Abstract
Alloreactivity is the response of T cells to MHC molecules not encountered during thymic development. A small population (1–8%) of peripheral T cells in mice and humans express two TCR due to incomplete allelic exclusion of TCRα, and we hypothesized they are highly alloreactive. FACS analysis of mouse T cell MLR revealed increased dual TCR T cells amongst alloreactive cells. Quantitative assessment of the alloreactive repertoire demonstrated a nearly 50% reduction in alloreactive T cell frequency amongst T cells incapable of expressing secondary TCR. We directly demonstrated expansion of the alloreactive T cell repertoire at the single cell level by identifying a dual TCR T cell with distinct alloreactivities for each TCR. The importance of dual TCR T cells is clearly demonstrated in a parent into F1 model of GvHD, where dual TCR T cells comprised up to 60% of peripheral activated T cells, demonstrating a disproportionate contribution to disease.
Keywords: T cell receptor, graft versus host disease, alloreactivity
Introduction
An unresolved question in alloreactivity involves how the naive T cell repertoire, shaped during thymic development to interact with self MHC, reacts 100- to 1000-fold more frequently with allogeneic MHC. The strong reactivity toward allogeneic MHC has led to many models proposed to explain the nature of this interaction, ranging from degenerate recognition of MHC by germline TCR elements (1, 2) to focus on presented peptides (3). Several studies have provided evidence for TCR germline affinity for MHC (1, 2, 4) though we have shown that alloreactive TCRs are capable of polyspecificity, recognizing multiple distinct peptides presented by allogeneic MHC (5). The few available TCR-peptide-allogeneic MHC co-crystal structures demonstrate that alloreactivity is generally similar to conventional recognition of foreign peptide presented by self MHC (6, 7), though functional analysis demonstrates increased flexibility in docking angle and energetic footprint (8, 9). This breadth of potential interactions helps explain the high alloreactivity of naive T cells.
A small population (1–8%) of peripheral T cells in humans and mice express a second TCRα capable of pairing with the same TCRβ to form functional secondary TCR heterodimers detectable on the cell surface (10–12). Though determination of the precise number of dual TCR T cells in the periphery is hampered by limited anti-Vα monoclonal antibodies (mAbs), these studies clearly demonstrate that a small number of peripheral T cells defy the “one cell, one receptor” maxim. Expression of 2 TCRα results from continuous rearrangement of both TCRα loci throughout T cell development until positive selection, contrasting with tight allelic exclusion of TCRβ (13–16). While it is estimated that 30% of mature peripheral T cells in humans and mice contain two successfully rearranged TCRα genes, only 1–8% express a secondary TCR at the cell surface (10–12, 17), attributed to preferential pairing between TCRα and β chains leading to a primary dominant TCR heterodimer and a rapidly degraded unpaired TCRα (11, 13, 14, 18). However, both TCRs on the surface of dual TCR T cells are functional and capable of contributing to immune responses (10, 19). We hypothesize that secondary TCRs have a substantial role in broadening the alloreactive T cell repertoire; not only could expression of a second TCR double the chance to recognize allogeneic MHC, but secondary TCR are not required to undergo thymic selection, giving them a potentially broader repertoire (10, 19–21).
Materials and methods
Mice
B6 (H-2b), B6.Ly5.1, B6.K (H-2k), BALB/c (H-2d), I/LnJ (H-2j), RIIIS/J (H-2r), SM/J (H-2v), DBA1/J (H-2q), PL/J (H-2u), B6.P (H-2p), and B6.G7 (H-2g7) mice were originally purchased from The Jackson Laboratory. Mice heterozygous for TCRA-C, incapable of expressing 2 TCRα chains, were derived by crossing TCRA-C−/− B6 mice with B6 mice. All mice were bred and housed in specific pathogen-free conditions at the animal facility at the Washington University Medical Center. All use of laboratory animals was approved and performed in accordance with the Washington University Division of Comparative Medicine.
Mixed lymphocyte culture
T cells from B6 and B6.Cα+/− mice were purified from collected splenocytes and lymph node cells by magnetic bead separation using anti-CD4 and anti-CD8 microbeads and LS positive selection columns according to manufacturer protocol (Miltenyi Biotech). Purified T cells (1 × 106) were labeled with 5 μM CFSE (Invitrogen) and cultured at 1:1 ratio with irradiated B6.K and BALB/c splenocytes or with 0.1 μg plate-bound anti-CD3 (2C11) and anti-CD28 (37.51) mAbs (Biolegend) in 2 ml RPMI 1640 (Invitrogen) supplemented with 10% FCS (Hyclone) for 4 days. Cells were subsequently analyzed by flow cytometry or ELISPOT.
Flow cytometry
Collected cells were labeled with PE-labeled anti-CD8 (53-6.7), AlexaFluor 647-labeled anti-Vα2 (B20.1) (Biolegend), and AlexaFluor 750-labeled anti-CD4 (RM4.5, eBioscience), and biotinylated anti-Vα3 (RR3-16), -Vα8 (KT50), or -Vα11 (RR8-1) in conjunction with streptavadin-PE Cy7 (BD). Ly5.1 T cells were labeled with FITC-labeled anti-CD45.1+ (A20, Biolegend). All samples were analyzed using a FACSCanto (BD) with calculated compensation, and data were analyzed using FlowJo software (Tree Star, Inc.).
ELISPOT
Alloreactive T cell frequency was assessed by 48 h MLR performed in replicates of 6 in 96-well Multiscreen IP plates (Millipore) coated with purified anti-IFN-γ (RA-6A2, eBioscience), and labeled with biotinylated anti-IFN-γ (XMG1.2) and streptavadin-conjugated HRP (Southern Biotech). Plates were developed with BCIP/NBT (Sigma), read on a CTL Immunospot reader, and data analysis performed using CTL Immunospot 4.0 (Cellular Technology Ltd.).
Vα2 2.102Vβ TCR T cell hybrids
The secondary Vα2, Vβ1 TCR from the 2.102 T cell was cloned from cDNA library by PCR and the two TCR chains were linked by extension PCR to add cloning sites and viral P2A cleavage site (22). Polycistronic TCR construct was cloned into an IRES-linked GFP retroviral expression vector, GFP-RV (gift of Dr. K. Murphy, Washington University), and 30 μg DNA with Lipofectamine 2000 (Invitrogen) was transfected into Platinum-E packaging cell line (gift of Dr. T Kitamura, University of Tokyo) to generate retroviral TCR construct virus. Retroviral TCR construct virus was used to transfect 58α-β-CD4+ T cell hybrids, selected for GFP expression using a FACSVantage cell sorter (BD) at the Washington University Department of Pathology and Immunology Cell Sorting Facility. T cell hybrids were tested for alloreactivity by triplicate culture of 1.0×105 hybrid cells with 1.0 × 106 irradiated splenocytes for 24 hours and measurement of IL-2 by ELISA.
IL-2 measurement
IL-2 in culture supernatant was measured by ELISA, using 100μg/well capture anti-IL-2 mAb (JES6-1A12, Biolegend), 50μg/well biotinylated-anti-IL-2 detection mAb (JES6-5H4, Biolegend), 100μl/well 1/10,000 dilution streptavadin-HRP (Southern Biotech), developed using 100μl/well 1-Step-Ultra TMB substrate, stopped at 15 min by addition of 100μl/well 2 M sulfuric acid, and assessed using a Victor3 plate reader (Perkin Elmer). IL-2 concentrations were calculated by linear regression from concurrent IL-2 standard curves.
Graft-vs.-host disease
Graft-vs.-host disease was induced by intravenous transfer of 2.0 × 107 BMC and 2.5 × 107 splenocytes from 6–8 week B6 Ly5.1+ donor mice into lethally irradiated (10 Gy) B6 or (B6 × CBA)F1 recipients. Disease progression was monitored by daily observation and weekly measurement of mouse weight. Mice were sacrificed at 80% of original weight, and peripheral T cells were collected from spleens and lymph nodes and analyzed by flow cytometry.
Statistics
All data were analyzed non-parametrically by Mann-Whitney U-test using Prism 4 software (Graph Pad, Inc.). p values < 0.05 were considered significant.
Results and Discussion
Dual TCR T cells are involved in in vitro alloreactivity
To observe involvement of dual TCR T cells in alloreactivity in vitro, we FACS analyzed 4 day MLR cultures using the 4 existing anti-mouse Vα mAbs. CFSE-labeled T cells from B6 mice were stimulated with anti-CD3 and anti-CD28 mAbs or irradiated splenocytes from B6.K (H-2k) and BALB/c (H-2d) mice, and responding T cells stained with anti-Vα2 mAb in combination with anti-Vα3, anti-Vα8, or anti-Vα11 mAbs. Dual TCR T cells comprised a significant population among T cells that had divided during MLR in response to allogeneic stimulation with either H-2k or H-2d (Fig. 1A). To directly compare the frequency of dual TCR T cells, cell numbers were normalized to Vα2+ T cells, the largest population of T cells amongst the 4; significantly more alloreactive Vα2+ T cells expressed a second TCRα as compared to non-divided T cells (Fig. 1B). The increased frequency of dual TCR T cells among alloreactive T cells was consistent when examining CD4+ T cells (Fig 1C). MLR culture conditions, without the addition of IL-2, favor CD4+ T cells; therefore too few CD8+ T cells were observed amongst the alloreactive T cells precluding accurate assessment of dual TCR T cell frequency (data not shown). Increased dual TCRα frequency was not observed following stimulation with anti-CD3 and anti-CD28 mAbs (Figs. 1B and 1C, p = 0.18–0.10), demonstrating that this phenomenon is unique to alloreactivity. The consistent increase in dual TCR T cells among T cells dividing in response to stimulation with either H-2k or H-2d demonstrates that this reflects a fundamentally high degree of alloreactivity in dual TCR T cells not limited to a single MHC haplotype.
FIGURE 1.
Dual TCR T cells are increased among alloreactive T cells. CFSE-labeled T cells from B6 (H-2b) mice cultured in MLR with irradiated splenocytes from either CBA (H-2k) or BALB/c (H-2d) mice or anti-CD3 and anti-CD28 mAbs for 4 d were analyzed by flow cytometry for the presence of dual TCR T cells. Cells were gated on CD4+ and CD8+ T cells, and alloreactive T cells were distinguished by gating on cells with diluted CFSE. A, Dual TCR T cells were consistently observed in higher frequency among T cells that had divided in response to allogeneic stimulation as compared to undivided controls. FACS data of dual TCRα T cells from a representative experiment with allogeneic stimulation by B6.K splenocytes show the increased dual TCRα T cells among T cells that had divided in response to allogeneic stimulation as measured by CFSE dilution. B, To compare the frequency of T cells expressing dual TCRα between populations and 5 independent experiments, T cell numbers were normalized to Vα2+ cells, demonstrating a consistent and significant (mean ± s.d. * p < 0.05 ** p < 0.01) increase in dual TCR T cells among alloreactive T cells. C, Increased dual TCR T cells were also consistently and significantly (mean ± s.d. * p < 0.05 ** p < 0.01) observed among alloreactive CD4+Vα2+ T cells. Stimulation with anti-CD3 and anti-CD28 mAbs did not result in significantly increased dual TCRα T cells (p = 0.18–0.10).
Dual TCR T cells contribute disproportionately to the alloreactive T cell repertoire
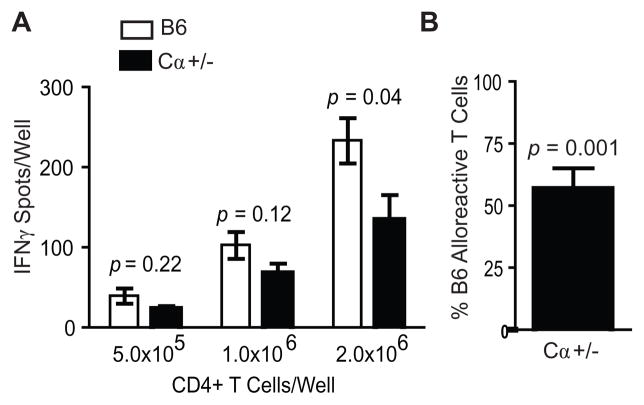
To assess quantitatively the contribution of secondary TCRα to the alloreactive T cell repertoire, we compared the frequency of alloreactive T cells from mice incapable of expressing two functional TCRα chains (B6.Cα+/−, heterozygous for a knockout allele of the gene encoding the constant region of TCRα) with the frequency of alloreactive T cells from B6 mice. Alloreactive CD4+ T cell frequency was assessed by ELISPOT for IFN-γ production following MLR against B6.K splenocytes. T cells from B6.Cα+/− mice consistently demonstrated a 35–65% decrease in alloreactive frequency (representative experiment shown Fig. 2A). Integration of multiple responder T cell concentrations from six independent experiments demonstrated a profound and highly statistically significant decrease of nearly 50% of the naive alloreactive T cell frequency in B6.Cα+/− mice (Fig. 2B). The dramatic nature of the contribution by secondary TCRα was unexpected, as several previous reports (10–12) and our own observations (Fig. 1) demonstrated expression of secondary TCRα on the cell surface on only 1–8% of peripheral T cells.
FIGURE 2.
Secondary TCRα contribute nearly half of the naive alloreactive T cell repertoire. A, Alloreactive T cell frequency of CD4+ T cells from B6 mice and from B6.Cα+/−mice incapable of expressing secondary TCRα were measured by ELISPOT analysis for IFN-γ production following 48 h MLR with irradiated B6.K splenocytes. Data from a representative experiment illustrates the consistent 40–60% reduction in alloreactive T cell frequency among CD4+ T cells from B6.Cα+/−mice. B, Integration of multiple cell concentrations from 6 independent experiments by normalizing the frequency of CD4+ T cell alloreactivity as a percentage of the alloreactivity of CD4+ T cells from B6 mice demonstrates the consistent and significant (mean ± s.d. p = 0.001) reduction in alloreactivity among CD4+ T cells from B6.Cα+/− mice.
Demonstration of secondary TCR expanding the alloreactive repertoire
Expansion of the alloreactive T cell repertoire by secondary TCRα was explored directly at the single cell level using a T cell clone, 2.102, reactive to Hb/I-Ek and alloreactive to I-Ep (23). We initially identified a Vα2, Vβ1 TCR from the 2.102 T cell clone and expressed it transgenically in mice. However, T cells from the mouse were not reactive to Hb/I-Ek (24); we subsequently discovered that reactivity to Hb/I-Ek and Ep utilized a Vα4 TCRα pairing with the identified Vβ1 TCRβ. The Vα2, Vβ1 TCR was a secondary TCR expressed on 2.102 T cells, allowing us the opportunity to explore the role of a secondary TCR in alloreactivity at the single cell level.
To test the role of this secondary TCRα in positive selection, we crossed the Vα2, Vβ1 TCR transgenic mouse onto a B6.K RAG-1−/− background. Analysis of thymi from Vα2, Vβ1 B6.K RAG-1−/− mice demonstrated no SP thymocytes, and essentially all T cells were DP, indicating a lack of positive selection of the expressed Vα2 TCR (Figs. 3A and 3B). Consistent with the lack of positive selection, there were very few peripheral T cells (data not shown), providing direct evidence that the Vα2, Vβ1 TCR was not involved in positive selection of the 2.102 T cell.
FIGURE 3.
2.102 secondary TCRα generates a non-thymically-selected TCR with specific and distinct alloreactivity. A secondary Vα2, Vβ1 TCR was identified from the Vα4, Vβ1 expressing 2.102 T cell clone. A, The Vα2, Vβ1 secondary TCR is not thymically selected, as RAG−/− mice transgenic for this receptor demonstrate thymocytes arrested at the double positive stage of development, and no peripheral αβ T cells (data not shown). B, Double positive thymocytes express the transgenic Vα2. C, The secondary Vα2, Vβ1 TCR demonstrates specific alloreactivity against H-2d, distinct from the alloreactivity against H-2Ep demonstrated by the primary Vα4, Vβ1 2.102 TCR. T cell hybrids expressing the secondary Vα2, Vβ1 TCR were tested for reactivity against a panel of allogeneic irradiated splenocytes, and T cell hybrid reactivity was assessed by measurement of IL-2 production by ELISA. T cell hybrids expressing the Vα2 secondary TCR were specifically reactive to H-2d APC, but not to H-2p APC, demonstrating a distinct alloreactivity (mean ± s.d. p = 0.05).
To identify the potential alloreactivity of the Vα2, Vβ1 TCR, we expressed it in a TCR-negative hybridoma using a retrogenic TCR expression construct (22). The T cell hybrid expressing the Vα2, Vβ1 secondary TCR was tested for alloreactivity against a panel of 8 different allogeneic H-2 splenocytes (H-2b,d,g7,j,k,p,q,r,u,v), with the only alloreactivity observed against H-2d stimulation (data not shown). Remarkably, the Vα2, Vβ1 TCR was specifically alloreactive against H-2d, but not against any other allotype, including H-2p (Fig. 3C). Thus, the 2.102 T cell is positively selected by the Vα4, Vβ1 TCR, specific for Hb/I-Ek and alloreactive to I-Ep, while the secondary Vα2, Vβ1 TCR is not positively selected in an H-2k thymus, but is alloreactive to H-2d. These findings directly demonstrate expansion of the alloreactive repertoire of an individual T cell by expression of a secondary TCRα.
Dual TCR T cells are dramatically involved in graft vs. host disease
To investigate dual TCR T cells in in vivo alloreactivity, we utilized a parent into F1 model of GvHD. Lethally-irradiated (B6 × CBA)F1 or B6 mice were reconstituted with 2.0 × 107 bone marrow cells (BMC) and 2.5 × 107 splenocytes from B6 Ly5.1+ mice. Within 4 weeks of cell transfer, F1 mice developed clinically-significant GvHD, manifested as weight loss, hunching, hair loss, and lymphocytic inflitration of the gastrointestinal tract (data not shown). Upon development of clinically significant GvHD, mice were sacrificed and T cells from spleens and lymph nodes were examined by FACS for dual TCRα. Dual TCR T cells were readily identified among transferred Ly5.1+ T cells in both F1 and control B6 mice, though F1 recipients had consistently more dual TCR T cells (Figs. 4A and 4C). Dual TCRα T cells from mice with GvHD were more likely to be activated, as evidenced by down regulation of TCR on the cell surface (Fig. 4A) and increased cell size (Fig. 4B). F1 recipients demonstrated a range of dual TCR T cells, with 6–75% of Vα2+Ly5.1+ T cells expressing a second TCRα, contrasting dramatically with only 1–6% of Vα2+Ly5.1+ T cells in B6 recipients expressing a second TCRα, similar to the normal frequency of dual TCR T cells in the periphery (Fig. 4C). This increased frequency of dual TCR T cells was consistent in both CD4+ (Fig. 4D) and CD8+ T cells (Fig. 4E) from mice with GvHD. The dramatic increase of dual TCR T cells, having an activated phenotype, specifically in mice with GvHD demonstrates that dual TCR T cells are involved in in vivo alloreactivity. While these findings demonstrate that dual TCRα T cells are significantly involved in GvHD, they do not preclude involvement from conventional single TCR T cells; T cells incapable of expressing dual TCRα still have a high, though reduced, frequency of alloreactive T cells (Fig. 2) expected to contribute to GvHD. The quantitative and qualitative contribution of dual TCRα T cells to GvHD are not clear, though our findings would predict that T cells incapable of expressing dual TCRα may have a reduced capacity to induce GvHD, owing to decreased alloreactive precursor frequency. Observing subtle differences in the development of clinical symptoms of GvHD is problematic, as differences in alloreactivity could be normalized during the 4 weeks required for development of clinical symptoms in our model. However, we predict that examination at earlier time points would reveal the contribution of dual TCR T cells to GvHD development, and are currently pursuing development of early markers of GvHD to facilitate this analysis.
FIGURE 4.
Dual TCR T cells are profoundly increased among T cells mediating GvHD. B6 Ly5.1+ bone marrow cells and splenocytes were transferred into lethally irradiated (B6 × CBA)F1 or control irradiated B6 mice. Lymphocytes were collected from spleens and lymph nodes and analyzed by flow cytometry using the 4 available Vα mAbs. A, Mice with GvHD demonstrated dramatically increased numbers of dual TCR T cells by FACS analysis compared to control B6 mice. FACS analysis of representative mice illustrate markedly increased dual TCR T cell frequency in mice with GvHD. Dual TCR T cells from mice with GvHD also exhibited down-regulation of their TCR, consistent with T cell activation. B, Dual TCR T cells from mice with GvHD are larger as measured by forward scatter, consistent with activation. C, Normalization of Vα2+ T cells expressing a second TCRα quantifies the dramatic increase in dual TCR T cells in mice with GvHD. Significantly more Vα2+ T cells from mice with GvHD expressed a second TCRα (mean ± s.d * p < 0.05 ** p < 0.01). D, The increase in dual TCR T cells was also observed among both alloreactive CD4+Vα2+ T cells and E, CD8+Vα2+ T cells.
Concluding remarks
Our alloreactivity model suggesting that energetic contributions to binding are required from both germline and non-germline TCR elements does not directly predict the origin of the paradoxically high degree of alloreactivity among naive T cells. We hypothesize that the high frequency of alloreactive T cells results from multiple sources, including degenerate interactions with allogeneic MHC and polyspecific peptide-MHC interactions (8). The work presented here describes a third contributor to the high frequency of alloreactive naive T cells, as non-thymically selected secondary TCR demonstrate a remarkably high frequency of alloreactivity and disproportionately contribute to the alloreactive T cell repertoire. The importance of alloreactivity is emphasized by the important influence of HLA matching and strong immunosuppression required for prevention of immunologic rejection in allogeneic transplantation. Advances in defining the molecular basis for alloreactivity and its relation to disease are necessary for continual improvement of clinical outcomes. Further testing of the mechanistic role of dual TCR T cells in mediating in vivo alloreactivity is warranted, both for improving our fundamental understanding of the nature of disease mediated by alloreactivity as well as potential for a target for immune manipulation during transplantation.
Acknowledgments
This work was supported by the National Institutes of Health AI-061173 (P.M.A.) and the W.M.Keck Foundation Fellowship in Molecular Medicine (G.P.M.).
We thank C. Hsieh, B. Sleckman, E. Unanue, D. Donermeyer, C. Hickman, and S. Weber for critical review of the manuscript, T. Mohanakumar for use of the Elispot Reader, T. Stappenbeck for assistance in pathology analysis, and D. Kreamalmeyer for maintenance of the mouse colony.
References
- 1.Zerrahn J, Held W, Raulet DH. The MHC reactivity of the T cell repertoire prior to positive and negative selection. Cell. 1997;88:627–636. doi: 10.1016/s0092-8674(00)81905-4. [DOI] [PubMed] [Google Scholar]
- 2.Huseby ES, White J, Crawford F, Vass T, Becker D, Pinilla C, Marrack P, Kappler JW. How the T cell repertoire becomes peptide and MHC specific. Cell. 2005;122:247–260. doi: 10.1016/j.cell.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Bevan M. High determinant density may explain the phenomenon of alloreactivity. Immunol Today. 1984;5:128–130. doi: 10.1016/0167-5699(84)90233-0. [DOI] [PubMed] [Google Scholar]
- 4.Ignatowicz L, Kappler J, Marrack P. The repertoire of T cells shaped by a single MHC/peptide ligand. Cell. 1996;84:521–529. doi: 10.1016/s0092-8674(00)81028-4. [DOI] [PubMed] [Google Scholar]
- 5.Felix NJ, Donermeyer DL, Horvath S, Walters JJ, Gross ML, Suri A, Allen PM. Alloreactive T cells respond specifically to multiple distinct peptide-MHC complexes. Nat Immunol. 2007;8:388–397. doi: 10.1038/ni1446. [DOI] [PubMed] [Google Scholar]
- 6.Housset D, Malissen B. What do TCR-pMHC crystal structures teach us about MHC restriction and alloreactivity? Trends Immunol. 2003;24:429–437. doi: 10.1016/s1471-4906(03)00180-7. [DOI] [PubMed] [Google Scholar]
- 7.Rudolph MG, Stanfield RL, Wilson IA. How TCRs bind MHCs, peptides, and coreceptors. Annu Rev Immunol. 2006;24:419–466. doi: 10.1146/annurev.immunol.23.021704.115658. [DOI] [PubMed] [Google Scholar]
- 8.Felix NJ, Allen PM. Specificity of T-cell alloreactivity. Nat Rev Immunol. 2007;7:942–953. doi: 10.1038/nri2200. [DOI] [PubMed] [Google Scholar]
- 9.Colf LA, Bankovich AJ, Hanick NA, Bowerman NA, Jones LL, Kranz DM, Garcia KC. How a single T cell receptor recognizes both self and foreign MHC. Cell. 2007;129:135–146. doi: 10.1016/j.cell.2007.01.048. [DOI] [PubMed] [Google Scholar]
- 10.Padovan E, Casorati G, Dellabona P, Meyer S, Brockhaus M, Lanzavecchia A. Expression of two T cell receptor α chains: dual receptor T cells. Science. 1993;262:422–424. doi: 10.1126/science.8211163. [DOI] [PubMed] [Google Scholar]
- 11.Alam SM, Gascoigne NR. Posttranslational regulation of TCR Vα allelic exclusion during T cell differentiation. J Immunol. 1998;160:3883–3890. [PubMed] [Google Scholar]
- 12.Corthay A, Nandakumar KS, Holmdahl R. Evaluation of the percentage of peripheral T cells with two different T cell receptor α-chains and of their potential role in autoimmunity. J Autoimmun. 2001;16:423–429. doi: 10.1006/jaut.2001.0504. [DOI] [PubMed] [Google Scholar]
- 13.Alam SM, I, Crispe N, Gascoigne NR. Allelic exclusion of mouse T cell receptor α chains occurs at the time of thymocyte TCR up-regulation. Immunity. 1995;3:449–458. doi: 10.1016/1074-7613(95)90174-4. [DOI] [PubMed] [Google Scholar]
- 14.Malissen M, Trucy J, Letourneur F, Rebai N, Dunn DE, Fitch FW, Hood L, Malissen B. A T cell clone expresses two T cell receptor α genes but uses one αβ heterodimer for allorecognition and self MHC-restricted antigen recognition. Cell. 1988;55:49–59. doi: 10.1016/0092-8674(88)90008-6. [DOI] [PubMed] [Google Scholar]
- 15.Turka LA, Schatz DG, Oettinger MA, Chun JJ, Gorka C, Lee K, McCormack WT, Thompson CB. Thymocyte expression of RAG-1 and RAG-2: termination by T cell receptor cross-linking. Science. 1991;253:778–781. doi: 10.1126/science.1831564. [DOI] [PubMed] [Google Scholar]
- 16.Petrie HT, Livak F, Schatz DG, Strasser A, Crispe IN, Shortman K. Multiple rearrangements in T cell receptor α chain genes maximize the production of useful thymocytes. J Exp Med. 1993;178:615–622. doi: 10.1084/jem.178.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casanova JL, Romero P, Widmann C, Kourilsky P, Maryanski JL. T cell receptor genes in a series of class I major histocompatibility complex-restricted cytotoxic T lymphocyte clones specific for a Plasmodium berghei nonapeptide: implications for T cell allelic exclusion and antigen-specific repertoire. J Exp Med. 1991;174:1371–1383. doi: 10.1084/jem.174.6.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuida K, Furutani-Seiki M, Saito T, Kishimoto H, Sano K, Tada T. Post-translational attainment of allelic exclusion of the T cell receptor α chain in a T cell clone. Int Immunol. 1991;3:75–82. doi: 10.1093/intimm/3.1.75. [DOI] [PubMed] [Google Scholar]
- 19.He X, Janeway CA, Jr, Levine M, Robinson E, Preston-Hurlburt P, Viret C, Bottomly K. Dual receptor T cells extend the immune repertoire for foreign antigens. Nat Immunol. 2002;3:127–134. doi: 10.1038/ni751. [DOI] [PubMed] [Google Scholar]
- 20.Hardardottir F, Baron JL, Janeway CA., Jr T cells with two functional antigen-specific receptors. Proc Natl Acad Sci U S A. 1995;92:354–358. doi: 10.1073/pnas.92.2.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zal T, Weiss S, Mellor A, Stockinger B. Expression of a second receptor rescues self-specific T cells from thymic deletion and allows activation of autoreactive effector function. Proc Natl Acad Sci U S A. 1996;93:9102–9107. doi: 10.1073/pnas.93.17.9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holst J, Szymczak-Workman AL, Vignali KM, Burton AR, Workman CJ, Vignali DA. Generation of T-cell receptor retrogenic mice. Nat Protoc. 2006;1:406–417. doi: 10.1038/nprot.2006.61. [DOI] [PubMed] [Google Scholar]
- 23.Daniel C, Grakoui A, Allen PM. Inhibition of an in vitro CD4+ T cell alloresponse using altered peptide ligands. J Immunol. 1998;160:3244–3250. [PubMed] [Google Scholar]
- 24.Hsu BL, Evavold BD, Allen PM. Modulation of T cell development by an endogenous altered peptide ligand. J Exp Med. 1995;181:805–810. doi: 10.1084/jem.181.2.805. [DOI] [PMC free article] [PubMed] [Google Scholar]