer patients enrolled in a randomized clinical trial of adjuvant chemotherapy was analyzed. A subanalysis by KRAS mutation status was also done because studies have suggested that statin use may inhibit RAS signaling.
Contribution
No differences between disease-free, recurrence-free, or overall survival between statin users and nonusers were observed in the general study population or in a subanalysis by KRAS mutation status.
Implications
Statin use during and after adjuvant chemotherapy for the treatment of colon cancer may not improve survival outcomes. A randomized placebo-controlled prevention trial to investigate the effect of statin use on colon cancer risk and recurrence is currently underway.
Limitations
The study of statin use depended on patient self-reports in response to a questionnaire. Also, the impact of different statins on patient outcome could not be determined.
From the Editors
Statins (3-hydroxy-3-methylglutaryl coenzyme A [HMG-CoA] reductase inhibitors) are widely prescribed for the treatment of hypercholesterolemia and have been shown to reduce cardiovascular events and mortality in several randomized clinical trials (1–3). Statins inhibit the conversion of HMG-CoA to the cholesterol precursor mevalonate, which is the rate-limiting step in cholesterol biosynthesis. Mevalonate is also the precursor compound for other isoprenoids, including farnesyl pyrophosphate and geranylgeranyl pyrophosphate, which are critical for posttranslational modification of proteins involved in cell growth, including both the RAS and RHO oncogenes (4,5). Consequently, statins are hypothesized to have antineoplastic benefits, possibly through inhibition of RAS signaling.
Several epidemiological studies have evaluated the association between statin use and the risk of colorectal cancer and yielded inconsistent results. A large retrospective case–control study in Israel demonstrated a 47% reduction in the risk of colorectal cancer after 5 years of statin use (6). Likewise, two large meta-analyses reported 12% and 13% reductions in colorectal cancer risk (7,8), respectively, after as little as 6 months of use. Nonetheless, several retrospective and prospective studies have offered conflicting results, and two other meta-analyses failed to confirm that statin use is associated with a reduction in the risk of cancer overall (9) or colorectal cancer specifically (10).
Beyond studies assessing the chemopreventative role of statins, the relationship between statin use and colon cancer patient outcome is unknown. Because of continued interest in the role of statin use in cancer risk and outcome, the National Surgical Adjuvant Breast and Bowel Project initiated the Statin Polyp Prevention Trial (protocol P-5), which is currently randomly assigning patients with resected stage I or II colon cancer to rosuvastatin (10 mg daily) or placebo treatment arms for a period of 5 years. The primary endpoint of this study is adenomatous polyp formation, metachronous colorectal carcinoma, or colon cancer recurrence. Results from this trial are not anticipated for several years. Therefore, to address the gap in knowledge, we prospectively examined the relationship between statin use and cancer recurrence or death from any cause, cancer recurrence only, and overall mortality in stage III colon cancer patients enrolled in a completed National Cancer Institute–sponsored clinical trial of adjuvant chemotherapy. In addition, given the hypothesis that statin use may inhibit RAS signaling, we also investigated the association between statin use and patient outcome according to tumoral KRAS mutation status.
Methods
Study Population
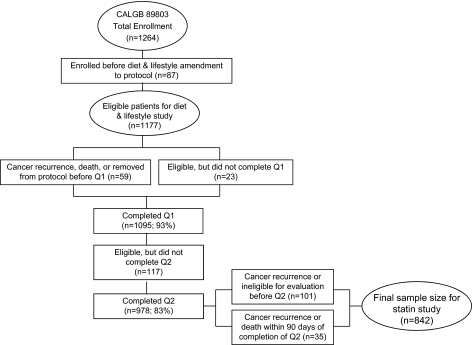
Patients in this prospective cohort study were participants in the Cancer and Leukemia Group B (CALGB) trial for stage III colon cancer (89 803) that compared adjuvant bolus 5-fluorouracil and leucovorin with the combination of irinotecan, bolus 5-fluorouracil, and leucovorin (11). Patients were enrolled from April 1999 to May 2001. A self-administered questionnaire that captured diet and lifestyle habits was completed by patients midway through their therapy (4 months after surgery, Questionnaire 1) and again 6 months after the completion of treatment (14 months after surgery; Questionnaire 2). The protocol amendment to survey diet and lifestyle was activated after the first 87 patients were enrolled; therefore, 1177 patients were eligible for the companion study (Figure 1).
Figure 1.
Flow chart of patient selection from the Cancer and Leukemia Group B (CALGB) trial 89803 for inclusion in the study cohort. Q1 = Questionnaire 1, Q2 = Questionnaire 2.
Patients were eligible for the treatment trial if they had undergone a complete surgical resection of their primary tumor within 56 days of study entry and had regional lymph node metastases but no distant metastases. Patients were required to have a baseline Eastern Cooperative Oncology Group performance status of 0–2 and adequate bone marrow, renal, and hepatic function. Median household income was estimated using concurrent census data determined by the patient zip code. Race/ethnicity was self-reported and recorded in the hospital database at each participating center. Classifications included white, Hispanic, black, Asian, Native Hawaiian, Native American, Indian, Filipino, other, and unknown. These and other demographic information were reported by each participating center to the CALGB Statistical Center. All patients gave informed consent, and the study protocol was approved by the review board of each institution.
Statin Assessment
Statin use was assessed by Questionnaire 2 after the completion of adjuvant chemotherapy. Only patients who did not experience disease recurrence or death before the second questionnaire were included in the analyses. Participants were asked whether they take any of several medications, including statin cholesterol–lowering drugs (eg, lovastatin [Mevacor], pravastatin [Pravachol], simvastatin [Zocor], and atorvastatin [Lipitor]). Those who reported current statin use were asked to provide the number of years of use (0–2, 3–5, or ≥6 years). We excluded patients whose cancer recurred or who died within 90 days of their statin assessment to avoid potential bias related to an underlying illness.
KRAS Mutation Assessment
Methods for determining the presence of a KRAS mutation have been previously described (12). Briefly, polymerase chain reactions and pyrosequencing spanning KRAS codons 12 and 13 were performed and validated against Sanger sequencing method (13). Polymerase chain reaction amplification primers for KRAS pyrosequencing were: KRAS-F, forward, 5’-nnn ggc ctg ctg aaa atg act gaa-3’ and KRAS-R, reverse biotinylated primer, 5’-tta gct gta tcg tca agg cac tct-3’. Sequencing primers were 5’-tgt ggt agt tgg agc tg-3’ and 5’-tgt ggt agt tgg agc t-3’. Similar baseline characteristics were observed for patients with available KRAS data and those without available tumor tissue. Moreover, similar tumor recurrence and mortality rates were observed among these two populations (13).
Study Endpoints
Study endpoints were calculated from the time of completion of statin assessment on Questionnaire 2 rather than from the start of trial treatment to avoid any biases from altered medication use and/or exposure during the period of active chemotherapy. The primary endpoint was disease-free survival (DFS), defined as the time from the completion of Questionnaire 2 to tumor recurrence, occurrence of a new primary colon cancer, or death from any cause. Recurrence-free survival (RFS) was defined as the time from the completion of Questionnaire 2 to tumor recurrence, death with evidence of recurrence, or occurrence of a new primary colon tumor; patients who died without known recurrence were censored at the last documented evaluation. Finally, overall survival (OS) was defined as the time from completion of Questionnaire 2 to death from any cause.
Statistical Analyses
Results from the CALGB trial for stage III colon cancer comparing adjuvant bolus 5-fluorouracil and leucovorin with the combination of bolus 5-fluorouracil, leucovorin, and irinotecan have been previously described (11), and results from the two chemotherapy treatment arms were similar; thus, data for patients were combined and analyzed according to categories of statin use after adjuvant chemotherapy for this study. Baseline characteristics were compared between statin users and nonusers using Fisher exact test for 2 × 2 categorical comparisons, a χ2 test for other categorical comparisons, and the Kruskal–Wallis test for continuous variables. The median follow-up time was determined by calculating the median survival time of patients still alive on March 31, 2009.
Multivariable models were adjusted for age (in years, as a continuous variable), sex (male or female), family history of colorectal cancer (yes or no), and Eastern Cooperative Oncology Group performance status at the initiation of chemotherapy (0 or 1–2). A performance status of 0 indicated that a patient was fully active, a status of 1 indicated that the patient was restricted in physically strenuous activity but ambulatory and able to carry out light work, and a status of 2 indicated that the patient was ambulatory and capable of all self-care but unable to carry out any work activities but up and about for more than 50% of the waking hours. We adjusted for the depth of invasion through the bowel wall by assigning T1 or T2 when the level of invasion was through the bowel wall but not beyond the muscle layer, whereas T3 or T4 was assigned when the level of invasion through the bowel wall was beyond the muscle layer. The number of positive lymph nodes (1–3 or ≥4), perineural invasion (yes or no), and extravascular invasion (yes or no) were other pathological variables that were included in the model. Finally, we also adjusted for the level of postoperative carcinoembryonic antigen present in serum before the initiation of chemotherapy (<5 or ≥5 ng/mL), the treatment arm (bolus 5-fluorouracil plus leucovorin or the combination of irinotecan, bolus 5-fluorouracil, and leucovorin), body mass index (BMI) at the initiation of chemotherapy (as a continuous variable), physical activity (metabolic equivalent task hours per week, as a continuous variable), Western pattern diet (as a continuous variable), and consistent aspirin use (any aspirin use on both Questionnaires 1 and 2). Continuous variables with missing values were imputed with the median value (n = 2 for BMI and for physical activity), and categorical covariables with missing values were coded with indicator variables. DFS, RFS, and OS were examined using Kaplan–Meier curves (14) and the log-rank test (15) in the overall population and in patients stratified by KRAS mutation status. Cox proportional hazards regression was used to determine the simultaneous impact of potential confounders (16). The proportionality of hazards assumption for the effect of statin use was satisfied by examining it as a time-dependent covariable in the model (17). The time-dependent statin covariable was non-statistically significant, indicating that the assumption of proportional hazards was appropriate. We tested for linear trend by entering the median value of each category of duration of statin use as a continuous variable in the model (18). Statistical interactions between statin use and potentially modifying covariables were assessed using Wald test of cross-product terms. Adverse events were graded according to the National Cancer Institute Common Toxicity Criteria version 2.0, and logistic regression was performed to examine the association between statins and grade 3 and higher toxic effects.
All statistical tests were two-sided and P values less than .05 were considered statistically significant. The sample size for the cohort was determined by the chemotherapy treatment trial, which had 82% power to detect a hazard ratio (HR) for death from any cause of 0.77 on the basis of an estimated 356 deaths among 1260 patients. In a post hoc calculation of power on the basis of the known sample size and the number of cancer recurrence or death events for this analysis, we had 80% power to detect a hazard ratio of 0.55 for cancer recurrence or death.
Patient registration and clinical data collection were conducted by the CALGB Statistical Center, and analyses were performed in conjunction with CALGB statisticians on the basis of the database freeze date of March 31, 2009. Using Clark C (19), the completeness of follow-up for this study was 84.2%; applying Wu modification (20) to adjust for unreported deaths, a more realistic assessment was 85.9%.
Results
Baseline Characteristics According to Statin Use
The questionnaire completion rates between the two treatment arms of the trial were similar. Baseline characteristics for the patients for whom data on statin use were captured are presented in Table 1. Among 842 patients who completed the second questionnaire 6 months after completion of adjuvant chemotherapy, 134 (15.9%) reported statin use. The median time from study entry (which had to be within 8 weeks of surgery) to statin assessment was 13.5 months. Patients who reported statin use were older, had slightly higher BMI (P = .07), and were more likely to report consistent aspirin use. Other potentially prognostic patient and tumor characteristics were similar among statin users compared with nonusers.
Table 1.
Baseline characteristics by statin use reported after adjuvant chemotherapy in patients from the Cancer and Leukemia Group B trial 89803*
| Characteristic | Statin use after completion of adjuvant chemotherapy |
||
| No (n = 708) | Yes (n = 134) | P† | |
| Median age (range), y | 59.0 (21–85) | 64.0 (47–80) | <.001 |
| Sex, No. (%) | 708 (100) | 134 (100) | .11 |
| Male | 390 (55.1) | 84 (62.7) | |
| Female | 318 (44.9) | 50 (37.3) | |
| Race/ethnicity, No. (%) | 705 (99.6) | 134 (100) | .42 |
| White | 628 (89.1) | 121 (90.3) | |
| Black | 50 (7.1) | 6 (4.5) | |
| Other | 27 (3.8) | 7 (5.2) | |
| Median household income (range), US dollars‡ | 40 742 (17 963–122 234) | 40 708 (21 203–122 956) | .69 |
| Family history of colorectal cancer, No. (%) | 708 (100) | 134 (100) | .63 |
| Yes | 137 (19.4) | 23 (17.2) | |
| No | 571 (80.6) | 111 (82.8) | |
| Baseline ECOG performance status, No. (%) | 692 (97.7) | 133 (99.3) | .91 |
| 0 | 524 (75.8) | 100 (75.2) | |
| 1–2 | 168 (24.3) | 33 (24.8) | |
| Invasion through bowel wall (T stage), No. (%) | 691 (97.6) | 133 (99.3) | .08 |
| T1 and T2 | 97 (14.0) | 27 (20.3) | |
| T3 and T4 | 594 (86.0) | 106 (79.7) | |
| Positive lymph nodes (N stage), No. of patients (%) | 693 (97.9) | 133 (99.3) | .48 |
| 1–3 (N1) | 465 (67.1) | 94 (70.7) | |
| ≥4 (N2) | 228 (32.9) | 39 (29.3) | |
| Grade of differentiation, No. (%) | 693 (97.9) | 133 (99.3) | .17 |
| Well differentiated | 45 (6.5) | 5 (3.8) | |
| Moderately differentiated | 488 (70.4) | 104 (78.2) | |
| Poorly differentiated or undifferentiated | 160 (23.1) | 24 (18.0) | |
| Lymphovascular invasion, No. of patients (%) | 686 (96.9) | 128 (95.5) | .67 |
| Yes | 190 (27.7) | 38 (29.7) | |
| No | 496 (72.3) | 90 (70.3) | |
| Perineural invasion, No. of patients (%) | 676 (95.5) | 126 (94) | 1.00 |
| Yes | 40 (5.9) | 7 (5.6) | |
| No | 636 (94.1) | 119 (94.4) | |
| Extravascular invasion, No. of patients (%) | 677 (95.6) | 125 (93.3) | .73 |
| Yes | 58 (8.6) | 9 (7.2) | |
| No | 619 (91.4) | 116 (92.8) | |
| KRAS mutation, No. of patients (%) | 326 (46.0) | 68 (50.7) | .68 |
| Yes | 117 (35.9) | 22 (32.4) | |
| No | 209 (64.1) | 46 (67.6) | |
| Clinical bowel perforation, No. of patients (%) | 686 (96.9) | 130 (97.0) | .32 |
| Yes | 24 (3.5) | 7 (5.4) | |
| No | 662 (96.5) | 123 (94.6) | |
| Clinical bowel obstruction, No. of patients (%) | 692 (97.7) | 132 (98.5) | .14 |
| Yes | 159 (23.0) | 22 (16.7) | |
| No | 533 (77.0) | 110 (83.3) | |
| Postoperative CEA, ng/mL, No. of patients (%) | 660 (93.2) | 124 (92.5) | .82 |
| <5 | 628 (95.0) | 119 (96.0) | |
| ≥5 | 32 (5.0) | 5 (4.0) | |
| Adjuvant chemotherapy arm, No. of patients (%) | 708 (100) | 134 (100) | .09 |
| 5-FU/LV | 365 (51.6) | 58 (43.3) | |
| IFL | 343 (48.4) | 76 (56.7) | |
| Median body mass index (range), kg/m2 | 28.3 (16.8−54.5) | 30.0 (17.6−45.1) | .07 |
| Median physical activity (range), MET h/wk | 7.6 (0−125.4) | 7.7 (0−168.7) | .67 |
| Western dietary pattern, median score (range) | −0.20 (−1.73 to 9.35) | −0.07 (−1.60 to 4.31) | .22 |
| Consistent aspirin use, No. of patients (%) | 698 (98.6) | 125 (93.3) | .001 |
| Yes | 53 (7.6) | 22 (17.6) | |
| No | 645 (92.4) | 103 (82.4) | |
| Regular COX-2 (PTGS2) inhibitor use, No. of patients (%) | 702 (99.2) | 131 (97.8) | .28 |
| Yes | 33 (4.7) | 9 (6.9) | |
| No | 669 (95.3) | 122 (93.1) | |
CEA = carcinoembryonic antigen; COX = cyclooxygenase; ECOG = Eastern Cooperative Oncology Group; 5-FU/LV = bolus 5-fluorouracil and leucovorin; IFL = bolus 5-fluorouracil, leucovorin, and irinotecan; IU = international units; MET = metabolic equivalent task; PTGS2 = prostaglandin–endoperoxide synthase.
P values were calculated by Fisher exact test for 2 × 2 categorical comparisons including sex (male or female), family history of colorectal cancer (yes or no), Eastern Cooperative Oncology Group performance status at the initiation of chemotherapy (0 or 1–2), depth of invasion into the bowel wall (T1 and T2 or T3 and T4), the number of positive lymph nodes (1–3 or ≥4), lymphovascular invasion (yes or no), perineural invasion (yes or no), extravascular invasion (yes or no), KRAS mutation (yes or no), clinical bowl perforation (yes or no), clinical bowel obstruction (yes or no), CEA serum concentration (<5 or ≥5 ng/mL), adjuvant chemotherapy arm, consistent aspirin use (any aspirin use on both Questionnaires 1 and 2, yes or no), and regular COX-2 (PTGS2) use (≥3 tablets of Celebrex or Vioxx per week, yes or no). A χ2 test was used to calculate P for other categorical comparisons including race/ethnicity (white, black, or a combined category that included Hispanic, Asian, Native Hawaiian, Native American, Indian, Filipino, other, and unknown race/ethnicity) and grade of differentiation (well differentiated, moderately differentiated, or poorly differentiated and undifferentiated). The Kruskal–Wallis test was used to calculate P values for continuous variables including age (in years), median household income, body mass index at the initiation of chemotherapy (kg/m2), physical activity (metabolic equivalent task hours per week), and Western pattern diet. All statistical tests were two-sided.
Analysis was on the basis of 1999 US census data as determined by patient zip code.
Impact of Statin Use on Cancer Recurrence and Death in the Overall Population
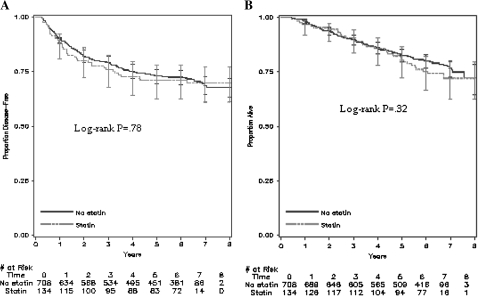
After a median follow-up of 6.5 years (10th and 90th percentiles: 4.4 and 7.3 years, respectively), 198 of the 842 eligible patients recurred and 177 died. Compared with nonusers, statin users had similar DFS (log-rank P = .78, Figure 2, A), RFS (P = .73, data not shown), and OS (P = .32, Figure 2, B). Similar outcomes were again observed after adjusting for other predictors of cancer recurrence when statin users and nonusers were compared (Table 2). Compared with patients who did not report statin use following adjuvant chemotherapy, statin users had similar DFS (multivariable HR of cancer recurrence or death = 1.04, 95% confidence interval [CI] = 0.73 to 1.49). Also, statin use was not associated with a statistically significant improvement in RFS (adjusted HR of cancer recurrence = 1.14, 95% CI = 0.77 to 1.69) or OS (adjusted HR of death = 1.15, 95% CI = 0.77 to 1.71). Our results remained unchanged when we additionally adjusted our model for median household income (data not shown) and KRAS mutation status (Table 2).
Figure 2.
Survival outcomes of statin users and nonusers from the Cancer and Leukemia Group B (CALGB) trial 89803. Kaplan–Meier curves of A) disease-free survival and B) overall survival of patients (n = 842) after a median follow-up of 6.5 years. Error bars represent 95% confidence intervals. Statistical significance was measured by the log-rank test. All P values were two-sided.
Table 2.
Relationship between statin use and colon cancer recurrence and mortality in patients from the Cancer and Leukemia Group B trial 89803 (n = 842)
| Outcome | Statin use reported after adjuvant chemotherapy |
|||||
| All |
Wild-type KRAS |
KRAS mutation |
||||
| No | Yes | No | Yes | No | Yes | |
| Cancer recurrence or death from any cause | ||||||
| No. of patients at risk | 708 | 134 | 209 | 46 | 117 | 22 |
| No. of events | 201 | 38 | 57 | 9 | 35 | 7 |
| Unadjusted HR (95% CI) | 1.0 (referent) | 1.05 (0.74 to 1.49) | 1.0 (referent) | 0.73 (0.36 to 1.47) | 1.0 (referent) | 1.09 (0.48 to 2.44) |
| Adjusted HR (95% CI)* | 1.0 (referent) | 1.04 (0.73 to 1.49)† | 1.0 (referent) | 0.83 (0.41 to 1.71)‡ | 1.0 (referent) | 1.21 (0.47 to 3.13)‡ |
| Additionally adjusted for KRAS mutation status | 1.0 (referent) | 1.05 (0.73 to 1.51) | Pinteraction = .84§ | |||
| Cancer recurrence | ||||||
| No. of patients at risk | 708 | 134 | 209 | 46 | 117 | 22 |
| No. of events | 166 | 32 | 46 | 9 | 32 | 5 |
| Unadjusted HR (95% CI) | 1.0 (referent) | 1.07 (0.73 to 1.56) | 1.0 (referent) | 0.91 (0.45 to 1.86) | 1.0 (referent) | 0.86 (0.33 to 2.20) |
| Adjusted HR (95% CI)* | 1.0 (referent) | 1.14 (0.77 to 1.69)† | 1.0 (referent) | 1.07 (0.51 to 2.22)‡ | 1.0 (referent) | 0.91 (0.30 to 2.79)‡ |
| Additionally adjusted for KRAS mutation status | 1.0 (referent) | 1.15 (0.78 to 1.70) | Pinteraction = .67§ | |||
| Overall mortality | ||||||
| No. of patients at risk | 708 | 134 | 209 | 46 | 117 | 22 |
| No. of events | 145 | 32 | 42 | 8 | 23 | 5 |
| Unadjusted HR (95% CI) | 1.0 (referent) | 1.22 (0.83 to 1.78) | 1.0 (referent) | 0.87 (0.41 to 1.85) | 1.0 (referent) | 1.26 (0.48 to 3.33) |
| Adjusted HR (95% CI)* | 1.0 (referent) | 1.15 (0.77 to 1.71)† | 1.0 (referent) | 0.88 (0.39 to 1.97)‡ | 1.0 (referent) | 1.18 (0.38 to 3.69)‡ |
| Additionally adjusted for KRAS mutation status | 1.0 (referent) | 1.16 (0.77 to 1.72) | Pinteraction = .98§ | |||
Multivariable HRs and 95% CIs were calculated by Cox proportional hazards models. CI = confidence interval; HR = hazard ratios.
Adjusted for age (in years as a continuous variable), sex (male or female), family history of colorectal cancer (yes or no), baseline performance status (0 or 1–2), depth of invasion through bowel wall (T1, T2, T3, or T4), number of positive lymph nodes (1–3 or ≥4), perineural invasion (yes or no), extravascular invasion (yes or no), postoperative carcinoembryonic antigen (<5 or ≥5), treatment arm (bolus 5-fluorouracil and leucovorin or bolus 5-fluorouracil, leucovorin, and irinotecan), body mass index (in kg/m2 as a continuous variable), physical activity (in metabolic equivalent task hours per week as a continuous variable), Western pattern diet (as a continuous variable), and consistent aspirin use (any aspirin use on both questionnaires).
Adjusted for age (in years as a continuous variable), sex (male or female), family history of colorectal cancer (yes or no), baseline performance status (0 or 1–2), depth of invasion through bowel wall (T1–T2 or T3–T4), number of positive lymph nodes (1–3 or ≥4), postoperative carcinoembryonic antigen serum levels (<5 or ≥5 ng/mL), treatment arm (bolus 5-fluorouracil and leucovorin or the combination of irinotecan, bolus 5-fluorouracil, and leucovorin), body mass index (in kg/m2 as a continuous variable), physical activity (in metabolic equivalent task hours per as a continuous variable), Western pattern diet (as a continuous variable), and consistent aspirin use (any aspirin use on both questionnaires).
Wald test of cross-product terms was used to calculate Pinteraction and was two-sided.
To address the possibility that excluding recurrences and deaths within 90 days of statin assessment masked a potential benefit of statin use, we repeated the analysis using all patients who had completed Questionnaire 2, and again DFS, RFS, and OS were similar. Furthermore, after extending the exclusion period from 90 to 180 days, DFS, RFS, and OS were again similar for statin users and nonusers (data not shown).
We also investigated the effect of the duration of statin use on patient outcome (Table 3). Recent statin use as reflected by reported use for 2 years or less did not lead to statistically significantly improved DFS (adjusted HR of cancer recurrence or death = 1.12, 95% CI = 0.68 to 1.86), RFS (adjusted HR of cancer recurrence = 1.27, 95% CI = 0.74 to 2.18), or OS (adjusted HR of death = 1.25, 95% CI = 0.71 to 2.19). Moreover, increasing duration of use was not associated with patient survival (Ptrend = .63 for DFS, .63 for RFS, and .59 for OS).
Table 3.
The association between statin use and colon cancer recurrence and mortality by the number of years of statin use in patients from the Cancer and Leukemia Group B trial 89803 (n = 839)*
| Outcome | Statin use, y | Ptrend† | |||
| 0 | ≤2 | 3–5 | ≥6 | ||
| Disease-free survival | |||||
| No. of patients at risk | 708 | 54 | 42 | 35 | — |
| No. of events | 201 | 17 | 10 | 11 | — |
| Unadjusted HR (95% CI) | 1.0 (referent) | 1.19 (0.72 to 1.95) | 0.84 (0.45 to 1.59) | 1.20 (0.65 to 2.19) | .80 |
| Adjusted HR (95% CI) | 1.0 (referent) | 1.12 (0.68 to 1.86) | 0.83 (0.43 to 1.58) | 1.25 (0.67 to 2.32) | .63 |
| Recurrence-free survival | |||||
| No. of patients at risk | 708 | 54 | 42 | 35 | — |
| No. of events | 166 | 15 | 8 | 9 | — |
| Unadjusted HR (95% CI) | 1.0 (referent) | 1.26 (0.74 to 2.14) | 0.82 (0.41 to 1.67) | 1.17 (0.60 to 2.28) | .86 |
| Adjusted HR (95% CI) | 1.0 (referent) | 1.27 (0.74 to 2.18) | 0.89 (0.43 to 1.84) | 1.28 (0.64 to 2.54) | .63 |
| Overall survival | |||||
| No. of patients at risk | 708 | 54 | 42 | 35 | — |
| No. of events | 145 | 14 | 9 | 9 | — |
| Unadjusted HR (95% CI) | 1.0 (referent) | 1.34 (0.77 to 2.31) | 1.07 (0.54 to 2.09) | 1.32 (0.67 to 2.58) | .41 |
| Adjusted HR (95% CI) | 1.0 (referent) | 1.25 (0.71 to 2.19) | 0.94 (0.47 to 1.91) | 1.28 (0.64 to 2.57) | .59 |
Multivariable HRs and 95% CIs were calculated by Cox proportional hazards regression models and were adjusted for age (in years as a continuous variable), sex (male or female), family history of colorectal cancer (yes or no), baseline performance status (0 or 1–2), depth of invasion through bowel wall (T1, T2, T3, or T4), number of positive lymph nodes (1–3 or ≥4), perineural invasion (yes or no), extravascular invasion (yes or no), postoperative carcinoembryonic antigen (<5 or ≥5), treatment arm (bolus 5-fluorouracil and leucovorin or bolus 5-fluorouracil, leucovorin, and irinotecan), body mass index (in kg/m2 as a continuous variable), physical activity (in metabolic equivalent task hours per week as a continuous variable), Western pattern diet (as a continuous variable), and consistent aspirin use (any aspirin use on both questionnaires). CI = confidence interval; HR = hazard ratios.
A linear test for trend was performed by entering the median value of each category of duration of statin use as a continuous variable in the model.
Impact of Statin Use on Cancer Recurrence and Death According to KRAS Mutation Status
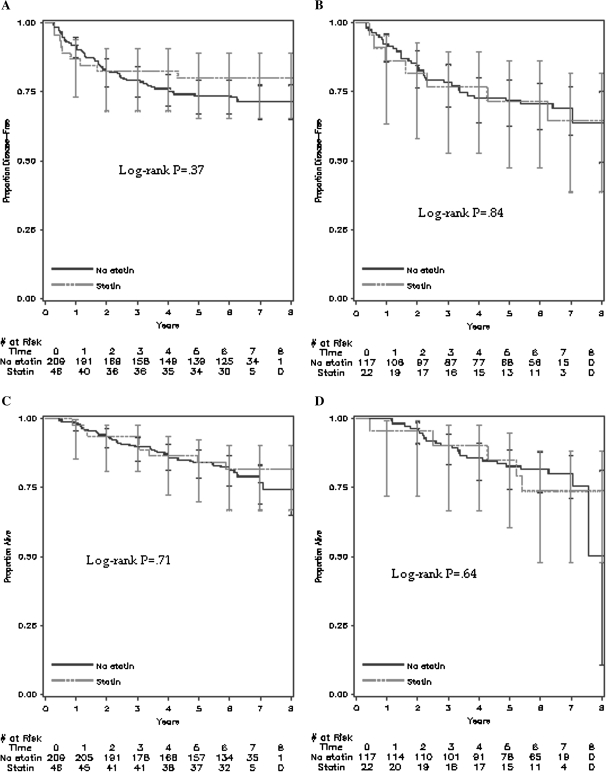
Among the 842 patients who reported on statin use following adjuvant chemotherapy, 394 (47%) had tumor tissue available for KRAS sequencing and 139 (35.3%) of these patients carried a KRAS mutation. Of the 139 tumors carrying a KRAS mutation, 96 (69%) had a KRAS mutation in codon 12 and 43 (31%) had a mutation in codon 13. DFS among statin users and nonusers, for both KRAS mutant and wild-type tumors, was similar (KRAS mutant tumors, adjusted HR of cancer recurrence or death = 1.21, 95% CI = 0.47 to 3.13; wild-type tumors, adjusted HR of cancer recurrence or death = 0.83, 95% CI = 0.41 to 1.71; Pinteraction = .84) (Figure 3, A and B, Table 2). RFS (KRAS mutant tumors, adjusted HR of cancer recurrence = 0.91, 95% CI = 0.30 to 2.79; wild-type tumors, adjusted HR of cancer recurrence = 1.07, 95% CI = 0.51 to 2.22; Pinteraction = .67) (Table 2) and OS (KRAS mutant tumors, adjusted HR of death = 1.18, 95% CI = 0.38 to 3.69; wild-type tumors, adjusted HR of survival = 0.88, 95% CI = 0.39 to 1.97; Pinteraction = .98) (Table 2 and Figure 3, C and D) were also similar among statin users and nonusers.
Figure 3.
Survival outcomes of statin users and nonusers from the Cancer and Leukemia Group B (CALGB) trial 89803 stratified by KRAS mutation status. Kaplan–Meier curves of A) disease-free survival in KRAS wild-type patients, B) disease-free survival in KRAS mutant patients, C) overall survival in KRAS wild-type patients, and D) overall survival in KRAS mutant patients in those with tissue available for KRAS mutation testing (n = 394) after a median follow-up of 6.5 years. Error bars represent 95% confidence intervals. Statistical significance was measured by the log-rank test. All P values were two-sided.
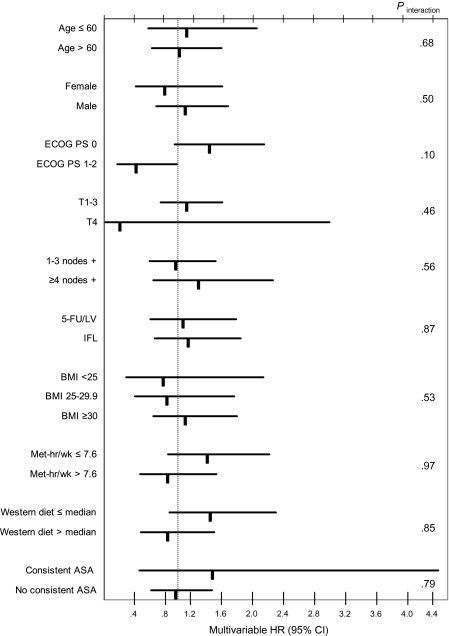
Impact of Statin Use Across Strata of Other Predictors of Patient Outcome
We examined the influence of statin use during adjuvant chemotherapy on DFS across strata of other predictors of cancer outcome (Figure 4). The relationship between statin use and DFS was similar across strata of age, sex, Eastern Cooperative Oncology Group performance status, the number of positive lymph nodes, depth of invasion through the bowel wall, treatment arm, BMI, physical activity, and Western pattern diet. Notably, the association between statin use and DFS was also similar among consistent and inconsistent aspirin users (Pinteraction = .79). Moreover, regular use of cyclooxygenase-2 inhibitors (also known as prostaglandin–endoperoxide synthase-2 inhibitors) did not modify the relationship between statin use and patient outcome (Pinteraction = .56) (data not shown).
Figure 4.
Risk of cancer recurrence and death among statin users and nonusers across strata of predictors of cancer outcome. Multivariable hazard ratios (HRs) and 95% confidence intervals (CIs) for cancer recurrence and death (disease-free survival) were calculated by Cox proportional hazards regression models. Wald test of cross-product terms was used to calculate Pinteraction and was two-sided. ASA = aspirin; BMI = body mass index; ECOG = Eastern Cooperative Oncology Group; IFL = irinotecan, bolus 5-FU, LV; MET = metabolic equivalent task; PS = performance status; T = depth of invasion through bowel wall; 5-FU/LV = bolus 5-fluorouracil/leucovorin; .
Relationship between Statin Use and Toxicity
We explored the influence of statins on the occurrence of the most common grade 3 or higher toxic effects seen in the treatment trial, as well as on cardiovascular events (Table 4) (11). The likelihood of developing grade 3 or higher toxic effects was similar for statin users of duration 2 years or more compared with nonusers after adjusting for potential confounding factors leukopenia (odds ratio [OR] = 1.10, 95% CI = 0.46 to 2.62), neutropenia (OR = 1.32, 95% CI = 0.71 to 2.44), nausea (OR = 0.68, 95% CI = 0.30 to 1.56), vomiting (OR = 0.91, 95% CI = 0.37 to 2.22), diarrhea (OR = 0.91, 95% CI = 0.54 to 1.55), and fatigue (OR = 0.77, 95% CI = 0.32 to 1.88). Also, the likelihood of developing grade 3 or higher cardiovascular toxicity was similar for statin users and nonusers, although the number of such events was small (n = 7 for nonusers and n = 1 for statin users; adjusted OR = 1.08, 95% CI = 0.13 to 9.08).
Table 4.
Association between statin use and the incidence of selected grade 3 or higher toxicities in patients from the Cancer and Leukemia Group B trial 89803 (n = 784)*
| Toxicity | Statin use reported after adjuvant chemotherapy, y |
|
| 0 | ≥2 | |
| Leukopenia | ||
| No. of patients at risk, n | 707 | 77 |
| No. of events, n (%) | 56 (8) | 7 (9) |
| Unadjusted HR (95% CI) | 1.0 (referent) | 1.16 (0.51 to 2.65) |
| Adjusted HR (95% CI) | 1.0 (referent) | 1.10 (0.46 to 2.62) |
| Neutropenia | ||
| No. of patients at risk, n | 707 | 77 |
| No. of events, n (%) | 160 (23) | 20 (26) |
| Unadjusted HR (95% CI) | 1.0 (referent) | 1.20 (0.70 to 2.06) |
| Adjusted HR (95% CI) | 1.0 (referent) | 1.32 (0.71 to 2.44) |
| Nausea | ||
| No. of patients at risk, n | 707 | 77 |
| No. of events, n (%) | 96 (14) | 7 (9) |
| Unadjusted HR (95% CI) | 1.0 (referent) | 0.64 (0.28 to 1.43) |
| Adjusted HR (95% CI) | 1.0 (referent) | 0.68 (0.30 to 1.56) |
| Vomiting | ||
| No. of patients at risk, n | 707 | 77 |
| No. of events, n (%) | 68 (10) | 6 (8) |
| Unadjusted HR (95% CI) | 1.0 (referent) | 0.79 (0.33 to 1.90) |
| Adjusted HR (95% CI) | 1.0 (referent) | 0.91 (0.37 to 2.22) |
| Diarrhea | ||
| No. of patients at risk, n | 707 | 77 |
| No. of events, n (%) | 236 (33) | 23 (30) |
| Unadjusted HR (95% CI) | 1.0 (referent) | 0.85 (0.51 to 1.42) |
| Adjusted HR (95% CI) | 1.0 (referent) | 0.91 (0.54 to 1.55) |
| Fatigue | ||
| No. of patients at risk, n | 707 | 77 |
| No. of events, n (%) | 66 (9) | 6 (8) |
| Unadjusted HR (95% CI) | 1.0 (referent) | 0.82 (0.34 to 1.96) |
| Adjusted HR (95% CI) | 1.0 (referent) | 0.77 (0.32 to 1.88) |
| Cardiovascular† | ||
| No. of patients at risk, n | 707 | 77 |
| No. of events, n (%) | 7 (1) | 1 (1) |
| Unadjusted HR (95% CI) | 1.0 (referent) | 1.32 (0.16 to 10.84) |
| Adjusted HR (95% CI) | 1.0 (referent) | 1.08 (0.13 to 9.08) |
| Any of the above toxicities | ||
| No. of patients at risk, n | 707 | 77 |
| No. of events, n (%) | 379 (54) | 41 (53) |
| Unadjusted HR (95% CI) | 1.0 (referent) | 0.99 (0.62 to 1.58) |
| Adjusted HR (95% CI) | 1.0 (referent) | 1.04 (0.64 to 1.71) |
Adverse events were rated according to the National Cancer Institute Common Toxicity Criteria version 2.0. Multivariable HRs and 95% CIs were calculated by logistic regression models. Models were adjusted for age (in years as a continuous variable), sex (male or female), baseline Eastern Cooperative Oncology Group performance status (0 or 1–2), treatment arm (bolus 5-fluorouracil and leucovorin or the combination of irinotecan, bolus 5-fluorouracil, and leucovorin), body mass index (in kg/m2 as a continuous variable), and physical activity (in metabolic equivalent task hours per week as a continuous variable). CI = confidence interval; HR = hazard ratios.
This category includes cardiac ischemia and/or infarction and cerebrovascular ischemia.
Discussion
In this large cohort of stage III colon cancer patients treated with surgery and adjuvant chemotherapy, DFS, RFS, and OS were similar for statin users and nonusers. Recent statin use during the period of cancer diagnosis and treatment conferred no improvement in DFS, RFS, or OS, and long-term regular statin use was similarly not associated with any benefit to survival. Moreover, the relationship between statin use and patient outcome was not modified by KRAS mutation status. Nonetheless, statin use did not increase the likelihood of chemotherapy-related adverse events. To our knowledge, this is the first study to prospectively examine the relationship between statin use and survival among patients with established colon cancer.
Because statins may interact with diverse signaling pathways that are critical for colon cancer development and progression, there has been intense interest in the potential of statins as chemopreventative and antitumor agents. A large observational study of 1953 patients with colorectal cancer and 2015 control subjects demonstrated a statistically significant reduction in the risk of colorectal cancer with 5 or more years of statin use (6), as did two recent meta-analyses (7,8). Unfortunately, these findings were not confirmed in a large meta-analysis of epidemiological studies and randomized controlled trials (10) nor in several smaller observational studies (21–28).
In contrast, very few studies have addressed the role of statins among patients with established colorectal cancer. Siddiqui et al. (29) found that statin use was statistically significantly associated with less-advanced tumor stage and improved 5-year survival; however, statin use was assessed retrospectively (29). A single-arm, multicenter phase II study of infusional 5-fluorouracil, leucovorin, and irinotecan plus simvastatin in previously untreated metastatic colorectal cancer patients has also been reported demonstrating reasonable tolerability, an overall response rate of 47%, median time to progression of 9.9 months, and median survival of 21.8 months (30). However, the added contribution of simvastatin to chemotherapy is impossible to determine in the context of the trial’s single-arm design. Finally, National Surgical Adjuvant Breast and Bowel Project protocol P-5, the Statin Polyp Prevention Trial, was recently activated and is currently enrolling patients. This large study plans to randomize 1740 patients with resected stage I or II colon cancer to rosuvastatin vs placebo for 5 years, with a primary endpoint of adenomatous polyp development, metachronous colorectal cancer, or colon cancer recurrence. Data will not be available for several years.
There are several mechanisms through which statin exposure may influence survival after a diagnosis of colon cancer. Statins have been shown to inhibit proliferation (31,32), induce apoptosis (33,34), inhibit angiogenesis (35,36), affect cell–cell adhesion (37), prevent metastasis (38), and decrease inflammation (39, 40). A leading hypothesis to explain the antitumor activity of statins in colorectal cancer revolves around the suppression of farnesylation of RAS, thus preventing RAS activation, a key oncogenic event. In fact, several pharmacological inhibitors of farnesylation have been examined in patients with KRAS-driven malignancies (41–44). However, in our analysis, statin use was not associated with improved survival in patients with either KRAS-mutated or wild-type colon tumors. Moreover, our analysis found that the relationship between statin use and patient outcome did not differ by aspirin or cyclooxygenase-2 inhibitor use.
There are several advantages to using a cohort within a clinical trial sponsored by the National Cancer Institute. First, all patients had stage III cancer, reducing the impact of heterogeneity by disease stage. Second, treatment and follow-up were standardized, and the date and nature of recurrence were recorded prospectively. Finally, detailed information on prognostic factors was routinely collected.
Several potential limitations deserve comment. First, the number of statin users in our cohort was small. However, the rate of statin use in our analysis is consistent with, if not higher than rates seen in other studies (6,27). Moreover, we had adequate power to detect a statistically significant treatment effect. The hazard ratios we obtained were in the 1.0–1.1 range, and no trends toward a positive or negative association were detected. Second, patients who enroll in randomized trials may differ from the population at large. However, because the study included patients from community and academic centers throughout North America, our findings should reflect the general US population. Third, because we relied on self-reported statin use, misclassification of exposure is possible. However, previous studies have demonstrated that such data are reliable (6). Furthermore, statin use was recorded before any knowledge of cancer-related outcomes, thus reducing the likelihood of reporting biases. Fourth, patients who are prescribed statins may differ from the general population by socioeconomic status (45); lifestyle factors such as diet, BMI, and physical activity; and utilization of medical care and preventative health practices (46). Our study was controlled for median household income, dietary pattern, BMI, physical activity, performance status, and other potentially prognostic variables. However, residual confounding from unknown variables is possible.
Another possible limitation is that we were unable to assess the individual impact of different statins on patient outcome, because information on the type of statin used by each patient was not collected on the questionnaires. Although some hypotheses suggest that lipophilic and hydrophilic statins may have distinct effects, previous studies have not been able to show a differential impact on colorectal cancer risk, although this has not been well studied. Finally, although many studies have reported that the presence of any KRAS mutation is associated with resistance to antibodies against the epidermal growth factor receptor, such as cetuximab (47–50), recent data indicate that KRAS codon 12 and 13 mutations may result in biologically and functionally distinct proteins, with different treatment responses to cetuximab (51). Unfortunately, we were unable to evaluate the effect of statins among tumors with specific KRAS mutations because of the limited number of patients with available KRAS data.
In conclusion, our large prospective study of stage III colon cancer patients found that DFS, RFS, and OS were similar for statin users and nonusers, independent of KRAS mutation. Importantly, statin use was not associated with increased toxicity in the patient population. We eagerly await the results of the Statin Polyp Prevention Trial (protocol P-5) initiated by the National Surgical Adjuvant Breast and Bowel Project. Additional studies are also needed to elucidate the potential role of statin use in colon cancer recurrence and patient outcome.
Funding
This work was supported by the National Cancer Institute at the National Institutes of Health (P50CA127003 and T32CA009001-34 to K.N.); American Society of Clinical Oncology Career Development Award (K.N.); The Charles A. King Trust Postdoctoral Fellowship Award, Bank of America, Co-Trustee (K.N.); as well as by Pharmacia & Upjohn Company (now Pfizer Oncology). The research for CALGB 89803 was also supported, in part, by grants from the National Cancer Institute at the National Institutes of Health (CA31946) to the Cancer and Leukemia Group B (Richard L. Schilsky, Chairman) and to the CALGB Statistical Center (Stephen George, CA33601).
Notes
The sponsors did not participate in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the article. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health, American Society of Clinical Oncology (ASCO), or the ASCO Foundation. DH, whose role in the study was to confirm and validate the data analyses, owns stock in Pfizer, Inc (New York, NY), the makers of irinotecan.
Primary contacts and clinical centers that participated in the CALGB clinical trial included Lee S. Schwartzberg, Baptist Cancer Institute Community Clinical Oncology Program, TN (supported by CA71323); Stephen Grubbs, Christiana Care Health Services, Inc, Community Clinical Oncology Program, Wilmington, DE (supported by CA45418); Harold J. Burstein, Dana-Farber Cancer Institute, Boston, MA (supported by CA32291); Konstantin Dragnev, Dartmouth Medical School, Norris Cotton Cancer Center, Lebanon, NH (supported by CA04326); Jeffrey Crawford, Duke University Medical Center, Durham, NC (supported by CA47577); Minetta C. Liu, Georgetown University Medical Center, Washington, DC (supported by CA77597); Jeffrey K. Giguere, Cancer Centers of the Carolinas, Greenville, SC (supported by CA29165); Jeffrey Kirshner, Hematology-Oncology Associates of Central New York Community Clinical Oncology Program, Syracuse, NY (supported by CA45389); Kanti R. Rai, Long Island Jewish Medical Center, Lake Success, NY (supported by CA35279); Jeffrey W. Clark, Massachusetts General Hospital, Boston, MA (supported by CA32291); Clifford A. Hudis, Memorial Sloan-Kettering Cancer Center, New York, NY (supported by CA77651); Lewis R. Silverman, .D., Mount Sinai School of Medicine, New York, NY (supported by CA04457); John A. Ellerton, Nevada Cancer Research Foundation CCOP, Las Vegas, NV (supported by CA35421); William Sikov, Rhode Island Hospital, Providence, RI (supported by CA08025); Ellis Levine, Roswell Park Cancer Institute, Buffalo, NY (supported by CA59518); James N. Atkins, Southeast Cancer Control Consortium, Inc, CCOP, Goldsboro, NC (supported by CA45808); Stephen L. Graziano, State University of New York Upstate Medical University, Syracuse, NY (supported by CA21060); Clara D. Bloomfield, The Ohio State University Medical Center, Columbus, OH (supported by CA77658); Barbara A. Parker, University of California at San Diego, San Diego, CA (supported by CA11789); Hedy L. Kindler, University of Chicago, Chicago, IL (supported by CA41287); David J. Peace, University of Illinois MBCCOP, Chicago, IL (supported by CA74811); Daniel A. Vaena, University of Iowa, Iowa City, IA (supported by CA47642); Martin Edelman, University of Maryland Greenebaum Cancer Center, Baltimore, MD (supported by CA31983); William V. Walsh, University of Massachusetts Medical School, Worcester, MA (supported by CA37135); Bruce A. Peterson, University of Minnesota, Minneapolis, MN (supported by CA16450); Michael C. Perry, University of Missouri/Ellis Fischel Cancer Center, Columbia, MO (supported by CA12046); Anne Kessinger, University of Nebraska Medical Center, Omaha, NE (supported by CA77298); Thomas C. Shea, University of North Carolina at Chapel Hill, Chapel Hill, NC (supported by CA47559); Harvey B. Niell, University of Tennessee Memphis, Memphis, TN (supported by CA47555); Marc S. Greenblatt, University of Vermont, Burlington, VT (supported by CA77406); David D. Hurd, Wake Forest University School of Medicine, Winston-Salem, NC (supported by CA03927); Brendan M. Weiss, Walter Reed Army Medical Center, Washington, DC (supported by CA26806); Nancy Bartlett, Washington University School of Medicine, St. Louis, MO (supported by CA77440); John Leonard, Weill Medical College of Cornell University, New York, NY (supported by CA07968).
References
- 1.Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335(14):1001–1009. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 2.The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339(19):1349–1357. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- 3.Serruys PW, de Feyter P, Macaya C, et al. Fluvastatin for prevention of cardiac events following successful first percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002;287(24):3215–3222. doi: 10.1001/jama.287.24.3215. [DOI] [PubMed] [Google Scholar]
- 4.Casey PJ. Protein lipidation in cell signaling. Science. 1995;268(5208):221–225. doi: 10.1126/science.7716512. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343(6257):425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 6.Poynter JN, Gruber SB, Higgins PD, et al. Statins and the risk of colorectal cancer. N Engl J Med. 2005;352(21):2184–2192. doi: 10.1056/NEJMoa043792. [DOI] [PubMed] [Google Scholar]
- 7.Samadder NJ, Gupta A, Rennert G, et al. Statins and the risk of colorectal cancer: a meta-analysis of 22 observational studies. San Antonio, TX: American College of Gastroenterology; 2010. Abstract 1481. [Google Scholar]
- 8.Ditah I. Statin use and the risk of colorectal cancer: has recent evidence shifted our opinion? A meta-analysis involving more than 1.7 million participants. San Antonio, TX: American College of Gastroenterology; 2010. Abstract 383. [Google Scholar]
- 9.Bonovas S, Filioussi K, Tsavaris N, Sitaras NM. Statins and cancer risk: a literature-based meta-analysis and meta-regression analysis of 35 randomized controlled trials. J Clin Oncol. 2006;24(30):4808–4817. doi: 10.1200/JCO.2006.06.3560. [DOI] [PubMed] [Google Scholar]
- 10.Bonovas S, Filioussi K, Flordellis CS, Sitaras NM. Statins and the risk of colorectal cancer: a meta-analysis of 18 studies involving more than 1.5 million patients. J Clin Oncol. 2007;25(23):3462–3468. doi: 10.1200/JCO.2007.10.8936. [DOI] [PubMed] [Google Scholar]
- 11.Saltz LB, Niedzwiecki D, Hollis D, et al. Irinotecan fluorouracil plus leucovorin is not superior to fluorouracil plus leucovorin alone as adjuvant treatment for stage III colon cancer: results of CALGB 89803. J Clin Oncol. 2007;25(23):3456–3461. doi: 10.1200/JCO.2007.11.2144. [DOI] [PubMed] [Google Scholar]
- 12.Ogino S, Kawasaki T, Brahmandam M, et al. Sensitive sequencing method for KRAS mutation detection by pyrosequencing. J Mol Diagn. 2005;7(3):413–421. doi: 10.1016/S1525-1578(10)60571-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogino S, Meyerhardt JA, Irahara N, et al. KRAS mutation in stage III colon cancer and clinical outcome following intergroup trial CALGB 89803. Clin Cancer Res. 2009;15(23):7322–7329. doi: 10.1158/1078-0432.CCR-09-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–481. [Google Scholar]
- 15.Therneau T, Grambsch P. Modeling Survival Data. New York, NY: Springer; 2000. [Google Scholar]
- 16.Jones MP, Crowley J. A general class of nonparametric tests for survival analysis. Biometrics. 1989;45(1):157–170. [PubMed] [Google Scholar]
- 17.Kleinbaum DG, Klein M. Survival Analysis: A Self-Learning Text. 2nd ed. New York, NY: Springer Science + Business Media, LLC; 2005. [Google Scholar]
- 18.Meyerhardt JA, Heseltine D, Niedzwiecki D, et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol. 2006;24(22):3535–3541. doi: 10.1200/JCO.2006.06.0863. [DOI] [PubMed] [Google Scholar]
- 19.Clark TG, Altman DG, De Stavola BL. Quantification of the completeness of follow-up. Lancet. 2002;359(9314):1309–1310. doi: 10.1016/s0140-6736(02)08272-7. [DOI] [PubMed] [Google Scholar]
- 20.Wu Y, Takkenberg JJ, Grunkemeier GL. Measuring follow-up completeness. Ann Thorac Surg. 2008;85(4):1155–1157. doi: 10.1016/j.athoracsur.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 21.Flick ED, Habel LA, Chan KA, et al. Statin use and risk of colorectal cancer in a cohort of middle-aged men in the US: a prospective cohort study. Drugs. 2009;69(11):1445–1457. doi: 10.2165/00003495-200969110-00004. [DOI] [PubMed] [Google Scholar]
- 22.Singh H, Mahmud SM, Turner D, Xue L, Demers AA, Bernstein CN. Long-term use of statins and risk of colorectal cancer: a population-based study. Am J Gastroenterol. 2009;104(12):3015–3023. doi: 10.1038/ajg.2009.574. [DOI] [PubMed] [Google Scholar]
- 23.Yang YX, Hennessy S, Propert K, Hwang WT, Sarkar M, Lewis JD. Chronic statin therapy and the risk of colorectal cancer. Pharmacoepidemiol Drug Saf. 2008;17(9):869–876. doi: 10.1002/pds.1599. [DOI] [PubMed] [Google Scholar]
- 24.Boudreau DM, Koehler E, Rulyak SJ, Haneuse S, Harrison R, Mandelson MT. Cardiovascular medication use and risk for colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2008;17(11):3076–3080. doi: 10.1158/1055-9965.EPI-08-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vinogradova Y, Hippisley-Cox J, Coupland C, Logan RF. Risk of colorectal cancer in patients prescribed statins, nonsteroidal anti-inflammatory drugs, and cyclooxygenase-2 inhibitors: nested case-control study. Gastroenterology. 2007;133(2):393–402. doi: 10.1053/j.gastro.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 26.Coogan PF, Smith J, Rosenberg L. Statin use and risk of colorectal cancer. J Natl Cancer Inst. 2007;99(1):32–40. doi: 10.1093/jnci/djk003. [DOI] [PubMed] [Google Scholar]
- 27.Jacobs EJ, Rodriguez C, Brady KA, Connell CJ, Thun MJ, Calle EE. Cholesterol-lowering drugs and colorectal cancer incidence in a large United States cohort. J Natl Cancer Inst. 2006;98(1):69–72. doi: 10.1093/jnci/djj006. [DOI] [PubMed] [Google Scholar]
- 28.Robertson DJ, Riis AH, Friis S, Pedersen L, Baron JA, Sorensen HT. Neither long-term statin use nor atherosclerotic disease is associated with risk of colorectal cancer. Clin Gastroenterol Hepatol. 2010;8(12):1056–1061. doi: 10.1016/j.cgh.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 29.Siddiqui AA, Nazario H, Mahgoub A, Patel M, Cipher D, Spechler SJ. For patients with colorectal cancer, the long-term use of statins is associated with better clinical outcomes. Dig Dis Sci. 2009;54(6):1307–1311. doi: 10.1007/s10620-009-0790-8. [DOI] [PubMed] [Google Scholar]
- 30.Lee J, Jung KH, Park YS, et al. Simvastatin plus irinotecan, 5-fluorouracil, and leucovorin (FOLFIRI) as first-line chemotherapy in metastatic colorectal patients: a multicenter phase II study. Cancer Chemother Pharmacol. 2009;64(4):657–663. doi: 10.1007/s00280-008-0913-5. [DOI] [PubMed] [Google Scholar]
- 31.Rao S, Porter DC, Chen X, Herliczek T, Lowe M, Keyomarsi K. Lovastatin-mediated G1 arrest is through inhibition of the proteasome, independent of hydroxymethyl glutaryl-CoA reductase. Proc Natl Acad Sci U S A. 1999;96(14):7797–7802. doi: 10.1073/pnas.96.14.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ukomadu C, Dutta A. p21-dependent inhibition of colon cancer cell growth by mevastatin is independent of inhibition of G1 cyclin-dependent kinases. J Biol Chem. 2003;278(44):43586–43594. doi: 10.1074/jbc.M307194200. [DOI] [PubMed] [Google Scholar]
- 33.Agarwal B, Bhendwal S, Halmos B, Moss SF, Ramey WG, Holt PR. Lovastatin augments apoptosis induced by chemotherapeutic agents in colon cancer cells. Clin Cancer Res. 1999;5(8):2223–2229. [PubMed] [Google Scholar]
- 34.Wu J, Wong WW, Khosravi F, Minden MD, Penn LZ. Blocking the Raf/MEK/ERK pathway sensitizes acute myelogenous leukemia cells to lovastatin-induced apoptosis. Cancer Res. 2004;64(18):6461–6468. doi: 10.1158/0008-5472.CAN-04-0866. [DOI] [PubMed] [Google Scholar]
- 35.Weis M, Heeschen C, Glassford AJ, Cooke JP. Statins have biphasic effects on angiogenesis. Circulation. 2002;105(6):739–745. doi: 10.1161/hc0602.103393. [DOI] [PubMed] [Google Scholar]
- 36.Alber HF, Dulak J, Frick M, et al. Atorvastatin decreases vascular endothelial growth factor in patients with coronary artery disease. J Am Coll Cardiol. 2002;39(12):1951–1955. doi: 10.1016/s0735-1097(02)01884-3. [DOI] [PubMed] [Google Scholar]
- 37.Nubel T, Dippold W, Kleinert H, Kaina B, Fritz G. Lovastatin inhibits Rho-regulated expression of E-selectin by TNFalpha and attenuates tumor cell adhesion. FASEB J. 2004;18(1):140–142. doi: 10.1096/fj.03-0261fje. [DOI] [PubMed] [Google Scholar]
- 38.Broitman SA, Wilkinson JIV, Cerda S, Branch SK. Effects of monoterpenes and mevinolin on murine colon tumor CT-26 in vitro and its hepatic “metastases” in vivo. Adv Exp Med Biol. 1996;401:111–130. doi: 10.1007/978-1-4613-0399-2_9. [DOI] [PubMed] [Google Scholar]
- 39.Hakamada-Taguchi R, Uehara Y, Kuribayashi K, et al. Inhibition of hydroxymethylglutaryl-coenzyme a reductase reduces Th1 development and promotes Th2 development. Circ Res. 2003;93(10):948–956. doi: 10.1161/01.RES.0000101298.76864.14. [DOI] [PubMed] [Google Scholar]
- 40.Demierre MF, Higgins PD, Gruber SB, Hawk E, Lippman SM. Statins and cancer prevention. Nat Rev Cancer. 2005;5(12):930–942. doi: 10.1038/nrc1751. [DOI] [PubMed] [Google Scholar]
- 41.Rao S, Cunningham D, de Gramont A, et al. Phase III double-blind placebo-controlled study of farnesyl transferase inhibitor R115777 in patients with refractory advanced colorectal cancer. J Clin Oncol. 2004;22(19):3950–3957. doi: 10.1200/JCO.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 42.Sharma S, Kemeny N, Kelsen DP, et al. A phase II trial of farnesyl protein transferase inhibitor SCH 66336, given by twice-daily oral administration, in patients with metastatic colorectal cancer refractory to 5-fluorouracil and irinotecan. Ann Oncol. 2002;13(7):1067–1071. doi: 10.1093/annonc/mdf173. [DOI] [PubMed] [Google Scholar]
- 43.Macdonald JS, McCoy S, Whitehead RP, et al. A phase II study of farnesyl transferase inhibitor R115777 in pancreatic cancer: a Southwest oncology group (SWOG 9924) study. Invest New Drugs. 2005;23(5):485–487. doi: 10.1007/s10637-005-2908-y. [DOI] [PubMed] [Google Scholar]
- 44.Adjei AA, Mauer A, Bruzek L, et al. Phase II study of the farnesyl transferase inhibitor R115777 in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2003;21(9):1760–1766. doi: 10.1200/JCO.2003.09.075. [DOI] [PubMed] [Google Scholar]
- 45.Thomsen RW, Johnsen SP, Olesen AV, et al. Socioeconomic gradient in use of statins among Danish patients: population-based cross-sectional study. Br J Clin Pharmacol. 2005;60(5):534–542. doi: 10.1111/j.1365-2125.2005.02494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neutel CI, Morrison H, Campbell NR, de Groh M. Statin use in Canadians: trends, determinants and persistence. Can J Public Health. 2007;98(5):412–416. doi: 10.1007/BF03405430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lievre A, Bachet JB, Le Corre D, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66(8):3992–3995. doi: 10.1158/0008-5472.CAN-06-0191. [DOI] [PubMed] [Google Scholar]
- 48.Di Fiore F, Blanchard F, Charbonnier F, et al. Clinical relevance of KRAS mutation detection in metastatic colorectal cancer treated by cetuximab plus chemotherapy. Br J Cancer. 2007;96(8):1166–1169. doi: 10.1038/sj.bjc.6603685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khambata-Ford S, Garrett CR, Meropol NJ, et al. Expression of epiregulin and amphiregulin and K-ras mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. J Clin Oncol. 2007;25(22):3230–3237. doi: 10.1200/JCO.2006.10.5437. [DOI] [PubMed] [Google Scholar]
- 50.Benvenuti S, Sartore-Bianchi A, Di Nicolantonio F, et al. Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti-epidermal growth factor receptor antibody therapies. Cancer Res. 2007;67(6):2643–2648. doi: 10.1158/0008-5472.CAN-06-4158. [DOI] [PubMed] [Google Scholar]
- 51.De Roock W, Jonker DJ, Di Nicolantonio F, et al. Association of KRAS p.G13D mutation with outcome in patients with chemotherapy-refractory metastatic colorectal cancer treated with cetuximab. JAMA. 2011;304(16):1812–1820. doi: 10.1001/jama.2010.1535. [DOI] [PubMed] [Google Scholar]