Abstract
The use of animal models (including the 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine (MPTP) mouse model) to mimic dopaminergic (DAergic) cell loss and striatal DA depletion, as seen in Parkinson’s disease (PD), has implicated a multitude of factors that might be associated with DAergic cell death in PD including excitotoxicity, inflammation, and oxidative stress. All of these factors have been shown to be reduced by administration of histone deacetylase (HDAC) inhibitors (HDACis) resulting in some degree of neuroprotection in various models of neurodegenerative disease including in Huntington’s disease and amyotrophic lateral sclerosis. However, there is limited information of effects of HDACis in PD models. We have previously shown HDACis to be partially protective against 1-methyl-4-phenylpyridinium (MPP+) mediated cell loss in vitro. The present study was conducted to extend these findings to an in vivo PD model. The HDACi valproic acid (VPA) was co-administered with MPTP for 5 days to male FVBn mice and continued for an additional 2 weeks, throughout the period of active neurodegeneration associated with MPTP-mediated DAergic cell loss. VPA was able to partially prevent striatal dopamine depletion and almost completely protect against substantia nigra DAergic cell loss. These results suggest that VPA may be a potential disease modifying therapy for PD.
Keywords: HDAC inhibitor, dopamine, neuroprotection, valproate
1. Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder characterized by the progressive loss of dopaminergic (DAergic) neurons in the substantia nigra pars compacta (SNc) and the loss of dopamine (DA) terminals in the striatum. Although effective symptomatic therapies exist for PD, there is no current treatment that has been shown to unequivocally slow or stop the progression of the disease. As such, new disease modifying therapies are needed to address the inexorable progression of PD.
The 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine (MPTP) mouse model of PD has been a useful screening tool for identification of compounds that may potentially interfere with substantia nigra (SN) DA cell death and consequent striatal DA depletion. There are different MPTP mouse models that utilize different toxin administration protocols, result in different modes of cell death and respond differently to putative neuroprotective agents (Anderson et al., 2006). The sub-acute MPTP mouse model has been associated with primarily apoptotic cell death (Tatton and Kish, 1997), presumably as a result of impaired activity of complex I in the mitochondria leading to oxidative stress and free radical generation (Singer et al., 1987, Langston, 1996, Speciale, 2002). Additionally, glial cells (both astrocytes and microglia) become activated in the SN (and striatum) further contributing to the toxic milieu in the nigrostriatal system (Hirsch et al., 2003). This increase in inflammatory cytokines and potentially excitotoxic compounds may perpetuate the initial insult from MPTP and exacerbate DAergic cell loss. Drugs that are able to inhibit one or more of these mechanisms of toxicity may be able to confer protection against MPTP-mediated damage.
Valproic acid (VPA) has been used clinically in the treatment of epilepsy and bipolar disorder for over 30 years (Bowden and McElroy, 1995). Its exact mechanisms of action are not completely understood but VPA is thought to modulate levels of the inhibitory neurotransmitter γ-amino butyric acid (GABA) as well as limit glycogen synthase kinase-3β (GSK3-β) activation (reviewed by (Gurvich and Klein, 2002). More recently, VPA has been shown to inhibit HDACs (Phiel et al., 2001). As an HDACi, VPA is effective in limiting the excitotoxic response to glutamate in primary cortical cultures (Leng et al., 2008) and induces apoptosis in normal microglial cultures (Chen et al., 2006) while suppressing lipopolysaccharide mediated activation of microglia in mixed cultures (Peng et al., 2005) suggesting it could possibly limit microgliosis in vivo. VPA has also been shown to increase transcription of anti-apoptotic proteins and free radical scavengers (Ryu et al., 2003, Kim et al., 2007). In addition, we have previously shown that HDACis including VPA can partially protect DAergic cells from 1-methyl-4-phenylpyridinium (MPP+, the active metabolite of MPTP) toxicity in vitro (Kidd and Schneider, 2010). The ability of VPA to act through a variety of potential protective mechanisms likely contributes to its ability to be protective in multiple neurodegeneration models.
The present study was conducted to extend previous in vitro findings and assess the potential of the HDACi, VPA, to protect against DAergic cell loss and loss of striatal DA in a mouse MPTP model of Parkinsonism.
2. Experimental Procedures
2.1. Animals and drug administration
All procedures used in this study were approved by the Thomas Jefferson University Institutional Animal Care and Use Committee, and studies were conducted in accord with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Male FVBn mice 8–10 weeks of age (Charles River Labs, Wilmington, MA) were housed 3–5 per cage with ad libitum access to food and water for one week prior to injection with VPA (Sigma-Aldrich, St. Louis, MO). The dosing paradigm for VPA (400mg/kg i.p.) was selected based on existing studies in which hyperacetylation was shown to occur in the rodent brain at this dose (Rouaux et al., 2007, Lv et al., 2011). Either saline or VPA was administered 30 min prior to administration of MPTP-HCl (Sequoia Research Products Ltd, Pangbourne, UK). MPTP was administered twice daily (25 mg/kg, s.c., 4 hours apart) for 5 consecutive days. Animals continued to receive VPA or saline once daily for 2 weeks after the final MPTP injection. Three hours after the final VPA or saline injection, mice were euthanized by decapitation and striatal tissue was rapidly removed and frozen for catecholamine analysis. Frontal cortex was removed and frozen for western blot analysis. The remaining tissue was post-fixed in 4% parafomaldehyde for 72hrs.
2.2. High pressure liquid chromatographic (HPLC) analysis of tissue catecholamines
Striatal samples were sonicated in 0.4M percholoric acid and centrifuged at 15000 rpm for 5 minutes at 4°C. Supernatant was removed for analysis by HPLC as previously described (Anderson et al., 2008), using isoproterenol (Sigma-Aldrich) as an internal standard. Samples were analyzed on a C18 column with a 20 µL injection loop using MDTM mobile phase (ESA Inc., Chemlsford, MS) on a Coulochem III system with an electrochemical detector (ESA Inc.). Peak heights were compared with internal standard values to determine the concentration DA and its metabolites (EZchrome V3.1, Agilent Technologies, Santa Clara, CA).
2.3. Immunohistochemistry and unbiased stereology
Fixed tissue blocks were immersed in 30% sucrose as a cryoprotectant and sectioned frozen on a sliding microtome (30µm section thickness) through the rostro-caudal extent of the substantia nigra pars compacta (SNc). Every third section was processed for tyrosine hydroxylase (TH) immunohistochemistry (rabbit anti-TH, 1:1000, Pel-freez, Rodgers, AZ) and adjacent sections were stained with cresyl violet as previously described (Anderson et al., 2008). Cells (both TH+ and cresyl violet stained (Nissl+)) were counted using unbiased stereology (SteroInvestigator, MBFbioscience, Williston, VT) on an Olympus BX-60 microscope equipped with a Ludl motorized stage. The region of interest (i.e., SNc) was outlined under low magnification (4×) and a grid measuring 195µm × 85µm was randomly placed over the region. Cells were then counted at high power (100×) using a counting frame measuring 40µm2. A cell was counted only if a nucleus was clearly identifiable and the cell was completely within the counting frame. This process was repeated for each section in the series for a given animal and a total of 10 total sections/animal were analyzed. Nissl+ cells were counted by overlaying the region of interest outline from the adjacent TH+ section and sampling was accomplished using the same parameters described above.
2.4. Immunofluorescence
Mice were injected with VPA or saline for 1 week as described above. Sections were washed in PBS, blocked in 5% non-fat milk containing 0.3% triton x-100 for 1hr and incubated in primary antibody (rabbit anti-AcH3, 1:1,000in block, Millepore, Billerica, MA), overnight at 4°C. Primary antibody was removed, sections were rinsed, and secondary antibody (488 DyLight goat anti rabbit, 1:500, Jackson ImmunoResearch Labs Inc., West Grove, PA) was added and sections were incubated in the dark for 2 hours. Sections were then washed multiple times, mounted and cover-slipped using Aqua-perm mounting media (Thermo Fisher, Waltham, MA) and stored at 4°C until imaged. Images were collected on a Zeiss Axioplan 2 confocal microscope.
2.5. Western Blot
Tissue samples were homogenized in hypotonic lysis buffer (10mM Hepes, 1.5mM MgCl2 and 10mM KCl, pH 7.9 with 1x HALT protease inhibitor, Thermo Fisher Scientific) using 5mm stainless steel beads and using a Qialyser (50 Hz for 3 min, Qiagen Inc., Germantown, MD). Homogenized samples were transferred to fresh tubes and 1M HCl was added to a final concentration of 0.2M HCl. Following 30min incubation on ice samples were centrifuged at 15000rpm for 10min and the resulting supernatant was used for western blot analysis. Ten micrograms of protein were loaded onto 4–12% bis-tris gels (Invitrogen Inc., Carlsbad, CA) and proteins were separated by electrophoresis in MOPS SDS running buffer (Invitrogen Inc.) for 1 hour at 200V. Proteins were transferred to 0.2µm nitrocellulose using semi-dry transfer (15V for 15min/membrane). Membranes were equilibrated in Tris buffered saline containing 0.1% Tween-20 (T-TBS) and then blocked for 1hr in 5% non-fat milk in T-TBS. Primary antibody (rabbit anti-acetyl H3 Lys 9 (1:10,000) or rabbit anti-β–actin (1:2,000) (Imgenex, San Diego, CA) was added for 1hr. Membranes were washed in T-TBS and exposed to secondary HRP-conjugated goat anti-rabbit (1:40,000 and 1:20,000 respectively, Thermo Fisher Scientific) in T-TBS for 1 hr. After washing in T-TBS to remove residual antibody, blots were developed using chemluminesence (Thermo Fisher Scientific) and were quantified using densitometry software (MCID Basic V 7.2).
2.6. Statistical analysis
All data are presented as mean ± standard error. Statistical significance was determined (defined as p < 0.05) using a one way analysis of variance with either Bonferonni or Dunnett post-hoc comparisons where appropriate (unless otherwise specified).
3. Results
3.1. Valproate treatment promotes histone hyperacetylation in the brain
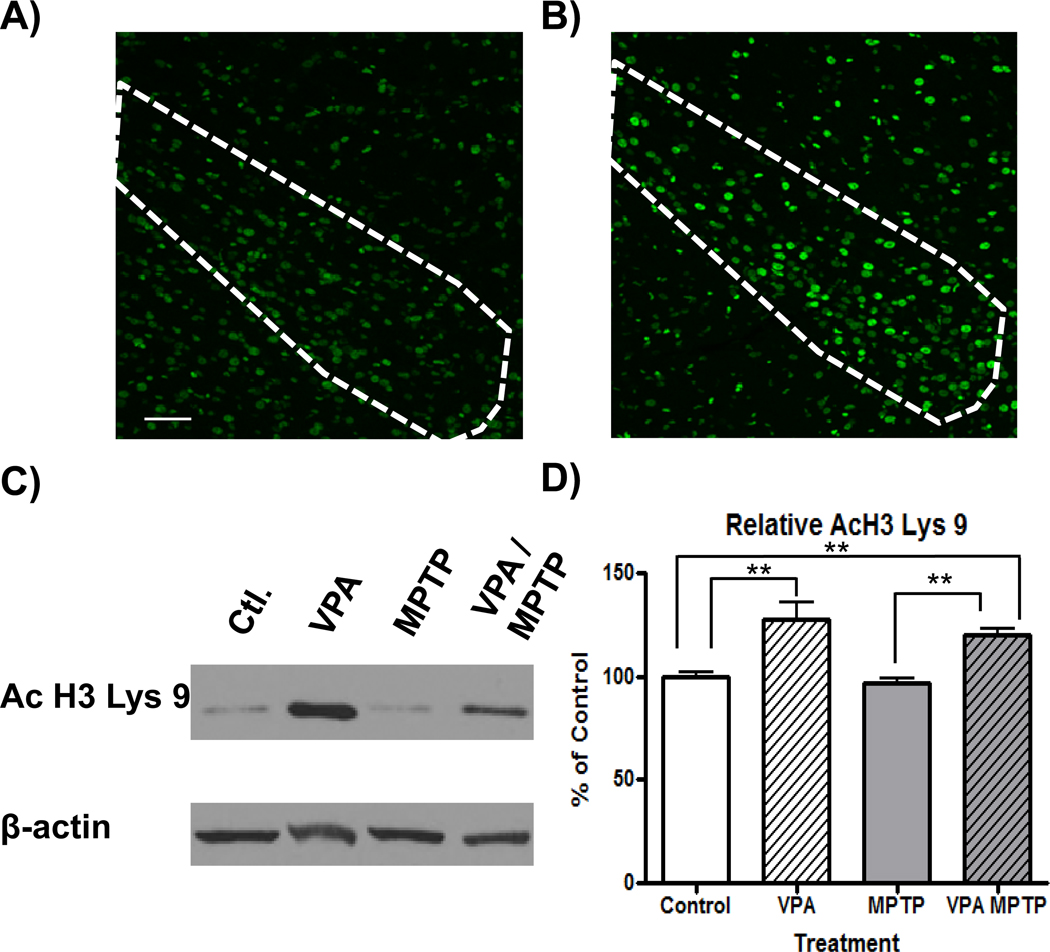
Systemic administration of VPA resulted in increased levels of acetylated histone 3 lysine 9 (AcH3 Lys 9) in the brain. In addition to inducing hyperacetylation in the SNc (Figure 1 A and B), VPA administration significantly increased the ratio of acetylated H3 Lys 9 to β-actin in the frontal cortex of control mice as well as animals exposed to MPTP (127.1 ± 7.9 and 120.1 ± 2.8 % respectively, F(3,18)=13.14; P<0.001, q=4.702, 3.393 p<0.01 vs. control, Figure 1 C and D). .
Figure 1.
Systemic valproate administration promotes histone hyperacetylation in the brain. Immunoflourescent images show the presence of acetylated histone 3 Lys9 (AcH3 Lys 9) in the substantia nigra (outline) of saline (A) or VPA treated (B) mice (scale bar = 100µm). Representative western blots show increased acetylation of AcH3 Lys 9 in frontal cortex in animals receiving VPA as well as VPA and MPTP, compared to control animals (C). Densitometric analysis showed significant increases in AcH3 Lys 9 (relative to the level of β-actin in each sample) in VPA and VPA/MPTP-treated animals, compared to their respective controls (D). **: p<0.01.
3.2. Valproate partially protects the nigrostriatal system from the effects of MPTP
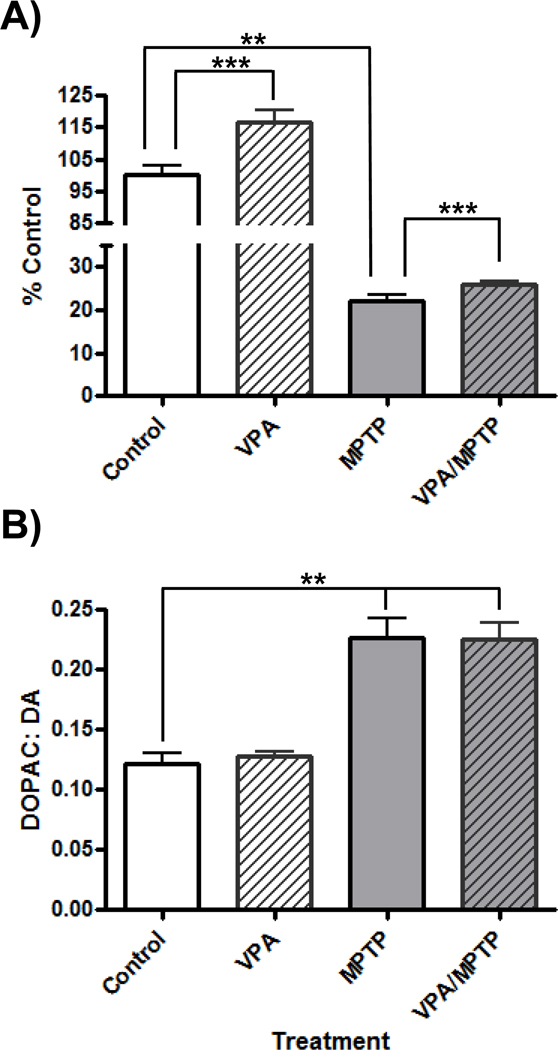
Treatment with MPTP significantly affected striatal DA levels (Kruskal-Wallis statistic = 31.07, P<0.0001) and DA turnover (F(3,38)=21.34, P<0.001). Sub-acute administration of MPTP resulted in a 77.0 ± 1.3% reduction in striatal DA levels (p<0.01, Figure 2A). Treatment with VPA resulted in significantly increased striatal DA levels compared to saline-treated control mice (10.21 ± 0.36 and 8.77 ± .25 µg/g wet tissue respectively; p< 0.001). Mice that received VPA and MPTP also had significantly higher striatal DA levels than animals that received MPTP and saline (2.24 ± 0.07 and 1.94 ± 0.11 µg/g respectively; p< 0.001). Dopamine turnover (DOPAC: DA) was increased 185.7 ±14.2 % following MPTP exposure (t= 6.17, p<0.01, Figure 2B). There was no difference in DA turnover between mice receiving MPTP or MPTP/VPA (0.23 ± 0.02 and 0.23 ± 0.01 respectively).
Figure 2.
Effects of Valproate (VPA) on striatal dopamine (DA) and DA turnover following administration of 1-methyl-4-phenyl-1,2,3,6 tetrahydropyridine (MPTP). Treatment with VPA increased striatal DA levels (A), as measured by high pressure liquid chromatography (HPLC) 2 weeks after the final MPTP injection, but had no effect on DA turnover (B) compared to control mice. . **p< 0.01, ***: p< 0.001.
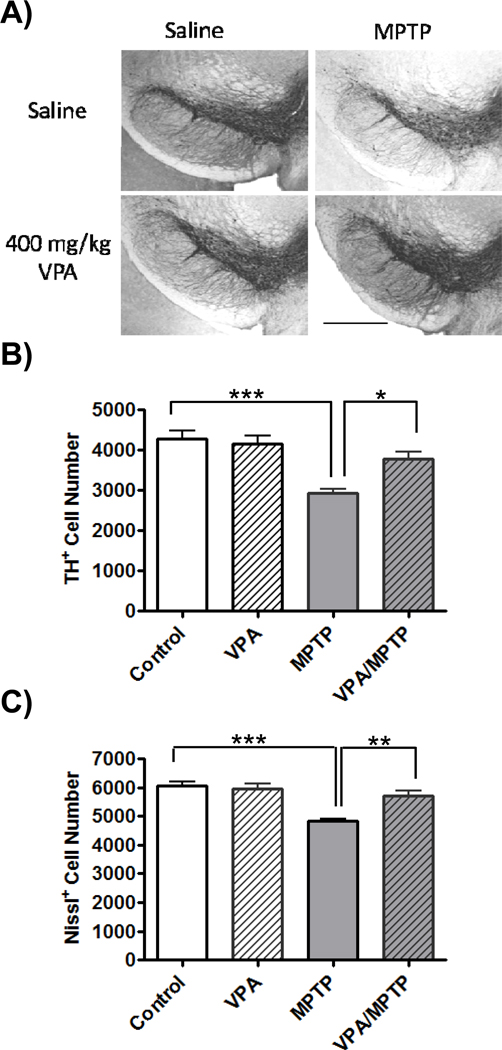
Treatment with MPTP significantly affected both DAergic (F(3,20)= 12.55, P<0.001) and total cell (F (3,20)= 14.7, P<0.001) numbers in the SNc. Specifically, the number of SNc DAergic neurons was reduced by 31.9 ± 2.3% (t= 5.56, p<0.001) following treatment with MPTP (Figure 3B), slightly more than the estimated loss of SNc neurons (20.3 ± 1.6%, t=5.95, p<0.001) obtained from counts of Nissl+ sections (Figure 3C). There were significantly more TH+ neurons in the SNc in animals treated with VPA and MPTP than in MPTP/saline treated animals (88.1 ± 4.4 and 68.1 ± 2.3 % of saline/saline TH+ neurons, respectively, t= 3.48 p< 0.05) There was also a significant increase in the number Nissl+ cells in the SNc of VPA/MPTP mice compared to saline/MPTP animals (94.6 ± 2.6 and 79.7 ± 1.6 % of saline/ saline Nissl+ cells respectively, t=4.36 p< 0.01).
Figure 3.
Effects of valproate (VPA) treatment on substantia nigra pars compacta (SNc) neuronal survival following 1-methyl-4-phenyl-1,2,3,6 tetrahydropyridine (MPTP) treatment. A) Photomicrographs of tyrosine hydroxlase (TH) staining in the SNc (scale bar = 2mm) B) Stereological estimates confirmed a loss of TH immunopositive (TH+) cells in the SNc following MPTP administration. The MPTP-induced decrease in TH+ cells was prevented by VPA administration. C) Stereological estimates confirmed a loss of neurons (Nissl+ cells) in the SNc as a result of the MPTP exposure. Mice receiving VPA and MPTP show a smaller amount of cell loss than animals that received MPTP without VPA. *: p< 0.05, **p< 0.01, ***: p< 0.001.
4. Discussion
The present results demonstrated that VPA was able to partially protect the nigrostriatal DA system from MPTP toxicity and that systemic treatment with VPA resulted in a significant increase of acetylated histone 3 in the substantia nigra as well as in non-basal ganglia regions of the mouse brain (i.e., frontal cortex). This hyperacetylation event was associated with greater striatal DA levels (both in normal as well as MPTP-treated mice) as well as increased survival of SNc neurons following MPTP exposure, compared to lesioned control animals. The observed protective effects of VPA appeared to be greater at the level of the cell bodies in the SNc than at the DA terminals in the striatum. However, considering the short time frame of this study (2 weeks following the last toxin administration) it is possible that given a longer post-lesion survival time, a greater increase in striatal DA levels may be observed as SNc neurons re-establish connections to the striatum. It is unlikely that the observed protective effects of VPA in these mice were due to interference with the acute toxicity of MPTP. Valproate does not impair DA transporter function (Chen et al., 2006, Kidd and Schneider, 2010) therefore the uptake of MPP+ into DAergic cells via the DA transporter should not be affected by VPA. In addition, VPA unlikely inhibited the enzyme responsible for the conversion of MPTP to MPP+, monoamine oxidase B, at the dose at which it was used in this study (400mg/kg or 2,406µmol/kg). The IC50 for VPA-mediated inhibition of monoamine oxidase B is >10,000 µmol/L (Fisar et al., 2010) and the amount of VPA that enters the brain is only a small fraction of the peripheral dose administered (for review see Loscher, 1999).
Inhibition of HDACs in the brain has been associated with the increased transcription of many factors that may contribute to the protection of DAergic neurons following MPTP exposure, including free radical scavengers (Faraco et al., 2006, Petri et al., 2006), heat-shock proteins (Kim et al., 2007, Marinova et al., 2009, Leng et al., 2010) and anti-apoptotic bcl-2 family members (Faraco et al., 2006, Lv et al., 2011). Oxidative stress is thought to play a significant role in both PD and MPTP-mediated parkinsonism (Jenner, 2003). Given the ability of VPA to increase the activity of superoxide dismutase and catalase resulting in reduced levels of superoxide radicals (Jornada et al., 2011) it may be able to reduce the oxidative burden on SNc neurons allowing them to survive the toxic insult from MPTP. In addition to reducing oxidative stress, the increase in transcript levels of inhibitors of apoptosis (Ryu et al., 2003) and anti-apoptotic bcl-2 family members (Petri et al., 2006, Rouaux et al., 2007) that result from HDAC inhibition may reduce mitochondrial stress caused by MPTP-induced inhibition of complex I, further contributing the survival of DAergic cells. Valproate-mediated hyperacetylation also increases transcription of HSP-70 (Marinova et al., 2009, Leng et al., 2010), induction of which has been shown to protect against excitotoxic stress (Marinova et al., 2009) and MPTP toxicity (Shen et al., 2005). VPA may also reduce inflammatory mediators by triggering apoptosis of microglia cells (Suuronen et al., 2003, Chen et al., 2007). Both astrocytes and microglia are activated in the sub-acute MPTP mouse model and likely contribute to the loss of DA in the nigrostriatal system via excitoxicity and the release of inflammatory cytokines (Hirsch et al., 2003). Inhibiting glial mediated inflammation may protect SNc neurons from MPTP-mediated cell death as long as therapy is continued throughout the time course of neurodegeneration (Aubin et al., 1998, Ferger et al., 1999, Mohanakumar et al., 2000, Du et al., 2001, Wu et al., 2002). Thus, there are multiple possible mechanisms through which VPA-mediated HDAC inhibition could confer protection to SNc neurons against MPTP-mediated toxicity.
The currently observed effects of VPA on the MPTP-damaged DA system might involve other mechanisms in addition to HDAC inhibition. For example, one of the primary means by which VPA is thought to inhibit epileptic activity is via the modulation of the inhibitory neurotransmitter GABA which results in accumulation of GABA in several brain regions including the basal ganglia (Loscher, 1989). MPTP depletes striatonigral GABA levels (Kuriyama et al., 1990) which may impair the ability of the basal ganglia to control motor function and the pharmacological modulation of GABA (ex. by modafinil) has been shown to confer some DAergic neuroprotection in MPTP models (Fuxe et al., 1992, Jenner et al., 2000). Thus, it is possible that VPA-mediated neuroprotection may to some extent involve increases in GABA in the basal ganglia. Another possible mechanism of VPA-mediated neuroprotection may relate to its ability to inhibit GSK3-β (Chen et al., 2007, Leng et al., 2008). Both in vitro and in vivo studies using Parkinson-producing toxins (i.e. rotenone, MPP+ and 6-OHDA) have implicated GSK3-β in DAergic cell death and have shown that inhibition of GSK3-β to be partially protective in these models (Chen et al., 2004, Wang et al., 2007, Chen et al., 2008). However, studies by Melamed et al (Melamed et al., 1986) and Lagrue et al (Lagrue et al., 2007) in which low levels of VPA were administered (below that needed to induce histone hyperacetylation) showed no protective effects against an acute MPTP-induced toxicity. This may suggest that a critical concentration of VPA (possibly that which is needed for HDAC inhibition) may be needed for VPA to protect against MPTP-induced parkinsonism.
In summary, VPA administration, at the dosing regimen used in the current study, partially protected the nigrostriatal dopamine system from injury in a mouse model of sub-acute MPTP toxicity. Additional studies using other PD model systems and other VPA dosing regimens are warranted in order to more fully define the potential neuroprotective activity of this drug. However, considering the extensive clinical experience with VPA (in patients with epilepsy and bipolar disorder) and its good clinical safety profile, clinical studies examining VPA-mediated HDAC inhibition as a potential disease modifying therapy in PD may be warranted.
Highlights.
Systemic administration of valproate promotes hyperacetylation of histone 3 lysine 9 in the mouse substantia nigra of FVBn mice
Valproate increases striatal dopamine levels in saline and MPTP treated mice
Valproate partially protects against MPTP-mediated dopamine cell loss in the substantia nigra of FVBn mice while maintaining dopamine phenotype
Acknowledgments
This research was funded by F.M. Kirby Foundation and NIEHS training grant T32 ES07282
Abbreviations
- AcH3 Lys 9
acetylated histone 3 lysine 9
- DA
dopamine
- DAergic
dopaminergic
- GABA
γ-amino butyric acid
- GSK3-β
glycogen synthase kinase-3β
- HDAC
histone deacetylase
- HDACi
histone deacetylase inhibitor
- HPLC
high pressure liquid chromatography
- MPP+
1-methyl-4-phenylpyridinium
- MPTP
1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine
- PD
Parkinson’s disease
- SN
substantia nigra
- SNc
substantia nigra pars compacta
- TH
tyrosine hydroxylase
- VPA
valproic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson DW, Bradbury KA, Schneider JS. Neuroprotection in Parkinson models varies with toxin administration protocol. Eur J Neurosci. 2006;24:3174–3182. doi: 10.1111/j.1460-9568.2006.05192.x. [DOI] [PubMed] [Google Scholar]
- Anderson DW, Bradbury KA, Schneider JS. Broad neuroprotective profile of nicotinamide in different mouse models of MPTP-induced parkinsonism. European Journal of Neuroscience. 2008;28:610–617. doi: 10.1111/j.1460-9568.2008.06356.x. [DOI] [PubMed] [Google Scholar]
- Aubin N, Curet O, Deffois A, Carter C. Aspirin and salicylate protect against MPTP-induced dopamine depletion in mice. J Neurochem. 1998;71:1635–1642. doi: 10.1046/j.1471-4159.1998.71041635.x. [DOI] [PubMed] [Google Scholar]
- Bowden CL, McElroy SL. History of the development of valproate for treatment of bipolar disorder. J Clin Psychiatry. 1995;56 Suppl 3:3–5. [PubMed] [Google Scholar]
- Chen G, Bower KA, Ma C, Fang S, Thiele CJ, Luo J. Glycogen synthase kinase 3beta (GSK3beta) mediates 6-hydroxydopamine-induced neuronal death. FASEB J. 2004;18:1162–1164. doi: 10.1096/fj.04-1551fje. [DOI] [PubMed] [Google Scholar]
- Chen PS, Peng GS, Li G, Yang S, Wu X, Wang CC, Wilson B, Lu RB, Gean PW, Chuang DM, Hong JS. Valproate protects dopaminergic neurons in midbrain neuron/glia cultures by stimulating the release of neurotrophic factors from astrocytes. Mol Psychiatry. 2006;11:1116–1125. doi: 10.1038/sj.mp.4001893. [DOI] [PubMed] [Google Scholar]
- Chen PS, Wang CC, Bortner CD, Peng GS, Wu X, Pang H, Lu RB, Gean PW, Chuang DM, Hong JS. Valproic acid and other histone deacetylase inhibitors induce microglial apoptosis and attenuate lipopolysaccharide-induced dopaminergic neurotoxicity. Neuroscience. 2007;149:203–212. doi: 10.1016/j.neuroscience.2007.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YY, Chen G, Fan Z, Luo J, Ke ZJ. GSK3beta and endoplasmic reticulum stress mediate rotenone-induced death of SK-N-MC neuroblastoma cells. Biochem Pharmacol. 2008;76:128–138. doi: 10.1016/j.bcp.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Du Y, Ma Z, Lin S, Dodel RC, Gao F, Bales KR, Triarhou LC, Chernet E, Perry KW, Nelson DL, Luecke S, Phebus LA, Bymaster FP, Paul SM. Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson's disease. Proc Natl Acad Sci U S A. 2001;98:14669–14674. doi: 10.1073/pnas.251341998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraco G, Pancani T, Formentini L, Mascagni P, Fossati G, Leoni F, Moroni F, Chiarugi A. Pharmacological inhibition of histone deacetylases by suberoylanilide hydroxamic acid specifically alters gene expression and reduces ischemic injury in the mouse brain. Mol Pharmacol. 2006;70:1876–1884. doi: 10.1124/mol.106.027912. [DOI] [PubMed] [Google Scholar]
- Ferger B, Teismann P, Earl CD, Kuschinsky K, Oertel WH. Salicylate protects against MPTP-induced impairments in dopaminergic neurotransmission at the striatal and nigral level in mice. Naunyn Schmiedebergs Arch Pharmacol. 1999;360:256–261. doi: 10.1007/s002109900079. [DOI] [PubMed] [Google Scholar]
- Fisar Z, Hroudova J, Raboch J. Inhibition of monoamine oxidase activity by antidepressants and mood stabilizers. Neuro Endocrinol Lett. 2010;31:645–656. [PubMed] [Google Scholar]
- Fuxe K, Janson AM, Rosen L, Finnman UB, Tanganelli S, Morari M, Goldstein M, Agnati LF. Evidence for a protective action of the vigilance promoting drug modafinil on the MPTP-induced degeneration of the nigrostriatal dopamine neurons in the black mouse: an immunocytochemical and biochemical analysis. Exp Brain Res. 1992;88:117–130. doi: 10.1007/BF02259133. [DOI] [PubMed] [Google Scholar]
- Gurvich N, Klein PS. Lithium and valproic acid: parallels and contrasts in diverse signaling contexts. Pharmacol Ther. 2002;96:45–66. doi: 10.1016/s0163-7258(02)00299-1. [DOI] [PubMed] [Google Scholar]
- Hirsch EC, Breidert T, Rousselet E, Hunot S, Hartmann A, Michel PP. The role of glial reaction and inflammation in Parkinson's disease. Ann N Y Acad Sci. 2003;991:214–228. doi: 10.1111/j.1749-6632.2003.tb07478.x. [DOI] [PubMed] [Google Scholar]
- Jenner P. Oxidative stress in Parkinson's disease. Ann Neurol. 2003;53 Suppl 3:S26–S36. doi: 10.1002/ana.10483. discussion S36–28. [DOI] [PubMed] [Google Scholar]
- Jenner P, Zeng BY, Smith LA, Pearce RK, Tel B, Chancharme L, Moachon G. Antiparkinsonian and neuroprotective effects of modafinil in the mptp-treated common marmoset. Exp Brain Res. 2000;133:178–188. doi: 10.1007/s002210000370. [DOI] [PubMed] [Google Scholar]
- Jornada LK, Valvassori SS, Steckert AV, Moretti M, Mina F, Ferreira CL, Arent CO, Dal-Pizzol F, Quevedo J. Lithium and valproate modulate antioxidant enzymes and prevent ouabain-induced oxidative damage in an animal model of mania. Journal of Psychiatric Research. 2011;45:162–168. doi: 10.1016/j.jpsychires.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Kidd SK, Schneider JS. Protection of dopaminergic cells from MPP+-mediated toxicity by histone deacetylase inhibition. Brain Res. 2010;1354:172–178. doi: 10.1016/j.brainres.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Rowe M, Ren M, Hong J-S, Chen P-S, Chuang D-M. Histone Deacetylase Inhibitors Exhibit Anti-Inflammatory and Neuroprotective Effects in a Rat Permanent Ischemic Model of Stroke: Multiple Mechanisms of Action. J Pharmacol Exp Ther. 2007;321:892–901. doi: 10.1124/jpet.107.120188. [DOI] [PubMed] [Google Scholar]
- Kuriyama T, Taguchi J, Kuriyama K. Functional alterations in striatal cholinergic and striato-nigral gabaergic neurons following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) administration. Neurochem Int. 1990;16:319–329. doi: 10.1016/0197-0186(90)90109-7. [DOI] [PubMed] [Google Scholar]
- Lagrue E, Chalon S, Bodard S, Saliba E, Gressens P, Castelnau P. Lamotrigine is neuroprotective in the energy deficiency model of MPTP intoxicated mice. Pediatr Res. 2007;62:14–19. doi: 10.1203/PDR.0b013e31806790d7. [DOI] [PubMed] [Google Scholar]
- Langston JW. The etiology of Parkinson's disease with emphasis on the MPTP story. Neurology. 1996;47:S153–S160. doi: 10.1212/wnl.47.6_suppl_3.153s. [DOI] [PubMed] [Google Scholar]
- Leng Y, Liang MH, Ren M, Marinova Z, Leeds P, Chuang DM. Synergistic neuroprotective effects of lithium and valproic acid or other histone deacetylase inhibitors in neurons: roles of glycogen synthase kinase-3 inhibition. J Neurosci. 2008;28:2576–2588. doi: 10.1523/JNEUROSCI.5467-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng Y, Marinova Z, Reis-Fernandes MA, Nau H, Chuang DM. Potent neuroprotective effects of novel structural derivatives of valproic acid: potential roles of HDAC inhibition and HSP70 induction. Neurosci Lett. 2010;476:127–132. doi: 10.1016/j.neulet.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loscher W. Valproate enhances GABA turnover in the substantia nigra. Brain Res. 1989;501:198–203. doi: 10.1016/0006-8993(89)91044-5. [DOI] [PubMed] [Google Scholar]
- Loscher W. Valproate: a reappraisal of its pharmacodynamic properties and mechanisms of action. Prog Neurobiol. 1999;58:31–59. doi: 10.1016/s0301-0082(98)00075-6. [DOI] [PubMed] [Google Scholar]
- Lv L, Sun Y, Han X, Xu CC, Tang YP, Dong Q. Valproic acid improves outcome after rodent spinal cord injury: Potential roles of histone deacetylase inhibition. Brain Res. 2011;1396:60–68. doi: 10.1016/j.brainres.2011.03.040. [DOI] [PubMed] [Google Scholar]
- Marinova Z, Ren M, Wendland JR, Leng Y, Liang MH, Yasuda S, Leeds P, Chuang DM. Valproic acid induces functional heat-shock protein 70 via Class I histone deacetylase inhibition in cortical neurons: a potential role of Sp1 acetylation. J Neurochem. 2009;111:976–987. doi: 10.1111/j.1471-4159.2009.06385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melamed E, Martinovits G, Pikarsky E, Rosenthal J, Uzzan A. Diphenylhydantoin and phenobarbital suppress the dopaminergic neurotoxicity of MPTP in mice. Eur J Pharmacol. 1986;128:255–257. doi: 10.1016/0014-2999(86)90773-9. [DOI] [PubMed] [Google Scholar]
- Mohanakumar KP, Muralikrishnan D, Thomas B. Neuroprotection by sodium salicylate against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity. Brain Research. 2000;864:281–290. doi: 10.1016/s0006-8993(00)02189-2. [DOI] [PubMed] [Google Scholar]
- Peng GS, Li G, Tzeng NS, Chen PS, Chuang DM, Hsu YD, Yang S, Hong JS. Valproate pretreatment protects dopaminergic neurons from LPS-induced neurotoxicity in rat primary midbrain cultures: role of microglia. Brain Res Mol Brain Res. 2005;134:162–169. doi: 10.1016/j.molbrainres.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Petri S, Kiaei M, Kipiani K, Chen J, Calingasan NY, Crow JP, Beal MF. Additive neuroprotective effects of a histone deacetylase inhibitor and a catalytic antioxidant in a transgenic mouse model of amyotrophic lateral sclerosis. Neurobiol Dis. 2006;22:40–49. doi: 10.1016/j.nbd.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Phiel CJ, Zhang F, Huang EY, Guenther MG, Lazar MA, Klein PS. Histone Deacetylase Is a Direct Target of Valproic Acid, a Potent Anticonvulsant, Mood Stabilizer, and Teratogen. J Biol Chem. 2001;276:36734–36741. doi: 10.1074/jbc.M101287200. [DOI] [PubMed] [Google Scholar]
- Rouaux C, Panteleeva I, Rene F, Gonzalez de Aguilar JL, Echaniz-Laguna A, Dupuis L, Menger Y, Boutillier AL, Loeffler JP. Sodium valproate exerts neuroprotective effects in vivo through CREB-binding protein-dependent mechanisms but does not improve survival in an amyotrophic lateral sclerosis mouse model. J Neurosci. 2007;27:5535–5545. doi: 10.1523/JNEUROSCI.1139-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu H, Lee J, Olofsson BA, Mwidau A, Dedeoglu A, Escudero M, Flemington E, Azizkhan-Clifford J, Ferrante RJ, Ratan RR. Histone deacetylase inhibitors prevent oxidative neuronal death independent of expanded polyglutamine repeats via an Sp1-dependent pathway. Proc Natl Acad Sci U S A. 2003;100:4281–4286. doi: 10.1073/pnas.0737363100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HY, He JC, Wang Y, Huang QY, Chen JF. Geldanamycin induces heat shock protein 70 and protects against MPTP-induced dopaminergic neurotoxicity in mice. J Biol Chem. 2005;280:39962–39969. doi: 10.1074/jbc.M505524200. [DOI] [PubMed] [Google Scholar]
- Singer TP, Castagnoli N, Jr, Ramsay RR, Trevor AJ. Biochemical events in the development of parkinsonism induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. J Neurochem. 1987;49:1–8. doi: 10.1111/j.1471-4159.1987.tb03384.x. [DOI] [PubMed] [Google Scholar]
- Speciale SG. MPTP: insights into parkinsonian neurodegeneration. Neurotoxicol Teratol. 2002;24:607–620. doi: 10.1016/s0892-0362(02)00222-2. [DOI] [PubMed] [Google Scholar]
- Suuronen T, Huuskonen J, Pihlaja R, Kyrylenko S, Salminen A. Regulation of microglial inflammatory response by histone deacetylase inhibitors. J Neurochem. 2003;87:407–416. doi: 10.1046/j.1471-4159.2003.02004.x. [DOI] [PubMed] [Google Scholar]
- Tatton NA, Kish SJ. In situ detection of apoptotic nuclei in the substantia nigra compacta of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice using terminal deoxynucleotidyl transferase labelling and acridine orange staining. Neuroscience. 1997;77:1037–1048. doi: 10.1016/s0306-4522(96)00545-3. [DOI] [PubMed] [Google Scholar]
- Wang W, Yang Y, Ying C, Li W, Ruan H, Zhu X, You Y, Han Y, Chen R, Wang Y, Li M. Inhibition of glycogen synthase kinase-3beta protects dopaminergic neurons from MPTP toxicity. Neuropharmacology. 2007;52:1678–1684. doi: 10.1016/j.neuropharm.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Wu DC, Jackson-Lewis V, Vila M, Tieu K, Teismann P, Vadseth C, Choi DK, Ischiropoulos H, Przedborski S. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J Neurosci. 2002;22:1763–1771. doi: 10.1523/JNEUROSCI.22-05-01763.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]