Abstract
Objectives
To estimate neuropathic sign/symptom rates with initiation of combination antiretroviral therapy (cART) in HIV-infected ART-naive patients, and to investigate risk factors for: peripheral neuropathy and symptomatic peripheral neuropathy (SPN), recovery from peripheral neuropathy/SPN after neurotoxic ART (nART) discontinuation, and the absence of peripheral neuropathy/SPN while on nART.
Design
AIDS Clinical Trials Group (ACTG) Longitudinal Linked Randomized Trial participants who initiated cART in randomized trials for ART-naive patients were annually screened for symptoms/signs of peripheral neuropathy. ART use and disease characteristics were collected longitudinally.
Methods
Peripheral neuropathy was defined as at least mild loss of vibration sensation in both great toes or absent/hypoactive ankle reflexes bilaterally. SPN was defined as peripheral neuropathy and bilateral symptoms. Generalized estimating equation logistic regression was used to estimate associations.
Results
Two thousand, one hundred and forty-one participants were followed from January 2000 to June 2007. Rates of peripheral neuropathy/SPN at 3 years were 32.1/8.6% despite 87.1% with HIV-1RNA 400 copies/ml or less and 70.3% with CD4 greater than 350 cells/µl. Associations with higher odds of peripheral neuropathy included older patient age and current nART use. Associations with higher odds of SPN included older patient age, nART use, and history of diabetes mellitus. Associations with lower odds of recovery after nART discontinuation included older patient age. Associations with higher odds of peripheral neuropathy while on nART included older patient age and current protease inhibitor use. Associations with higher odds of SPN while on nART included older patient age, history of diabetes, taller height, and protease inhibitor use.
Conclusion
Signs of peripheral neuropathy remain despite virologic/immunologic control but frequently occurs without symptoms. Aging is a risk factor for peripheral neuropathy/SPN.
Keywords: aging, antiretroviral therapy, HIV, neurological, peripheral neuropathy, risk factors
Introduction
Combination antiretroviral (cART) therapy has resulted in declines in the incidences of HIV-associated dementia and central nervous system (CNS) opportunistic infections. However, sensory neuropathies are still prevalent as the most frequent neurological disorder associated with HIV infection and its treatment with ART [1–3]. Ellis et al. [4] reports that distal sensory polyneuropathy is prevalent (57%) in the cART era.
There are two major types of HIV-associated distal sensory peripheral neuropathies: primary HIV-associated distal sensory polyneuropathy (HIV-DSP) and ART toxic neuropathy (ATN), together which affect approximately 30–67% of patients with advanced HIV disease [5,6]. HIV-DSP is the most common sensory neuropathy in HIV infection with a reported 1-year incidence in an advanced patient cohort selected for neurologic disease risk of 36/21% in the pre-cART/cART eras, respectively [7]. ATN is associated with neurotoxic ART (nART), for example, dideoxynucleoside analogs and shares most clinical features of HIV-DSP except that neuropathy develops while taking the nART. ATN is the most common toxicity of ART therapy in sub-Saharan Africa [5,6,8–10].
The pathology of HIV-DSP involves a length-dependent degeneration of both small and large peripheral nerve fibers but the pathogenesis is unknown [6,11,12]. The pathogenesis of ATN is believed to reflect inhibition of gamma DNA polymerase by nARTs leading to reduced mitochondrial DNA content and therefore to mitochondrial dysfunction [13]. Reduced mitochondrial DNA levels in subcutaneous fat obtained by punch skin biopsies have been associated with ATN [14,15].
The signs/symptoms of HIV-DSP and ATN resemble common neuropathies encountered in clinical practice including diabetic and alcohol-associated neuropathy. Symptoms include numbness, paresthesia, burning sensation, and stabbing pain. Common signs include reduced or absent ankle reflexes relative to patellar reflexes, reduced or absent vibration sensation in the toes, and decreased pin and temperature sensation in a stocking/glove distribution. No FDA-approved therapies exist for HIV-associated sensory neuropathies with treatment limited to symptomatic measures with limited efficacy [1].
Higher plasma HIV-1 RNA and lower CD4+ cell counts before the initiation of ART increased the risk of HIVDSP in the pre-cART era [16,17]. Risk factors in the cART era have been investigated [7,18–23]. Data suggest that risk factors include prolonged exposure to cART [24], protease inhibitor exposure [25] and lipid-lowering drugs (statins and fibrates) [26]. Diabetes mellitus is a common cause of sensory neuropathy, and ART therapy, especially protease inhibitors, is associated with diabetes mellitus [27]. Ances et al. [28] reports that the risk for HIV-DSP is associated with diabetes and hypertriglyceridemia but not by other metabolic syndrome components. The interaction between metabolic syndrome and HIV is unclear. Height has been identified as a risk factor for peripheral neuropathy in diabetes [29] and recently in HIV infection when using nARTs [30].
We evaluated potential risk factors for peripheral neuropathy and symptomatic peripheral neuropathy (SPN) using data from more than 2000 ART-naive patients initiating cART in six randomized AIDS Clinical Trials Group (ACTG) trials.
Methods
Participants were selected from the ACTG Longitudinal Linked Randomized Trials (ALLRT), a meta-study of participants prospectively enrolled into randomized clinical trials of cART (at least three ARTs) regimens. Study participants include those that agreed to be followed long term for the purpose of evaluating clinical, virologic, immunologic, and neurologic outcomes associated with treatment of HIV with cART. All available data for ART-naive participants for which neuropathy-related data had been collected were included. Demographics and ART use were also collected. Study participants had variable length of follow-up and variable timing (relative to initiation of cART) for their first evaluations. Participants from six randomized trials [ACTG trials 347 (n = 19 [31]), 384 (n = 566 [32]), 388 (n = 214 [33]), A5014 (n = 38 [34]), A5095 (n = 771 [35]), and A5142 (n = 533 [36])] were analyzed.
Neuropathy data
The Brief Peripheral Neuropathy Screen (BPNS) was administered in ALLRT every 48 weeks by trained non-neurologist site personnel. The BPNS assesses signs (vibration sensation at the feet and ankle reflexes) and symptoms (pain, ‘pins and needles’ sensation, and numbness). The performance characteristics of the BPNS have been reported [37,38].
Peripheral neuropathy was defined as at least mild loss of vibration sensation in both great toes or absent or hypoactive ankle reflexes bilaterally relative to knees. SPN was defined as peripheral neuropathy and any bilateral symptoms. Use of nART or protease inhibitors was defined as at least 4 weeks of use within the last 6 months of the evaluation.
Objectives
Objectives of this study include the following:
estimating the prevalence of neuropathic signs and symptoms at cART initiation in ART-naive patients,
investigating risk factors for peripheral neuropathy and SPN,
investigating predictors for recovery from peripheral neuropathy and SPN (i.e. transitioning from peripheral neuropathy or SPN to no peripheral neuropathy or SPN) after nART discontinuation, and
investigating risk factors for peripheral neuropathy and SPN while on nART.
Statistical methods
Descriptive statistics are used to describe the study sample. Longitudinal plots display the prevalence of peripheral neuropathy, SPN, neuropathic signs and symptoms, and ART use over time. Multivariate logistic regression models [logistic generalized estimating equation (GEE)] were used to estimate the association [i.e. odds ratios (ORs) and associated confidence intervals (CIs)] between potential risk factors with peripheral neuropathy and SPN. Multivariate models included all of the risk variables described below since there was interest in estimating the association between each of these variables with peripheral neuropathy/SPN. Model fit was also assessed using methods described in Evans and Li [39]. Forest plots summarize these OR estimates and associated CIs. Nonsignificant P values should not be interpreted as ‘no association’. Instead the CIs was used to ‘rule out’ associations with reasonable confidence.
Reporting model results are from ‘full’ models including all covariates (except for evaluation of associations with recovery from SPN after discontinuation of nART for which univariate results are reported), and thus OR estimates are adjusted for other variables. Variables included in the models were demographics, HIV disease characteristics, ART use at the time of the evaluation, concomitant therapy use, and other patient characteristics.
Demographic variables included: age at the time of evaluation (scaled such that ORs are interpreted for a 10-year increment), race [white (reference), black, Hispanic, other], sex (reference = men), and height at cART initiation (scaled such that ORs are interpreted for a 5 cm increment). HIV disease characteristics variables included: log10 (HIV-1 RNA) at cART initiation, CD4 at cART initiation: [categories: ≤ 200, 201–350, 351–500, ≥501 (reference)], HIV-1 RNA at the time of evaluation [categories: ≤ 400 (reference), >400], CD4 at the time of evaluation: [categories: ≤ 200, 201–350, 351–500, ≥501 (reference)], years since cART initiation, and years since cART discontinuation. ART use at the time of the evaluation variables included: nART use (i.e. use of stavudine, didanosine, or zalcitabine) and protease inhibitor use. Concomitant therapy use variables included: the use of a statin drug within the previous 21 days of the evaluation, the use of a nonstatin lipid-lowering drug within the previous 21 days of the evaluation, the use of an insulin drug within the previous 21 days of the evaluation and the use of a glucose-lowering drug (noninsulin) within the previous 21 days of the evaluation. Other patient characteristics included reported history of diabetes, hepatitis C virus (HCV) seropositivity and history of intravenous (i.v.) drug use.
Models to investigate predictors for recovery from peripheral neuropathy/SPN after nART discontinuation were simplified to obtain better fit. Specifically ‘Hispanic’ was collapsed into ‘other’ race, and baseline CD4 and CD4 nadir were converted to continuous variables scaled such that odds ratios are interpreted for a 50 cell increment.
Estimates from GEE models have a population average interpretation (i.e. an OR of 2 for a binary covariate is interpreted as the odds of the outcome is two times higher for a population with the characteristic than without).
Results
Demographics and baseline characteristics
Two thousand one hundred and forty-one ART-naïve participants (81% men, 44% white, 32% black, median age = 39 years, median log10 HIV-1 RNA = 4.90 copies/ml and median CD4 cell count = 206 cell/µl at cART initiation) were analyzed (Table 1).
Table 1.
Demographics and baseline characteristics.
| Characteristic | ART naive (N=2141) |
Model_1 (N=1923) |
Model_2 PN (N=116) |
Model_2 SPN (N=55) |
Model_3 (N=573) |
|---|---|---|---|---|---|
| Age at ALLRT initiation | |||||
| 10–19 years | 13 (1%) | 8 (0%) | 0 (0%) | 0 (0%) | 2 (0%) |
| 20–29 years | 353 (16%) | 308 (16%) | 16 (14%) | 4 (7%) | 96 (17%) |
| 30–39 years | 906 (42%) | 805 (42%) | 48 (41%) | 26 (47%) | 244 (43%) |
| 40–49 years | 632 (30%) | 577 (30%) | 32 (28%) | 13 (24%) | 167 (29%) |
| 50–59 years | 191 (9%) | 181 (9%) | 16 (14%) | 9 (16%) | 49 (9%) |
| Over 60 years | 46 (2%) | 44 (2%) | 4 (3%) | 3 (5%) | 15 (3%) |
| Sex | |||||
| Men | 1742 (81%) | 1577 (81%) | 98 (84%) | 47 (85%) | 458 (80%) |
| Women | 399 (19%) | 366 (19%) | 18 (16%) | 8 (15%) | 115 (20%) |
| Race/ethnicity | |||||
| White | 952 (44%) | 873 (45%) | 59 (51%) | 29 (53%) | 269 (47%) |
| Black | 693 (32%) | 610 (32%) | 30 (26%) | 15 (27%) | 165 (29%) |
| Hispanic | 441 (21%) | 392 (20%) | 21 (18%) | 9 (16%) | 123 (21%) |
| Asian | 42 (2%) | 36 (2%) | 4 (3%) | 2 (4%) | 13 (2%) |
| Native American | 7 (0%) | 7 (0%) | 2 (2%) | 0 (0%) | 2 (0%) |
| Other | 6 (0%) | 5 (0%) | 0 (0%) | 0 (0%) | 1 (0%) |
| IV drug history | |||||
| No | 1933 (90%) | 1752 (91%) | 104 (90%) | 51 (93%) | 519 (91%) |
| Yes | 208 (10%) | 171 (9%) | 12 (10%) | 4 (7%) | 54 (9%) |
| HCV seropositivity | |||||
| Positive ever | 210 (10%) | 199 (10%) | 14 (12%) | 3 (5%) | 61 (11%) |
| Negative | 1768 (83%) | 1724 (90%) | 102 (88%) | 52 (95%) | 512 (89%) |
| Not available | 163 (8%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Baseline CD4 cell count (cells/µl) | |||||
| N | 2139 | 1923 | 116 | 55 | 573 |
| Mean (SD) | 236 (199) | 234 (197) | 261 (194) | 278 (219) | 222 (185) |
| Min, Max | 0, 1513 | 0, 1513 | 1, 891 | 11, 891 | 0, 938 |
| Median | 206 | 206 | 235 | 236 | 184 |
| Q1, Q3 | 60, 351 | 62, 347 | 97, 383 | 94, 423 | 57, 344 |
| Baseline log10(RNA VL) (copies/ml) | |||||
| N | 2141 | 1923 | 116 | 55 | 573 |
| Mean (SD) | 5.0 (0.8) | 5.0 (0.8) | 5.0 (0.8) | 5.1 (0.7) | 5.0 (0.8) |
| Min, Max | 2.0, 7.1 | 2.0, 7.1 | 2.2, 6.6 | 3.8, 6.6 | 2.2, 6.9 |
| Median | 4.9 | 4.9 | 5.0 | 5.1 | 5.1 |
| Q1, Q3 | 4.5, 5.5 | 4.5, 5.5 | 4.5, 5.6 | 4.6, 5.6 | 4.5, 5.6 |
Model_1 = Risk factors for PN and SPN (multivariate GEE model). Model_2 PN = Risk factors for recovery from PN after nART (multivariate GEE model). Model_2 SPN = Risk factors for recovery from SPN after nART (univariate GEE model). Model_3 = Risk factors for PN and SPN while on nART (multivariate GEE model). HCV, hepatitis C virus; IV, intravenous; PN, peripheral neuropathy; SPN, symptomatic peripheral neuropathy; VL, viral load.
Prevalence
Prior to cART initiation, the prevalence and 95% CI of peripheral neuropathy and SPN were 22.6% (19.0%, 26.4%) and 4.3% (2.7%, 6.4%), respectively.
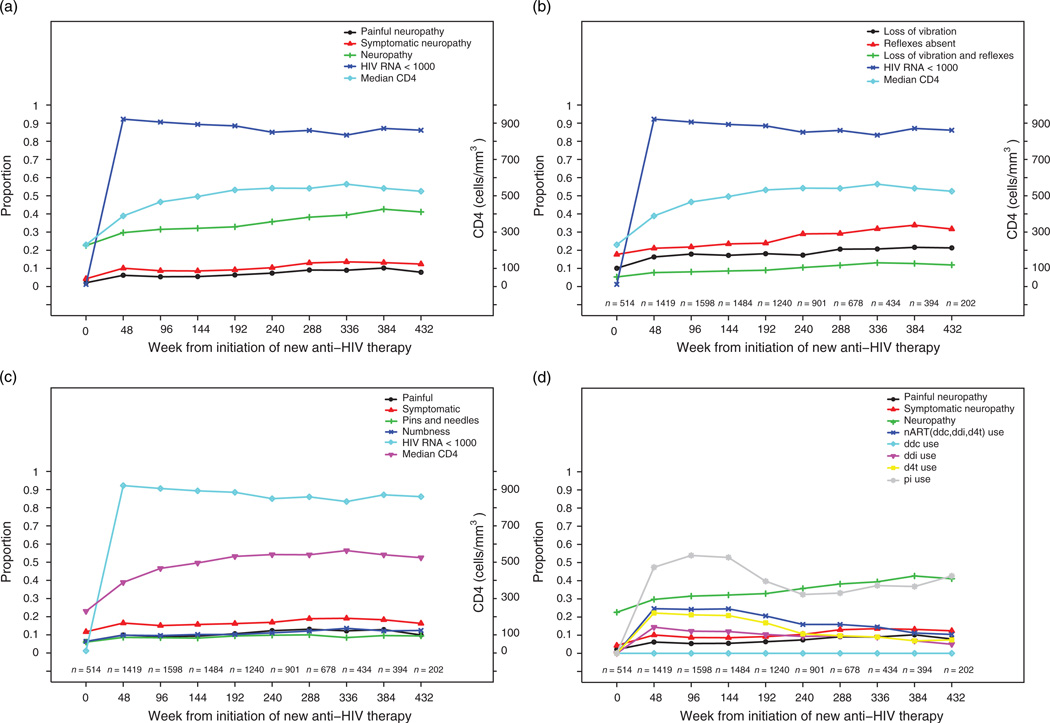
The treated cohort displayed viral control (i.e. HIV-1 RNA ≤ 400 copies/ml) in 82% (7258 of 8864) of patient visits. Signs of peripheral neuropathy persist despite viral control and improved immune function with initiation of combination ARTs (Fig. 1a–c, Table 2).
Fig. 1. Longitudinal characterisitics of peripheral neuropathy and combination antiretroviral therapy initiation.
(a) Peripheral neuropathy over time. (b) Neuropathic signs over time. (c) Neuropathic symptoms over time. (d) Antiretroviral therapy (ART) use over time. d4t, stavudine; ddc, zalcitabine; ddi, didanosine; nART, neurotoxic antiretroviral therapy.
Table 2.
Prevalence of peripheral neuropathy and symptomatic peripheral neuropathy over time.
| CD4>350 cells/µl |
VL≤400 copies/ml |
All patients |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Week since ART initiation |
N | Neuropathy [% (95% CI)] |
Symptomatic neuropathy [% (95% CI)] |
N | Neuropathy [% (95% CI)] |
Symptomatic neuropathy [% (95% CI)] |
N | Neuropathy [% (95% CI)] |
Symptomatic neuropathy [% (95% CI)] |
| 0 | 143 | 25.9 (18.9–33.9) | 2.8 (0.8–7.0) | 0 | – | – | 514 | 22.6 (19.0–26.4) | 4.3 (2.7–6.4) |
| 48 | 785 | 25.1 (22.1–28.3) | 8.0 (6.2–10.2) | 1276 | 29.1 (26.5–31.6) | 10.0 (8.4–11.7) | 1419 | 29.7 (27.3–32.1) | 10.1 (8.6–11.8) |
| 96 | 1069 | 29.2 (26.5–32.0) | 7.7 (6.1–9.4) | 1420 | 31.4 (29.1–33.9) | 8.5 (7.1–10.1) | 1598 | 31.5 (29.3–33.9) | 8.7 (7.4–10.2) |
| 144 | 1043 | 29.2 (26.3–31.9) | 7.5 (6.0–9.2) | 1293 | 31.9 (29.4–34.6) | 8.5 (7.0–10.2) | 1484 | 32.1 (29.8–34.6) | 8.6 (7.2–10.2) |
| 192 | 917 | 32.1 (29.5–35.2) | 8.5 (6.8–10.5) | 1083 | 32.6 (29.8–35.5) | 9.3 (7.7–11.2) | 1240 | 32.9 (30.3–35.6) | 9.2 (7.6–10.9) |
| 240 | 690 | 34.2 (30.7–37.9) | 9.6 (7.5–12.0) | 751 | 36.4 (32.9–39.9) | 10.7 (8.5–13.1) | 901 | 35.7 (32.6–39.0) | 10.4 (8.5–12.6) |
| 288 | 506 | 37.0 (32.7–41.3) | 11.5 (8.8–14.6) | 568 | 40.7 (36.6–44.8) | 13.7 (11.0–16.8) | 678 | 38.2 (34.5–42.0) | 13.0 (10.5–15.7) |
| 336 | 315 | 36.8 (31.5–42.4) | 12.1 (8.7–16.2) | 357 | 39.5 (34.4–44.8) | 13.2 (9.8–17.1) | 434 | 39.4 (34.8–44.2) | 13.6 (10.5–17.2) |
| 384 | 294 | 40.5 (34.8–46.3) | 13.3 (9.6–17.7) | 337 | 43.6 (38.3–49.1) | 13.9 (10.4–18.1) | 394 | 42.6 (37.7–47.7) | 13.2 (10.0–16.9) |
| 432 | 138 | 37.0 (28.9–45.6) | 10.9 (6.2–17.3) | 173 | 39.9 (32.5–47.6) | 12.1 (7.7–18.0) | 202 | 41.1 (34.2–48.2) | 12.4 (8.2–17.7) |
VL, viral load.
Peripheral neuropathy appears to increase over time after initiation of cARTs despite decline in nART use (Fig. 1d).
Symptoms
Most peripheral neuropathy signs occur in the absence of symptoms. Of 2815 patient visits with peripheral neuropathy, 2255 (80.1%) reported a pain level of zero, 2327 (82.7%) reported complete absence of ‘pins and needles’ sensation, and 2230 (79.2%) reported complete absence of numbness.
Associations with peripheral neuropathy and symptomatic peripheral neuropathy
Evaluation of the associations with peripheral neuropathy and SPN is based on data from 1923 patients (76 with diabetes) with appropriate data, consisting of 7699 patient visits. The median (Q1, Q3) visit per patient was 4 (3, 5).
Associations with peripheral neuropathy
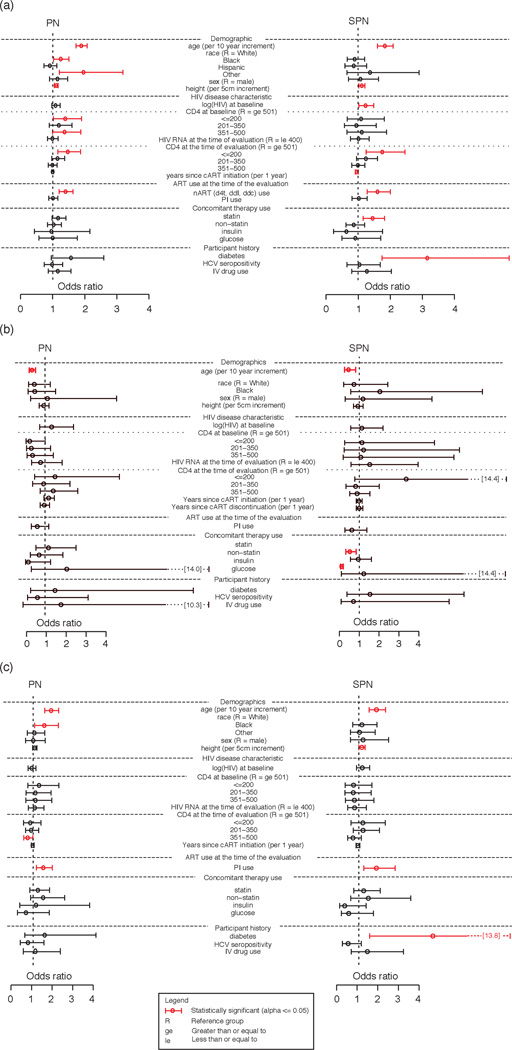
The following variables were associated with a higher odds of peripheral neuropathy in a model simultaneously evaluating all factors: older patient age [OR = 1.89, 95% CI = (1.73–2.07), P < 0.001], baseline CD4 200 or less compared to CD4 at least 501 [OR = 1.39, 95% CI = (1.02–1.90), P = 0.121], current CD4 200 or less compared to CD4 at least 501 [OR = 1.47, 95% CI = (1.16–1.87), P = 0.007], current nART use [OR = 1.40, 95% CI = (1.20–1.63), P < 0.001], taller height [OR = 1.11, 95% CI = (1.05–1.17), P < 0.001], black race compared to white race [OR = 1.25, 95% CI = (1.03–1.51), P = 0.004], and other race compared to white race [OR = 1.96, 95% CI = (1.21–3.19), P = 0.004]. A history of diabetes [OR = 1.57, 95% CI = (0.96–2.59), P = 0.080] and use of a statin drug [OR = 1.17, 95% CI = (0.98–1.41), P = 0.097] also trended towards significance. Viral suppression (HIV-1 RNA ≤ 400 copies/ml) was not associated with peripheral neuropathy [OR = 0.99, 95% CI = (0.84–1.17), P = 0.902] (Fig. 2a).
Fig. 2. Estimates of association with peripheral neuropathy and symptomatic peripheral neuropathy.
(a) Odds ratio estimates [95% confidence interval (CI)] for peripheral neuropathy (PN) and symptomatic PN (SPN). (b) Odds ratio estimates (95% CI) for PN and SPN after neurotoxic antiretroviral therapy (nART) discontinuation. (c) Odds ratio estimates (95% CI) for PN and SPN while on nART. d4t, stavudine; ddc, zalcitabine; ddi, didanosine; ge, greater than or equal to; HCV, hepatitis C virus; IV, intravenous; le, less than or equal to; R, reference group. *Statistically significant (α ≤ 0.05).
Associations with symptomatic peripheral neuropathy
The following variables were associated with higher odds of SPN in a model simultaneously evaluating all factors: older patient age [OR = 1.83, 95% CI = (1.60–2.09), P < 0.001], current CD4 200 or less compared to CD4 at least 501 [OR = 1.75, 95% CI = (1.25–2.46), P = 0.011], higher log10 (HIV-1 RNA) at baseline [OR = 1.23, 95% CI = (1.02–1.48), P = 0.030], nART use [OR = 1.60, 95% CI = (1.28–2.00), P < 0.001], history of diabetes [OR = 3.15, 95% CI = (1.74–5.70), P = 0.001], taller height [OR = 1.11, 95% CI = (1.02–1.21), P = 0.014], use of a statin drug [OR = 1.45, 95% CI = (1.15–1.82), P = 0.004], and fewer years since cART initiation [OR = 0.95, 95% CI = (0.91–0.99), P = 0.046]. Viral suppression (HIV-1 RNA ≤ 400 co-copies/ml) was not associated with SPN [OR = 1.01, 95% CI = (0.76–1.34), P = 0.931] nor was baseline CD4 (P = 0.811) (Fig. 2a).
Associations with recovery after discontinuation of neurotoxic antiretroviral therapy
Patients that had peripheral neuropathy (and SPN) while on nART and later withdrew nART were followed to evaluate recovery. A 54.1% (44.8%, 63.2%) of patients continued to have peripheral neuropathy during all remaining follow-up, whereas 18% (11.7%, 26.0%) of patients had no peripheral neuropathy during all remaining follow-up. 36.8% (24.5%, 50.7%) of patients continued to have SPN during all remaining follow-up, whereas 43.9% (30.7%, 57.6%) of patients had no SPN during all remaining follow-up.
Associations with recovery from peripheral neuropathy
Evaluation of the associations with peripheral neuropathy is based on data from 116 patients (7 with diabetes) with appropriate data, consisting of 338 patient visits. The median (Q1, Q3) visit per patient was 3 (1, 4).
Higher patient age was associated with a lower odds of recovery [OR = 0.33, 95% CI = (0.21, 0.53), P < 0.001]. Viral suppression (HIV-1 RNA ≤ 400 copies/ml) was not associated with peripheral neuropathy [OR = 0.77, 95% CI = (0.32–1.86), P = 0.554] nor was baseline CD4 (P = 0.389) (Fig. 2b).
Associations with recovery from symptomatic peripheral neuropathy
Evaluation of the associations with SPN is based on data from 55 patients (5 with diabetes) with appropriate data, consisting of 162 patient visits. The median (Q1, Q3) visit per patient was 3 (1, 4).
Multivariate models for recovery from SPN were not conducted due to small numbers. Univariate associations are thus reported. Higher patient age was associated with a lower odds of recovery [OR = 0.46, 95% CI = (0.26–0.82), P = 0.006]. HCV was also associated with recovery but the association was not directly estimable using these models. Thirteen of 13 [95% CI = (0.75–1.00)] HCV-positive patient visits had SPN, whereas 74/159 [95% CI = (0.38–0.55)] HCV-negative had SPN. Viral suppression (HIV-1 RNA ≤ 400 copies/ml) was not associated with SPN [OR = 1.53, 95% CI = (0.59–3.96), P = 0.353] nor was baseline CD4 (P = 0.996) (Fig. 2b).
Associations while on neurotoxic antiretroviral therapy
Evaluation of the associations with peripheral neuropathy and SPN are based on data from 573 patients (33 with diabetes) with appropriate data, consisting of 1566 patient visits. The median (Q1, Q3) visit per patientwere 2 (1, 4). 27.4% (23.8%, 31.1%) of patients had peripheral neuropathy during all follow-up, whereas 42.1% (38.0%, 46.1%) of patients had no peripheral neuropathy during all remaining follow-up. 9.8% (7.3%, 12.2%) of patients had SPN during all follow-up, whereas 75.2% (71.7%, 78.8%) of patients had no SPN during all remaining follow-up.
Associations with peripheral neuropathy
The following variables were associated with higher odds of peripheral neuropathy while on nART: older patient age [OR = 1.87, 95% CI = (1.56–2.24), P < 0.001], protease inhibitor use [OR = 1.49, 95% CI = (1.15–1.93), P = 0.003], black race [OR = 1.54, 95% CI = (1.07–2.22)] compared to white race, and current CD4 between 351 and 500 compared to current CD4 at least 501. Viral suppression (HIV-1 RNA ≤ 400 copies/ml) was not associated with peripheral neuropathy [OR = 1.08, 95% CI = (0.76–1.53), P = 0.672] nor was baseline CD4 (P = 0.826) (Fig. 2c).
Associations with symptomatic peripheral neuropathy
The following variables were associated with a higher odds of SPN while on nART: older patient age [OR = 1.86, 95% CI = (1.50–2.30), P < 0.001], history of diabetes [OR = 4.59, 95% CI = (1.53–13.83), P = 0.046], taller height [OR = 1.14, 95% CI = (1.01–1.30), P = 0.038], and protease inhibitor use [OR = 1.85, 95% CI = (1.24–2.76), P = 0.003]. Viral suppression (HIV-1 RNA ≤ 400 copies/ml) was not associated with SPN [OR = 0.80, 95% CI = (0.46–1.37), P = 0.402] nor was baseline CD4 (P = 0.898) (Fig. 2c).
Discussion
Peripheral neuropathy in HIV patients persists despite improved immunologic function and virologic control associated with cART and decreased nART use. Our data span studies in which nART was initiated to recent studies avoiding nART. nART use has become uncommon in the developed world yet remains in resource-limited settings due to low cost, making these observations relevant. Additionally, this study considers the impact of diabetes, statins, and protease inhibitors, all of which are central issues for HIV care.
Damage to peripheral nerves may be insidious but the persistence of dysfunction once nerves are damaged suggests that it is important to acknowledge this developing toxicity and to seek means of minimizing it. Evidence of peripheral nerve damage is easily detectable on an exam by trained non-neurologist staff as was done in this study. It is likely that the asymptomatic neuropathy patients will be more likely to become symptomatic when challenged with nART or other risks for peripheral neuropathy, if asymptomatic neuropathy is ignored. Our observations suggest that in treatment-naïve HIV patients receiving state of the ART therapy, peripheral neuropathy is found in more than 30%, whereas about a third of that number will also have symptoms. Sicker patients not enrolled in clinical trials, those with nutritional deficiency or more complications are likely at higher risk. However, diagnostic characteristics of our instrument [37,38] indicate that prevalence may be substantially lower. Using sensitivity/specificity estimates of 73/68% [37] derived from reference comparison to a neurologist total neuropathy score (TNS) evaluation, an observed prevalence of 40% from our study, suggests a prevalence of 20% had the TNS evaluation been performed.
Our findings support other observations about the importance of age to neuropathy in HIV [40,41]. Peripheral nerves are long, large, and metabolically stressed cells that are likely particularly vulnerable to toxicity and damage. Aging appears to increase this vulnerability making age one of the most notable and consistent risk factors for peripheral neuropathy. Given the rapidly aging HIV population due to successful therapy, the intersection of aging and increased risk of neuropathy portends ongoing challenges from this complication for HIV therapeutics.
Early studies emphasized CD4 and viral load as risk factors, but with successful therapy these associations have become less important. Specifically viral suppression did not decrease the odds of peripheral neuropathy or SPN. However, underlying HIV disease impacts the risk of neuropathy with evidence that successful HIV treatment as reflected by higher CD4 cell count is associated with a lower risk of neuropathy than found in those whose CD4 responses are poorer (Fig. 2a,b). Current CD4 200 cells/µl or less, reflecting advanced disease is a risk for peripheral neuropathy/SPN. Nadir CD4, in this treatment-naive cohort represented by the baseline CD4, also is associated with greater risk of peripheral neuropathy (but not SPN) [41]. In contrast, current viral load data failed to be associated with neuropathy outcomes.
Validation of our observations is found with confirmation that nART increases the risk of neuropathy. This is an important reason that alternatives to nART should be made available to HIV patients worldwide. A more subtle and uncertain area of ART neurotoxicity surrounds the status of protease inhibitors. Clinical and in-vitro studies have supported the possibility of protease inhibitor-associated neuropathy. Experienced clinicians have generally not recognized this association. The CHARTER study found only weak evidence for any of the protease inhibitor drugs contributing to neuropathy [41]. Our analyses do not support the claim that protease inhibitor use is associated with increased risk of neuropathy overall. However, our data do not fully exonerate protease inhibitor risk, as there appears an association of increased neuropathy risk with protease inhibitor used in conjunction with nART. Given the observational nature of the study, it may be that patients on nART and protease inhibitors vs. patients on nART without protease inhibitors are inherently different, potentially biasing the estimate of the protease inhibitor association. However, the protease inhibitor association was estimated while controlling for disease characteristics, demographics, and concomitant therapy. This will be critically important as second-line therapies are introduced in developing world sites where it may be common to pair nART with protease inhibitor in salvage regimens. Our data suggest that such combinations would be more likely to induce neuropathy.
Our data reinforce the interaction of glucose intolerance/diabetes with neuropathy risk. Many HIV neuropathy studies exclude diabetics so the degree of interaction of these problems has not been well described. Whereas the numbers of diabetic patients in our study is modest, our data suggest that diabetes is capable of substantially raising the risk of SPN. This is a very serious finding given the increasing impact of insulin resistance and diabetes in the setting of HIV infection.
Our study confirms the clinical impression that neuropathy in HIV is length-dependent, with a small, but consistently significant contribution in taller persons, which is consistent with a recent study [29] that associates patient height and risk of developing ATN in an international study. However, the impact of height is modest compared to aging and glucose intolerance. Hepatitis C is a frequent and serious coinfection of HIV patients, and the possibility that it influences neurological outcomes continues to be a concern. Our analyses support other observations about the lack of association of HCV coinfection with neuropathy [42].
Statin drugs are very widely used in the developed world and particularly in HIV populations with elevated cholesterols and enhanced cardiovascular risk. Statins have been implicated as causing neuropathy in other populations [43], although subsequent studies have not confirmed these findings. Our data suggest that this is an area in which more attention is needed in HIV therapeutics, since we found symptomatic neuropathy to be enhanced with concurrent statin use. However, statins did not seem to augment risk of neuropathy in patients on nART. Ongoing studies have shown an association between triglycerides and sensory neuropathy. Individuals taking statins are more likely to have elevated triglyceride levels, potentially mediating the association between statins and neuropathy in HIV [27].
Whereas there is considerable information confirming the risk of neuropathy with use of nART, traditionally reversal of neuropathy with discontinuation of nART was used to confirm the causative association of the drug for neuropathy. Clinical experience that some patients developing such neuropathy have recovery has not been carefully studied in large populations. We evaluated factors predicting recovery from neuropathy. The results show a strong influence with advancing age reducing the risk of recovery. It is also notable that statin use appears to decrease the chance of recovery from symptomatic neuropathy, a factor that could be of considerable clinical importance. Diabetes reflected by insulin use also predicts less chance for reversal of symptomatic neuropathy, suggesting that combining the diabetic risk with nART-induced mitochondrial deficits may be a particularly troublesome neurotoxic situation. HIV affects diverse populations worldwide. Racial disparity with regard to complications remains an intriguing and important area for thoughtful observations. African American (Black) patients in our cohort had increased risk of peripheral neuropathy which did not extend to SPN. The cause of this apparent risk is unknown, but higher risk of diabetes, hypertension, and potentially other genetic or pharmacokinetic risks could be considered.
Limitations of this study include its observational nature with the potential for informative drop-out/in, self-selection issues in ARTand concomitant medication use, and that the observed association may not be causal. There is a potential for selection bias as the analyses are restricted to clinical trial volunteers that were willing and able to return for follow-up visits. Results could be biased if these patients are very different than patients not included in analyses. Estimated association with variables subject to self-selection should be interpreted with caution. Nonsignificant P values should not be interpreted as ‘no association’. Instead the CIs should be used to ‘rule out’ associations with reasonable confidence. Some effect estimates, although not significant, cannot rule out potentially large associations.
Acknowledgements
The work was supported by National Institute of Health (NIH) grants including the Neurologic AIDS Research Consortium grant NS32228 from NINDS, the AIDS Clinical Trials Grant AI068636 from NIAID, and the Statistical and Data Management Center of the Adult AIDS Clinical Trials Group grant 1 U01 068634. The authors acknowledge the generous dedication of the many participants volunteering for the ALLRT study, and for the contributions of the contributing AIDS Clinical Trials Units, their investigators and staffs, that collected the samples and clinical data used for this analysis.
References
- 1.Keswani SC, Pardo CA, Cherry CL, Hoke A, McArthur JC. HIV-associated sensory neuropathies. AIDS. 2002;16:2105–2117. doi: 10.1097/00002030-200211080-00002. [DOI] [PubMed] [Google Scholar]
- 2.Bacellar H, Munoz A, Miller EN, Cohen BA, Besley D, Selnes OA, et al. Temporal trends in the incidence of HIV-1-related neurologic diseases: Multicenter AIDS Cohort Study, 1985–1992. Neurology. 1994;44:1892–1900. doi: 10.1212/wnl.44.10.1892. [DOI] [PubMed] [Google Scholar]
- 3.McArthur JC, Brew BJ, Nath A. Neurological complications of HIV infection. Lancet Neurol. 2005;4:543–555. doi: 10.1016/S1474-4422(05)70165-4. [DOI] [PubMed] [Google Scholar]
- 4.Ellis R, Rosario D, Clifford D, McArthur J, Simpson D, Alexander T, et al. CROI. Montreal, Canada: 2009. Persisting high prevalence of HIV distal sensory peripheral neuropathy in the era of cART: correlates in the CHARTER study. [Paper #461] [Google Scholar]
- 5.Wulff EA, Wang AK, Simpson DM. HIV-associated peripheral neuropathy: epidemiology, pathophysiology and treatment. Drugs. 2000;59:1251–1260. doi: 10.2165/00003495-200059060-00005. [DOI] [PubMed] [Google Scholar]
- 6.Cornblath DR, McArthur JC. Predominantly sensory neuropathy in patients with AIDS and AIDS-related complex. Neurology. 1988;38:794–796. doi: 10.1212/wnl.38.5.794. [DOI] [PubMed] [Google Scholar]
- 7.Schifitto G, McDermott MP, McArthur JC, Marder K, Sacktor N, McClernon DR, et al. Markers of immune activation and viral load in HIV-associated sensory neuropathy. Neurology. 2005;64:842–848. doi: 10.1212/01.WNL.0000152981.32057.BB. [DOI] [PubMed] [Google Scholar]
- 8.Forna F, Liechty C, Solberg P, Asiimwe F, Were W, Mermin J, et al. CROI. Denver, CO: 2006. Early clinical toxicity to nonnucleoside reverse transcriptase inhibitor-based HAART in a home-based AIDS care program in rural Uganda. [Abstract #142] [Google Scholar]
- 9.Kim A, Ngan’ga L, Macharia D, Wangai M, Ilako F, Isavwa A, et al. CROI. Denver, CO: 2006. Adverse events in HIV-infected patients receiving ART in a treatment program in a Nairobi slum, Kenya, 2003 to 2005. [Abstract #143] [Google Scholar]
- 10.Simpson DM, Kitch D, Evans SR, McArthur JC, Asmuth DM, Cohen B, et al. HIV neuropathy natural history cohort study: assessment measures and risk factors. Neurology. 2006;66:1679–1687. doi: 10.1212/01.wnl.0000218303.48113.5d. [DOI] [PubMed] [Google Scholar]
- 11.Barohn RJ, Gronseth GS, LeForce BR, McVey AL, McGuire SA, Butzin CA. Peripheral nervous system involvement in a large cohort of human immunodeficiency virus-infected individuals. Arch Neurol. 1993;50:167–171. doi: 10.1001/archneur.1993.00540020045016. [DOI] [PubMed] [Google Scholar]
- 12.Griffin JW, Crawford TO, McArthur JC. Peripheral neuropathies associated with HIV infection. In: Gendelman HE, Epstein E, Lipton SA, Swindells S, editors. The neurology of AIDS. New York: Chapman & Hall; 1998. pp. 275–291. [Google Scholar]
- 13.Brinkman K, Hofstede J, Burger D, Smeitink J, Koopmans P. Adverse effects of reverse transcriptase inhibitors: mitochondrial toxicity as common pathway. AIDS. 1998;12:1735–1744. doi: 10.1097/00002030-199814000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Brew BJ, Tisch S, Law M. Lactate concentrations distinguish between nucleoside neuropathy and HIV neuropathy. AIDS. 2003;17:1094–1096. doi: 10.1097/00002030-200305020-00024. [DOI] [PubMed] [Google Scholar]
- 15.Cherry CL, Gahan ME, McArthur J, Lwein SR, Hoy JF, Wesselingh SL. Exposure to dideoxynucleosides is reflected in lowered mitochondrial DNA in subcutaneous fat. J Acquir Immune Defic Syndr. 2002;30:271–277. doi: 10.1097/00126334-200207010-00002. [DOI] [PubMed] [Google Scholar]
- 16.Childs EA, Lyles RH, Selnes OA, Chen B, Miller EN, Cohen BA, et al. Plasma viral load and CD4 lymphocytes predict HIV-associated dementia and sensory neuropathy. Neurology. 1999;52:607–613. doi: 10.1212/wnl.52.3.607. [DOI] [PubMed] [Google Scholar]
- 17.Tagliati M, Grinnell J, Godbold J, Simpson DM. Peripheral nerve function in HIV infection: clinical, electrophysiologic, and laboratory findings. Arch Neurol. 1999;56:84–89. doi: 10.1001/archneur.56.1.84. [DOI] [PubMed] [Google Scholar]
- 18.Cherry CL, Skolasky RL, Lal L, Creighton J, Hauer P, Raman SP, et al. Antiretroviral use and other risks for HIV-associated neuropathies in an international cohort. Neurology. 2006;66:867–873. doi: 10.1212/01.wnl.0000203336.12114.09. [DOI] [PubMed] [Google Scholar]
- 19.Lichtenstein KA, Armon C, Buchacz K, Chmiel JS, Moorman AC, Wood KC, et al. Initiation of antiretroviral therapy at CD4 cell counts ≥350 cells/mm3 does not increase incidence or risk of peripheral neuropathy, anemia, or renal insufficiency. J Acquir Immune Defic Syndr. 2008;47:27–35. doi: 10.1097/QAI.0b013e31815acacc. [DOI] [PubMed] [Google Scholar]
- 20.Lichtenstein KA, Armon C, Baron A, Moorman AC, Wood KC, Holmberg SD. Modification of the incidence of drug-associated symmetrical peripheral neuropathy by host and disease factors in the HIV outpatient study cohort. Clin Infect Dis. 2005;40:148–157. doi: 10.1086/426076. [DOI] [PubMed] [Google Scholar]
- 21.Morgello S, Estanislao L, Simpson D, Geraci A, DiRocco A, Gerits P, et al. HIV-associated distal sensory polyneuropathy in the era of highly active antiretroviral therapy: the Manhattan HIV Brain Bank. Arch Neurol. 2004;61:546–551. doi: 10.1001/archneur.61.4.546. [DOI] [PubMed] [Google Scholar]
- 22.Smyth K, Affandi JS, McArthur JC, Bowtell-Harris C, Mijch AM, Watson K, et al. Prevalence of and risk factors for HIV-associated neuropathy in Melbourne, Australia 1993–2006. HIV Med. 2007;8:367–373. doi: 10.1111/j.1468-1293.2007.00478.x. [DOI] [PubMed] [Google Scholar]
- 23.Schifitto G, McDermott M, McArthur J, Marder K, Sacktor N, Epstein L. Incidence of and risk factors for HIV-associated distal sensory polyneuropathy. Neurology. 2002;58:1764–1768. doi: 10.1212/wnl.58.12.1764. [DOI] [PubMed] [Google Scholar]
- 24.Sacktor N, Lyles RH, Skolasky R, Kleeberger C, Selnes OA, Miller EN, et al. HIV-associated neurologic disease incidence changes: multicenter AIDS Cohort Study, 1990–1998. Neurology. 2001;56:257–260. doi: 10.1212/wnl.56.2.257. [DOI] [PubMed] [Google Scholar]
- 25.Pettersen JA, Jones G, Worthington C, Krentz HB, Keppler OT, Hoke A, et al. Sensory neuropathy in human immunodeficiency virus/acquired immunodeficiency syndrome patients: protease inhibitor-mediated neurotoxicity. Ann Neurol. 2006;59:816–824. doi: 10.1002/ana.20816. [DOI] [PubMed] [Google Scholar]
- 26.Corrao G, Zambon A, Bretu L, Botteri E, Leoni O, Contiero P. Lipid lowering drug prescription and the risk of peripheral neuropathy: an exploratory case-control study using automated databases. J Epidemiol Community Health. 2004;58:1047–1051. doi: 10.1136/jech.2003.013409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banerjee S, McCutchan JA, Ances BM, Deutsch R, Riggs PK, Way L, Ellis RJ. Hypertriglyceridemia in combination antiretroviral-treated HIV-positive individuals: potential impact on HIV sensory polyneuropathy. AIDS. 2011;25:F1–F6. doi: 10.1097/QAD.0b013e328341dd68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ances B, Rosario D, Vaida F, Marquie-Beck J, Ellis R, Simpson D, et al. CROI. Montreal, Canada: 2009. Metabolic syndrome components as risk factors for distal sensory polyneuropathy. [Paper #463] [Google Scholar]
- 29.Sosenko JM, Boulton AJM, Gadia MT, Ward JD, Skylar JS. The association between symptomatic sensory neuropathy and body stature in diabetic patients. Diabetes Res Clin Pract. 1988;4:95–98. doi: 10.1016/s0168-8227(88)80003-2. [DOI] [PubMed] [Google Scholar]
- 30.Cherry CL, Affandi JS, Imran D, Yunihastuti E, Smyth K, Vanar S, et al. Age and height predict neuropathy risk in patients with HIV prescribed stavudine. Neurology. 2009;73:315–320. doi: 10.1212/WNL.0b013e3181af7a22. [DOI] [PubMed] [Google Scholar]
- 31.Eron J, Smeaton L, Fiscus S, Gulick R, Currier J, Lennox J, et al. The effects of protease inhibitor therapy on human immunodeficiency virus type 1 levels in semen (AIDS clinical trials group protocol 850) Am J Infect Dis. 2000;181:1622–1628. doi: 10.1086/315447. [DOI] [PubMed] [Google Scholar]
- 32.Robbins G, DeGruttola V, Shafer R, Smeaton L, Snyder S, Pettinelli C, et al. Comparison of sequential three-drug regimens as initial therapy for HIV-1 infection. N Engl J Med. 2003;349:2293–2303. doi: 10.1056/NEJMoa030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fischl M, Ribaudo H, Collier A, Erice A, Giuliano M, Dehlinger M, et al. A randomized trial of 2 different 4-drug antiretroviral regimens versus a 3-drug regimen, in advanced human immunodeficiency virus disease. Am J Infect Dis. 2003;188:625–634. doi: 10.1086/377311. [DOI] [PubMed] [Google Scholar]
- 34.Landay A, Spritzler J, Kessler H, Mildvan D, Pu M, Fox L, et al. Immune reconstitution is comparable in antiretroviral-naive subjects after 1 year of successful therapy with a nucleoside reverse-transcriptase inhibitor- or protease inhibitor-containing antiretroviral regimen. Am J Infect Dis. 2003;188:1444–1454. doi: 10.1086/379041. [DOI] [PubMed] [Google Scholar]
- 35.Gulick R, Ribaudo H, Shikuma C, Lustgarten S, Squires K, Meyer W, et al. Triple-nucleoside regimens versus efavirenz-containing regimens for the initial treatment of HIV-1 infection. N Engl J Med. 2004;350:1850–1861. doi: 10.1056/NEJMoa031772. [DOI] [PubMed] [Google Scholar]
- 36.Riddler S, Haubrich R, DiRienzo G, Peeples L, Powderly W, Klingman K, et al. Class-sparing regimens for initial treatment of HIV-1 infection. N Engl J Med. 2008;358:2095–2106. doi: 10.1056/NEJMoa074609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simpson DM, Kitch D, Evans SR, McArthur J, Asmuth DM, Cohen B, et al. HIV Neuropathy Natural History Cohort Study: assessment measures and risk factors. Neurology. 2006;66:1679–1687. doi: 10.1212/01.wnl.0000218303.48113.5d. [DOI] [PubMed] [Google Scholar]
- 38.Ellis R, Evans SR, Clifford D, Moo L, McArthur J, Collier A, et al. Clinical validation of the neuroscreen. J Neurovirol. 2005;11:503–511. doi: 10.1080/13550280500384966. [DOI] [PubMed] [Google Scholar]
- 39.Evans S, Li L. A comparison of goodness of fit tests for the logistic GEE model. Statist Med. 2005;24:1245–1261. doi: 10.1002/sim.2023. [DOI] [PubMed] [Google Scholar]
- 40.Watters MR, Poff PW, Shiramizu BT, Holck PS, Fast KM, Shikuma CM, et al. Symptomatic distal sensory polyneuropathy in HIV after age 50. Neurology. 2004;62:1378–1383. doi: 10.1212/01.wnl.0000120622.91018.ea. [DOI] [PubMed] [Google Scholar]
- 41.Ellis RJ, Rosario D, Clifford DB, McArthur JC, Simpson D, Alexander T, et al. Continued high prevalence and adverse clinical impact of human immunodeficiency virus-associated sensory neuropathy in the era of combination antiretroviral therapy. Arch Neurol. 2010;67:552–558. doi: 10.1001/archneurol.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cherry CL, Affandi JS, Brew BJ, Creighton J, Djauzi S, Hooker DJ, et al. Hepatitis C seropositivity is not a risk factor for sensory neuropathy among patients with HIV. Neurology. 2010;74:1538–1542. doi: 10.1212/WNL.0b013e3181dd436d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gaist D, Jeppesen U, Andersen M, García Rodríguez LA, Hallas J, Sindrup SH. Statins and risk of polyneuropathy: a case-control study. Neurology. 2002;58:1333–1337. doi: 10.1212/wnl.58.9.1333. [DOI] [PubMed] [Google Scholar]