Abstract
A short, high yielding protocol has been developed for the highly diastereoselective (dr >20:1) and general synthesis of primary β-fluoroamines by the enantioselective α-fluorination of aldehydes, conversion into the N-sulfinyl aldimine, nucleophilic addition of various organometallic species and 1° amine liberation.
β-Fluoroamines are common moieties in drug candidates as the β-fluorine atom can lower pKa (diminish ancillary pharmacology), enhance binding interactions, improve metabolic stability and increase CNS penetration.1–6 Despite their value, few methods exist for the general and stereoselective synthesis of primary β-fluoroamines outside of the classical DAST approaches which are plagued with rearranged and dehydrated products.7–10 Alternate routes have emerged based on the diastereoselective reduction of α-fluoroimines, hydrofluorination of epoxides with further manipulation to provide β-fluoroamines, or tandem conjugate addition-fluorination sequences; however, all of these routes lack generality (inability to incorporate diversity) or display preference for either the anti or syn products.11–14
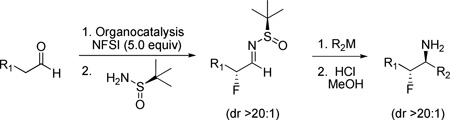
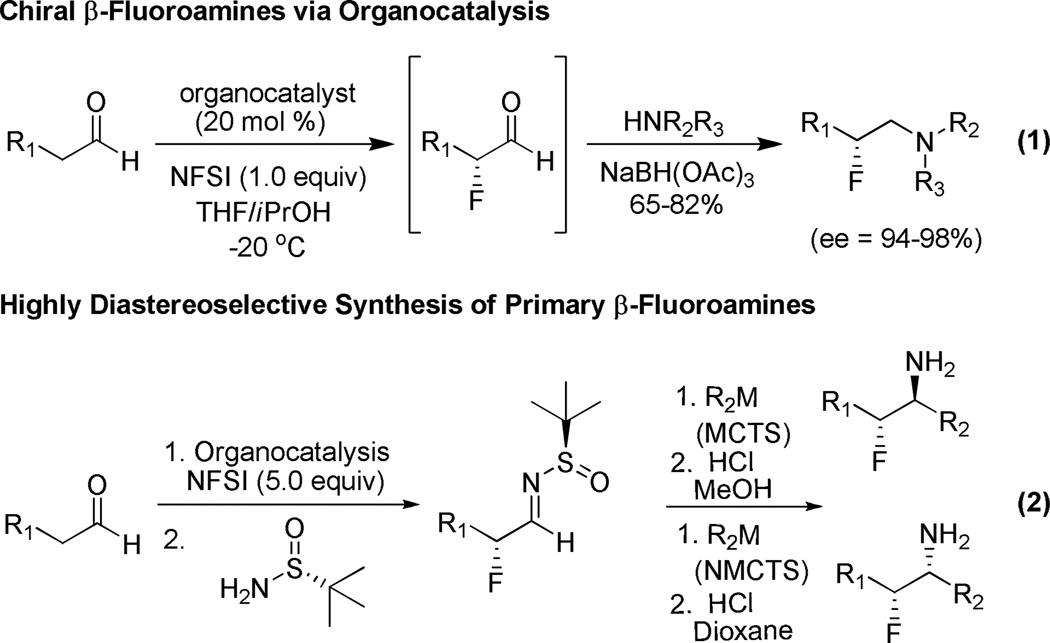
Recently, we reported a one-pot protocol to provide pharmaceutically relevant chiral β-fluoroamines via organocatalysis (Figure 1, eq 1).15 In this Letter, we describe a novel methodology for the general synthesis of primary β-fluoroamines in high yield and diasteroselectivity employing organocatalytic α-fluorination of aldehydes,16 Ellman N-sulfinyl aldimine technology,17–21 nucleophilic addition of organometallic species22–25 and 1° amine liberation (Figure 1, eq 2). This methodology affords complete control of the two contiguous stereogenic centers based on the chirality of the organocatalyst and sulfimine employed as well as on the transiton state (metal-chelate versus non-metal chelate transition states, MCTS and NMCTS, respectively) that the organometallic species adopts.
Figure 1.
Organocatalytic Approach to Chiral β-Fluoroamines and Envisioned Route to Chiral Primary β-Fluoroamines.
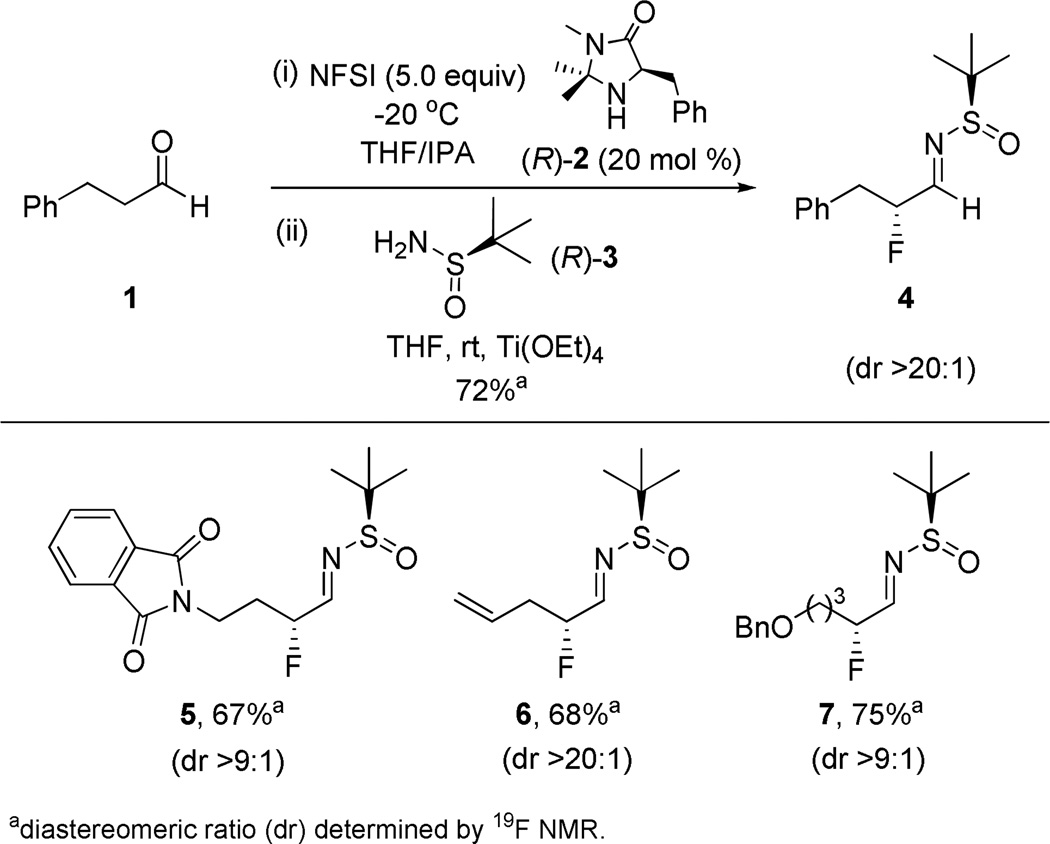
Key to the success of this approach is the ability to trap the incipient chiral α-fluoroaldehyde with the Ellman tert-butanesulfonamide to form the corresponding α-fluoro-N-sulfinylaldimine in high diastereomeric ratio (dr). A number of conditions were surveyed (time, dessicant, equivalents of NFSI) for the conversion of hydrocinnamaldehyde 1 to β-fluoro-N-sulfinylaldimine 4 (Scheme 1). Ultimately, we found that 5.0 equivalents of NFSI, 20 mol% (R)-2, Ti(OEt)4 as dessicant with (R)-tert-butanesulfinamide 3 and a five hour reaction time was optimial to deliver 4 in >20:1 dr (as judged by 19F NMR). Employing these conditions, a variety of diverse aldehydes could be converted into their corresponding β-fluoro-N-sulfinylaldimines 5–7 in good isolated yield (67–75%) for the two steps and with up to >20:1 d.r. (Figure 1). Here, substrates with handles for further elaboration were tolerated, such as the phthalimide congener 5, a progenitor for basic amine analogues, the olefin in 6 for subsequent manipulations and a benzyl protected alcohol congener 7.
Scheme 1.
Optimization of α-Fluoro-N-Sulfinyl Aldimine 4 Formation and Substrate Scope.
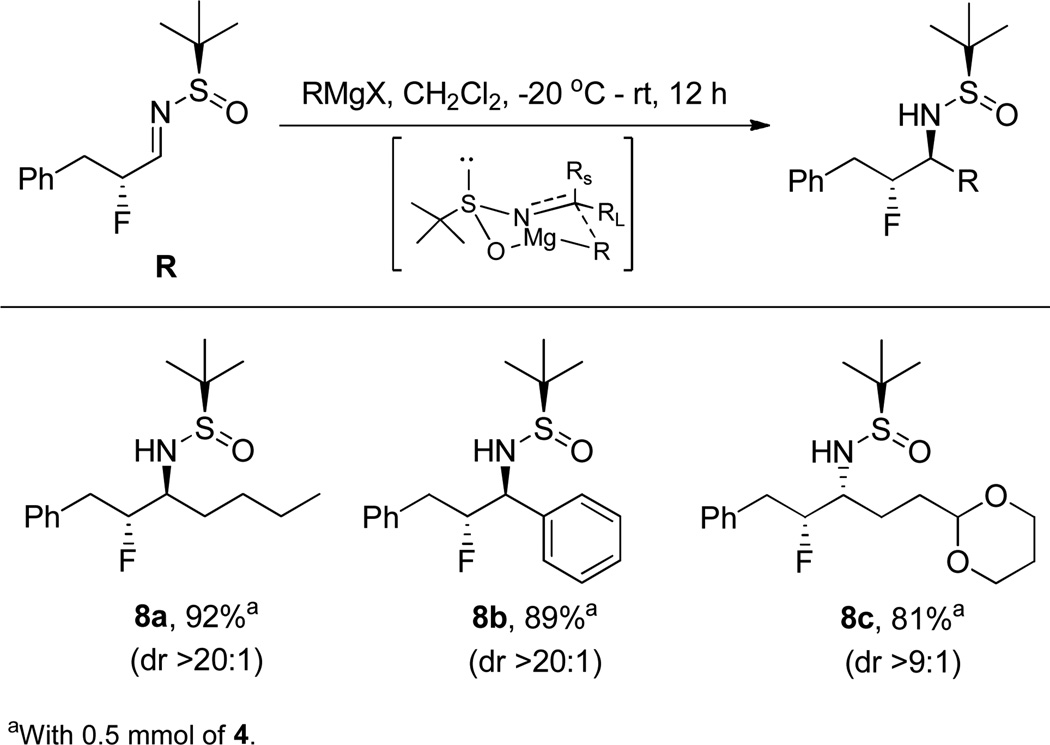
With α-fluoro-N-sulfinylaldimine 4 in hand, we examined the additon of a number of organometallic nucleophiles to add unprecendented diversity to the primary β-fluoroamine scaffold and access derivatives previously unavailable. We first explored Grignard reagents (Scheme 2), and found a wide range of diverse R groups were tolerated affording the sulfinamide-protected primary β-fluoroamines 8a–c in good isolated yields (72–92%) and >20:1 dr (as determined by 19F NMR).22 The anti-diastereomer of the β-fluoroamine formed predominantly as expected due to the known metal-chelated transition state (see inset under arrow) for 8a and 8b. The syn-diastereomer was formed in the case of 8c, as a result of additional coordination to the acetal oxygen, in accordance with the work of Ellman.23
Scheme 2.
Scope of Grignard Additon to 4 to Deliver Sulfinamide-Protected Primary β-Fluoroamines 8a–c.
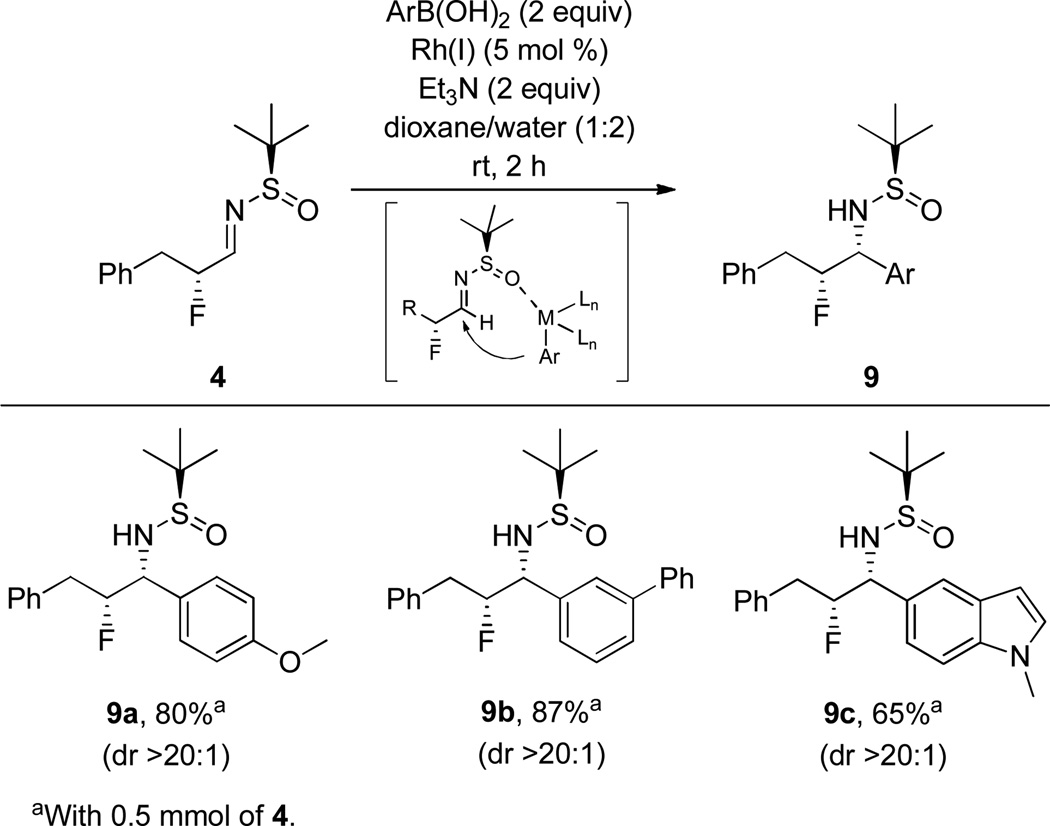
Due to the extensive diversity and availability of functionalized boronic acids, we next explored boronic acid additions. Under Batey’s mild Rh(I) catalysis protocol,24 a diverse group of aryl boronic acids (electron-rich, biaryl, and heterocyclic congeners), smoothly afforded the target adducts 9a–c in 65–91% yield and once again in >20:1 dr (Scheme 3). In this instance, the syn-diastereomers of the β-fluoroamines are formed based on the known addition of the aryl rhodium species through a non-metal chelated transition state.24
Scheme 3.
Scope of Boronic Acid Additon to 4 to Deliver Sulfinamide-Protected Primary β-Fluoroamines 9a–e.
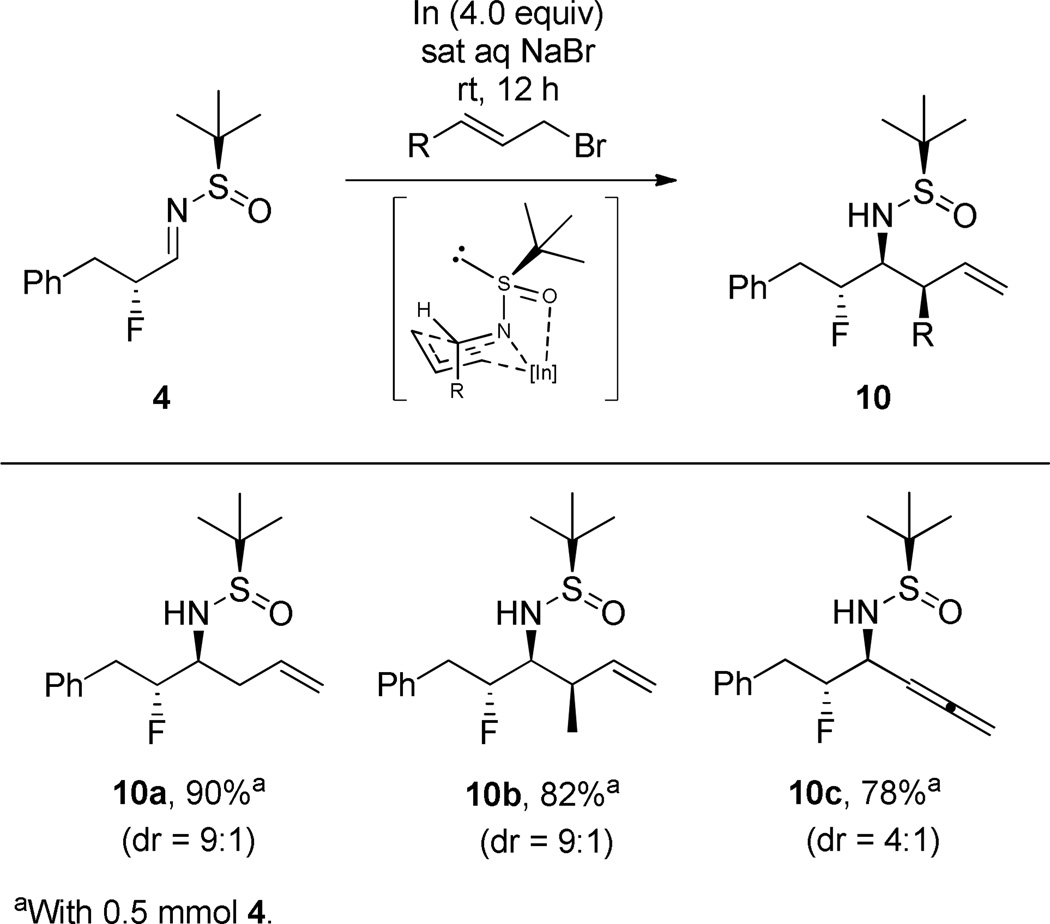
Lin and co-workers previously demonstrated that indium-mediated allylations (In(0), allyl bromide in aqueous NaBr) of (R)-N-tert-butanesulfinylimines provides the (S)-adducts as confirmed by single X-ray crystallography.25 To further add structural diversity to the primary β-fluoroamine scaffolds accessible through this approach, we employed Lin’s protocol with our β-fluoro-N-sulfinylaldimine 4 (Scheme 4). As expected, anti-diastereomers of the sulfinamide-protected β-fluoroamine products 10 were produced in good yields (66–90%) and up to 9:1 dr. Here, we could install additional stereogenic centers (10b) and allene moieties (10c).
Scheme 4.
Scope of In-Mediated Allylation of 4 to Deliver Sulfinamide-Protected Primary β-Fluoroamines 10a–c.
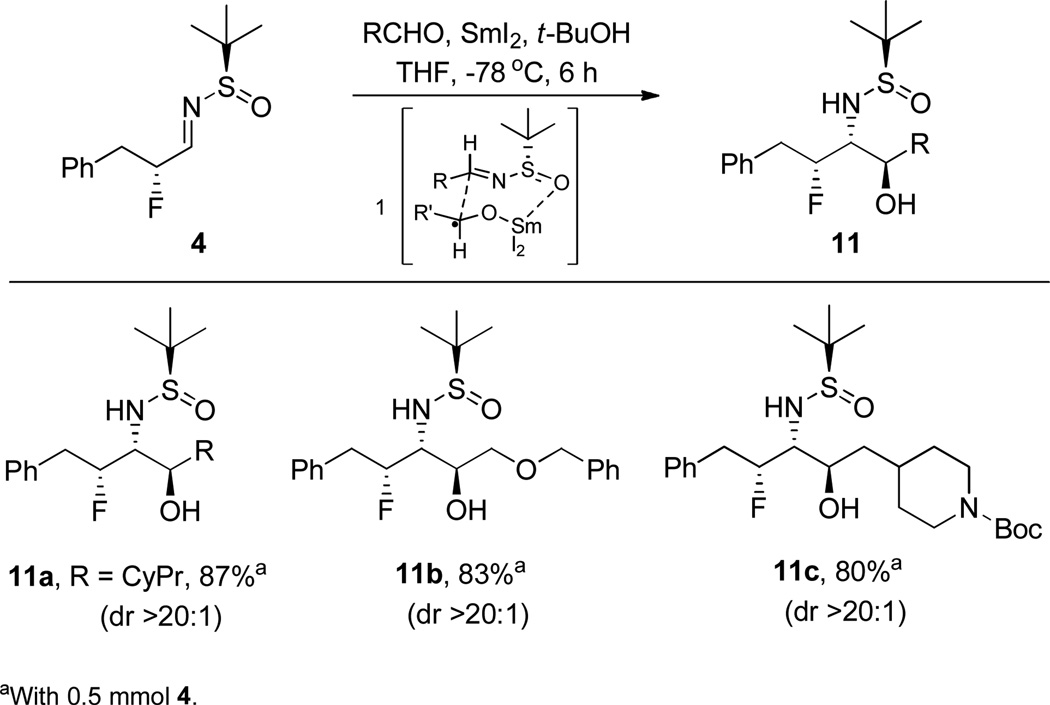
We also hoped to expand this protocol to include β-amino-alcohols. Following the work of Xu and Lin,26 the SmI2-induced reductive aldehyde cross-coupling with our β-fluoro-N-sulfinylaldimine 4 (Scheme 5) produced the target sulfinamide-protected primary β-fluoro-β’-amino-alcohols 11a–c in excellent yields (80–87%) and once again with high dr (>20:1).
Scheme 5.
Scope of SmI2-Induced Reductive Aldehyde Coupling of 4 to Deliver Sulfinamide-Protected Primary β-Fluoro-β’-Amino Alcohols 11a–c.
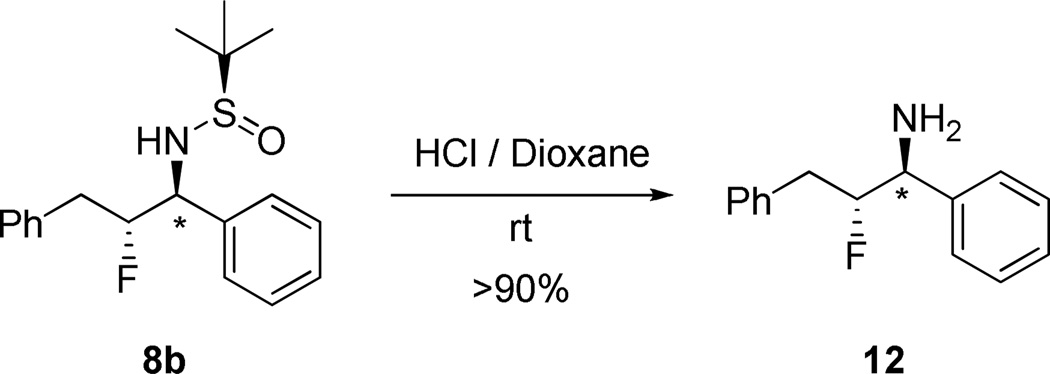
An attractive feature of the Ellman sulfinamide-protected primary amines is the generality and ease of deprotection.17–26 Treatment of a representative example 8b with HCl in dioxane at room temperature smoothly affords the desired primary β-fluoroamine congener 12 in greater than 90% yield and without loss of sterochemical integrity (Scheme 6). In our drug discovery efforts, we have deprotected numerous congeners of 8b, and have never observed issues with stereochemical integrity or clean deprotections.
Scheme 6.
Deprotection to Afford 1° β-Fluoroamine 12
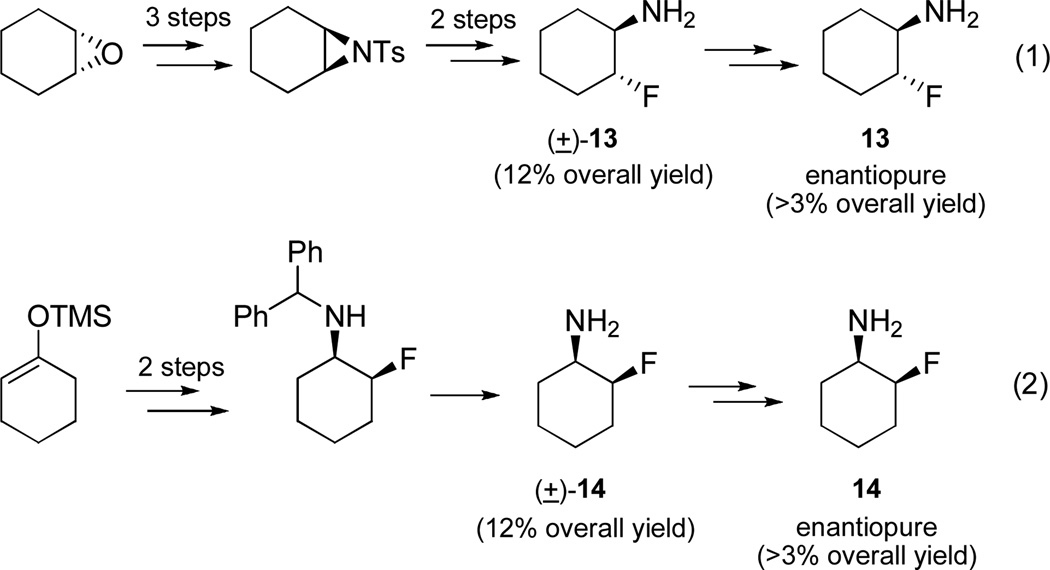
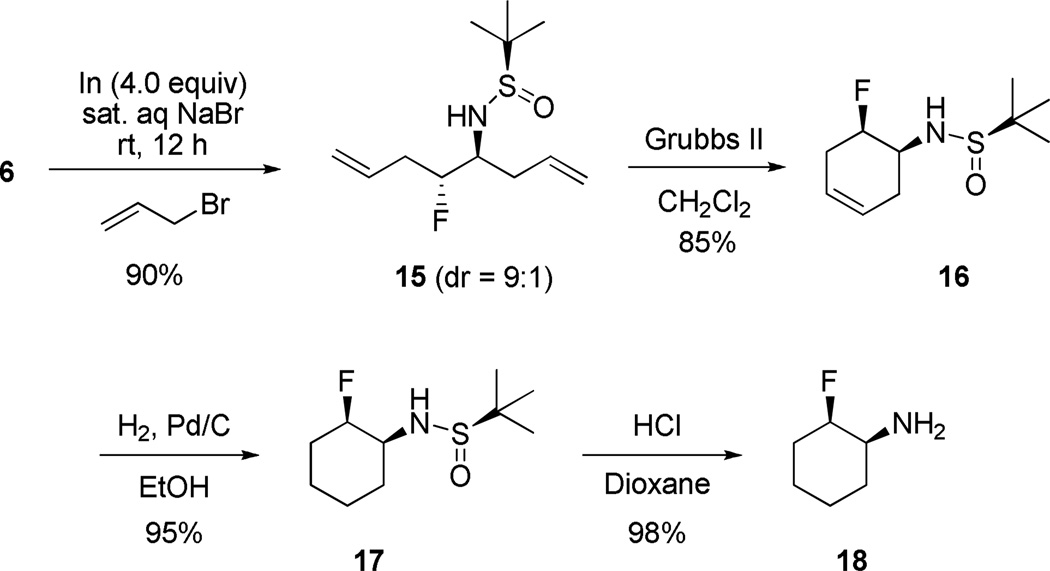
Finally, we wanted to apply this methodology to cyclic primary β-fluoroamines, as this pharmacophore has proven to be an important component of therapeutics, such as BACE inhibitors.27,28 Classically, cyclic, racemic trans-primary β-fluoroamines (±)-13 are prepared by the ring opening of aziridines with nucleophilic fluoride, followed by deprotection (Figure 2, eq 1).8,27,28 Cyclic, racemic cis-primary β-fluoroamines (±)-14 are prepared by fluorination of a silyl enol ether, followed by reductive amination and deprotection (Figure 2, eq 1).27,28 Overall, yields are modest to low (average 12% overall) for the racemic, cyclic primary β-fluoroamines and require several steps. In order to access enantiopure derivatives, heroic chiral chromatography is performed on either the free amine, or more likely, a protected form that introduces a chromophore, followed by chiral chromatography and deprotection. Ultimately, pure enantiomers of trans-13 and cis-14 are arrived at in typically less than 3% overall yield. In contrast, our methodology can provide rapid, high yielding access to either cis- or trans-cyclic primary β-fluoroamines with high enantioselectivity based on the chirality of the organocatalyst and the sulfinylamine employed. For example (Scheme 7), an indium-mediated allylation of 6 with allyl bromide gives 15 in 90% yield and 9:1 dr (if allyl Grignard employed, comparable yield, but >20:1 dr). Ring closing metathesis with Grubbs II affords cyclohexene 16 in 85% yield. Hydrogenation and standard acid-mediated deprotection provides chiral cyclic primary β-fluoroamine 18 in 70% overall yield for the four step sequence, a notable improvement over the classical route. The relative stereochemistry of 18 was confirmed by correlation with previously reported 19F NMR shifts for cis- (−197 ppm) and trans-cyclic β-fluoroamines (−180 ppm), wherein 18 dsiplayed a singlet at −198 ppm.29
Figure 2.
Standard Synthetic Route to trans- and cis-Racemic and Enantiopure Cyclic Primary β-Fluoroamines.
In summary, we have developed a highly diastereoselective and general synthesis of primary β-fluoroamines using a combination of organocatalysis and Ellman N-sulfinylaldimine technology to enable the preparation of all possible stereoisomers in high isolated yields. Importantly, this methodology provides access to primary β-fluoramine chemotypes that are generally not accessible, or difficult to prepare, through current chemistries, as well as providing a significant improvement for the synthesis of cyclic primary β-fluoroamines. Further refinements are in progess and will be reported in due course.
Supplementary Material
Scheme 7.
Access to Chiral, Cyclic 1° β-Fluoroamine 18.
Acknowledgment
This work was supported, in part, by the Department of Pharmacology and Vanderbilt University. Funding for the NMR instrumentation was provided in part by a grant from NIH (S10 RR019022).
Footnotes
Supporting Information Available: Experimental procedures, characterization data, chiral HPLC traces and 1H, 13C and 19F NMR spectra for all new compounds. This material is available free of charge via the internet at http://pubs.acs.org.
References
- 1.Hagmann WK. J. Med. Chem. 2008;51:4359–4369. doi: 10.1021/jm800219f. [DOI] [PubMed] [Google Scholar]
- 2.Muller K, Mainfisch P, Altmann KH, Schlosser M. ChemBio-Chem. 2004;5:559–572. [Google Scholar]
- 3.Bohm H-J, Banner D, Bendels S, Kansy M, Kuhn B, Muller K, Obst-Sander U, Stahl M. ChemBioChem. 2004;5:637–643. doi: 10.1002/cbic.200301023. [DOI] [PubMed] [Google Scholar]
- 4.Kirk KL. Curr. Top. Med. Chem. 2006;6:1445–1543. doi: 10.2174/156802606777951073. [DOI] [PubMed] [Google Scholar]
- 5.Kirk KL. Curr. Top. Med. Chem. 2006;6:1013–1029. doi: 10.2174/156802606777951073. [DOI] [PubMed] [Google Scholar]
- 6.Morgenthaler M, Schweizer E, Hoffman-Roder F, Benini F, Martin RE, Jaeschke G, Wagner B, Fischer H, Bendels S, Zimmerili D, Schneider J, Hiedrich F, Kansy M, Muller K. ChemMedChem. 2007;2:1100–1115. doi: 10.1002/cmdc.200700059. [DOI] [PubMed] [Google Scholar]
- 7.Percy JM. Sci. Synth. 2005;34:379–416. [Google Scholar]
- 8.Alvenrhe GM, Lacombe S, Laurent AJ. Tetrahderon Lett. 1980;21:289–293. [Google Scholar]
- 9.Thiabaudeau S, Martin-Mingot A, Jouannetaud M-P, Karam O, Zunino F. Chem. Commun. 2007:3198–3200. doi: 10.1039/b703629a. [DOI] [PubMed] [Google Scholar]
- 10.Hsin L-W, Chang L-T, Rothman RB, Dersch CM, Jacobson AE, Rice KC. J. Med. Chem. 2008;51 doi: 10.1021/jm701270n. 2795-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Creswell AJ, Davies SG, Lee JA, Roberts PM, Russell AJ, Thomson JE, Tyte MJ. Org. Lett. 2010;12:2936–2939. doi: 10.1021/ol100862s. [DOI] [PubMed] [Google Scholar]
- 12.Duggan PJ, Johnston M, March TL. J. Org. Chem. 2010;75:7365–7372. doi: 10.1021/jo101600c. [DOI] [PubMed] [Google Scholar]
- 13.Appayee C, Brenner-Moyer SE. Org. Lett. 2010;12:3356–3359. doi: 10.1021/ol101167z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malamakal RM, Hess WR, Davis TA. Org. Lett. 2010;12:2186–2189. doi: 10.1021/ol100647b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fadeyi OO, Lindsley CW. Org. Lett. 2009;11:943–946. doi: 10.1021/ol802930q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beeson TD, MacMillan DWC. J. Am. Chem. Soc. 2005;127:8826–8828. doi: 10.1021/ja051805f. [DOI] [PubMed] [Google Scholar]
- 17.Cogan DA, Ellman JA. J. Am. Chem. Soc. 1999;121:269–269. [Google Scholar]
- 18.Liu G, Cogan DA, Owens TD, Tang TP, Ellman TP. J. Org,. Chem. 1999;64:1278–1284. [Google Scholar]
- 19.Tang TP, Ellman JA. J. Org. Chem. 2002;67:7819–7832. doi: 10.1021/jo025957u. [DOI] [PubMed] [Google Scholar]
- 20.Weix DJ, Shi Y, Ellman JA. J. Am. Chem. Soc. 2005;127:1092–1093. doi: 10.1021/ja044003d. [DOI] [PubMed] [Google Scholar]
- 21.Brak K, Ellman JA. J. Am. Chem. Soc. 2009;131:3850–3851. doi: 10.1021/ja9002603. [DOI] [PubMed] [Google Scholar]
- 22.Brinner KM, Ellman JA. Org. Biomol. Chem. 2005;3:2109–2113. doi: 10.1039/b502080h. [DOI] [PubMed] [Google Scholar]
- 23.Cogan DA, Liu G, Ellman JA. Tetrahedron. 1999;55:8883–8904. [Google Scholar]
- 24.Bolshan Y, Batey RA. Org. Lett. 2005;7:1481–1484. doi: 10.1021/ol050014f. [DOI] [PubMed] [Google Scholar]
- 25.Sun X-W, Liu M, Xu M-H, Lin G-Q. Org. Lett. 2008;10:1259–1262. doi: 10.1021/ol8001514. [DOI] [PubMed] [Google Scholar]
- 26.Zhong Y-W, Dong Y-Z, Fang K, Izumi K, Xu M-H, Lin G-Q. J. Am. Chem. Soc. 2005;127:11956–11957. doi: 10.1021/ja054401w. [DOI] [PubMed] [Google Scholar]
- 27.Merck & Co. 2007 WO011810. [Google Scholar]
- 28.Merck & Co: WO011833. 2007 [Google Scholar]
- 29.Toulgui Ch, Chaabouni MM, Baklouti K. J. Fluorine. Chem. 1990;46:385–391. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.