Abstract
Interactions of copper and membranes with α-synuclein have been implicated in pathogenic mechanisms of Parkinson’s disease, yet work examining both concurrently is scarce. We have examined the effect of copper(II) on protein/vesicle binding and found that both the copper(II) affinity and α-helical content are enhanced for the membrane-bound protein.
α-Synuclein (α-syn), an amyloidogenic protein implicated in Parkinson’s disease (PD), localizes near synaptic vesicles and mitochondrial membranes in vivo.1, 2 Despite the attention given to this protein, its native function and pathological role in PD remain ill-defined. Recently, it was established that α-syn acts as a chaperone, facilitating the rapid reassembly of the SNARE complex which is needed for neurotransmitter release from presynaptic vesicles.3 While protein-vesicle interactions are involved in the physiological function of α-syn, it also has been suggested that α-syn-membrane association promotes aggregation, where the membrane surface serves as an initiation site or “seed” for amyloid (fibril) formation.4, 5 Through detailed studies employing a variety of spectroscopic methods such as NMR,6–8 EPR,9–11 and fluorescence,12–14 two different membrane-bound α-syn conformations (extended helix vs. an antiparallel arrangement of two helices) have been reported, however a consensus has yet to be achieved.
Alterations in metal homeostasis with age, specifically pertaining to copper accumulation, also has been a topic relevant to PD etiology that is progressively gaining attention in the literature.15–21 Copper, is a redox-active biometal that can easily promote aberrant oxidative chemistry through abnormal metal-protein interactions. In fact, we previouly reported evidence for dityrosine crosslinks within α-syn fibrils that resulted from copper(I)/O2 chemistry.22 Moreover, amongst an array of metals examined, copper(II) has been shown to accelerate the fibrillation process of α-syn.20, 23
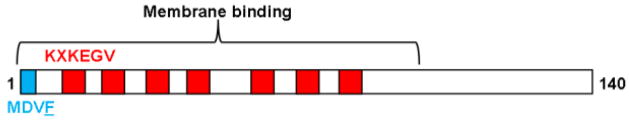
The focus of this study is to examine the effect of copper(II) on α-syn/lipid interactions and to test whether the phospholipid vesicles can modulate the copper(II) binding properties. Copper(II) is known to coordinate to soluble α-syn within the first four residues (MDVF) through the N-terminal amine and backbone amide chelation.24–29 The predicted geometry of this high affinity metal binding site is square planar. Markedly, the N-terminal region is also anticipated to interact electrostatically with cellular membranes through seven imperfect amino acid repeats (KXKEGV) in the primary amino acid sequence (Fig. 1).30 Hence, our studies were restricted to the N-terminus of α-syn since it contains the copper(II) binding site and is thought to be the anchoring region for α-syn/membrane interactions.8
Fig. 1.
Schematic representation of the human α-syn primary amino acid sequence. The N-terminal copper(II)-binding sequence (cyan) and the seven imperfect amino acid repeats (red) are highlighted. Location of Trp mutation within the α-syn variant (F4W) is underlined.
The N-terminal Trp-containing variant of α-syn, F4W, is excellent for investigating the effect of extrinsic environmental factors such as copper- or membrane-protein interactions by monitoring changes in the W4 fluorescence properties.27, 31, 32 Specifically, our prior work has shown that the F4W mutant exhibits similar membrane and copper binding properties as the wild-type protein. To conduct this study, synthetic membranes were prepared from a 1:1 molar ratio of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphate (POPA) and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC). The average hydrodynamic radius of these phospholipid vesicles was estimated as ~50 nm from dynamic light scattering measurements.
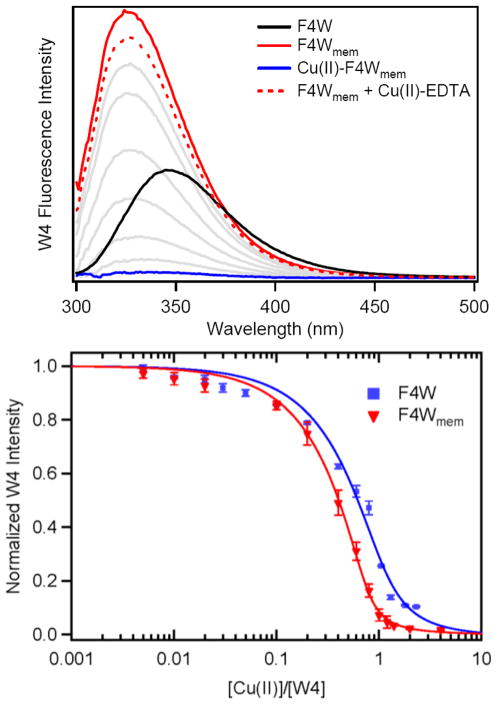
Consistent with our previous work, addition of phospholipid vesicles to a pH 7 buffer solution (20 mM MOPS, 100 mM NaCl) of F4W (5 μM) results in a pronounced blue shift of the W4 spectrum (Δλmax = 25 nm), shifting from 350 nm to 325 nm, and a greater than 3-fold increase in quantum yield upon saturation ([liposomes] ~ 1.9 mM; lipid-to-protein ratio ~ 380; Fig. 2 Top).32 In general, the respective data reveal that the environment surrounding W4 has increased hydrophobicity and decreased dynamic motion, indicating that the indole sidechain has become more restricted. These spectroscopic signatures can be easily rationalized by the N-terminus of F4W α-syn inserting into the phospholipid bilayer, therefore transitioning from water- to membrane-exposed.
Fig. 2.
Copper(II) binding of membrane-bound α-syn. (Top) W4 spectroscopic changes in the presence of POPA:POPC vesicles ([liposomes] = 1.9 mM, [α-syn] = 5 μM, red) and subsequent copper(II) additions (5 nM to 5 μM, grey and blue). Lipid background spectra have been subtracted. (Bottom) Comparison of copper(II) titration curves in the presence (red) and absence (blue) of vesicles ([liposomes] = 1.9 mM, [α-syn] = 1 μM).
Saturation of the W4 intensity at λmax = 325 nm with addition of synthetic vesicles indicates that the membrane-bound protein (F4Wmem) has fully formed. Small quantities (5 nM – 20 μM; 0.001 – 4 eqs.) of copper(II) were then titrated into the sample containing 5 μM F4Wmem, which resulted in quenching of the W4 emission as a function of [CuII] (Fig. 2 Top). The W4 fluorescence intensity was fully quenched with addition of ~1 eq. copper(II), indicating direct CuII/F4Wmem and a 1:1 metal to F4Wmem binding stoichiometry.
In order to verify that the copper-bound protein is still vesicle bound and this metal-association is reversible, a strong copper(II) chelator, ethylenediaminetetraacetic acid (EDTA), was added to extract the metal ion from CuII–F4Wmem. Compared to the W4 fluorescence intensity measured in the absence of copper(II), the F4Wmem recovery yield was determined as ~ 90%. Strikingly, this result suggests that α-syn remains membrane-bound with coordination and release of copper(II). Therefore, the N-terminus must reversibly permeate the membrane surface in order to facilitate copper(II) binding since metal ions cannot enter the phospholipid bilayer.
Copper(II) titrations also were conducted at lower protein concentrations ([F4Wmem] = 1 μM), enabling a more accurate measurement of the apparent dissociation constant (Kd(app)). A single-site binding model (CuII + F4Wmem ⇆ CuII – F4Wmem) was employed and Kd(app) ~ 30 nM was determined (Fig. 2 Bottom). Previously, we reported the Kd(app) for copper(II) binding to soluble F4W as ~ 100 nM under anaerobic conditions. It is noteworthy that these values have some uncertainty since the protein concentrations are greater than Kd(app). Regardless, the results indicate that the copper(II) binding affinity of F4W α-syn is enhanced when the protein is membrane-bound. Consistent with our result, Paik and coworkers previously found that copper(II) localization to α-syn was increased in the presence of anionic phospholipids.33
α-Syn is known to bind anionic detergent micelles and phospholipid vesicles resulting in a conformational change from unstructured to α-helical based on in vitro studies.30, 32 As shown by circular dichroism (CD) spectroscopic measurements in Fig. 3, addition of phospholipid vesicles to a solution at pH 7 ([α-syn] = 5 μM, 20 mM MOPS, 100 mM NaCl) induces a secondary structural transition from unfolded to α-helical. Typical features with negative maxima at 208 and 222 nm were observed with an isodichroic point at 203 nm, consistent with a two-state model. The maximum total α-helical content is approximated to be 69% with a mean residue ellipticity ([Θ]222nm) of −23,800 deg cm2 dmol−1. Remarkably, based on complementary CD data analysis of wild-type α-syn, the maximum α-helical content increases by 7%, [Θ]222nm = −26,700 deg cm2 dmol−1, with addition of ~1 eq. of copper(II), i.e. formation of CuII–α-synmem; the signal saturates at 1 eq and does not change upon further copper(II) addition. Removal of the metal ion by EDTA results in the membrane-bound protein returning to the initial unmetallated conformation (α-synmem).
Fig. 3.
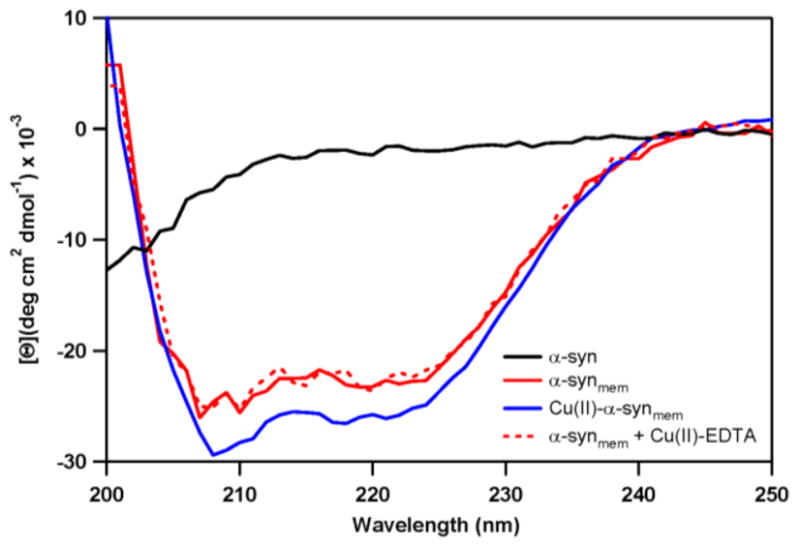
Changes in α-syn secondary structure upon vesicle association and the effect of copper(II) binding, as determined by CD spectroscopy. In solution, α-syn is unstructured (black) and in the presence of POPA:POPC vesicles, the protein adopts an α-helical conformation (solid red). This helical structure increases with coordination of 1 eq. copper(II) (blue). Removal of copper(II) by EDTA reverses this transformation back to the unmetallated membrane-bound protein (red dashes).
Metal coordination in a square planar geometry, as is predicted for CuII-α-syn, is ideal for stabilizing an α-helix based on structural information gained from the de novo design of proteins or peptides.34–36 Specific functions or desired properties can be promoted through controlled transition metal binding that induces select conformational modes. For example, the α-helical amphiphilic cell-lytic peptide, mastoparan X, has been engineered to bind divalent cations resulting in an increase in helical propensity, enhanced lytic potential, and thermodynamic stabilization of the protein structure.36 Therefore, coordination of copper(II) at the N-terminus of α-syn could lock the protein backbone in an α-helical conformation explaining our observed increase in helical content.
Notably, Lansbury and coworkers have shown that toxic variants of α-syn generally have a higher propensity to form an α-helical structure.37 Moreover, Brown and coworkers have reported that α-syn cellular toxicity requires copper(II) binding to the protein.16, 38 This toxicity was also correlated with an increase in the formation of oligomeric species. In separate work, such oligomeric or protofibrillar forms of α-syn were capable of permeabilizing vesicles through the formation of pores in the membrane.39 Therefore, an important direction for future work is to examine the effect of copper binding on membrane integrity.
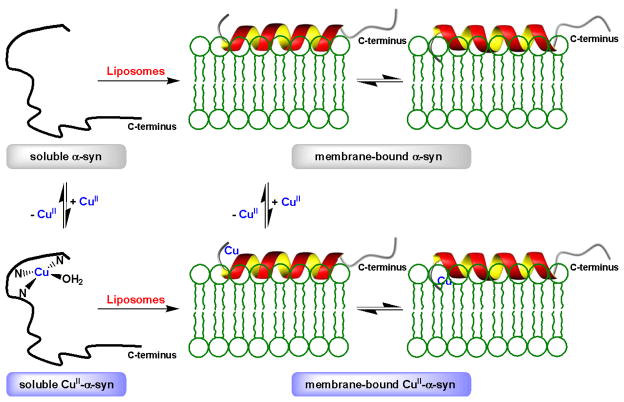
In summary, we show that copper(II) binding to the N-terminus of α-syn affects its helical propensity. Furthermore, this work demonstrates that N-terminal membrane association is a dynamic process at least in regards to the copper-binding site penetrating the vesicle surface (Fig. 4). Since α-syn/membrane interactions are relevant to the protein’s native function, it is possible that alterations in cellular copper levels with age could influence protein conformation leading to increased toxicity. Given that the C-terminus of α-syn is important for assembly of the SNARE complex, this process should not be affected by copper(II) binding. Nevertheless, further work is necessary to probe the effects of metal-induced membrane leakage and/or lipid oxidation that could result from CuI/O2 redox chemistry. Failure of membrane integrity could ultimately result in changes in cellular homeostasis, leading to cell dysfunction, as potential relevance to PD onset and other synucleinopathies.
Fig. 4.
Schematic representation of soluble and membrane-bound α-synuclein (α-syn) and interaction with copper(II). In the soluble form, the N-terminus binds copper(II) in a square planar geometry ligated by the α-amino group and backbone amides. Water is shown as an exogeneous ligand. Upon membrane interaction, α-syn adopts a helical conformation. The N-terminus that comprise the copper-binding site reversibly permeate the membrane surface in order for the vesicle-bound polypeptide to associate copper(II).
Supplementary Material
Acknowledgments
Supported by the Intramural Research Program of the National Institutes of Health, National Heart, Lung, and Blood Institute. We also acknowledge Dr. Duck-Yeon Lee (Biochemistry Core Facility) and Dr. Grzegorz Piszczek (Biophysical Facility) for technical assistance.
Footnotes
Electronic Supplementary Information (ESI) available: [materials and methods]. See DOI: 10.1039/b000000x/
References
- 1.Clayton DF, George JM. J Neurosci Res. 1999;58:120–129. [PubMed] [Google Scholar]
- 2.Maroteaux L, Campanelli JT, Scheller RH. J Neurosci. 1988;8:2804–2815. doi: 10.1523/JNEUROSCI.08-08-02804.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burré J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Sudhof TC. Science. 2010;329:1663–1667. doi: 10.1126/science.1195227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu M, Li J, Fink AL. J Biol Chem. 2003;278:40186–40197. doi: 10.1074/jbc.M305326200. [DOI] [PubMed] [Google Scholar]
- 5.Lee HJ, Choi C, Lee SJ. J Biol Chem. 2002;277:671–678. doi: 10.1074/jbc.M107045200. [DOI] [PubMed] [Google Scholar]
- 6.Bussell R, Jr, Eliezer D. J Biol Chem. 2001;276:45996–46003. doi: 10.1074/jbc.M106777200. [DOI] [PubMed] [Google Scholar]
- 7.Ulmer TS, Bax A, Cole NB, Nussbaum RL. J Biol Chem. 2005;280:9595–9603. doi: 10.1074/jbc.M411805200. [DOI] [PubMed] [Google Scholar]
- 8.Bodner CR, Dobson CM, Bax A. J Mol Biol. 2009;390:775–790. doi: 10.1016/j.jmb.2009.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jao CC, Hegde BG, Chen J, Haworth IS, Langen R. Proc Natl Acad Sci U S A. 2008;105:19666–19671. doi: 10.1073/pnas.0807826105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Georgieva ER, Ramlall TF, Borbat PP, Freed JH, Eliezer D. J Am Chem Soc. 2008;130:12856–12857. doi: 10.1021/ja804517m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drescher M, Veldhuis G, van Rooijen BD, Milikisyants S, Subramaniam V, Huber M. J Am Chem Soc. 2008;130:7796. doi: 10.1021/ja801594s. [DOI] [PubMed] [Google Scholar]
- 12.Trexler AJ, Rhoades E. Biochemistry. 2009;48:2304–2306. doi: 10.1021/bi900114z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferreon ACM, Gambin Y, Lemke EA, Deniz AA. Proc Natl Acad Sci U S A. 2009;106:5645–5650. doi: 10.1073/pnas.0809232106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rhoades E, Ramlall TF, Webb WW, Eliezer D. Biophys J. 2006;90:4692–4700. doi: 10.1529/biophysj.105.079251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spasojevic I, Mojovic M, Stevic Z, Spasic SD, Jones DR, Morina A, Spasic MB. Redox Rep. 2010;15:29–35. doi: 10.1179/174329210X12650506623087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X, Moualla D, Wright JA, Brown DR. J Neurochem. 2010;113:704–714. doi: 10.1111/j.1471-4159.2010.06638.x. [DOI] [PubMed] [Google Scholar]
- 17.Brown DR. Metallomics. 2010;2:186–194. doi: 10.1039/b912601e. [DOI] [PubMed] [Google Scholar]
- 18.Matusch A, Depboylu C, Palm C, Wu B, Hoglinger GU, Schafer MK, Becker JS. J Am Soc Mass Spectrom. 2010;21:161–171. doi: 10.1016/j.jasms.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 19.Barnham KJ, Bush AI. Curr Opin Chem Biol. 2008;12:222–228. doi: 10.1016/j.cbpa.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 20.Santner A, Uversky VN. Metallomics. 2010;2:378–392. doi: 10.1039/b926659c. [DOI] [PubMed] [Google Scholar]
- 21.Wang CS, Liu L, Zhang L, Peng Y, Zhou FM. Biochemistry. 2010;49:8134–8142. doi: 10.1021/bi1010909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lucas HR, Debeer S, Hong MS, Lee JC. J Am Chem Soc. 2010;132:6636–6637. doi: 10.1021/ja101756m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Binolfi A, Rasia RM, Bertoncini CW, Ceolin M, Zweckstetter M, Griesinger C, Jovin TM, Fernandez CO. J Am Chem Soc. 2006;128:9893–9901. doi: 10.1021/ja0618649. [DOI] [PubMed] [Google Scholar]
- 24.Binolfi A, Rodriguez EE, Valensin D, D’Amelio N, Ippoliti E, Obal G, Duran R, Magistrato A, Pritsch O, Zweckstetter M, Valensin G, Carloni P, Quintanar L, Griesinger C, Fernandez CO. Inorg Chem. 2010;49:10668–10679. doi: 10.1021/ic1016752. [DOI] [PubMed] [Google Scholar]
- 25.Drew SC, Leong SL, Pham CL, Tew DJ, Masters CL, Miles LA, Cappai R, Barnham KJ. J Am Chem Soc. 2008;130:7766–7773. doi: 10.1021/ja800708x. [DOI] [PubMed] [Google Scholar]
- 26.Jackson MS, Lee JC. Inorg Chem. 2009;48:9303–9307. doi: 10.1021/ic901157w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lucas HR, Lee JC. J Inorg Biochem. 2010;104:245–249. doi: 10.1016/j.jinorgbio.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rasia RM, Bertoncini CW, Marsh D, Hoyer W, Cherny D, Zweckstetter M, Griesinger C, Jovin TM, Fernandez CO. Proc Natl Acad Sci U S A. 2005;102:4294–4299. doi: 10.1073/pnas.0407881102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kowalik-Jankowska T, Rajewska A, Wisniewska K, Grzonka Z, Jezierska J. J Inorg Biochem. 2005;99:2282–2291. doi: 10.1016/j.jinorgbio.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 30.Davidson WS, Jonas A, Clayton DF, George JM. J Biol Chem. 1998;273:9443–9449. doi: 10.1074/jbc.273.16.9443. [DOI] [PubMed] [Google Scholar]
- 31.Lee JC, Gray HB, Winkler JR. J Am Chem Soc. 2008;130:6898–6899. doi: 10.1021/ja711415b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pfefferkorn CM, Lee JC. J Phys Chem B. 2010;114:4615–4622. doi: 10.1021/jp908092e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee EN, Lee SY, Lee D, Kim J, Paik SR. J Neurochem. 2003;84:1128–1142. doi: 10.1046/j.1471-4159.2003.01612.x. [DOI] [PubMed] [Google Scholar]
- 34.Ghadiri MR, Choi C. J Am Chem Soc. 1990;112:1630–1632. [Google Scholar]
- 35.Shields SB, Franklin SJ. Biochemistry. 2004;43:16086–16091. doi: 10.1021/bi048555k. [DOI] [PubMed] [Google Scholar]
- 36.Signarvic RS, DeGrado WF. J Am Chem Soc. 2009;131:3377–3384. doi: 10.1021/ja809580b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vamvaca K, Volles MJ, Lansbury PT. J Mol Biol. 2009;389:413–424. doi: 10.1016/j.jmb.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wright JA, Wang XY, Brown DR. FASEB J. 2009;23:2384–2393. doi: 10.1096/fj.09-130039. [DOI] [PubMed] [Google Scholar]
- 39.Volles MJ, Lansbury PT. Biochemistry. 2002;41:4595–4602. doi: 10.1021/bi0121353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.