Abstract
Scintigraphic colonic transit measurement has emerged as a unique disease-related biomarker pertinent to drug development and personalized therapy. This surrogate offers reproducible and accurate performance across a spectrum of common colonic motility disorders, linking colonic transit measurements to biological processes and clinical end points. The pathobiologic validity, prognostic utility, performance characteristics, evidence-based linkage of therapeutic intervention with outcome, applicability across distinct pharmacodynamic profiles, and potential for individualizing patient management are all attributes of this gastrointestinal imaging paradigm. The cumulative evidence suggests that the integration of colonic transit measurement as a biomarker would accelerate the development and regulatory approval of therapeutic agents for the treatment of colonic dysmotility.
BACKGROUND AND DEFINITIONS
As a result of a consensus conference of the US Food and Drug Administration and the National Institutes of Health, a biomarker was defined as “a characteristic that is measured and evaluated as an indicator of normal biologic processes, pathogenic processes, or pharmacological responses to a therapeutic intervention.”1 A surrogate end point (SEP) was defined as a biomarker that is intended to be a substitute for a clinical end point; prediction of clinical benefit is based on epidemiologic, therapeutic, or pathophysiologic evidence. Although SEPs have been used in oncology, infectious disease, cardiology, and bone health,2 there have been no such advances in the field of colonic motility and functional gastrointestinal disorders (FGID). Biomarkers include the following:
Target-engagement, or proximal, biomarkers, which provide information on physical or biological interactions with the molecular target or mechanism of a drug.
Disease-related, or distal, biomarkers,2 which come into play late in the pathophysiological cascade and are capable of predicting clinical benefit. These biomarkers qualify as SEPs if they demonstrate3 (i) apparent biologic plausibility, (ii) prognostic value for the outcome of the disease, and (iii) an association between changes in the SEP and outcome with therapeutic intervention.
This article reviews the evidence, based on a literature search conducted using PubMed, that scintigraphic imaging of colonic transit has a role as a disease-related biomarker and should be considered a valuable tool for drug development because it fulfills all the required criteria: known performance characteristics, reproducible and accurate data over a range of conditions, and evidence of linkage to biological processes and clinical end points. The combined evidence provides convincing arguments that colonic transit measurement could be cited as a biomarker in new drug applications pertaining to colonic motility disorders. Because the biomarker has not been used as the basis for seeking approval of a drug, it cannot yet be designated an SEP. However, scintigraphic colonic transit has the potential to fulfill the criteria proposed for SEPs.3
THE METHOD
Colonic transit is usually measured as part of a gastrointestinal and colonic transit test using radioscintigraphy. The method is summarized in Figure 1a.
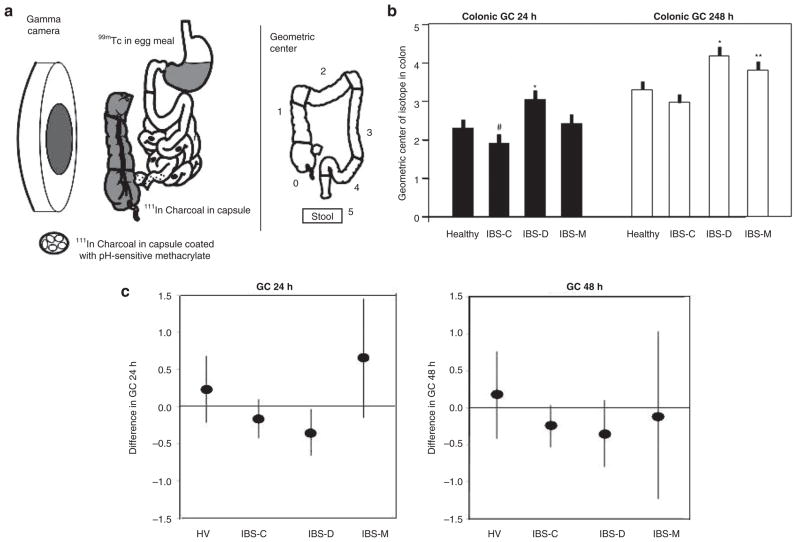
Figure 1.
Colonic transit measurement by scintigraphy in health and subgroups of IBS. (a) Method to measure colonic transit: radioisotope-labeled activated charcoal particles delivered in a pH-sensitive methacrylate-coated capsule to the ileocolonic region. Methacrylate dissolves at pH >6.4, which is achieved in the distal small intestine. The radiolabeled particles traverse the colon in the stool, and the counts in each of five regions are used to summarize the location of the weighted average or geometric center at specified times after capsule ingestion, e.g., at 24 and 48 h. More frequent imaging allows accurate estimation of the ascending colon emptying time. The ascending colon emptying time is significantly correlated with stool consistency. (b) Comparison of colonic geometric center at 24 and 48 h in healthy controls and in IBS patients with different types of bowel dysfunction. #P = 0.078; *P < 0.05; **P = 0.056. Reproduced from ref. 6. (c) Changes in geometric center in various subgroups expressed as means and 95% confidence intervals. Note that the 95% confidence interval for the IBS-D subgroup does not cross the zero line, indicating a significant difference in colonic transit time for the IBS-D subgroup but not for the other groups. Reproduced from ref. 9. GC, geometric center; HV, healthy volunteers; IBS, irritable bowel syndrome; IBS-C, constipation-predominant IBS; IBS-D, diarrhea-predominant IBS; IBS-M, IBS with mixed bowel function.
Procedure
After an overnight fast, a capsule containing 111In adsorbed on activated charcoal is administered. The capsule is coated with methacrylate (Eudragit S-100; Dow Chemical, Midland, MI) and dissolves in a pH-sensitive manner when it reaches the alkaline terminal ileum, releasing the radioisotope into the lumen and thereby allowing assessment of colonic transit. After the capsule has emptied from the stomach, documented by its position relative to a radioisotope marker placed on the right anterior iliac crest, participants ingest a 99mTc-labeled breakfast (315 kcal; two scrambled eggs, one slice of whole wheat bread, and one glass of whole milk). The 99mTc-sulfur colloid in the breakfast is used to estimate the gastric emptying and colonic filling profile at specified times; colonic filling at 6 h is a surrogate for small-bowel transit time.
Using a gamma camera, anterior and posterior abdominal scans, each of 2-min duration, are carried out immediately after ingestion of the radiolabeled breakfast and at 4, 6, 8, 24, and 48 h after the breakfast to assess small-bowel and colonic transit. Two standard meals (a 462-kcal lunch and a 607-kcal dinner) are ingested at 4 and 8 h, respectively, after the 99mTc-labeled breakfast. The only definite contraindication for the test is pregnancy.
Data analysis
99mTc counts are quantified within a 140-keV (±20%) window and 111In counts within a 247-keV (±20%) window. Count corrections are made for isotope decay, tissue attenuation, and downscatter of the 111In in the 99mTc window. A variable region-of-interest program is used to quantitate counts in each colonic segment: ascending colon (AC), transverse colon (TC), descending colon (DC), and rectosigmoid (RS), numbered as segments 1–4, respectively. Segment 5 refers to the expelled stool.
End points summarizing colonic transit
Two primary end points are used to summarize colonic transit: (i) overall colonic transit, expressed as geometric center, and (ii) emptying of the ascending colon.
Overall colonic transit is expressed as the geometric center (GC), which is the weighted average of the isotope distribution within the colon and stool and is calculated as the sum of the products of the proportion of 111In counts in each segment and its weighting factor:
The emptying of the AC is summarized as the t1/2 (time for 50% emptying) as calculated by linear interpolation of values on the AC emptying curve. The delayed-release capsule facilitates this measurement by delivering the radiolabeled charcoal to the ileocolonic region before dissolution of the capsule allows the particles to disperse. Given that the emptying of the ileum into the colon occurs as bolus movements,4 there is relatively clear accumulation of 111In-charcoal in the AC.
BIOLOGIC PLAUSIBILITY
Whole colonic transit and AC emptying have been reported to be abnormal in several diseases of colonic motility, including idiopathic constipation, functional diarrhea, carcinoid diarrhea,5 and several subtypes of irritable bowel syndrome (IBS) based on predominant bowel dysfunction.6 It is therefore plausible that colonic transit measurement may serve as a biological marker of colonic function in disease and as a biomarker in the evaluation of drug therapy.
For example, in a study of the highly prevalent, multifactorial clinical condition of IBS,6 32% of patients had abnormal colonic transit: 16% of the patients with constipation-predominant IBS (IBS-C) had slow transit, and 46% of those with diarrhea-predominant IBS (IBS-D) had fast transit, relative to normal transit duration. In addition, 14% of patients with IBS with mixed bowel function had fast transit at 48 h. These data from 120 patients6 are summarized in Figure 1b. Colonic transit measurement detects group differences in subtypes of IBS based on the predominant bowel function as reported by the patients.
These observations were consolidated in a recent analysis7 of 287 patients with lower FGID: IBS-C or functional constipation (n = 118), IBS-D or functional diarrhea (n = 139), and IBS with mixed bowel function (n = 30). There were 170 healthy controls.7 Overall colonic transit was estimated at time points of 8, 24, and 48 h. Abnormal colonic transit at 24 h (GC 24 of <1.50 or >3.86) was observed in 29.7% of all patients with lower FGID. There were significant (P < 0.01 for all) overall associations between colonic transit time and subject group (healthy controls and FGID subgroups) at the 8-, 24-, and 48-h time points. Significant associations were particularly evident at the 24- and 48-h time points for patients with diarrhea or constipation (P < 0.05), and these associations remained significant after adjusting for age, gender, and body mass index.
PERFORMANCE CHARACTERISTICS
The performance characteristics have been appraised in two publications that involved healthy participants and patients with IBS.
Cremonini et al. reported on 37 healthy volunteers (mean age 39 ± 11 years; 10 women) who underwent scintigraphic measurement of gastrointestinal and colonic transit over a period of 48 h.8 Three weeks after the initial evaluation, the scintigraphic transit test was repeated in 21 of the 37 volunteers (mean age 36 ± 9 years; 8 women), using the same procedure. Colonic measurements varied by >1 geometric center unit in 37% of the subjects at 24 h and in 26% of the subjects at 48 h. The interindividual coefficients of variation (COV) for 21 participants were 37 and 24% at 24 and 48 h, respectively; the intraindividual COVs were 28 and 14%, respectively. There were no significant effects of age and gender on these transit summaries in healthy adults, whose ages ranged between 18 and 65 years.
More recently, Deiteren et al.9 assessed inter- and intraindividual variations of scintigraphic colonic transit measurements, after either a short interval (within 3 weeks) or a long interval (median 2.0 years (range 0.1, 11.0)) in 86 IBS patients and 17 healthy subjects. There was limited overall intraindividual variation over the short-term period of <3 weeks (COV 31% at 24 h, 27% at 48 h) and over a median interval of 2.0 years (COV 38% at 24 h, 30% at 48 h) in healthy persons and in those with IBS-C. The COV reflects the physiological variation in colonic motor function that also manifests as natural variation in stool frequency and consistency in IBS-D patients.
Significant intraindividual differences in GC at 24 h were observed only in patients with IBS-D, in whom the mean differences at 24 and 48 h were −0.35 ± 1.19 (SD) and 0.35 ± 0.90, respectively. Figure 1c shows the change in GC (expressed as mean and 95% confidence intervals) over the long term in the various subgroups of patients and healthy controls. Note that the 95% confidence interval for the IBS-D subgroup does not cross the zero line, indicating a significant difference in colonic transit time for the IBS-D subgroup but not for the other groups.
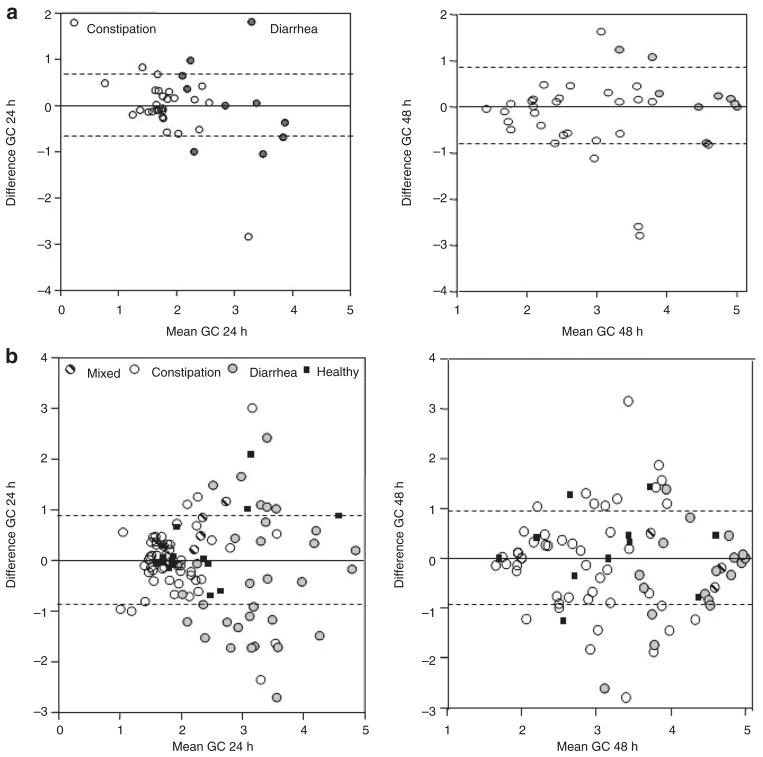
Bland–Altman plots (Figure 2) from the study by Deiteren et al.9 show the intraindividual variation for repeat measurements conducted within 3 weeks or after a median of 2.0 years; the data suggest that the degree of variation is similar across different mean values of colonic transit, and the vast majority of individuals have replicate values within one GC unit of baseline measurement.
Figure 2.
Intrasubject variation in colonic transit over short- and long term. (a) Bland–Altman plots showing short-term (median 3 weeks) intrasubject variation in colonic transit at 24 and 48 h in IBS patients with diarrhea or constipation. Plot shows 1 SD as interrupted lines. Note that most of the data are well within 1 SD, which is ~0.7 GC units (y axis). Reproduced from ref. 9. (b) Bland–Altman plots showing long-term (median 2 years) intrasubject variation in colonic transit at 24 and 48 h in participants in the various subgroups. The plot shows 1 SD as interrupted lines. Note that most of the data are well within 1 SD, which is ~0.9 GC units (y axis). The greatest variation occurs in IBS patients with diarrhea, and the variation is greater at 24 than at 48 h. Reproduced from ref. 9. GC, geometric center; IBS, irritable bowel syndrome.
In summary, the biomarker is relatively stable at median 3 weeks and at median 2 years, and the significant difference in the GC at 24 h in patients with IBS-D reflects the higher colonic transit values at 24 and 48 h and the natural variation in overall bowel function among patients with chronic diarrheal disorders. The documented COV has to be factored into the planned size of the sample in each treatment group so as to exclude a type II error.
CLINICAL TRIAL EVIDENCE: COLONIC TRANSIT BIOMARKER RESPONSE PREDICTS THERAPEUTIC OUTCOME
The measurements of colonic transit in response to drugs in development have generally predicted the responses to treatment observed in phase IIB or III clinical trials (Table 1, refs. 10–36). For instance, scintigraphic colonic transit has correctly predicted clinical efficacy with medications targeting a variety of mechanisms, including prokinetics (e.g., 5-HT4 agonists, bisacodyl, and neurotrophin-3), medications that retard transit (5-HT3 antagonists and CCK1 antagonists), and secretagogues (e.g., linaclotide and lubiprostone). Equally important, there have been clinical trials of IBS in which the measurement of colonic transit time showed no significant effects of the drug on colonic transit and thereby correctly predicted lack of efficacy of the medication in ameliorating bowel dysfunction. Examples include trials with a CRF1-antagonist33,34 and solabegron (a β3-adrenergic agonist35,36).
Table 1.
Evidence of clinical efficacy predicted by colonic transit as measured by scintigraphy
| Drug class | Pharmacodynamics (intestine or colon) | Clinical efficacy: phase IIB or II studies | Reference |
|---|---|---|---|
| 5-HT3-antagonist, alosetron | 1 mg b.i.d. delayed the rapid colonic transit in IBS-D | IIB, III studies in thousands of patients with non-IBS-C or IBS-D showed adequate relief of pain and discomfort of IBS, bowel dysfunction (including diarrhea), and urgency | 10 |
| 5-HT4-agonist, tegaserod | 2 mg b.i.d. accelerated SB transit and colonic transit in healthy persons and in patients with IBS-C (without evacuation disorder) | IIB, III studies in several thousands of patients with IBS-C and CC showed relief of pain and discomfort of IBS and bowel dysfunction | 11 |
| 5-HT4-agonist, prucalopride | Increased SB and colon motility and transit in healthy controls and in patients with CC | IIB and III in thousands of patients with CC showed improvement in BM frequency and satisfaction with bowel function | 12,13 |
| 5-HT4-agonist, velusetrag | Caused dose-related increases in SB and colon transit in healthy controls | IIB dose-ranging study in 401 patients with CC showed increased in BM frequency and proportion, with adequate relief | 14,15 |
| Bisacodyl | Accelerated colon transit in healthy controls | Showed relief of constipation after acute administration | 16,17 |
| Recombinant human neurotrophin (NT)-3 | NT-3 accelerated colonic transit in patients with CC | NT-3, administered TTW, increased stool frequency, enhanced colon transit, and improved symptoms of CC | 18,19 |
| C1–C2 channel activator, lubiprostone | Accelerated SB and colonic transit in healthy controls | Two phase III trials in several hundred patients with CC and IBS-C: efficacious in relief of pain and bowel dysfunction | 20–24 |
| Guanylate cyclase-C agonist, linaclotide | Accelerated AC transit and altered bowel function in 36 female patients with IBS-C | IIA and IIB studies in patients with CC or IBS-C showed increased BM frequency and relief from bloating and abdominal discomfort | 25,26 |
| κ-Opioid agonist, asimadoline | Showed no significant effect on colonic transit in healthy volunteers | On-demand dosing not effective in reducing severity of abdominal pain in 100 patients with IBS; IIB dose-ranging study on 596 patients with IBS: post hoc subgroup analysis showed benefit in average moderate pain in patients with IBS-D and IBS-Alt | 27–29 |
| CCK1-antagonist, dexloxiglumide | Showed slower AC emptying with no effect on overall colonic transit in patients with IBS-C | Two initial II B or III trials: not efficacious in patients with IBS-C; a randomized withdrawal design trial showed longer time to loss of therapeutic response, longer for dexloxiglumide | 30–32 |
| CRH1-antagonist, pexacerfont | Showed no effect on colonic transit and bowel function in patients with IBS-D | One phase IIB study showed that GW876008 showed no significant difference from placebo in the global improvement scale, daily self-assessment of IBS pain/discomfort, and individual lower GI symptoms | 33,34 |
| β3-Adrenergic agonist, solabegron | Showed no significant effect on GI or colonic transit | One phase IIB study showed no significant change in bowel symptoms, although there was adequate relief of IBS pain and discomfort | 35,36 |
AC, ascending colon; BM, bowel movement; Cl–C2, chloride channel type 2; CC, chronic constipation; CCK, cholecystokinin; CRH, corticotropin-releasing hormone; GI, gastrointestinal; 5-HT, 5-hydroxytryptamine; IBS, irritable bowel syndrome; IBS-Alt, alternating IBS; IBS-C, constipation-predominant IBS; IBS-D, diarrhea-predominant IBS; SB, small bowel; TTW, three times per week.
USE OF THE BIOMARKER IN PHARMACOGENETIC STUDIES: POTENTIAL USE IN INDIVIDUALIZED MEDICINE
We have demonstrated that genetic variation in the 5-HTTLPR gene (which encodes the serotonin transporter protein SLC6A4) influences the effect of the 5-HT3 antagonist alosetron on colonic transit in patients with IBS-D. The homozygous long genotype, as compared with the short allele, was associated with a greater retardation of colonic transit by alosetron.37 This observation suggests that colonic transit may also serve as a biomarker to select the patients who are likely to respond to medication, a priority for individualized medicine.
CONCLUSION
In summary, there is overwhelming evidence of scintigraphic colonic transit’s biologic plausibility, association with the clinical manifestations of the diseases that affect colonic motility, performance characteristics, correct prediction of positive and negative outcomes in therapeutic interventions, and potential use as a marker in support of individualized medicine. Scintigraphic colonic transit is an important disease-related biomarker, as demonstrated by the fact that colonic transit at 24 h is abnormal in 29.7% of all patients with lower FGID and in 46% of those with diarrhea-related lower FGID. In addition, results of pharmacodynamic studies demonstrate either acceleration of transit (in disorders associated with diarrhea) or retardation of transit (in disorders associated with constipation); therefore, the same biomarker serves as a viable biomarker for testing drug efficacy in the wide spectrum of colonic motility disorders that manifest with abnormal transit. Pharmacodynamic studies using this relatively simple biomarker correctly predict efficacy or lack of efficacy (using patients’ symptom-based end points) observed in clinical trials of medications with diverse mechanisms of action.
The results of scintigraphic transit measurements were included in the new drug applications for several medications that have been approved by the US Food and Drug Administration or the European Medicines Agency. These include alosetron, tegaserod, prucalopride, and lubiprostone. It is therefore appropriate to propose scintigraphic colonic transit as a biomarker in the appraisal and development of drugs for the treatment of colonic motility and lower FGID, and to continue validation to fulfill criteria as an SEP.3
Acknowledgments
The author gratefully acknowledges the support of the National Institutes of Health (NIH) (DK86182, DK79866, and DK54681) and a Mayo Clinic CTSA grant from the NIH (RR24150).
Footnotes
CONFLICT OF INTEREST
The author declared no conflict of interest.
References
- 1.Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 2.Wagner JA. Biomarkers: principles, policies, and practice. Clin Pharmacol Ther. 2009;86:3–7. doi: 10.1038/clpt.2009.77. [DOI] [PubMed] [Google Scholar]
- 3.Lathia CD, et al. The value, qualification, and regulatory use of surrogate end points in drug development. Clin Pharmacol Ther. 2009;86:32–43. doi: 10.1038/clpt.2009.69. [DOI] [PubMed] [Google Scholar]
- 4.Spiller RC, Brown ML, Phillips SF. Emptying of the terminal ileum in intact humans. Influence of meal residue and ileal motility. Gastroenterology. 1987;92:724–729. doi: 10.1016/0016-5085(87)90024-2. [DOI] [PubMed] [Google Scholar]
- 5.von der Ohe MR, Camilleri M, Kvols LK, Thomforde GM. Motor dysfunction of the small bowel and colon in patients with the carcinoid syndrome and diarrhea. N Engl J Med. 1993;329:1073–1078. doi: 10.1056/NEJM199310073291503. [DOI] [PubMed] [Google Scholar]
- 6.Camilleri M, et al. Prospective study of motor, sensory, psychologic, and autonomic functions in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2008;6:772–781. doi: 10.1016/j.cgh.2008.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manabe N, Wong BS, Camilleri M, Burton D, McKinzie S, Zinsmeister AR. Lower functional gastrointestinal disorders: evidence of abnormal colonic transit in a 287 patient cohort. Neurogastroenterol Motil. 2009 doi: 10.1111/j.1365-2982.2009.01442.x. e-pub ahead of print 21 December 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cremonini F, Mullan BP, Camilleri M, Burton DD, Rank MR. Performance characteristics of scintigraphic transit measurements for studies of experimental therapies. Aliment Pharmacol Ther. 2002;16:1781–1790. doi: 10.1046/j.1365-2036.2002.01344.x. [DOI] [PubMed] [Google Scholar]
- 9.Deiteren A, et al. Performance characteristics of scintigraphic colon transit measurement in health and irritable bowel syndrome and relationship to bowel functions. Neurogastroenterol Motil. 2009 doi: 10.1111/j.1365-2982.2009.01441.x. e-pub ahead of print 18 December 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andresen V, Montori VM, Keller J, West CP, Layer P, Camilleri M. Effects of 5-hydroxytryptamine (serotonin) type 3 antagonists on symptom relief and constipation in nonconstipated irritable bowel syndrome: a systematic review and meta-analysis of randomized controlled trials. Clin Gastroenterol Hepatol. 2008;6:545–555. doi: 10.1016/j.cgh.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prather CM, Camilleri M, Zinsmeister AR, McKinzie S, Thomforde G. Tegaserod accelerates orocecal transit in patients with constipation-predominant irritable bowel syndrome. Gastroenterology. 2000;118:463–468. doi: 10.1016/s0016-5085(00)70251-4. [DOI] [PubMed] [Google Scholar]
- 12.Bouras EP, Camilleri M, Burton DD, Thomforde G, McKinzie S, Zinsmeister AR. Prucalopride accelerates gastrointestinal and colonic transit in patients with constipation without a rectal evacuation disorder. Gastroenterology. 2001;120:354–360. doi: 10.1053/gast.2001.21166. [DOI] [PubMed] [Google Scholar]
- 13.Camilleri M, Kerstens R, Rykx A, Vandeplassche L. A randomized, placebo-controlled trial to evaluate the efficacy, safety and effect on quality of life of prucalopride in severe chronic constipation. N Engl J Med. 2008;358:2344–2354. doi: 10.1056/NEJMoa0800670. [DOI] [PubMed] [Google Scholar]
- 14.Manini ML, et al. Effects of Velusetrag (TD-5108) on gastrointestinal transit and bowel function in health and pharmacokinetics in health and constipation. Neurogastroenterol Motil. 2010;22:42–9. e7. doi: 10.1111/j.1365-2982.2009.01378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldberg MR, Li YP, Pitzer K, Johanson JF, Mangel AW, Kitt MM. T1389 TD-5108, a selective 5-HT4 agonist, is consistently better than placebo regardless of response definition in patients with chronic constipation. Gastroenterology. 2008;134:A545. [Google Scholar]
- 16.Manabe N, Cremonini F, Camilleri M, Sandborn WJ, Burton DD. Effects of bisacodyl on ascending colon emptying and overall colonic transit in healthy volunteers. Aliment Pharmacol Ther. 2009;30:930–936. doi: 10.1111/j.1365-2036.2009.04118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kienzle-Horn S, Vix JM, Schuijt C, Peil H, Jordan CC, Kamm MA. Efficacy and safety of bisacodyl in the acute treatment of constipation: a double-blind, randomized, placebo-controlled study. Aliment Pharmacol Ther. 2006;23:1479–1488. doi: 10.1111/j.1365-2036.2006.02903.x. [DOI] [PubMed] [Google Scholar]
- 18.Coulie B, et al. Recombinant human neurotrophic factors accelerate colonic transit and relieve constipation in humans. Gastroenterology. 2000;119:41–50. doi: 10.1053/gast.2000.8553. [DOI] [PubMed] [Google Scholar]
- 19.Parkman HP, et al. Functional Constipation Study Investigators. Neurotrophin-3 improves functional constipation. Am J Gastroenterol. 2003;98:1338–1347. doi: 10.1111/j.1572-0241.2003.t01-1-07477.x. [DOI] [PubMed] [Google Scholar]
- 20.Camilleri M, et al. Effect of a selective chloride channel activator, lubiprostone, on gastrointestinal transit, gastric sensory, and motor functions in healthy volunteers. Am J Physiol Gastrointest Liver Physiol. 2006;290:G942–G947. doi: 10.1152/ajpgi.00264.2005. [DOI] [PubMed] [Google Scholar]
- 21.Johanson JF, Morton D, Geenen J, Ueno R. Multicenter, 4-week, double-blind, randomized, placebo-controlled trial of lubiprostone, a locally-acting type-2 chloride channel activator, in patients with chronic constipation. Am J Gastroenterol. 2008;103:170–177. doi: 10.1111/j.1572-0241.2007.01524.x. [DOI] [PubMed] [Google Scholar]
- 22.Johanson JF, Ueno R. Lubiprostone, a locally acting chloride channel activator, in adult patients with chronic constipation: a double-blind, placebo-controlled, dose-ranging study to evaluate efficacy and safety. Aliment Pharmacol Ther. 2007;25:1351–1361. doi: 10.1111/j.1365-2036.2007.03320.x. [DOI] [PubMed] [Google Scholar]
- 23.Johanson JF, Drossman DA, Panas R, Wahle A, Ueno R. Clinical trial: phase 2 study of lubiprostone for irritable bowel syndrome with constipation. Aliment Pharmacol Ther. 2008;27:685–696. doi: 10.1111/j.1365-2036.2008.03629.x. [DOI] [PubMed] [Google Scholar]
- 24.Drossman DA, Chey W, Panas R, Wahle A, Scott C, Ueno R. Lubiprostone significantly improves symptom relief rates in adults with IBS. Gastroenterology. 2007;132:2586–2587. [Google Scholar]
- 25.Andresen V, et al. Effect of 5 days linaclotide on transit and bowel function in females with constipation-predominant irritable bowel syndrome. Gastroenterology. 2007;133:761–768. doi: 10.1053/j.gastro.2007.06.067. [DOI] [PubMed] [Google Scholar]
- 26.Johnston JM, et al. Pilot study on the effect of linaclotide in patients with chronic constipation. Am J Gastroenterol. 2009;104:125–132. doi: 10.1038/ajg.2008.59. [DOI] [PubMed] [Google Scholar]
- 27.Delvaux M, Beck A, Jacob J, Bouzamondo H, Weber FT, Frexinos J. Effect of asimadoline, a kappa opioid agonist, on pain induced by colonic distension in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2004;20:237–246. doi: 10.1111/j.1365-2036.2004.01922.x. [DOI] [PubMed] [Google Scholar]
- 28.Szarka LA, et al. Efficacy of on-demand asimadoline, a peripheral kappa-opioid agonist, in females with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2007;5:1268–1275. doi: 10.1016/j.cgh.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mangel AW, et al. Clinical trial: asimadoline in the treatment of patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2008;28:239–249. doi: 10.1111/j.1365-2036.2008.03730.x. [DOI] [PubMed] [Google Scholar]
- 30.Cremonini F, et al. Effect of CCK-1 antagonist, dexloxiglumide, in female patients with irritable bowel syndrome: a pharmacodynamic and pharmacogenomic study. Am J Gastroenterol. 2005;100:652–663. doi: 10.1111/j.1572-0241.2005.41081.x. [DOI] [PubMed] [Google Scholar]
- 31.D’Amato M, Whorwell PJ, Thompson DG. The efficacy and safety of the CCKA-receptor antagonist dexloxiglumide in IBS. Gut. 1999;45 (suppl 5):A258. [Google Scholar]
- 32.Whorwell PJ, et al. A phase III, 6-month, double-blind, placebo-controlled, randomized withdrawal trial of the selective CCK-1 antagonist dexloxiglumide in constipation-predominant IBS: the Darwin study. Gastroenterology. 2008;134:A157. [Google Scholar]
- 33.Sweetser S, et al. Do corticotropin releasing factor-1 receptors influence colonic transit and bowel function in women with irritable bowel syndrome? Am J Physiol Gastrointest Liver Physiol. 2009;296:G1299–G1306. doi: 10.1152/ajpgi.00011.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duke GE, Mayer EA, Kelleher DL, Hicks KJ, Boardley RL, Alpers DH. A randomised, double blind, placebo (PLA) controlled, crossover study to evaluate the efficacy and safety of the corticotrophin releasing factor 1 (CRF1) receptor antagonist (RA) GW876008 in irritable bowel syndrome (IBS) patients. Neurogastroenterol Motil. 21(suppl):84. (abstract, 2009) [Google Scholar]
- 35.Grudell AB, et al. Dose-response effect of a beta3-adrenergic receptor agonist, solabegron, on gastrointestinal transit, bowel function, and somatostatin levels in health. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1114–G1119. doi: 10.1152/ajpgi.00051.2008. [DOI] [PubMed] [Google Scholar]
- 36.Kelleher DL, Hicks KJ, Cox DS, Williamson RR, Alpers DH, Dukes GE. Randomized, double-blind, placebo (PLA)-controlled, crossover study to evaluate efficacy and safety of the beta 3-adrenergic receptor agonist solabegron (SOL) in patients with irritable bowel syndrome (IBS) Neurogastroenterol Motil. 20(suppl 1):131–132. (abstract, 2008) [Google Scholar]
- 37.Camilleri M, et al. Serotonin-transporter polymorphism pharmacogenetics in diarrhea-predominant irritable bowel syndrome. Gastroenterology. 2002;123:425–432. doi: 10.1053/gast.2002.34780. [DOI] [PubMed] [Google Scholar]