Abstract
Vesicle trafficking is a highly regulated process that transports proteins and other cargoes through eukaryotic cells while maintaining cellular organization and compartmental identity. In order for cargo to reach the correct destination, each step of trafficking must impart specificity. During vesicle formation, this is achieved by coat proteins, which selectively incorporate cargo into the nascent vesicle. Classically, vesicle coats are thought to dissociate shortly after budding. However, recent studies suggest that coat proteins can remain on the vesicle en route to their destination, imparting targeting specificity by physically and functionally interacting with Rab-regulated tethering systems. This review focuses on how interactions among Rab GTPases, tethering factors, SNARE proteins, and vesicle coats contribute to vesicle targeting, fusion, and coat dynamics.
Keywords: Rab GTPase, tether, vesicle trafficking, adaptor, vesicle coat, uncoating
1. Overview of vesicle trafficking
Vesicular traffic allows eukaryotic cells to transport lipids and proteins while preserving organelle identity. Trafficking can be divided into four precisely choreographed stages: budding from a donor membrane; movement through the cell; tethering and docking at a target membrane; and finally, membrane fusion. Budding initiates when coat complexes are recruited to the donor membrane. The three most studied vesicle coats are COPI (coat protein complex I), COPII, and clathrin [1–3]. COPI mediates intra-Golgi traffic and retrograde traffic from the Golgi to the ER. COPII mediates anterograde traffic from the ER to the Golgi, and clathrin coats mediate traffic throughout the endocytic system. Each coat is composed of an outer coat and an inner adaptor coat (Table 1). Adaptors directly couple cargo selection to vesicle formation by binding proteins that contain sorting motifs and by recruiting an outer coat that aids in the initiation and stabilization of membrane curvature. While the outer coats of COPI and COPII each bind a single set of adaptors, clathrin binds various heterotetrameric APs (adaptor protein complexes) and alternative monomeric adaptors (Table 1). Once formed, the vesicle pinches off from the donor membrane and moves through the cell, often via cytoskeleton-motor systems [4]. Conventionally, vesicle uncoating is thought to occur concurrently with budding or shortly thereafter, to expose the tethering and fusion machinery. At the target membrane, tethering factors make initial contacts between membranes. Tethering is followed by docking. During docking, SNARE (soluble N-ethylmaleimide sensitive factor attachment protein receptor) proteins on both membranes zipper together into a tight four helix bundle, or trans-SNARE complex, which initiates fusion by triggering lipid and content mixing [5,6].
Table 1. Coat subunits.
COPI, COPII, and clathrin are composed of outer coats and inner adaptor coats, whereas retromer is composed of a cargo recognition complex and sorting nexins. Superscript letters denote coat subunits that interact with tethers or a Rab GEF (a, TRAPPI; b, TRAPPII; c, COG; d, Dsl1; e, HOPS; f, Grhl1; g, p115; h, R6IP1; I, hRME-6). In addition to APs, clathrin also interacts with alternative adaptors (GGA1-3, Hrs, and Dab2) and CLASPs (clathrin-associated sorting proteins) that function in concert with AP-2 (not shown; reviewed in [144]). CHC, clathrin heavy chain; LC, light chain; GGA, Golgi-localized gamma-ear-containing Arf-binding protein
| COPII | COPI | Clathrin | Retromer | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mammals | Yeast | Mammals | Yeast | Mammals | Yeast | Mammals | Yeast | ||
|
|
|
||||||||
| Outer Coat | Sec13 | Sec13 | α-COP | Cop1b,d | CHC | Chc1 | Sorting Nexins | SNX1h | Vps5 |
| Sec31 | Sec31 | β′-COP | Sec27 | LC | Clc1 | SNX2 | |||
| ε-COP | Sec28 | SNX5 | Vps17 | ||||||
| SNX6 | |||||||||
|
|
|
||||||||
| Inner Adaptor Coat | AP1-4: | AP1-3: | Cargo Recognition Complex | VPS26 | Vps26 | ||||
| Sec23a | Sec23 a, f | β-COP c, g | Sec26 | β1-4 | Apl1, Apl2, | VPS29 | Vps29 | ||
| Sec24 | Sec24 f | δ-COP | Ret2 d | μ1-4 | Apl6 | VPS35 | Vps35 | ||
| γ-COP b | Sec21 c | γ, α i, δ, ε | Apm1-3 | ||||||
| ζ-COP | Ret3 | σ1-4 | Apl3, Apl4, Apl5 e | ||||||
| Alternative Adaptors | GGA1-3 | Aps1-3 | |||||||
| Hrs | |||||||||
| Dab2 | Gga1, Gga2 | ||||||||
| Vps27 | |||||||||
Vesicles are targeted to specific destinations through interactions with the tethering and fusion machinery. Besides the tethers themselves, tethering usually involves small G-proteins of the Rab/Ypt family. Each Rab has a characteristic membrane localization within the cell and regulates specific tethering and docking events [7]. Rabs are molecular switches that cycle through inactive GDP-bound and active GTP-bound states; this cycle is catalyzed by GEFs (guanine nucleotide exchange factors) and GAPs (GTPase activating proteins) [8]. Rabs also cycle between the cytosol and membranes through interactions with the cytosolic chaperone GDI (GDP dissociation inhibitor). GTP binding activates the Rab, allowing it to bind effector proteins including tethers. Tethers are long coiled-coil proteins or large multi-subunit tethering complexes (MTCs) thought to function as physical bridges between two membranes. Although many putative tethers promote tethering in vivo or in cell-free assays that employ purified organelles, few have been rigorously shown to mechanically stabilize membrane-membrane contacts in chemically-defined systems. Proteins known to directly tether membranes include the HOPS (homotypic fusion and vacuole protein sorting) complex [9,10] and the coiled-coil tether GMAP-210 [11]. Tethers are also thought to impart specificity to targeting by forming a network of interactions between Rabs, SNAREs, and specific lipids. SNAREs provide a final layer of specificity [12], as only certain cognate SNAREs catalyze fusion [13].
Vesicle coats may also impart specificity to vesicle targeting. Most adaptor coats are recruited to the membrane primarily through interactions with activated Arf or Sar small GTPases [14–22]. As Arf/Sar activation results in COPI and COPII coat assembly, uncoating was thought to be a direct consequence of GAP-stimulated GTP hydrolysis and a prerequisite for tethering and fusion. This model was largely based on cell-free assays of COPI and COPII trafficking, which demonstrated that fusion could not occur if coats were stabilized by preventing hydrolysis of Arf-GTP or Sar-GTP [3,23–26]. It was inferred that vesicle coats occluded factors required for tethering and fusion. Similarly, outer clathrin coats are disassembled by auxilin and the ATPase Hsp70 [27]. Cells lacking auxilin accumulate clathrin-coated vesicles (CCVs) [28], suggesting that clathrin must also be removed prior to tethering and fusion. However, recent evidence suggests that some vesicles retain at least portions of their coats throughout trafficking [29–31], with targeting specificity achieved through interactions between vesicle coats and the docking and fusion machinery. Here, we focus on Rabs and Rab tethers that interact with coat proteins and how these interactions influence tethering and uncoating.
2. Multi-subunit Rab tethers
Several MTCs have been identified in eukaryotes, including TRAPPI-III, HOPS, CORVET, Exocyst, COG, GARP, and Dsl1 [32]. Each MTC peripherally associates with membranes, controls specific trafficking pathways, and has been hypothesized or demonstrated to act as a tether. With the exception of Dsl1, MTCs bind Rabs as effectors, GEFs, or both. Most MTCs also interact with SNAREs or SNARE-binding SM (Sec1/Munc18) proteins to facilitate docking. TRAPPI, TRAPPII, COG, Dsl1, and HOPS also have been shown to interact with adaptor coats (Figure 1, Table 2), suggesting that MTCs are central organizers of vesicle consumption at target membranes.
Figure 1. Overview of known coat-tether-Rab interactions.
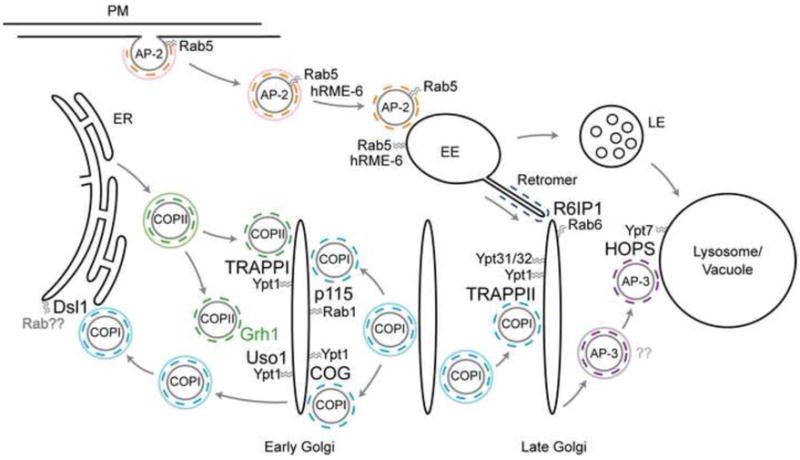
Coat-tether interactions have been studied in both yeast and mammalian cells and are presented as a composite model. While not depicted, TRAPP is required for homotypic COPII vesicle fusion and subsequent VTC formation in mammalian cells [37]. Rabs are listed as the yeast homolog except in cases where the interaction between the Rab, tether, and coat have been identified only in mammalian cells. Solid and dashed colored circles correspond to outer and adaptor coats, respectively. PM, plasma membrane; EE, early endosome; LE, late endosome.
Table 2. Tethers in yeast and mammals.
This table lists tethers discussed in this review along with known Rab and coat interactions. Coat subunits in parentheses are reported to bind tethers, although it is unclear whether these interactions are direct. Rab1, Rab5, Rab6, Rab7, and Rab11 are mammalian homologs of yeast Ypt1, Vps21, Ypt6, Ypt7, and Ypt31/32
| Yeast
|
Mammals
|
||||||
|---|---|---|---|---|---|---|---|
| Tether | Rab | Coat | Tether | Rab | Coat | ||
| Multi-Subunit Tethers (MTCs) | TRAPPI | Ypt1 | COPII: Sec23 | TRAPPI? | Rab1? | COPII: Sec23 | |
| TRAPPII | Ypt1, Ypt31, Ypt32 | COPI: (Cop1) | TRAPPII | Rab1 | COPI: γ-COP | ||
| COG | Ypt1 | COPI: Sec21 | COG | Rab1, Rab6? | COP: (β-COP) | ||
| Dsl1 | ? | COPI: Cop1, Ret2 | Syntaxin-18 | Rab6? | ? | ||
| HOPS | Ypt7 | AP-3: Apl5 | HOPS | Rab5, Rab7 | ? | ||
|
| |||||||
| Coiled-Coil Tethers | Grh1 | ? | COPII: (Sec23, Sec24) | GRASP65 | Rab1 | ? | |
| Uso1 | Ypt1 | ? | p115 | Rab1 | COPI: β-COP | ||
| ? | ? | ? | R6IP1 | Rab6, Rab11? | Retromer; SNX1 | ||
2.1. TRAPPI - COPII
Three TRAPP (transport protein particle) complexes (I–III) have been identified in yeast. Although the TRAPP subunits are evolutionarily conserved, only TRAPPII has been characterized in mammalian cells. Each TRAPP complex comprises six core subunits: Trs20, Trs23, Trs31, Trs33, Bet3, and Bet5. Trs85, originally considered a TRAPP core subunit, was recently shown to be a unique subunit of TRAPPIII, which promotes autophagosome formation [33]. Yeast TRAPPI facilitates transport of ER-derived COPII vesicles to the early Golgi [34,35]. It consists solely of the TRAPP core, with two copies of Bet3 and a single copy of the remaining subunits. TRAPPI resembles a dumbbell, with a copy of Bet3 at each end [36].
Bet3 binds directly to the COPII adaptor coat subunit, Sec23, and in an in vitro reconstitution, excess Sec23 blocks vesicle tethering at the Golgi [31]. These observations suggest models in which COPII remains on the vesicle throughout trafficking and binds TRAPPI at the target membrane. The role of Bet3 in binding COPII may explain its symmetric distribution in TRAPPI. In mammals, TRAPP promotes homotypic fusion of COPII vesicles to form VTCs (vesicular tubular clusters) [37], and each copy of Bet3 likely binds Sec23 found on opposing vesicles. It is less clear how such a mechanism would support heterotypic fusion of COPII vesicles at the Golgi. While many tethering complexes are Rab effectors, TRAPPI has only been shown to act as a Ypt1 GEF [38,39]. Four of six core subunits are required for GEF activity: Trs23, Trs31, Bet3, and Bet5 [40]. In addition to Rab activation and COPII-binding, Bet3 is required for TRAPPI membrane localization [36,41]. The involvement of Bet3 in adaptor binding, membrane association, and nucleotide exchange suggest that these events may be closely coupled.
Unlike other multisubunit tethers, TRAPPI is not known to interact with SNAREs or SNARE cofactors. TRAPPI may indirectly promote SNARE function by recruiting the coiled-coil tether Uso1, an ortholog of mammalian p115 (See Section 3.2). Thus, TRAPPI and Uso1/p115 may have distinct, but coupled, roles in COPII targeting.
2.2 TRAPPII - COPI
TRAPPII comprises the TRAPP core plus Trs65, Trs120, and Trs130 [38]and is thought to mediate trafficking within the Golgi, from late Golgi, and from early endosomes to Golgi [40,42]. Impaired Trs130 function results in vesicle accumulation in yeast and mammalian cells [43,44]. It remains unclear whether TRAPPII acts as a GEF for Ypt1/Rab alone [40,44,45] or also for Ypt31 and Ypt32, Rabs required for trafficking to the late Golgi [39,42,46]. This discrepancy might be explained by the co-purification of additional proteins, such as the sedlin family protein, Tca17 [47].
Even if each TRAPP is a Ypt1 GEF, targeting specificity may be achieved through binding to unique adaptors. Mammalian and yeast TRAPPII bind COPI [43,44]. In mammalian cells, Trs130 and Trs120 directly bind the COPI adaptor γ-COP [44]. However, TRAPPII, the sole TRAPP complex characterized in mammalian cells, may tether both COPI and COPII vesicles. Mammalian Bet3 directly binds Sec23 and is required for homotypic COPII fusion in vitro [31,37].
2.3 COG - COPI
The COG (conserved oligomeric Golgi) complex comprises 8 subunits (Cog1-8). COG mediates retrograde traffic from the late to early Golgi and regulates Golgi morphology [48,49]. COG interacts with Ypt1/Rab1 as an effector [48], and may also interact with Rab6 [50]. The yeast COG subunit Cog2 binds the COPI adaptor subunit Sec21, and Cog3 binds β-COP in mammalian cells [48,51]. Mutations in yeast COG, or knockdown of mammalian Cog3, result in accumulation of small vesicles, and a subset of vesicles appear to be coated with COPI in mammalian cells [51,52]. Yeast and mammalian COG bind SNARE complexes containing Sed5/Syntaxin5. Cog7 knockdown results in reduced SNARE complex abundance [53], likely due to defects in SNARE complex assembly or stability.
COG is also implicated in COPII trafficking. COG binds p115/Uso1 [49], and Cog2 is required for Uso1-mediated COPII transport [54]. The involvement of COG in both COPI and COPII transport is supported by its ability to bind SNAREs involved in intra-Golgi and ER-Golgi transport [48,53]. While the function of COG in COPII trafficking remains unclear, its interaction with p115/Uso1 may modulate the affinity of COG for different SNAREs or vesicle coats.
2.4 Dsl1 - COPI
The Dsl1 complex is a yeast MTC required for trafficking of COPI vesicles from the Golgi to the ER [55–57] and is homologous to the mammalian syntaxin 18 complex [58]. The smallest of the MTCs, it comprises 3 subunits (Dsl1, Sec39, Tip20) that stably interact with 3 SNAREs (Sec20, Ufe1, Use1) [57]. Crystal structures of the Dsl1 subunits have been modeled into a single-particle EM density map of the complex [59,60]. The complex resembles a clothespin in which Tip20 and Sec39 bind membrane-bound SNAREs, while Dsl1 bridges the membrane distal regions of Tip20 and Sec39 [59]. Dsl1 projects ~200 Å from the membrane and contains a flexible loop that binds COPI [30,61]. Dsl1 also contains a flexible hinge, consistent with EM images showing the complex in “closed” and “open” conformations. The Ds1l complex can catalyze SNARE complex formation, so this flexibility might be used to assemble SNAREs on the ER membrane [59]. Alternatively, this flexibility may be harnessed to pull the COPI vesicle closer to the ER membrane.
While most tether-coat interactions involve direct interactions with adaptors, Dsl1 binds both the COPI adaptor subunit Ret2 and the outer coat subunit Cop1 [30,61]. This may be explained by the unique dynamics of COPI. Unlike COPII and clathrin, which undergo stepwise assembly of the inner and outer coats, COPI is recruited en bloc as a stable complex [62]. Thus, it may be expected that COPI dissociates from the vesicle as an intact complex. Yeast Cop1 also binds TRAPPII, suggesting that all COPI tethers (Dsl1, TRAPPII, COG, p115) interact with both adaptor and outer coats [43]. Dsl1 has not been shown to directly catalyze COPI uncoating, but Dsl1 knockdown results in accumulation of COPI coated vesicles [30]. Dsl1 binds a region of Cop1 that interacts with other COPI subunits [30,63] and might promote uncoating by disrupting inter- or intra- molecular interactions among COPI subunits.
The Dsl1 complex is the only MTC not known to interact with a Rab. Ypt1 is implicated in COPI trafficking to the ER [64], but it remains unclear whether the Dsl1 complex binds Ypt1. Another possible candidate is Rab6/Ypt6. Rab6 is required for Golgi fragmentation that results from knockdown of ZW10, the mammalian Dsl1 ortholog [50]. The lack of an identified Rab in Dsl1 complex tethering raises two important questions: what is the function of Rabs in tethering, and can proper tethering occur in absence of a Rab? Besides mediating membrane association of tethers, Rabs can impose temporal regulation on a tethering event. As the Dsl1 complex stably associates with SNAREs on the ER, it is unclear if the Dsl1 complex recycles on and off the membrane. The Dsl1 complex may be regulated in another manner (e.g., phosphorylation) to avoid constitutive activation of tethering.
2.5 HOPS - AP-3
HOPS is a hexameric tethering complex composed of four core subunits (Vps11, Vps16, Vps18, and Vps33) and two additional proteins, Vps39 and Vps41 [65–67]. In yeast, HOPS is required for most fusion events at the vacuolar lysosome, including homotypic fusion between vacuoles and heterotypic fusion with autophagosomes, late endosomes, and AP-3 vesicles [65,68]. HOPS tethers isolated vacuoles [69] and vacuole-like proteoliposomes [9,10]. The Rab Ypt7, along with phosphoinositides, recruits HOPS to membranes. Vps41 directly binds Ypt7-GTP [70,71]. Vps39 was reported to acts at as a Ypt7 GEF [66,72], though highly purified Vps39 and HOPS fail to show GEF activity, suggesting that additional factors are required (Merz lab, unpublished data). HOPS functions in docking by promoting trans-SNARE assembly [9,69,73–76]. HOPS is the only MTC that contains a SNARE-interacting SM protein (Vps33), yet it is unclear how Vps33 contributes to SNARE complex assembly or stabilization [74,75,77]. HOPS may also shield trans-SNARE complexes from disassembly by Sec17 (α-SNAP) and Sec18 (NSF), allowing the complexes to persist until they initiate lipid and content mixing [76,78–80].
The Rab-binding subunit Vps41 also binds Apl5, a subunit of the AP-3 adaptor coat [29,81,82]. In yeast, AP-3 mediates trafficking from the late Golgi to the vacuole. Because AP-3 trafficking is clathrin-independent in yeast and Vps41 contains a region homologous to clathrin heavy chain [83], it was hypothesized that Vps41, independently of HOPS, might form a clathrin-like outer coat [81,82]. However, subsequent studies demonstrated that yeast Apl5 preferentially binds Vps41 within the context of HOPS rather than as a monomer [29]. Similarly, HOPS and AP-3 co-immunoprecipitate from mammalian cells [84]. Furthermore, yeast cells lacking Vps41 accumulate AP-3 coated vesicles [29]. These results suggest that HOPS tethers AP-3 vesicles at the vacuole target membrane, with the AP-3 coat retained on the vesicle at least until tethering has occurred.
The Yck3 kinase and Vps41 are the only proteins, other than AP-3 itself, known to have selective roles in AP-3 cargo sorting from the Golgi to the vacuole [82,85,86]. Phosphorylation of Vps41 by Yck3 results in loss of HOPS from membranes [71,87–89]. While cells lacking Yck3 exhibit normal or hyperactive vacuole fusion, they also accumulate endosomal structures adjacent to the vacuole and are partially defective in AP-3 trafficking [71,86,87,89]. These data suggest that phosphorylation of Vps41 by Yck3, and subsequent loss of HOPS from the vacuole, promotes heterotypic fusion while attenuating homotypic fusion. Although HOPS does not detectably localize to AP-3 vesicles [29], it is possible that AP-3 recruits sub-stoichiometric amounts of cytosolic HOPS to the vesicle, and tethering proceeds through HOPS oligomerization.
3. Coiled-coil Rab tethers
Coiled-coil tethers are proteins with extended α-helical structures that often dimerize with themselves and other coiled-coil tethers. Many are found at the Golgi, where they are required for proper Golgi morphology and stack formation. Similar to the MTCs, coiled-coil tethers also interact with Rabs, SNAREs, and vesicle coats (Figure 1, Table 2).
3.1. Grh1 - COPII
Grh1 is the yeast ortholog of the mammalian coiled-coil tether GRASP65. GRASP65 recruits GM130, another coiled-coil tether, to the early Golgi, where they promote a variety of tethering events [90]. Yeast lack a clear GM130 homolog, but Grhl binds another coiled-coil protein, Bug1 [91]. GRASP65 and GM130 bind Rab1-GTP [92], but it is unknown whether Grh1 and Bug1 interact with Ypt1. Similar to TRAPPI, COG, and p115/Uso1, Grh1 is required for COPII tethering. Grh1 co-purifies with the COPII adaptor subunits Sec23 and Sec24, and COPII vesicles formed in vitro contain Grh1, suggesting that this coat-tether interaction recruits Grh1 to ER-derived vesicles. Deletion of Grh1 or Bug1 does not affect COPII budding, but reduces the amount of vesicles involved in Uso1-dependent tethering [91]. SLY1-20, a dominant allele encoding an SM protein that promotes SNARE pairing, rescues the lethality of mutants lacking Uso1 [93] or Ypt1 [94]. However, in absence of Bug1 or Grh1, Sly1-20 no longer rescues [91]. While Sly1-20 may compensate for a SNARE-related docking function of Uso1, Grh1 may be needed to mechanically tether membranes. However, the requirement for Grh1 in the presence of wild type Uso1 suggests that the function of Grh1 is not redundant, but additive, with Uso1.
3.2. p115 - COPI
Like COG, p115 is implicated in both COPI and COPII tethering [95–97]. The COPI subunit β-COP structurally resembles the large subunits of the AP clathrin adaptors. It consists of a conserved N-terminal trunk that interacts with the other adaptor subunits and a C-terminal “ear” domain attached to the N-terminal domain through a flexible linker. Both β-COP and AP ear domains contain F/W motifs that bind accessory proteins [98]. p115 directly binds the F/W motif on β-COP [99]. Like knockdown of p115, expression of β-COP with a mutation in its F/W motif results in coalescence of early and medial Golgi markers and defective anterograde trafficking.
p115/Uso1 is also required for heterotypic fusion of COPII vesicles at the Golgi [95] and homotypic tethering of COPII vesicles [100]. p115 binds Rab1-GTP [96], and in vitro, GDI-mediated Ypt1 extraction releases Uso1 from membranes [95]. These findings suggest that activation of Rab1/Ypt1, presumably by TRAPPI, is required for p115/Uso1 membrane recruitment. While p115/Uso1 promotes packaging of SNAREs and other cargo into COPII vesicles [96,101], it also stimulates SNARE assembly at the target membrane. Uso1-deficient yeast cannot form SNARE complexes required for COPII fusion [93]. In mammals, p115 interacts with SNAREs, catalyzes SNARE complex formation [102], and is required for proper Golgi assembly and anterograde trafficking [103]. Despite the role of p115/Uso1 in COPII trafficking, it is unknown whether p115/Uso1 directly interacts with COPII.
The ability of p115 to bind COPI and COPII vesicles may be influenced by additional tethers. p115 not only binds the coiled-coil tethers giantin and GM130, but also COG [49,104]. COPI and COG bind distinct regions on p115 [49,99], suggesting that these interactions could occur simultaneously. Similarly, another coiled-coil tether, CASP, may promote tethering of both COPI and COPII vesicles. Golgi-localized CASP tethers uncoated COPI vesicles that contain the coiled-coil tether golgin-84 [105,106], but CASP also copurifies with the COPII adaptor Sec23 [105]. The ability of a tether to function in more than one context does not necessarily preclude a role in targeting specificity, as interactions with other factors may modulate its ability to target specific vesicle populations.
3.3. R6IP1 - retromer
Retromer is a heteropentameric coat complex involved in retrograde transport from endosomes to the TGN (trans-Golgi network) [107]. Like other coats, retromer is capable of cargo sorting, self-assembly, and membrane deformation. However, instead of vesicles, retromer drives the extrusion of endosomal membrane tubules [108]. Retromer is composed of two subcomplexes not readily categorized as adaptors or outer coats. Sorting nexins mediate binding of the cargo recognition complex (Vps26, Vps29, and Vps35) to the Golgi. While one pair of sorting nexins (Vps5 and Vps17) provides this function in yeast, mammalian cells contain two orthologs of each protein (SNX1, SNX2 and SNX5, SNX6; Table 1) [107,109]. Sorting nexins contain BAR (Bin/Amphiphysin/Rvs) domains that dimerize to create a concave surface that contacts the membrane [110]. In addition to recruitment of the cargo recognition complex, SNX1 induces tubulation of endosomes in vitro [111].
R6IP1 (Rab6 interacting protein 1) is a predicted coiled-coil protein recruited to Golgi by Rab6-GTP [112,113]. R6IP1 also binds Rab11-GTP [112], but R6IP1 has not been detected on recycling endosomes. Overexpression of the Rab6 binding domain of R6IP1 inhibits retrograde transport from endosomes to the TGN [113]. R6IP1 directly interacts with the retromer subunit SNX1. Knockdown of R6IP1 results in dispersal of SNX1 positive endosomes from areas adjacent to the TGN to the cell periphery [109], implying that R6IP1 acts at the TGN to tether retromer-coated tubules. It is unclear whether the R6IP1-SNX1 interaction modulates the ability of SNX1 to bind or deform membranes. As yeast contains retromer but lacks obvious R6IP1 homologs, it is unknown which yeast proteins provide this tethering function. Overall, these data suggest that interactions between coats and tethers may be widely employed to correctly traffic cargo, regardless of the trafficking modality.
4. Rab-mediated uncoating
Rabs are implicated at almost every stage of trafficking. In addition to the recruitment of tethers, Rabs regulate cargo recruitment during budding and facilitate connections between vesicles and cytoskeleton motors [7]. More recently, Rabs have been implicated in vesicle uncoating. These studies suggest that Rabs are key regulators of vesicle trafficking and may couple vesicle uncoating to tethering and fusion at target membranes.
4.1. hRME-6 and AP-2
The AP-2 adaptor mediates clathrin-dependent internalization of endocytic cargo from the plasma membrane [114]. Unlike other adaptors, AP-2 is not recruited to the membrane by an activated Arf/Sar GTPase, but by direct binding of AP-2 α and μ2 subunits to cargo and PtdIns(4,5)P2 [115,116]. Upon membrane binding, μ2 is phosphorylated by AAK1 (adaptor associated kinase 1) [117]. Phosphorylated μ2 binds both PtdIns(4,5)P2 and cargo with increased affinity, thereby stabilizing AP-2 on the membrane [118,119]. Protein phosphatase 2A (PP2A) dephosphorylates μ2 in vivo [120] and promotes AP-2 uncoating in vitro [121]. Thus, the membrane association of AP-2 is controlled by its phosphorylation state, as opposed to the nucleotide state of Arf/Sar. This phosphorylation is further regulated by Rab5 and its GEF, hRME-6 (human receptor mediated endocytosis 6) [122]. hRME-6 competes with AAK1 for a site on the α-ear, resulting in decreased μ2 phosphorylation and weakened membrane association. α-ear binding stimulates the Rab5 GEF activity of hRME-6. A dominant negative Rab5 increases PtdIns(4,5)P2 levels, suggesting that activated Rab5 likely downregulates PtdIns (4,5)P2 levels by recruiting a lipid phosphatase (e.g. synaptojanin). Thus it appears that both Rab activation and downstream Rab functions contribute to uncoating. Additional experiments will be needed to determine when uncoating occurs, as both hRME-6 and Rab5 are present on CCVs and early endosomes [123,124]. Rabs may also regulate the membrane association of other adaptors. Coats are stabilized on membranes by an array of interactions (see Section 4.2). A Rab or Rab GEF’s ability to compete for any of these interactions may stimulate coat release. Since Rab activation is often a prerequisite for tethering and fusion, it is tempting to hypothesize that uncoating is coupled to these events at the target membrane.
4.2. Implications for uncoating
Coat-tether interactions and the accumulation of coated vesicles in the absence of tethering have expanded our view of when and where uncoating occurs (Figure 2). The historical view of vesicle uncoating occurring concurrently with or immediately after budding (Figure 2a) is not necessarily at odds with recent experiments; it remains unclear whether coat-tether interactions recruit tethers to vesicles or whether coats interact with tethers exclusively at target membranes. Moreover, some trafficking events probably require a tether-coat interaction, while others do not.
Figure 2. Models of vesicle uncoating.
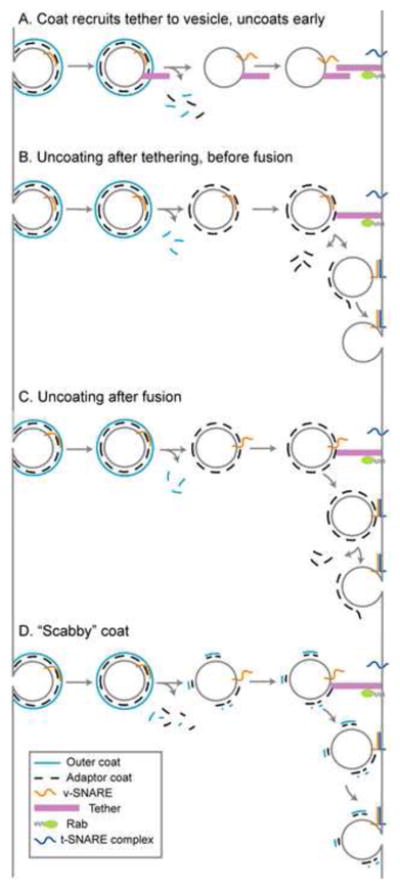
See Section 4.2 for discussion. v-SNARE, vesicle SNARE; t-SNARE, target SNARE.
Pivotal studies demonstrated that blocking GTP hydrolysis inhibits uncoating and subsequent fusion [3,23–26]. However, other evidence suggests that GTP hydrolysis is required early in budding for cargo sorting [125–127] and membrane deformation [128]. Furthermore, purified CCVs contain little Arf1 [129], and COPII coated vesicles formed in vitro remain coated, even though they contain almost no Sar1 [3]. Thus, if coats are retained on vesicles throughout trafficking (Figure 2b–d), retention is likely achieved through Arf/Sar-independent interactions with cargo, SNAREs, lipids, and other factors [130,131]. For some trafficking events, calcium may play a role in coat retention. Calcium chelation results in decreased retention of the COPII outer coat subunit Sec31 but not the inner coat subunit Sec23 [132]. Chelation of calcium also results in the loss of COPI from the Golgi in vivo and loss of COPI from vesicles formed in vitro [133]. Coat retention might be mediated by calcium sensing proteins, such as ALG-2 on the vesicle surface [132], though the relevant mechanisms of calcium release and detection are unclear.
If coats remain on vesicles throughout trafficking, uncoating may be coupled to tethering, docking, or fusion (Figure 2b). Dsl1 likely plays a key role in COPI uncoating (see Section 2.4). Similar to hRME-6 binding AP-2, binding of tethers to adaptor ears may activate other functions of the tether or compete with binding of accessory proteins. HOPS and p115 bind ear domains within AP-3 and COPI, however, further work is needed to sort out the functional consequences of these interactions. SNARE complex formation may also occur in the presence of adaptor coats. SNAREs can stably pair once membranes approach to within ~80 Å [134,135]. On COPII vesicles, Sec23/24 adaptor coats extend ~40–60 Å from the vesicle surface [136], suggesting that SNARE zippering might initiate prior to uncoating. However, it remains unclear whether SNAREs can pair under these conditions. SNAREs are loaded into vesicles by adaptor coats, and some of these interactions involve the membrane distal N-termini of SNAREs [137]. Furthermore, if adaptors continue to bind SNAREs through tethering and docking, SNARE pairing might be coupled to uncoating. Formation of trans-SNARE complexes may weaken interactions between unpaired SNAREs and adaptors, contributing to uncoating. It remains possible that full uncoating does not occur until after SNARE mediated fusion (Figure 2c). In this scenario, uncoating may be catalyzed by decreased membrane curvature, changes in phosphoinositide composition, phosphorylation of proteins and lipids, or other factors at the target membrane.
To what extent is a vesicle coated, and would the presence of a coat prevent tethering and fusion? Outer coats seem to form a complete shell around the vesicle, but the stoichiometry of adaptor subunits to outer coat subunits is less clear. Formation of CCVs in vitro requires a 1:1 stoichiometry of clathrin and adaptors [138], and EM analyses of CCVs suggested that adaptor layers are tightly packed [139]. However, higher resolution EM data lacked the AP density expected for a stoichiometric interaction [140]. This result is supported by fluorescence microscopy of clathrin coats. Clathrin and AP-2 are evenly distributed at the beginning of CCV formation, but AP-2 subsequently becomes polarized away from the site of vesicle formation [141]. Thus, adaptors do not necessarily form an unbroken mesh around the membrane that would occlude the tethering and fusion machinery.
It is possible that a majority of the adaptor coat is shed concurrently with the outer coat, leaving a “scabby coat” required for tethering (Figure 2d). This scabby coat may consist of only the adaptor or both adaptor and outer coats. Clathrin adaptors were reported to remain on vesicles after clathrin uncoating [142], but in more recent experiments, clathrin and AP-2 appeared to uncoat together [143]. Furthermore, TRAPPI interacts with Sec23 on COPII vesicles that still contain detectable amounts of the outer coat subunit Sec31 [31]. As coat formation appears to be asymmetrical, the scabby coat might correspond to a special region of the coat that was deposited during the initiation or termination of coat formation and thereby marked for retention on the vesicle.
5. Conclusions
Tethers are emerging as nexuses that link Rabs, SNAREs, and vesicle coats to promote membrane association, SNARE assembly, and ultimately, targeting specificity. However, the spatiotemporal dynamics and consequences of these interactions remain unclear. Does the coat simply recruit a tether to the vesicle, or does the coat directly participate in tethering by interacting with tethers on the target membrane? Several pathways employ more than one tether. One possibility is that different combinations of tethers contribute to trafficking specificity by binding a subset of vesicles containing specific cargo. Conversely, tether-tether interactions could increase reliability by providing backup mechanisms for vesicles that have fully shed their coats. A third possibility is that each tether executes diverse functions. For example, TRAPPI provides Ypt1 GEF activity and coat binding, while Uso1 promotes SNARE pairing. For at least a subset of trafficking pathways, tethering is a prerequisite for uncoating. However, it is generally unclear whether uncoating is directly coupled to tethering or whether it occurs during, or after, fusion. Furthermore, the involvement of Rabs in tethering and uncoating suggest that they may link these processes to enhance spatiotemporal regulation throughout trafficking.
Acknowledgments
We thank Drs. C. Barlowe, J. Hay, T. Kirchhausen, G. Payne, and our colleagues in the Merz lab for insightful discussions and critical readings of the manuscript. Our work is funded by NIH grant GM077349. AJM is an American Cancer Society Research Scholar.
Abbreviations
- AP
adaptor protein complex
- CCV
clathrin-coated vesicle
- COG
conserved oligomeric Golgi
- COP
coat protein complex
- GAP
GTPase activating protein
- GEF
guanine nucleotide exchange factor
- HOPS
homotypic fusion and vacuole protein sorting
- hRME-6
human receptor mediated endocytosis 6
- MTC
multi-subunit tethering complex
- R6IP1
Rab6 interacting protein 1
- SM
Sec1/Munc18
- SNARE
soluble N-ethylmaleimide sensitive factor attachment protein receptor
- TGN
trans-Golgi network
- TRAPP
transport protein particle
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pearse BM. Coated vesicles from pig brain: purification and biochemical characterization. J Mol Biol. 1975;97:93–98. doi: 10.1016/s0022-2836(75)80024-6. [DOI] [PubMed] [Google Scholar]
- 2.Malhotra V, Serafini T, Orci L, Shepherd JC, Rothman JE. Purification of a novel class of coated vesicles mediating biosynthetic protein transport through the Golgi stack. Cell. 1989;58:329–36. doi: 10.1016/0092-8674(89)90847-7. [DOI] [PubMed] [Google Scholar]
- 3.Barlowe C, Orci L, Yeung T, Hosobuchi M, Hamamoto S, Salama N, et al. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994;77:895–907. doi: 10.1016/0092-8674(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 4.Hehnly H, Stamnes M. Regulating cytoskeleton-based vesicle motility. FEBS Lett. 2007;581:2112–18. doi: 10.1016/j.febslet.2007.01.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, et al. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–72. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- 6.Sutton RB, Fasshauer D, Jahn R, Brunger AT. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature. 1998;395:347–53. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- 7.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–25. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 8.Barr F, Lambright DG. Rab GEFs and GAPs. Curr Opin Cell Biol. 2010 doi: 10.1016/j.ceb.2010.04.007. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stroupe C, Hickey CM, Mima J, Burfeind AS, Wickner W. Minimal membrane docking requirements revealed by reconstitution of Rab GTPase-dependent membrane fusion from purified components. Proc Natl Acad Sci U S A. 2009;106:17626–33. doi: 10.1073/pnas.0903801106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hickey CM, Wickner W. HOPS Initiates Vacuole Docking by Tethering Membranes Prior to trans-SNARE Complex Assembly. Mol Biol Cell. 2010;21:2297–305. doi: 10.1091/mbc.E10-01-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drin G, Morello V, Casella JF, Gounon P, Antonny B. Asymmetric tethering of flat and curved lipid membranes by a golgin. Science. 2008;320:670–73. doi: 10.1126/science.1155821. [DOI] [PubMed] [Google Scholar]
- 12.Sollner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, et al. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–24. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- 13.McNew JA, Parlati F, Fukuda R, Johnston RJ, Paz K, Paumet F, et al. Compartmental specificity of cellular membrane fusion encoded in SNARE proteins. Nature. 2000;407:153–59. doi: 10.1038/35025000. [DOI] [PubMed] [Google Scholar]
- 14.Nakano A, Muramatsu M. A novel GTP-binding protein, Sar1p, is involved in transport from the endoplasmic reticulum to the Golgi apparatus. J Cell Biol. 1989;109:2677–91. doi: 10.1083/jcb.109.6.2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donaldson JG, Cassel D, Kahn RA, Klausner RD. ADP-ribosylation factor, a small GTP-binding protein, is required for binding of the coatomer protein beta-COP to Golgi membranes. Proc Natl Acad Sci U S A. 1992;89:6408–12. doi: 10.1073/pnas.89.14.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barlowe C, Schekman R. SEC12 encodes a guanine-nucleotide-exchange factor essential for transport vesicle budding from the ER. Nature. 1993;365:347–49. doi: 10.1038/365347a0. [DOI] [PubMed] [Google Scholar]
- 17.Stamnes MA, Rothman JE. The binding of AP-1 clathrin adaptor particles to Golgi membranes requires ADP-ribosylation factor, a small GTP-binding protein. Cell. 1993;73:999–1005. doi: 10.1016/0092-8674(93)90277-w. [DOI] [PubMed] [Google Scholar]
- 18.Traub LM, Ostrom JA, Kornfeld S. Biochemical dissection of AP-1 recruitment onto Golgi membranes. J Cell Biol. 1993;123:561–73. doi: 10.1083/jcb.123.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palmer DJ, Helms JB, Beckers CJ, Orci L, Rothman JE. Binding of coatomer to Golgi membranes requires ADP-ribosylation factor. J Biol Chem. 1993;268:12083–89. [PubMed] [Google Scholar]
- 20.Ooi CE, Dell’Angelica EC, Bonifacino JS. ADP-Ribosylation factor 1 (ARF1) regulates recruitment of the AP-3 adaptor complex to membranes. J Cell Biol. 1998;142:391–402. doi: 10.1083/jcb.142.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faundez V, Horng JT, Kelly RB. A function for the AP3 coat complex in synaptic vesicle formation from endosomes. Cell. 1998;93:423–32. doi: 10.1016/s0092-8674(00)81170-8. [DOI] [PubMed] [Google Scholar]
- 22.Boehm M, Aguilar RC, Bonifacino JS. Functional and physical interactions of the adaptor protein complex AP-4 with ADP-ribosylation factors (ARFs) EMBO J. 2001;20:6265–76. doi: 10.1093/emboj/20.22.6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Antonny B, Madden D, Hamamoto S, Orci L, Schekman R. Dynamics of the COPII coat with GTP and stable analogues. Nat Cell Biol. 2001;3:531–37. doi: 10.1038/35078500. [DOI] [PubMed] [Google Scholar]
- 24.Melancon P, Glick BS, Malhotra V, Weidman PJ, Serafini T, Gleason ML, et al. Involvement of GTP-binding “G” proteins in transport through the Golgi stack. Cell. 1987;51:1053–62. doi: 10.1016/0092-8674(87)90591-5. [DOI] [PubMed] [Google Scholar]
- 25.Tanigawa G, Orci L, Amherdt M, Ravazzola M, Helms JB, Rothman JE. Hydrolysis of bound GTP by ARF protein triggers uncoating of Golgi-derived COP-coated vesicles. J Cell Biol. 1993;123:1365–71. doi: 10.1083/jcb.123.6.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barlowe C. Coupled ER to Golgi transport reconstituted with purified cytosolic proteins. J Cell Biol. 1997;139:1097–108. doi: 10.1083/jcb.139.5.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ungewickell E, Ungewickell H, Holstein SE, Lindner R, Prasad K, Barouch W, et al. Role of auxilin in uncoating clathrin-coated vesicles. Nature. 1995;378:632–35. doi: 10.1038/378632a0. [DOI] [PubMed] [Google Scholar]
- 28.Pishvaee B, Costaguta G, Yeung BG, Ryazantsev S, Greener T, Greene LE, et al. A yeast DNA J protein required for uncoating of clathrin-coated vesicles in vivo. Nat Cell Biol. 2000;2:958–63. doi: 10.1038/35046619. [DOI] [PubMed] [Google Scholar]
- 29.Angers CG, Merz AJ. HOPS interacts with Apl5 at the vacuole membrane and is required for consumption of AP-3 transport vesicles. Mol Biol Cell. 2009;20:4563–74. doi: 10.1091/mbc.E09-04-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zink S, Wenzel D, Wurm CA, Schmitt HD. A link between ER tethering and COP-I vesicle uncoating. Dev Cell. 2009;17:403–16. doi: 10.1016/j.devcel.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 31.Cai H, Yu S, Menon S, Cai Y, Lazarova D, Fu C, et al. TRAPPI tethers COPII vesicles by binding the coat subunit Sec23. Nature. 2007;445:941–44. doi: 10.1038/nature05527. [DOI] [PubMed] [Google Scholar]
- 32.Koumandou VL, Dacks JB, Coulson RM, Field MC. Control systems for membrane fusion in the ancestral eukaryote; evolution of tethering complexes and SM proteins. BMC Evol Biol. 2007;7:29. doi: 10.1186/1471-2148-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lynch-Day MA, Bhandari D, Menon S, Huang J, Cai H, Bartholomew CR, et al. Trs85 directs a Ypt1 GEF, TRAPPIII, to the phagophore to promote autophagy. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1000063107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sacher M, Jiang Y, Barrowman J, Scarpa A, Burston J, Zhang L, et al. TRAPP, a highly conserved novel complex on the cis-Golgi that mediates vesicle docking and fusion. Embo J. 1998;17:2494–503. doi: 10.1093/emboj/17.9.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rossi G, Kolstad K, Stone S, Palluault F, Ferro-Novick S. BET3 encodes a novel hydrophilic protein that acts in conjunction with yeast SNAREs. Mol Biol Cell. 1995;6:1769–80. doi: 10.1091/mbc.6.12.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim YG, Raunser S, Munger C, Wagner J, Song YL, Cygler M, et al. The architecture of the multisubunit TRAPP I complex suggests a model for vesicle tethering. Cell. 2006;127:817–30. doi: 10.1016/j.cell.2006.09.029. [DOI] [PubMed] [Google Scholar]
- 37.Yu S, Satoh A, Pypaert M, Mullen K, Hay JC, Ferro-Novick S. mBet3p is required for homotypic COPII vesicle tethering in mammalian cells. J Cell Biol. 2006;174:359–68. doi: 10.1083/jcb.200603044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sacher M, Barrowman J, Wang W, Horecka J, Zhang Y, Pypaert M, et al. TRAPP I implicated in the specificity of tethering in ER-to-Golgi transport. Mol Cell. 2001;7:433–42. doi: 10.1016/s1097-2765(01)00190-3. [DOI] [PubMed] [Google Scholar]
- 39.Jones S, Newman C, Liu F, Segev N. The TRAPP complex is a nucleotide exchanger for Ypt1 and Ypt31/32. Mol Biol Cell. 2000;11:4403–11. doi: 10.1091/mbc.11.12.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cai Y, Chin HF, Lazarova D, Menon S, Fu C, Cai H, et al. The structural basis for activation of the Rab Ypt1p by the TRAPP membrane-tethering complexes. Cell. 2008;133:1202–13. doi: 10.1016/j.cell.2008.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim YG, Sohn EJ, Seo J, Lee KJ, Lee HS, Hwang I, et al. Crystal structure of bet3 reveals a novel mechanism for Golgi localization of tethering factor TRAPP. Nat Struct Mol Biol. 2005;12:38–45. doi: 10.1038/nsmb871. [DOI] [PubMed] [Google Scholar]
- 42.Morozova N, Liang Y, Tokarev AA, Chen SH, Cox R, Andrejic J, et al. TRAPPII subunits are required for the specificity switch of a Ypt-Rab GEF. Nat Cell Biol. 2006;8:1263–69. doi: 10.1038/ncb1489. [DOI] [PubMed] [Google Scholar]
- 43.Cai H, Zhang Y, Pypaert M, Walker L, Ferro-Novick S. Mutants in trs120 disrupt traffic from the early endosome to the late Golgi. J Cell Biol. 2005;171:823–33. doi: 10.1083/jcb.200505145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamasaki A, Menon S, Yu S, Barrowman J, Meerloo T, Oorschot V, et al. mTrs130 is a component of a mammalian TRAPPII complex, a Rab1 GEF that binds to COPI-coated vesicles. Mol Biol Cell. 2009;20:4205–15. doi: 10.1091/mbc.E09-05-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang W, Ferro-Novick S. A Ypt32p exchange factor is a putative effector of Ypt1p. Mol Biol Cell. 2002;13:3336–43. doi: 10.1091/mbc.01-12-0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jedd G, Mulholland J, Segev N. Two new Ypt GTPases are required for exit from the yeast trans-Golgi compartment. J Cell Biol. 1997;137:563–80. doi: 10.1083/jcb.137.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Montpetit B, Conibear E. Identification of the novel TRAPP associated protein Tca17. Traffic. 2009;10:713–23. doi: 10.1111/j.1600-0854.2009.00895.x. [DOI] [PubMed] [Google Scholar]
- 48.Suvorova ES, Duden R, Lupashin VV. The Sec34/Sec35p complex, a Ypt1p effector required for retrograde intra-Golgi trafficking, interacts with Golgi SNAREs and COPI vesicle coat proteins. J Cell Biol. 2002;157:631–43. doi: 10.1083/jcb.200111081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sohda M, Misumi Y, Yoshimura S, Nakamura N, Fusano T, Ogata S, et al. The interaction of two tethering factors, p115 and COG complex, is required for Golgi integrity. Traffic. 2007;8:270–84. doi: 10.1111/j.1600-0854.2006.00530.x. [DOI] [PubMed] [Google Scholar]
- 50.Sun Y, Shestakova A, Hunt L, Sehgal S, Lupashin V, Storrie B. Rab6 regulates both ZW10/RINT-1 and conserved oligomeric Golgi complex-dependent Golgi trafficking and homeostasis. Mol Biol Cell. 2007;18:4129–42. doi: 10.1091/mbc.E07-01-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zolov SN, Lupashin VV. Cog3p depletion blocks vesicle-mediated Golgi retrograde trafficking in HeLa cells. J Cell Biol. 2005;168:747–59. doi: 10.1083/jcb.200412003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wuestehube LJ, Duden R, Eun A, Hamamoto S, Korn P, Ram R, et al. New mutants of Saccharomyces cerevisiae affected in the transport of proteins from the endoplasmic reticulum to the Golgi complex. Genetics. 1996;142:393–406. doi: 10.1093/genetics/142.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shestakova A, Suvorova E, Pavliv O, Khaidakova G, Lupashin V. Interaction of the conserved oligomeric Golgi complex with t-SNARE Syntaxin5a/Sed5 enhances intra-Golgi SNARE complex stability. J Cell Biol. 2007;179:1179–92. doi: 10.1083/jcb.200705145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.VanRheenen SM, Cao X, Lupashin VV, Barlowe C, Waters MG. Sec35p, a novel peripheral membrane protein, is required for ER to Golgi vesicle docking. J Cell Biol. 1998;141:1107–19. doi: 10.1083/jcb.141.5.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Andag U, Neumann T, Schmitt HD. The coatomer-interacting protein Dsl1p is required for Golgi-to-endoplasmic reticulum retrieval in yeast. J Biol Chem. 2001;276:39150–60. doi: 10.1074/jbc.M105833200. [DOI] [PubMed] [Google Scholar]
- 56.Reilly BA, Kraynack BA, VanRheenen SM, Waters MG. Golgi-to-endoplasmic reticulum (ER) retrograde traffic in yeast requires Dsl1p, a component of the ER target site that interacts with a COPI coat subunit. Mol Biol Cell. 2001;12:3783–96. doi: 10.1091/mbc.12.12.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kraynack BA, Chan A, Rosenthal E, Essid M, Umansky B, Waters MG, et al. Dsl1p, Tip20p, and the novel Dsl3(Sec39) protein are required for the stability of the Q/t-SNARE complex at the endoplasmic reticulum in yeast. Mol Biol Cell. 2005;16:3963–77. doi: 10.1091/mbc.E05-01-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hirose H, Arasaki K, Dohmae N, Takio K, Hatsuzawa K, Nagahama M, et al. Implication of ZW10 in membrane trafficking between the endoplasmic reticulum and Golgi. EMBO J. 2004;23:1267–78. doi: 10.1038/sj.emboj.7600135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ren Y, Yip CK, Tripathi A, Huie D, Jeffrey PD, Walz T, et al. A structure-based mechanism for vesicle capture by the multisubunit tethering complex Dsl1. Cell. 2009;139:1119–29. doi: 10.1016/j.cell.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tripathi A, Ren Y, Jeffrey PD, Hughson FM. Structural characterization of Tip20p and Dsl1p, subunits of the Dsl1p vesicle tethering complex. Nat Struct Mol Biol. 2009;16:114–23. doi: 10.1038/nsmb.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Andag U, Schmitt HD. Dsl1p, an essential component of the Golgi-endoplasmic reticulum retrieval system in yeast, uses the same sequence motif to interact with different subunits of the COPI vesicle coat. J Biol Chem. 2003;278:51722–34. doi: 10.1074/jbc.M308740200. [DOI] [PubMed] [Google Scholar]
- 62.Hara-Kuge S, Kuge O, Orci L, Amherdt M, Ravazzola M, Wieland FT, et al. En bloc incorporation of coatomer subunits during the assembly of COP-coated vesicles. J Cell Biol. 1994;124:883–92. doi: 10.1083/jcb.124.6.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eugster A, Frigerio G, Dale M, Duden R. COP I domains required for coatomer integrity, and novel interactions with ARF and ARF-GAP. EMBO J. 2000;19:3905–17. doi: 10.1093/emboj/19.15.3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kamena F, Diefenbacher M, Kilchert C, Schwarz H, Spang A. Ypt1p is essential for retrograde Golgi-ER transport and for Golgi maintenance in S. cerevisiae. J Cell Sci. 2008;121:1293–302. doi: 10.1242/jcs.016998. [DOI] [PubMed] [Google Scholar]
- 65.Seals DF, Eitzen G, Margolis N, Wickner WT, Price A. A Ypt/Rab effector complex containing the Sec1 homolog Vps33p is required for homotypic vacuole fusion. Proc Natl Acad Sci U S A. 2000;97:9402–07. doi: 10.1073/pnas.97.17.9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wurmser AE, Sato TK, Emr SD. New component of the vacuolar class C-Vps complex couples nucleotide exchange on the Ypt7 GTPase to SNARE-dependent docking and fusion. J Cell Biol. 2000;151:551–62. doi: 10.1083/jcb.151.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nickerson DP, Brett CL, Merz AJ. Vps-C complexes: gatekeepers of endolysosomal traffic. Curr Opin Cell Biol. 2009;21:543–51. doi: 10.1016/j.ceb.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rieder SE, Emr SD. A novel RING finger protein complex essential for a late step in protein transport to the yeast vacuole. Mol Biol Cell. 1997;8:2307–27. doi: 10.1091/mbc.8.11.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stroupe C, Collins KM, Fratti RA, Wickner W. Purification of active HOPS complex reveals its affinities for phosphoinositides and the SNARE Vam7p. EMBO J. 2006;25:1579–89. doi: 10.1038/sj.emboj.7601051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Price A, Seals D, Wickner W, Ungermann C. The docking stage of yeast vacuole fusion requires the transfer of proteins from a cis-SNARE complex to a Rab/Ypt protein. J Cell Biol. 2000;148:1231–38. doi: 10.1083/jcb.148.6.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brett CL, Plemel RL, Lobinger BT, Vignali M, Fields S, Merz AJ. Efficient termination of vacuolar Rab GTPase signaling requires coordinated action by a GAP and a protein kinase. J Cell Biol. 2008;182:1141–51. doi: 10.1083/jcb.200801001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Binda M, Peli-Gulli MP, Bonfils G, Panchaud N, Urban J, Sturgill TW, et al. The Vam6 GEF controls TORC1 by activating the EGO complex. Mol Cell. 2009;35:563–73. doi: 10.1016/j.molcel.2009.06.033. [DOI] [PubMed] [Google Scholar]
- 73.Collins KM, Wickner WT. Trans-SNARE complex assembly and yeast vacuole membrane fusion. Proc Natl Acad Sci U S A. 2007;104:8755–60. doi: 10.1073/pnas.0702290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sato TK, Rehling P, Peterson MR, Emr SD. Class C Vps protein complex regulates vacuolar SNARE pairing and is required for vesicle docking/fusion. Mol Cell. 2000;6:661–71. doi: 10.1016/s1097-2765(00)00064-2. [DOI] [PubMed] [Google Scholar]
- 75.Dulubova I, Yamaguchi T, Wang Y, Sudhof TC, Rizo J. Vam3p structure reveals conserved and divergent properties of syntaxins. Nat Struct Biol. 2001;8:258–64. doi: 10.1038/85012. [DOI] [PubMed] [Google Scholar]
- 76.Collins KM, Thorngren NL, Fratti RA, Wickner WT. Sec17p and HOPS, in distinct SNARE complexes, mediate SNARE complex disruption or assembly for fusion. EMBO J. 2005;24:1775–86. doi: 10.1038/sj.emboj.7600658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Laage R, Ungermann C. The N-terminal domain of the t-SNARE Vam3p coordinates priming and docking in yeast vacuole fusion. Mol Biol Cell. 2001;12:3375–85. doi: 10.1091/mbc.12.11.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sollner T, Bennett MK, Whiteheart SW, Scheller RH, Rothman JE. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell. 1993;75:409–18. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- 79.Ungermann C, Nichols BJ, Pelham HR, Wickner W. A vacuolar v-t-SNARE complex, the predominant form in vivo and on isolated vacuoles, is disassembled and activated for docking and fusion. J Cell Biol. 1998;140:61–69. doi: 10.1083/jcb.140.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xu H, Jun Y, Thompson J, Yates J, Wickner W. HOPS prevents the disassembly of trans-SNARE complexes by Sec17p/Sec18p during membrane fusion. EMBO J. 2010;29:1948–60. doi: 10.1038/emboj.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rehling P, Darsow T, Katzmann DJ, Emr SD. Formation of AP-3 transport intermediates requires Vps41 function. Nat Cell Biol. 1999;1:346–53. doi: 10.1038/14037. [DOI] [PubMed] [Google Scholar]
- 82.Darsow T, Katzmann DJ, Cowles CR, Emr SD. Vps41p function in the alkaline phosphatase pathway requires homo-oligomerization and interaction with AP-3 through two distinct domains. Mol Biol Cell. 2001;12:37–51. doi: 10.1091/mbc.12.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ybe JA, Brodsky FM, Hofmann K, Lin K, Liu SH, Chen L, et al. Clathrin self-assembly is mediated by a tandemly repeated superhelix. Nature. 1999;399:371–75. doi: 10.1038/20708. [DOI] [PubMed] [Google Scholar]
- 84.Salazar G, Zlatic S, Craige B, Peden AA, Pohl J, Faundez V. Hermansky-Pudlak syndrome protein complexes associate with phosphatidylinositol 4-kinase type II alpha in neuronal and non-neuronal cells. J Biol Chem. 2009;284:1790–802. doi: 10.1074/jbc.M805991200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cowles CR, Snyder WB, Burd CG, Emr SD. Novel Golgi to vacuole delivery pathway in yeast: identification of a sorting determinant and required transport component. Embo J. 1997;16:2769–82. doi: 10.1093/emboj/16.10.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Anand VC, Daboussi L, Lorenz TC, Payne GS. Genome-wide analysis of AP-3-dependent protein transport in yeast. Mol Biol Cell. 2009;20:1592–604. doi: 10.1091/mbc.E08-08-0819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.LaGrassa TJ, Ungermann C. The vacuolar kinase Yck3 maintains organelle fragmentation by regulating the HOPS tethering complex. J Cell Biol. 2005;168:401–14. doi: 10.1083/jcb.200407141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hickey CM, Stroupe C, Wickner W. The major role of the Rab Ypt7p in vacuole fusion is supporting HOPS membrane association. J Biol Chem. 2009;284:16118–25. doi: 10.1074/jbc.M109.000737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cabrera M, Ostrowicz CW, Mari M, LaGrassa TJ, Reggiori F, Ungermann C. Vps41 phosphorylation and the Rab Ypt7 control the targeting of the HOPS complex to endosome-vacuole fusion sites. Mol Biol Cell. 2009;20:1937–48. doi: 10.1091/mbc.E08-09-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Barr FA, Puype M, Vandekerckhove J, Warren G. GRASP65, a protein involved in the stacking of Golgi cisternae. Cell. 1997;91:253–62. doi: 10.1016/s0092-8674(00)80407-9. [DOI] [PubMed] [Google Scholar]
- 91.Behnia R, Barr FA, Flanagan JJ, Barlowe C, Munro S. The yeast orthologue of GRASP65 forms a complex with a coiled-coil protein that contributes to ER to Golgi traffic. J Cell Biol. 2007;176:255–61. doi: 10.1083/jcb.200607151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Moyer BD, Allan BB, Balch WE. Rab1 interaction with a GM130 effector complex regulates COPII vesicle cis--Golgi tethering. Traffic. 2001;2:268–76. doi: 10.1034/j.1600-0854.2001.1o007.x. [DOI] [PubMed] [Google Scholar]
- 93.Sapperstein SK, Lupashin VV, Schmitt HD, Waters MG. Assembly of the ER to Golgi SNARE complex requires Uso1p. J Cell Biol. 1996;132:755–67. doi: 10.1083/jcb.132.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dascher C, Ossig R, Gallwitz D, Schmitt HD. Identification and structure of four yeast genes (SLY) that are able to suppress the functional loss of YPT1, a member of the RAS superfamily. Mol Cell Biol. 1991;11:872–85. doi: 10.1128/mcb.11.2.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cao X, Ballew N, Barlowe C. Initial docking of ER-derived vesicles requires Uso1p and Ypt1p but is independent of SNARE proteins. EMBO J. 1998;17:2156–65. doi: 10.1093/emboj/17.8.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Allan BB, Moyer BD, Balch WE. Rab1 recruitment of p115 into a cis-SNARE complex: programming budding COPII vesicles for fusion. Science. 2000;289:444–48. doi: 10.1126/science.289.5478.444. [DOI] [PubMed] [Google Scholar]
- 97.Waters MG, Clary DO, Rothman JE. A novel 115-kD peripheral membrane protein is required for intercisternal transport in the Golgi stack. J Cell Biol. 1992;118:1015–26. doi: 10.1083/jcb.118.5.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hoffman GR, Rahl PB, Collins RN, Cerione RA. Conserved structural motifs in intracellular trafficking pathways: structure of the gammaCOP appendage domain. Mol Cell. 2003;12:615–25. doi: 10.1016/j.molcel.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 99.Guo Y, Punj V, Sengupta D, Linstedt AD. Coat-tether interaction in Golgi organization. Mol Biol Cell. 2008;19:2830–43. doi: 10.1091/mbc.E07-12-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bentley M, Liang Y, Mullen K, Xu D, Sztul E, Hay JC. SNARE status regulates tether recruitment and function in homotypic COPII vesicle fusion. J Biol Chem. 2006;281:38825–33. doi: 10.1074/jbc.M606044200. [DOI] [PubMed] [Google Scholar]
- 101.Morsomme P, Riezman H. The Rab GTPase Ypt1p and tethering factors couple protein sorting at the ER to vesicle targeting to the Golgi apparatus. Dev Cell. 2002;2:307–17. doi: 10.1016/s1534-5807(02)00133-8. [DOI] [PubMed] [Google Scholar]
- 102.Shorter J, Beard MB, Seemann J, Dirac-Svejstrup AB, Warren G. Sequential tethering of Golgins and catalysis of SNAREpin assembly by the vesicle-tethering protein p115. J Cell Biol. 2002;157:45–62. doi: 10.1083/jcb.200112127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Puthenveedu MA, Linstedt AD. Gene replacement reveals that p115/SNARE interactions are essential for Golgi biogenesis. Proc Natl Acad Sci U S A. 2004;101:1253–56. doi: 10.1073/pnas.0306373101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sonnichsen B, Lowe M, Levine T, Jamsa E, Dirac-Svejstrup B, Warren G. A role for giantin in docking COPI vesicles to Golgi membranes. J Cell Biol. 1998;140:1013–21. doi: 10.1083/jcb.140.5.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gillingham AK, Pfeifer AC, Munro S. CASP, the alternatively spliced product of the gene encoding the CCAAT-displacement protein transcription factor, is a Golgi membrane protein related to giantin. Mol Biol Cell. 2002;13:3761–74. doi: 10.1091/mbc.E02-06-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Malsam J, Satoh A, Pelletier L, Warren G. Golgin tethers define subpopulations of COPI vesicles. Science. 2005;307:1095–98. doi: 10.1126/science.1108061. [DOI] [PubMed] [Google Scholar]
- 107.Seaman MN, McCaffery JM, Emr SD. A membrane coat complex essential for endosome-to-Golgi retrograde transport in yeast. J Cell Biol. 1998;142:665–81. doi: 10.1083/jcb.142.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Arighi CN, Hartnell LM, Aguilar RC, Haft CR, Bonifacino JS. Role of the mammalian retromer in sorting of the cation-independent mannose 6-phosphate receptor. J Cell Biol. 2004;165:123–33. doi: 10.1083/jcb.200312055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wassmer T, Attar N, Harterink M, van Weering JR, Traer CJ, Oakley J, et al. The retromer coat complex coordinates endosomal sorting and dynein-mediated transport, with carrier recognition by the trans-Golgi network. Dev Cell. 2009;17:110–22. doi: 10.1016/j.devcel.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Peter BJ, Kent HM, Mills IG, Vallis Y, Butler PJ, Evans PR, et al. BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science. 2004;303:495–99. doi: 10.1126/science.1092586. [DOI] [PubMed] [Google Scholar]
- 111.Carlton J, Bujny M, Peter BJ, Oorschot VM, Rutherford A, Mellor H, et al. Sorting nexin-1 mediates tubular endosome-to-TGN transport through coincidence sensing of high- curvature membranes and 3-phosphoinositides. Curr Biol. 2004;14:1791–800. doi: 10.1016/j.cub.2004.09.077. [DOI] [PubMed] [Google Scholar]
- 112.Miserey-Lenkei S, Waharte F, Boulet A, Cuif MH, Tenza D, El Marjou A, et al. Rab6-interacting protein 1 links Rab6 and Rab11 function. Traffic. 2007;8:1385–403. doi: 10.1111/j.1600-0854.2007.00612.x. [DOI] [PubMed] [Google Scholar]
- 113.Monier S, Jollivet F, Janoueix-Lerosey I, Johannes L, Goud B. Characterization of novel Rab6-interacting proteins involved in endosome-to-TGN transport. Traffic. 2002;3:289–97. doi: 10.1034/j.1600-0854.2002.030406.x. [DOI] [PubMed] [Google Scholar]
- 114.Ahle S, Mann A, Eichelsbacher U, Ungewickell E. Structural relationships between clathrin assembly proteins from the Golgi and the plasma membrane. EMBO J. 1988;7:919–29. doi: 10.1002/j.1460-2075.1988.tb02897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gaidarov I, Chen Q, Falck JR, Reddy KK, Keen JH. A functional phosphatidylinositol 3,4,5-trisphosphate/phosphoinositide binding domain in the clathrin adaptor AP-2 alpha subunit. Implications for the endocytic pathway. J Biol Chem. 1996;271:20922–29. doi: 10.1074/jbc.271.34.20922. [DOI] [PubMed] [Google Scholar]
- 116.Rohde G, Wenzel D, Haucke V. A phosphatidylinositol (4,5)-bisphosphate binding site within mu2-adaptin regulates clathrin-mediated endocytosis. J Cell Biol. 2002;158:209–14. doi: 10.1083/jcb.200203103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Conner SD, Schmid SL. Identification of an adaptor-associated kinase, AAK1, as a regulator of clathrin-mediated endocytosis. J Cell Biol. 2002;156:921–29. doi: 10.1083/jcb.200108123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fingerhut A, von Figura K, Honing S. Binding of AP2 to sorting signals is modulated by AP2 phosphorylation. J Biol Chem. 2001;276:5476–82. doi: 10.1074/jbc.M009516200. [DOI] [PubMed] [Google Scholar]
- 119.Ricotta D, Conner SD, Schmid SL, von Figura K, Honing S. Phosphorylation of the AP2 mu subunit by AAK1 mediates high affinity binding to membrane protein sorting signals. J Cell Biol. 2002;156:791–95. doi: 10.1083/jcb.200111068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ricotta D, Hansen J, Preiss C, Teichert D, Honing S. Characterization of a protein phosphatase 2A holoenzyme that dephosphorylates the clathrin adaptors AP-1 and AP-2. J Biol Chem. 2008;283:5510–17. doi: 10.1074/jbc.M707166200. [DOI] [PubMed] [Google Scholar]
- 121.Ghosh P, Kornfeld S. AP-1 binding to sorting signals and release from clathrin-coated vesicles is regulated by phosphorylation. J Cell Biol. 2003;160:699–708. doi: 10.1083/jcb.200211080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Semerdjieva S, Shortt B, Maxwell E, Singh S, Fonarev P, Hansen J, et al. Coordinated regulation of AP2 uncoating from clathrin-coated vesicles by rab5 and hRME-6. J Cell Biol. 2008;183:499–511. doi: 10.1083/jcb.200806016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Horiuchi H, Giner A, Hoflack B, Zerial M. A GDP/GTP exchange-stimulatory activity for the Rab5-RabGDI complex on clathrin-coated vesicles from bovine brain. J Biol Chem. 1995;270:11257–62. doi: 10.1074/jbc.270.19.11257. [DOI] [PubMed] [Google Scholar]
- 124.Sato M, Sato K, Fonarev P, Huang CJ, Liou W, Grant BD. Caenorhabditis elegans RME-6 is a novel regulator of RAB-5 at the clathrin-coated pit. Nat Cell Biol. 2005;7:559–69. doi: 10.1038/ncb1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Malsam J, Gommel D, Wieland FT, Nickel W. A role for ADP ribosylation factor in the control of cargo uptake during COPI-coated vesicle biogenesis. FEBS Lett. 1999;462:267–72. doi: 10.1016/s0014-5793(99)01543-4. [DOI] [PubMed] [Google Scholar]
- 126.Pepperkok R, Whitney JA, Gomez M, Kreis TE. COPI vesicles accumulating in the presence of a GTP restricted arf1 mutant are depleted of anterograde and retrograde cargo. J Cell Sci. 2000;113:135–44. doi: 10.1242/jcs.113.1.135. [DOI] [PubMed] [Google Scholar]
- 127.Tabata KV, Sato K, Ide T, Nishizaka T, Nakano A, Noji H. Visualization of cargo concentration by COPII minimal machinery in a planar lipid membrane. EMBO J. 2009;28:3279–89. doi: 10.1038/emboj.2009.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bielli A, Haney CJ, Gabreski G, Watkins SC, Bannykh SI, Aridor M. Regulation of Sar1 NH2 terminus by GTP binding and hydrolysis promotes membrane deformation to control COPII vesicle fission. J Cell Biol. 2005;171:919–24. doi: 10.1083/jcb.200509095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhu Y, Traub LM, Kornfeld S. ADP-ribosylation factor 1 transiently activates high-affinity adaptor protein complex AP-1 binding sites on Golgi membranes. Mol Biol Cell. 1998;9:1323–37. doi: 10.1091/mbc.9.6.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Forster R, Weiss M, Zimmermann T, Reynaud EG, Verissimo F, Stephens DJ, et al. Secretory cargo regulates the turnover of COPII subunits at single ER exit sites. Curr Biol. 2006;16:173–79. doi: 10.1016/j.cub.2005.11.076. [DOI] [PubMed] [Google Scholar]
- 131.Schmid EM, Ford MG, Burtey A, Praefcke GJ, Peak-Chew SY, Mills IG, et al. Role of the AP2 beta-appendage hub in recruiting partners for clathrin-coated vesicle assembly. PLoS Biol. 2006;4:e262. doi: 10.1371/journal.pbio.0040262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bentley M, Nycz DC, Joglekar A, Fertschai I, Malli R, Graier WF, et al. Vesicular Calcium Regulates Coat Retention, Fusogenicity, and Size of Pre-Golgi Intermediates. Mol Biol Cell. 2010 doi: 10.1091/mbc.E09-10-0914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ahluwalia JP, Topp JD, Weirather K, Zimmerman M, Stamnes M. A role for calcium in stabilizing transport vesicle coats. J Biol Chem. 2001;276:34148–55. doi: 10.1074/jbc.M105398200. [DOI] [PubMed] [Google Scholar]
- 134.Li F, Pincet F, Perez E, Eng WS, Melia TJ, Rothman JE, et al. Energetics and dynamics of SNAREpin folding across lipid bilayers. Nat Struct Mol Biol. 2007;14:890–96. doi: 10.1038/nsmb1310. [DOI] [PubMed] [Google Scholar]
- 135.Schwartz ML, Merz AJ. Capture and release of partially zipped trans-SNARE complexes on intact organelles. J Cell Biol. 2009;185:535–49. doi: 10.1083/jcb.200811082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bi X, Corpina RA, Goldberg J. Structure of the Sec23/24-Sar1 pre-budding complex of the COPII vesicle coat. Nature. 2002;419:271–77. doi: 10.1038/nature01040. [DOI] [PubMed] [Google Scholar]
- 137.Mossessova E, Bickford LC, Goldberg J. SNARE selectivity of the COPII coat. Cell. 2003;114:483–95. doi: 10.1016/s0092-8674(03)00608-1. [DOI] [PubMed] [Google Scholar]
- 138.Zaremba S, Keen JH. Assembly polypeptides from coated vesicles mediate reassembly of unique clathrin coats. J Cell Biol. 1983;97:1339–47. doi: 10.1083/jcb.97.5.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Smith CJ, Grigorieff N, Pearse BM. Clathrin coats at 21 A resolution: a cellular assembly designed to recycle multiple membrane receptors. EMBO J. 1998;17:4943–53. doi: 10.1093/emboj/17.17.4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cheng Y, Boll W, Kirchhausen T, Harrison SC, Walz T. Cryo-electron tomography of clathrin-coated vesicles: structural implications for coat assembly. J Mol Biol. 2007;365:892–99. doi: 10.1016/j.jmb.2006.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Saffarian S, Kirchhausen T. Differential evanescence nanometry: live-cell fluorescence measurements with 10-nm axial resolution on the plasma membrane. Biophys J. 2008;94:2333–42. doi: 10.1529/biophysj.107.117234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hannan LA, Newmyer SL, Schmid SL. ATP- and cytosol-dependent release of adaptor proteins from clathrin-coated vesicles: A dual role for Hsc70. Mol Biol Cell. 1998;9:2217–29. doi: 10.1091/mbc.9.8.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ehrlich M, Boll W, Van Oijen A, Hariharan R, Chandran K, Nibert ML, et al. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell. 2004;118:591–605. doi: 10.1016/j.cell.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 144.Traub LM. Common principles in clathrin-mediated sorting at the Golgi and the plasma membrane. Biochim Biophys Acta. 2005;1744:415–37. doi: 10.1016/j.bbamcr.2005.04.005. [DOI] [PubMed] [Google Scholar]


