Abstract
Background
Parainfluenza virus (PIV) infections are an important cause of morbidity in children with upper or lower respiratory tract infection (URTI and LRTI respectively). However, the epidemiology of PIV infections in children with cancer has not been well studied.
Methods
This retrospective study sought to determine the epidemiology of PIV infections and risk factors for progression to a LRTI in 1,381 children diagnosed with leukemia or lymphoma, between 2000 and 2009.
Results
PIV infections were diagnosed in 83 (10%) of 820 children tested for respiratory infections. PIV-3 accounted for 49 (61%) of the PIV infections. Of the 83 infections, 75 (90%) were community-acquired. Children less than 2 years of age were more likely to have PIV infection (p=0.002, odds ratio 2.69; 95% CI 1.5–4.8). PIV infections were more common in children with acute lymphoblastic leukemia as compared with other malignancies (p<0.0001, odds ratio 4.13; 95% CI 2.37–7.21). The majority of patients, 66 (80%) had URTI. Children with LRTI had a median age of 27 months as compared with 56 months for children with URTI (p=0.005). Fever with severe neutropenia was more common in patients with LRTI than with URTI (p=0.02). LRTI was significantly associated with ANC<500 cells/µL (p=0.002) and ALC<100 cells/µL (p=0.008) at onset of PIV infection. There was no mortality attributed to PIV infections, although 3 children required mechanical ventilation for respiratory failure due to PIV infection.
Conclusions
PIV was the second most common respiratory viral infection in this population after influenza (A and B). Young children were more likely to have PIV infection and LRTI. Severe neutropenia and lymphopenia was associated with LRTI.
Keywords: Parainfluenza virus, Infections, Children, Cancer
INTRODUCTION
The incidence of parainfluenza virus (PIV) infections and its natural course in children with cancer is not well known. Leukemia and lymphoma are the most common malignancies in children.1 Respiratory syncytial virus (RSV) and influenza virus infections have been the focus of research in children with cancer and respiratory virus (RV) infections.2 PIV has been an under-appreciated cause of RV infections, although it may be second only to RSV in children younger than 5 years of age, with upper respiratory tract infection (URTI) or lower respiratory tract infection (LRTI), in the United States.3
This retrospective study includes a large cohort of children with leukemia, lymphoma and PIV infection, who were symptomatic with URTI or LRTI, with or without fever. The epidemiology of these infections and risk factors for progression to LRTI are described.
PATIENTS AND METHODS
Patient population
This retrospective cohort study included 1,381 children diagnosed with leukemia or lymphoma during a 10 year period, between January 2000 and December 2009 at St. Jude Children’s Research Hospital (SJCRH). Of these 1381 children, a total of 820 children were tested for respiratory infections. The study was approved by the Institutional Review Board at SJCRH. SJCRH is a tertiary care children’s research hospital which treats approximately 3,000 patients with cancer each year referred immediately after diagnosis of their underlying malignancy, from across the US, but mostly from within a 600 mile radius of Memphis. The typical duration of follow-up is for 10 years after diagnosis, or until 18 years of age, whichever is later.
Virologic records were reviewed and patients in whom PIV 1–4 was detected by culture, direct fluorescent antibody (DFA) testing or polymerase chain reaction (PCR), from respiratory tract secretions were identified. Medical record review included analysis of clinical variables including age, race, gender, date of onset of respiratory symptoms, duration of viral shedding, URTI or LRTI, hospital or community-acquired infection, presence of fever, need for oxygen, intensive care unit admission, mechanical ventilation, co-pathogens, absolute neutrophil count (ANC) and absolute lymphocyte count (ALC) at the time of infection.
Virology and microbiology procedures
A nasopharyngeal swab or wash for culture and viral DFA staining was performed on all patients with clinically suspected URTI or LRTI. Samples were inoculated to tissue culture containing rhesus monkey kidney, A549 and Madin-Darby canine kidney (MDCK) cells. Upper respiratory cultures were kept for 14 days before reporting as negative. Virus causing hemadsorption with guinea pig red cells on day 7 and 14 or cytopathic effect at any time was confirmed as PIV 1–4 using type-specific respiratory DFA smears prepared using commercially available type-specific antisera against PIV 1–4 (Bartels, County Wicklow Ireland; Pathodx, KS,USA; Diagnostic Hybrid, OH,USA). In addition to culture and DFA, PCR was performed on respiratory samples obtained after February 2007, using a laboratory developed test panel of individual real-time PCR assays, targeting PIV 1–3, run on SmartCycler real-time PCR instrumentation (Cepheid, Sunnyvale, CA).
Definitions
PIV infection was defined as an URTI when signs and symptoms were consistent with the diagnosis, with a normal chest examination and chest roentgenogram. PIV infection was defined as a LRTI when symptoms were accompanied by signs on lung auscultation or when a new pulmonary infiltrate as defined by a plain chest radiograph, high resolution computerized-tomography or both, unexplained by a non-infectious etiology, was present. The day of onset of PIV infection was defined as the day when the first positive diagnostic sample was collected. Mortality was considered attributable to PIV, if the patient died from a LRTI, as a result of respiratory failure, with isolation of PIV from a respiratory specimen, and with no other ascertainable cause. The presence of a co-pathogen was defined by the isolation or detection of pathogenic bacterial or fungal species or by the identification of other opportunistic pathogens in addition to PIV from any site. Hospital-acquired infections (HAI) were defined as those occurring 3 days or more after admission to the inpatient unit. Duration of viral shedding was defined as the interval between the day of the first positive culture or diagnostic test and the first negative culture or diagnostic test. New infections were defined by isolation or detection of PIV from the same patient, 14 days or more after the last negative culture or diagnostic test. Only the first infection from each patient was included in the analyses.
Management
Children diagnosed with PIV infection were placed in respiratory isolation. Nasopharyngeal swabs or washes were obtained once weekly in most patients, to document clearance of the virus.
Statistical analysis
Descriptive statistics were obtained for the demographic variables of children with leukemia or lymphoma and PIV infection. The marginal association between PIV infection and independent variables were determined by Fischer’s exact test and Kruskal-Wallis test. Multiple exact logistic regression models were further used to examine the association between LRTI and ANC, ALC, age after adjusting for potential confounders, such as admission to the intensive care unit, oxygen requirement, and mechanical ventilation. Wilcoxon rank sum test was used to compare continuous variables between two groups. All analyses were performed in statistical software package SAS 9.2 (SAS Institute, Inc., Cary, NC).
RESULTS
PIV infection was diagnosed in 83 (10%) of 820 children tested, and in 3% of the 2773 respiratory samples collected from these patients between January 2000 and December 2009. PIV was the second most common cause of respiratory viral infection during this 10 year period, after influenza which was diagnosed in 119 (14.5%) patients. Influenza A occurred in 80 (9.7%) and influenza B in 39 (4.8%) patients. RSV infections were seen in 59 (7.1%), cytomegalovirus in 26 (3.1%), human metapneumovirus in 12 (1.5%), adenovirus in 11 (1.3%), HSV in 9 (1.1%), rhinovirus in 7 (0.9%), and human enterovirus in 3 (0.4%) patients.
The number of PIV infections increased from 19 infections out of 961 respiratory samples collected from 366 (5%) patients in 2000–2004 to 64 infections out of 1812 respiratory samples collected from 454 (14%) patients in 2005–2009 (p< 0.0001).
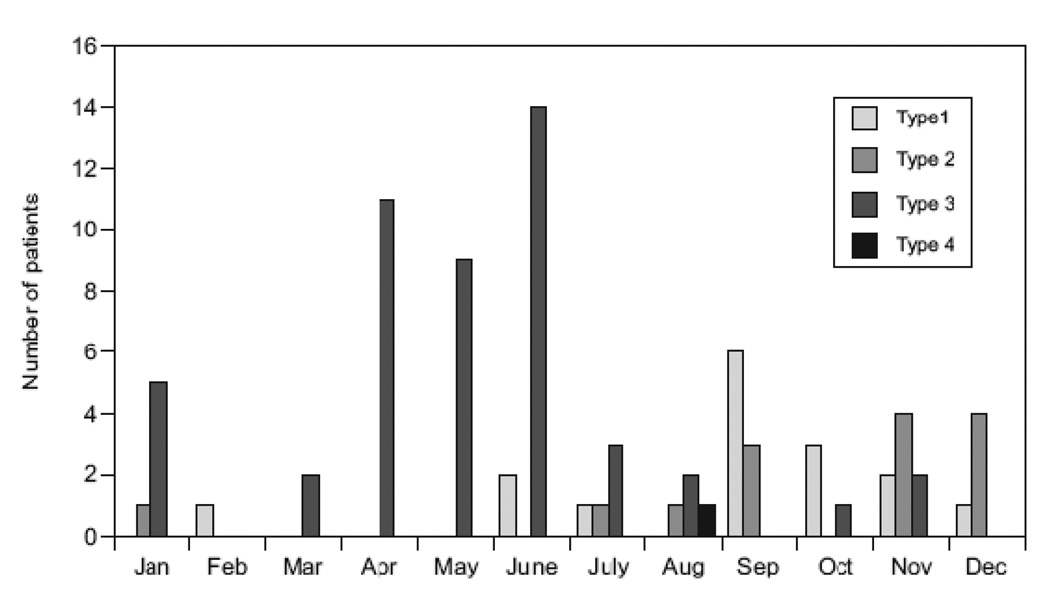
PIV-3 accounted for the majority of PIV infections (49 out of 80 patients or 61%). PIV-1 was seen in 16 (20%), PIV-2 in 14 (18%) and PIV-4 in 1 patient. PIV was not typed in 3 patients. The majority of PIV-3 infections occurred in spring and summer (41 of 49 or 84%) while 12 (75%) of PIV-1 and 12 (86%) of PIV-2 infections occurred in fall and winter (Fig 1). PIV-1 infections occurred mostly in 2003, 2005 and 2007. Of the 83 infections, 75 (90%) were community-acquired (CA) and 8(10%) were HAl. HAI were interspersed among CA infections, with no type-specific predilection. No apparent outbreaks of PIV infection were observed.
FIGURE 1.
Monthly distribution of the incidence of parainfluenza virus respiratory infections in children with leukemia or lymphoma between 2000 and 2009.
Clinical features
The clinical characteristics of children with hematological malignancies with and without PIV infection are represented in Table, Supplemental Digital Content (SDC) 1. The entire cohort of 1381 patients was followed for a total of 6861.8 years giving an incidence rate of 12.1 cases of PIV infection per 1000 patient years. The median age of the children was 4 years and 5 months (range 6 months to 17 years and 6 months). The mean age of children with PIV infection was significantly lower than of children without PIV infection (p< 0.0001; Table, SDC 1). Children less than 2 years of age were more likely to have PIV infection as compared with children 2–18 years of age (p=0.002; odds ratio 2.69; 95% CI 1.5–4.8). Children with acute lymphoblastic leukemia (ALL) were more likely to have PIV infection as compared with children with other malignancies (p<0.0001; odds ratio 4.13; 95% CI 2.37–7.21).
The majority of patients 77 (93%) were in cancer remission. Approximately one-half of the infections, 42 (51%) occurred in the first 9 months of therapy during the phases of induction, consolidation and re-intensification while the remainder, 41 (49%) occurred in the maintenance phase of therapy. All the infections occurred within 5 years from diagnosis (p<0.0001; Table, SDC 1).
Of the 83 patients, the majority 66 (80%) had URTI. The most common symptoms were cough and rhinorrhea. No child had croup caused by PIV infection. Sinusitis accompanied URTI in 3 patients and 2 patients had unilateral parotitis. LRTI was seen in 17 (20%) patients, confirmed by lung auscultation and chest radiography, with unilateral alveolar infiltrates in 3 patients and peribronchial thickening or bilateral interstitial or perihilar infiltrates in 11 patients. Bilateral nodular infiltrates were demonstrated by computerized tomography of the chest in 3 patients. LRTI was preceded by URTI in all patients. Fever with severe neutropenia (ANC<500 cells/µL) was seen in 28 (34%) patients with PIV infection. Of the 17 patients with LRTI, 10 (59%) had fever with severe neutropenia (p=0.02; Table 1). LRTI was significantly associated with ANC<500 cells/µL (p=0.002) and ALC<100 cells/µL (p=0.008; Table 1). After adjusting for potential confounders, such as admission to the intensive care unit, oxygen requirement, and mechanical ventilation, an increased risk for LRTI was associated with an ANC<500 cells/µL (p=0.01, odds ratio 5.52; 95% CI 1.29–33.74), ALC<100 cells/µL (p=0.03, odds ratio 8.0; 95% CI 1.18–63.58) and age (p=0.01). The median age for patients with LRTI was 27 months as compared with 56 months for patients with URTI (p=0.005; Table 1). PIV-3 infections were not associated with an increased incidence of LRTI (p=0.28). Of the 17 patients with LRTI, 8 (47%) received steroids in the 2 weeks prior to onset of infection as compared with 26 of 66 (39%) patients with URTI. Steroid use preceding the infection was not associated with progression to LRTI (p=0.59; Table 1). Other factors not associated with progression to LRTI included race, gender, underlying diagnosis, HAI and presence of co-pathogens.
TABLE 1.
Risk factors for LRTI from PIV infections in children with cancer
| Characteristic | LRTI n=17 |
URTI n= 66 |
p-value |
|---|---|---|---|
| Median age (months) | 27 | 56 | 0.005 |
| Fever with ANC<500 cells/µL | 10 | 18 | 0.02 |
| Absence of fever and neutropenia | 7 | 48 | |
| ANC<500 cells/µL | 14 | 26 | 0.002 |
| ANC>500 cells/µL | 3 | 40 | |
| ALC<100 cells/µL | 5 | 3 | 0.008 |
| ALC>100 cells/µL | 12 | 63 | |
| Use of steroids prior to infection | 9 | 40 | 0.59 |
| No steroid use prior to infection | 8 | 26 |
LRTI, lower respiratory tract infection; URTI, upper respiratory tract infection; PIV, parainfluenza virus; ANC, absolute neutrophil count; ALC, absolute lymphocyte count
There was no mortality attributed to PIV infection. Three patients required mechanical ventilation for respiratory failure due to PIV infection for 20, 17 and 9 days. LRTI in these patients was caused by PIV-4, PIV-1 and PIV-3, respectively. None of the patients requiring mechanical ventilation had co-pathogens detected. None of these patients received treatment with ribavirin.
Diagnosis of PIV infection
The median duration between onset of symptoms and collection of a diagnostic specimen was 2 days (range 1–14 days). The median duration of viral shedding evaluated in 62 patients was 8 days (range 2–44 days). The duration of viral shedding was not significantly different between patients who had URTI or LRTI (p=0.27). The virus was isolated by culture in all but 4 patients. In 2 of these DFA was positive. Of the 35 patients tested concurrently with culture and PCR, culture tested negative in 4 patients who were positive for PIV by PCR. Of these 4 patients, 2 were positive by DFA. The PCR test result was negative in 4 patients who were culture positive. Of these 4 patients, 3 were positive by DFA. Co-pathogens were detected in a total of 5 patients. These included RSV, CMV, HHV-6, influenza A and Klebsiella pneumoniae from respiratory specimens, in 1 patient each. Three patients had initial infection with PIV serotype 3, and a new infection with PIV serotype 1, serotype 2 and serotype 3 respectively; 30, 27 and 15 months after the first infection.
DISCUSSION
The human PIVs belong to the family Paramyxoviridae and are enveloped single stranded RNA viruses. PIV infections have been less well studied in comparison to RSV and influenza virus infections in healthy children. PIV was isolated from 286 out of 5099 (5.6%) respiratory samples collected from 1429 children over a 20 year period, using standard virologic methods.4 They were reported to cause 64% of croup, 22% of LRTI and 18% of URTI in otherwise healthy preschool children with PIV infection.4 PIV was the second most common cause of respiratory viral infection in our population after influenza (A and B). This is similar to the observation by Henrickson et al.5 who reported influenza (A and B) to be the commonest cause of respiratory viral infection in 46 (34%) of 134 immune-compromised children, followed by PIV in 45 (33%) of patients, using molecular and standard virologic methods.
PIV caused respiratory viral illness in 10% of the patients in our cohort, higher than the incidence of 6.2% reported in adult leukemia patients, using standard virologic methods, where it was isolated in 47 of 770 patients.6 PIV-3 accounted for 61% of PIV infections followed by PIV-1 in 18% and PIV-2 in 20%. PIV-4 was diagnosed in 1 patient. This is similar to the incidence in healthy children4, but contrasts with the experience in adult leukemic patients where PIV-3 accounted for 83% of the infections.6 PIV infections were more common in patients with ALL as compared with children with AML or lymphoma. They occurred as frequently in the intense phases of treatment as during remission. All infections occurred within five years after diagnosis of malignancy.
In otherwise healthy children, PIV-1and PIV-2 tends to occur biennially in the fall of odd-numbered years, while PIV-3 occurs throughout the year with a peak in spring and early summer.4 In our population, PIV maintained this seasonal and temporal distribution. Most of the infections (90%) were community-acquired. No clustering of infections or outbreaks was observed. Hospital acquired outbreaks of PIV infection have been reported in adult HSCT patients.7
LRTI was seen in 17 (20%) of the patients in our series, as compared with 26 of 47 (55%) adult leukemic patients.6 This difference may in part be attributed to the presence of co-infections which was seen in only 5 (29%) of our patients compared with 16 of 26 (62%) adults. The incidence of LRTI is similar to that seen with PIV infections in healthy children, although none of our patients developed croup which was seen in 64% of healthy children with PIV infection.4 LRTI was confirmed by the presence of bilateral infiltrates in the majority of patients and nodular infiltrates on chest CT in two patients. Multiple bilateral small nodules <10mm in diameter have been associated with a diagnosis of viral pneumonitis.8 Parotitis which was seen in two of our patients is known to be associated with PIV infection.9
LRTI was significantly associated with severe neutropenia and lymphopenia. The association of LRTI with lymphopenia has been observed in adult leukemic and HSCT patients with PIV infections.6,10 This emphasizes the importance of pulmonary cytotoxic T lymphocyte responses for clearance of respiratory viral infections.11
All patients with PIV LRTI survived in the present study as compared with a mortality of 15% in adult leukemic patients. The increased mortality in adult patients with PIV infection may be related to the presence of co-morbidities with advancing age. Lymphopenia and LRTI were significantly associated with the high mortality reported in adults.10 Use of steroids at the time of onset of infection was associated with progression to LRTI and mortality from PIV infection in studies of adult HSCT patients10 but not in our study. The only case of PIV-4 infection, in our series, required mechanical ventilation for 3 weeks and had the most severe course. PIV-4 has been shown to cause severe respiratory disease in immune-competent children12 and in an adult recipient13 of HSCT.
Infants and toddlers were more likely to have LRTI from PIV in our series, as compared with older children. This association has not been reported previously in immune-compromised patients with PIV infection. Age < 2 years was associated with progression to LRTI with RSV infection.14 An association between higher RSV viral loads and younger age has been reported.15 RSV viral load has been shown to predict disease severity in previously healthy infants.16 Further studies are needed to show if the increased incidence of LRTI in infants due to PIV is related to an increased viral load.
Febrile infections are the most common complications of therapy in children with cancer.17 Respiratory viral (RV) infections have been found in a third of the episodes in febrile children with cancer.18 In a prospective study, RV infections, including 4 episodes of PIV infection, were found in 44% of the febrile neutropenic episodes in 51 children with leukemia.19 Studies of RV infections in children with cancer have focused on febrile neutropenic patients.18,20 However, only 34% of the patients with PIV infection in our series had fever with neutropenia. Hence, PIV infections in children with cancer may be relatively underestimated if only febrile neutropenic episodes are studied.
PCR has been found to be more sensitive than culture and DFA for detection of PIV.21 Of the 35 patients tested concurrently with culture, DFA and PCR, in 4 patients the diagnosis was made by PCR alone and 4 culture-positive patients were PCR negative. In a prospective study of respiratory viral infections using molecular methods, PIV 1–3 was detected by culture and PCR in 28 and 41specimens respectively.20 In our series, 4 PIV-3 isolates were detected by culture and not by PCR. With the use of culture and DFA alone, 2 patients would have remained undiagnosed. Only 35 patients were tested by culture, DFA and PCR, hence a method comparison of the sensitivity of PCR with DFA and culture cannot be made. The small number of patients tested concurrently by both molecular and standard virologic methods, and the retrospective nature of the study, may account for the lower detection rates with PCR.
Our study has several limitations, including its retrospective nature and small number of events per variable. There is ascertainment bias, as only children with respiratory symptoms were screened, at the discretion of the treating physician.
This study represents the largest cohort of children with cancer and PIV infection studied to date. PIV was the second most common cause of respiratory viral infection in this population. The incidence of LRTI is similar to that seen with PIV infections in healthy preschool children, but lower than that seen in adults with hematological malignancies. Croup was noticeably absent in this population. No mortality was seen, in contrast to previous studies on adults with PIV infection and leukemia. Younger age, severe lymphopenia and neutropenia were associated with LRTI. Prospective studies in children with cancer and respiratory viral infections are needed to confirm the trend towards an increasing incidence. This may most likely represent an increase in the usage of modern molecular diagnostic assays.
Supplementary Material
ACKNOWLEDGEMENT
Grant funding:
This work was supported by National Cancer Institute Cancer Center CORE Support Grant P30 CA 21765 and by the American Lebanese Syrian Associated Charities.
The authors thank Mark Mestemacher from the clinical laboratory and Harry R. McKeon from the cancer center administration, at SJCRH, Memphis, TN, for assistance in data collection.
Footnotes
Presentation of the above work:
This work was presented at the 52th annual meeting of the American Society of Hematology, December 2010, Orlando, FL (Abstract #3909)
REFERENCES
- 1.Xie Y, Davies SM, Xiang Y, et al. Trends in leukemia incidence and survival in the US (1973–1998) Cancer. 2003;97:2229–2235. doi: 10.1002/cncr.11316. [DOI] [PubMed] [Google Scholar]
- 2.Hall CB, Powell KR, MacDonald NE, et al. Respiratory syncytial viral infection in children with compromised immune function. N Engl J Med. 1986;315:77–81. doi: 10.1056/NEJM198607103150201. [DOI] [PubMed] [Google Scholar]
- 3.Weinberg GA, Hall CB, Iwane MK, et al. Parainfluenza Virus Infection of Young Children: Estimates of the population-based burden of hospitalization. J Pediatr. 2009;154:694–699. doi: 10.1016/j.jpeds.2008.11.034. [DOI] [PubMed] [Google Scholar]
- 4.Reed G, Jewett PH, Thompson J, et al. Epidemiology and clinical impact of parainfluenza virus infections in otherwise healthy infants and young children <5 years old. J Infect Dis. 1997;175:807–813. doi: 10.1086/513975. [DOI] [PubMed] [Google Scholar]
- 5.Henrickson KJ, Hoover S, Kehl KS, et al. National disease burden of respiratory viruses detected in children by polymerase chain reaction. Pediatr Infect Dis J. 2004;23:S11–S18. doi: 10.1097/01.inf.0000108188.37237.48. [DOI] [PubMed] [Google Scholar]
- 6.Marcolini JA, Malik S, Suki D, Whimbey E, Bodey GP. Respiratory Disease Due to Parainfluenza Virus in Adult Leukemia Patients. Eur J Clin Microbiol Infect Dis. 2003;22:79–84. doi: 10.1007/s10096-002-0864-4. [DOI] [PubMed] [Google Scholar]
- 7.Cortez KJ, Erdman DD, Peret TC, et al. Outbreak of human parainfluenza virus 3 infections in a hematopoietic stem cell transplant population. J Infect Dis. 2001;184:1093–1097. doi: 10.1086/322041. [DOI] [PubMed] [Google Scholar]
- 8.Franquet T, Müller NL, Giménez A, et al. Infectious pulmonary nodules in immunocompromised patients: usefulness of computed tomography in predicting their etiology. J Comput Assist Tomogr. 2003;27:461–468. doi: 10.1097/00004728-200307000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Lange T, Franke G, Niederwieser D. Parotitis associated with a parainfluenza virus type 3 infection during aplasia after unrelated allogeneic stem cell transplantation. Leuk Lymphoma. 2006;47:1714–1715. doi: 10.1080/10428190600648606. [DOI] [PubMed] [Google Scholar]
- 10.Nichols WG, Corey L, Gooley T, Davis C, Boeckh M. Parainfluenza virus infections after hematopoietic stem cell transplantation: risk factors, response to antiviral therapy, and effect on transplant outcome. Blood. 2001;98:573–578. doi: 10.1182/blood.v98.3.573. [DOI] [PubMed] [Google Scholar]
- 11.Welliver TP, Garobalo RP, Hosakote Y, et al. Severe human lower respiratory tract illness caused by respiratory syncytial virus and influenza virus is characterized by the absence of pulmonary cytotoxic lymphocyte responses. J Infect Dis. 2007;195:1126–1136. doi: 10.1086/512615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindquist SW, Darnule A, Istas A, Demmler GJ. Parainfluenza virus type 4 infections in pediatric patients. Pediatr Infect Dis J. 1997;16:34–38. doi: 10.1097/00006454-199701000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Chiu CY, Rouskin S, Koshy A. Microarray Detection of Human Parainfluenzavirus 4 Infection Associated with Respiratory Failure in an Immunocompetent Adult. Clin Infect Dis. 2006;43:71–76. doi: 10.1086/507896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saleeby CM, Somes GW, DeVincenzo JP, Gaur AH. Risk factors for severe respiratory virus disease in children with cancer. The importance of lymphopenia and young age. Pediatrics. 2008;121:235–243. doi: 10.1542/peds.2007-1102. [DOI] [PubMed] [Google Scholar]
- 15.Kuypers J, Wright N, Morrow R. Evaluation of quantitative and type specific real time RT-PCR assays for detection of respiratory syncytial virus in respiratory specimens from children. J Clin Virol. 2004;31:123–129. doi: 10.1016/j.jcv.2004.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeVincenzo JP, Saleeby CM, Bush AJ. RSV viral load predicts disease severity in previously healthy infants. J Infect Dis. 2005;191:1861–1868. doi: 10.1086/430008. [DOI] [PubMed] [Google Scholar]
- 17.Pizzo PA. Fever in immunocompromised patients. N Engl J Med. 1999;341:893–900. doi: 10.1056/NEJM199909163411207. [DOI] [PubMed] [Google Scholar]
- 18.Arola M, Ruuskanen O, Ziegler T, et al. Respiratory virus infections during anticancer treatment in children. Pediatr Infect Dis J. 1995;14:690–694. doi: 10.1097/00006454-199508000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Koskenvuo M, Möttönen M, Rahiala J, et al. Respiratory Viral Infections in Children With Leukemia. Pediatr Infect Dis J. 2008;27:974–980. doi: 10.1097/INF.0b013e31817b0799. [DOI] [PubMed] [Google Scholar]
- 20.Möttönen M, Uhari M, Lanning M, et al. Prospective survey of viral infections in children with acute lymphoblastic leukemia during chemotherapy. Cancer. 1995;75:1712–1717. doi: 10.1002/1097-0142(19950401)75:7<1712::aid-cncr2820750724>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 21.Weinberg GA, Erdman DD, Edwards KM, et al. Superiority of Reverse-Transcription Polymerase Chain Reaction to Conventional Viral Culture in the Diagnosis of Acute Respiratory Tract Infections in Children. J Infect Dis. 2004;189:706–710. doi: 10.1086/381456. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.