Fig. 3.
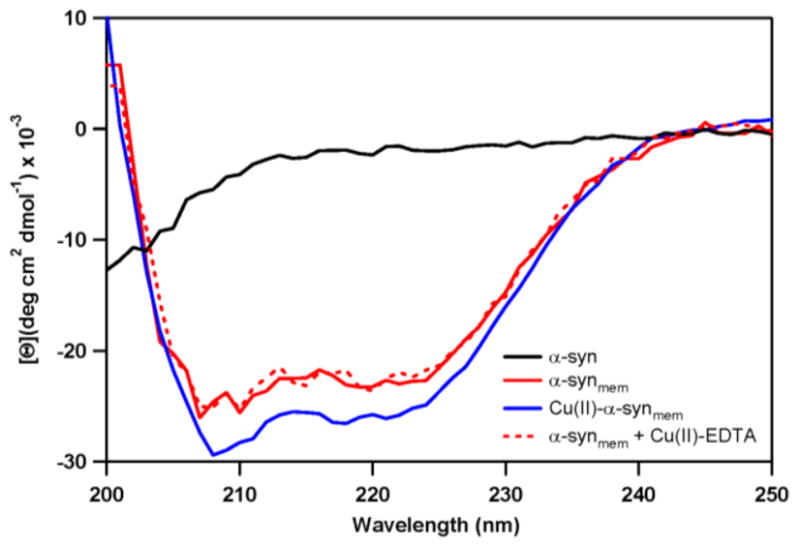
Changes in α-syn secondary structure upon vesicle association and the effect of copper(II) binding, as determined by CD spectroscopy. In solution, α-syn is unstructured (black) and in the presence of POPA:POPC vesicles, the protein adopts an α-helical conformation (solid red). This helical structure increases with coordination of 1 eq. copper(II) (blue). Removal of copper(II) by EDTA reverses this transformation back to the unmetallated membrane-bound protein (red dashes).
