Abstract
Background
Increased age is a major risk factor for stroke incidence, post-ischemic mortality, and severe and long-term disability. Stroke outcome is considerably influenced by post-ischemic mechanisms. We hypothesized that the inflammatory response following an ischemic injury is altered in aged organisms.
Methods and Results
To that end, we analyzed the expression pattern of pro-inflammatory cytokines (TNF, IL-1α, IL-1β, IL-6), anti-inflammatory cytokines (IL-10, TGFβ1), and chemokines (Mip-1α, MCP-1, RANTES) of adult (2 months) and aged (24 months) mice brains at different reperfusion times (6 h, 12 h, 24 h, 2 d, 7 d) following transient occlusion of the middle cerebral artery. The infarct size was assessed to monitor possible consequences of an altered inflammatory response in aged mice. Our data revealed an increased neuro-inflammation with age. Above all, we found profound age-related alterations in the reaction to stroke. The response of pro-inflammatory cytokines (TNF, and IL-1β) and the level of chemokines (Mip-1α, and MCP-1) were strongly diminished in the aged post-ischemic brain tissue. IL-6 showed the strongest age-dependent decrease in its post-ischemic expression profile. Anti-inflammatory cytokines (TGFβ1, and IL-10) revealed no significant age dependency after ischemia. Aged mice brains tend to develop smaller infarcts.
Conclusion
The attenuated inflammatory response to stroke in aged animals may contribute to their smaller infarcts. The results presented here highlight the importance of using aged animals to investigate age-associated diseases like stroke, and should be considered as a major prerequisite in the development of age-adjusted therapeutic interventions.
Introduction
In general, the interplay between the peripheral immune system and the brain is well balanced in young organisms but becomes unfavorable with ageing [1]. Impaired immune homeostasis in aged organisms is associated with a decline in the ability to adapt to environmental stress. The consequence of this is a significant higher vulnerability for diseases and their impacts in the elderly. Stroke is the most common and most important vascular disease of the cerebral nervous system. Cerebral infarcts are the second leading cause of death worldwide and the leading cause of adult disability. Increased age is a major risk factor for stroke incidence, post-ischemic mortality, and severe and long-term disability [2], [3], [4]. Although stroke is an extremely important health issue, most pharmaceutical companies have terminated their research programs due to the failure of previous studies [5]. One explanation for the inability to transfer experimental results to the clinic may be the predominant incorporation of young rodents in basic research. Several initial indications that the response of the central nervous system to an ischemic event is age dependent have been reported [6], [7], [8], [9]. However, post-ischemic mechanisms are complex and more fundamental studies are required to understand the impact of age on these processes.
Following cerebral ischemia, a cascade of inflammatory mediators including cytokines (TNF, IL-1α, IL-1β, IL-6, IL-10, TGFβ1) and chemokines (MCP-1 [CCL2], Mip-1α [CCL3], RANTES [CCL5]) is initiated. Cytokines and chemokines have complex, overlapping and pleiotropic functions that may be both beneficial and deleterious. The temporal expression profile of each inflammatory mediator, its specific cell source and target, and their coordinated interaction are important for stroke recovery. The complex as well as dual role (beneficial versus deleterious) of the inflammatory response to stroke has been extensively discussed [10], [11], [12], [13]. Ageing is associated with alterations of the cerebral inflammatory system [14], [15], [16], [17]. In particular, TNF, IL-1, and IL-6 have been found to be increased with age [7], [18]. The inflammatory reaction of aged brains to cerebral injuries has received little attention to date [19], [20], [21], [22]. Indeed, the response to stroke is currently unknown. It is assumed that the inflammatory reaction influences the dimension of the injury [20]. Previous studies investigating the infarct size in old mice have revealed conflicting results; there are reports of increased, decreased or similar infarct sizes in aged brains [8].
We hypothesized that the extent and the progression of the immune response following an ischemic injury are altered in the elderly and that this age-dependent inflammatory reaction is a determinant of the outcome following stroke. The aim of the present study was to systematically characterize the age-dependent changes of the infarct size and the inflammatory response in an experimental stroke model of mice. Transient occlusion of the middle cerebral artery (MCAO) – a model that closely resembles human stroke [23] – was used to induce cerebral infarction. The infarct size was analyzed at three different reperfusion times up to 7 days. The expression patterns of pro-inflammatory cytokines (TNF, IL-1α, IL-1β, IL-6), anti-inflammatory cytokines (IL-10, TGFβ1), and chemokines (Mip-1α, MCP-1, RANTES) were investigated at different reperfusion times (6 h, 12 h, 24 h, 2 d, 7 d).
Materials and Methods
All investigated mice were taken from a C57/BL6 inbred mice strain, originally obtained from Jackson Laboratory, and randomly assigned to the groups (described in more detail in Supplement S1). Adult (2 months), middle-aged (9 months), and aged (15 months and 24 months) male native mice were used to investigate age-related neuro-inflammation (n = 4, each). The age-dependent inflammatory response following induction of a cerebral infarct (6 h, 12 h, 24 h, 2 d, and 7 d) was studied using adult (2 months) and aged (24 months) mice that had undergone MCAO (n = 4, each). Infarct volumes were analyzed 2 h, 2 d, and 7 d after MCAO (n = 4, each). MCAO was performed as described previously [24]. Briefly, a monofilament was introduced into the internal carotid artery through an incision of the left common carotid artery. In this position, the middle cerebral artery was occluded for 30 min. Up to 7 d after reperfusion, the mortality rates were 7% and 39% in adult and aged mice, respectively. The effect of the surgical procedure was controlled using sham-operated mice (n = 4, each). These animals underwent anesthesia and surgical procedures similar to the MCAO group but without occlusion of the middle cerebral artery. In a pre-study, the cerebral blood flow (CBF) was measured by laser Doppler flowmetry (LDF) (Peri Flux System 5000, Perimed, Sweden) in adult (n = 10) and aged (n = 9) mice. Rectal temperature was measured using the DC Temperature Control System (FHC Inc., USA) in adult (n = 6) and aged (n = 8) mice. Animals were maintained with access to water and food ad libitum. All animal procedures were approved by the local government (Thueringer Landesamt für Lebensmittelsicherheit und Verbraucherschutz [TLLV], Dep.2, Gesundheitlicher Verbraucherschutz, Veterinärwesen, Pharmazie, Germany, Approval ID's 02-20/05 and 02-028/10) and conformed to international guidelines on the ethical use of animals. All surgeries were performed under deep anesthesia (isoflurane).
Sample preparation
Infarct volumes were analyzed on Map2 immunostained slices [2 h, 2 d, and 7 d after reperfusion, as described in detail by us previously [25]]. Deeply anesthetized mice were fixed by perfusion through the ascending aorta with 4% paraformaldehyde. Brains were removed, cryoprotected in 0.2 M phosphate-buffered saline containing 30% sucrose, and stored at −80°C. Coronal sections (40-µm thick) were cut with a freezing microtome (MH400, Microm International GmbH).
For mRNA and protein quantification, a separate group of mice underwent MCAO. Animals were decapitated under deep anesthesia and brains were removed at 6 h, 12 h, 24 h, 2 d, and 7 d. Using a Precision Brain Slicer (BS-2000C Adult Mouse, Braintree Scientific Inc.), three coronal sections were dissected cutting +2.8 and +0.8 mm to bregma (rostral slice), +0.8 and −1.2 mm to bregma (middle slice), and −1.2 and −3.2 mm to bregma (caudal slice). The ipsilateral (ischemic tissue) and contralateral (non-ischemic tissue) hemispheres of the middle brain slice were separated and snap-frozen (for details see Supplement S1). Adjacent slices were used for infarct validation (Map2 immunohistochemistry), to select mice with similar lesion size for mRNA and protein quantification.
Tissue samples of native mice (right hemisphere, +0.8 and −1.2 mm to bregma) were used to study the age-related cerebral inflammation.
For quantitative PCR (qPCR), tissue samples were homogenized in 1 ml QIAzol Lysis Reagent and total RNA was isolated using the RNeasy Lipid Tissue Mini Kit (Qiagen GmbH). For cytokine bead assay (CBA), tissue samples were homogenized in 400 µl ice-cold lysis buffer (0.32 M sucrose; 4 mM Tris-HCl, pH 7.4; 1 mM EDTA; and 0.25 mM dithiothreitol) containing protease inhibitors Complete Mini (Roche) and proteins were isolated as previously described [26].
Immunohistochemistry (Map2)
Free-floating sections (cerebrum, 40 µm thick) were incubated at 4°C overnight with antibody against Map2 (monoclonal mouse anti-MAP2 (2a+2b), clone AP-20, Sigma-Aldrich, 1∶1,000), then further processed by the Vectastain Elite ABC Kit (Vector Laboratories) using a biotinylated secondary antibody (donkey anti-mouse, Dianova, 1∶500). Immunoreactivity was developed in 3,3′-diaminobenzidine tetrahydrochloride (DAB; Sigma).
qPCR
For qPCR, equal amounts of total RNA (1 µg) were transcribed in cDNA with RevertAid First Strand cDNA synthesis kit (Fermentas [27]). Amplification products were analyzed using gel electrophoresis, melting curve analysis, and sequencing to confirm the PCR product specificity. PCR was performed in a volume of 20 µl containing Brilliant® II SYBR Green® qPCR Master Mix (Stratagene), cDNA (equivalent to 25 ng reverse transcribed RNA), and specific primers (Table 1) each at a final concentration of 500 nM. Amplification was performed using the Rotor-Gene 6000 (Corbett Life Science) applying the following cycle conditions: 10 min polymerase activation, 40 amplification cycles (95°C for 30 s, 60°C for 30 s, 72°C for 30 s), and melting curve.
Table 1. Primer sequences.
| Primer | Sequence (5′→3′) | GenBank accession number |
| Gapdh forward | CAACAGCAACTCCCACTCTTC | NM_008084.2 |
| Gapdh reverse | GGTCCAGGGTTTCTTACTCCTT | |
| Hmbs forward | GAAATCATTGCTATGTCCACCA | NM_013551.2 |
| Hmbs reverse | GCGTTTTCTAGCTCCTTGGTAA | |
| TNFα forward | GTCTACTGAACTTCGGGGTGAT | NM_013693.2 |
| TNFα reverse | ATGATCTGAGTGTGAGGGTCTG | |
| IL-1α forward | GCCTTATTTCGGGAGTCTAT | NM_010554.4 |
| IL-1α reverse | TAGGGTTTGCTCTTCTCTTACA | |
| IL-1β forward | GAAGAGCCCATCCTCTGTGA | NM_008361.3 |
| IL-1β reverse | TTCATCTCGGAGCCTGTAGTG | |
| IL-6 forward | ACAAAGCCAGAGTCCTTCAGAG | NM_031168.1 |
| IL-6 reverse | CATTGGAAATTGGGGTAGGA | |
| IL-10 forward | ATGGTGTCCTTTCAATTGCTCT | NM_010548.1 |
| IL-10 reverse | AGGATCTCCCTGGTTTCTCTTC | |
| TGFβ1 forward | TGCTTCAGCTCCACAGAGAA | NM_011577.1 |
| TGFβ1 reverse | TACTGTGTGTCCAGGCTCCA | |
| Mip-1α forward | TGGAACTGAATGCCTGAGAGT | NM_011337.2 |
| Mip-1α reverse | TAGGAGATGGAGCTATGCAGGT | |
| MCP-1 forward | AGGTGTCCCAAAGAAGCTGTAG | NM_011333.3 |
| MCP-1 reverse | AATGTATGTCTGGACCCATTCC | |
| RANTES forward | CCAGAGAAGAAGTGGGTTCAAG | NM_013653.2 |
| RANTES reverse | AAGCTGGCTAGGACTAGAGCAA |
The GenBank accession numbers were obtained from the NCBI (March 2010).
Cytokine bead assay (CBA)
The protein expression pattern was measured by a CBA with TNF, IL-1α, IL-1β, IL-6, IL-10, Mip-1α, MCP-1, and RANTES mouse Flex Sets (BD Bioscience). No antibody was available for TGFβ1. The procedure was performed according to the manufacturer's instructions with the following modifications. To ensure a valid analysis of proteins below 10 pg/ml (the default outlined limit for quantification), (i) 300 µg whole protein was dissolved in 50 µl lysis buffer, (ii) the standard curve was constructed from 1.25–156 pg/ml (dissolved in lysis buffer), and (iii) samples as well as standards were washed twice at the end of the procedure to reduce background signals. Standards and test samples were analyzed using the Cytomics FC500 Flow Cytometry System (Beckman Coulter) with its settings optimized to ensure valid quantifications even for proteins present in very low amounts.
Data analysis
qPCR
External standard curves of purified PCR products (five 10-fold dilution series) were applied for absolute quantification, as described previously [27]. The numbers of transcripts were calculated per 1,000 transcripts of Gapdh by including the criterion of the length of each specific amplicon. Hmbs was used as a second internal standard. Both Gapdh and Hmbs were stably expressed under the conditions of investigation.
CBA
The raw data from the Cytomics FC500 Flow Cytometry System were analyzed using FCAP Array™ software (SoftFlow). Protein concentrations of the test samples were calculated using a corresponding standard curve (supplied recombinant protein with known concentration).
Statistical analysis
Differences between the infarct volumes of adult (2 months) and aged (24 months) mice at different time points (2 h, 2 d, and 7 d) were analyzed with ANOVA and post-hoc Tukey test. Significant differences of mRNA and protein expression values dependent on ageing per se (versus 2-month-old controls) were calculated with ANOVA and post-hoc Tukey test (& p≤0.05, && p≤0.01, &&& p≤0.001). Correlation of the expression level with age (R, Pearson correlation) is indicated by + p≤0.05, ++ p≤0.01, and +++ p≤0.001. Significant post-ischemic differences of the expressions levels (ipsi versus contra) at several time points (6 h, 12 h, 24 h, 2 d and 7 d) were calculated with paired two-way ANOVA and post-hoc Tukey test. Analyses were done separately for adult (* p≤0.05, ** p≤0.01, *** p≤0.001) and aged (# p≤0.05, ## p≤0.01, ### p≤0.001) mice. Significant age-dependent differences of absolute expression within the ipsilateral hemisphere were calculated within a further two-way ANOVA and are displayed by † p≤0.05, †† p≤0.01, ††† p≤0.001. All tests were performed with SigmaStat (software version 3.5).
Results
Almost all the tested inflammatory mediators showed increased expression values during ageing per se. The pro-inflammatory cytokines, particularly TNF, as well as the chemokines Mip-1α and MCP-1 showed a distinct up-regulation with age. Following stroke, the expression of almost all the tested inflammatory mediators was significantly up-regulated in ischemic tissues. However, a clearly attenuated inflammatory response was apparent in aged brains following the ischemic insult. TNF, IL-1α, IL-1β, IL-6, Mip-1α, and MCP-1 displayed an age-related decreased post-ischemic expression, with IL-6 exhibiting the strongest decrease. Aged mice brains also exhibited smaller infarcts. The sham procedure had no substantial effect on the expression of all the tested cytokines and chemokines. No significant differences of the cerebral blood flow and the body temperature were observed between adult and aged mice during or after MCAO (data not shown).
Infarct volume
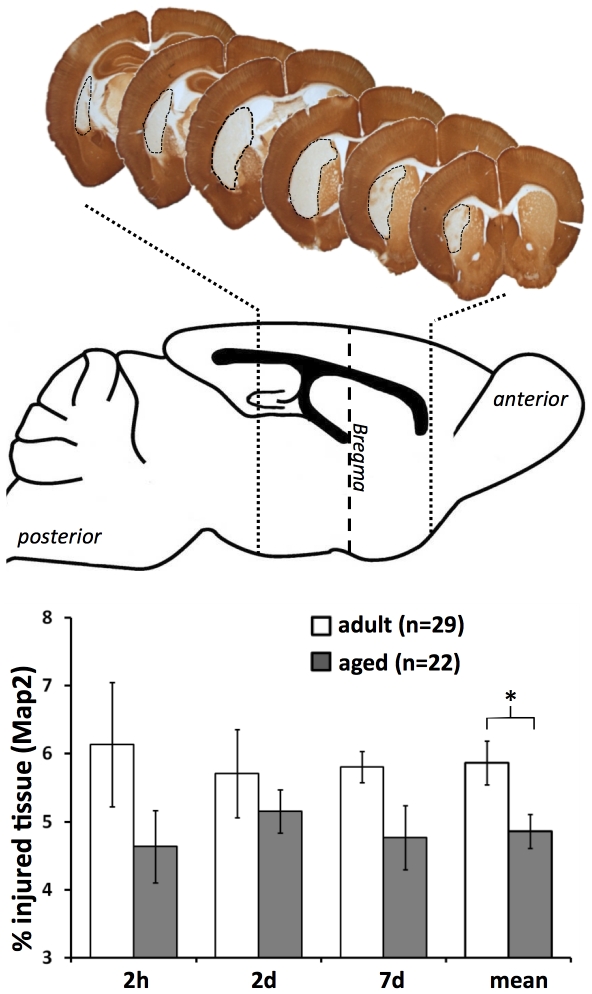
Occlusion of the middle cerebral artery for 30 min led to an ischemic injury mainly restricted to striatal regions of the mice brains. At different reperfusion times (2 h, 2 d, and 7 d) the infarct size tend to be smaller in aged mice. Taking all time points together, the mean infarct volume of aged mice was significantly smaller (Figure 1, Map2 immunohistochemistry, ANOVA with post-hoc Tukey test, p≤0.05).
Figure 1. Infarct volumes at different ages.
The mean infarct volume (±SEM) was significantly smaller in aged mice brains (Map2 immunohistochemistry, ANOVA with post-hoc Tukey test, * p≤0.05).
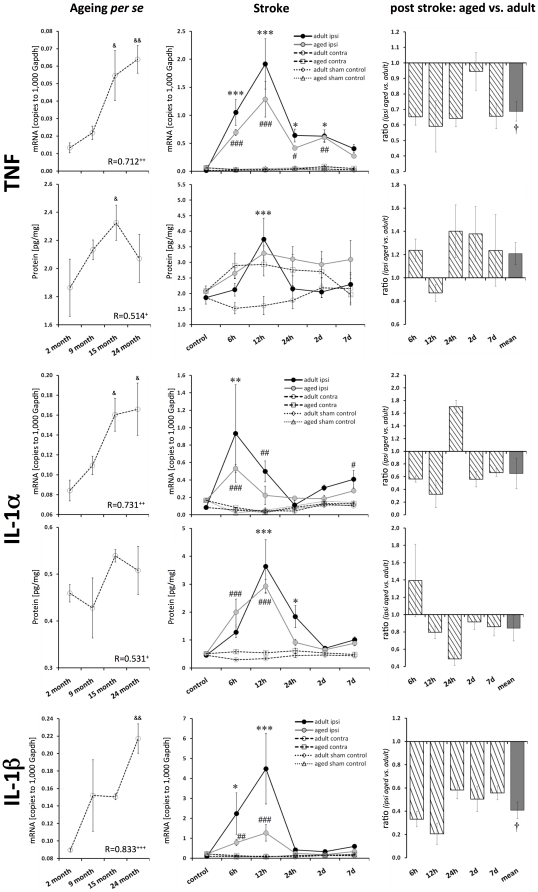
Pro-inflammatory cytokines TNF, IL-1α, and IL-1β
All the tested major pro-inflammatory cytokines were significantly elevated with ageing per se regarding their mRNA level (TNF 5-fold, p≤0.01; IL-1α ∼1.9-fold, p≤0.05; IL-1β ∼2.4-fold, p≤0.01; Figure 2). This up-regulation was reflected in the level of protein, though it was not as pronounced (TNF 1.1-fold, n.s.; IL-1α 1.1-fold, n.s.; Figure 2).
Figure 2. Age- and stroke-dependent expression of TNF, IL-1α, and IL-1β.
TNF, IL-1α, and IL-1β displayed significantly elevated expression levels with age. All these pro-inflammatory cytokines showed a distinct response following stroke, which was attenuated in aged brains. Expression values are shown as mean ± SEM and ratios as geometric mean ± SEM. Significant age-related differences (versus 2-month-old native mice) are indicated by & p≤0.05, && p≤0.01, and &&& p≤0.001 (ANOVA and post-hoc Tukey test). Correlation of the cytokine expression level with age is indicated by + p≤0.05, ++ p≤0.01, and +++ p≤0.001 (R, Pearson correlation). Significant post-stroke differences (ipsi versus contra) are indicated by adult: * p≤0.05, ** p≤0.01, and *** p≤0.001; and aged: # p≤0.05, ## p≤0.01, and ### p≤0.001 (paired two-way ANOVA and post-hoc Tukey test). Significant age-dependent differences after stroke (ipsi aged versus ipsi adult) are displayed by † p≤0.05, †† p≤0.01, and ††† p≤0.001 (two-way ANOVA).
Following ischemia in adult mice brains, the transcript expression of all the tested pro-inflammatory cytokines was clearly elevated (TNF up to 61-fold, p≤0.001; IL-1α up to 13-fold, p≤0.01; IL-1β up to 37-fold, p≤0.001; Figure 2). This massive inflammatory response in adult ischemic brains was markedly attenuated in aged brains (by 41% for TNF; by 68% for IL-1α; by 79% for IL-1β).
The up-regulation of TNF mRNA and protein peaked at 12 h after reperfusion in adult mice (mRNA 56-fold, protein 2.3-fold; p≤0.001). The TNF protein level in aged mice brains following stroke showed an elevated expression but was not significantly affected. The extent of the post-ischemic response was approximately the same for IL-1α mRNA and protein, but a delayed peak expression of IL-1α protein was observed (mRNA peak at 6 h, protein peak at 12 h after reperfusion). The protein level of IL-1β could not be reliably quantified (data not shown).
Cytokine IL-6
The increased IL-6 mRNA level significantly correlated with age (Pearson, p≤0.05), though this was not reflected in its protein level (Figure 3). The post-ischemic IL-6 expression of adult mice was up-regulated by up to 69-fold for its mRNA level (peak at 12 h after reperfusion, p≤0.001) and by up to 11-fold for its protein level (peak at 24 h after reperfusion, p≤0.001; Figure 3). The ischemic response in aged brains was attenuated by 82% at the transcript level (at 12 h) and by 85% at the protein level (at 24 h). In adult mice, the mRNA expression of IL-6 increased from 6 h to 12 h when it reached its peak expression. In aged mice, IL-6 mRNA expression peaked at 6 h and then displayed a rapid decay.
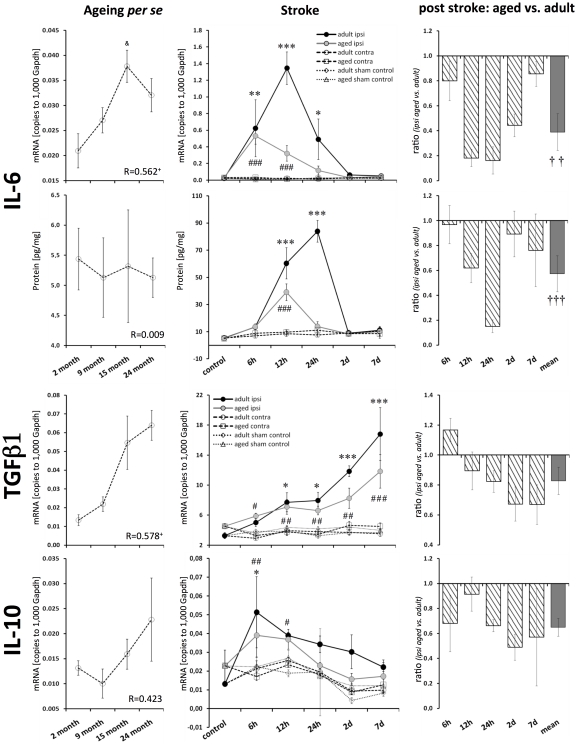
Figure 3. Age- and stroke-dependent expression of IL-6, TGFβ1 and IL-10.
IL-6 transcript expression significantly correlated with age; however, this correlation was not reflected in the expression of its protein. The post-ischemic IL-6 response was attenuated in aged brains (mRNA and protein). TGFβ1 mRNA significantly correlated with age. IL-10 mRNA tended to be elevated in aged brains. TGFβ1 transcript expression in the ischemic tissue increased continuously with reperfusion time at both ages. The up-regulation of TGFβ1 and IL-10 following ischemia tended to be attenuated in aged brains. Expression values are shown as mean ± SEM and ratios as geometric mean ± SEM. For details of the statistical analysis, see Figure 2's legend or the Materials and Methods section.
Anti-inflammatory cytokines IL-10 and TGFβ1
TGFβ1 transcript levels correlated significantly with age (Pearson, p≤0.05; Figure 3). In contrast to all the other tested cytokines, the post-ischemic TGFβ1, expression increased continuously with time (up to 7 d) in adult and aged brains (4.5-fold and 2.5-fold, respectively; p≤0.001). However, the up-regulation of TGFβ1 was attenuated in aged mice by 33% (7 d after reperfusion; Figure 3).
The expression of IL-10 mRNA tended to be elevated during ageing per se, but it was not significantly up-regulated (1.5-fold, n.s.; Figure 3). Following stroke, the IL-10 transcript expression peaked at 6 h after reperfusion for both ages of mice (2.1-fold, adult p≤0.05, aged p≤0.01). The post-ischemic IL-10 mRNA response was not found to be significantly altered in aged brains (Figure 3).
The protein expression of IL-10 and TGFβ1 could not be validated. The IL-10 protein levels in the test samples have been found below the sensitivity of the BD Bioscience Flex Set. In the case of TGFβ1, an appropriate antibody was not provided by the BD Bioscience Flex Set.
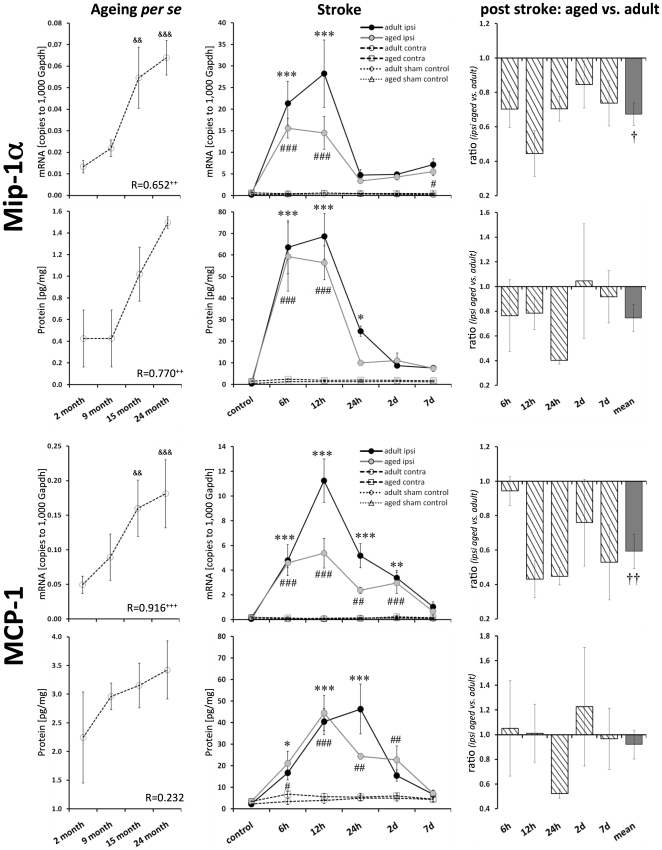
Chemokines Mip-1α, MCP-1, and RANTES
The transcript levels of all the tested chemokines increased with ageing per se. The strongest increase was observed for Mip-1α mRNA (3.5-fold, p≤0.001; Figure 4) and MCP-1 mRNA (3.6-fold, p≤0.001; Figure 4). The protein levels of Mip-1α and RANTES correlated significantly with age (Pearson, p≤0.01 and p≤0.05, respectively; Figures 4 and 5). The protein level of MCP-1 tended to be increased; however, it failed to reach significance (Figure 4).
Figure 4. Age- and stroke-dependent expression of Mip-1α and MCP-1.
Mip-1α and MCP-1 displayed significantly elevated expression levels with age. Mip-1α and MCP-1 transcript and protein expression increased significantly following stroke. This up-regulation was attenuated in aged brains. Expression values are shown as mean ± SEM and ratios as geometric mean ± SEM. For details of the statistical analysis, see Figure 2's legend or the Materials and Methods section.
Figure 5. Age- and stroke-dependent expression of RANTES.
The expression of RANTES significantly correlated with age. After ischemia, the level of RANTES mRNA continuously increased up to 7 d in adult brains, whereas no up-regulation was observed in aged brains. Protein levels of RANTES were up-regulated in adult and aged brains. Expression values are shown as mean ± SEM and ratios as geometric mean ± SEM. For details of the statistical analysis, see Figure 2's legend or the Materials and Methods section.
Mip-1α mRNA expression peaked 12 h after ischemia in adult mice (Figure 4). Aged mice displayed a significant attenuated response of Mip-1α transcript expression at 12 h after reperfusion (by 55%) and over all time points (by 33%). This post-ischemic diminished Mip-1α response was observed to be less pronounced at the protein level (by 25%, n.s.).
The transcript expression of MCP-1 peaked at 12 h after ischemia in adult mice brains (up to 102-fold, p≤0.001; Figure 4). However, this strong response was significantly attenuated in aged mice brains (up to 57%). The post-ischemic protein expression peaked at 12 h and 24 h in aged and adult mice brains, respectively (up to 9-fold, adults, p≤0.001). MCP-1 mRNA response seems also be attenuated, in aged mice brains (by 48%, 24 h after reperfusion).
The expression of RANTES mRNA increased continuously up to 7 d following ischemia in adult brains (3.5-fold, p≤0.001; Figure 5). Aged brains showed no obvious post-ischemic increase in RANTES transcript expression in the ipsilateral hemisphere; however, the contralateral hemisphere displayed a decrease (Figure 5). Therefore, the ratio of ipsilateral versus contralateral RANTES mRNA resulted in a significant ischemic effect (Figure 5). The level of RANTES protein peaked at 24 h after reperfusion. RANTES did not display a significant age-dependent post-ischemic response (Figure 5).
Discussion
We found profound age-related alterations in the reaction to stroke. The response of pro-inflammatory cytokines and the level of chemokines were strongly diminished in the aged post-ischemic brain tissue (summarized in Figure 6). Anti-inflammatory cytokines (TGFβ1, and IL-10) revealed no significant age dependency after ischemia. In concordance, the mean infarct volume of aged mice brains was found to be smaller.
Figure 6. Schematic diagram summarizing the post-ischemic expression profiles of all the tested inflammatory mediators.
The relative expression profiles (%) of all the tested inflammatory mediators in adult (continuous line, colored area) and aged brains (dotted line, light colored area) are shown (mRNA, left panel; protein, right panel). The post-ischemic response of TNF, IL-1α, IL-1β, IL-6, Mip-1α, MCP-1, and TGFβ1 was considerably attenuated with age, with IL-6 exhibiting the strongest age-dependent effect.
Age dependence of infarct size
Previous studies reported contradictory results on the age dependence of stroke size. Most studies found no age dependence or rather smaller infarct sizes, in experimental [28], [29], [30] as well as in clinical [31], [32] observations. Larger stroke sizes in aged animals were reported after permanent MCAO at early times (6 h [33], 24 h [34], and 3 d [35]) while this was not observed at later times (7 d [35]), indicating that the dynamics of stroke development following permanent MCAO might be different in aged animals. Stroke size might also depend on the gender of aged animals and the hormone level. Female aged rodents had larger infarcts whereas ovariectomized rats had smaller infarcts [36], [37]. Altogether, our result of a tendency to smaller infarcts in aged mice following transient MCAO is in agreement with most of the previous experimental studies [28], [29], [30] and in line with clinical observations [31], [32].
Elevated cerebral inflammation with age
The expression levels of inflammatory mediators in the brain are low due to the immune privilege of the central nervous system [38]. We therefore carefully established our qPCR settings to guarantee high sensitivity [27]. Protein expression was determined by flow cytometry with a bead-based immunoassay to minimize technical variations and to guarantee a high performance of each antibody in a multiplex scenario.
With the systematic approach used in this study, we showed that baseline expression levels of all the tested inflammatory mediators increased with age. Transcripts of the pro-inflammatory cytokines IL-1α, IL-1β, IL-6, and notably TNF as well as transcripts of the chemokines Mip-1α, MCP-1, and RANTES were up-regulated. Moreover, we found increased levels of the anti-inflammatory cytokines IL-10 and TGFβ1, with the transcript of TGFβ1 exhibiting the more prominent increase with age. These data are in agreement with the findings of previous studies, which reported similar tendencies for selected inflammatory mediators using different techniques [7], [9], [17], [18], [39]. With the exception of IL-6 and MCP-1, the protein expression of the inflammatory mediators increased with age, similar to their mRNA profiles. Although several methodological precautions were applied, the physiological protein levels were at (IL-6 and MCP-1) or below (IL-1β and IL-10) the limit of quantification. As mentioned above, an appropriate TGFβ1 antibody was not available.
Attenuated inflammatory response after ischemic injury in aged brains
The post-ischemic response of the pro-inflammatory cytokines TNF, IL-1β, and particularly IL-6 as well as of the chemokines Mip-1α and MCP-1 was significantly attenuated in aged brains. The anti-inflammatory capacity (TGFβ1, and IL-10) revealed no significant age related differences after ischemia.
In line with our findings, an attenuated inflammatory response in aged brains was also observed following NMDA-induced brain injury (striatal TNF and cortical IL-1β [7]) or after whole brain irradiation (hippocampal TNF, IL-1β, IL-6, and MCP-1 [40]). In contrast, elevated pro-inflammatory reactions were observed in aged brains after mechanical injuries [9], [41], intracerebral hemorrhage [42], or ischemia [43]. Therefore, post-injury inflammatory mechanisms do not follow a general pattern, but appear to depend on the type and extent of stimulation.
Potential reasons for the age-related attenuated post-ischemic inflammatory response
Elevated pro-inflammatory cytokine levels in aged brains may mediate a kind of preconditioning similar to the situation where administration of low levels of TNF and IL-1 leads to ischemic tolerance [44], [45]. In such a scenario, greater levels of stimulation would be required to induce a comparable inflammatory response. However, if and to what extent the attenuated post-ischemic immune response is due to elevated baseline levels of pro-inflammatory cytokines in aged brains cannot be determined from our results.
IL-6 exhibited the strongest decrease in its expression profile following stroke in aged mice brains. IL-6 is a pleiotropic cytokine that coordinates inflammatory processes between the periphery and the central nervous system, and can be released by various cell types in response to injuries. Moreover, IL-6 influences the expression and function of several other inflammatory mediators. Therefore, IL-6 may be a key mediator of the reduced inflammatory reaction following stroke in aged mice.
Similar to IL-6, all the other tested inflammatory mediators interact with each other. TNFα, for instance, influences several other inflammatory mediators, e.g. IL-6 and IL-1β [46], [47]. Also, IL-1β can stimulate cells to express MCP-1 [48]. Conversely, MCP-1-deficient mice express less IL-1β after permanent MCAO, indicating a signaling in both directions [49]. Therefore, a down-regulation of some of the key players of the post-stroke immune response may subsequently lead to a general reduction of the involved inflammatory mediators.
Potential functional consequences of an attenuated inflammatory response
Pro-inflammatory cytokines are generally considered to mediate detrimental effects following ischemia. Administration of TNF or IL-1 exacerbates damage [19], [50], and inhibition of TNF or IL-1 as well as IL-1 deficiency lead to reduced ischemic injuries [19].
IL-6 as a pleiotropic mediator can potentially exert detrimental (early phase) or beneficial effects (late phase) following ischemia. Post-ischemic injection of recombinant IL-6 mediates neuroprotection and reduces the injury, whereas administration of anti-mouse IL-6 receptor monoclonal antibody or the use of IL-6-knockout mice increases infarct size [51].
Anti-inflammatory cytokines such as TGFβ1 and IL-10 have a beneficial function after ischemia. IL-10 deficiency exacerbates damage, whereas its over-expression reduces infarct volumes after ischemic brain injury [52]. Both of these anti-inflammatory cytokines are able to block NF-kappa B activation and thereby the expression of transcripts involved in immune and inflammatory responses [53], [54].
CC chemokines or β-chemokines recruit immune competent cells to injured tissue [55]. Studies which inhibited MCP-1 in the rat brain or used MCP-1-deficient mice reported smaller ischemic lesions, whereas over-expression exacerbated damage [19]. Inhibition of MCP-1 and Mip-1α signaling leads to reduced infarct volumes following MCAO [56]. In concordance, the administration of Mip-1α has been found to increase the infarct volume [56]. RANTES-deficient mice exhibited decreased leukocyte adhesion, diminished blood-brain barrier permeability, and less tissue infarction [57].
The present study has revealed an attenuated response of inflammatory mediators in aged brains following stroke. Moreover, we observed smaller infarcts in aged brains. Most studies in rodents as well as in humans have reported that cerebral infarcts in aged organisms are not different from those in adults [8]. The smaller infarcts in aged brains observed here could be a consequence of the diminished pro-inflammatory response. This hypothesis is in line with the literature which reports a detrimental effect of pro-inflammatory cytokines and chemokines on the extent of injuries (as outlined above). The attenuated expression of IL-6 in aged post-ischemic brains may be harmful in the late ischemic phase.
Conclusions and future perspectives
From a theoretical point of view, attenuated post-lesional levels of pro-inflammatory cytokines and chemokines may be considered as a neuroprotective mechanism in aged brains. The smaller infarcts in aged brains could potentially be a consequence of this neuroprotection. However, reasons for the attenuated age-related stroke response cannot yet be specified. Increased baseline inflammation in aged brains may influence post-ischemic inflammatory processes. Modulation of the age-related neuro-inflammation (e.g. by pharmacological intervention or physical activity) with subsequent stroke induction would provide an opportunity to clarify this issue. Furthermore, investigations involving aged transgenic mice with altered inflammatory brain responses are necessary to unravel the functional relevance of our findings.
Supporting Information
(DOC)
Acknowledgments
The authors thank Madlen Guenther, Svetlana Tausch, and Ina Ingrisch for their excellent technical assistance and Martin Brehm, Matthias Kohl and Mario Walther for his insightful comments.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The study was funded by the European Union (STREP, FP6, ‘Age-dependent inflammatory response after stroke’ – ARGES [LSHB-CT-2006-018936]) and is part of the research program of the Jena centre for systems biology of ageing – Jenage (BMBF 0315581). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Dilger RN, Johnson RW. Aging, microglial cell priming, and the discordant central inflammatory response to signals from the peripheral immune system. J Leukoc Biol. 2008;84:932–939. doi: 10.1189/jlb.0208108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Truelsen T, Piechowski-Jozwiak B, Bonita R, Mathers C, Bogousslavsky J, et al. Stroke incidence and prevalence in Europe: a review of available data. Eur J Neurol. 2006;13:581–598. doi: 10.1111/j.1468-1331.2006.01138.x. [DOI] [PubMed] [Google Scholar]
- 3.Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008;371:1612–1623. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- 4.Saposnik G, Black SE, Hakim A, Fang J, Tu JV, et al. Age disparities in stroke quality of care and delivery of health services. Stroke. 2009;40:3328–3335. doi: 10.1161/STROKEAHA.109.558759. [DOI] [PubMed] [Google Scholar]
- 5.O'Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, et al. 1,026 experimental treatments in acute stroke. Ann Neurol. 2006;59:467–477. doi: 10.1002/ana.20741. [DOI] [PubMed] [Google Scholar]
- 6.Buga AM, Sascau M, Pisoschi C, Herndon JG, Kessler C, et al. The genomic response of the ipsilateral and contralateral cortex to stroke in aged rats. J Cell Mol Med. 2008;12:2731–2753. doi: 10.1111/j.1582-4934.2008.00252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campuzano O, Castillo-Ruiz MM, Acarin L, Castellano B, Gonzalez B. Increased levels of proinflammatory cytokines in the aged rat brain attenuate injury-induced cytokine response after excitotoxic damage. J Neurosci Res. 2009;87:2484–2497. doi: 10.1002/jnr.22074. [DOI] [PubMed] [Google Scholar]
- 8.Petcu EB, Sfredel V, Platt D, Herndon JG, Kessler C, et al. Cellular and molecular events underlying the dysregulated response of the aged brain to stroke: a mini-review. Gerontology. 2008;54:6–17. doi: 10.1159/000112845. [DOI] [PubMed] [Google Scholar]
- 9.Sandhir R, Puri V, Klein RM, Berman NE. Differential expression of cytokines and chemokines during secondary neuron death following brain injury in old and young mice. Neurosci Lett. 2004;369:28–32. doi: 10.1016/j.neulet.2004.07.032. [DOI] [PubMed] [Google Scholar]
- 10.Dirnagl U, Klehmet J, Braun JS, Harms H, Meisel C, et al. Stroke-induced immunodepression: experimental evidence and clinical relevance. Stroke. 2007;38:770–773. doi: 10.1161/01.STR.0000251441.89665.bc. [DOI] [PubMed] [Google Scholar]
- 11.Feuerstein GZ, Wang X. Inflammation and stroke: benefits without harm? Arch Neurol. 2001;58:672–674. doi: 10.1001/archneur.58.4.672. [DOI] [PubMed] [Google Scholar]
- 12.Whitaker JN. The potential for harm or benefit from inflammatory processes in stroke. Arch Neurol. 2001;58:674. doi: 10.1001/archneur.58.4.674. [DOI] [PubMed] [Google Scholar]
- 13.Schroeter M, Jander S, Witte OW, Stoll G. Local immune responses in the rat cerebral cortex after middle cerebral artery occlusion. J Neuroimmunol. 1994;55:195–203. doi: 10.1016/0165-5728(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 14.Grubeck-Loebenstein B, Wick G. The aging of the immune system. Adv Immunol. 2002;80:243–284. doi: 10.1016/s0065-2776(02)80017-7. [DOI] [PubMed] [Google Scholar]
- 15.Makinodan T, Kay MM. Age influence on the immune system. Adv Immunol. 1980;29:287–330. doi: 10.1016/s0065-2776(08)60047-4. [DOI] [PubMed] [Google Scholar]
- 16.Rink L, Cakman I, Kirchner H. Altered cytokine production in the elderly. Mech Ageing Dev. 1998;102:199–209. doi: 10.1016/s0047-6374(97)00153-x. [DOI] [PubMed] [Google Scholar]
- 17.Felzien LK, McDonald JT, Gleason SM, Berman NE, Klein RM. Increased chemokine gene expression during aging in the murine brain. Brain Res. 2001;890:137–146. doi: 10.1016/s0006-8993(00)03090-0. [DOI] [PubMed] [Google Scholar]
- 18.Sheng JG, Mrak RE, Griffin WS. Enlarged and phagocytic, but not primed, interleukin-1 alpha-immunoreactive microglia increase with age in normal human brain. Acta Neuropathol. 1998;95:229–234. doi: 10.1007/s004010050792. [DOI] [PubMed] [Google Scholar]
- 19.Vexler ZS, Tang XN, Yenari MA. Inflammation in adult and neonatal stroke. Clin Neurosci Res. 2006;6:293–313. doi: 10.1016/j.cnr.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nilupul Perera M, Ma HK, Arakawa S, Howells DW, Markus R, et al. Inflammation following stroke. J Clin Neurosci. 2006;13:1–8. doi: 10.1016/j.jocn.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Barone FC, Kilgore KS. Role of inflammation and cellular stress in brain injury and central nervous system diseases. Clinical Neuroscience Research. 2006;6:329–356. [Google Scholar]
- 22.del Zoppo G, Ginis I, Hallenbeck JM, Iadecola C, Wang X, et al. Inflammation and stroke: putative role for cytokines, adhesion molecules and iNOS in brain response to ischemia. Brain Pathol. 2000;10:95–112. doi: 10.1111/j.1750-3639.2000.tb00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ginsberg MD, Busto R. Rodent models of cerebral ischemia. Stroke. 1989;20:1627–1642. doi: 10.1161/01.str.20.12.1627. [DOI] [PubMed] [Google Scholar]
- 24.Sieber MW, Guenther M, Kohl M, Witte OW, Claus RA, et al. Inter-age variability of bona fide unvaried transcripts Normalization of quantitative PCR data in ischemic stroke. Neurobiol Aging. 2010;31:654–664. doi: 10.1016/j.neurobiolaging.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 25.Popp A, Jaenisch N, Witte OW, Frahm C. Identification of ischemic regions in a rat model of stroke. PLoS One. 2009;4:e4764. doi: 10.1371/journal.pone.0004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jaenisch N, Witte OW, Frahm C. Downregulation of potassium chloride cotransporter KCC2 after transient focal cerebral ischemia. Stroke. 2009;41:e151–159. doi: 10.1161/STROKEAHA.109.570424. [DOI] [PubMed] [Google Scholar]
- 27.Sieber MW, Recknagel P, Glaser F, Witte OW, Bauer M, et al. Substantial performance discrepancies among commercially available kits for reverse transcription quantitative polymerase chain reaction: a systematic comparative investigator-driven approach. Anal Biochem. 2010;401:303–311. doi: 10.1016/j.ab.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Popa-Wagner A, Schroder E, Walker LC, Kessler C. beta-Amyloid precursor protein and ss-amyloid peptide immunoreactivity in the rat brain after middle cerebral artery occlusion: effect of age. Stroke. 1998;29:2196–2202. doi: 10.1161/01.str.29.10.2196. [DOI] [PubMed] [Google Scholar]
- 29.Zhang L, Zhang RL, Wang Y, Zhang C, Zhang ZG, et al. Functional recovery in aged and young rats after embolic stroke: treatment with a phosphodiesterase type 5 inhibitor. Stroke. 2005;36:847–852. doi: 10.1161/01.STR.0000158923.19956.73. [DOI] [PubMed] [Google Scholar]
- 30.Zhao CS, Puurunen K, Schallert T, Sivenius J, Jolkkonen J. Effect of cholinergic medication, before and after focal photothrombotic ischemic cortical injury, on histological and functional outcome in aged and young adult rats. Behav Brain Res. 2005;156:85–94. doi: 10.1016/j.bbr.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 31.Nakayama H, Jorgensen HS, Raaschou HO, Olsen TS. The influence of age on stroke outcome. The Copenhagen Stroke Study. Stroke. 1994;25:808–813. doi: 10.1161/01.str.25.4.808. [DOI] [PubMed] [Google Scholar]
- 32.Engelter ST, Provenzale JM, Petrella JR, DeLong DM, Alberts MJ. Infarct volume on apparent diffusion coefficient maps correlates with length of stay and outcome after middle cerebral artery stroke. Cerebrovasc Dis. 2003;15:188–191. doi: 10.1159/000068826. [DOI] [PubMed] [Google Scholar]
- 33.Davis M, Mendelow AD, Perry RH, Chambers IR, James OF. Experimental stroke and neuroprotection in the aging rat brain. Stroke. 1995;26:1072–1078. doi: 10.1161/01.str.26.6.1072. [DOI] [PubMed] [Google Scholar]
- 34.Doyle KP, Cekanaviciute E, Mamer LE, Buckwalter MS. TGFbeta signaling in the brain increases with aging and signals to astrocytes and innate immune cells in the weeks after stroke. J Neuroinflammation. 7:62. doi: 10.1186/1742-2094-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Popa-Wagner A, Badan I, Walker L, Groppa S, Patrana N, et al. Accelerated infarct development, cytogenesis and apoptosis following transient cerebral ischemia in aged rats. Acta Neuropathol. 2007;113:277–293. doi: 10.1007/s00401-006-0164-7. [DOI] [PubMed] [Google Scholar]
- 36.Rosen CL, Dinapoli VA, Nagamine T, Crocco T. Influence of age on stroke outcome following transient focal ischemia. J Neurosurg. 2005;103:687–694. doi: 10.3171/jns.2005.103.4.0687. [DOI] [PubMed] [Google Scholar]
- 37.Dubal DB, Wise PM. Neuroprotective effects of estradiol in middle-aged female rats. Endocrinology. 2001;142:43–48. doi: 10.1210/endo.142.1.7911. [DOI] [PubMed] [Google Scholar]
- 38.Neumann H, Takahashi K. Essential role of the microglial triggering receptor expressed on myeloid cells-2 (TREM2) for central nervous tissue immune homeostasis. J Neuroimmunol. 2007;184:92–99. doi: 10.1016/j.jneuroim.2006.11.032. [DOI] [PubMed] [Google Scholar]
- 39.Sierra A, Gottfried-Blackmore AC, McEwen BS, Bulloch K. Microglia derived from aging mice exhibit an altered inflammatory profile. Glia. 2007;55:412–424. doi: 10.1002/glia.20468. [DOI] [PubMed] [Google Scholar]
- 40.Lee WH, Sonntag WE, Lee YW. Aging attenuates radiation-induced expression of pro-inflammatory mediators in rat brain. Neurosci Lett. 2010;476:89–93. doi: 10.1016/j.neulet.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kyrkanides S, O'Banion MK, Whiteley PE, Daeschner JC, Olschowka JA. Enhanced glial activation and expression of specific CNS inflammation-related molecules in aged versus young rats following cortical stab injury. J Neuroimmunol. 2001;119:269–277. doi: 10.1016/s0165-5728(01)00404-0. [DOI] [PubMed] [Google Scholar]
- 42.Lee JC, Cho GS, Choi BO, Kim HC, Kim WK. Aging exacerbates intracerebral hemorrhage-induced brain injury. J Neurotrauma. 2009;26:1567–1576. doi: 10.1089/neu.2008.0630. [DOI] [PubMed] [Google Scholar]
- 43.Dinapoli VA, Benkovic SA, Li X, Kelly KA, Miller DB, et al. Age exaggerates proinflammatory cytokine signaling and truncates signal transducers and activators of transcription 3 signaling following ischemic stroke in the rat. Neuroscience. 2010;170:633–644. doi: 10.1016/j.neuroscience.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nawashiro H, Tasaki K, Ruetzler CA, Hallenbeck JM. TNF-alpha pretreatment induces protective effects against focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 1997;17:483–490. doi: 10.1097/00004647-199705000-00001. [DOI] [PubMed] [Google Scholar]
- 45.Ohtsuki T, Ruetzler CA, Tasaki K, Hallenbeck JM. Interleukin-1 mediates induction of tolerance to global ischemia in gerbil hippocampal CA1 neurons. J Cereb Blood Flow Metab. 1996;16:1137–1142. doi: 10.1097/00004647-199611000-00007. [DOI] [PubMed] [Google Scholar]
- 46.Sawada M, Suzumura A, Marunouchi T. TNF alpha induces IL-6 production by astrocytes but not by microglia. Brain Res. 1992;583:296–299. doi: 10.1016/s0006-8993(10)80037-x. [DOI] [PubMed] [Google Scholar]
- 47.Tuttolomondo A, Di Raimondo D, di Sciacca R, Pinto A, Licata G. Inflammatory cytokines in acute ischemic stroke. Curr Pharm Des. 2008;14:3574–3589. doi: 10.2174/138161208786848739. [DOI] [PubMed] [Google Scholar]
- 48.Che X, Ye W, Panga L, Wu DC, Yang GY. Monocyte chemoattractant protein-1 expressed in neurons and astrocytes during focal ischemia in mice. Brain Res. 2001;902:171–177. doi: 10.1016/s0006-8993(01)02328-9. [DOI] [PubMed] [Google Scholar]
- 49.Cartier L, Hartley O, Dubois-Dauphin M, Krause KH. Chemokine receptors in the central nervous system: role in brain inflammation and neurodegenerative diseases. Brain Res Brain Res Rev. 2005;48:16–42. doi: 10.1016/j.brainresrev.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 50.Barone FC, Arvin B, White RF, Miller A, Webb CL, et al. Tumor necrosis factor-alpha. A mediator of focal ischemic brain injury. Stroke. 1997;28:1233–1244. doi: 10.1161/01.str.28.6.1233. [DOI] [PubMed] [Google Scholar]
- 51.Suzuki S, Tanaka K, Suzuki N. Ambivalent aspects of interleukin-6 in cerebral ischemia: inflammatory versus neurotrophic aspects. J Cereb Blood Flow Metab. 2009;29:464–479. doi: 10.1038/jcbfm.2008.141. [DOI] [PubMed] [Google Scholar]
- 52.Planas AM, Gorina R, Chamorro A. Signalling pathways mediating inflammatory responses in brain ischaemia. Biochem Soc Trans. 2006;34:1267–1270. doi: 10.1042/BST0341267. [DOI] [PubMed] [Google Scholar]
- 53.Kaltschmidt B, Widera D, Kaltschmidt C. Signaling via NF-kappaB in the nervous system. Biochim Biophys Acta. 2005;1745:287–299. doi: 10.1016/j.bbamcr.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 54.Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 55.Ziebell JM, Morganti-Kossmann MC. Involvement of pro- and anti-inflammatory cytokines and chemokines in the pathophysiology of traumatic brain injury. Neurotherapeutics. 2010;7:22–30. doi: 10.1016/j.nurt.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takami S, Minami M, Nagata I, Namura S, Satoh M. Chemokine receptor antagonist peptide, viral MIP-II, protects the brain against focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2001;21:1430–1435. doi: 10.1097/00004647-200112000-00007. [DOI] [PubMed] [Google Scholar]
- 57.Terao S, Yilmaz G, Stokes KY, Russell J, Ishikawa M, et al. Blood cell-derived RANTES mediates cerebral microvascular dysfunction, inflammation, and tissue injury after focal ischemia-reperfusion. Stroke. 2008;39:2560–2570. doi: 10.1161/STROKEAHA.107.513150. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)