Abstract
Purpose
The development of new effective therapeutic agents with minimal side effects for prostate cancer treatment is much needed. Indirubin, an active molecule identified in the traditional Chinese herbal medicine – Qing Dai (Indigo Naturalis), has been used to treat leukemia for decades. However, the anti-cancer properties of Natura-alpha, an indirubin derivative, are not well studied in solid tumors, particularly in prostate cancer.
Experimental Design
Human prostate cancer cell lines were treated with or without Natura-alpha followed by cell growth and invasion assays measured. The anti-tumor effects of Natura-alpha were examined in nude mice tumor xenograft models, as well as in a patient with advanced hormone refractory metastatic prostate cancer. Signal network proteins targeted by Natura-alpha were analyzed using Proteomic Pathway Array Analysis (PPAA) on xenografts.
Results
Natura-alpha inhibited the growth of both androgen-dependent (LNCaP), and androgen-independent (LNCaP-AI, PC-3, and DU145) prostate cancer cells with IC50 between 4 to 10 Μm, also inhibits invasion of androgen-independent prostate cancer cells. Its anti-tumor effects were further evident in vivo tumor reduction in androgen-dependent and -independent nude mice tumor xenograft models as well as reduced tumor volume in the patient with hormone refractory metastatic prostate cancer. PPAA revealed that anti-proliferative and anti-invasive activities of Natura-alpha on prostate cancer might primarily be through its down-regulation of Forkhead box M1 (FOXM1) protein. Forced over-expression of FOXM1 largely reversed the inhibition by Natura-alpha.
Conclusion
Natura-alpha could serve as a novel and effective therapeutic agent for treatment of both hormone sensitive and hormone refractory prostate cancer with minimal side effects.
Introduction
Prostate cancer (PC) is the most common cancer in men in the United States, and was expected to cause 192,280 new cases and 27,360 deaths in 2009 (1). Androgen ablation is the most common therapy for advanced prostate cancer. The treatment failure of prostate cancer (PC) lies in the fact that, after androgen ablation therapy, the disease inevitably progresses from androgen-dependence to androgen-independence. For patients who are not cured by local treatment with ensuing metastasizes, neither androgen ablation nor chemotherapy can extend their survival time. Thus, the development of new effective therapeutic agents with minimal side effects is highly warranted.
Cancer is increasingly being viewed as a cell cycle disease since deregulation in the cell cycle machinery can be found in most cancers (2–4). Major components in the cell cycle machinery are cyclin dependent kinases (cdks) and their interacting partners, the cyclins and the endogenous inhibitors (e.g., cdki). Defects have been described in the components of the cell cycle machinery itself, or the checkpoint components that ensure orderly advancement through the cell cycle phases, or in upstream signaling that triggers cell cycle events (5–6). Strategies have been developed and intensified in the last few years by directly or indirectly targeting cdks and these have been reviewed extensively (3, 7–9). The first two cdk inhibitors, Flavopiridol and UCN-01 have been in clinical trials alone, or in combination with other chemotherapeutic agents, and have shown promising results with evidence of antitumor activity (10–12).
Indirubin, an active molecule identified in the traditional Chinese herbal medicine – Qing Dai (Indigo Naturalis), has been used to treat leukemia for decades (13–14). In recent years, there has been a dramatic renewal of the interest in indirubin due to the discovery of its great pharmacological potential (15). Increasing evidences demonstrate that indirubin, and its derivatives and analogues, target various important signal pathways involved in cancer, including inhibition of cyclin-dependent kinases (cdks) (16). Natura-alpha (NTI-Onco2008-1), an indirubin derivative, demonstrates an ability to arrest leukemia cells at G1 phase, inhibit expression of the oncogene c-Myb, and induce cell differentiation and maturation at low concentration, in which cell growth is completely inhibited without decrease in cell viability. At higher concentration, this agent blocks tumor cells at M/G2 phases.
To further evaluate its potential clinical application and to explore mechanisms of its anticancer action, in this study we examined therapeutic activities of Natura-alpha on androgen-dependent and -independent prostate in vitro and in vivo, as well as in a patient with advanced hormone refractory metastatic prostate cancer. Natura-alpha showed strong inhibition of cell proliferation and invasion in various human prostate cancer cell lines and tumor growth in nude mouse xenografts. Most importantly, the patient with hormone refractory metastatic prostate achieved stable disease in response to Natura-alpha’s with his liver metastatic tumors reduced by approximately 26% using guidelines of RECIST (17). Further study indicated that the suppression of FOXM1 was the primary target of inhibition of proliferation and invasion by Natura-alpha.
Materials and methods
Reagents
The chemical name of Natura-alpha is N-methyl-Δ3, 3'-dihydroindole-2, 2' diketone. Natura-alpha was provided by Natrogen Therapeutics International, Inc (New York, NY). It was synthesized under cGMP conditions, and structure confirmed by IR, MNR, and Mass spectrometry with a purity ≥98.00%.
Cell culture and cell proliferation assays
LNCaP and DU145 cells were maintained in RPMI 1640 (GIBCO, Gaithersburg, MD) and PC3 cells were cultured in 50% RPMI 1640 and 50% F2 GIBCO, Gaithersburg, MD) with 10% heat-inactivated bovine serum (FBS). The androgen-independent LNCaP-AI cells, a derivative of LNCaP (18–20), were maintained in RPMI 1640 medium containing 10% charcoal-stripped, heat-inactivated FBS (CSFBS) (Hyclone Laboratories, Inc., Logan, UT) and 5 µg/ml of insulin, as described previously (18). Cell proliferation was determined by MTT as described previously (21–22). Anchorage-independent cell growth in soft agar was performed in triplicate with cells (1 × 104) suspended in 2 ml of medium containing 0.35% agar (Becton Dickinson) spread on top of 5 ml of 0.7% solidified agar (23). Colony volume was calculated from the average radius of representative colonies.
Matrigel invasion assays
Effect of Natura-alpha on invasive activity of LNCaP and LNCaP-AI cells was determined via BD Matrigel invasion assay (Growth Factor Reduced Matrigel Invasion Chamber) as described (24). After rehydration of the insert with medium for 2 hrs, LNCaP and LNCaP-AI cells at their exponential growth phases were added to the upper chamber at the density of 1×104 cells in 500ul medium in the presence or absence of indicated concentration of Natura-alpha and incubated at 37° C for 48 hrs. Data was adjusted by growth condition, and expressed as mean of migrating cells in 3 fields ± SD.
Western blot analysis
Whole cell or cell fraction extracts were subjected to SDS-PAGE and transferred to a nitrocellulose membrane for western blot analysis. Blots were incubated with primary antibodies including FOXM1, cyclin D1, cyclin B, cyclin E, and β-actin (Cell Signaling Technology, Inc., Danvers, MA) for 2hrs at room temperature, washed with TBS-T, and incubated for 1.5hrs with horseradish peroxidase-conjugated secondary antibody (1:5,000, Amersham Biosciences). The protein bands were detected by an enhanced chemiluminesence kit (Amersham Biosciences).
Nude mice xenografts
Androgen-dependent LNCaP and -independent LNCaP-AI prostate cancer cells, mixed with Matrigel (Becton Dickinson, Bedford, MA) at a ratio of 1:1 were inoculated into the bilateral flanks of 4–5 week-old male Nu/Nu Balb/c athymic nude mice by subcutaneous injection. The tumor growth and volume was monitored every 3 days. When the prostate tumor grew to a diameter of 4–8 mm (4–5 weeks), animals were randomly divided into 2 groups, 10 mice each, according to tumor size. One group of animals was treated with drug vehicle only as control, and another group was treated with Natura-alpha at dose of 100mg/kg by gavage, once a day, 5 days a week until the diameter of tumors in control group reached approximately 15 mm. The tumor growth was monitored daily and tumor size recorded every three days. The tumor volume was calculated as l × d × h × 0.52 (25).
Proteomic Pathway Array Analysis
Total cellular proteins were extracted from xenograft tumors (3 mm3) using a lysis buffer containing 20mmol/L Tris-HCl (pH 7.5), 20mmol/L sodium Pyrophosphate, 40mmol/L B-glycerophosphate, 30mmol/L Sodium Fluoride, 2mmol/L EGTA, 10mmol/L NaCl, and 0.5% NP-40. The lysate was sonicated three times for 15 seconds each time, and then centrifuged (14,000 rpm, 30 minutes, 4°C). The tubes were kept on ice throughout the process. The protein concentration was determined with the BCA Protein Assay kit (PIERCE, Rockford, IL). Isolated proteins were separated by SDS-PAGE (10% acrylamide). Three hundred (300) µg of protein extracts were loaded in a well across the entire width of gel for SDS-PAGE, followed by electro-transferring to a nitrocellulose membrane. The membrane was then blocked for 1 hr with 5% milk or 3% BSA, and clamped on to a Mini-PROTEAN II Multiscreen apparatus that isolates 20 channels across the membrane (Bio-Rad, Hercules, CA). Two or three antibodies were added to each channel and incubated overnight at 4°C. Different sets of antibodies were used for each membrane after stripping the previous set of antibodies. Antibodies were purchased either from Cell Signaling Technology, Inc. (Boston, MA) or from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA) (Supplementary Table S1). The pathway array analysis was run in duplicate for each sample in each set of antibodies and protein levels were normalized using beta-actin and GAPDH as standards. Chemiluminescence signals were captured using the ChemiDoc XRS System. Differences in protein levels were determined by densitometric scanning and normalized to internal standards.
FDA and IRB approved single patient clinical trial
An 86 year old patient with advanced androgen-independent metastatic prostate cancer was consented for the Natura-alpha trial therapy for his disease with approval from the FDA (IND# 104191) and IRB (IntegReview NTI-2007-1-PC). Natura-alpha was administered orally with increasing doses from 40mg, 80mg, 160 to 200 mg per day every two weeks and 200 mg later on for three months.
Statistical analysis
The growth inhibition (1-T/C, Effect), median effect dose (Dm), and combination indexes (CI) were calculated, and analyzed using the computer program, CalcuSyn, of Biosoft edited by T.C. Chou, Memorial Sloan-Kettering Cancer Center, New York, and M.P. Hayball, of Biosoft, Cambridge, UK, (21, 26). The combination index (CI) was used to evaluate the results of the combinations. A CI greater than 1 indicates the combination is antagonistic, CI equal to 1 indicates the combination is additive, and CI smaller than 1 indicates that the combination is synergistic (26).
Results
Effects of Natura-alpha on prostate cancer growth and invasion in vitro
A response of different human prostate cancer cells to the treatment of Natura-alpha was obtained using colorimetric MTT and/or SRB methods after three days of exposure. Table 1 showed the IC50 of Natura-alpha on hormone dependent and independent human prostate cancer cell lines ranged between 3.96 to 9.39µM. The IC50 data pertains to the concentration of the tested drug that inhibits 50% cell growth in in vitro growth kinetic studies. The data of Table 1 are mean+SD from three independent experiments.
Table 1.
IC50 of Natura in hormone dependent and independent human cancer cell lines
| Cell Lines | IC50 |
|---|---|
| LNCaP | 4.54±0.73 |
| LNCaP-AI | 6.89±0.57 |
| DU145 | 9.39±0.23 |
| PC-3 | 3.96±0.58 |
To explore if Natura-alpha is able to enhance activity of clinical available chemotherapeutic drugs used for prostate cancer, the commonly used antimicrotubule agent, paclitaxel (Taxol), was combined with Natura-alpha in three different sequential exposures. In the first combination, LNCaP-AI cells were treated with Natura-alpha and Taxol simultaneously for 6 days; In the second combination, the cells were treated with Natura-alpha for the first 3 days followed by treatment with Taxol for additional 3 days (NTI→ Taxol); In the third regimen, the cells were treated with Taxol for the first 3 days followed by treatment with Natura-alpha for additional 3 days (Taxol→NTI). Exposure of the cells to either Natura-alpha or Taxol alone served as controls. After treatment, cell growth was determined by MTT assays with the growth inhibition (1-T/C, Effect), median effect dose (Dm), and combination indexes (CI) calculated, and analyzed as described in the Materials and Methods.
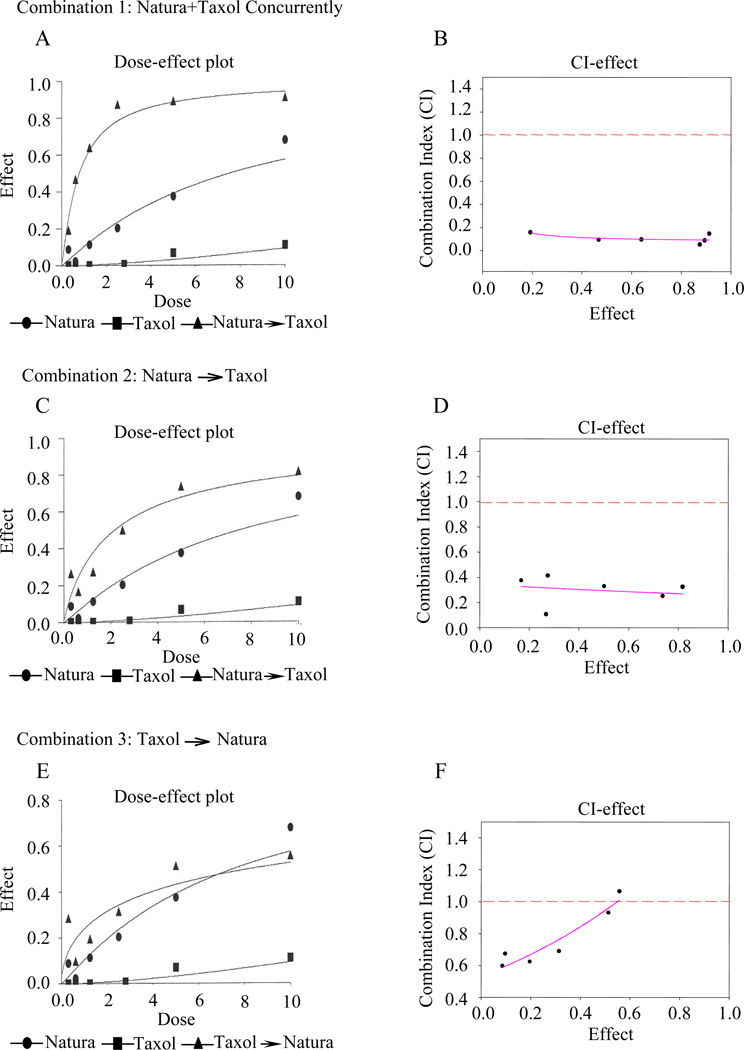
The concurrent combination of Natura-alpha, with the anti-microtubule agent, Taxol achieved a strong synergistic effect on LNCaP-AI prostate cancer growth. For example, the calculated Dm of Natura-alpha and Taxol alone against LNCaP-AI cells was found to be approximately 7.54 µM and 41.57 nM, respectively. However, when the two drugs were applied to the cancer cells simultaneously at ratio of 1000:1 (Natura-alpha:Taxol), Dm was significantly reduced to 0.78 µM and 0.78 nM, respectively (Supplementary Table S2) and CI was well below 1 (Fig. 1A and B). One-way ANOVA test showed p<0.001 indicating that there is statistically significant difference between the combination and the two individual agent.
Figure 1.
Effects of Natura-alpha (NTI) and Taxol on cell growth of androgen-independent prostate cancer (LNCaP-AI) with different treatment regimens. A, C, and E are Dose-effect curves, and B, D, and F are CI-effect curves. Dash line in B, D, and F (CI=1) is the divider of outcomes of two-drug combination. CI value above the dash line indicates an antagonistic effect, below the line is a synergistic effect, and on the line implies an additive effect. A regression analysis of CI values versus effects in B, D, and F were performed by Sigma plot 8, and the line direction indicates trend of a combination. Androgen-independent LNCaP-AI cells were treated simultaneously with NTI and Taxol (A and B); C and D (Combination 2, NTI → Taxol): the cells were exposed to NTI first for 3 days followed by Taxol treatment for additional 3 days; Panel E&F (Combination 3, Taxol → NTI): the cells were treated with Taxol first for 3 days followed by NTI treatment for additional 3 days.
Interestingly, the effects of the combination are highly dependent on the sequence of the drug exposure indicated by the CI (Fig. 1) and Dm values (Supplementary Table S2). A strong synergistic growth inhibitory effect of LNCaP-AI cells was achieved when the cancer cells were exposed to Natura-alpha and Taxol concurrently (Fig. 1A and B), where CI at each concentration points were well below 1, whereas only a moderate synergism was observed when the cells were treated with Natura-alpha first for 3 days followed by Taxol treatment for additional 3 days. Notably, the trend of the combination became antagonistic when the cancer cells were exposed to Taxol for the first 3 days followed by exposure to Natura-alpha for an additional 3 days (Fig. 1E and F). Similar results were also obtained in LNCaP cells (data not shown).
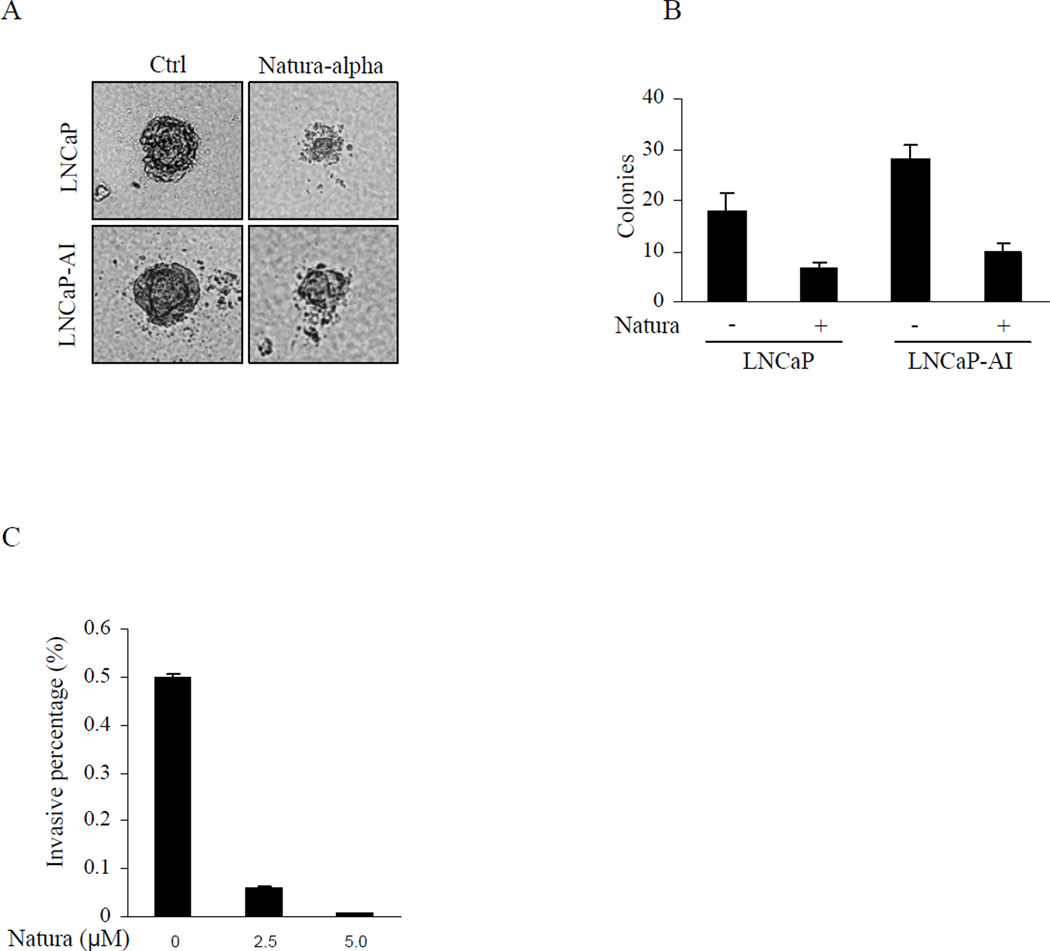
Growth inhibition of Natura-alpha on prostate cancer cells was further supported by anchorage independent assay. While both LNCaP and LNCaP-AI cells could easily form colonies in soft agar in the absence of Natura-alpha (Fig. 2A), LNCaP-AI cells showed stronger capacity of colony formation. However, the colony formation of both LNCaP and LNCaP-AI cells was significantly inhibited by Natura-alpha (final concentration 2.5 µM) as reflected by remarkable decrease in numbers and size of colonies under the same experimental conditions (Fig. 2B).
Figure 2.
Inhibition of prostate cancer anchorage independent growth and invasion. A and B: Anchorage independent cell growth in soft agar was performed in triplicate with cells (1 × 104) suspended in 2 mL of medium containing 0.35% agar (Becton Dickinson) spread on top of 5 mL of 0.7% solidified agar. Colony volume was calculated from the average radius of representative colonies. C: LNCaP-AI cells at their exponential growth phases were added to the upper chamber at density of 1×104 cells in 500ul medium in the presence or absence of indicated concentrations of Natura-alpha and incubated at 37° C for 48 hrs, and the invading cells adherent at the bottom of the membrane were fixed, stained, and counted by tallying the number of cells in 3 random fields under the microscope. Data were adjusted by growth condition, and expressed as mean of migrating cells in 3 fields ±SD.
To examine whether Natura-alpha inhibits the invasive potential of prostate cancer cells, invasive activity of LNCaP and LNCaP-AI cells was determined via the BD Matrigel invasion assay. Results showed that invasive capacity of LNCaP cells were highly limited. Only a few cells migrated (data not shown). In contrast, LNCaP-AI cells demonstrated strong invasive potential. Over 4000 cells invaded per high power field during 48 hrs culture in the presence of androgen. Interestingly, the invasive capacity of LNCaP-AI cells was strongly blocked by Natura-alpha in a concentration-dependent manner. Inhibitions of invasive LNCaP-AI cells reached over 87% and 99% at concentrations of 2.5µM and 5.0µM of Natura-alpha, respectively (Fig. 2C).
Inhibition of prostate tumor growth in vivo by Natura-alpha
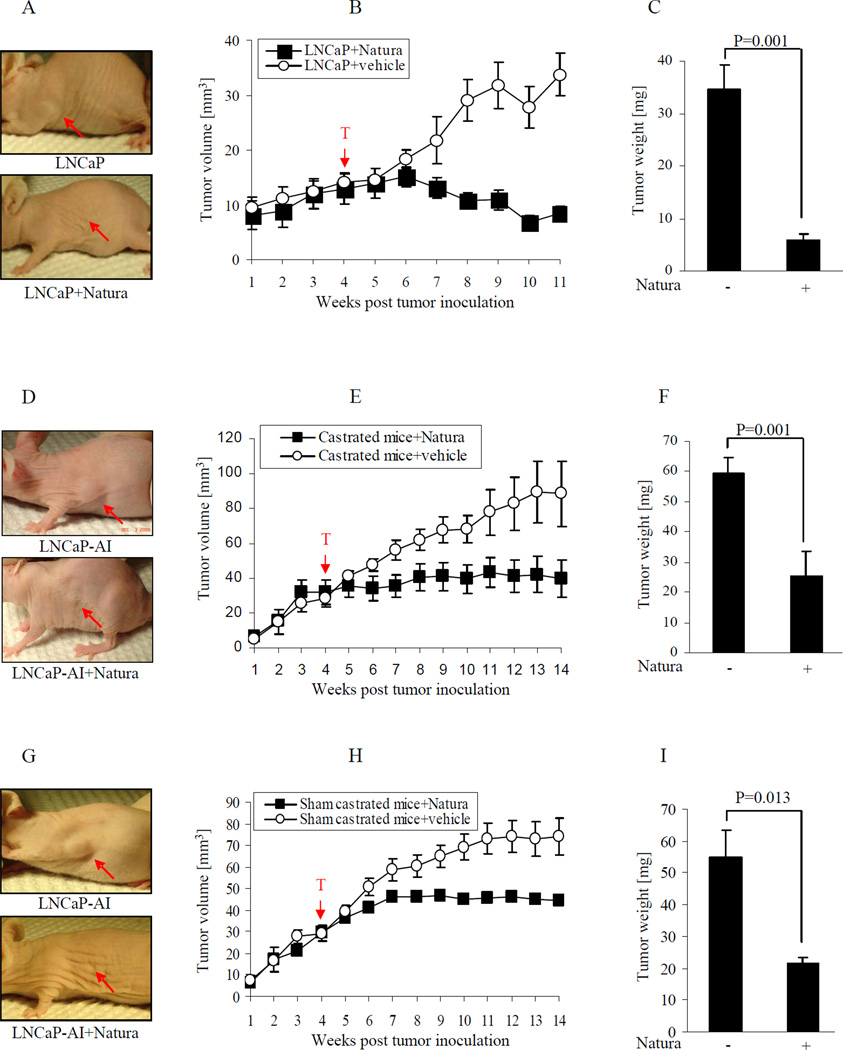
In an androgen-dependent (LNCaP) xenograft model, prostate cancer cells were injected subcutaneously into the flank region of male nude mice. When the prostate tumor grew for 4 – 5 weeks (20 to 30 mm3), animals were randomly divided into two groups, 10 animals each, according to tumor size. A suspension of Natura-alpha was given at dose of 100mg/kg by gavages once a day for 5 days a week. Mice fed with equal volume of solution of 0.05% Tween 20 in water (a solution used in preparing Natura-alpha suspension) served as vehicle controls. The tumor size was measured every 3 days, and tumor growth curves (tumor size versus time) were plotted. As shown in Fig. 3A and B, treating with Natura-alpha, starting at week 5, slowed tumor growth compared to the control group. By week 6, tumor growth in the Natura-alpha treated group almost completely halted, whereas tumors in the vehicle treated group increasingly grew. Continued feeding with Natura-alpha not only completely halted tumor growth, but significantly reduced the tumor volume. For example, on day 78, the average volume of tumors in the Natura-alpha treated group was reduced by 53% (p=0.035). Additionally, after dissection, tumor weight from the Natura-alpha treated group was reduced about 6 folds as compared with the control group (p=0.001) and hazard ratio is 0.168 (Fig. 3C).
Figure 3.
Natura-alpha inhibits prostate cancer growth in vivo. Prostate cancer cells (LNCaP or LNCaP-AI) were injected subcutaneously into the flank region of male nude mice. After the prostate tumor grew for 4 – 5 weeks (20 to 30 mm3), animals were randomly divided into two groups according to tumor size, 10 animals each. A suspension of Natura-alpha (or equal volume of the vehicle) was given at a dose of 100mg/kg by gavage once per day for 5 days a week. The tumor size was measured every 3 days, and tumor growth curves (tumor size versus time) were plotted. A, B and C: LNCaP tumors in mice without castration. D, E and F: LNCaP-AI tumors in castrated mice. G, H and I: LNCaP-AI tumors in sham castrated mice.
To determine the effects of Natura-alpha on androgen-independent prostate cancer, we developed a xenograft model using androgen-independent LNCaP-AI cells, with castration or sham castration. After 4 weeks of prostate tumor growth (20 to 30 mm3 in size), animals were castrated or sham- castrated, and randomly divided into four groups, 10 animals each, on the basis of tumor size. Group A and B consist of castrated mice fed with Natura-alpha or with equal volume of vehicle as control respectively. Group C and D consist of sham castrated mice fed with Natura-alpha or with equal volume of vehicle as control respectively. A suspension of Natura-alpha or equal volume of vehicle was given at dose of 100mg/kg by gavage, once a day and 5 days a week starting on day 28.
As shown in Fig. 3D, E, F and G, H, I, tumor volumes of castrated or sham castrated mice from both vehicle groups showed constant growth. In contrast, the growth of tumors in the Natura-alpha treated group was much slower. The reduction of tumor volume between Natura-alpha and the vehicle treated group was found to be statistically significant (Fig. 3E, p=0.019 and Fig. 3H, p=0.026) starting at week 7. The tumor weight from the Natura-alpha treated group was reduced approximately 2.33 folds (Castrated group) (Fig. 3F) and 2.6 folds (Sham castrated group) (Fig. 3I) as compared with the control group. The hazard ratios are 0.429 (Castrated group) and 0.385 (Sham castrated group), respectively.
In an effort to determine whether Natura-alpha would prevent tumor growth, we fed mice with Natura-alpha two weeks prior to LNCaP-AI cell transplantation. After tumor cell injection, the mice were fed continuously with Natura-alpha until dissection. As showed in supplementary Fig. S1, tumor growth from the pre-feeding group mice stopped by week 3 and did not grow any further. Tumor volume from the pre-feeding group was reduced more than 3.5 folds as compared with that of the vehicle control group (p<0.001). Additionally, pre-feeding reduced tumor volume almost 2 folds when compared with that of mice fed with Natura-alpha beginning at week 5 post-injection (p<0.001).
Natura-alpha reduces tumor burden in a patient with hormone refractory metastatic prostate cancer
An 86-year old patient with advanced hormone refractory and metastatic prostate cancer (liver metastasis confirmed by biopsy) who had failed previous chemotherapy, was put on Natura-alpha therapy for his disease with permission from the FDA with three treatment cycles (4 weeks per cycle). During the three treatment cycles allowed by IRB, laboratory tests and imaging examinations have been performed at the end of each treatment cycle (Supplementary Table S3).
Biological response: the value of alkaline phosphotase (APL) generally decreased during treatment period. For example on December 28, 2008 it was 377 U/L, and it decreased to 123 U/L on March 30, 2009. The decrease of APL may reflect improvement of liver and bone metastases. There was, however, no significant improvement in his serum PSA after Natura-alpha treatment. Serum PSA initially was at 270 ng/mL on January 2, 2009, decreased to 160ng/mL on January 20, 2009 and elevated to 294 ng/mL on March 20, 2009.
Assessment of target response: target response has been evaluated by anterior and posterior whole body images (bone scan) and a CT scan of the chest, abdomen, and pelvis at the end of each cycle. These studies showed that overall tumor burden was reduced. Using the "Guidelines to Evaluate the Response to Treatment in Solid Tumors (RECIST)"(17), five liver metastatic tumors at end of third cycle were compared with their baseline before Natura-alpha treatment. Multiple metastatic lesions within the liver were unchanged in number but decreased in size. As shown in supplementary Fig. S2, a 26% decrease in a sum of the longest diameters of 5 tumors was achieved, indicating Natura-alpha treatment stabilized the disease condition. A bone scan at the end of each cycle showed mostly unchanged as compared with the baseline before the study except for the following lesions where the radiotracer uptake was slightly decreased: anterior left second rib, upper thoracic spine, and posterior upper ribs. Unfortunately, the patient expired 10 months after 3-cycle Natura-alpha treatment.
Signal Network Proteins Targeted by Natura-alpha by Pathway Array Analysis on Xenograft Tumors
To further explore the mechanism of tumor inhibition by Natura-alpha, we performed Proteomic Pathway Array analysis (PPAA) using tumor samples from androgen-dependent LNCaP and -independent LNCaP-AI xenografts with or without treatments of Natura-alpha.
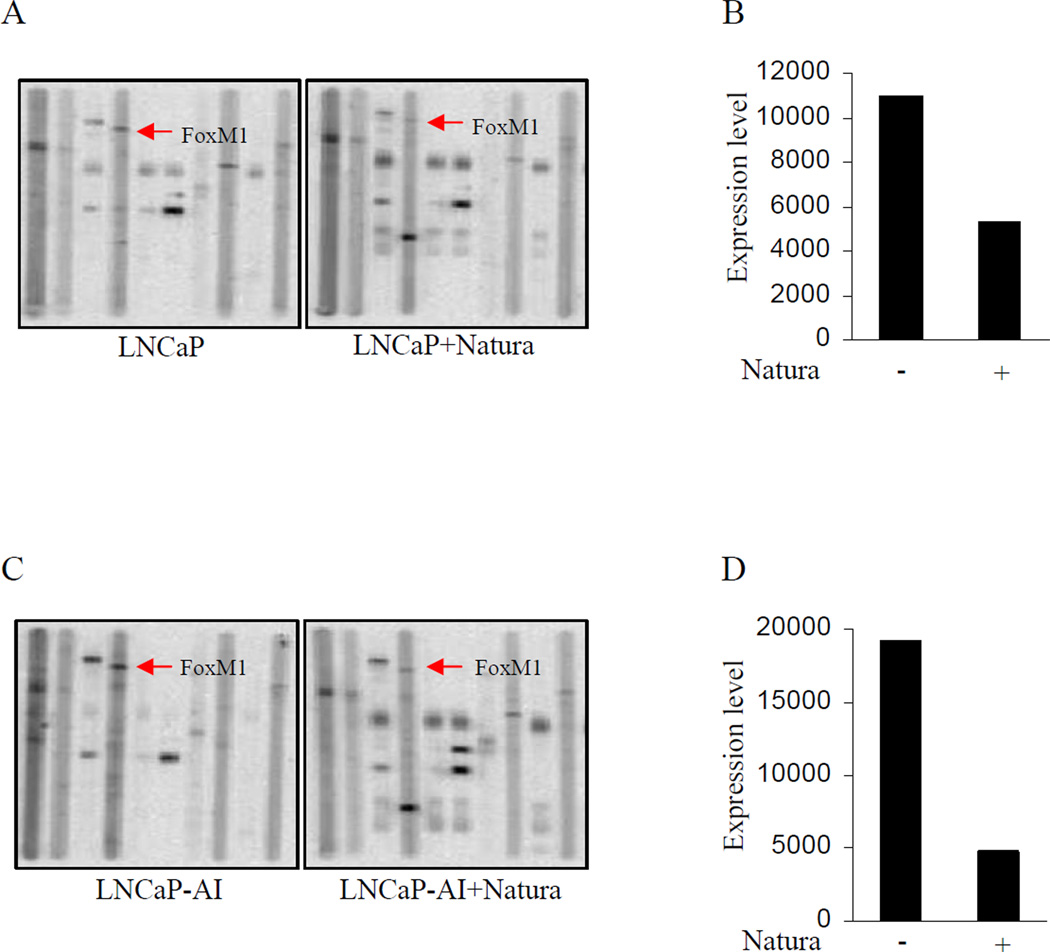
PPAA showed that Natura-alpha significantly affects molecules involved in regulating cell proliferation and migration/invasion, or metastasis. Natura-alpha significantly (at least > 2 folds) inhibited expression and activations of cyclin dependent kinases, such as cdk2, cdk6, p-cdc2Tyr15, and pRBSer780, which confirmed our previous observations in vitro (16). As an inhibitor of cdks, it seems that Natura-alpha's inhibition of cdk activity (i.e. phosphorylation) was stronger than its reduction of protein expression. For example, only 2 to 3 fold decreases in levels of cdk2 and cdk6 were achieved, whereas almost complete inhibition of p-cdc2Tyr15 was obtained by the compound. Natura-alpha showed little effects on expression of cyclin D1 and E. Another key cell cycle regulator, Forkhead box M1 (FOXM1), however, is also significantly inhibited by Natura-alpha (Fig. 4).
Figure 4.
Proteomic Pathway Array Analysis of Xenograft Tumors treated with Natura-alpha. A and B: expression of FOXM1 in samples from LNCaP xenograft tumors; Panel C and D: expression of FOXM1 in samples from LNCaP-AI xenograft tumors.
Natura-alpha also significantly affected the expression of two important molecules, E-cadherin and Mesothelin, in LNCaP xenografts (Supplementary Fig. S3). These proteins are involved in adhesion, migration, and invasion/metastasis. Natura-alpha strongly up-regulated expression of E-cadherin (<10-folds) while considerably inhibited expression of Mesothelin (>2-folds) in LNCaP xenograft tumors.
In addition, PPAA study also showed that Natura-alpha significantly (>2.5-folds) inhibited activations of various protein kinases, including p-PKCα, p-PKCδ, p-ERK and p-p38. Since overactivation of these protein kinases have been demonstrated to be involved in prostate tumor growth, progression, and drug resistance (27–29), inhibitions of Natura-alpha on these protein kinases may also play an important role in suppressing tumor growth and metastasis. Moreover, p-ERK and p-p38 are also involved in lipopolysaccharide (LPS)-mediated inflammatory signaling (30), suggesting inhibition of activation of p-ERK and p-p38 may also play a role in the anti-inflammatory activities of Natura-alpha.
FOXM1 mediates inhibition of prostate cancer growth and invasion by Natura-alpha
As mentioned above, the PPAA revealed that Natura-alpha significantly inhibited expression of cell cycle regulator Forkhead box M1 (FOXM1). As showed in Fig. 4A and B, expression of FOXM1 was reduced over 3 folds by Natura-alpha in tumor samples from androgen-dependent LNCaP xenografts. Similarly, Natura-alpha also repressed expression of FOXM1 approximately 3 folds in tumor samples from androgen-independent LNCaP-AI xenografts (Fig. 4C and D). The PPAA results suggest that Natura-alpha could be an effective inhibitor of FOXM1 expression, resulted in repressing the FOXM1 pathway-mediated the tumor growth promotion.
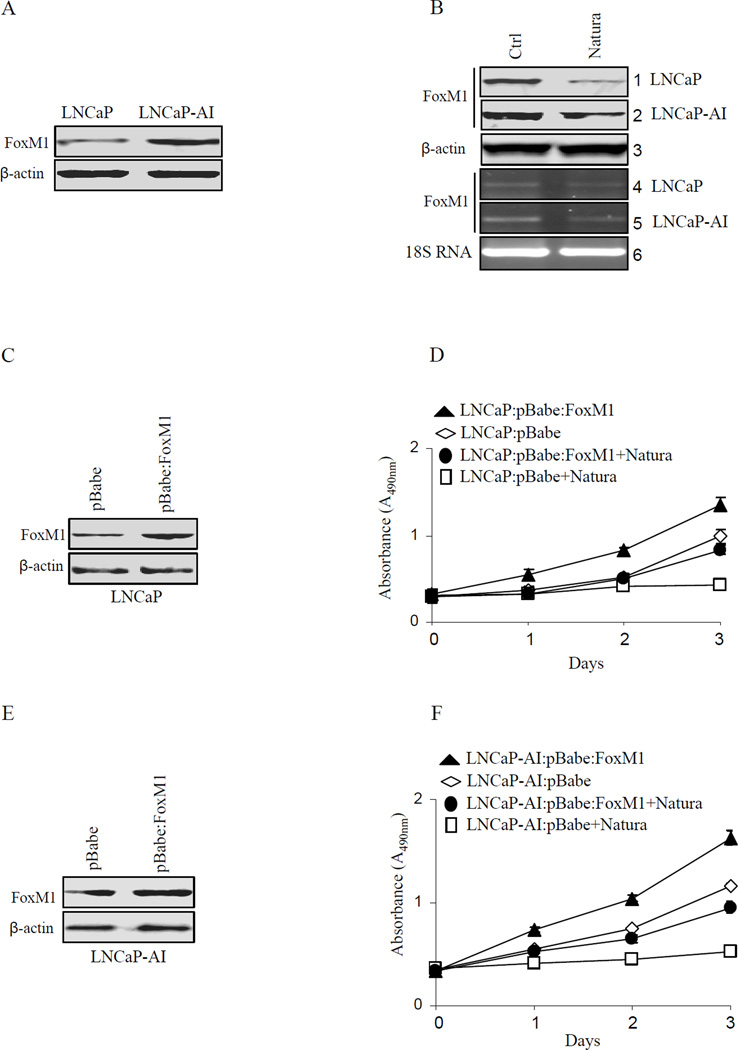
Since repression of FOXM1 was seen in vivo from LNCaP and LNCaP-AI xenografts by Natura-alpha, we investigated in vitro expression of FOXM1 in LNCaP and LNCaP-AI cells. As showed in Fig. 5A, endogenous FOXM1 was expressed in both LNCaP and LNCaP-AI cells, however about 2 fold higher expression was seen in LNCaP-AI cells compared to LNCaP cells. Next, we examined the effects of Natura-alpha on FOXM1 expression in both LNCaP and LNCaP-AI cells by incubating these cells in media containing 5 µM Natura-alpha for 24 hours. FOXM1 expression was reduced over 3 folds in both LNCaP and LNCaP-AI cells treated with Natura-alpha as compared to the control group (Fig. 5B panel 1 and 2). RT-PCR also revealed that Natura-alpha repressed FOXM1 expression at the transcriptional level (Fig. 5B panel 4 and 5).
Figure 5.
Effects of Natura-alpha on FOXM1 expression in vitro. A: endogenous expression of FOXM1 in both LNCaP and LNCaP-AI cells. B: Analysis of FOXM1 expression by RT-PCR and Western blot in LNCaP and LNCaP-AI. Lanes 1, 4: LNCaP, Lanes 2, 5: LNCaP-AI, Lanes 3, 6: Beta-actin and 18S RNA loading controls. C, D, E and F: expression of FOXM1 and cell proliferation in LNCaP and LNCaP-AI cells transfected with FOXM1 or control plasmid in response to Natura-alpha treatment; G and H: expression of FOXM1 and invasion in cells transfected with FOXM1 or control plasmid in response to Natura-alpha treatment; I: effects of Natura-alpha on the expression of cyclin B1, D1, and cyclin E in LNCaP-AD and LNCaP-AI cells.
To examine whether FOXM1 governs cell cycle progression in both LNCaP and LNCaP-AI cells, we performed FOXM1 knockdown using siRNA and observed that cell cycle was arrested upon FOXM1 knockdown in both LNCaP and LNCaP-AI cells (Supplementary Fig. S4). This observation indicated that FOXM1 plays a key role in cell cycle progression which is consistent with previous report (31).
To further explore whether Natura-alpha mediated repression of FOXM1 would cause cell cycle arrest, stable transfected cell lines of LNCaP and LNCaP-AI with overexpression of FOXM1 were established by retrovirus system (Fig. 5C and E), and their proliferations were measured. Forced expression of FOXM1 was found to promote cell proliferation in both LNCaP and LNCaP-AI cell lines. Moreover, the overexpressed FOXM1 in both cell lines largely reversed the growth inhibition by Natura-alpha, indicating that repression of FOXM1 mediated by Natura-alpha was a primary cause of cell cycle arrest by the compound (Fig. 5D and F).
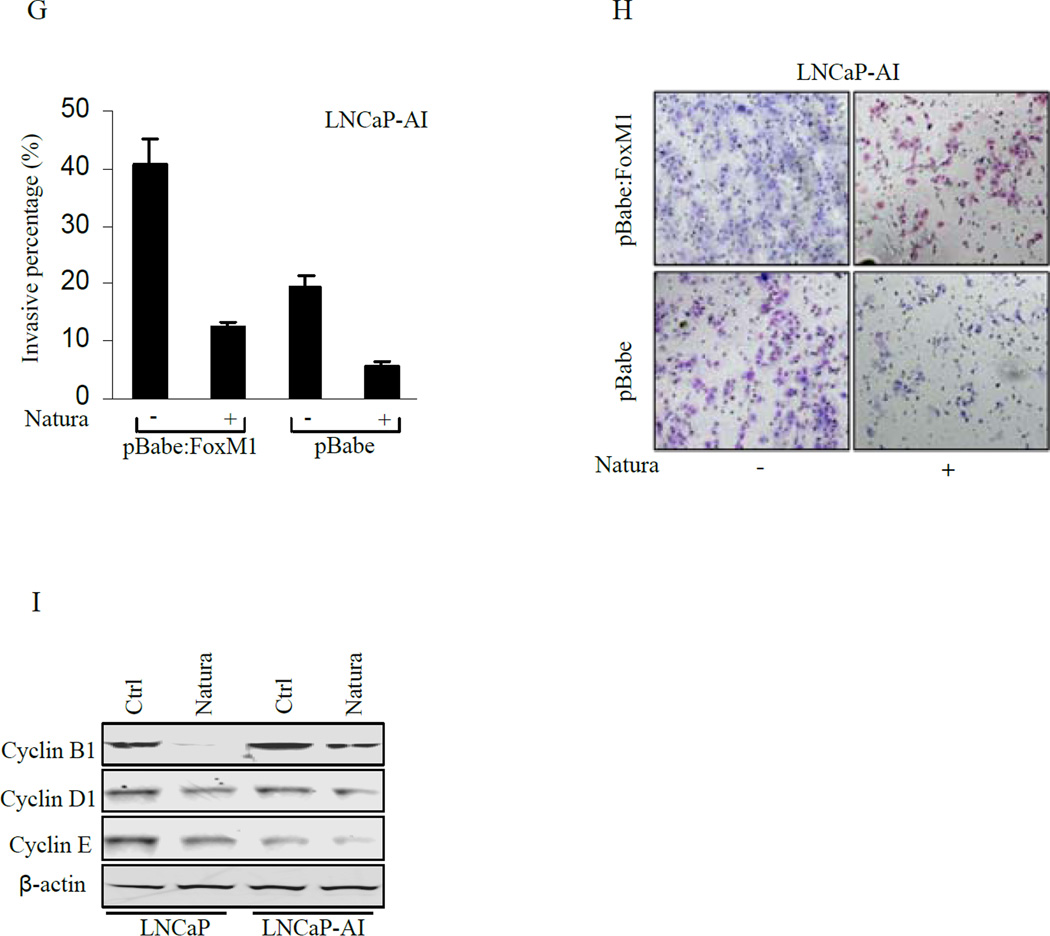
Since invasion of LNCaP-AI cells was inhibited by Natura-alpha (see Fig. 1), we examined whether over-expression of FOXM1 played a role in the invasion of LNCaP-AI cells. As showed in Fig. 5G and H, the over-expression of FOXM1 resulted in over 2 fold increase in the invasion capability of LNCaP-AI cells as compared with the control (Fig. 5G column 1 and 3). Although Natura-alpha inhibited the invasion in both LNCaP-AI cells and FOXM1-overexpressed LNCaP-AI cells, the inhibitory effect of the compound on invasion, however, was diminished to some extent by the over-expression of FOXM1 (Fig. 5G column 2 and 4).
FOXM1 promotes cell cycle progression at both G1/S- and G2/M-transitions, through regulating its direct target genes (cyclin B1, cyclin D1, cyclin E, cdc23B) and indirectly-regulated genes (cyclin A, cyclin F, cdc20 ect.) (31). To further explore the mechanisms of Natura-alpha on inhibition of cell proliferation and invasion, we investigated expression of several downstream genes of FOXM1 in response to Natura-alpha treatment. We found that Natura-alpha slightly decreased the expression of cyclin D1 and cyclin E which is consistent with our PPAA results. Interestingly, Natura-alpha dramatically inhibited expression of FOXM1 direct targeted gene cyclin B1, indicating that Natura-alpha probably blocks cell cycle progression through FOXM1-mediated down-regulation of cyclin B1 (Fig. 5I).
Discussion
The development of new therapeutic drugs that simultaneously target both cell proliferation and inflammation would be ideal since it may not only suppress cancer growth, but also prevent tumor metastasis. Natura-alpha has anti-proliferative and anti-inflammatory activities both in vitro and in vivo via inhibition of CDKs and pro-inflammatory cytokines (16, 32). In this study, our data showed that Natura-alpha inhibited growth of prostate cancer cells LNCaP, LNCaP-AI, PC3, and DU145 by MTT and anchorage independent assay. Matrigel assays also showed that Natura-alpha potentially inhibited invasive capability of cancer cells in vitro. In particular, a significant synergistic effect was also seen when Natura-alpha was concurrently combined with the anti-microtubule agent, paclitaxel at a ratio of 1000:1 (NTI-Onco2008-1:Paclitaxel) in androgen-dependent LNCaP and -independent LNCaP-AI, indicating that Natura-alpha was able to enhance activity of a clinically available chemotherapeutic drug for prostate cancer.
Mechanisms by which different sequential combinations of Natura-alpha and Taxol produce different outcomes are currently not clear. It may depend on the timing of cell cycle progression affected by the two drugs. Natura-alpha and taxol target two different cell proliferative pathways, the former inhibiting molecules in cell cycle regulation (it may arrest cells at G1, S, and G2+M phase) and the later interfering with microtubule stability and mostly arresting cells at G2+M phase. Thus, different sequential combinations may lead to different timing of cell cycle progression dynamics by the two drugs, which may affect their ability to reduce growth. For example, if the cells are quickly arrested at G1, then the numbers of cells entering G2+M phase will be reduced, and therefore the G2+M phase target drug, taxol’s activity will be reduced (less cells it can attack), and so, a less additive effect will occur. However, if cells in G1 and G2+M are simultaneously attacked, theoretically, at least an additive effect or synergism will occur. Thus, our in vitro data clearly demonstrated Natura-alpha’s activity against prostate cancer.
The above in vitro results are substantiated by in vivo experiments using mouse models with xenograft tumors from androgen-dependent (LNCaP) and -independent (LNCaP-AI) prostate cancer cells. Continued treatment with the compound completely halted tumor growth from LNCaP cells and tumor volume was significantly reduced as compared with the control group. Most encouragingly, with the permission of FDA, one patient with advanced metastatic prostate cancer achieved stable disease condition after treatment with Natura-alpha for three months. All of his liver metastatic tumors reduced in size by approximately 26% as determined using RECIST (17). Of note, although this patient showed response in liver metastases, PSA value remained the same. This phenomena needs to be further studied. In general, the amount of PSA produced increases with the size of the tumor, thus PSA is widely used as a biomarker for prostate cancer as well as a biomarker for treatment response. However, there is a poor correlation between the PSA level and the actual size of the tumor (33). In addition, prostate cancer progression may occur in the presence of undetectable or low serum prostate-specific antigen level (34). A proof of concept clinical trial of Natura-alpha in treating patients with advanced metastatic prostate cancer is currently under preparation.
PPAA showed that two categories of molecules involved in regulating cell proliferation, and migration/invasion or metastasis were found to be significantly affected by Natura-alpha. Natura-alpha inhibited expression and activation of cyclin dependent kinases, such as cdk2, cdk6, p-cdc2Tyr15, and p-RBSer780 as well as FOXM1, which confirmed some of our previous observations in vitro (16). E-cadherin and Mesothelin, two critical molecules involved in cell adhesion, migration, invasion or metastasis were also significantly impacted by Natura-alpha treatment, which provided strong molecular basis for Natura-alpha in suppression of tumor proliferation and metastasis observed both in vitro and in vivo, as well as in one human case. As tumors become more aggressive with the ability to metastasize, E-cadherin is commonly lost, as in prostate cancer (35–38). There is evidence that mesothelin may have potential as a new cancer diagnostic marker and a novel molecular target for gene therapy (39–41). Changes of these molecules in response to Natura-alpha treatments may not only help us to understand mechanisms of its activities on prostate cancer invasion/metastasis, but may also make E-cadherin and mesothelin valuable biological markers to monitor Natura’s therapeutic activities clinically.
Our most important finding in this study is that Natura-alpha significantly inhibited FOXM1 in vivo as revealed by PPAA study demonstrating that significant repression of FOXM1 by Natura-alpha may be the primary cause of cell cycle arrest and inhibition of invasion by over-expression or siRNA to knockdown expression of FOXM1 in both LNCaP and LNCaP- AI cells. FOXM1 is a human proto-oncogene (42) and shown to regulate expression of a large array of G2/M-specific genes, such as Plk1, cyclin B2, Nek2 and CENPF, and plays an important role in maintenance of chromosomal segregation and genomic stability (43). In addition, FOXM1 interacts with the cell cycle-inhibitory pocket protein pRb and the cdk-activating phosphatase cdc25B in G1 and in G1/S, respectively (44). Since FOXM1 is essential for cancer cell viability, targeting FOXM1 has become a new strategy for developing novel anticancer drugs (45–46). Recent study showed that Iressa (Gefitinib) represses FOXM1 expression in breast cancer (47). Repression of FOXM1 by Natura-alpha primarily resulted in cell cycle arrest and inhibition of invasion, thus, FOXM1 may be one of critical targets of Natura-alpha against prostate cancer. Our data together strongly suggest that Natura-alpha could become a novel and effective therapeutic agent in treating hormone dependent and hormone refractory prostate cancer with minimal side effect.
Translational Relevance.
Prostate cancer (PC) is the most common cancer in men in the United States. The development of new effective therapeutic agents with minimal side effects for prostate cancer treatment is much needed. In this study, we demonstrate anti-prostate cancer properties of Natura-alpha in vitro, in vivo using nude mice xenograft models, as well as in a patient with advanced hormone refractory metastatic prostate cancer. Our findings revealed that anti-proliferation and anti-invasion activities of Natura-alpha on prostate cancer might primarily be through its down-regulation of Forkhead box M1 (FOXM1) protein. This study provides comprehensive evidence to support that Natura-alpha could serve as a novel and effective therapeutic agent for treatment of both hormone sensitive and hormone refractory prostate cancer in near future with minimal side effects.
Supplementary Material
Acknowledgements
We would like to thank Ms. X. Zhou for technical assistance. The study is supported by NYUSOM Urologic Center of Excellence and CTSI (1UL1RR029893) funds to PL; NYU CTSI TL1 fellowship (1UL1RR029893) to GD; Natrogen Therapeutics International, Inc. and James McCarron Jr. Medical Foundation.
Footnotes
Conflict of interest disclosure: Longgui Wang and Simon Mencher are employee of Natrogen Therapeutics.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009 doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Hartwell LH, Kastan MB. Cell cycle control and cancer. Science. 1994;266:1821–1828. doi: 10.1126/science.7997877. [DOI] [PubMed] [Google Scholar]
- 3.Spugnini EP, Campioni M, D'Avino A, Caruso G, Citro G, Baldi A. Cell-cycle molecules in mesothelioma: an overview. J Exp Clin Cancer Res. 2007;26:443–449. [PubMed] [Google Scholar]
- 4.Sharma PS, Sharma R, Tyagi R. Inhibitors of cyclin dependent kinases: useful targets for cancer treatment. Curr Cancer Drug Targets. 2008;8:53–75. doi: 10.2174/156800908783497131. [DOI] [PubMed] [Google Scholar]
- 5.Strauss M, Lukas J, Bartek J. Unrestricted cell cycling and cancer. Nat Med. 1995;1:1245–1246. doi: 10.1038/nm1295-1245. [DOI] [PubMed] [Google Scholar]
- 6.Boxem M. Cyclin-dependent kinases in C. elegans. Cell Div. 2006;1:6. doi: 10.1186/1747-1028-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee JC, Adams JL. Inhibitors of serine/threonine kinases. Curr Opin Biotechnol. 1995;6:657–661. doi: 10.1016/0958-1669(95)80108-1. [DOI] [PubMed] [Google Scholar]
- 8.Gray N, Detivaud L, Doerig C, Meijer L. ATP-site directed inhibitors of cyclin-dependent kinases. Curr Med Chem. 1999;6:859–875. [PubMed] [Google Scholar]
- 9.Chu IM, Hengst L, Slingerland JM. The Cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat Rev Cancer. 2008;8:253–267. doi: 10.1038/nrc2347. [DOI] [PubMed] [Google Scholar]
- 10.Senderowicz AM. Cyclin-dependent kinases as targets for cancer therapy. Cancer Chemother Biol Response Modif. 2002;20:169–196. [PubMed] [Google Scholar]
- 11.Godman J, Balk J. Genome analysis of Chlamydomonas reinhardtii reveals the existence of multiple, compartmentalized iron-sulfur protein assembly machineries of different evolutionary origins. Genetics. 2008;179:59–68. doi: 10.1534/genetics.107.086033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tse AN, Carvajal R, Schwartz GK. Targeting checkpoint kinase 1 in cancer therapeutics. Clin Cancer Res. 2007;13:1955–1960. doi: 10.1158/1078-0432.CCR-06-2793. [DOI] [PubMed] [Google Scholar]
- 13.Han R. Highlight on the studies of anticancer drugs derived from plants in China. Stem Cells. 1994;12:53–63. doi: 10.1002/stem.5530120110. [DOI] [PubMed] [Google Scholar]
- 14.Ji XJ, Liu XM, Li K, Chen RH, Wang LG. Pharmacological studies of meisoindigo: absorption and mechanism of action. Biomed Environ Sci. 1991;4:332–337. [PubMed] [Google Scholar]
- 15.Meijer LGN, Skaltsounis LA, Eisenbrand G, editors. Life in Progress. Roscoff, France: Place Georges Teissier; 2006. Indirbin, the red shade of indigo in editions. [Google Scholar]
- 16.Wang LG. Multiple faces of indirubin and its analogues in fighting cancer. Roscoff, France: 2006. aMS. [Google Scholar]
- 17.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 18.Gao M, Ossowski L, Ferrari AC. Activation of Rb and decline in androgen receptor protein precede retinoic acid-induced apoptosis in androgen-dependent LNCaP cells and their androgen-independent derivative. J Cell Physiol. 1999;179:336–346. doi: 10.1002/(SICI)1097-4652(199906)179:3<336::AID-JCP11>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 19.Wang LG, Ossowski L, Ferrari AC. Overexpressed androgen receptor linked to p21WAF1 silencing may be responsible for androgen independence and resistance to apoptosis of a prostate cancer cell line. Cancer Res. 2001;61:7544–7551. [PubMed] [Google Scholar]
- 20.Li Y, Wang L, Zhang M, Melamed J, Liu X, Reiter R, et al. LEF1 in androgen-independent prostate cancer: regulation of androgen receptor expression, prostate cancer growth, and invasion. Cancer Res. 2009;69:3332–3338. doi: 10.1158/0008-5472.CAN-08-3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu XM, Jiang JD, Ferrari AC, Budman DR, Wang LG. Unique induction of p21(WAF1/CIP1)expression by vinorelbine in androgen-independent prostate cancer cells. Br J Cancer. 2003;89:1566–1573. doi: 10.1038/sj.bjc.6601317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang LG, Liu XM, Chiao JW. Repression of androgen receptor in prostate cancer cells by phenethyl isothiocyanate. Carcinogenesis. 2006;27:2124–2132. doi: 10.1093/carcin/bgl075. [DOI] [PubMed] [Google Scholar]
- 23.Peng Y, Chen F, Melamed J, Chiriboga L, Wei J, Kong X, et al. Distinct nuclear and cytoplasmic functions of androgen receptor cofactor p44 and association with androgen-independent prostate cancer. Proc Natl Acad Sci U S A. 2008;105:5236–5241. doi: 10.1073/pnas.0712262105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cai CQ, Peng Y, Buckley MT, Wei J, Chen F, Liebes L, et al. Epidermal growth factor receptor activation in prostate cancer by three novel missense mutations. Oncogene. 2008;27:3201–3210. doi: 10.1038/sj.onc.1210983. [DOI] [PubMed] [Google Scholar]
- 25.Taneja S, MacGregor J, Markus S, Ha S, Mohr I. Enhanced antitumor efficacy of a herpes simplex virus mutant isolated by genetic selection in cancer cells. Proc Natl Acad Sci U S A. 2001;98:8804–8808. doi: 10.1073/pnas.161011798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chou TC, Motzer RJ, Tong Y, Bosl GJ. Computerized quantitation of synergism and antagonism of taxol, topotecan, and cisplatin against human teratocarcinoma cell growth: a rational approach to clinical protocol design. J Natl Cancer Inst. 1994;86:1517–1524. doi: 10.1093/jnci/86.20.1517. [DOI] [PubMed] [Google Scholar]
- 27.Tatsuda Y, Iguchi K, Usui S, Suzui M, Hirano K. Protein kinase C is inhibited by bisphosphonates in prostate cancer PC-3 cells. Eur J Pharmacol. 2009 doi: 10.1016/j.ejphar.2009.10.067. [DOI] [PubMed] [Google Scholar]
- 28.Willey CD, Xiao D, Tu T, Woon Kim K, Moretti L, Niermann KJ, et al. Enzastaurin (LY317615), a Protein Kinase C Beta Selective Inhibitor, Enhances Antiangiogenic Effect of Radiation. Int J Radiat Oncol Biol Phys. 2009 doi: 10.1016/j.ijrobp.2009.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007;1773:1263–1284. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sartor RB. Mechanisms of disease: pathogenesis of Crohn's disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:390–407. doi: 10.1038/ncpgasthep0528. [DOI] [PubMed] [Google Scholar]
- 31.Wierstra I, Alves J. FOXM1, a typical proliferation-associated transcription factor. Biol Chem. 2007;388:1257–1274. doi: 10.1515/BC.2007.159. [DOI] [PubMed] [Google Scholar]
- 32.Glatigny S, Blaton MA, Mencher SK, Mistou S, Lucas B, Fournier C, et al. Treatment of collagen-induced arthritis by Natura-alpha via regulation of Th-1/Th-17 responses. Eur J Immunol. 40:460–469. doi: 10.1002/eji.200939566. [DOI] [PubMed] [Google Scholar]
- 33.Lee M. Basic skills in interpreting laboratory data. 4th ed. Bethesda, Md.: American Society of Health-System Pharmacists; 2009. American Society of Health-System Pharmacists. [Google Scholar]
- 34.Leibovici D, Lindner A, Stay K, Zisman A. [Management of prostate cancer with indolent biological potential: from watchful waiting to active surveillance] Harefuah. 2006;145:763–767. 81, 80. [PubMed] [Google Scholar]
- 35.Bussemakers MJ, Van Bokhoven A, Tomita K, Jansen CF, Schalken JA. Complex cadherin expression in human prostate cancer cells. Int J Cancer. 2000;85:446–450. [PubMed] [Google Scholar]
- 36.Day ML, Zhao X, Vallorosi CJ, Putzi M, Powell CT, Lin C, et al. E-cadherin mediates aggregation-dependent survival of prostate and mammary epithelial cells through the retinoblastoma cell cycle control pathway. J Biol Chem. 1999;274:9656–9664. doi: 10.1074/jbc.274.14.9656. [DOI] [PubMed] [Google Scholar]
- 37.Tran NL, Nagle RB, Cress AE, Heimark RL. N-Cadherin expression in human prostate carcinoma cell lines. An epithelial-mesenchymal transformation mediating adhesion withStromal cells. Am J Pathol. 1999;155:787–798. doi: 10.1016/S0002-9440(10)65177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arya M, Bott SR, Shergill IS, Ahmed HU, Williamson M, Patel HR. The metastatic cascade in prostate cancer. Surg Oncol. 2006;15:117–128. doi: 10.1016/j.suronc.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 39.Hassan R, Bera T, Pastan I. Mesothelin: a new target for immunotherapy. Clin Cancer Res. 2004;10:3937–3942. doi: 10.1158/1078-0432.CCR-03-0801. [DOI] [PubMed] [Google Scholar]
- 40.Hassan R, Ho M. Mesothelin targeted cancer immunotherapy. Eur J Cancer. 2008;44:46–53. doi: 10.1016/j.ejca.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ordonez NG. Application of mesothelin immunostaining in tumor diagnosis. Am J Surg Pathol. 2003;27:1418–1428. doi: 10.1097/00000478-200311000-00003. [DOI] [PubMed] [Google Scholar]
- 42.Myatt SS, Lam EW. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 2007;7:847–859. doi: 10.1038/nrc2223. [DOI] [PubMed] [Google Scholar]
- 43.Laoukili J, Kooistra MR, Bras A, Kauw J, Kerkhoven RM, Morrison A, et al. FoxM1 is required for execution of the mitotic programme and chromosome stability. Nat Cell Biol. 2005;7:126–136. doi: 10.1038/ncb1217. [DOI] [PubMed] [Google Scholar]
- 44.Major ML, Lepe R, Costa RH. Forkhead box M1B transcriptional activity requires binding of Cdk-cyclin complexes for phosphorylation-dependent recruitment of p300/CBP coactivators. Mol Cell Biol. 2004;24:2649–2661. doi: 10.1128/MCB.24.7.2649-2661.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Radhakrishnan SK, Bhat UG, Hughes DE, Wang IC, Costa RH, Gartel AL. Identification of a chemical inhibitor of the oncogenic transcription factor forkhead box M1. Cancer Res. 2006;66:9731–9735. doi: 10.1158/0008-5472.CAN-06-1576. [DOI] [PubMed] [Google Scholar]
- 46.Gartel AL. FoxM1 inhibitors as potential anticancer drugs. Expert Opin Ther Targets. 2008;12:663–665. doi: 10.1517/14728222.12.6.663. [DOI] [PubMed] [Google Scholar]
- 47.McGovern UB, Francis RE, Peck B, Guest SK, Wang J, Myatt SS, et al. Gefitinib (Iressa) represses FOXM1 expression via FOXO3a in breast cancer. Mol Cancer Ther. 2009;8:582–591. doi: 10.1158/1535-7163.MCT-08-0805. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.