Abstract
In a prior study, we have shown that tumor necrosis factor (TNF)-α neutralization improves inflammatory markers and total adiponectin in patients with the metabolic syndrome, without improving insulin sensitivity. In this study, we sought to extend our understanding of the effects of TNF-α neutralization in this human model of obesity by investigating the responses of high-molecular-weight (HMW) adiponectin, resistin, leptin, and muscle adiposity to etanercept in patients with the metabolic syndrome. Fifty-six men and women with the metabolic syndrome enrolled in a double-blind randomized placebo-controlled trial. Circulating concentrations of total and HMW adiponectin, resistin, and leptin were determined at baseline and after 4 wk of treatment with etanercept. Muscle adiposity was measured by computed tomography (CT). Although etanercept increased total adiponectin concentration, the HMW form, which is thought to mediate insulin sensitivity, was unchanged. Thus the ratio of HMW to total adiponectin decreased following etanercept treatment compared with placebo (−0.03 ± 0.03 vs. 0.06 ± 0.03, P = 0.02). Resistin tended to decrease in the etanercept-treated group compared with placebo (−0.6 ± 0.7 vs. 1.2 ± 0.7 ng/ml, P = 0.06), whereas leptin was not altered. Etanercept decreased muscle attenuation on CT [−0.61 ± 0.64 Hounsfield units (HU) vs. 1.54 ± 0.77 HU in placebo, P = 0.04], suggesting an increase in muscle adiposity. Together, these results demonstrate that neutralization of TNF-α in obese humans results in differential effects on critical adipokines and body composition indexes. These findings may help to explain the lack of effect on insulin sensitivity and extend our knowledge of the biological effects of TNF-α neutralization in obesity.
Keywords: tumor necrosis factor-α, adiponectin, resistin, muscle adiposity, metabolic syndrome
We have previously reported the effects of tumor necrosis factor (TNF)-α blockade using etanercept in human participants with the metabolic syndrome (4). Although the etanercept-treated group showed favorable improvements in inflammatory markers, including a significant decrease in C-reactive protein and an increase in total adiponectin, insulin sensitivity did not differ from participants that received placebo (4). These data suggest the effects of TNF-α neutralization on insulin sensitivity in obese humans may be complex. To answer this question, we have analyzed new data from this study, investigating the effects of TNF-α neutralization on high-molecular-weight (HMW) adiponectin, the proportion of HMW to total adiponectin, resistin, and leptin, and muscle adiposity measured noninvasively by computed tomography (CT) muscle attenuation.
Adipose tissue secretes several cytokines and hormones that affect insulin sensitivity. One such cytokine, TNF-α, is over-expressed in adipose tissue and muscle of obese and insulin-resistant humans (18, 24, 44). This proinflammatory cytokine causes insulin resistance via effects on insulin-mediated cellular signaling pathways (19). TNF-α neutralization in obese Zucker (fa/fa) rats increased insulin-stimulated peripheral glucose utilization rate (20). In patients with rheumatoid arthritis in one study, decreases in insulin levels and improvement in homeostasis model assessment of insulin resistance (HOMA-IR) and the quantitative insulin sensitivity check index (QUICKI) indexes were demonstrated after a single intravenous infusion of infliximab (13). In another study in patients with rheumatoid arthritis and ankylosing spondylitis treated with intravenous infliximab for 6 mo, significant decreases in HOMA-IR and QUICKI were found in patients in the highest tertile of insulin resistance (25). However, a few other studies in humans with obesity to date have not demonstrated improvement in insulin sensitivity with TNF-α neutralization (4, 7, 40). Some key differences exist between these studies and are worth mentioning. The studies demonstrating a positive effect on insulin sensitivity were performed in patients with rheumatologic disease requiring therapy and used infliximab, whereas the studies that did not demonstrate an improvement were performed in obese patients, rather than rheumatologic patients, and used etanercept or lenercept (Ro 45-2081). In a prior study, we did not show an effect of TNF-α neutralization on insulin sensitivity with etanercept, suggesting that other mechanisms and effects of TNF-α inhibition might be operative to oppose this effect.
Adipocyte production of adiponectin, resistin, leptin, and free fatty acids is regulated by TNF-α in vitro (11, 22, 24, 31, 47, 49). These factors may be affected by neutralization of TNF-α in vivo and in turn affect insulin sensitivity in peripheral tissues. In the serum, adiponectin circulates as multimeric complexes of different sizes, including a lower molecular weight (LMW) hexamer form and a HMW form consisting of six trimers (37). Some studies suggest that the proportion of the HMW form of adiponectin may be more closely linked to insulin sensitivity (37, 38). The effect of TNF-α neutralization on HMW adiponectin concentration or the ratio of HMW-to-total adiponectin is not yet known.
Resistin, a hormone secreted by adipocytes identified by Steppan and colleagues (50), is increased in obesity and decreased by thiazolidinediones. In vitro, resistin gene expression has been found to be altered by TNF-α in opposite directions in different cell types. Shojima and colleagues (47) showed resistin mRNA and protein expression to be suppressed by TNF-α in 3T3-L1 adipocytes. In human peripheral blood mononuclear cells, on the other hand, Kaser and colleagues (22) found that TNF-α increased resistin mRNA expression. However, the effect of TNF-α neutralization on resistin levels has not yet been examined in humans in vivo. Similarly, leptin gene expression can be affected by TNF-α. TNF-α was shown to increase leptin levels in humans, and inflammatory cytokines have been shown to increase leptin expression in animals (15, 45, 59).
In addition to adipocyte-produced peptides, intramyocellular lipid accumulation has also been associated with insulin resistance (27). The effect of TNF-α neutralization on muscle lipid content is not yet known. As a noninvasive assessment of muscle adiposity, muscle attenuation measurements by CT decrease with rising skeletal muscle lipid content (14). In this study, we investigated the effects of TNF-α neutralization on adipokine concentrations and body composition indexes. The data from this study extend our knowledge of the physiological effects of TNF-α neutralization in obesity.
MATERIALS AND METHODS
The 56 participants had a mean age 45.6 ± 1.1 yr, body mass index (BMI) 36.5 ± 0.7 kg/m2, and waist-to-hip ratio (WHR) 0.96 ± 0.009 (mean ± SE) and met the modified World Health Organization criteria of the metabolic syndrome with either hyperinsulinemia (≥10 µU/ml) or impaired glucose tolerance (fasting glucose 110–125 mg/dl) and two of three additional criteria as follows: 1) WHR >0.90 for men and >0.85 for women or BMI >30 kg/m2, 2) serum triglycerides ≥150 mg/dl or high-density lipoprotein <35 mg/dl for men and <39 mg/dl for women, and 3) blood pressure ≥140/90 mmHg or receiving antihypertensive medication were enrolled in a randomized double-blind placebo-controlled trial and seen at the General Clinical Research Center of the Massachusetts Institute of Technology and Massachusetts General Hospital.
All subjects gave written informed consent, and the study was approved by the Human Research Committee of the Massachusetts General Hospital (MGH), the Committee on the Research of Human Subjects at Massachusetts Institute of Technology and the Food and Drug Administration (IND BB-IND no. 11463). This clinical trial is registered (ClinicalTrials.gov Identifier NCT00409318).
Each participant underwent a baseline visit after an overnight fast. Height, weight, and BMI were determined. Fasting blood was drawn. Single-slice cross-sectional CT scans of the abdomen and of the thigh were performed. Participants were randomized to receiving 50 mM subcutaneous weekly etanercept (Enbrel; Amgen, Thousand Oaks, CA) in two 25-mg injections or identical placebo for 4 wk. All tests obtained at the baseline visit before randomization were repeated on day 25, 3 days after the fourth and final dose of the study drug. In addition, 34 healthy male control volunteers had single-slice CT scans of the thigh. Mean thigh muscle attenuation of healthy controls was compared with muscle attenuation of patients with the metabolic syndrome.
Serum chemistry
Insulin levels were measured in serum using an RIA (Diagnostic Products, Los Angeles, CA). Interassay coefficients of variation (CV) range from 4.9 to 10.0%. Glucose concentrations were measured using a glucose hexokinase method at MGH. The HOMA-IR score was calculated by the following formula: fasting serum insulin (µU/ml) × fasting plasma glucose (mmol/l)/22.5 as described by Matthews and coworkers (32). Total adiponectin concentrations were originally measured using an RIA by Linco as previously described (4). To determine the ratio of HMW to total adiponectin (HMW/total adiponectin), total and HMW adiponectin were simultaneously measured by Alpco enzyme immunoassay as described in the literature (CV 4.3–5.2%; sensitivity 0.04 ng/ml; see Refs. 8 and 34; Alpco, Salem, NH). Results of total adiponectin by the Alpco and Linco assays were similar (r = 0.74 and P <0.0001). The ratio of HMW to total adiponectin has previously been termed the adiponectin sensitivity index (38).
Sandwich enzyme immunoassay technique was used to measure TNF-α (CV 5.3– 8.8%; sensitivity 0.12 pg/ml), soluble TNF-α receptor (sTNFR) 1 (CV 3.6 –5.0%; sensitivity 0.001 ng/ml), and sTNFR2 (CV 2.6 – 4.8%; sensitivity 0.001 ng/ml; R&D Systems, Minneapolis, MN). Resistin and leptin were measured using ELISA kits from Linco Research (St. Charles, MO; CV 3.7– 6.5% and sensitivity 0.1 ng/ml for resistin; CV 3.5–5.8% and sensitivity 0.5 ng/ml for leptin; see Ref. 41).
Body composition and muscle attenuation by CT scan
Weight was determined in the morning, before breakfast. A cross-sectional CT scan at the level of the L4 pedicle was performed to determine abdominal subcutaneous and visceral fat areas (SAT and VAT, respectively). A single 1-cm slice CT through the left midfemur was also performed, equidistant between the articular surfaces of femoral head and medial femoral condyle. Scan parameters for each image were standardized (144 cm table height, 80 kV, 70 mA, 2 s, 1.0 cm slice thickness, 48 cm field of view). Fat attenuation coefficients were set at −50 to −250 Hounsfield units (HU; see Ref. 5). The cross-sectional area and mean attenuation values in HU of the whole leg, the anterior muscles, and the posterior muscles were assessed using commercial software (Alice; Parexel, Waltham, MA) by manually tracing the anterior and posterior thigh muscle compartments. The mean leg muscle attenuation was calculated by taking the average of the anterior and posterior muscle attenuation values. The CV for the measurement of muscle attenuation in our radiology department is 2.4% (9). Total fat was measured by dual energy X-ray absorptiometry (DEXA; Hologic, Waltham, MA) with a precision error of 1.7% for fat in our laboratory.
Statistical analysis
Student’s t-test was used for baseline comparisons between two groups for continuous variables. Change from the baseline to end of study was compared between the etanercept and placebo-treated groups by Student’s t-test also. Paired t-test was used to compare between baseline and posttreatment values within the etanercept-treated group and within the placebo-treated group. Pearson correlation coefficient was used to report relationships between continuous variables unless otherwise indicated. Intention-to-treat analyses were performed for all variables. Missing data for assessments at week 4 were imputed with the use of the last-observation-carried forward method. Results are reported as means ± SE unless otherwise indicated. All reported P values are two-sided, and all statistical analyses were performed using SAS JMP statistics software version 5.1. (SAS Institute, Cary, NC).
RESULTS
Characteristics of study participants
At baseline, the age of the study participants was 45.6 ± 1.1 (SE) yr, and BMI was 36.5 ± 0.7 (SE) kg/m2. Thirty participants were male and 26 were female; 37 were Caucasian, 17 were Black, and 2 were Hispanic. Baseline values for glucose, insulin, HOMA-IR, leptin, resistin, total adiponectin, HMW adiponectin, %HMW to total adiponectin, leg muscle attenuation, VAT, SAT, total body fat, and lean body mass are shown in Table 1. These measurements were similar at baseline between the placebo and etanercept-treated groups. In the group randomized to receive etanercept, two subjects discontinued intervention after baseline treatment. In the placebo group, one subject discontinued before completion of the baseline visit, and one discontinued intervention after baseline treatment.
Table 1.
Effects of etanercept treatment on metabolic parameters, adipocytokines, inflammatory markers, and muscle attenuation
| Placebo (n = 28) | Etanercept (n = 28) | ||||
|---|---|---|---|---|---|
| Baseline | Change from baseline | Baseline | Change from baseline | P | |
| Glucose, mg/dl | 91±3 | 8±6 | 97±5 | 4±6 | 0.61 |
| Insulin, µIU/ml | 12.6±1.5 | 1.2±1.7 | 16.8±2.4 | 0.6±1.7 | 0.81 |
| HOMA-IR | 2.9±0.4 | 0.9±0.7 | 4.2±0.7 | 0.2±0.5 | 0.47 |
| Leptin, ng/ml | 45.1±7.3 | −1.8±3.0 | 39.7±6.7 | −3.4±3.1 | 0.72 |
| Resistin, ng/ml | 14.2±1.0 | 1.2±0.7 | 15.0±1.2 | −0.6±0.7 | 0.06 |
| Total adiponectin (Linco), ng/ml | 7600±700 | −300±300 | 7000±700 | 800±400* | 0.02 |
| HMW adiponectin, ng/ml | 1256±174 | 97±68 | 1193±163 | 79±53 | 0.83 |
| HMW-to-total adiponectin ratio | 0.38±.03 | 0.06±.03* | 0.43±.03 | −0.02±.03 | 0.03 |
| Leg muscle attenuation, HU | 44.3±1.2 | 1.5±0.8 | 44.1±1.2 | −0.6±0.6 | 0.04 |
| VAT, cm2 | 201.9±13.4 | 0.2±7.0 | 213.8±15.0 | 2.7±5.1 | 0.76 |
| SAT, cm2 | 421.4±32.6 | 2.1±5.6 | 497.5±36.1 | −5.9±6.8 | 0.37 |
| Total fat by DEXA, kg | 38.0±2.1 | 0.1±0.2 | 40.7±2.8 | 0.0±0.2 | 0.66 |
| Total lean mass by DEXA, kg | 64.0±2.5 | −0.2±0.3 | 67.3±2.4 | −0.1±0.3 | 0.87 |
Results are reported as means ± SE; n, no. of subjects. HOMA-IR, homeostasis model assessment of insulin resistance; HMW, high molecular weight; HU, Hounsfield units; VAT, visceral fat; SAT, subcutaneous area; DEXA, dual-energy X-ray absorptiometry. Baseline comparisons all P > 0.05. International system of units conversion factors: for conversion from mg/dl to mmol/l for glucose, multiply by 0.0555; for conversion from µIU/ml to pmol/l for insulin, multiply by 6.945. P value reported for Student’s t-test comparing changes in the placebo vs. etanercept-treated group are shown.
Statistically significant change from baseline by paired t-test within placebo or etanercept-treated group.
Baseline correlation analysis of adipocytokines
At baseline, HOMA-IR increased with BMI (P = 0.02 across BMI quartiles with means ranging from 2.0 ± 0.3 to 4.8 ± 0.9 kg/m2 in the lowest to highest quartile, respectively). At baseline, HOMA-IR correlated significantly with resistin concentration (r = 0.35, P = 0.009; Table 2). Total adiponectin correlated with leptin (r = 0.36, P = 0.007), sTNFR1 (r = 0.32, P = 0.02), and sTNFR2 (r = 0.32, P = 0.02). In contrast, HMW adiponectin did not correlate significantly with sTNFR1 or sTNFR2 and tended to correlate with leptin (r = 0.25, P = 0.07). sTNFR1 correlated with total adiponectin and leptin (r = 0.48, P = 0.0002) as well as BMI (r = 0.35, P = 0.008) and total body fat (r = 0.50, P = 0.0002) but not with insulin (r = 0.13, P = 0.35) or HOMA-IR (r = 0.12, P = 0.38). sTNFR2 also correlated with total adiponectin, leptin (r = 0.43, P = 0.001), BMI (r = 0.40, P = 0.003) and total body fat (r = 0.50, P = 0.0002) but not with insulin (r = 0.17, P = 0.23) or HOMA-IR (r = 0.13, P = 0.35). The ratio of HMW to total adiponectin did not correlate significantly with HOMA-IR (r = −0.1167, P = 0.40).
Table 2.
Baseline associations of adipocytokines to insulin sensitivity and markers of TNF-α activation in the metabolic syndrome
| Total Adiponectin | HMW Adiponectin | Resistin | Leptin | |||||
|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | |
| Marker of insulin resistance | ||||||||
| HOMA-IR | −0.11 | 0.44 | −0.19 | 0.16 | 0.35 | 0.009 | 0.06 | 0.68 |
| Markers of TNF-α activation | ||||||||
| TNF-α | 0.02 | 0.87 | 0.03 | 0.85 | 0.04 | 0.77 | 0.10 | 0.49 |
| sTNFR1 | 0.32 | 0.02 | 0.10 | 0.48 | 0.20 | 0.15 | 0.48 | 0.0002 |
| sTNFR2 | 0.32 | 0.02 | 0.10 | 0.45 | 0.18 | 0.19 | 0.43 | 0.001 |
TNF-α, tumor necrosis factor-α; sTNFR1 and -2, soluble TNF-α receptor types 1 and 2, respectively.
Muscle attenuation in subjects with the metabolic syndrome compared with control subjects
Mean muscle attenuation was significantly lower in patients with the metabolic syndrome (n = 56, 44.2 ± 0.9 HU) than healthy controls (n = 34, 48.5 ± 0.7 HU) by Student’s t-test (P = 0.0002). The healthy control group was similar in age to the study participants with the metabolic syndrome (mean age of controls 46.9 ± 1.5 yr), but the control group had lower mean BMI (25.7 ± 0.56 kg/m2). Muscle attenuation was inversely correlated with leptin (r = −0.37, P = 0.007), %body fat by DEXA (r = −0.56, P = <0.0001), total body fat by DEXA (r = −0.42, P = 0.002), and VAT (r = −0.41, P = 0.002) but not significantly with SAT (r = −0.25, P = 0.07) among patients with the metabolic syndrome.
Effects of etanercept on metabolic parameters, adiponectin, resistin, and muscle adiposity
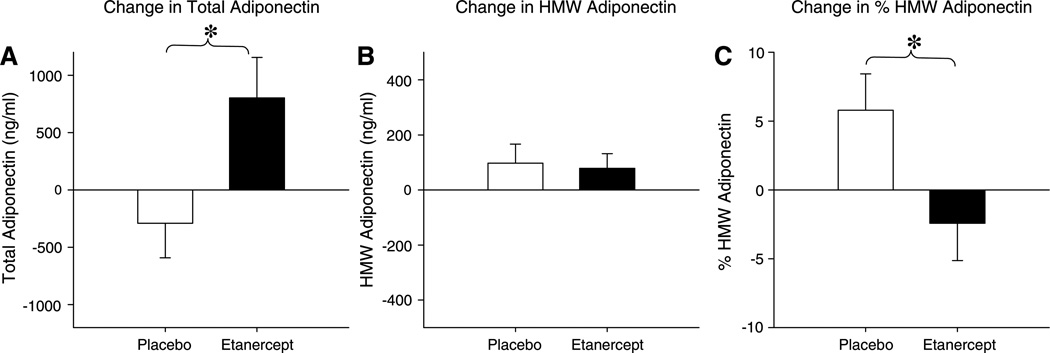
Treatment with etanercept did not affect glucose, insulin, or HOMA-IR (Table 1). Similarly, no effect on insulin sensitivity determined by frequently sampled intravenous glucose tolerance test was observed (0.33 ± 0.2 vs. 0.76 ± 0.34 × 10−4 × min−1 × µIU/ml, P = 0.23, etanercept vs. placebo), as previously reported (4). In the current analysis, we found that, although etanercept increased total adiponectin, HMW adiponectin did not significantly increase in the etanercept-treated group compared with placebo (104 ± 64 vs. 83 ± 70 ng/ml; P = 0.82); therefore, we found a resulting decrease in the HMW adiponectin-to-total adiponectin ratio in the etanercept-treated group compared with placebo (−0.03 ± 0.03 vs. 0.06 ± 0.03; P = 0.03; Fig. 1). Resistin tended to decrease with etanercept treatment compared with placebo (−0.6 ± 0.7 vs. 1.2 ± 0.7 ng/ml, P = 0.06). Leptin did not change in response to etanercept (−3.4 ± 3.1 vs. −1.8 ± 3.0 ng/ml; P = 0.72). Muscle attenuation decreased in the etanercept-treated participants after 4 wk treatment with etanercept compared with placebo (−0.6 ± 0.6 vs. 1.5 ± 0.8 HU; P = 0.04). The change in muscle attenuation was significantly related to change in plasma free fatty acids (β = 7.5 HU·meq−1 · l−1, P = 0.02) among the etanercept-treated group but was not related to change in adiponectin (β= −0.36, P = 0.30).
Fig. 1.
Effects of etanercept treatment on total adiponectin, high-molecular-weight (HMW) adiponectin, and %HMW adiponectin. Mean change is represented by bar, and SE of the mean is represented by error bar. *P < 0.05 by Student’s t-test.
DISCUSSION
Cytokines and hormones secreted by adipose tissue can influence energy metabolism and may be directly involved in pathological processes in obesity. Inflammation has long been linked to obesity and insulin resistance. Prior studies have demonstrated that the adipose tissue-derived proinflammatory cytokine TNF-α is a mediator of insulin resistance and provided insight into the pathophysiological link of inflammatory pathways with insulin resistance in obesity (20). In vitro and in vivo in animal models, TNF-α has been shown to modulate the production of other adipokines, including adiponectin, leptin, and resistin (10, 22, 26, 31, 47, 54). What are the effects of blocking TNF-α activity on these adipokines in humans? We present evidence here that TNF-α neutralization by etanercept in humans with the metabolic syndrome leads to an increase in total adiponectin but not HMW adiponectin, thereby decreasing the HMW-to-total adiponectin ratio. TNF-α neutralization did not affect circulating leptin levels; however, it tended to decrease circulating resistin. Furthermore, treatment with etanercept was associated with increased skeletal muscle adiposity as measured by CT muscle attenuation. Causes of insulin resistance in the metabolic syndrome are multifactorial, and our study further underscores the notion that TNF-α-mediated inflammation is but only one of the multiple possible mechanisms of insulin resistance in obese humans.
TNF-α exerts its effects by binding to two different cell-surface receptors that have been identified: TNFR1 or TNFR2 (30, 52). TNF-α-converting enzyme (TACE/ADAM-17) is a transmembrane metalloproteinase-disintegrin that cleaves and releases the extracellular domain of TNFR1 and TNFR2, thus releasing soluble TNF-α receptors sTNFR1 and sTNFR2 (43). Etanercept is a soluble TNF-α receptor fusion protein (p75 TNF-α receptor 2 fused to Fc fragment of human immunoglobulin G1) that binds TNF-α, blocks its interaction with cell surface receptors, and therefore reduces the biological activity of TNF-α (33). Etanercept prolongs the half-life of TNF-α (with a subsequent rise in measured serum TNF-α levels), yet etanercept renders TNF-α biologically inactive and unavailable to bind to its receptor. Given the difficulties of interpreting various TNF-α assays (43), measurements of sTNFR1 and sTNFR2 may be more reliable in the assessment of TNF-α activity. According to the manufacturer, etanercept is not well distributed in adipose tissue.
Hotamisligil and coworkers (17) have previously found sTNFR2 circulating levels to be significantly elevated in obese female subjects compared with lean control subjects, and their expression levels in adipose tissue of TNFR2 were elevated as well. They showed that TNFR2 expression levels in adipose tissue were strongly correlated with BMI, degree of hyperinsulinemia, and level of TNF-α mRNA expression in fat tissue. In our study, we also found that baseline circulating sTNFR2 correlated with BMI, total body fat, and subcutaneous abdominal fat as did sTNFR1, but we did not find a significant relationship between sTNFR1 or sTNFR2 with insulin levels.
Notably, levels of sTNFR1 and sTNFR2 before etanercept administration correlated with total adiponectin levels but not with HMW adiponectin. In addition, we also found sTNFR1 and sTNFR2 correlated with leptin. Kirchgessner and colleagues (26) had previously shown that TNF-α regulates leptin secretion posttranslationally in cultured adipocytes and in mice in a secretagogue-like fashion. In contrast, etanercept may not affect leptin in vivo, as it sequesters TNF-α in the circulation and may not be well-distributed in adipose tissue.
Maeda and colleagues (31) have previously shown in vitro that TNF-α reduced the expression and secretion of adiponectin in 3T3-L1 adipocytes in a dose-dependent manner. Simons and colleagues (48) have also demonstrated that TNF-α suppressed total adiponectin secretion in cultured human adipocytes in vitro, but they found the amount of secreted HMW complexes were not altered by TNF-α. Consistent with these in vitro results, we have previously shown that total adiponectin increases with TNF-α blockade (4). Upon further investigation, we now find that etanercept causes an increase in circulating total adiponectin levels but not the HMW form. The baseline significant correlation of total adiponectin, but not HMW adiponectin, to sTNFR1 and sTNFR2 is also consistent with our finding that TNF-α neutralization affected total adiponectin levels and not HMW adiponectin.
Although many studies have demonstrated a significant role for adiponectin in obesity and insulin sensitivity (3, 57), other studies have shown a lack of a relationship between total circulating adiponectin and obesity or insulin sensitivity (36). Although thiazolidinedione treatment upregulates mRNA expression and plasma concentrations of adiponectin (6, 31), another insulin-sensitizing agent, metformin, does not affect adiponectin concentrations (6). Both metformin and adiponectin can increase hepatic insulin sensitivity via activation of AMP-activated protein kinase (56, 58). Therefore, thiazolidinediones may increase hepatic insulin sensitivity via raising adiponectin, whereas metformin has direct effects on AMP-activated protein kinase downstream of adiponectin. Levels of HMW adiponectin or the proportion of HMW adiponectin to total adiponectin may possibly be more representative of adiponectin’s biologic activity. Pajvani and colleagues (38) have previously shown that db/db mice have a lower proportion of circulating adiponectin in the HMW form but similar total adiponectin levels compared with wild-type mice. In addition, they also demonstrated that diabetic patients have decreased HMW-to-total adiponectin ratios compared with lean controls. Furthermore, they found the ratio of HMW to total adiponectin to correlate better with insulin sensitivity. Thiazolidinediones have been shown to increase HMW adiponectin (38). In support of these findings, Waki and colleagues (53) found that mutations in the human adiponectin gene, G84R and G90S, which cause impaired multimerization of adiponectin, are associated with diabetes. T-cadherin has been identified to be a receptor for HMW and hexameric adiponectin (21). HMW adiponectin levels have been shown to be inversely related to insulin resistance in patients with the metabolic syndrome traits (16, 29). In our study, the lack of increase in HMW adiponectin and/or the decrease in the HMW-to-LMW ratio may help explain the lack of improvement in insulin sensitivity with etanercept.
In this cohort of patients with the metabolic syndrome, we found that resistin was the adipocytokine that best correlated with insulin resistance. Resistin is a hormone produced by white adipose tissue that is induced during adipocyte differentiation and is reduced by thiazolidinediones (50). The role of resistin as a mediator of insulin resistance in rodents is well-established (2, 50). However, whether resistin is involved in glucose regulation in humans remains controversial (1). On the other hand, resistin has been linked to inflammation in humans (28, 51). These findings are consistent with our results in which there was a trend of etanercept to lower resistin levels.
In vitro data have shown that TNF-α affects resistin mRNA expression and protein secretion. Fasshauer and colleagues (11) have found that resistin mRNA expression and protein secretion were inhibited by 70–90% in 3T3-L1 adipocytes after treatment with TNF-α in a time- and dose-dependent fashion. These results were corroborated by Shojima and others (47) in 3T3-L1 adipocytes. Paradoxically, Kaser and others (22) have found that TNF-α increased resistin mRNA expression in human peripheral blood mononuclear cells. The latter finding may be more directly applicable to humans, since resistin expression appears to be predominantly in mononuclear cells and low/absent in adipocytes in humans, contrary to rodents (46). Therefore, our finding of a trend toward a decrease in resistin with etanercept is biologically plausible. Interestingly, the converse also occurs, since human recombinant resistin has been shown to induce the secretion of TNF-α in macrophages. This mutual amplification may play a role in the inflammation-hormonal signaling interaction.
Neutralization of TNF-α resulted in an increase in thigh muscle adiposity as evidenced by decreasing muscle attenuation among patients with the metabolic syndrome treated with etanercept compared with placebo. Because Goodpaster and colleagues (14) have validated that skeletal muscle attenuation determined by CT is related to muscle lipid content, the decrease in muscle attenuation with etanercept treatment suggests muscle lipid content increased with TNF-α neutralization. The mechanism for the possible increase in muscle lipid content by blocking TNF-α is unknown; however, decreased lipolysis by inhibition of TNF-α within muscle tissue may be a potential mechanism.
In this study, we also showed that muscle attenuation is reduced, indicating more muscle adiposity, in patients with the metabolic syndrome compared with a control population. At baseline, increased indexes of total body and regional fat were associated with muscle attenuation, indicating muscle adiposity is most strongly related to total body and visceral fat in the metabolic syndrome.
In adipose tissue, TNF-α induces lipolysis by decreasing lipoprotein lipase activity and possibly stimulating hormone sensitive lipase. TNF-α decreased transcription of adipocyte lipoprotein lipase gene in vitro (23). In human adipose tissue, Kern and colleagues (24) demonstrated that TNF-α expression inversely correlated with lipoprotein lipase activity. Starnes and colleagues (49) administered recombinant human TNF-α intravenously to patients as part of an antineoplastic trial and found >80% increase in glycerol turnover and >60% increase in free fatty acid turnover, indicating increased whole body lipolysis. TNF-α is expressed in muscle cells, and higher expression of TNF-α occurs in muscle tissue and cultured muscle cells from insulin-resistant and diabetic patients (44); however, the effect of TNF-α or TNF-α blockade on muscle lipolysis is unknown. Our data showing a possible increase in muscle adiposity by etanercept raises the question whether TNF-α blockade decreases lipolysis within muscle.
Increased triglyceride deposition in skeletal muscle is correlated with insulin resistance (27, 39, 42). Possible augmentation of muscle lipid content in the etanercept-treated group may be related to the lack of improvement in insulin sensitivity, contrary to animal data. Hotamisligil and colleagues demonstrated an increase in insulin sensitivity using a euglycemic clamp in mice after TNF-α neutralization for 3 days (20). Perhaps this duration of TNF-α neutralization in rats did not provide enough time for intramyocellular lipids to accumulate. To see if there was a difference in insulin sensitivity after a shorter duration of TNF-α blockade in our study, we analyzed HOMA in the patients that received etanercept at 1 wk after treatment, and there was no improvement when compared with baseline HOMA. Furthermore, single-dose intravenous infusion of a recombinant TNF-α receptor-IgG fusion protein in humans did not improve insulin sensitivity as measured by euglycemic clamp in obese insulin-resistant patients (40), nor did recombinant human TNF-α neutralizing antibody (CDP571) affect insulin sensitivity in obese type 2 diabetic patients (35). In contrast to the animal studies, the lack of improvement in insulin sensitivity in humans could also be the result of differences in the regulation of skeletal muscle fatty acid metabolism between rodent models and humans.
Surprisingly, muscle attenuation decreased despite a significant rise in plasma total adiponectin levels with etanercept treatment. Animal data have shown adiponectin can enhance lipid oxidation and reduce muscle triglycerides (12, 57). Weiss and coworkers (55) have found obese adolescents to have reduced adiponectin levels, and they found that adiponectin levels were inversely related to intramyocellular lipid accumulation, independent of percentage total body fat and central adiposity. In our current study, etanercept likely directly increased adiponectin through effects of blocking TNF-α but simultaneously increased muscle adiposity by decreasing muscle lipolysis. Because etanercept increased adiponectin and simultaneously increased adiposity, our data suggest that the changes in muscle adiposity resulting from etanercept were not related to an effect of adiponectin. In contrast, the significant correlation between decreased free fatty acids and increased muscle adiposity in response to etanercept supports a potential effect of etanercept to decrease lipolysis and increase muscle adiposity through this mechanism. Furthermore, we saw no overall increase in BMI with etanercept treatment in the primary study; thus, increased muscle adiposity could not be attributed to increased overall body fat. In addition, it is unlikely that muscle attenuation changed because of a change in muscle mass or muscle volume, since total lean body mass measured by DEXA showed no change with etanercept treatment (4).
Our study has several potential limitations. The dosing duration was relatively short but adequate to result in significant alterations in adipocytokine concentrations. The effects of longer-term administration of etanercept on glucose homeostasis in humans are not known. Although muscle fat content can be approximated by X-ray attenuation by CT, intramyocellular lipid content was not directly measured. It is uncertain if the muscle attenuation decreased because of extramyocellular fat interspersed within the muscle tissue or lipid within the myocyte.
In conclusion, we investigated the novel effects of etanercept on circulating adipocytokines and muscle adiposity in patients with the metabolic syndrome. We have previously reported that etanercept significantly improved inflammatory markers, including c-reactive protein, total adiponectin, and fibrinogen, but had no effects on insulin resistance in patients with the metabolic syndrome (4). In this experimental paradigm, we extend these findings to demonstrate a decrease in the HMW-to-total adiponectin ratio and an increase in thigh muscle adiposity among patients with the metabolic syndrome treated with etanercept compared with placebo over a short 4-wk period. These negative effects on HMW adiponectin ratio and on muscle adiposity may tend to counteract potential beneficial effects of etanercept on insulin resistance and explain the absent effect of etanercept on insulin sensitivity in this and other short-term human studies. Etanercept appears to inhibit the TNF-α-mediated inflammatory cascade on obesity, but further long-term studies with more direct endpoints are required to study the effects on adipocytokines and muscle fat and clinical consequences of these changes.
ACKNOWLEDGMENTS
We thank the nursing and bionutrition staff of the Massachusetts General Hospital (MGH) and Massachusetts Institute of Technology General Clinical Research Center, the radiology department staff, and especially all the volunteers who participated in this study.
GRANTS
This work was supported by National Institutes of Health Grants M01 RR-01066-25S1 (MGH General Clinical Research Center), T32 HD-052961-01A (S. K. Grinspoon, J. Lo), K30 RR-022292-07 (J. Lo), and P01DK-49210 (R. S. Ahima, M. B. Jackson).
Footnotes
DISCLOSURE
The randomized trial was supported by Amgen, Inc.
REFERENCES
- 1.Arner P. Resistin: yet another adipokine tells us that men are not mice. Diabetologia. 2005;48:2203–2205. doi: 10.1007/s00125-005-1956-3. [DOI] [PubMed] [Google Scholar]
- 2.Banerjee RR, Rangwala SM, Shapiro JS, Rich AS, Rhoades B, Qi Y, Wang J, Rajala MW, Pocai A, Scherer PE, Steppan CM, Ahima RS, Obici S, Rossetti L, Lazar MA. Regulation of fasted blood glucose by resistin. Science. 2004;303:1195–1198. doi: 10.1126/science.1092341. [DOI] [PubMed] [Google Scholar]
- 3.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 4.Bernstein LE, Berry J, Kim S, Canavan B, Grinspoon SK. Effects of etanercept in patients with the metabolic syndrome. Arch Intern Med. 2006;166:902–908. doi: 10.1001/archinte.166.8.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borkan GA, Gerzof SG, Robbins AH, Hults DE, Silbert CK, Silbert JE. Assessment of abdominal fat content by computed tomography. Am J Clin Nutr. 1982;36:172–177. doi: 10.1093/ajcn/36.1.172. [DOI] [PubMed] [Google Scholar]
- 6.Combs TP, Wagner JA, Berger J, Doebber T, Wang WJ, Zhang BB, Tanen M, Berg AH, O’Rahilly S, Savage DB, Chatterjee K, Weiss S, Larson PJ, Gottesdiener KM, Gertz BJ, Charron MJ, Scherer PE, Moller DE. Induction of adipocyte complement-related protein of 30 kilodaltons by PPARgamma agonists: a potential mechanism of insulin sensitization. Endocrinology. 2002;143:998–1007. doi: 10.1210/endo.143.3.8662. [DOI] [PubMed] [Google Scholar]
- 7.Dominguez H, Storgaard H, Rask-Madsen C, Steffen Hermann T, Ihlemann N, Baunbjerg Nielsen D, Spohr C, Kober L, Vaag A, Torp-Pedersen C. Metabolic and vascular effects of tumor necrosis factor-alpha blockade with etanercept in obese patients with type 2 diabetes. J Vasc Res. 2005;42:517–525. doi: 10.1159/000088261. [DOI] [PubMed] [Google Scholar]
- 8.Ebinuma H, Miyazaki O, Yago H, Hara K, Yamauchi T, Kadowaki T. A novel ELISA system for selective measurement of human adiponectin multimers by using proteases. Clin Chim Acta. 2006;372:47–53. doi: 10.1016/j.cca.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 9.Fairfield WP, Treat M, Rosenthal DI, Frontera W, Stanley T, Corcoran C, Costello M, Parlman K, Schoenfeld D, Klibanski A, Grinspoon S. Effects of testosterone and exercise on muscle leanness in eugonadal men with AIDS wasting. J Appl Physiol. 2001;90:2166–2171. doi: 10.1152/jappl.2001.90.6.2166. [DOI] [PubMed] [Google Scholar]
- 10.Fasshauer M, Klein J, Neumann S, Eszlinger M, Paschke R. Hormonal regulation of adiponectin gene expression in 3T3–L1 adipocytes. Biochem Biophys Res Commun. 2002;290:1084–1089. doi: 10.1006/bbrc.2001.6307. [DOI] [PubMed] [Google Scholar]
- 11.Fasshauer M, Klein J, Neumann S, Eszlinger M, Paschke R. Tumor necrosis factor alpha is a negative regulator of resistin gene expression and secretion in 3T3–L1 adipocytes. Biochem Biophys Res Commun. 2001;288:1027–1031. doi: 10.1006/bbrc.2001.5874. [DOI] [PubMed] [Google Scholar]
- 12.Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erickson MR, Yen FT, Bihain BE, Lodish HF. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci USA. 2001;98:2005–2010. doi: 10.1073/pnas.041591798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez-Gay MA, De Matias JM, Gonzalez-Juanatey C, Garcia-Porrua C, Sanchez-Andrade A, Martin J, Llorca J. Anti-tumor necrosis factor-alpha blockade improves insulin resistance in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2006;24:83–86. [PubMed] [Google Scholar]
- 14.Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol. 2000;89:104–110. doi: 10.1152/jappl.2000.89.1.104. [DOI] [PubMed] [Google Scholar]
- 15.Grunfeld C, Zhao C, Fuller J, Pollack A, Moser A, Friedman J, Feingold KR. Endotoxin and cytokines induce expression of leptin, the ob gene product, in hamsters. J Clin Invest. 1996;97:2152–2157. doi: 10.1172/JCI118653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hara K, Horikoshi M, Yamauchi T, Yago H, Miyazaki O, Ebinuma H, Imai Y, Nagai R, Kadowaki T. Measurement of the high-molecular weight form of adiponectin in plasma is useful for the prediction of insulin resistance and metabolic syndrome. Diabetes Care. 2006;29:1357–1362. doi: 10.2337/dc05-1801. [DOI] [PubMed] [Google Scholar]
- 17.Hotamisligil GS, Arner P, Atkinson RL, Spiegelman BM. Differential regulation of the p80 tumor necrosis factor receptor in human obesity and insulin resistance. Diabetes. 1997;46:451–455. doi: 10.2337/diab.46.3.451. [DOI] [PubMed] [Google Scholar]
- 18.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hotamisligil GS, Budavari A, Murray D, Spiegelman BM. Reduced tyrosine kinase activity of the insulin receptor in obesity-diabetes. Central role of tumor necrosis factor-alpha. J Clin Invest. 1994;94:1543–1549. doi: 10.1172/JCI117495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 21.Hug C, Wang J, Ahmad NS, Bogan JS, Tsao TS, Lodish HF. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci USA. 2004;101:10308–10313. doi: 10.1073/pnas.0403382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaser S, Kaser A, Sandhofer A, Ebenbichler CF, Tilg H, Patsch JR. Resistin messenger-RNA expression is increased by proinflammatory cytokines in vitro. Biochem Biophys Res Commun. 2003;309:286–290. doi: 10.1016/j.bbrc.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Kawakami M, Pekala PH, Lane MD, Cerami A. Lipoprotein lipase suppression in 3T3–L1 cells by an endotoxin-induced mediator from exudate cells. Proc Natl Acad Sci USA. 1982;79:912–916. doi: 10.1073/pnas.79.3.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kern PA, Saghizadeh M, Ong JM, Bosch RJ, Deem R, Simsolo RB. The expression of tumor necrosis factor in human adipose tissue. Regulation by obesity, weight loss, and relationship to lipoprotein lipase. J Clin Invest. 1995;95:2111–2119. doi: 10.1172/JCI117899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiortsis DN, Mavridis AK, Vasakos S, Nikas SN, Drosos AA. Effects of infliximab treatment on insulin resistance in patients with rheumatoid arthritis and ankylosing spondylitis. Ann Rheum Dis. 2005;64:765–766. doi: 10.1136/ard.2004.026534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirchgessner TG, Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Tumor necrosis factor-alpha contributes to obesity-related hyperleptinemia by regulating leptin release from adipocytes. J Clin Invest. 1997;100:2777–2782. doi: 10.1172/JCI119824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krssak M, Falk Petersen K, Dresner A, DiPietro L, Vogel SM, Rothman DL, Roden M, Shulman GI. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia. 1999;42:113–116. doi: 10.1007/s001250051123. [DOI] [PubMed] [Google Scholar]
- 28.Kusminski CM, da Silva NF, Creely SJ, Fisher fM, Harte AL, Baker AR, Kumar S, McTernan PG. The in vitro effects of resistin on the innate immune signaling pathway in isolated human subcutaneous adipocytes. J Clin Endocrinol Metab. 2007;92:270–276. doi: 10.1210/jc.2006-1151. [DOI] [PubMed] [Google Scholar]
- 29.Lara-Castro C, Luo N, Wallace P, Klein RL, Garvey WT. Adiponectin multimeric complexes and the metabolic syndrome trait cluster. Diabetes. 2006;55:249–259. [PubMed] [Google Scholar]
- 30.Loetscher H, Pan YCE, Lahm HW, Gentz R, Brockhaus M, Tabuchi H, Lesslauer W. Molecular cloning and expression of the human 55 kd tumor necrosis factor receptor. Cell. 1990;61:351–359. doi: 10.1016/0092-8674(90)90815-v. [DOI] [PubMed] [Google Scholar]
- 31.Maeda N, Takahashi M, Funahashi T, Kihara S, Nishizawa H, Kishida K, Nagaretani H, Matsuda M, Komuro R, Ouchi N, Kuriyama H, Hotta K, Nakamura T, Shimomura I, Matsuzawa Y. PPARgamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes. 2001;50:2094–2099. doi: 10.2337/diabetes.50.9.2094. [DOI] [PubMed] [Google Scholar]
- 32.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 33.Mohler KM, Torrance DS, Smith CA, Goodwin RG, Stremler KE, Fung VP, Madani H, Widmer MB. Soluble tumor necrosis factor (TNF) receptors are effective therapeutic agents in lethal endotoxemia and function simultaneously as both TNF carriers and TNF antagonists. J Immunol. 1993;151:1548–1561. [PubMed] [Google Scholar]
- 34.Nakano Y, Tajima S, Yoshimi A, Akiyama H, Tsushima M, Tanioka T, Negoro T, Tomita M, Tobe T. A novel enzyme-linked immunosorbent assay specific for high-molecular-weight adiponectin. J Lipid Res. 2006;47:1572–1582. doi: 10.1194/jlr.D600010-JLR200. [DOI] [PubMed] [Google Scholar]
- 35.Ofei F, Hurel S, Newkirk J, Sopwith M, Taylor R. Effects of an engineered human anti-TNF-alpha antibody (CDP571) on insulin sensitivity and glycemic control in patients with NIDDM. Diabetes. 1996;45:881–885. doi: 10.2337/diab.45.7.881. [DOI] [PubMed] [Google Scholar]
- 36.Owecki M, Miczke A, Pupek-Musialik D, Bryl W, Cymerys M, Nikisch E, Sowinski J. Circulating serum adiponectin concentrations do not differ between obese and non-obese caucasians and are unrelated to insulin sensitivity. Horm Metab Res. 2007;39:25–30. doi: 10.1055/s-2007-957343. [DOI] [PubMed] [Google Scholar]
- 37.Pajvani UB, Du X, Combs TP, Berg AH, Rajala MW, Schulthess T, Engel J, Brownlee M, Scherer PE. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications fpr metabolic regulation and bioactivity. J Biol Chem. 2003;278:9073–9085. doi: 10.1074/jbc.M207198200. [DOI] [PubMed] [Google Scholar]
- 38.Pajvani UB, Hawkins M, Combs TP, Rajala MW, Doebber T, Berger JP, Wagner JA, Wu M, Knopps A, Xiang AH, Utzschneider KM, Kahn SE, Olefsky JM, Buchanan TA, Scherer PE. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem. 2004;279:12152–12162. doi: 10.1074/jbc.M311113200. [DOI] [PubMed] [Google Scholar]
- 39.Pan DA, Lillioja S, Kriketos AD, Milner MR, Baur LA, Bogardus C, Jenkins AB, Storlien LH. Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes. 1997;46:983–988. doi: 10.2337/diab.46.6.983. [DOI] [PubMed] [Google Scholar]
- 40.Paquot N, Castillo MJ, Lefebvre PJ, Scheen AJ. No increased insulin sensitivity after a single intravenous administration of a recombinant human tumor necrosis factor receptor: Fc fusion protein in obese insulin-resistant patients. J Clin Endocrinol Metab. 2000;85:1316–1319. doi: 10.1210/jcem.85.3.6417. [DOI] [PubMed] [Google Scholar]
- 41.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350:664–671. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phillips DI, Caddy S, Ilic V, Fielding BA, Frayn KN, Borthwick AC, Taylor R. Intramuscular triglyceride and muscle insulin sensitivity: evidence for a relationship in nondiabetic subjects. Metabolism. 1996;45:947–950. doi: 10.1016/s0026-0495(96)90260-7. [DOI] [PubMed] [Google Scholar]
- 43.Reddy P, Slack JL, Davis R, Cerretti DP, Kozlosky CJ, Blanton RA, Shows D, Peschon JJ, Black RA. Functional analysis of the domain structure of tumor necrosis factor-alpha converting enzyme. J Biol Chem. 2000;275:14608–14614. doi: 10.1074/jbc.275.19.14608. [DOI] [PubMed] [Google Scholar]
- 44.Saghizadeh M, Ong JM, Garvey WT, Henry RR, Kern PA. The expression of TNFalpha by human muscle. Relationship to insulin resistance. J Clin Invest. 1996;97:1111–1116. doi: 10.1172/JCI118504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sarraf P, Frederich RC, Turner EM, Ma G, Jaskowiak NT, Rivet DJ, 3rd, Flier JS, Lowell BB, Fraker DL, Alexander HR. Multiple cytokines and acute inflammation raise mouse leptin levels: potential role in inflammatory anorexia. J Exp Med. 1997;185:171–175. doi: 10.1084/jem.185.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Savage DB, Sewter CP, Klenk ES, Segal DG, Vidal-Puig A, Considine RV, O’Rahilly S. Resistin/Fizz3 expression in relation to obesity and peroxisome proliferator-activated receptor-gamma action in humans. Diabetes. 2001;50:2199–2202. doi: 10.2337/diabetes.50.10.2199. [DOI] [PubMed] [Google Scholar]
- 47.Shojima N, Sakoda H, Ogihara T, Fujishiro M, Katagiri H, Anai M, Onishi Y, Ono H, Inukai K, Abe M, Fukushima Y, Kikuchi M, Oka Y, Asano T. Humoral regulation of resistin expression in 3T3–L1 and mouse adipose cells. Diabetes. 2002;51:1737–1744. doi: 10.2337/diabetes.51.6.1737. [DOI] [PubMed] [Google Scholar]
- 48.Simons PJ, van den Pangaart PS, Aerts JM, Boon L. Pro-inflammatory delipidizing cytokines reduce adiponectin secretion from human adipocytes without affecting adiponectin oligomerization. J Endocrinol. 2007;192:289–299. doi: 10.1677/JOE-06-0047. [DOI] [PubMed] [Google Scholar]
- 49.Starnes HF, Jr, Warren RS, Jeevanandam M, Gabrilove JL, Larchian W, Oettgen HF, Brennan MF. Tumor necrosis factor and the acute metabolic response to tissue injury in man. J Clin Invest. 1988;82:1321–1325. doi: 10.1172/JCI113733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 51.Szapary PO, Bloedon LT, Samaha FF, Duffy D, Wolfe ML, Soffer D, Reilly MP, Chittams J, Rader DJ. Effects of pioglitazone on lipoproteins, inflammatory markers, and adipokines in nondiabetic patients with metabolic syndrome. Arterioscler Thromb Vasc Biol. 2006;26:182–188. doi: 10.1161/01.ATV.0000195790.24531.4f. [DOI] [PubMed] [Google Scholar]
- 52.Tartaglia LA, Weber RF, Figari IS, Reynolds C, Palladino MA, Jr, Goeddel DV. The two different receptors for tumor necrosis factor mediate distinct cellular responses. Proc Natl Acad Sci USA. 1991;88:9292–9296. doi: 10.1073/pnas.88.20.9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Waki H, Yamauchi T, Kamon J, Ito Y, Uchida S, Kita S, Hara K, Hada Y, Vasseur F, Froguel P, Kimura S, Nagai R, Kadowaki T. Impaired multimerization of human adiponectin mutants associated with diabetes: molecular structure and multimer formation of adiponectin. J Biol Chem. 2003;278:40352–40363. doi: 10.1074/jbc.M300365200. [DOI] [PubMed] [Google Scholar]
- 54.Wang B, Jenkins JR, Trayhurn P. Expression and secretion of inflammation-related adipokines by human adipocytes differentiated in culture: integrated response to TNF-α. Am J Physiol Endocrinol Metab. 2005;288:E731–E740. doi: 10.1152/ajpendo.00475.2004. [DOI] [PubMed] [Google Scholar]
- 55.Weiss R, Dufour S, Groszmann A, Petersen K, Dziura J, Taksali SE, Shulman G, Caprio S. Low adiponectin levels in adolescent obesity: a marker of increased intramyocellular lipid accumulation. J Clin Endocrinol Metab. 2003;88:2014–2018. doi: 10.1210/jc.2002-021711. [DOI] [PubMed] [Google Scholar]
- 56.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 57.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 58.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zumbach MS, Boehme MWJ, Wahl P, Stremmel W, Ziegler R, Nawroth PP. Tumor necrosis factor increases serum leptin levels in humans. J Clin Endocrinol Metab. 1997;82:4080–4082. doi: 10.1210/jcem.82.12.4408. [DOI] [PubMed] [Google Scholar]