Abstract
In neurons, the axon initial segment (AIS) is a specialised region near the start of the axon that is the site of action potential initiation1-6. The precise location of the AIS varies across and within different neuronal types7,8, and has been linked to cells’ information-processing capabilities8; however, the factors determining AIS position in individual neurons remain unknown. Here we show that changes in electrical activity can alter the location of the AIS. In dissociated hippocampal cultures, chronic depolarization with high potassium moves multiple components of the AIS, including voltage-gated sodium channels, up to 17 μm away from the soma of excitatory neurons. This movement reverses when neurons are returned to non-depolarized conditions, and depends upon the activation of T- and/or L-type voltage-gated calcium channels. The AIS also moved distally when we combined long-term LED (light-emitting diode) photostimulation with sparse neuronal expression of the light-activated cation channel channelrhodopsin-2; here, burst patterning of activity was successful where regular stimulation at the same frequency failed. Furthermore, changes in AIS position correlate with alterations in current thresholds for action potential spiking. Our results show that neurons can regulate the position of an entire subcellular structure according to their ongoing levels and patterns of electrical activity. This novel form of activity-dependent plasticity may fine-tune neuronal excitability during development.
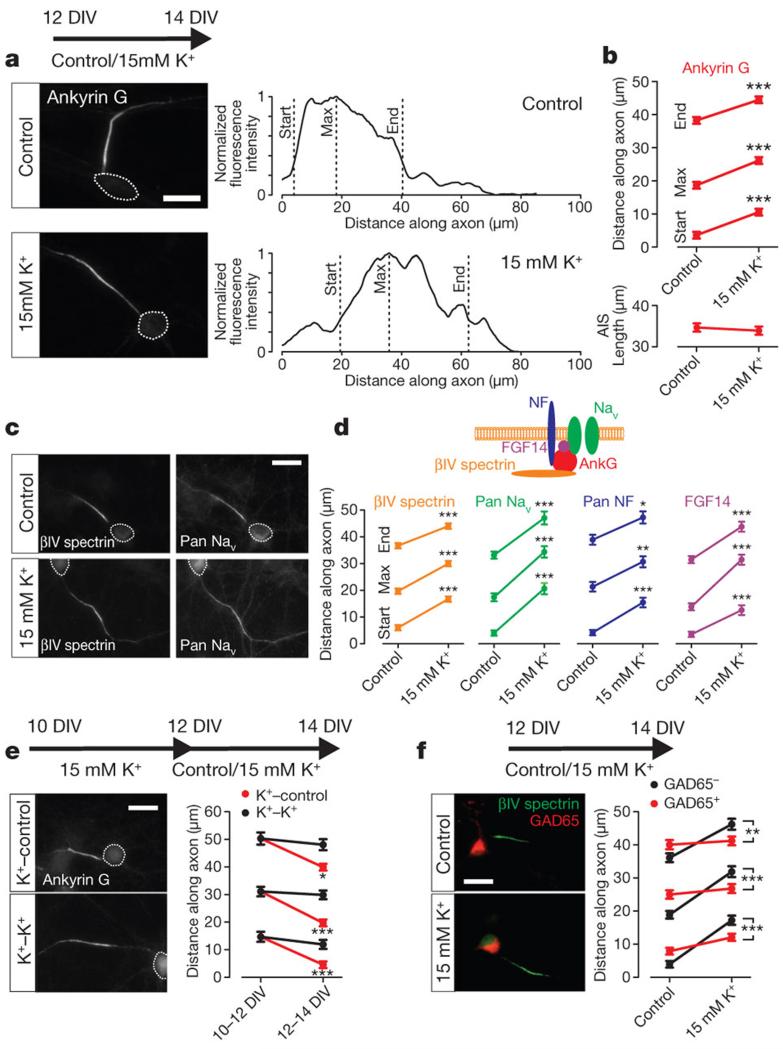
In dissociated hippocampal neurons, global depolarization with 15 mM extracellular potassium from 12 to 14 days in vitro (DIV) produced a significant distal shift in AIS location (Fig. 1a-d and Supplementary Figs 1-3). Labelling for the AIS scaffolding protein ankyrin G9, showed that start, maximum, and end AIS positions were all significantly relocated away from the soma (Fig. 1a,b; start: Mann-Whitney U-test, P < 0.0001; maximum: P < 0.0001; end: P < 0.0001; n = 885 cells, 36 coverslips), leaving the length of the AIS unchanged (Fig. 1a,b; P = 0.11). We also observed significant activity-dependent distal shifts in other AIS-specific proteins10,11, including the scaffolding protein βIV spectrin (Fig. 1c,d; start: Mann-Whitney U-test, P < 0.0001; maximum: P < 0.0001; end: P < 0.0001; n = 1065 cells, 44 coverslips), the extracellular matrix binding protein neurofascin (Fig. 1d; start: Mann-Whitney U-test, P < 0.0001; maximum: P = 0.002; end: P = 0.01; n = 96 cells, 4 coverslips), the ion channel-associated protein FGF14 (Fig. 1d; start: Mann-Whitney U-test, P < 0.0001; maximum: P < 0.0001; end: P < 0.0001; n = 89 cells, 4 coverslips), and, vitally, the voltage-gated sodium channels (VGSCs) essential for action potential initiation, stained with a Pan-VGSC (PanNav) antibody4,12 (Fig. 1c,d; PanNav start: Mann-Whitney U-test, P < 0.0001; maximum: P < 0.0001; end: P < 0.0001; n = 95 cells, 4 coverslips). This distal shift of up to 17 μm (Fig. 1d) was not accompanied by any changes in PanNav immunofluorescence intensity (Supplementary Fig. 4). In addition, AIS relocation occurs without any changes in soma size (Supplementary Fig. 5), requires long-term depolarization (Supplementary Fig. 6), and is sensitive to K+ changes over a relatively small range (Supplementary Fig. 7). It also operates throughout development: activity-dependent relocation occurred at 5-7 DIV (Supplementary Fig. 8), only shortly after the AIS has properly formed13.
Figure 1. Activity-dependent changes in AIS position.
a, Ankyrin G label in control and 15 mM K+ conditions. Right, fluorescence intensity along the axon. Dotted lines indicate soma. b, Ankyrin G positions and length (885 cells, 36 coverslips). c, βIV-spectrin and PanNav label. d, Positions for βIV-spectrin (1065 cells, 44 coverslips), PanNav (95 cells, 4 coverslips), Pan-neurofascin (NF; 96 cells, 4 coverslips), and FGF-14 (89 cells, 4 coverslips). e, Ankyrin G label after recovery from (K+-control), or continued (K+-K+) depolarization (194 cells, 8 coverslips). f, βIV spectrin label in GAD65+positive neurons (200 cells, 8 coverslips). All scale bars indicate 20 μm; *P < 0.05; **, P < 0.01; ***, P < 0.001. All plots show mean ± s.e.m.
The proximal position of the AIS in our control cultures precluded attempts to move the structure proximally with decreasing activity. However, after 15 mM K+ treatment from 10 to12 DIV, (start, maximum and end positions ≥ 11 μm distal vs control, Mann-Whitney U-test, P < 0.0001), returning cultures to control medium (5 mM K+) from 12 to 14 DIV produced significant proximal AIS movement (Fig. 1e; ankyrin G start: Dunn post test vs 10-12 DIV K+ following Kruskal-Wallis ANOVA, P < 0.0001; maximum: P < 0.0001; end: P < 0.05; n = 194 cells, 8 coverslips). Neurons can therefore respond to both increases and decreases in ongoing activity levels with distal or proximal changes in AIS position.
AIS plasticity was much more marked in excitatory neurons, with 15 mM K+ treatment producing significantly larger distal shifts in AIS position in non-GABAergic (GAD65-negative) cells (Fig. 1f;. βIV spectrin start: effect of cell type×treatment interaction in 2-way ANOVA on ranks14, P < 0.0001; maximum: P < 0.0001; end: P = 0.006; n = 200 cells, 8 coverslips). This may have important implications for AIS plasticity in epileptiform models15.
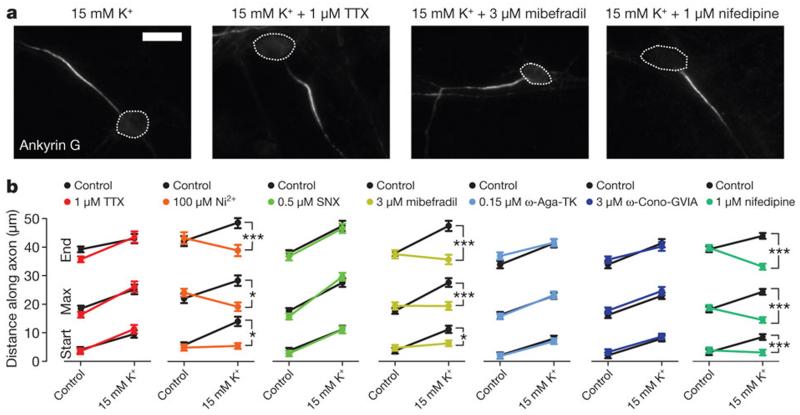
What are the sensors of neuronal activity responsible for AIS plasticity? Surprisingly, blocking VGSCs with tetrodotoxin (TTX, 1μM) did not affect the distal AIS movement produced by 15 mM K+ treatment (Fig. 2a,b; ankyrin G start: effect of drug×treatment interaction in 2-way ANOVA on ranks, P = 0.16; max: P = 0.17; end: P = 0.19; n = 392 cells, 16 coverslips). Indeed, on-cell recordings of spontaneous spiking activity showed that the ~10 mV chronic depolarization in 15mM K+ conditions produced sufficient VGSC inactivation to completely block spontaneous firing (Supplementary Fig. 2b,c). 15mM K+ depolarization does, however, produce a chronic increase in neuronal [Ca2+]i (Supplementary Fig. 2d), and particular blockers of voltage-gated calcium channels (VGCCs) could prevent activity-dependent AIS relocation. Preferentially blocking L-type calcium currents with 1 μM nifedipine16 completely abolished activity-dependent AIS movement (Fig. 2a,b; start: P < 0.0001; maximum: P < 0.0001; end: P < 0.0001; n = 298 cells, 12 coverslips). The AIS contains R-and T-type VGCCs which locally shape action potential properties17, and preferentially blocking R- and T-type channels with a low concentration of Ni2+ (100 μM18) also prevented all distal AIS movement after 15 mM K+ treatment (Fig. 2a,b; start: P = 0.03; maximum: P = 0.01; end: P = 0.002; n = 193 cells, 8 coverslips). Furthermore, although the R-type antagonist SNX-482 (0.5 μM) had no effect on activity-dependent AIS relocation (Fig. 2b; start: P = 0.25; maximum: P = 0.12; end: P = 0.74; n = 247 cells, 10 coverslips), the T/L-type blocker mibefradil (3 μM16) prevented all AIS movement (Fig. 2a,b; P = 0.01; maximum: P < 0.0001; end: P < 0.0001; n = 246 cells, 10 coverslips). In contrast, the P/Q-type blocker ω-agatoxin-TK (0.15 μM; Fig. 2b; start: P = 0.97; maximum: P = 0.62; end: P = 0.51; n = 199 cells, 8 coverslips), and the N-type antagonist ω-conotoxin-GVIA (3 μM; Fig. 2b; start: P = 0.43; maximum: P = 0.84; end: P = 0.38; n = 199 cells, 8 coverslips) had no effect on activity-dependent AIS movement. The sensor for neuronal activity necessary for AIS relocation is therefore likely to be a combination of T- and/or L-type VGCCs.
Figure 2. T- and/or L-type calcium channels mediate activity-dependent changes in AIS position.
a, Ankyrin G label after 12-14 DIV, 15 mM K+ -treatment with different voltage-gated ion channel antagonists. Scale bar, 20 μm; dotted line, soma. b, Mean ankyrin G positions (TTX, 392 cells, 16 coverslips; Ni2+, 193 cells, 8 coverslips; SNX, 247 cells, 10 coverslips; mibefradil, 246 cells, 10 coverslips; ω-agatoxin-TK, 199 cells, 8 coverslips; ω-conotoxin-GVIA, 199 cells, 8 coverslips; nifedipine, 200 cells, 8 coverslips). *, P < 0.05; ***, P < 0.001; error bars, s.e.m.
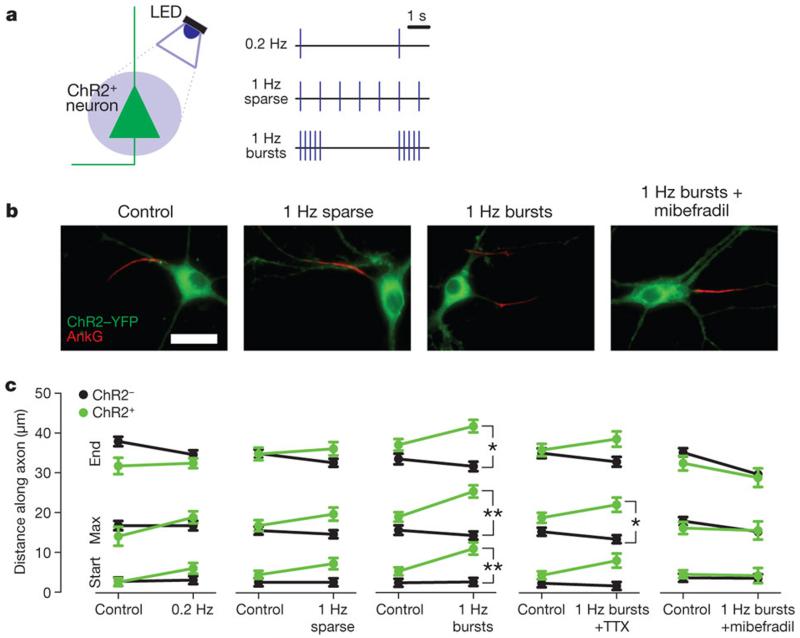
Although chronic depolarization with 15 mM K+ is a reliable technique for altering neuronal activity, it gives no indication of the temporal structure of activity responsible for AIS movement. To improve on this, we sparsely transfected neurons with channelrhodopsin-2 (ChR2), allowing photostimulation-based depolarization with millisecond resolution19. We delivered varying temporal patterns of 20 ms-duration blue LED light pulses from 12 to 14 DIV (Fig. 3a and Supplementary Fig. 9). Steady stimulation at 0.2 Hz or 1 Hz produced no significant change in AIS position (Fig. 3b,c; 0.2 Hz ankyrin G start: effect of cell-type×photostimulation interaction in 2-way ANOVA on ranks, P = 0.10; maximum: P = 0.12; end: P = 0.28; n = 133 cells, 4 coverslips; 1 Hz sparse start: P = 0.31; maximum: P = 0.19; end: P = 0.14; n = 274 cells, 8 coverslips). However, keeping the overall 1 Hz stimulation frequency constant but grouping flashes into bursts (5 flashes at 20 Hz every 5 s) produced a significant distal shift in AIS position that was specific to ChR2-expressing neurons (Fig. 3c; start: P = 0.001; maximum: P = 0.002; end: P = 0.01; n = 310 cells, 8 coverslips) and showed that activity-dependent AIS relocation can be cell-autonomous. We also saw no significant difference in PanNav immunofluorescence intensities between ChR2-positive and neighbouring ChR2-negative neurons (Supplementary Fig. 4). The effects of 1 Hz burst photostimulation were partially blocked by TTX (1 μM; Fig. 3c; start: P = 0.06; maximum: P = 0.03; end: P = 0.09; n = 154 cells, 4 coverslips), but entirely blocked by mibefradil (3 μM; Fig. 3b,c; start: P = 0.67; maximum: P = 0.30; end: P = 0.60; n = 134 cells, 4 coverslips). Indeed, although the 1Hz burst stimulus produced a lower overall spike rate than 1Hz sparse stimulation (Supplementary Fig. 9a,b), and although the plateaued [Ca2+]i increase in response to 1Hz sparse stimulation was qualitatively more similar to that caused by 15mM K+ treatment (Supplementary Fig. 9c and Supplementary Fig. 2d), peak [Ca2+]i transients were significantly greater under 1Hz burst conditions (Supplementary Fig. 9c,d). We therefore propose that AIS relocation results from activity patterns with two important properties: increases in [Ca2+]i must exceed a certain threshold and occur regularly over an extended period of time. Frequent, high frequency bursts of activity, as seen in the hippocampus during normal development or pathological states20,21, can therefore produce AIS relocation where steady stimulation at the same overall frequency does not.
Figure 3. Changes in AIS position with patterned ChR2 photostimulation.
a, LED photostimulation of ChR2-positive neurons. b, Ankyrin G label in ChR2-positive neurons after different photostimulation conditions. Scale bar, 20 μm. c, Mean ankyrin G positions for ChR2-positive (green) and ChR2-negative (black) neurons after different photostimulation conditions (0.2 Hz, 133 cells, 4 coverslips; 1 Hz Sparse, 274 cells, 8 coverslips; 1 Hz Bursts, 310 cells, 8 coverslips; 1 Hz Bursts + TTX, 154 cells, 4 coverslips; 1 Hz Bursts + mibefradil, 134 cells, 4 coverslips). *, P < 0.05; **, P < 0.01; error bars, s.e.m.
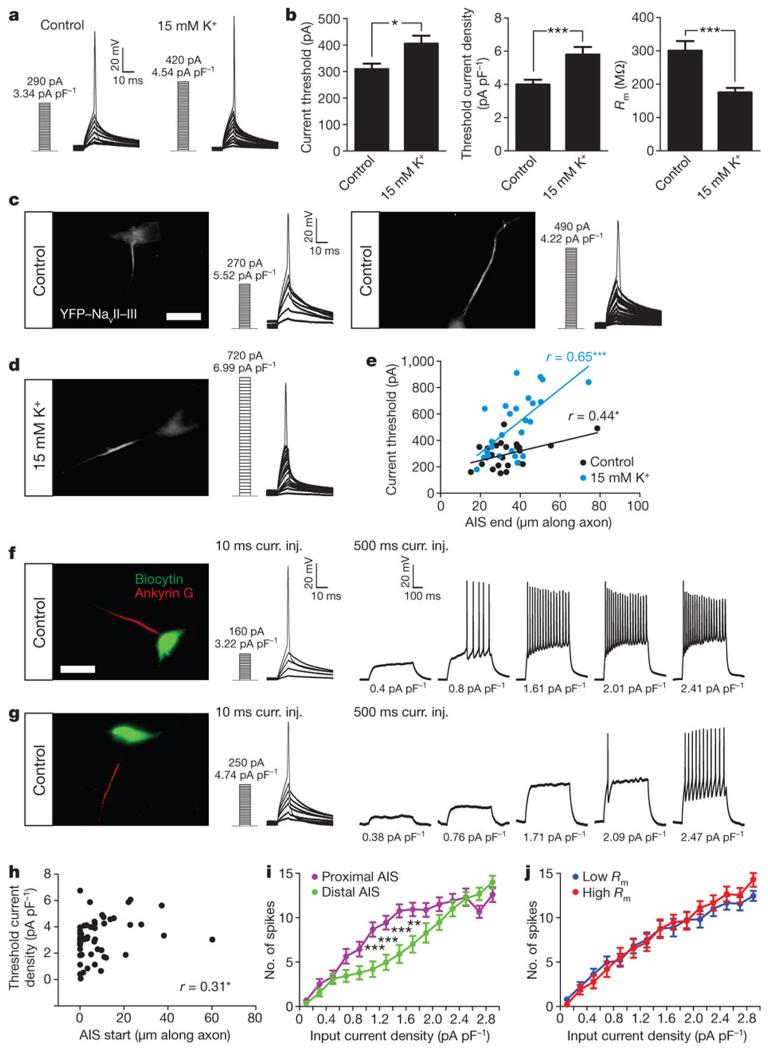
We next investigated the functional consequences of activity-dependent AIS relocation. Patch-clamp recordings in identical external solutions showed that a 48 h treatment with 15 mM K+ decreased neuronal excitability (Fig. 4a), resulting in a significant increase in threshold current and threshold current density for firing an action potential (Fig. 4a,b; current, Mann-Whitney U-test, P = 0.01; current density, P = 0.0003; n = 41 cells, 3 cultures; Supplementary Table 1)22. Chronic depolarization additionally lowered membrane resistance (Rm; Fig. 4b; Mann-Whitney U-test, P < 0.0001; n = 41 cells, 3 cultures; Supplementary Table 1)22, but simple calculations suggest that this change alone can only account for ~43 pA of the ~100 pA increased threshold current that we observed (see Methods)
Figure 4. Differences in AIS position are associated with differences in neuronal excitability.
a, Current-clamp traces in control and 15 mM K+ conditions Numbers indicate threshold current (pA) and threshold current density (pA pF−1). b, Threshold current, threshold current density and Rm (41 cells, 3 cultures). c,d, YFP-NavII-III label and current-clamp traces e, AIS end position versus current threshold (control, 24 cells, 3 cultures; AIS end mean 33.51 ± 2.63 μm; 15 mM K+, 26 cells, 1 culture; AIS end mean 37.02 ± 2.40 μm). Lines indicate linear regression. f, g, Left: biocytin and ankyrin G label. Middle and right panels show current-clamp traces for 10 ms and 500 ms current injections. . Numbers indicate input pA pF−1. h, AIS start position versus threshold current density for 10ms current injections (53 cells, 2 cultures). i, Current density versus spike number for 500ms current injections, sample split by AIS start position. j, As for i, but sample split by Rm. All scalebars indicate 20 μm; *, P < 0.05; **, P < 0.01; ***, P < 0.001. All plots show mean ± s.e.m.
To link AIS position more directly to variations in neuronal excitability we added an AIS-targeting domain23 to YFP to create a live marker of AIS position (see Methods and Supplementary Fig. 10). Patch-clamp recordings of control and 15 mM K+ -treated neurons uncovered significant correlations between AIS end position and current threshold (Fig. 4c,e; control, Pearson r = 0.43, P = 0.03; n = 24 cells, 3 cultures; 15 mM K+, r = 0.65, P = 0.0003; n = 26 cells, 1 culture) However, in these samples, other important functional parameters such as Rm and cell capacitance (Cm) also co-correlated with both AIS position and current threshold (Pearson r ≥ 0.29). We therefore took a parallel approach to linking AIS position and excitability in individual control neurons, filling recorded cells with biocytin and subsequently processing them for ankyrin G immunofluorescence (Fig. 4f,g). This method enabled accurate measurements of AIS start position (see Methods), which, unlike Rm or Cm in this sample (Rm, Pearson r = 0.15, P = 0.28; Cm, Pearson r = −0.24, P = 0.08; Supplementary Table 2), correlated positively and significantly with threshold current density (Fig. 4h; Spearman r = 0.31, P = 0.03; Supplementary Table 2). Responses to longer, 500 ms current pulses revealed further relationships between AIS position and spiking behaviour (Fig. 4f,g). The 50 % of cells with the most proximal AIS start positions fired more spikes to 1-2 pA pF−1 inputs than the 50 % of neurons with the most distal AIS start positions (Fig. 4i). This difference was not due to co-correlations with Rm (Fig. 4j). Both sets of cells reached indistinguishable firing levels at high input currents (Fig. 4i) and were not significantly different for low input currents (Fig. 4i), likely due to the limited range in spike number for small stimuli. Cells’ input-output relationships also showed a strong negative correlation between AIS start position and curve steepness (Supplementary Fig. 11d; Spearman r = −0.52, P < 0.0001), showing that neurons with more proximal AISs not only spike in response to lower current input, they also add more spikes in response to input current increases. In agreement with this, mathematical models also predict that AIS location is an important parameter controlling neuronal excitability (Supplementary Fig. 12) 8,24.
We have described a novel mechanism of activity-dependent plasticity whereby the excitability of a neuron can be regulated by changes in the location of an entire subcellular compartment – the AIS. During development, this mechanism may play a vital role as neurons establish themselves within functional networks, allowing cells to fine-tune their excitability according to ongoing levels of input activity25-27. In a similar fashion to other forms of adaptation28, this mechanism would compensate for chronic alterations in activity by shifting the input-output function of a neuron along its input axis. This is in contrast to synaptic mechanisms of homeostatic scaling, which scale the gain of synaptic inputs and result in shifts along a cell’s input-output curve25 (Supplementary Fig. 1). Together, these two forms of plasticity provide an important toolbox that a neuron can draw upon to stabilize its excitability and maximize its information processing capacity. Furthermore, because the AIS not only determines action potential initiation, but also controls protein transport into the axon29,30, changes in its position may also have implications for neuronal compartmentalization and neurite identity. Unravelling the pathways linking calcium entry to AIS relocation could uncover potential new targets for epilepsy treatment, where fine-scale control over neuronal excitability represents a major therapeutic challenge.
Supplementary Material
Methods summary.
We used dissociated cultures prepared from E 18 rat pups according to standard protocols. Lipofectamine transfections occurred at 7 DIV, and all treatments were carried out for 48 h between 12-14 DIV, unless otherwise stated. AIS-targeted constructs were made by fusing the sequence for the II-III intracellular loop of the sodium channel subunit Nav1.2 to the carboxy terminus of YFP. Photostimulation of ChR2-expressing neurons took place in a standard cell culture incubator, with collimator-fitted blue LEDs positioned directly under coverslips kept in their ‘home’ plates. AIS proteins were labelled using standard techniques for immunofluorescence, and specificity of AIS label was confirmed for each marker by co-labelling with βIV spectrin. All imaging and image analysis was carried out blind to experimental group. Images were obtained with a x100 oil immersion objective coupled to a CCD camera, and image analysis was carried out on 16-bit exported TIFF images with custom routines written in Matlab. The ‘maximum’ position of the AIS was determined by the distance from the start of the axon where the smoothed and normalised profile of fluorescence intensity reached its peak; the ‘start’ and ‘end’ positions were the proximal and distal axonal points, respectively, at which the profile dipped to 33 % of its peak. Whole-cell patch-clamp recordings were made using a HEPES-buffered saline external solution and a K+-gluconate-based internal solution. Synaptic inputs were blocked with gabazine, 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and dl-2-amino-5-phosphonovaleric acid (AP5), and current clamp recordings were obtained at a Vhold of −60 mV. Current threshold was measured as the lowest input amplitude that reliably evoked an action potential.
Acknowledgements
We thank M. Komada for the anti-βIV-spectrin antibody, K. Deisseroth for the ChR2 construct, N. Ben Fredj for assistance with cell culture, S. Poopalasundaram for help with molecular biology, P. Degenaar and N. Grossman for assistance with LED photostimulation, and I. Thompson and K. Bender for comments on the manuscript. This work was supported by the Wellcome Trust and a Lister Institute Research Prize to JB.
Appendix
Methods
Dissociated Hippocampal Cultures
We prepared dissociated mixed neuronal and glial cultures from E 18 Sprague-Dawley rat hippocampi according to standard protocols. Briefly, hippocampi were dissected in HBSS, digested in trypsin (Worthington; 0.5 mg/ml; 15 min 37 °C), triturated through Pasteur pipettes of increasingly narrow diameter, and plated at 90,000 cells ml−1 on glass coverslips pre-coated with PDL (50 μg ml−1) and laminin (40 μg ml−1). Cultures were incubated (37 °C, 6 % CO2) in a 1:1 mixture of neurobasal medium plus B27 supplement (2 %) and neurobasal medium plus foetal calf serum (2 %), with additional glutamax (500 μM) and penicillin-streptomycin (100 μg ml−1). At 7 DIV half of this medium was replaced with neurobasal medium plus B27 supplement. Unless otherwise specified, all culture reagents were from Invitrogen.
Chronic depolarization and pharmacology
We depolarized our cultured neurons by increasing the KCl content of the culture medium from 5 mM to 15 mM. For recovery experiments, coverslips were removed from 15 mM KCl medium, washed briefly in PBS, and moved to 5 mM KCl medium that had supported sister cultures for the entire culture period. Pharmacological agents were added from frozen stock solutions at the following concentrations: 1 μM TTX (Alomone Labs), 100 μM NiCl2 (Sigma), 3 μM mibefradil (Tocris Bioscience), 0.5 μM SNX-482 (Tocris Bioscience), 1 μM nifedipine (Sigma), 0.15 μM ω-agatoxin-TK (Tocris Bioscience), 3 μM ω-conotoxin-GVIA (Tocris Bioscience).
Molecular Biology and Transfections
We made the YFP-NavII-III construct by cloning the II-III intracellular loop sequence of Nav1.2 (ref. 23) (SSFSSDNLAATDDDNEMNNLQIAVGRMQKGIDFVKRKIREFIQKAFVRKQKALDEIKPLEDLNNKKDSCISNH TTIEIGKDLNYLKDGNGTTSGIGSSVEKYVVDESDYMSFINNPSLTVTVPIALGESDFENLNTEEFSSESDMEESK EKLNATSSSEGSTVDIGAPAEGEQPEAEPEESLEPEACFTEDCVRKFKCCQISIEEGKGKLWWNLRKTCYKX;MWG) into the BsrgI site found at the C-terminal end of YFP in the pEYFP-N1 plasmid (Clontech). The appropriate base pairs were added to complete the YFP sequence before the NavII-III sequence began. Correct insertion was verified with sequencing. The ChR2-YFP construct was pLenti-Synapsin-hChR2(H134R)-EYFP-WPRE, a gift from Karl Deisseroth (www.optogenetics.org). Cells were transfected at 7 DIV using lipofectamine2000 (Invitrogen).
ChR2 Photostimulation
Cultures sparsely transfected with ChR2-YFP were prepared 2 h prior to photostimulation by supplementing their culture medium with a cocktail of additional antioxidants (110 μM vitamin C, 100 μM Trolox, 2.3 μM vitamin E, 77 nM superoxide dismutase, 3.2 μM glutathione, 10 nm catalase; all from Sigma), and by blocking excitatory synaptic transmission with 20 μM 6-Cyano-7-nitroquinoxaline-2,3-dione (CNQX; Sigma). The antioxidant supplements alone, CNQX alone, or antioxidants + CNQX, did not produce any significant change in AIS position (Supplementary Fig. 13). For long-term photostimulation, we placed 12-well culture plates directly onto a bed of collimator-topped blue LEDs (Royal Blue Luxeon K2, Philips LumiLEDs, UK; 455 ± 10 nm; ~1 mW.mm−2 at the coverslip surface), powered by a custom-made driver (a gift from N. Grossman and P. Degenaar) and controlled by a Master-8 stimulator (AMPI). Flash duration was 20ms, ensuring at least one spike per stimulus in all ChR2-positive neurons (Supplementary Fig. 9). All coverslips in a given plate received the same pattern of photostimulation; control plates were loosely covered in tin foil.
Immunocytochemistry
We used the following primary antibodies: mouse monoclonal anti-ankyrin G (clone N106/36; 1:500; NeuroMab), chicken polyclonal anti-βIV spectrin (1:500; a gift from M. Komada), mouse monoclonal anti-PanNav (1:100; Sigma), mouse monoclonal anti-Pan-neurofascin (1:1000; NeuroMab), mouse monoclonal anti-FGF14 (1:500; NeuroMab), mouse monoclonal anti-GAD65 (1:500; Developmental Studies Hybridoma Bank), rabbit polyclonal anti-MAP2 (1:1000; Abcam). For ankyrin G and most βIV spectrin labelling, cultures were fixed with 4 % paraformaldehyde (TAAB Laboratories; in 3 % sucrose, 60 mM PIPES, 25 mM HEPES, 5 mM EGTA, 1 mM MgCl2; pH 7.4; 20 min at room temperature, 23 °C) and incubated in primary antibody for 1 h at room temperature; for other AIS antigens we used a 1 % PFA fixation (20 min at room temperature) and incubated in primary antibody for 3 h at room temperature. Otherwise, all labelling steps were identical. After washing in PBS, permeabilising in 0.25 % Triton X-100 (Sigma; 5 min room temperature), and blocking in 3 % BSA (Sigma; 1 h room temperature), cultures were placed in primary antibody solution (in 3 % BSA), washed, placed in secondary antibody solution (Alexa Fluor goat anti-mouse 488 or 555, with/without goat anti-chicken 488 or 568; Invitrogen; 1:1000; 1 h room temperature), washed again, and mounted in Mowiol (Calbiochem).
Image Analysis
All image acquisition and analysis was performed blind to experimental group. Neurons with AISs of obvious soma origin were imaged using a Xenon-arc lamp (Lambda-LS, Sutter Instruments) with appropriate excitation and emission filters (Chroma Tech. Corp.), an Olympus 100x oil immersion objective and a CCD camera (CoolSnap HQ, Photometrics) coupled to Slidebook software (1392×1040 pixels with 2×2 pixel binning; 0.5 μm z-axis steps). Image stacks were converted into single maximum intensity z-axis projections, exported as raw 16-bit TIFF files, and imported into Matlab (Mathworks) for analysis using custom-written functions. We drew a line profile starting at the soma that extended down the axon, through and past the AIS. At each pixel (1 pixel = 0.129 μm) along this profile, fluorescence intensity values were averaged over a 3×3 pixel square centred on the pixel of interest. Averaged profiles were then smoothed using a 40-point (~5 μm) sliding mean, and normalised between 1 (maximum smoothed fluorescence, location of the AIS max position) and 0 (minimum smoothed fluorescence). AIS start and end positions were obtained at the proximal and distal axonal positions, respectively, where the normalised and smoothed profile declined to 0.33. These positions agreed extremely well (Spearman r > 0.8) with AIS start and end points plotted by eye, and varying the threshold value between 0.1 and 0.9 did not affect the reported pattern of results. Owing to contamination from soma fluorescence, we used only the AIS ‘end’ measure in YFP-NavII-III-expressing neurons. Fluorescence intensity measurements for PanNav staining were obtained from the same axonal profiles, non-normalised and unsmoothed, but background-subtracted using the final 20 pixels (2.6 μm) of each profile as axon baseline staining. Peak fluorescence was taken as the maximum value of each profile, integrated AIS fluorescence was calculated as the area underneath the profile between the AIS start and end positions, and AIS fluorescence density was simply the integrated AIS fluorescence divided by AIS length. We used ImageJ (NIH, USA) to measure soma area in neurons stained for the somatodendritic marker MAP2.
Electrophysiology
For assessments of neuronal exctability, we made visually targeted whole-cell patch-clamp recordings from 11 to 14 DIV cultures maintained in an HBS extracellular solution (pH 7.4, ~280 mOsm, ~23 °C) that contained (in mM): 136 NaCl, 2.5 KCl, 10 HEPES, 10 d-glucose, 2 CaCl2, 1.3 MgCl2, 0.01 SR-95531 (gabazine, Sigma) 0.02 CNQX (Sigma), 0.025 dl-2-amino-5-phosphonovaleric acid (APV, Sigma). Pipettes were pulled from borosilicate glass (outer diameter 1.5 mm, inner diameter 1.17 mm, Harvard Apparatus), with a resistance of 3-7 MΩ, and were filled with an internal solution containing (in mM): 130 K-gluconate, 10 NaCl, 1 EGTA, 0.133 CaCl2, 2 MgCl2, 10 HEPES, 3.5 MgATP, 1 NaGTP. Recordings were obtained with a Heka EPC10/2 amplifier coupled to Pulse acquisition software. Signals were Bessel filtered at 10 kHz (filter 1), and 2.9 kHz (filter 2), digitized, and sampled at 25-50 kHz (20-40 μs sample interval). Fast capacitance was compensated in the on-cell configuration. With slow capacitance compensation inactive in voltage-clamp mode, we used responses to a 10 mV hyperpolarisation step to estimate the series resistance (Rs) of the recording (<18 MΩ for all cells; from the current peak), and the neuron’s membrane resistance (Rm; from the steady holding current at the new voltage) and membrane capacitance (Cm; from the area under the exponentially-decaying current from peak to holding). Current thresholds for action potential firing were measured in current clamp with Vhold set to −60 mV (uncorrected, as for all voltages reported in the paper, for an estimated liquid junction potential of ~15 mV). We injected 10-ms-duration current steps of increasing amplitude (increments of 5, 10, or 20 pA) until we reached current threshold, at which the neuron reliably fired an action potential (Vm > 0 mV). Experiments involving 500-ms-duration current steps followed the same protocol. All subsequent analysis focused on the rising + plateau phase of the current vs spike number relationship: we discarded all data points for very high current input where spike number fell below maximum due to sodium channel inactivation. To compare current density vs spike number in sub-groups of recorded cells, neurons were ranked on the basis of their AIS start position or Rm, and divided at the median into 2 equally-sized sub-populations. Input pA pF−1 vs spike number data in each sub-population were then sorted into 0.2 pA pF−1 bins, and mean binned values were compared across input magnitudes using a 2-way ANOVA. To describe input-output spiking relationships, plots of current density versus spike number for each neuron were fitted using a least-squares algorithm (Prism, GraphPad, USA) with the following ‘plateau followed by one-phase association’ equation:
Where y0 is baseline, ymax is the maximum number of spikes, x0 is the departure point of the curve from baseline, and k is the rate constant of the exponential. Neurons for which fitting was ambiguous, with very wide confidence intervals for the fitted parameters (Prism, GraphPad), were removed from our analyses.
In some experiments the internal solution also contained 0.15% biocytin (Sigma). Filled cells were fixed immediately after recording in 4% paraformaldehyde (20 min, room temperature), and subsequently processed for ankyrin G immunofluorescence (see above) along with biocytin label using streptavidin-conjugated Alexa 488 (Invitrogen).
The resting membrane potential of 12 DIV neurons in control and 15 mM K+ conditions was measured at I = 0 in current clamp immediately after membrane rupture, using the K-gluconate-based internal solution described above, and the respective neurobasal-based culture media as external solutions.
On-cell configuration recordings for spiking behaviour at 12-13 DIV also used the above K-gluconate-based internal solution, and the respective 5 mM or 15mM K+ neurobasal-based culture media as external solutions (34-36 °C, maintained via a heated chamber TC-344B, Harvard Apparatus). Seal resistance (control 9.13 ± 2.84 GΩ, 15mM K+ 5.29 ± 1.03 GΩ, Mann-Whitney U-test P = 0.59), and RMS noise (control 1.10 ± 0.03 pA, 15mM K+ 1.14 ± 0.08 pA, Mann-Whitney U-test, P = 0.81) did not differ between control and 15mM K+ recordings. Spikes were identified by their characteristic biphasic waveform, and were analysed off-line using MiniAnalysis (Synaptosoft). For on-cell recordings of ChR2-induced spiking, photostimulation was delivered using exactly the same LED, collimator, driver, and Master-8 timer as used for our chronic photostimulation experiments (see above), and 20 μM CNQX was present in the external medium. No directly- or indirectly-driven spikes were observed following LED stimulation in neighbouring ChR2-negative neurons (n = 3).
We used the following equation to predict the current I required in order to change a cell’s membrane potential by ΔV:
Where t is time of current pulse, and τ is Rm•Cm. For current threshold, ΔV = Vthresh – Vhold . With Vhold at −60 mV, t at 10 ms, and Vthresh, Cm, and Rm at their measured control mean values of −29.48 mV, 80.86 pF and 300.87 MΩ, respectively, I = 301.16 pA. Keeping all other values constant, but changing Rm to its measured 15 mM K+ treatment mean value of 175 MΩ, I = 344.4 pA.
Calcium Imaging
We loaded 12-13 DIV neurons with the ratiometric calcium indicator fura-2-AM (Invitrogen; 5 μM in HBS for 30 min at room temperature, followed by wash, and 30 min in medium at 37 °C). Cells were maintained in a steady gravity-fed flow of phenol red-free neurobasal medium (Invitrogen; 34-36 °C maintained with in-line heater SH-27B, Harvard Apparatus). For high K+ wash-in, single z-axis images were captured for both 340 nm and 380 nm excitation wavelengths at 0.5 Hz using an Olympus 40x oil immersion objective and a CCD camera coupled to Slidebook software (see above; 2×2 pixel binning). Fura-2 340/380 ratios were then calculated before and during 15 mM K+ wash-in, using fluorescence intensities averaged across cell body regions of interest (ROIs) and normalised for pre-wash-in photobleaching. For ChR2-induced calcium responses, stimulating with LED light made it impossible to collect useful fura-2 data during the stimulus itself. Instead, we used 4×4 pixel binning, a soma-restricted ROI, and a single 340 nm excitation wavelength to image continuously at 16 Hz, enabling us to capture the ‘tail’ of ChR2-induced calcium responses immediately after LED offset. A combination of 340 nm excitation and a 60% neutral density filter kept ChR2 stimulation by the excitation light to a minimum. Photostimulation was delivered using exactly the same LED, collimator, driver, and Master-8 timer as used for our chronic photostimulation experiments (see above), and, as in those experiments, 20 μM CNQX was present in the external medium.
Statistics
Statistical analysis was carried out in Prism (GraphPad) or SPSS (SPSS Inc.). n values reported throughout the text refer to the total number of cells and coverslips in each experiment. Most data sets were non-normally distributed, and had significant heterogeneity in variance. For these reasons, we used non-parametric group comparisons throughout our study: Mann-Whitney U-tests for 2 independent samples, Kruskal-Wallis tests for >2 independent samples (followed by Dunn post-hoc pairwise tests), and non-parametric 2-way ANOVAs on sample ranks14. Linear regression was performed on parameters that were Normally distributed, or could be made Normal by a logarithmic transform. For other parameters, we used non-parametric Spearman correlations. All tests were two-tailed, with the level of significance (α) set at 0.05.
Mathematical Modelling
We used a previously published model of action potential initiation in CA1 neurons31 using the simulation software NEURON (v7.0)32. All parameters from this model were kept intact, except for a spacer region with passive membrane properties, which was added to introduce varying distances between the AIS and the soma. No active conductances were present in this section of the axon. The passive properties of the neuron included specific membrane capacitance, membrane resistivity, and the resistivity of the cytoplasm, and were set to 0.75 μF cm−2, 15,000 Ωcm2 and 100 Ωcm, respectively in the soma, the AIS and the spacer. In other compartments these properties were varied according to the original model31. Leak currents were assumed to have a reversal potential of −70 mV. The conductances included in each section of the model neuron are shown in Supplementary Table 3.
The graph in Supplementary Figure 11 was produced by injecting a 1 ms current pulse and recording the change in voltage. The threshold for firing an action potential was established for different distances between the AIS and the soma by increasing the length of the spacer region. The current threshold when the AIS is right next to the soma (D = 0 μm) is taken as zero.
- 31.Royeck M, et al. Role of axonal NaV1.6 sodium channels in action potential initiation of CA1 pyramidal neurons. J Neurophysiol. 2008;100:2361–80. doi: 10.1152/jn.90332.2008. [DOI] [PubMed] [Google Scholar]
- 32.Hines ML, Carnevale NT. The NEURON simulation environment. Neural Comput. 1997;9:1179–1209. doi: 10.1162/neco.1997.9.6.1179. [DOI] [PubMed] [Google Scholar]
Footnotes
Author information
Reprints and permissions information is available at www.nature.com/reprints.
References
- 1.Coombs JS, Curtis DR, Eccles JC. The generation of impulses in motoneurones. J Physiol. 1957;139:232–49. doi: 10.1113/jphysiol.1957.sp005888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palmer LM, Stuart GJ. Site of action potential initiation in layer 5 pyramidal neurons. J Neurosci. 2006;26:1854–63. doi: 10.1523/JNEUROSCI.4812-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meeks JP, Mennerick S. Action potential initiation and propagation in CA3 pyramidal axons. J Neurophysiol. 2007;97:3460–72. doi: 10.1152/jn.01288.2006. [DOI] [PubMed] [Google Scholar]
- 4.Kole MH, et al. Action potential generation requires a high sodium channel density in the axon initial segment. Nat Neurosci. 2008;11:178–86. doi: 10.1038/nn2040. [DOI] [PubMed] [Google Scholar]
- 5.Foust A, et al. Action potentials initiate in the axon initial segment and propagate through axon collaterals reliably in cerebellar Purkinje neurons. J Neurosci. 2010;30:6891–902. doi: 10.1523/JNEUROSCI.0552-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palmer MN, et al. Initiation of simple and complex spikes in cerebellar Purkinje neurons. J Physiol. 2010;588:1709–17. doi: 10.1113/jphysiol.2010.188300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fried SI, Lasker AC, Desai NJ, Eddington DK, Rizzo JF., 3rd. Axonal sodium-channel bands shape the response to electric stimulation in retinal ganglion cells. J Neurophysiol. 2009;101:1972–87. doi: 10.1152/jn.91081.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuba H, Ishii TM, Ohmori H. Axonal site of spike initiation enhances auditory coincidence detection. Nature. 2006;444:1069–72. doi: 10.1038/nature05347. [DOI] [PubMed] [Google Scholar]
- 9.Zhou D, et al. AnkyrinG is required for clustering of voltage-gated Na channels at axon initial segments and for normal action potential firing. J Cell Biol. 1998;143:1295–304. doi: 10.1083/jcb.143.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogawa Y, Rasband MN. The functional organization and assembly of the axon initial segment. Curr Opin Neurobiol. 2008;18:307–13. doi: 10.1016/j.conb.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Lorincz A, Nusser Z. Cell-type-dependent molecular composition of the axon initial segment. J Neurosci. 2008;28:14329–40. doi: 10.1523/JNEUROSCI.4833-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu W, et al. Distinct contributions of Na(v)1.6 and Na(v)1.2 in action potential initiation and backpropagation. Nat Neurosci. 2009;12:996–1002. doi: 10.1038/nn.2359. [DOI] [PubMed] [Google Scholar]
- 13.Yang Y, Ogawa Y, Hedstrom KL, Rasband MN. betaIV spectrin is recruited to axon initial segments and nodes of Ranvier by ankyrinG. J Cell Biol. 2007;176:509–19. doi: 10.1083/jcb.200610128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akritas MG. The rank transform method in some two-factor designs. J Amer Statistical Assoc. 1990;85:73–78. [Google Scholar]
- 15.Cendes F. Progressive hippocampal and extrahippocampal atrophy in drug resistant epilepsy. Curr Opin Neurol. 2005;18:173–7. doi: 10.1097/01.wco.0000162860.49842.90. [DOI] [PubMed] [Google Scholar]
- 16.Lee TS, et al. Actions of mibefradil, efonidipine and nifedipine block of recombinant T- and L-type Ca channels with distinct inhibitory mechanisms. Pharmacology. 2006;78:11–20. doi: 10.1159/000094900. [DOI] [PubMed] [Google Scholar]
- 17.Bender KJ, Trussell LO. Axon initial segment Ca2+ channels influence action potential generation and timing. Neuron. 2009;61:259–71. doi: 10.1016/j.neuron.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magee JC, Johnston D. Characterization of single voltage-gated Na+ and Ca2+ channels in apical dendrites of rat CA1 pyramidal neurons. J Physiol. 1995;487(Pt 1):67–90. doi: 10.1113/jphysiol.1995.sp020862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–8. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 20.Leinekugel X, et al. Correlated bursts of activity in the neonatal hippocampus in vivo. Science. 2002;296:2049–52. doi: 10.1126/science.1071111. [DOI] [PubMed] [Google Scholar]
- 21.Khalilov I, Holmes GL, Ben-Ari Y. In vitro formation of a secondary epileptogenic mirror focus by interhippocampal propagation of seizures. Nat Neurosci. 2003;6:1079–85. doi: 10.1038/nn1125. [DOI] [PubMed] [Google Scholar]
- 22.O’Leary T, van Rossum MC, Wyllie DJ. Homeostasis of intrinsic excitability in hippocampal neurones: dynamics and mechanism of the response to chronic depolarization. J Physiol. 2010;588:157–70. doi: 10.1113/jphysiol.2009.181024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garrido JJ, et al. A targeting motif involved in sodium channel clustering at the axonal initial segment. Science. 2003;300:2091–4. doi: 10.1126/science.1085167. [DOI] [PubMed] [Google Scholar]
- 24.Kress GJ, Dowling MJ, Eisenman LN, Mennerick S. Axonal sodium channel distribution shapes the depolarized action potential threshold of dentate granule neurons. Hippocampus. 2010;20:558–71. doi: 10.1002/hipo.20667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turrigiano GG, Nelson SB. Hebb and homeostasis in neuronal plasticity. Curr Opin Neurobiol. 2000;10:358–64. doi: 10.1016/s0959-4388(00)00091-x. [DOI] [PubMed] [Google Scholar]
- 26.Burrone J, Murthy VN. Synaptic gain control and homeostasis. Curr Opin Neurobiol. 2003;13:560–7. doi: 10.1016/j.conb.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Marder E, Prinz AA. Modeling stability in neuron and network function: the role of activity in homeostasis. Bioessays. 2002;24:1145–54. doi: 10.1002/bies.10185. [DOI] [PubMed] [Google Scholar]
- 28.Laughlin SB. The role of sensory adaptation in the retina. J Exp Biol. 1989;146:39–62. doi: 10.1242/jeb.146.1.39. [DOI] [PubMed] [Google Scholar]
- 29.Winckler B, Forscher P, Mellman I. A diffusion barrier maintains distribution of membrane proteins in polarized neurons. Nature. 1999;397:698–701. doi: 10.1038/17806. [DOI] [PubMed] [Google Scholar]
- 30.Song AH, et al. A selective filter for cytoplasmic transport at the axon initial segment. Cell. 2009;136:1148–60. doi: 10.1016/j.cell.2009.01.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.