Abstract
Avian influenza A/H9N2 viruses can infect people and are viruses considered to be a potential pandemic threat. Prior studies with an inactivated G1 clade H9N2 vaccine reported that persons born before 1968 were more likely to have an immune response than younger subjects. We performed a randomized, double-blind trial to evaluate whether immune responses following immunization with an inactivated, unadjuvanted influenza G9 H9N2 vaccine prepared from A/chicken/Hong Kong/G9/97 virus were more frequent in persons born in 1964 or earlier (44–59 years) than in those born in 1970 or later (18–38 years). One hundred twenty one persons were randomized to receive two doses of either 7.5- or 30-mcg of hemagglutinin intramuscularly. Post-vaccination serum antibody responses as measured by hemagglutination inhibition and microneutralization were either similar in the two age cohorts or greater in the younger age group. Persons born before 1968 were not more likely to respond to a G9 H9N2 influenza vaccine than persons born in 1970 or later.
1. Introduction
Influenza A/H9N2 viruses are panzootic in poultry in Eurasia, and several different clades have been identified, represented by G1, Y280, and Korean strains [1,2]. These clades can be differentiated by phylogenetic analysis of the hemagglutinin gene as well as by antigenic analyses. Hemagglutination inhibition (HAI) assays using polyclonal antisera to H9N2 isolates identify low levels of cross-reactive antibody between clades while high levels are present within a clade [2]. Pools of monoclonal antibodies that recognize clade-specific epitopes also can be used to characterize H9N2 isolates [1]. Because of the ability of H9N2 viruses to cause infection in people, these viruses are considered potential pandemic threats [3,4]. Vaccination is the primary method for prevention of influenza, and the development of immunogenic vaccines and vaccination schedules against potential pandemic viruses is a cornerstone of pandemic preparedness plans [5].
Several studies of candidate influenza A/H9N2 vaccines have been reported [6–10]. In one of these, Stephenson et al. [10] evaluated the safety and immunogenicity of two doses of whole virus or subunit influenza A/Hong Kong/1073/99 (H9N2) vaccine containing 7.5, 15 or 30mcg of hemagglutinin (HA) given 21 days apart among healthy adults; the vaccine strain was a G1 clade virus. There were no differences in seroconversion frequencies between groups given whole virus and subunit virus vaccines, but persons born after 1969 (<35 years of age) were less likely to respond. Only 14% of subjects born in 1969 or later responded to a single dose of subvirion vaccine compared to 63% of subjects born before 1969. These findings suggested that persons born before 1969 were primed for responding to the vaccine strain. Nicholson et al. subsequently reported that persons born before 1969 were also more likely to respond to a single dose of whole virus G1 H9N2 vaccine with or without alum adjuvant than were younger study participants [9]. Their proposal was that the older persons were infected with an A/H2N2 influenza virus that had primed them for an antibody response to an A/H9N2 virus [10].
We previously described the immunogenicity of an inactivated purified surface hemagglutinin H9N2 vaccine containing a G9 strain (belonging to the Y280 clade) among younger adults born in 1970 or later [6]. The vaccine was safe and well-tolerated, and vaccine adjuvanted with MF59 was more immunogenic than non-adjuvanted vaccine. In the present study, we examined whether persons born in 1970 or later were less likely to respond to the unadjuvanted H9N2 vaccine containing a G9 virus than persons born before 1965, as had been reported for vaccines containing a G1 clade strain. Such a response pattern could convey the public health benefit of need for a single booster dose only to older persons should a need for immunization for an A/H9N2 pandemic threat emerge. Thus, the primary objective of the study was to determine whether persons 44–59 years of age were more likely to have a four-fold or greater antibody rise to a single dose of the G9 strain of A/H9 vaccine virus than persons 18–38 years of age.
2. Materials and Methods
2.1 Subjects
Study participants were healthy, non-pregnant adults who were divided into two age groups: persons between the ages of 18 and 38 years and those between the ages of 44 and 59 years. The younger group included persons born in 1970 or later, while the older group included persons born in 1964 or earlier. Exclusion criteria included the following: allergy to eggs or other vaccine components; positive urine pregnancy test; breast-feeding; immunosuppression due to underlying illness or treatment; active neoplastic disease or history of hematologic malignancy; use of oral, parenteral or high-dose inhaled steroids; receipt of immunoglobulin or other blood product within 3 months; receipt of any inactivated vaccine within 2 weeks or of any live vaccine within 4 weeks; chronic medical condition, including diabetes mellitus, chronic liver disease, significant renal disease, progressive neurological disorder; history of severe reactions to influenza vaccines; acute illness, including oral temperature >38°C, within past week; receipt of experimental agent within 1 month of study vaccination; prior receipt of H9N2 vaccine; participation in another clinical trial; known active infection with HIV, hepatitis B or hepatitis C; history of alcohol or drug abuse within 5 years; history of Guillain-Barré syndrome; current or past significant psychiatric illness, including need for hospitalization, suicide attempt, use of psychiatric drugs (except stable use of a single antidepressant for at least 3 months); and travel outside the US in the first 8 weeks of the study. Eligible persons were screened and enrolled after providing written informed consent in accordance with protocols approved by the Texas A&M and Baylor College of Medicine Institutional Review Boards (Clinicaltrials.gov NCT00617331).
2.2 Vaccines
The vaccine was a monovalent subvirion influenza A/H9N2 vaccine (with the HA of A/chicken/Hong Kong/G9/1997) prepared by Novartis, as described previously [6]. Prefilled, single dose syringes contained one of two dosage levels, 7.5 or 30-mcg HA per 0.5 mL, as determined by single radial immunodiffusion. The vaccine was prepared by the manufacturer, and Fisher Bioservices (Rockville, MD) coded the study vaccine and provided it to the study site.
2.3 Study procedures
After informed consent was obtained, subjects were screened for good health by obtaining a health history and performing a targeted physical examination. Inclusion and exclusion criteria were reviewed to ensure good health, and urine pregnancy tests were performed on female subjects capable of bearing children. Eligible subjects were stratified by age and randomized using the Internet Data Entry System (EMMES Corporation). Either 7.5- or 30-mcg HA dose of study vaccine was administered as a 0.5 mL injection into the deltoid muscle by an unblinded vaccinator who was not otherwise involved in the evaluation of study participants. All other study procedures were performed by blinded personnel, and the study subjects were blinded to the vaccine dosage received. A second dose was administered four weeks later into the opposite arm of subjects who continued to meet eligibility criteria. Subjects remained in the clinic area for at least 20 min after each injection for evaluation of immediate reactogenicity. For seven days after each vaccination the subjects maintained a memory aid to record a daily oral temperature and to capture information about injection site and systemic symptoms. The memory aids were reviewed in the clinic 8 days after each vaccination. Serum samples for antibody assays were collected prior to each vaccination (days 0 and 28) and 4 weeks (day 56) after the second vaccination.
Information on adverse events was collected at each clinic visit. Injection site (pain, tenderness) and systemic (feverishness, headache, malaise, myalgia or nausea) symptoms were graded on a scale of 0 to 3, with 0 indicating absence of the symptom, 1 graded as mild (easily tolerated), 2 graded as moderate (interferes with activity) and 3 graded as severe (incapacitating). Unsolicited adverse events were graded in the same fashion. Fever was defined as an oral temperature of 38.0 C or higher. Injection site erythema and induration were also graded on a 0 to 3 scale, with 0 being absence of the finding, 1 being small (<20 mm), 2 being medium (20–50 mm), and 3 being large (>50 mm). Serious adverse events (SAEs) were defined as life threatening adverse events, significant or persistent disability, hospitalization or death, and information on possible SAEs was solicited at each clinic visit and with a phone call 6 months after the second vaccination.
2.4 Serology
Serum hemagglutination inhibition (HAI) and microneutralization (MNt) assays were performed as previously described [11–13]. Influenza A/chicken/Hong Kong/G9/1997 and A/quail/Hong Kong/G1/1997 viruses were used as antigens. A 4-fold or greater increase in HAI or neutralizing antibody titer from the baseline pre-vaccination level was considered a seroresponse.
2.5 Statistical procedures
Categorical data were compared with Fisher’s exact test or chi-square. Proportions and 95% CIs also were used to describe the following immunogenicity endpoints: frequency of ≥4-fold increase in titer between pre- and post-immunization serum samples as measured by HAI or MNt (seroconversion rate, or SCR), and the proportion of persons achieving a serum HAI titer ≥32 following each vaccination. Additional HAI and MNt immunogenicity measures included geometric mean titers (GMTs) at each study time point and geometric mean fold rise (seroconversion factor, or SCF) from pre-vaccination to each post-vaccination sample, with corresponding 95% CIs. Logistic regression analyses were performed to evaluate reactogenicity and to examine the effect of age cohort and dosage level on SCR. The primary study endpoint was the SCR following a single dose of study vaccine, and with 60 persons per age cohort the study had 80% power to detect a difference in response frequency of 12% or more between the two groups.
3. Results
3.1 Study subjects
One hundred twenty one persons were enrolled in the study between February and June 2008. The demographics of the study population stratified by age group and vaccine dosage group are shown in Table 1. A higher frequency of non-white race was observed among subjects enrolled in the older age group (25% vs. 5% non-white, older vs. younger, P=0.002, Fisher’s exact test).
Table 1.
Demographics of the study population
| Younger age group | Older age group | |||||
|---|---|---|---|---|---|---|
| 7.5 mcg | 30 mcg | All | 7.5 mcg | 30 mcg | All | |
| Number | 31 | 30 | 61 | 28 | 32 | 60 |
| Age in years | ||||||
| Mean (SD) | 27.0 (4.8) | 25.7 (4.7) | 26.4 (4.8) | 51.7 (5.0) | 50.5 (4.1) | 51.0 (4.5) |
| Median (range) | 26.3 (20.4, 38.1) | 24.2 (20.6, 37.7) | 25.2 (20.4, 38.1) | 50.9 (44.1, 59.8) | 50.4 (44.2, 56.4) | 50.6 (44.1, 59.8) |
| Race/Ethnicity (No.) | ||||||
| African- American | 0 | 0 | 0 | 3 | 5 | 8 |
| Asian | 2 | 0 | 2 | 0 | 3 | 3 |
| Mixed/Other/Unknown | 0 | 1 | 1 | 2 | 2 | 4 |
| White | 29 | 29 | 58 | 23 | 22 | 45 |
| Ethnicity (No.) | ||||||
| Hispanic | 1 | 6 | 7 | 3 | 2 | 5 |
| Non-Hispanic | 30 | 24 | 54 | 25 | 30 | 55 |
| Sex (No.) | ||||||
| Female | 22 | 26 | 48 | 21 | 22 | 43 |
| Male | 9 | 4 | 13 | 7 | 10 | 17 |
3.2 Reactogenicity
The numbers of solicited adverse injection site and systemic events in the week following each vaccination are shown in Table 2. Mild injection site reactions (erythema, pain, tenderness) occurred predominantly in the first 2 days after vaccination in about half of subjects. Headache was the most common systemic reaction. Frequencies of reactions were similar in the two dosage groups (7.5 and 30 mcg); persons in the older age group were less likely to report any reaction than persons in the younger age group after each vaccination (logistic regression, OR 3.3 [95% CI 1.4, 7.4] and 3.1 [95% CI 1.4, 6.7] after first and second vaccinations, respectively). No serious adverse events related to the study vaccine occurred during the study.
Table 2.
Maximum intensity of injection site and systemic reactions during the week after immunization
| Dosage | 7.5 mcg | 7.5 mcg | 7.5 mcg | 30 mcg | 30 mcg | 30 mcg |
|---|---|---|---|---|---|---|
| Age group in years | 18–38 | 44–59 | All | 18–38 | 44–59 | All |
| Number of subjects | 31,27 | 28,26 | 59,53 | 30,29 | 32,32 | 62,61 |
| Local | ||||||
| Erythema | ||||||
| Any | 32,33* | 14,19 | 24,26 | 33,34 | 16,13 | 24,23 |
| Grade 2 or 3 | 3,0 | 0,0 | 2,0 | 7,7 | 0,0 | 3,3 |
| Induration | ||||||
| Any | 3,7 | 7,8 | 5,8 | 3,7 | 3,13 | 3,10 |
| Grade 2 or 3 | 0,0 | 0,0 | 0,0 | 0,0 | 0,0 | 0,0 |
| Pain | ||||||
| Any | 23,19 | 7,8 | 15,13 | 43,14 | 13,9 | 27,11 |
| Grade 2 or 3 | 0,0 | 0,0 | 0,0 | 0,0 | 0,0 | 0,0 |
| Tenderness | ||||||
| Any | 16,26 | 11,4 | 14,15 | 40,34 | 22,22 | 31,28 |
| Grade 2 or 3 | 0,0 | 0,0 | 0,0 | 0,0 | 0,0 | 0,0 |
| Any local | ||||||
| Any | 52,56 | 29,23 | 41,40 | 77,59 | 38,38 | 56,48 |
| Grade 2 or 3 | 3,0 | 0,0 | 2,0 | 7,7 | 0,0 | 3,3 |
| Systemic | ||||||
| Fever | ||||||
| Any | 3,0 | 0,0 | 2,0 | 3,0 | 0,0 | 2,0 |
| Grade 2 or 3 | 3,0 | 0,0 | 2,0 | 0,0 | 0,0 | 0,0 |
| Feverishness | ||||||
| Any | 13,0 | 7,0 | 10,0 | 13,3 | 0,0 | 6,2 |
| Grade 2 or 3 | 3,0 | 4,0 | 3,0 | 10,0 | 0,0 | 5,0 |
| Headache | ||||||
| Any | 48,26 | 32,12 | 41,19 | 37,24 | 13,13 | 24,18 |
| Grade 2 or 3 | 16,4 | 18,4 | 17,4 | 13,3 | 9,0 | 11,2 |
| Malaise | ||||||
| Any | 19,11 | 21,12 | 20,11 | 30,24 | 19,9 | 24,16 |
| Grade 2 or 3 | 10,4 | 4,0 | 7,2 | 17,0 | 9,3 | 13,2 |
| Myalgia | ||||||
| Any | 16,4 | 14,4 | 15,4 | 27,7 | 16,0 | 21,3 |
| Grade 2 or 3 | 10,0 | 0,0 | 5,0 | 10,0 | 6,0 | 8,0 |
| Nausea | ||||||
| Any | 10,7 | 11,4 | 10,6 | 17,7 | 13,3 | 15,5 |
| Grade 2 or 3 | 0,4 | 4,0 | 2,2 | 3,0 | 6,3 | 5,2 |
| Any systemic | ||||||
| Any | 52,33 | 46,19 | 49,26 | 50,38 | 31,16 | 40,26 |
| Grade 2 or 3 | 23,7 | 21,4 | 22,6 | 23,3 | 19,3 | 21,3 |
| Any local or systemic | ||||||
| Any | 84,74 | 64,38 | 75,57 | 80,69 | 53,50 | 66,59 |
| Grade 2 or 3 | 26,7 | 21,4 | 24,6 | 27,10 | 19,3 | 23,7 |
Numbers in the cell refer to the percentages of subjects per vaccine group experiencing reactions after first and second vaccine dose, respectively.
3.3 Pre-vaccination antibody levels
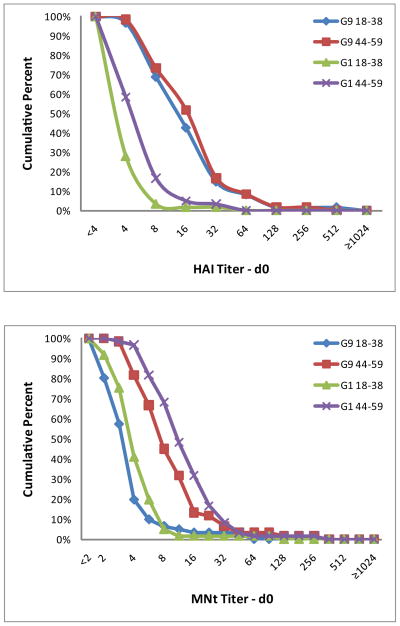
The pre-vaccination GMTs of the older cohort were significantly higher than those of the younger cohort for both HAI and MNt antibody titers (P≤0.001) except for the HAI titers to the A/H9N2 G9 antigen, against which titers were similar among the two age strata (Tables 3 and 4, Figure 1). Persons in both age cohorts were more likely to have baseline serum HAI antibody titers of >8 against the G9 strain than the G1 strain (43% vs 2%, respectively, for the 18–38 year old cohort and 52% vs 5%, respectively, for the 44–59 year old cohort). In contrast, baseline serum MNt antibody titers >8 occurred with similar frequency for the G9 and G1 strains but were more frequent in the older age cohort (5% vs 2%, respectively, for the 18–38 year old cohort and 32% vs 48%, respectively, for the 44–59 year old cohort).
Table 3.
Serum HAI and MNt antibody responses to the homologous vaccine antigen, influenza A/Chicken/Hong Kong/G9/97 (H9N2)
| Age group | 18–38 | 44–59 | ||||
|---|---|---|---|---|---|---|
| 7.5 mcg (n=31) | 30 mcg (n=30) | All (n=61) | 7.5 mcg (n=28) | 30 mcg (n=32) | All (n=60) | |
| HAI* | ||||||
| SCR (95% CI) | ||||||
| Day 28 | 40 (23, 59) | 70 (51, 85) | 55 (42, 68) | 61 (41, 79) | 41 (24, 59) | 50 (37, 63) |
| Day 56 | 44 (26, 65) | 72 (53, 87) | 59 (45, 87) | 54 (33, 73) | 56 (38, 74) | 55 (42, 68) |
| GMTs (95%CI) | ||||||
| Day 0 | 11 (8, 16) | 9 (6, 14) | 10 (8, 13) | 13 (9, 19) | 10 (8, 14) | 11 (9, 15) |
| Day 28 | 24 (18, 33) | 41 (27, 62) | 32 (24, 41) | 46 (28, 77) | 27 (21, 36) | 35 (27, 46) |
| Day 56 | 31 (22, 44) | 57 (39, 82) | 43 (33, 55) | 38 (23, 62) | 36 (27, 47) | 36 (28, 47) |
| SCF (95% CI) | ||||||
| Day 28 | 2.1 (1.6, 2.9) | 4.5 (3.2, 6.3) | 3.1 (2.4, 3.9) | 3.6 (2.5, 5.3) | 2.7 (1.8, 3.8) | 3.1 (2.4, 4.0) |
| Day 56 | 2.8 (1.9, 4.0) | 7.1 (4.6, 11.0) | 4.5 (3.3, 6.1) | 3.0 (2.0, 4.5) | 3.4 (2.4, 5.0) | 3.2 (2.5, 4.2) |
| % with titer ≥32 (95%CI) | ||||||
| Day 0 | 19 (7,37) | 10 (2, 27) | 15 (7, 26) | 18 (6,37) | 16 (5,33) | 17 (8, 29) |
| Day 28 | 50 (31, 69) | 63 (44, 80) | 57 (43, 69) | 75 (55, 89) | 56 (38, 74) | 65 (52, 77) |
| Day 56 | 67 (46, 84) | 86 (68, 96) | 77 (64, 87) | 62 (41, 80) | 69 (50, 84) | 66 (52, 78) |
| MNt | ||||||
| SCR | ||||||
| Day 28 | 63 (44, 80) | 70 (51, 85) | 67 (53, 78) | 36 (19, 56) | 44 (26, 62) | 40 (28, 54) |
| Day 56 | 93 (76, 99) | 97 (82, 100) | 95 (85, 99) | 58 (37, 77) | 75 (57, 89) | 67 (54, 79) |
| GMTs (95%CI) | ||||||
| Day 0 | 3 (2, 4) | 3 (2, 3) | 3 (2,3) | 8 (6, 12) | 6 (5, 8) | 7 (6, 9) |
| Day 28 | 13 (8, 19) | 20 (13, 31) | 16 (12, 21) | 29 (16, 53) | 21 (14, 30) | 24 (17, 34) |
| Day 56 | 36 (22, 58) | 69 (45, 105) | 50 (36, 69) | 37 (22, 61) | 44 (30, 66) | 41 (30, 55) |
| SCF (95% CI) | ||||||
| Day 28 | 4.2 (3.1, 5.8) | 7.9 (5.3, 11.9) | 5.8 (4.4, 7.5) | 3.4 (2.3, 5.2) | 3.3 (2.2, 4.8) | 3.3 (2.6, 4.4) |
| Day 56 | 12.4 (8.4, 18.2) | 30.1 (19.2, 47.4) | 19.6 (14.3, 26.9) | 4.4 (2.9, 6.6) | 6.9 (4.6, 10.5) | 5.7 (4.2, 7.6) |
Abbreviations: HAI = hemagglutination inhibition, GMT = geometric mean titer, SCF = seroconversion factor, SCR = seroconversion rate, MNt = microneutralization
Table 4.
Serum HAI and MNt antibody responses to the heterologous H9N2 antigen, influenza A/Quail/Hong Kong/G1/97 (H9N2)
| Age group | 18–38 | 44–59 | ||||
|---|---|---|---|---|---|---|
| 7.5 mcg (n=31) | 30 mcg (n=30) | All (n=61) | 7.5 mcg (n=28) | 30 mcg (n=32) | All (n=60) | |
| HAI* | ||||||
| SCR (95% CI) | ||||||
| Day 28 | 14 (4, 33) | 30 (15, 49) | 22 (13, 35) | 21 (8, 41) | 13 (4, 29) | 17 (8, 29) |
| Day 56 | 11 (2, 29) | 24 (10, 44) | 18 (9, 30) | 12 (2, 30) | 13 (4, 29) | 12 (5, 23) |
| GMTs (95%CI) | ||||||
| Day 0 | 2 (2, 3) | 3 (2, 3) | 3 (2, 3) | 4 (3, 5) | 3 (3, 4) | 4 (3, 4) |
| Day 28 | 4 (3, 5) | 5 (3, 7) | 4 (3, 5) | 7 (5, 9) | 6 (5, 7) | 6 (5, 8) |
| Day 56 | 3 (3, 4) | 4 (3, 5) | 4 (3, 4) | 5 (4, 7) | 5 (4, 7) | 5 (4, 6) |
| SCF (95% CI) | ||||||
| Day 28 | 1.6 (1.2, 2.0) | 1.8 (1.4, 2.4) | 1.7 (1.4, 2.0) | 1.8 (1.4, 2.3) | 1.8 (1.4, 2.3) | 1.8 (1.5, 2.1) |
| Day 56 | 1.4 (1.1, 1.7) | 1.7 (1.3, 2.1) | 1.5 (1.3, 1.8) | 1.4 (1.1, 1.8) | 1.6 (1.3, 2.0) | 1.5 (1.3, 1.8) |
| % with titer ≥32 (95%CI) | ||||||
| Day 0 | 0 (0, 11) | 3 (0, 17) | 2 (0,9) | 4 (0,18) | 3 (0,16) | 3 (0,12) |
| Day 28 | 0 (0, 12) | 7 (1, 22) | 3 (0, 12) | 7 (1, 24) | 6 (1, 21) | 7 (2, 16) |
| Day 56 | 0 (0, 13) | 0 (0, 12) | 0 (0, 6) | 4 (0, 20) | 3 (0, 16) | 3 (0, 12) |
| MNt | ||||||
| SCR | ||||||
| Day 28 | 3 (0, 17) | 30 (15, 49) | 17 (8, 29) | 4 (0, 18) | 9 (2, 25) | 7 (2, 16) |
| Day 56 | 22 (9, 42) | 52 (33, 71) | 38 (25, 52) | 12 (2, 30) | 9 (2, 25) | 10 (4, 21) |
| GMTs (95%CI) | ||||||
| Day 0 | 3 (2, 4) | 3 (2, 4) | 3 (3, 4) | 10 (7, 14) | 10 (8, 12) | 10 (8, 12) |
| Day 28 | 6 (5, 7) | 9 (7, 13) | 7 (6, 9) | 15 (10, 22) | 16 (11, 22) | 15 (12, 20) |
| Day 56 | 7 (5, 9) | 10 (8, 13) | 8 (7, 10) | 18 (12, 26) | 17 (13, 23) | 17 (14, 22) |
| SCF (95% CI) | ||||||
| Day 28 | 1.8 (1.6, 2.1) | 2.8 (2.2, 3.6) | 2.3 (2.0, 2.6) | 1.5 (1.2, 1.9) | 1.6 (1.1, 2.2) | 1.6 (1.3, 1.9) |
| Day 56 | 2.1 (1.7, 2.6) | 3.4 (2.7, 4.3) | 2.7 (2.3, 3.2) | 1.8 (1.5, 2.2) | 1.7 (1.3, 2.3) | 1.8 (1.5, 2.1) |
Abbreviations: HAI = hemagglutination inhibition, GMT = geometric mean titer, SCF = seroconversion factor, SCR = seroconversion rate, MNt = microneutralization
Figure 1.
Reverse cumulative distributions by age group and clade for HAI (A) and MNt (B) serologies measured at day 0. Data are shown for each age group (18–38 years and 44–59 years) and H9N2 clade (G9 and G1).
3.4 Post-vaccination antibody responses
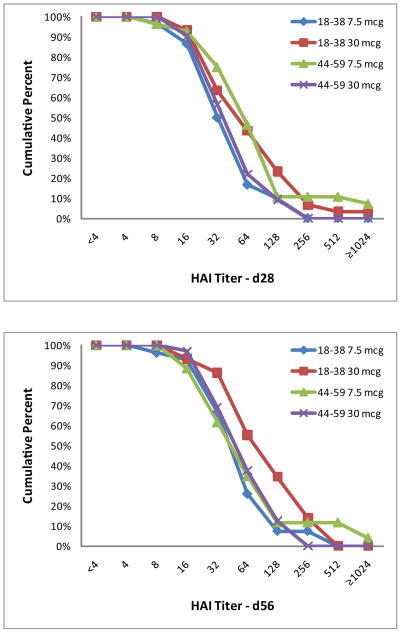
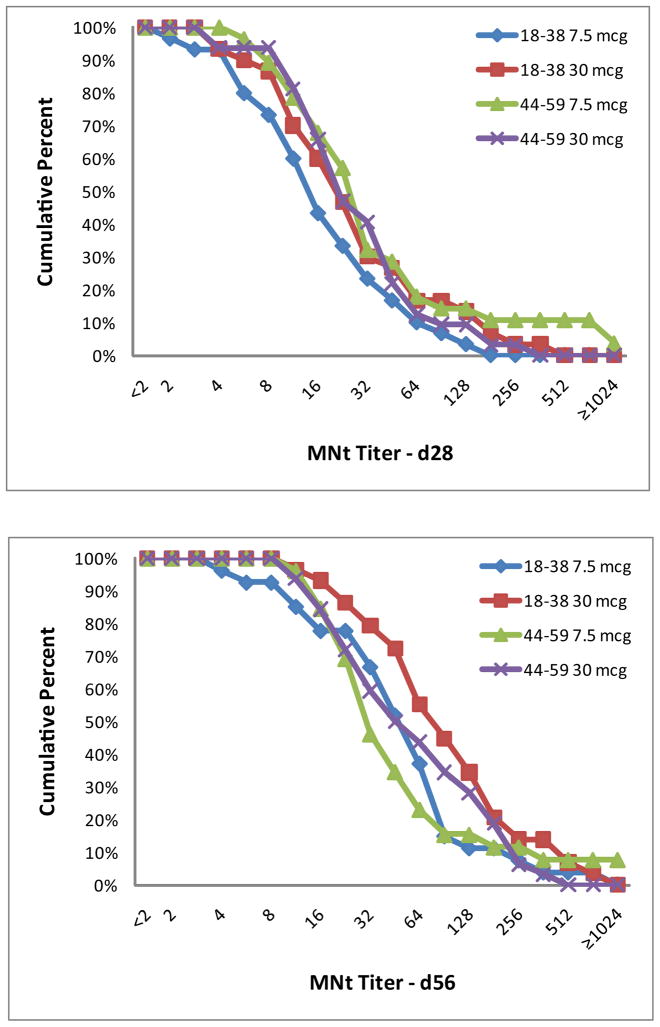
Significant increases in antibody levels compared to baseline were observed in both age cohorts following the first and second vaccinations and for both dosage levels (7.5 and 30 mcg) against the homologous G9 antigen (Table 3). SCRs were similar after both doses for the younger and older cohorts as measured by HAI to the G9 antigen, but were significantly higher (P≤0.006) in the younger age group when measured by MNt after each vaccination. Thus, for the primary endpoint, the older age cohort was not more likely to respond after a single dose of G9 H9N2 vaccine. Similar findings were present when measuring geometric mean fold rise (or SCF) in antibody levels; i.e., the younger age cohort had significantly larger increases in SCF by MNt (P≤ 0.004) but not by HAI 4 weeks after each vaccination. GMTs and reverse cumulative distributions (RCDs, Figure 2) were similar between age cohorts and dosage groups (7.5 and 30 mcg) at each post-vaccination time point. Antibody responses were lower in magnitude and less frequent for the heterologous G1 antigen (Table 4). SCF by HAI and MNt to the G1 antigen were similar for both age cohorts.
Figure 2.
Reverse cumulative distributions for the G9 strain by dosage and age group as measured by HAI (A and B) and MNt (C and D) for the day 28 (A and C) and day 56 (B and D) time points. Data are shown for each age group (18–38 years and 44–59 years) and dosage group (7.5 and 30 mcg).
In a logistic regression model examining the influence of age on MNt antibody responses to the homologous antigen, the younger age group was more likely to have a four fold rise following each vaccination (OR, 3.0 [95% CI 1.4, 6.5] and 7.9 {95% CI 2.1, 29.5] after first and second vaccinations, respectively) and to achieve a titer of 32 or greater after the second vaccination (OR 2.6, [95% CI 1.2, 5.9]) than the older age group.
4. Discussion
The principal goal of this study was to determine whether there were age related differences in immune responses to an influenza A/H9N2 G9 vaccine similar to what had been described previously for influenza A/H9N2 G1 vaccines. In our study, persons born before 1968 were not more likely to respond to a G9 H9N2 vaccine than younger persons, as had been described for a G1 H9N2 vaccine [9,10]. Instead, younger age and higher vaccine dosages were associated with improved serological responses following vaccination, as is observed following immunization with seasonal inactivated influenza vaccines [14].
Responses to a single vaccine dose in our study do not support greater priming in the older age group for all H9N2 viruses. The prevaccination serum MNt antibody levels were higher in the older age cohort (Figure 1b), but these differences were not associated with an age-related increase in seroconversion frequency following vaccination. One potential reason for the differences in observed responses to vaccines containing different H9N2 clades may be differences in priming against and seroprevalence to these viruses from different H9 clades. We observed serum HAI G9 H9N2 antibodies with a titer of 8 or higher in 43% of adults in the younger cohort, which is similar to the ~33% we and Karron et al. reported previously [6,8]. In contrast, only one person in this age cohort had this level of serum HAI antibody to the G1 H9N2 strain. Since A/H9N2 viruses have not been circulating in the population, this antibody must be cross reactive from another influenza infection. Although the cross-reactive antibody observed in the older age cohort might be due to a previous A/H2N2 infection as suggested in the reports of responses to a G1 H9N2 vaccine [9,10], this proposal cannot explain the presence of such antibody in the younger cohort born after A/H2N2 viruses were no longer circulating in the population. Cross-reactive heterosubtypic neutralizing antibodies that target conserved epitopes on the stem of the hemagglutinin have been described [15–17]; such antibodies may explain the higher levels of neutralizing activity in the preimmunization specimens from the older cohort. However, such antibodies would not explain the identification of cross-reactive HAI antibody, as HAI antibody targets the receptor-binding domains on the globular head of the hemagglutinin [18]. A clinical trial assessing the immunogenicity of the G1 and G9 H9N2 antigens in persons in both age groups evaluated in the current trial may provide additional insight into identifying the cross-reactive HAI and MNt epitopes found in G9 strains but not in G1 strains.
The frequencies and levels of HAI and MNt antibodies induced by the G9 H9N2 vaccine against a G1 H9N2 strain were low. Although a previous study in BALB/c mice suggested induction of low levels of HAI antibody against a G1 strain after vaccination with a G9 H9 antigen [19], a second study failed to detect cross-reactive HAI or Nt antibody [20]. Both mouse studies observed protection or partial protection against challenge with a G1 strain. However, the partial protection to a heterologous H9N2 challenge observed in the mouse studies in the absence of significant HAI or MNt antibody response cannot be extrapolated to protection in people [18]. Serum HAI or Nt antibody levels are the primary correlates of immunity following vaccination with an inactivated vaccine. In our previous study of a G9 H9N2 vaccine in young adults, responses to the G1 H9N2 virus were low, even when the vaccine was adjuvanted with MF59 [6].
As reported earlier, the vaccine was safe and well tolerated at both vaccine dosages, and the immunogenicity of the H9N2 vaccine in the younger cohort was similar to that which we observed in a similar age group to the same vaccine previously [6]. Immune responses cross-reactive with a G1 clade H9N2 strain were limited, which is similar to our previous observations. Poor induction of HAI or neutralizing antibody cross-reactive with viruses from other clades has also been observed for non-adjuvanted H5N1 vaccines [21–24].
In summary, adults born before 1968 and likely to have been infected with an influenza A/H2N2 virus were not more likely to have an immune response to an inactivated, unadjuvanted G9 H9N2 influenza vaccine. The differences in immune responses observed for G1 and G9 H9N2 influenza viruses highlight the need to improve our understanding of age-related patterns of vaccine responses to potential influenza pandemic virus.
Highlights.
Seroprevalence to a G9 influenza A/H9N2 strain was higher than against a G1 strain
Persons born after 1970 responded as well to a G9 vaccine as older persons
Immune responses following vaccination with G1 and G9 H9N2 strains differ
Acknowledgments
We thank the clinical staff of the VRPRU for help in performance of the study and the technical staff for performance of the HAI and MNt serologic assays, Robin Cessna of EMMES Corporation, members of the Safety Monitoring Committee (Drs. Patricia Winokur, ChrisAnna Mink, Rachel Bramson, and Richard Sutton), and the National Institute of Allergy and Infectious Diseases Influenza Team (Sonnie Kim-Grossman, Wendy Buchanan, Linda Lambert). This study was conducted with support from the National Institutes of Health (N01-AI-30039).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Choi YK, Ozaki H, Webby RJ, et al. Continuing evolution of H9N2 influenza viruses in Southeastern China. J Virol. 2004;78:8609–14. doi: 10.1128/JVI.78.16.8609-8614.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee YJ, Shin JY, Song MS, et al. Continuing evolution of H9 influenza viruses in Korean poultry. Virology. 2007;359:313–23. doi: 10.1016/j.virol.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 3.Butt KM, Smith GJ, Chen H, et al. Human infection with an avian H9N2 influenza A virus in Hong Kong in 2003. J Clin Microbiol. 2005;43:5760–7. doi: 10.1128/JCM.43.11.5760-5767.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peiris M, Yuen KY, Leung CW, et al. Human infection with influenza H9N2. Lancet. 1999;354:916–7. doi: 10.1016/s0140-6736(99)03311-5. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Responding to the avian influenza pandemic threat. Recommended strategic actions. Geneva: World Health Organization; 2005. Pamphlet no. WHO/CDS/CSR/GIP/2005.8. [Google Scholar]
- 6.Atmar RL, Keitel WA, Patel SM, et al. Safety and immunogenicity of nonadjuvanted and MF59-adjuvanted influenza A/H9N2 vaccine preparations. Clin Infect Dis. 2006;43:1135–42. doi: 10.1086/508174. [DOI] [PubMed] [Google Scholar]
- 7.Hehme N, Engelmann H, Kunzel W, Neumeier E, Sanger R. Pandemic preparedness: lessons learnt from H2N2 and H9N2 candidate vaccines. Med Microbiol Immunol (Berl ) 2002;191:203–8. doi: 10.1007/s00430-002-0147-9. [DOI] [PubMed] [Google Scholar]
- 8.Karron RA, Callahan K, Luke C, et al. A live attenuated H9N2 influenza vaccine is well tolerated and immunogenic in healthy adults. J Infect Dis. 2009;199:711–6. doi: 10.1086/596558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicholson KG, Thompson CI, Klap JM, et al. Safety and immunogenicity of whole-virus, alum-adjuvanted whole-virus, virosomal, and whole-virus intradermal influenza A/H9N2 vaccine formulations. Vaccine. 2009;28:171–8. doi: 10.1016/j.vaccine.2009.09.103. [DOI] [PubMed] [Google Scholar]
- 10.Stephenson I, Nicholson KG, Gluck R, et al. Safety and antigenicity of whole virus and subunit influenza A/Hong Kong/1073/99 (H9N2) vaccine in healthy adults: phase I randomised trial. Lancet. 2003;362:1959–66. doi: 10.1016/S0140-6736(03)15014-3. [DOI] [PubMed] [Google Scholar]
- 11.Dowdle WR, Kendal AP, Noble GR. Influenza viruses. In: Lennette EH, Schmidt NJ, editors. Diagnostic Procedures for Viral, Rickettsial, and Chlamydial Infections. 5. Washington, D.C: American Public Health Association; 1979. pp. 585–609. [Google Scholar]
- 12.Frank AL, Puck J, Hughes BJ, Cate TR. Microneutralization test for influenza A and B and parainfluenza 1 and 2 viruses that uses continuous cell lines and fresh serum enhancement. J Clin Microbiol. 1980;12:426–32. doi: 10.1128/jcm.12.3.426-432.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keitel WA, Atmar RL, Cate TR, et al. Safety of high doses of influenza vaccine and effect on antibody responses in elderly persons. Arch Intern Med. 2006;166:1121–7. doi: 10.1001/archinte.166.10.1121. [DOI] [PubMed] [Google Scholar]
- 14.Couch RB. Seasonal inactivated influenza virus vaccines. Vaccine. 2008;26 (Suppl 4):D5–D9. doi: 10.1016/j.vaccine.2008.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okuno Y, Isegawa Y, Sasao F, Ueda S. A common neutralizing epitope conserved between the hemagglutinins of influenza A virus H1 and H2 strains. J Virol. 1993;67:2552–8. doi: 10.1128/jvi.67.5.2552-2558.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang TT, Tan GS, Hai R, et al. Vaccination with a synthetic peptide from the influenza virus hemagglutinin provides protection against distinct viral subtypes. Proc Natl Acad Sci U S A. 2010;107:18979–84. doi: 10.1073/pnas.1013387107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corti D, Suguitan AL, Jr, Pinna D, et al. Heterosubtypic neutralizing antibodies are produced by individuals immunized with a seasonal influenza vaccine. J Clin Invest. 2010;120:1663–73. doi: 10.1172/JCI41902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dormitzer PR, Galli G, Castellino F, et al. Influenza vaccine immunology. Immunol Rev. 2011;239:167–77. doi: 10.1111/j.1600-065X.2010.00974.x. [DOI] [PubMed] [Google Scholar]
- 19.Chen H, Subbarao K, Swayne D, et al. Generation and evaluation of a high-growth reassortant H9N2 influenza A virus as a pandemic vaccine candidate. Vaccine. 2003;21:1974–9. doi: 10.1016/s0264-410x(02)00809-5. [DOI] [PubMed] [Google Scholar]
- 20.Lu X, Renshaw M, Tumpey TM, Kelly GD, Hu-Primmer J, Katz JM. Immunity to influenza A H9N2 viruses induced by infection and vaccination. J Virol. 2001;75:4896–901. doi: 10.1128/JVI.75.10.4896-4901.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langley JM, Frenette L, Ferguson L, et al. Safety and cross-reactive immunogenicity of candidate AS03-adjuvanted prepandemic H5N1 influenza vaccines: a randomized controlled phase 1/2 trial in adults. J Infect Dis. 2010;201:1644–53. doi: 10.1086/652701. [DOI] [PubMed] [Google Scholar]
- 22.Leroux-Roels I, Borkowski A, Vanwolleghem T, et al. Antigen sparing and cross-reactive immunity with an adjuvanted rH5N1 prototype pandemic influenza vaccine: a randomised controlled trial. Lancet. 2007;370:580–9. doi: 10.1016/S0140-6736(07)61297-5. [DOI] [PubMed] [Google Scholar]
- 23.Leroux-Roels I, Bernhard R, Gerard P, Drame M, Hanon E, Leroux-Roels G. Broad Clade 2 cross-reactive immunity induced by an adjuvanted clade 1 rH5N1 pandemic influenza vaccine. PLoS One. 2008;3:e1665. doi: 10.1371/journal.pone.0001665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stephenson I, Bugarini R, Nicholson KG, et al. Cross-reactivity to highly pathogenic avian influenza H5N1 viruses after vaccination with nonadjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a potential priming strategy. J Infect Dis. 2005;191:1210–5. doi: 10.1086/428948. [DOI] [PubMed] [Google Scholar]