Abstract
Context
Metabolic abnormalities such as hypertriglyceridemia remain a challenge for optimizing long-term health in HIV-infected patients.
Objective
Elevation of free fatty acids (FFAs) may contribute to hyperlipidemia and insulin resistance in HIV. We evaluated the efficacy and safety of chronic inhibition of lipolysis in HIV-infected men and women with hypertrigyceridemia. We hypothesized that acipimox would lead to significant reductions in triglycerides and improved insulin sensitivity, compared with placebo.
Design
A 3-month, randomized, double-blind, controlled trial of acipimox (250 mg thrice daily) vs. placebo was conducted in 23 HIV-infected men and women with hypertriglyceridemia (>150 mg/dl), abnormal fat distribution, and no current lipid-lowering therapy. The primary outcome variable was triglyceride concentration, and insulin sensitivity measured by hyperinsulinemic euglycemic clamp was a secondary outcome.
Setting
The study was conducted at an academic medical center.
Results
Acipimox resulted in significant reductions in FFAs [mean change −0.38 (0.06) vs. 0.08 (0.06) mEq/liter with placebo, −68 vs. +17% change from mean baseline, P < 0.0001], decreased rates of lipolysis (P < 0.0001), and a median triglyceride decrease from 238 mg/dl at baseline to 190 mg/dl, compared with an increase from 290 to 348 mg/dl in the placebo group (P = 0.01). Acipimox improved insulin sensitivity [acipimox +2.31 (0.74) vs. placebo −0.21 (0.90) mg glucose per kilogram lean body mass per minute, or +31 vs. −2% change from mean baseline values, P = 0.04]. Improvements in insulin sensitivity were significantly correlated with reductions in FFAs (r = −0.62, P = 0.003) and lipolysis (r = −0.59, P = 0.005).
Conclusions
Acipimox resulted in significant sustained reductions in lipolysis, improved glucose homeostasis, and significant but modest reductions in triglycerides in HIV-infected individuals with abnormal fat distribution and hypertriglyceridemia. Improvement in overall metabolic profile with acipimox suggests a potential clinical utility for this agent that requires further investigation.
There is an increased risk of cardiovascular disease associated with HIV infection and the use of antiretroviral therapy, and hyperlipidemia is one important contributing factor to this risk (1). Dyslipidemia is likely multifactorial and has been associated with HIV infection as well as the different components of antiretroviral therapy (2–6). However, attempts to achieve recommended lipid reductions with 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors and fibrates in HIV-infected patients on antiretroviral therapy have not been successful for the majority of patients (7).
One potential contributing factor to dyslipidemia in HIV-infected patients with lipodystrophy may be inappropriately elevated rates of lipolysis and increased circulating free fatty acids (FFAs) (8–12). Furthermore, dyslipidemia often coexists with insulin resistance in HIV-infected patients (13). Previously we demonstrated that individuals with HIV infection and abnormal fat distribution have increased levels of circulating FFAs, which are associated with visceral adiposity and predictive of insulin resistance (9). Acute inhibition of lipolysis and subsequent lowering of circulating FFAs improves insulin sensitivity in lean and obese adults (14, 15). In prior work, we found that acute inhibition of lipolysis in patients with HIV-associated lipodystrophy led to improved insulin sensitivity (16), but chronic administration of lipolytic blocking agents for hyperlipidemia has not been evaluated in the HIV-infected population.
Acipimox, a nicotinic acid analog and a potent inhibitor of lipolysis, is an established available therapy for dyslipidemia outside the United States, with known efficacy in the setting of familial hypercholesterolemia (17) and in association with type II diabetes mellitus (18, 19). For example, Fulcher et al. (18) showed improvement in lipids, as well as insulin action, in nonobese type 2 diabetics with acipimox. As a lipolytic blocking agent, acipimox results in decreases in circulating FFAs, which may in turn result in improvement in insulin sensitivity (20). In a recent study of individuals with a strong family history of type 2 diabetes mellitus, Bajaj et al. (20) found a significant correlation between reductions in plasma FFAs and improvement in whole-body glucose disposal after 7 d of acipimox administration.
We hypothesized that chronic administration of acipimox would improve hypertriglyceridemia and insulin sensitivity among HIV-infected patients experiencing metabolic disturbances. We completed a 3-month randomized, double-blind, placebo-controlled trial of acipimox in HIV-infected patients with hypertriglyceridemia and abnormal fat distribution. Acipimox administration resulted in significant improvements in triglycerides, sustained reductions in lipolysis, and improved glucose homeostasis.
Subjects and Methods
Subjects
Between July 2003 and November 2005, 80 HIV-infected men and women were screened for possible participation in the study. Eligible subjects were between 18 and 65 yr of age with documented HIV infection, no change in antiretroviral therapy for longer than 3 months, fasting triglyceride level greater than 150 mg/dl, and evidence of abnormal fat distribution. Abnormal fat distribution or lipodystrophy was defined as evidence of one or more of the following: increased abdominal or cervical fat and/or decreased sc fat of the face, arms, or legs based on physical exam and judged by a single investigator (J.L.). Subjects were excluded for current therapy with a lipid-lowering medication or treatment with these agents in the 3 months before enrollment, current use of hormone replacement therapy, oral contraceptives for women or supraphysiologic testosterone therapy in men, fasting triglycerides greater than 1000 mg/dl, active alcohol or substance abuse, active peptic ulcer disease, history of renal failure or serum creatinine greater than 1.5 mg/dl, serious opportunistic infection within the past 3 months, hemoglobin less than 11.0 g/dl, elevated transaminase levels (aspartate aminotransferase or alanine aminotransferase > 2 × the upper limit of normal), previously diagnosed diabetes mellitus, or receiving current treatment for diabetes. Written informed consent was obtained from all participants before study testing in accordance with the Massachusetts General Hospital and Massachusetts Institute of Technology institutional review boards. All research testing and visits were conducted at the Massachusetts Institute of Technology Clinical Research Center and in the Massachusetts General Hospital Radiology Department.
Study design
After eligibility was established, participants completed a detailed metabolic assessment before randomization and again 3 months after randomization. After the baseline studies, subjects were randomly assigned to acipimox (250 mg by mouth three times per day) or an identical placebo. Acipimox was provided by Pharmacia Italia (Milan, Italy) and used under a Food and Drug Administration Investigational New Drug (IND no. 59,526). Randomization was stratified by age (< vs. ≥ 40 yr) and sex. The MGH research pharmacy prepared and labeled all study medication; the protocol staff and subjects were unaware of treatment assignment during the entire study.
Fasting determination of blood lipids, hemoglobin, liver transaminase levels, nonesterified fatty acids, as well as CD4 count were obtained at baseline and 3 months. In addition, insulin sensitivity and lipolysis were determined by hyperinsulinemic euglycemic clamp testing and coadministration of stable isotope tracers. Assessment of lipolysis and clamp testing were completed after a 12-h overnight fast. The tracer infusion was started at 0700 h and continued for the remainder of study (180 min in the basal state and 120 min during the insulin clamp study). 2H5-glycerol, dissolved in normal saline, was administered at a rate of 0.11 µmol/kg·min for 300 min after a priming dose of 1.6 µmol/kg over 1–2 min. At 180 min, subjects received a primed infusion of 40 mU/m2·min of regular insulin (Humulin; Eli Lilly and Co., Indianapolis, IN) for 2 h. A variable rate of 20% dextrose was adjusted every 5 min as needed to maintain blood glucose levels at 5.0 mmol/liter (90 mg/dl). Retrograde iv blood samples obtained from a heated hand were used to approximate arterialized blood for determinations of glucose levels. Insulin sensitivity was calculated as M (milligrams glucose per kilogram lean body mass per minute) according to the technique of DeFronzo et al. (21) using data obtained during the final 20 min of the clamp procedure. The rate of appearance of glycerol (Ra glycerol) was calculated at two time points using the average of three to four samples taken every 10 min in the last 30 min of the 3-h basal state infusion and again in the last 20 min of the 2-h insulin clamp. Calculations were based on estimates of steady state using the following formula: Ra glycerol = F/(rsa – rbk) (1–0.00015)5 where F = infusion rate of tracer, rsa = enrichment in the sample at steady-state, and rbk = background enrichment of the sample before tracer administration (22). One subject (assigned to placebo) did not have clamp testing performed due to limited iv access.
Body composition evaluation
Computed tomography scans were used to measure sc and visceral abdominal fat cross-sectional area. A single-slice abdominal computed tomography scan at the level of the L4 pedicle was performed at baseline and 3 months. Scan parameters for each image were standardized (144 table height, 80 kV, 70 mA, 2 sec, 0.25 cm × 4-slice thickness, 48 field of view). Fat attenuation coefficients were set at −50 to −250 HU as described by Borkan et al. (23). Dual-energy x-ray absorptiometry (DEXA) was used to measure lean body mass for clamp calculations and fat mass (QDR 4500A; Hologic Inc., Bedford, MA).
Intramyocellular lipid (IMCL) content of the tibialis anterior was determined by 1H-MRS (magnetic resonance spectroscopy) in 20 subjects (10 in each treatment arm) at baseline and 3 months after randomization. All scans were performed using a 1.5-T system (Signa LX, version 8.3; GE Medical Systems, Milwaukee, WI), and fitting of all 1H-MRS data were performed using LCModel software (version 6.0-2; S. Provencher, Oakville, Ontario, Canada) running on a Linux (RedHat, Raleigh, NC) workstation. A detailed description of the technique for 1H-MRS data acquisition and analysis was reported previously (24, 25).
Subjects completed 4-d food records (Minnesota Data Nutrition Systems, Minneapolis, MN) and underwent indirect calorimetry (Deltatrac II; Sensormedics, Yorba Linda, CA) to determine overall nutritional status at baseline and 3 months. Weight, height, and waist to hip ratio (WHR) were measured at each visit. In addition to the baseline and 3-month assessments, participants were evaluated at 1 and 2 months after randomization. Subjects were assessed for potential side effects, and blood samples were obtained for fasting lipid profile, creatinine, and liver transaminases. Compliance with study medication was determined by pill count at each visit.
Bioassays
Total cholesterol, high density lipoprotein cholesterol, triglyceride, glucose, creatinine and alanine aminotransferase were measured using standard techniques. Insulin levels were measured in serum using RIA (Diagnostic Products Corp. Los Angeles, CA). Intra- and interassay coefficients of variation range from 3.1–9.3 and 4.9–10.0%, respectively. CD4+ count was determined by flow cytometry (Becton Dickinson Biosciences, San Jose, CA). Nonesterified fatty acids were measured with an in vitro enzymatic colorimetric assay kit (Wako Chemicals USA, Richmond, VA). The intraassay coefficients of variation for fatty acids ranged from 1.1 to 2.7%. Low-density lipoprotein cholesterol was measured directly (Genzyme Diagnostics, Cambridge, MA).
Statistical analyses
Baseline characteristics were compared between randomization groups using Student’s t test and χ2 statistics for noncontinuous variables. Intent-to-treat analyses were performed. For the one subject who did not complete the study, the data from an early termination visit were used for end-of-treatment values; however, an insulin clamp study and body composition assessments were not completed at this early termination visit. Triglyceride levels are presented as the median and inter-quartile range (IQR) due to nonnormal distribution. IMCL data were log transformed before analysis to approximate a normal distribution. The primary end point for the study was triglyceride concentration, and a repeated-measures analysis of covariance with baseline values as a covariate was used to evaluate the effect of treatment assignment on triglyceride levels after randomization over time. For each variable representing a secondary end point, change values from baseline to 3 months were calculated, and a t test was used to compare the difference in change scores between treatment groups. Univariate correlation coefficients were calculated between continuous variables to determine the relationship between changes in lipolysis, lipids, and insulin sensitivity. All values are presented as mean (se) unless otherwise indicated, and a two-level alpha of 0.05 was used to determine statistical significance. All statistical analyses were performed on SAS JMP software, version 6.0 (SAS Institute, Inc., Cary, NC).
Results
Thirty-one of the 80 subjects screened were deemed eligible for inclusion in the study, seven declined participation before randomization, and 24 were randomized. During the course of the study, one participant was noted to be using known lipid-lowering therapy (omega-3 fish oil and flax seed oil) and therefore was not included in the data analysis. No other subject was taking similar supplements. One subject randomized to placebo developed a creatinine level greater than 1.5 mg/dl, an a priori exclusion criterion, and therefore, participation was discontinued after approximately 2 months. There were no other dropouts or subjects lost to follow-up.
The baseline clinical characteristics of the two treatment groups are summarized in Tables 1 and 2. Both groups were similar in age, sex, duration of HIV infection, protease inhibitor exposure, and CD4 T cell count. The subject group assigned to acipimox had a higher body mass index (BMI), compared with the group assigned to placebo, which was associated with greater sc abdominal adipose tissue area. However, the two groups did not differ with respect to baseline triglycerides [median baseline for placebo 290 mg/dl, IQR 194–478, and for acipimox 238 mg/dl, IQR 139–300], FFA concentration, rates of lipolysis, or measures of insulin sensitivity.
TABLE 1.
Baseline clinical characteristics
| Placebo (n = 12) |
Acipimox (n = 11) |
P value | |
|---|---|---|---|
| Age (yr) | 46 (3) | 45 (2) | 0.7 |
| Sex (male/female) | 8/4 | 9/2 | 0.4 |
| Duration HIV (yr) | 11 (1) | 11 (1) | 0.8 |
| Current PI use (n) | 8 | 4 | 0.1 |
| PI (months) | 54 (9) | 43 (14) | 0.5 |
| CD4 T cell count (cells/mm3) | 417 (95) | 428 (61) | 0.9 |
| BMI (kg/m2) | 24 (1) | 29 (2) | 0.01 |
Values represent mean (SEM); P values represent result of Student’s t test between groups, except for categorical variables in which χ2 statistics were used. PI, Protease inhibitor.
TABLE 2.
Metabolic and body composition parameters: baseline and 3 months after acipimox vs. placebo
| Baseline visit | Mean and mean change at 3 months |
|||||
|---|---|---|---|---|---|---|
| Placebo | Acipimox | P valuea | Placebo | Acipimox | P valueb | |
| Lipid, glucose, lipolysis, and insulin resistance | ||||||
| Total cholesterol (mg/dl) | 220 (11) | 183 (12) | 0.03 | 218 (14) | 178 (11) | |
| Mean change from baseline | −2 (8) | −6 (6) | 0.7 | |||
| LDL cholesterol (mg/dl) | 120 (8) | 103 (9) | 0.2 | 115 (8) | 106 (10) | |
| Mean change from baseline | −5 (7) | +3 (5) | 0.4 | |||
| HDL cholesterol (mg/dl) | 35 (2) | 31 (3) | 0.3 | 34 (2) | 34 (5) | |
| Mean change from baseline | −2 (1) | +3 (2) | 0.07 | |||
| Fasting glucose (mg/dl) | 76 (3) | 82 (4) | 0.3 | 85 (4) | 80 (4) | |
| Mean change from baseline | +9 (4) | −2 (3) | 0.03 | |||
| Fasting insulin (µ IU/ml) | 11 (3) | 13 (3) | 0.7 | 12 (3) | 15 (3) | |
| Mean change from baseline | +1 (1) | +3 (2) | 0.6 | |||
| Fasting FFAs (mEq/liter) | 0.48 (0.06) | 0.56 (0.04) | 0.3 | 0.56 (0.05) | 0.18 (0.04) | |
| Mean change from baseline | +0.08 (0.06) | −0.38 (0.06) | <0.0001 | |||
| M (mg/kg LBM per minute) | 9.25 (1.08) | 7.40 (0.85) | 0.2 | 9.04 (0.67) | 9.71 (1.07) | |
| Mean change from baseline | −0.21 (0.90) | +2.31 (0.74) | 0.04 | |||
| Basal Ra glycerol (µ mol/kg min) | 2.86 (0.23) | 3.04 (0.30) | 0.7 | 3.33 (0.37) | 1.54 (0.20) | |
| Mean change from baseline | +0.46 (0.27) | −1.51 (0.28) | <0.0001 | |||
| Clamp Ra glycerol (µ mol/kg min) | 2.03 (0.31) | 1.70 (0.19) | 0.4 | 2.12 (0.28) | 1.48 (0.17) | |
| Mean change from baseline | +0.08 (0.18) | −0.22 (0.12) | 0.2 | |||
| Body composition and IMCL | ||||||
| Log IMCL (IU) | 1.50 (0.23) | 1.95 (0.19) | 0.2 | 1.89 (0.18) | 1.78 (0.21) | |
| Mean change from baseline | +0.39 (0.23) | −0.17 (0.16) | 0.06 | |||
| WHR | 1.00 (0.02) | 1.02 (0.01) | 0.3 | 1.00 (0.02) | 1.01 (0.01) | |
| Mean change from baseline | 0.00 (0.008) | −0.01 (0.008) | 0.2 | |||
| DEXA percent body fat | 21.3 (2.2) | 25.3 (2.5) | 0.2 | 21.0 (2.2) | 25.0 (2.8) | |
| Mean change from baseline | −0.3 (0.4) | −0.4 (0.4) | 0.9 | |||
| Abdominal VAT (cm2) | 147 (22) | 175 (22) | 0.4 | 152 (24) | 177 (21) | |
| Mean change from baseline | +5 (10) | +2 (14) | 0.9 | |||
| Abdominal SAT (cm2) | 173 (23) | 282 (49) | 0.06 | 156 (17) | 267 (48) | |
| Mean change from baseline | −17 (12) | −15 (8) | 0.9 | |||
Values represent mean (SEM). LBM, Lean body mass; LDL, low-density lipoprotein; DEXA, Dual-energy x-ray absorptiometry; VAT, visceral adipose tissue; SAT, sc adipose tissue. Repeat evaluations for body composition were not completed on one subject who terminated participation early; therefore, n = 11 for placebo and n = 11 for acipimox.
P values represent results of t tests between groups at baseline.
P values represent results of t tests between group differences in mean change from baseline.
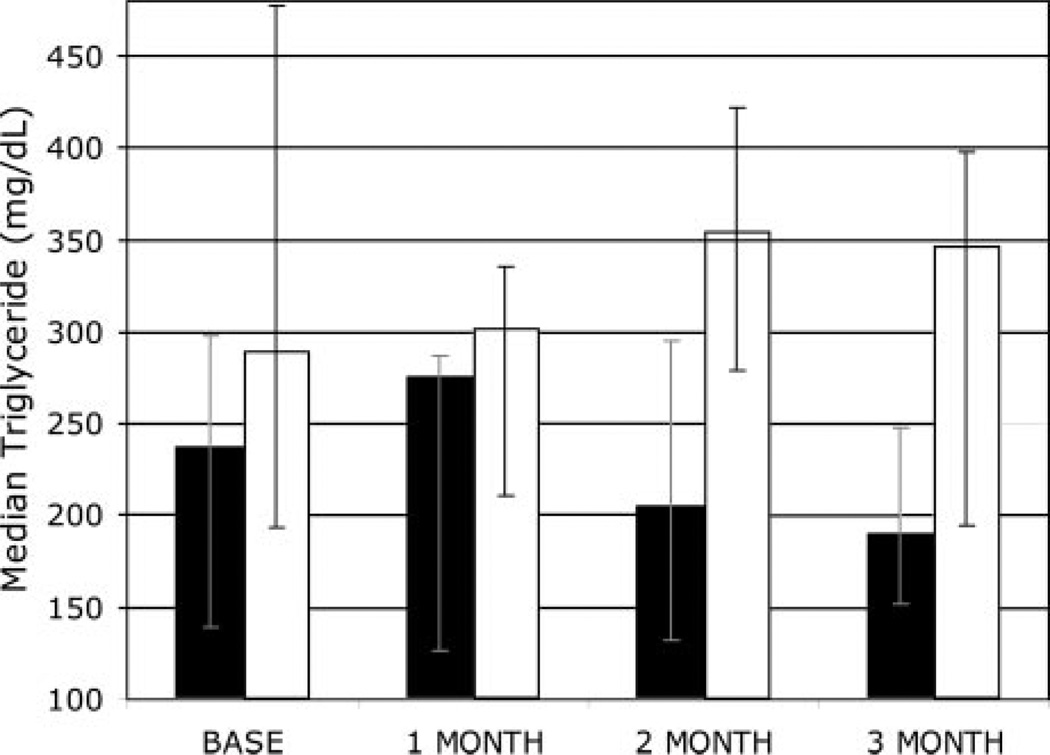
Three months of acipimox administration resulted in significant reductions in serum triglycerides (repeated measures ANOVA, P = 0.01 acipimox vs. placebo). At 3 months, subjects receiving acipimox experienced a median reduction in triglyceride of 48 mg/dl (or a 20% reduction from baseline), compared with a median increase of 58 mg/dl in subjects on placebo (a 20% increase from baseline; see Fig. 1 for monthly triglyceride medians and IQRs). This reduction in triglyceride concentration was accompanied by statistically significant reductions in FFAs and basal rates of lipolysis, compared with placebo (P < 0.001 for each, see Fig. 2 and Table 2). Fasting FFA levels were suppressed 68% with acipimox, compared with a 17% increase from baseline with placebo. Basal rates of lipolysis were suppressed by 50% with acipimox and increased 16% with placebo, compared with baseline.
Fig. 1.
Median triglyceride concentration (with error bars representing IQR) over 3 months, acipimox (solid bars, n = 11) and placebo (white bars, n = 12), and repeated-measures ANOVA (P = 0.01) between the two groups.
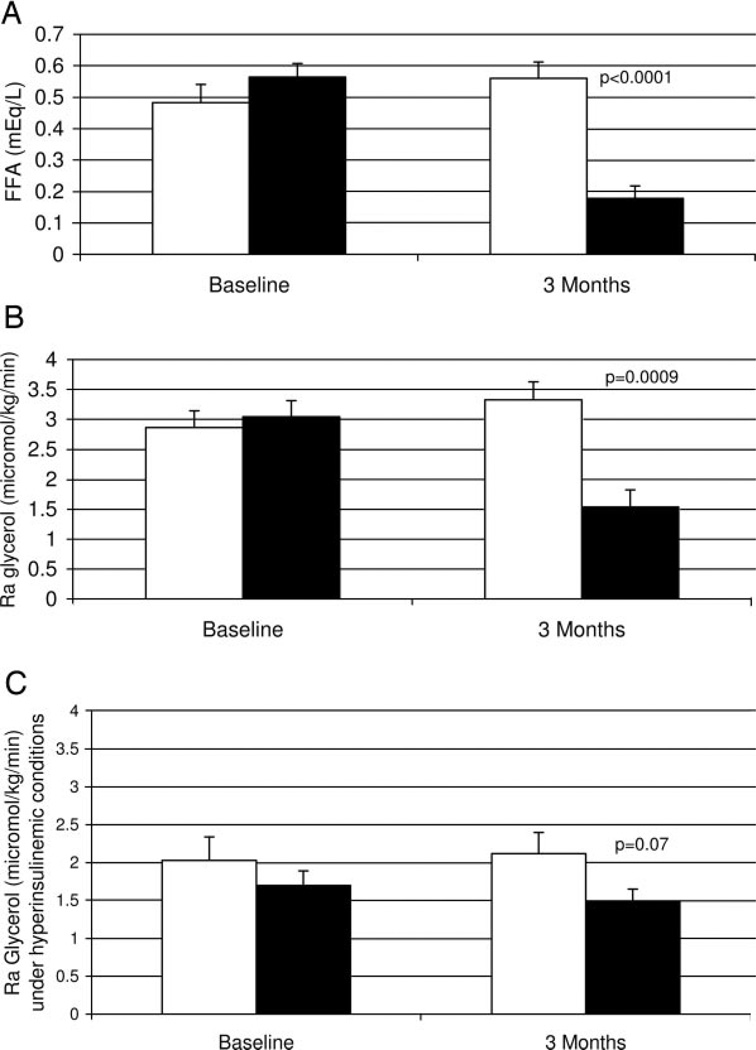
Fig. 2.
Baseline and 3-month FFA concentrations (A) and basal lipolysis (B) measured by Ra glycerol and Ra glycerol during hyperinsulinemic clamp (C) with placebo (open bars, n=10) and acipimox (solid bars, n=11). P values represent the results of t tests between groups at 3 months; there were no differences between groups at baseline.
Before randomization, insulin administration during the clamp resulted in partial suppression of lipolysis; the mean Ra glycerol in the basal state for all subjects was 2.96 (0.19) µmol/kg·min, and this decreased by 38% to 1.85 (0.18) µmol/kg·min during hyperinsulinemia. Administration of acipimox under hyperinsulinemic conditions resulted in a 12% greater reduction of Ra glycerol, compared with the preacipimox hyperinsulinemic condition, whereas placebo did not suppress lipolysis further during hyperinsulinemia (+4% in placebo). However, this difference was not statistically significant between groups (P = 0.2).
When Ra glycerol is calculated as micromoles per kilogram of body fat per minute rather than by total body mass, similar results are observed. At baseline both groups were similar [basal Ra glycerol: acipimox 13.5 (2.4) µmol/kg of body fat per minute vs. placebo: 15.4 (2.3) µmol/kg of body fat per minute, P = 0.6], and acipimox resulted in a statistically significant reduction in the basal rate of lipolysis (45% suppression vs. a 22% increase with placebo [Ra glycerol: acipimox 7.4 (1.8) µmol/kg of body fat per minute vs. placebo: 18.8 (3.5) µmol/kg of body fat per minute, P = 0.01, mean change with acipimox −6.1 (1.2) vs. placebo + 3.4 (1.8) µmol/kg of body fat per minute, P value for t test of change between groups = 0.0005].
Whereas there was a trend toward an improvement in high-density lipoprotein (HDL) cholesterol [mean change +3 (2) with acipimox vs. −2 (1) with placebo, P = 0.07], this was not statistically significant. There was no significant difference between treatment groups in the change in total or low-density lipoprotein cholesterol after 3 months.
Acipimox led to increased insulin sensitivity as measured by hyperinsulinemic-euglycemic clamp testing (P = 0.04, see Table 2). Fasting glucose decreased slightly in the acipimox group and increased in the placebo group, and this difference was statistically significant (P = 0.03, see Table 2). The change in insulin sensitivity (M) during hyperinsulinemic steady state was significantly correlated with the change in FFAs after 3 months (r = −0.62, P = 0.003). Similarly, there was a strong inverse correlation between the change in M and the change in basal rates of lipolysis (r = −0.59, P = 0.005).
IMCL content of the anterior tibialis decreased after acipimox administration, whereas subjects receiving placebo had an increase; the difference between the two treatment groups in change in IMCL at 3 months approached statistical significance (P = 0.06). There were, however, no significant changes in overall adiposity as measured by WHR, percent body fat, or abdominal visceral or sc adipose tissue area with acipimox over 3 months (Table 2). Similarly, there were no significant differences between treatments in change of energy intake or resting energy expenditure from baseline to 3 months (data not shown).
Acipimox was well tolerated with mild itching reported by only two subjects receiving active medication, and in both cases this resolved spontaneously within 3 weeks. One subject receiving acipimox had a single episode of flushing when a dose of study medication was taken on an empty stomach; this resolved without treatment and no other such episodes occurred during the 3 months. Mild gastrointestinal side effects (e.g. diarrhea, nausea, and abdominal discomfort) were reported with equal frequency by the two groups (six subjects on acipimox vs. five subjects on placebo). Compliance with the thrice-daily dosing of study medication was very good, with 92% compliance in the placebo group and 90% compliance in the acipimox group at 3 months according to pill count.
Discussion
Hypertriglyceridemia is a common problem in HIV and is estimated to occur in over 50% of HIV-infected patients treated with antiretroviral therapy and/or experiencing lipodystrophy (13, 26). Previous studies designed to evaluate conventional therapies for the reduction of hyperlipidemia and elevated triglycerides have met with modest or limited success (7, 27), particularly in the setting of continued use of antiretroviral therapies known to affect lipid metabolism. In the present study, we demonstrate modest but significant reductions in triglyceride concentration in conjunction with improvements in insulin sensitivity using a lipolytic blocking agent, acipimox, in HIV-infected patients with hypertriglyceridemia.
Previous research investigating lipid metabolism in HIV-infected patients, particularly in the setting of lipodystrophy (lipoatrophy and/or central adipose accumulation), showed increased rates of lipolysis and FFA release from adipose tissue (10, 12). Reeds et al. (11) also noted that HIV-infected men with dyslipidemia had increased FFA release, measured as the Ra of palmitate, as well as decreased very low-density lipoprotein-triglyceride clearance, compared with age-matched healthy controls. Abnormal triglyceride clearance has been observed in HIV-infected patients in several other studies (6, 28). In a rhesus monkey model, use of acipimox increased the fractional clearance rate of very low-density lipoprotein triglycerides (29). Therefore, suppression of lipolysis, reductions in FFA, and potential improvement in triglyceride clearance with acipimox is a rational therapeutic approach to HIV-associated hypertriglyceridemia.
In the current study, 3 months of acipimox was associated with significant suppression of lipolysis and a 68% reduction in circulating FFAs. This was accompanied by a median triglyceride reduction of 48 mg/dl in subjects receiving acipimox and significant improvements in insulin sensitivity. Similar to the observations of Bajaj et al. (20), who used acipimox in adults with a strong family history of type 2 diabetes, we also identified a strong inverse relationship between changes in plasma FFAs and improvements in whole-body glucose disposal. We observed an improvement of approximately 30% in M after 12 wk, and Bajaj et al. (20) found an 18% improvement in glucose disposal after 7 d of acipimox administration. These data are also consistent with observations on the short-term use of acipimox to reduce insulin levels in association with reduced FFA levels in obese adults (14).
Two open-label trials of an extended-release niacin preparation in HIV-infected patients with dyslipidemia reported significant reductions in triglycerides (30, 31). There was a substantial increase in homeostasis model assessment insulin resistance index, one of the measured indices of insulin resistance in one study (30), but Dube et al. (31) showed that early increases in glucose at 12 wk were transient and did not persist at 24 or 48 wk. The chronic use of nicotinic acid has been associated with rebound of plasma FFAs, and previously its use in diabetes has been limited due to potential deleterious effects on glucose control. However, the use of lower-dose, extended-release preparations of niacin (1000–1500 mg/d), which may avoid this rebound rise in FFAs, reduces triglycerides, increases HDL cholesterol, and has not consistently been associated with worsened glycemic control in type 2 diabetes (32).
Acipimox has a longer half-life than nicotinic acid, and in the dosing regimen of 250 mg three times daily, it has not been associated with worsened glucose metabolism. Fulcher et al. (18) demonstrated sustained improvement in triglycerides and insulin sensitivity among patients with type 2 diabetes after 3 months of acipimox treatment, compared with placebo. In addition, among patients with type 2 diabetes, Worm et al. (33) found that FFA levels rebounded after 3 d of acipimox, whereas glucose and insulin levels steadily improved. The 20% reduction in triglycerides observed in the present study is within the range of observed benefit in similar studies of acipimox in type 2 diabetes demonstrating 19–30% reductions in triglycerides (18, 19, 34). The continued exposure to antiretroviral therapy potentially contributing to increased lipolysis and hyperlipideima may account for the observed response to acipimox in the current study.
Increased IMCL content is associated with insulin resistance (35) and in several studies was found to be elevated in HIV-infected men and women (25, 36, 37). In the present study, suppression of lipolysis and a reduction in the free fatty acid pool was also associated with a trend in reduction of IMCL, compared with placebo. These data are in agreement with the observations of van Loon et al. (38), who identified significant reductions in intramuscular triglyceride content and increased intramuscular lipid use in healthy volunteers in the setting of a reduced FFA pool with acipimox. Chronic reduction in the FFA pool with acipimox may therefore lead to increased mobilization of IMCL, which in turn promotes increased insulin sensitivity.
There is evidence that acipimox directly affects lipolysis in sc adipose tissue. Using microdialysis techniques, Flechtner-Mors et al. (39) showed decreased glycerol outflow and therefore decreased lipolysis from sc fat depots among obese individuals after acipimox treatment. These data suggest a potential specific benefit of acipimox for HIV-infected patients in whom increased lipolytic rates in sc fat stores may contribute to increased FFA levels and to lipodystrophy. Furthermore, increased lipolysis in visceral adipose, coupled with increased rates of reesterification (12), may contribute to hypertriglyceridemia in HIV, and acipimox may lead to decreases in triglyceride via this mechanism. In the present study, we did not observe changes in sc fat or body fat distribution, but the ability to detect such changes may have been limited by the small sample size and the relative short duration of the study.
The present study was designed to stratify randomization by age and sex to achieve balanced distributions in the two treatment arms on these characteristics. Whereas this was achieved, there were unanticipated differences between the two treatment groups at baseline in BMI and total cholesterol. The subjects receiving acipimox had greater mean BMI and lower mean total cholesterol. The present study was also conducted in HIV-infected patients experiencing changes in body fat distribution or lipodystrophy. Indeed, a larger trial will be necessary to reproduce these results and to determine the generalizability of the benefits of acipimox on overweight and lean HIV-infected men and women. Also, subjects in the present study were not on lipid-lowering therapy, and therefore, the additive benefit of acipimox to 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors and fibrates should also be evaluated in this population in whom lipid management is challenging.
Hypertriglyceridemia and insulin resistance are frequently recognized in HIV-infected patients and confer significant increased risk of metabolic syndrome, diabetes, and cardiovascular disease (1, 40–42). Acipimox is a unique medication in its documented ability to improve hyperlipidemia and insulin resistance. In the present study, we demonstrate sustained benefits in triglyceride concentration and insulin sensitivity as well as a modest increase in HDL cholesterol using this lipolytic-blocking agent in HIV-infected men and women with hypertriglyceridemia and thereby improved their overall metabolic profile. Additional investigation is warranted to establish the long-term clinical benefits of acipimox in HIV-infected patients.
Acknowledgments
The authors thank the nursing and bionutrition staff of the MIT General Clinical Research Center for their ongoing dedication to patient care and patient-oriented research.
This work was supported by National Institutes of Health funding: Grants R21-HL73675, K23-DK-02844, MO1-RR-01066 (to Massachusetts Institute of Technology General Clinical Research Center), R01-DK-59535, and K23-RR-18715.
Abbreviations
- BMI
Body mass index
- FFA
free fatty acid
- HDL
high-density lipoprotein
- IMCL
intramyocellular lipid
- IQR
interquartile range
- M
insulin sensitivity
- Ra
rate of appearance
- WHR
waist to hip ratio
Footnotes
Disclosure statement: J.L. has equity interest in Hologic, Inc.
References
- 1.Data Collection on Adverse Events of Anti-HIV Drugs (DAD) Study Group. Friis-Moller N, Sabin CA, Weber R, d’Arminio Monforte A, El-Sadr WM, Reiss P, Thiebaut R, Morfeldt L, De Wit S, Pradier C, Calvo G, Law MG, Kirk O, Phillips AN, Lundgren JD. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349:1993–2003. doi: 10.1046/j.1468-1293.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- 2.Mulligan K, Grunfeld C, Tai VW, Algren H, Pang M, Chernoff DN, Lo JC, Schambelan M. Hyperlipidemia and insulin resistance are induced by protease inhibitors independent of changes in body composition in patients with HIV infection. J Acquir Immune Defic Syndr. 2000;23:35–43. doi: 10.1097/00126334-200001010-00005. [DOI] [PubMed] [Google Scholar]
- 3.Dube M, Parker RA, Tebas P, Grinspoon SK, Zackin RA, Robbins GK, Roubenoff R, Shafer RW, Wininger DA, Meyer WA, Snyder SW, Mulligan K. Glucose metabolism, lipid and body fat changes in antiretroviral-naive subjects randomized to nelfinavir or efavirenz plus dual nucleosides. AIDS. 2005;19:1807–1818. doi: 10.1097/01.aids.0000183629.20041.bb. [DOI] [PubMed] [Google Scholar]
- 4.Purnell JQ, Zambon A, Knopp RH, Pizzuti DJ, Achari R, Leonard JM, Locke C, Brunzell JD. Effect of ritonavir on lipids and post-heparin lipase activities in normal subjects. AIDS. 2000;14:51–57. doi: 10.1097/00002030-200001070-00006. [DOI] [PubMed] [Google Scholar]
- 5.Grunfeld C, Kotler DP, Hamadeh R, Tierney A, Wang J, Pierson RN. Hypertriglyceridemia in the acquired immunodeficiency syndrome. Am J Med. 1989;86:27–31. doi: 10.1016/0002-9343(89)90225-8. [DOI] [PubMed] [Google Scholar]
- 6.Grunfeld C, Pang M, Doerrler W, Shigenaga JK, Jensen P, Feingold KR. Lipids, lipoproteins, triglyceride clearance, and cytokines in human immunodeficiency virus infection and the acquired immunodeficiency syndrome. J Clin Endocrinol Metab. 1992;74:1045–1052. doi: 10.1210/jcem.74.5.1373735. [DOI] [PubMed] [Google Scholar]
- 7.ACTG 5087 Study Team. Aberg JA, Zackin RA, Brobst SW, Evans SR, Alston BL, Henry WK, Glesby MJ, Torriani FJ, Yang Y, Owens SI, Fichtenbaum CJ. A randomized trial of the efficacy and safety of fenofibrate versus pravastatin in HIV-infected subjects with lipid abnormalities: AIDS Clinical Trials Group Study 5087. AIDS Res Hum Retroviruses. 2005;21:757–767. doi: 10.1089/aid.2005.21.757. [DOI] [PubMed] [Google Scholar]
- 8.Vigouroux C, Gharakhanian S, Salhi Y, Nguyen TH, Chevenne D, Capeau J, Rozenbaum W. Diabetes, insulin resistance and dyslipidaemia in lipodystrophic HIV-infected patients on highly active antiretroviral therapy (HAART) Diabetes Metab. 1999;25:225–232. [PubMed] [Google Scholar]
- 9.Meininger G, Hadigan C, Laposata M, Brown J, Rabe J, Louca J, Aliabadi N, Grinspoon S. Elevated concentrations of free fatty acids are associated with increased insulin response to standard glucose challenge in human immunodeficiency virus-infected subjects with fat redistribution. Metabolism. 2002;51:260–266. doi: 10.1053/meta.2002.29999. [DOI] [PubMed] [Google Scholar]
- 10.Hadigan C, Borgonha S, Rabe J, Young V, Grinspoon S. Increased rates of lipolysis among HIV-infected men receiving highly active antiretroviral therapy. Metabolism. 2002;51:1143–1147. doi: 10.1053/meta.2002.34704. [DOI] [PubMed] [Google Scholar]
- 11.Reeds DN, Mittendorfer B, Patterson BW, Powderly WG, Yarasheski KE, Klein S. Alterations in lipid kinetics in men with HIV-dyslipidemia. Am J Physiol Endocrinol Metab. 2003;285:E490–E497. doi: 10.1152/ajpendo.00118.2003. [DOI] [PubMed] [Google Scholar]
- 12.Sekhar RV, Jahoor F, White AC, Pownall HJ, Visnegarwala F, Rodriguez-Barradas MC, Sharma M, Reeds PJ, Balasubramanyam A. Metabolic basis of HIV-lipodystrophy syndrome. Am J Physiol Endocrinol Metab. 2002;283:E332–E337. doi: 10.1152/ajpendo.00058.2002. [DOI] [PubMed] [Google Scholar]
- 13.Hadigan C, Meigs JB, Corcoran C, Rietschel P, Piecuch S, Basgoz N, Davis B, Sax P, Stanley T, Wilson PW, D’Agostino RB, Grinspoon S. Metabolic abnormalities and cardiovascular disease risk factors in adults with human immunodeficiency virus infection and lipodystrophy. Clin Infect Dis. 2001;32:130–139. doi: 10.1086/317541. [DOI] [PubMed] [Google Scholar]
- 14.Andreotti AC, Lanzi R, Manzoni MF, Caumo A, Moreschi A, Pontiroli AE. Acute pharmacologic blockade of lipolysis normalizes nocturnal growth hormone levels and pulsatility in obese subjects. Metabolism. 1994;43:1207–1213. doi: 10.1016/0026-0495(94)90212-7. [DOI] [PubMed] [Google Scholar]
- 15.Hennes MM, Dua A, Maas DL, Sonnenberg GE, Krakower GR, Kissebah AH. Relationships of plasma leptin levels to changes in plasma free fatty acids in women who are lean and women who are abdominally obese. Obes Res. 1997;5:442–446. doi: 10.1002/j.1550-8528.1997.tb00668.x. [DOI] [PubMed] [Google Scholar]
- 16.Hadigan C, Rabe J, Meininger G, Aliabadi N, Breu J, Grinspoon S. Inhibition of lipolysis improves insulin sensitivity in protease inhibitor-treated HIV-infected men with fat redistribution. Am J Clin Nutr. 2003;77:490–494. doi: 10.1093/ajcn/77.2.490. [DOI] [PubMed] [Google Scholar]
- 17.Stuyt PM, Kleinjans HA, Stalenhoef AF. Tolerability and effects of high doses acipimox as additional lipid-lowering therapy in familial hypercholesterolemia. Neth J Med. 1998;53:228–233. doi: 10.1016/s0300-2977(98)00076-x. [DOI] [PubMed] [Google Scholar]
- 18.Fulcher GR, Catalano C, Walker M, Farrer M, Thow J, Whately-Smith CR, Alberti KG. Adouble blind study of the effect of acipimox on serum lipids, blood glucose control and insulin action in non-obese patients with type 2 diabetes mellitus. Diabet Med. 1992;9:908–914. doi: 10.1111/j.1464-5491.1992.tb01730.x. [DOI] [PubMed] [Google Scholar]
- 19.Koev D, Zlateva S, Susic M, Babic D, Profozic V, Skrabalo Z, Langrova H, Cvrkalova AL, Rajecova E, Klimes I, Sebokova E, Hanzen E, Lacko A, Kreze A, Rybka J, Gus M, Kalits I, Karadi I, Romics L, Leowski J, Orlandini L. Improvement of lipoprotein lipid composition in type II diabetic patients with concomitant hyperlipoproteinemia by acipimox treatment. Results of a multicenter trial. Diabetes Care. 1993;16:1285–1290. doi: 10.2337/diacare.16.9.1285. [DOI] [PubMed] [Google Scholar]
- 20.Bajaj M, Suraamornkul S, Kashyap S, Cusi K, Mandarino L, DeFronzo RA. Sustained reduction in plasma free fatty acid concentration improves insulin action without altering plasma adipocytokine levels in subjects with strong family history of type 2 diabetes. J Clin Endocrinol Metab. 2004;89:4649–4655. doi: 10.1210/jc.2004-0224. [DOI] [PubMed] [Google Scholar]
- 21.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: A method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 22.Wolfe RR, Chinkes DL. Istope tracers in metabolic research. Hoboken, NJ: John Wiley and Sons, Inc; 2005. Calculation of substrate kinetics: single-pool model. [Google Scholar]
- 23.Borkan GA, Gerzof SG, Robbins AH, Hults DE, Silbert CK, Silbert JE. Assessment of abdominal fat content by computed tomography. Am J Clin Nutr. 1982;36:172–177. doi: 10.1093/ajcn/36.1.172. [DOI] [PubMed] [Google Scholar]
- 24.Torriani M, Thomas BJ, Halpern EF, Jensen ME, Rosenthal DI, Palmer WE. Intramyocellular lipid quantification: repeatability with 1H MR spectroscopy. Radiology. 2005;236:609–614. doi: 10.1148/radiol.2362041661. [DOI] [PubMed] [Google Scholar]
- 25.Torriani M, Thomas BJ, Barlow RB, Librizzi J, Dolan S, Grinspoon S. Increased intramyocellular lipid accumulation in HIV-infected women with fat redistribution. J Appl Physiol. 2006;100:609–614. doi: 10.1152/japplphysiol.00797.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carter VM, Hoy JF, Bailey M, Colman PG, Nyulasi I, Mijch AM. The prevalence of lipodystrophy in an ambulant HIV-infected population: it all depends on the definition. HIV Med. 2001;2:174–180. doi: 10.1046/j.1468-1293.2001.00073.x. [DOI] [PubMed] [Google Scholar]
- 27.Miller J, Brown D, Amin J, Kent-Hughes J, Law M, Kaldor J, Cooper DA, Carr A. A randomized, double-blind study of gemfibrozil for the treatment of protease inhibitor-associated hypertriglyceridaemia. AIDS. 2002;16:2195–2200. doi: 10.1097/00002030-200211080-00012. [DOI] [PubMed] [Google Scholar]
- 28.Schmitz M, Michl GM, Walli R, Bogner J, Bedynek A, Seidel D, Goebel FD, Demant T. Alterations of apolipoprotein B metabolism in HIV-infected patients with antiretroviral combination therapy. J Acquir Immune Defic Syndr. 2001;26:225–235. doi: 10.1097/00042560-200103010-00004. [DOI] [PubMed] [Google Scholar]
- 29.Hannah JS, Bodkin NL, Paidi MS, Anh-Le N, Howard BV, Hansen BC. Effects of Acipimox on the metabolism of free fatty acids and very low lipoprotein triglyceride. Acta Diabetol. 1995;32:279–283. doi: 10.1007/BF00576264. [DOI] [PubMed] [Google Scholar]
- 30.Gerber MT, Mondy KE, Yarasheski KE, Drechsler H, Claxton S, Stoneman J, DeMarco D, Powderly WG, Tebas P. Niacin in HIV-infected individuals with hyperlipidemia receiving potent antiretroviral therapy. Clin Infect Dis. 2004;39:419–425. doi: 10.1086/422144. [DOI] [PubMed] [Google Scholar]
- 31.Dube MP, Wu JW, Aberg JA, Deeg AM, McGovern ME, Alston BL, Shriver SL, Greenwald ML, Lee D, Stein JH. Safety and efficacy of extended-release niacin for the treatment of dyslipidemia in patients with HIV infection: a prospective multicentre study (ACTG 5148). Proc 7th International Workshop on Adverse Drug Reactions and Lipodystrophy in HIV; Dublin, Ireland. 2005. Nov, p. 10. L9 (Antiviral Therapy Abstract 12) [Google Scholar]
- 32.Diabetes Multicenter Research Group. Grundy SM, Vega GL, McGovern ME, Tulloch BR, Kendall DM, Fitz-Patrick D, Ganda OP, Rosenson RS, Buse JB, Robertson DD, Sheehan JP. Efficacy, safety, and tolerability of once-daily niacin for the treatment of dyslipidemia associated with type 2 diabetes. Arch Intern Med. 2002;162:1568–1576. doi: 10.1001/archinte.162.14.1568. [DOI] [PubMed] [Google Scholar]
- 33.Worm D, Henriksen JE, Vaag A, Thye-Ronn P, Melander A, Beck-Nielsen H. Pronounced blood glucose-lowering effect of the antilipolytic drug acipimox in noninsulin-dependent diabetes mellitus patients during a 3-day intensified treatment period. J Clin Endocrinol Metab. 1994;78:717–721. doi: 10.1210/jcem.78.3.8126147. [DOI] [PubMed] [Google Scholar]
- 34.Dean JD, McCarthy S, Betteridge DJ, Whately-Smith C, Powell J, Owens DR. The effect of acipimox in patients with type 2 diabetes and persistent hyperlipidemia. Diabet Med. 1992;9:611–615. doi: 10.1111/j.1464-5491.1992.tb01855.x. [DOI] [PubMed] [Google Scholar]
- 35.Krssak M, Falk Petersen K, Dresner A, DiPietro L, Vogel SM, Rothman DL, Roden M, Shulman GI. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia. 1999;42:113–116. doi: 10.1007/s001250051123. [DOI] [PubMed] [Google Scholar]
- 36.Luzi L, Perseghin G, Tambussi G, Meneghini E, Scifo P, Pagliato E, Del Maschio A, Testolin G, Lazzarin A. Intramyocellular lipid accumulation and reduced whole body lipid oxidation in HIV lipodystrophy. Am J Physiol Endocrinol Metab. 2003;284:E274–E280. doi: 10.1152/ajpendo.00391.2001. [DOI] [PubMed] [Google Scholar]
- 37.Gan SK, Samaras K, Thompson CH, Kraegen EW, Carr A, Cooper DA, Chisholm DJ. Altered myocellular and abdominal fat partitioning predict disturbance in insulin action in HIV protease inhibitor-related lipodystrophy. Diabetes. 2002;51:3163–3169. doi: 10.2337/diabetes.51.11.3163. [DOI] [PubMed] [Google Scholar]
- 38.van Loon LJ, Thomason-Hughes M, Constantin-Teodosiu D, Koopman R, Greenhaff PL, Hardie DG, Keizer HA, Saris WH, Wagenmakers AJ. Inhibition of adipose tissue lipolysis increases intramuscular lipid and glycogen use in vivo in humans. Am J Physiol Endocrinol Metab. 2005;289:E482–E493. doi: 10.1152/ajpendo.00092.2005. [DOI] [PubMed] [Google Scholar]
- 39.Flechtner-Mors M, Jenkinson CP, Alt A, Adler G, Ditschuneit HH. Effects of acipimox on the lipolysis rate in subcutaneous adipose tissue of obese subjects. Diabetes Metab Res Rev. 2001;17:387–390. doi: 10.1002/dmrr.219. [DOI] [PubMed] [Google Scholar]
- 40.Hadigan C, Meigs JB, Wilson PW, D’Agostino RB, Davis B, Basgoz N, Sax PE, Grinspoon S. Prediction of coronary heart disease risk in HIV-infected patients with fat redistribution. Clin Infect Dis. 2003;36:909–916. doi: 10.1086/368185. [DOI] [PubMed] [Google Scholar]
- 41.van Wijk JP, de Koning EJ, Cabezas MC, Joven J, op’t Roodt J, Rabelink TJ, Hoepelman AM. Functional and structural markers of atherosclerosis in human immunodeficiency virus-infected patients. J Am Coll Cardiol. 2006;47:1117–1123. doi: 10.1016/j.jacc.2005.09.073. [DOI] [PubMed] [Google Scholar]
- 42.Brown TT, Cole SR, Li X, Kingsley LA, Palella FJ, Riddler SA, Visscher BR, Margolick JB, Dobs AS. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med. 2005;165:1179–1184. doi: 10.1001/archinte.165.10.1179. [DOI] [PubMed] [Google Scholar]