Abstract
Recently, expression of glutamate decarboxylase-67 (GAD67), a key enzyme of GABA synthesis, was detected in the otherwise glutamatergic mossy fibers of the rat hippocampus. Synthesis of the enzyme was markedly enhanced after experimentally induced status epilepticus. Here, we investigated the expression of GAD67 protein and mRNA in 44 hippocampal specimens from patients with mesial temporal lobe epilepsy (TLE) using double immunofluorescence histochemistry, immunoblotting, and in situ hybridization. Both in specimens with (n = 37) and without (n = 7) hippocampal sclerosis, GAD67 was highly coexpressed with dynorphin in terminal areas of mossy fibers, including the dentate hilus and the stratum lucidum of sector CA3. In the cases with Ammon’s horn sclerosis, also the inner molecular layer of the dentate gyrus contained strong staining for GAD67 immunoreactivity, indicating labeling of mossy fiber terminals that specifically sprout into this area. Double immunofluorescence revealed the colocalization of GAD67 immunoreactivity with the mossy fiber marker dynorphin. The extent of colabeling correlated with the number of seizures experienced by the patients. Furthermore, GAD67 mRNA was found in granule cells of the dentate gyrus. Levels, both of GAD67 mRNA and of GAD67 immunoreactivity were similar in sclerotic and nonsclerotic specimens and appeared to be increased compared to post mortem controls. Provided that the strong expression of GAD67 results in synthesis of GABA in hippocampal mossy fibers this may represent a self-protecting mechanism in TLE. In addition GAD67 expression also may result in conversion of excessive intracellular glutamate to nontoxic GABA within mossy fiber terminals.
Keywords: GABA, GAD67, VGAT, inverse GABA transport, plasticity
INTRODUCTION
Mesial temporal lobe epilepsies (TLE) are among the most severe forms of focal epilepsies. Up to 80% of TLE patients do not adequately respond to therapies based on currently available antiepileptic drugs. Epilepsy surgery removing the epileptogenic tissue in the mesial temporal lobe offers a valuable treatment option for these patients with a seizure-free outcome or marked reduction of seizure frequency of more than 70–90% (Wiebe et al., 2001). The examination of tissue obtained from TLE patients at surgery and study of animal models of TLE have revealed widespread molecular, morphologic, and neurophysiological rearrangement of the epileptic hippocampus (Houser and Esclapez, 1994; Mikkonen et al., 1998; Mathern et al., 1999; Pirker et al., 2001). In particular, granule cells of the dentate gyrus display remarkable plasticity, which is accompanied by altered expression of numerous functionally relevant peptides and proteins including growth factors, neurotransmitter receptors, and ion channels. The axons of granule cells, the mossy fibers, sprout and aberrantly target the inner molecular layer of the dentate gyrus where they appear to substitute for innervation by associational/commissural fibers from hilar mossy cells, which degenerate in the epileptic hippocampus (Sutula et al., 1989).
Mossy fibers are excitatory and use glutamate as their principal neurotransmitter (Bramham et al., 1990). Mossy fiber terminals are rich in dense core vesicles that also contain neuropeptides, including dynorphin and chromogranins (Pirker et al., 2001). Using electron microscopy, Sandler and Smith (1991) demonstrated the presence of GABA in close vicinity to the synaptic vesicles of mossy fibers in the monkey hippocampus. Since then, several groups have reported that mossy fibers in rats and monkeys also express glutamate decarboxylase-67 (GAD67), which catalyzes the synthesis of GABA from glutamate, and that levels of GAD67 mRNA and protein are enhanced in granule cells/mossy fibers after sustained epileptic seizures in rats (Schwarzer and Sperk, 1995; Sloviter et al., 1996; Sperk et al., 2003). Subsequent studies indicated that stimulation of granule cells causes release of GABA from mossy fiber terminals, activating postsynaptic GABAA receptors and leading to hyperpolarization of CA3 neurons (Gutierrez and Heinemann, 2001; Walker et al., 2001). The data suggest that the synthesis and release of GABA from mossy fibers could be an endogenous mechanism for seizure termination or to convert excess glutamate into GABA (Sperk et al., 2003).
In the present study, we addressed the question of whether GAD67 is also expressed in mossy fibers of the human epileptic hippocampus. Using specimens from patients with TLE obtained at surgery we demonstrate coexpression of GAD67 with the mossy fiber marker dynorphin in terminal areas of mossy fibers (Houser et al., 1990), including the dentate hilus, the stratum lucidum, and the inner molecular layer, which becomes innervated by sprouted mossy fibers in Ammon’s horn sclerosis.
PATIENTS AND METHODS
Patients
This study conforms to The Code of Ethics of the World Medical Association (Declaration of Helsinki). Approval for this study was obtained from the Institutional Boards of the Medical Universities Vienna and Innsbruck, Austria and informed consent was obtained from all patients providing specimens. Specimens were obtained at surgery from 44 patients (24 females and 20 males) with chronic pharmaco-resistant TLE who had undergone unilateral selective amygdalo-hippocampectomy or anteromedial temporal lobe resection. Clinical data are shown in Table 1. Thirty seven specimens had moderate to severe, seven cases no or only minimal Ammon’s horn sclerosis (termed nonsclerotic). The age of patients at surgery ranged from 9 to 65 yrs (mean ± standard error of mean (SEM), 35 ± 1.9 yrs). The time between onset of epilepsy and surgery ranged from 3 to 51 yrs. The decision for surgery was based on converging evidence from EEG recordings during prolonged video-EEG monitoring with scalp and sphenoidal electrodes, ictal perfusion SPECT [single photon emission computed tomography (SPECT)] using [123I]HMPAO (hexamethyl propylene amine oxime), high-resolution magnetic resonance imaging (MRI), and formal neuropsychological tests indicating a unilateral and mesial temporal lobe onset of seizures.
TABLE 1.
Clinical Data of the 44 Patients Included in the Immunohistochemical Study: Comparison With Rating for Codistribution of GAD67 and Dynorphin in TLE Patients With And Without Hippocampal Sclerosis
| Patient no. | Age | Age at onset of epilepsy (years) |
Surgery (years after onset of epilepsy) |
Early risk factors |
Medications | No. of seizures/month |
Degeneration rating |
Granule cell dispersion rating |
GAD67/dyn score |
|---|---|---|---|---|---|---|---|---|---|
| Sclerotic specimens | |||||||||
| N3/99 | 27 | 22 | 5 | Febr.s. (13 m) | LTG | 4 | 2 | 2/D | 2.7 |
| N5/99 | 60 | 13 | 47 | no | CBZ, VGB | 4.5 | 4 | 2 | 3.0 |
| N6/99 | 33 | 23 | 10 | Febr.s. (18 m) | CBZ, LTG | 5.5 | 4 | 2 | 3.0 |
| N10/99 | 33 | 1 | 32 | no | CBZ, TGB, VGB | 4 | 3 | 1 | 2.5 |
| N15/99 | 30 | 23 | 7 | trauma (4 y) | CBZ | 5 | 2 | 1 | 2.8 |
| N16/99 | 51 | 31 | 20 | no | CBZ | 4.5 | 3 | 3 | 2.7 |
| N3/00 | 47 | 25 | 22 | no | CBZ, TPM | 5 | 4 | 1 | 2.2 |
| H2/01 | 39 | 12 | 27 | Febr.s. (3 y) | TPM, OXC | 12.5 | 4 | 3 | 2.3 |
| M8/01 | 38 | 4 | 34 | Febr.s. (9 m) | OXC | 3.5 | 4 | 2 | 2.2 |
| N1/01 | 41 | 1 | 40 | Febr.s. (12 m) | CBZ | 50 | 2 | 2/D | 0.8 |
| N2/01 | 17 | 1 | 16 | trauma (1y) | OXC, TPM | 18 | 4 | 3/D | 0.8 |
| N3/01 | 22 | 3 | 19 | no | LTG, TPM | 7.5 | 2 | 1/D | 3.0 |
| N5/01 | 35 | 20 | 15 | trauma (14 y) | CBZ | 2 | 2 | 3/D | 2.0 |
| M3/02 | 39 | 35 | 4 | no | OXC | ? | 3 | 2/D | 3.0 |
| M5/02 | 16 | 9 | 7 | Febr.s. (18 m) | OXC | 2 | 4 | 0 | 2.3 |
| M13/02 | 65 | 14 | 51 | no | CLB, PRD, OXC | 3 | 2 | 0 | 3.0 |
| M15/02 | 29 | 25 | 4 | trauma (7 y) | PHT, LEV | 0.2 | 3 | 0 | 1.7 |
| M17/03 | 55 | 27 | 28 | meningitis (1 y) | CBZ, LTG | 1.5 | 3 | 0 | 2.8 |
| M21/03 | 35 | 2 | 33 | Febr.s. (8 m), meningitis (3y) |
CBZ | 4.5 | 4 | 2 | 1.2 |
| M22/03 | 48 | 6 | 42 | no | LEV, PHT, GBP | 1 | 3 | 2 | 1.4 |
| M24/03 | 37 | 6 | 31 | trauma (baby) | LTG, CLB | 1.5 | 4 | 3 | 1.0 |
| M25/03 | 9 | 4 | 5 | Febr.s. (2 y) | OXC | 3 | 2 | 0 | 3.0 |
| M26/03 | 9 | 3 | 6 | Febr.s. (1 y) | VPA | 8 | 3 | 0 | 3.0 |
| E18 | 29 | 26 | 3 | trauma (21 y) | CBZ, GBP | 49 | 3 | 2 | 3.0 |
| E19 | 37 | 20 | 17 | Febr.s. (1 y) | PHT, GBP | 24 | 4 | 1 | 0.6 |
| E22 | 37 | 12 | 25 | no | PHT, GBP | 10.5 | 2 | 1 | 2.5 |
| E25 | 33 | 1 | 32 | meningitis (1 y) | CBZ | 0.5 | 4 | 0 | 2.5 |
| E30 | 45 | 1 | 44 | Febr.s. (1 y) | PRD | 4.5 | 2 | 3/D | 1.5 |
| E32 | 31 | 3 | 28 | no | CBZ, PHT | 15 | 4 | 0 | 3.0 |
| E34 | 29 | 11 | 18 | no | CBZ, VPA | 4.5 | 3 | 2 | 1.8 |
| E35 | 25 | 9 | 16 | no | CBZ, VPA | 12.5 | 2 | 1 | 1.8 |
| E36 | 41 | 25 | 16 | no | PHT | 32 | 2 | 0 | 1.3 |
| E39 | 32 | 14 | 18 | Febr.s. (4 y) | CBZ, VGB | 2.5 | 3 | 2 | 2.5 |
| E40 | 40 | 6 | 34 | trauma (2 y) | CBZ | 28 | 3 | 2 | 1.0 |
| E41 | 45 | 38 | 7 | trauma (25 y) | PHT, OXC, CLB | 4 | 3 | 2 | 3.0 |
| E45 | 27 | 6 | 21 | Febr.s. (6 m), trauma (4 y) |
CBZ | 3.5 | 3 | 3/D | 1.0 |
| N2/99 | 43 | 0 | 43 | no | CBZ | 2 | 2 | 1 | 2.0 |
| Non-sclerotic and modestly sclerotic specimens | |||||||||
| N9/99 | 35 | 10 | 25 | no | CBZ, TPM, CLB barbexaclon, | 4.5 | 0 | 0 | 2.0 |
| G1/00 | 56 | 45 | 11 | no | OXC | 17 | 0 | 0 | 0 |
| N13/99 | 48 | 36 | 12 | no | CBZ | 2 | 0 | 1 | 3.0 |
| K6/00 | 17 | 11 | 6 | trauma (10 y) | FBM, OXC, CLB | 7 | 1 | 0 | 2.5 |
| K10/01 | 26 | 2 | 24 | no | CBZ | 2.5 | 1 | 0 | 1.8 |
| H1/02 | 24 | 7 | 17 | no | OXC, TPM, LEV | 3.5 | 0 | 0 | 2.8 |
| E27 | 22 | 13 | 9 | no | LTG, VGB | 7 | 0 | 0 | 2.0 |
For rating scales see Methods. Abbreviations: Early risk factors: Febr.s., febrile seizures; in parenthesis the age of occurrence in years (y) or months (m) is given. Medication: CBZ, carbamazepine; CLB, clobazam; FBM, felbamate; GBP, gabapentin; LTG, lamotrigine; LEV, levetiracetam; OXC, oxcarbazepine; PHT, phenytoin; PRD, primidone; TGB tiagabin, TPM, topiramate; VPA, valproic acid; VGB, vigabatrine. For rating of degeneration and granule cell dispersion see Methods section. GAD67/dyn score: score for co-distribution of GAD67- and dynorphin-IR in terminal areas of mossy fibers (dentate gyrus, stratum lucidum CA3, inner molecular layer of the dentate gyrus in sclerotic cases). These values represent means of ratings obtained for the three areas by two independent observers. D in column granule cell dispersion denotes double layer.
Post Mortem Tissue From Nonepileptic Patients
Hippocampal tissue was obtained at routine autopsy from 11 patients (two females, nine males) with no known history of any neurological or psychiatric disease (Table 2). The time from death to snap freezing, respectively paraformaldehyde (PFA) fixation (contralateral side) of brain specimens ranged from 8 to16 h (11.6 ± 0.93 h). The mean age at death was 52.5 ± 5.6 yrs. Causes of death were pneumonia, pulmonary embolism, liver cirrhosis, cardiovascular arrest, leukemia, melanoma, lymphoma and cancer of the pharynx, stomach and breast.
TABLE 2.
Clinical Data of Post Mortem Controls
| Patient no. | Age at death | Sex | Cause of death | Time to autopsy |
|---|---|---|---|---|
| Controls | ||||
| C1 | 28 | m | pneumonia | 16 |
| C4 | 95 | m | pulmonary embolism | 8 |
| C6 | 58 | m | pharynx cancer | 11 |
| C7 | 54 | m | liver cirrhosis | 8 |
| C8 | 42 | m | leucemia | 9 |
| C9 | 46 | m | melanoma | 13.5 |
| C10 | 51 | f | breast cancer | 15 |
| C11 | 76 | f | myocardal infarct | 8 |
| C15 | 44 | m | lymphoma | 12.5 |
| C16 | 41 | m | liver cirrhosis | 15.5 |
| C18 | 42 | m | stomach cancer | 11 |
Preparation and Fixation of Specimens
Hippocampal specimens were rinsed in 50 mM phosphate-buffered saline, pH 7.4 (PBS) at 4°C and sectioned perpendicularly to the hippocampal axis into 5-mm blocks. Those obtained from the hippocampal body (middle segment) were included in the study. Tissue blocks were either rapidly snap frozen for immunoblotting (n = 8 TLE specimens and 8 post mortem controls) or fixed with PFA for immunohistochemistry and in situ hybridization (44 TLE specimens and 11 post mortem controls). These were immediately immersed in 4% paraformaldehyde in PBS for 4 to 5 days followed by stepwise immersion in sucrose (concentrations ranging from 5 to 20%) over 2 days and then snap frozen (−70°C cold isopentane, 3 min). After allowing the isopentane to evaporate for 24 h at −70°C, tissue samples were sealed in vials and stored at −70°C. For immunohistochemistry, microtome sections (40 μm) were collected and kept for up to 2 weeks in PBS containing 0.1% sodium azide at 5°C to reduce endogenous peroxidase activity. For in situ hybridization, 20-μm sections were cut, mounted on poly-l-lysine-coated slides and stored at −20°C.
Immunohistochemistry
Antibodies: GAD67-immunoreactivity (IR) was studied using a monoclonal mouse GAD67-specific antibody (1:10,000; IgG2α, clone1G10.2; Chemicon, MAB5406). The antibody was highly specific for GAD67 in immunoblots. A rabbit antiserum targeting dynorphin A (1–8) (1:20,000) (Pirker et al., 2001, 2009) was used to label mossy fibers. For labeling terminals of GABA-ergic neurons, an affinity-purified rabbit antiserum against a fusion protein of the N-terminal sequence of the vesicular GABA transporter (VGAT) with glutathione S-transferase (1:1,000) was applied (Chaudhry et al., 1998). For glutamate decarboxylase65 (GAD65) a commercially available rabbit antibody (1:5,000; AB 5,082, Chemicon; Millipore, Vienna, Austria) was used. For detection of neuronal nuclear protein (NeuN), a monoclonal mouse antibody (1:5,000; MAB 377; Chemicon, Millipore, Vienna, Austria) was used.
Adjacent free-floating 40-μm sections were used for immunohistochemistry. Sections were pretreated with target retrieval solution (pH 6.0; Dako, Vienna, Austria, 70°C, 20 min) and, after washing in 50 mM Tris-buffered saline pH 7.2 (TBS) for 5 min, with 0.6% H2O2, 20% methanol/TBS for 20 min to reduce endogenous peroxidase activity. They were then incubated in 10% normal horse serum (for GAD67 and NeuN) or 10% normal goat serum (for dynorphin, VGAT and GAD65; both VWR, Vienna, Austria) in TBS for 90 min and subsequently with the primary antiserum at 4°C for 48–72 h. The sections were then processed by the Vectastain ABC standard procedure using 1:200 dilutions of Vectastain PK4002 horse biotinylated antimouse antibody for detecting the GAD67 and NeuN mouse antibodies, and Vectastain PK4001 goat biotinylated antirabbit antibody for detecting the rabbit VGAT and dynorphin antibodies (Vector, Szabo-Scandic, Vienna, Austria). Incubations with the biotinylated secondary antibodies and subsequent incubations with the ABC reagent (mixture of avidin-biotin-horseradish peroxidase complex; 1:100) were done at room temperature for 60 min each. The resulting complex was labeled by reacting the peroxidase with 0.05% 3,3′-diaminobenzidine (Sigma, Munich, Germany) and 0.005% H2O2 in TBS for 4 min. Sections were mounted on slides, air-dried, dehydrated, and cover-slipped. After each incubation step (except pre-incubation with 10% normal horse serum), three 5 min washes with TBS were included. All buffers and antibody dilutions, except those for washing after target retrieval solution and peroxidase treatment, and the reaction with diaminobenzidine, contained 0.1% Triton X-100. Normal horse or goat serum (10%) was included in all antibody-containing buffers. Sections not exposed to the primary antibody were included as controls and did not show immunoreactive elements.
Double Immunofluorescence
Double immunofluorescence was performed on sections from six selected patients. Incubations were done using free-floating 40-μm sections. After subjecting them to target retrieval treatment and peroxidase block, incubations were processed as described above using concomitant incubations with the monoclonal GAD67 antibody (dilution of 1:5,000), and with the rabbit dynorphin A (1–8) antibody (1:10,000) or the VGAT antiserum (1:1,000). For colabeling of GAD67-IR and dynorphin-IR or GAD67 and VGAT, an Alexa 555-coupled goat antimouse antibody (IgG2α; 1:500; Molecular Probes; Eubio, Vienna, Austria) for detecting GAD67-IR and an Alexa AF488-coupled donkey antirabbit antibody (1:1,000; Molecular Probes; Eubio, Vienna, Austria) for detecting dynorphin and VGAT, respectively, were used as secondary antibodies. The sections were embedded in Vectashield (Vector Laboratories; Szabo-Scandic, Vienna, Austria) in 0.5% gelatin/0.05% chrome alum on uncoated slides. A Zeiss Axioplan-2 fluorescence microscope equipped with a CCD camera (AxioCam, Zeiss, Vienna) was used for recording images. These were analyzed and displayed using Openlab software (version 3.0.2; Improvision, Tübingen, Germany).
In Situ Hybridization
In situ hybridization was performed on 20-μm sections from 36 TLE patients and 9 post mortem controls. An oligonucleotide complementary to bases 203–252 of human GAD67 mRNA (Bu et al., 1992), (GenBank accession number NM_000817.2: AGCTGCTTCCTCGTTCTCGCTAGTTCCCTCTCGCACGCGAGCGCCAGGAG) was synthesized by Microsynth (Balgach, Switzerland). It was labeled with [35S] α-thio-dATP (1,300 Ci mmol−1; New England Nuclear, Boston, MA) on the 3′ end by reaction with terminal deoxynucleotidyl transferase (Roche, Mannheim, Germany), and in situ hybridization was performed as described previously (Furtinger et al., 2001). After washing stringently (four times in 50% formamide in 2× SSC; 42°C, 15 min), the sections were briefly rinsed in 1× SSC followed by water, dipped in 70% ethanol, dried, and exposed to BioMax MR films (Amersham Pharmacia Biotech, Buckinghamshire, UK) for 21 days. An excess of nonradioactive probe displaced the radioactive labeling and was used as control. The relative amount of GAD67-specific hybridization signal was determined over the granule cell layer by densitometric analysis of film autoradiograms using the ImageJ analysis system (written by Wayne Rasband at the U.S. National Institutes of Health and available from the Internet by anonymous FTP from zippy.nimh.nih.gov). Changes in hybridization signal intensity in TLE specimens were quantified by determining the percent difference in relative optic density (ROD) in control tissue sections relative to experimental tissue sections processed in parallel. Background values were determined in the adjacent white matter and subtracted from ROD values. Calibration and linearization of film autoradiograms were preformed relative to 14C-labeled autoradiographic standards (microscales, New England Nuclear, Boston, MA), which were exposed to each film simultaneously with hybridized tissue sections.
Neuronal Cell Counts
After autoradiography the sections were Nissl-stained and cell counts were performed in three different 250 × 100 μm2 areas of the granule cell layer, and values were averaged. An ocular grid (100 boxes of 2.5 × 2.5 μm2 each) was used at 400× magnification.
Neurodegeneration Rating
Neurodegeneration rating was performed independently by two blinded observers using Nissl-stained sections adjacent to the sections used for immunohistochemistry and the following rating scale (Davies et al., 1996): 0, no obvious neurodegeneration; 1, modest cell losses (up to about 10%) of pyramidal neurons (primarily CA1) and/or hilar interneurons; 2, minor but obvious cell losses of hilar interneurons and CA1 and CA3 pyramidal cells; 3, significant cell losses in the hilus of the dentate gyrus (only a few cells are visible), major parts of the CA1 and CA3 sector of the Ammon’s horn are affected; 4, clear-cut losses in dentate granule cells and CA2 neurons in addition to severe damage of hilar interneurons and CA1 and CA3 pyramidal neurons.
Rating of Granule Cell Dispersion
Granule cell dispersion was rated as follows: 0, no obvious dispersion; 1, modest dispersion; 2, more than 30% of the granule cell layer is broadened by ~50–100%; 3, more than half of the granule cell layer is affected and its width is significantly increased (generally by 100% or more). At ratings 2 and 3, the density of the granule cell layer often loosens and significant neurodegeneration can lead to segments of even reduced layer width. Double granule cell layer can be seen in over 20% of cases (labeled D in Table 1).
Evaluation of Codistribution of GAD67-IR and Dynorphin-IR After Conventional Immunohistochemistry
The distribution of GAD67-IR, dynorphin-IR, and VGAT-IR was assessed in adjacent sections processed by the avidin-biotin-peroxidase method. We assessed the extent of colabeling in the following terminal structures of mossy fibers: (i) the hilus of the DG (bundles of mossy fibers; plaques presumably originating from degenerating mossy fibers); (ii) the terminal area of mossy fibers in the stratum lucidum (no or minor overlap with VGAT-IR); (iii) the area of sprouted mossy fiber terminals in the inner molecular layer.
A rating scale for the likelihood of codistribution of GAD67-IR and dynorphin-IR was adapted. Two independent observers rated the patterns of GAD67 and dynorphin colabeling at all three sites using a four-point rating scale: 0, colabeling unlikely; 1, colabeling possible; 2, colabeling likely; 3, colabeling evident. Independent ratings were obtained for all three sites by the two observers, and mean values were obtained from these data.
Immunoblotting
Cryotome sections (20 μm) of snap frozen, unfixed hippocampal specimens were obtained from controls (n = 8) and sclerotic TLE patients (n = 8) and mounted on slides. For each specimen, the area of the dentate gyrus was scraped off from six sections using a spatula and collected in an Eppendorf vial. Tissues were homogenized in 10 mM HEPES pH 7.5, 1 mM EDTA, 1 mM benzamidine, 0.3 mM phenylmethylsulfonyl fluoride, 100 mg L−1 bacitracin (all Sigma, Munich, Germany) by mild sonication. Samples were centrifuged (8,000g, 20 min, 4°C). Supernatants were denatured (15 min, 65°C) in sample buffer (NP0007, Invitrogen, Lofer, Austria) under reducing conditions (10% reducing agent NP0001; Invitrogen). Samples (5 μg protein) were run on 4–12% NuPAGE Bis-Tris gels with a MOPS running buffer (NP0321, Invitrogen) and blotted onto polyvinylidene difluoride (PVDF) membranes (SequiBlot, BioRad, Vienna, Austria), according to the standard procedures of the manufacturers. Membranes were blocked (2 h, room temperature) in 2.5% skim milk in TBS containing 0.1% Tween 20 (Merck, Darmstadt, Germany) and then incubated with the primary GAD67 antibody (0.3 μg mL−1; IgG2α, clone1G10.2; Chemicon, MAB5406) in the same buffer at 4°C over night. Subsequently, labeling with a mouse monoclonal neuron-specific enolase (NSE) antibody (ab8197, Abcam, Cambridge, UK; 1:4,000, 1 h at room temperature) was performed as an internal standard. In both instances, a horseradish peroxidase-conjugated secondary antibody (rabbit antimouse IgG, P260, Dako, Vienna, Austria; 1:4,000, 90 min) was applied in TBS-Tween 20. Prestained size markers (See Blue Plus 2, LC 5,925, Invitrogen, Lofer, Austria) were used for calibration. Enhanced chemoluminescence was used for signal detection. Films were exposed for 5 s (NSE) and 5–10 min (GAD67), respectively, and then digitized. The ROD of bands corresponding to GAD67 and NSE were determined. Data are expressed as ratios of GAD67 and NSE values.
Determination of GABA Levels
Amino acid levels were determined in the dentate gyri of 10 sclerotic TLE patients and six post mortem controls. Hippocampal slices (40 μm) were cut in a microtome. The dentate gyri were removed under a dissection microscope, transferred to an Eppendorf tube, weighed, and frozen. The samples were then sonicated in 50 vol of 0.1 M perchloric acid, centrifuged for 15 min at 13,000g at 4°C, and the supernatants were frozen at −80°C until analysis. Samples were thawed and diluted with H2O (final dilution 1:2,000). Each sample (100 μL) was then mixed with 50 μL OPA-reagent (25 mg o-phthalaldehyde dissolved in a mixture of 0.5 mL methanol, 25 μL mercaptoethanol, and 4.5 mL 0.4-M sodium borate, pH 11), and after a reaction time of 2 min, 20 μL was injected by an autosampler (AS950, Jasco). The HPLC system consisted of a RP-18 column (Chromolith-Performance RP-18e, 100 × 4.6 mm2, Merck) in a column-heater set a 25°C (BFO-04 f1, W.O. Electronics), and an aqueous mobile phase containing 0.1 M sodium phosphate buffer, pH 6.0 and 0.13 mM EDTA mixed with methanol by a high-pressure gradient system at a flow-rate of 1.2 mL min−1 (two pumps, L-7,100, Merck-Hitachi) in a step-gradient of 18% methanol for 18 min and 38% methanol for the remainder of the 25-min run. Derivatized amino acids (GABA, alanine, serine, threonine, glycine, taurine, glutamate, glutamine, aspartate, or asparagine) were detected by a fluorescence detector (L-7,480, Merck-Hitachi) with excitation at 340 nm and emission at 440 nm. Ten major amino acids, including GABA, were detected and quantified. GABA levels were normalized to the alanine content, using alanine as an internal reference.
Evaluation of Loss in Immunoreactivity and mRNA in the Rat Brain Post Mortem
To evaluate the possible loss in immunoreactivity and mRNA post mortem, we performed an experiment in which rat brains were exposed for different time intervals to elevated (16°C) temperature prior to histochemistry. For immunohistochemistry rat brains were perfused with PBS (25 mL, room temperature) to remove blood cells and thus to avoid artifacts by endogenous peroxidase activity. They were kept within their sculls 0, 8, and 24 h (N = 4 for each group) and then fixed in 4% PFA as described for the human brain samples. For in situ hybridization a separate set of rat brains was obtained. These brains were not perfused and removed after the same time intervals from their sculls and snap frozen in −70°C isopentane. For in situ hybridization and immunohistochemistry the same protocols as for the human samples were used. A probe for rat GAD67 (GCAGGTTCTTGGAGGCCTGCCTCTCCCTGAAGGCGCTCAC; custom synthesized by Microsynth, Balgach, Switzerland) was applied.
Statistical Analyses
Data are presented as means ± SEM. They were analyzed for normal distribution and equal variances using GraphPad Prism software (Prism 5 for Macintosh, GraphPad Software, San Diego, CA). Statistical analyses were done by Student’s t test for data with normal distribution or Mann-Whitney test as a nonparametric test. Analysis of variance (ANOVA) was used for multiple comparisons (rat studies). Statistical analysis of GAD67/dynorphin codistribution with clinical data was done for sclerotic and nonsclerotic specimens by analysis of covariance (ANCOVA). GAD67/dynorphin codistribution rating was defined as the dependent variable and age, years after onset of epilepsy, age of seizure onset, number of antiepileptic drugs, degeneration score, granule cell dispersion, and number of seizures per month as covariates. ANCOVA was performed on SPSS 16.0 (SPSS, Chicago, IL).
RESULTS
Clinical Variables of Patients
Specimens were obtained from 44 TLE patients and 11 autopsy controls. Clinical variables are shown in Tables 1 and 2, respectively. Among the TLE patients 37 revealed hippocampal sclerosis, 7 were nonsclerotic. Patients with Ammon’s horn sclerosis had a significantly higher granule cell dispersion rating than nonsclerotic patients (P = 0.003) but did not significantly differ in time of onset of epilepsy (P = 0.450), duration of epilepsy (P = 0.227), number of seizures/month (P = 0.947), number of antiepileptic drugs in treatment before surgery (P = 0.338) or in GAD67/dynorphin codistribution rating (P = 0.746). The age at surgery did not differ between nonsclerotic (mean ± SEM: 32.6 ± 5.46) and sclerotic patients (35.4 ± 2.03) but was significantly higher in autopsy controls (52.5 ± 5.61; P < 0.01).
Post Mortem Changes in GAD67 Protein and mRNA Levels in the Rat
To examine the influence of time from death to fixation/snap freezing of the tissue we examined GAD67-IR and GAD67 mRNA levels in mossy fibers and granule cells, respectively at death and 8 and 24 h after storing the brains at 16°C. After 24 h, we observed a 28% decrease in immunoreactivity and a 24% decrease in mRNA levels (Fig. 1). The ratio of RODs GAD67/NSE in Western blotting was unchanged, although there was a 65% reduction in ROD values per μg protein for both NSE and GAD67 (data not shown). Interestingly, in the human brains ROD values/μg protein were identical for post mortem and epilepsy specimens, indicating a higher stability of proteins in the human brain than in rat brains at the indicated experimental conditions.
FIGURE 1.
Influence of post mortem time on GAD67-IR and mRNA levels in the rat brain. Rats were killed and decapitated. Their sculls were kept for 0, 8, and 24 h at 16°C. Their brains were then removed and either immersion fixed with paraformaldehyde for subsequent immunohistochemistry or snap frozen for in situ hybridization. In A, D, and G GAD67-IR is shown in the dentate gyrus at the post mortem intervals of 0, 8, and 24 h, respectively. In B, E, H, GAD67-IR is shown in the prefrontal cortex region revealing survival of GAD67-IR neurons at the same intervals. In C, F, I GAD67 mRNA is shown at post mortem intervals of 0, 8, and 24 h. In J and K relative optical density (ROD) values are shown for the mossy fibers in the tip of the hilus (A, D, G) and for the granule cell layer (C, F, I), respectively. The numbers of animals per group were 4. Statistical analysis was done by ANOVA with Bonferoni post hoc test. P refers to the nonexposed (0 h) group.
Distribution of GAD67 and Dynorphin Immunoreactivities
The distribution of GAD67-IR was heterogeneous in the TLE specimens. Strikingly, in most cases (38 of 44) labeling was considerably stronger in the terminal fields of mossy fibers (dentate hilus, stratum lucidum) than in areas associated with dense GABAergic axon terminals (e.g., the outer molecular layer of the DG, axons of GABA neurons passing the CA1 stratum pyramidale), (Fig. 2A). In all cases with moderate to strong hippocampal sclerosis (n = 36), the inner molecular layer of the DG, corresponding to the terminal area of sprouted mossy fibers, was also immunoreactive for GAD67 (Figs. 2A and 3A). In contrast, specimens without Ammon’s horn sclerosis (n = 8) revealed labeling in the hilus and in the stratum lucidum of CA3 but not in the inner molecular layer of the DG (Fig. 3D). Dense immunoreactivity was also present in the subiculum (Fig. 2D).
FIGURE 2.
Distribution of GAD67-, dynorphin-, VGAT-, and NeuN-IR in the hippocampus of a TLE patient with Ammon’s horn sclerosis (A–C, E, F; case E18) and of GAD67-IR in a specimen without hippocampal sclerosis (D; case K10/01). In the specimen with Ammon’s horn sclerosis, NeuN-IR (C) reveals cell losses in the hilus and in sectors CA1 and CA3, whereas the subiculum (S) and the dentate granule cell layer are largely preserved. Dynorphin-IR (B) and GAD67-IR (A) mainly followed the pattern of the terminal field of mossy fibers (hilus of the dentate gyrus, stratum lucidum) and labeled the inner molecular layer (iml), which is aberrantly innervated by mossy fibers (mf). GAD67-IR was also present in the subiculum and extended (in contrast to dynorphin-IR) from the stratum lucidum (s. luc) to the stratum pyramidale of sector CA1 (there labeling fibers of GABA neurons). VGAT-IR (E) and GAD65-IR (F) revealed an entirely different labeling pattern in adjacent sections of the same (sclerotic) specimen reflecting the distribution of fibers originating from GABA-ergic interneurons. In contrast to dynorphin-IR and GAD67-IR VGAT-IR (E) and GAD65-IR (F) are faint in all terminal areas of mossy fibers, but label GABA-ergic fibers e.g., in the (inner and outer) molecular layer, in the stratum pyramidale CA1 and in the subiculum (E, F). Presumably due to neurodegeneration, the dentate hilus is weakly labeled for both markers (E, F). In D GAD67-IR is shown for a specimen without hippocampal sclerosis. It reveals a similar labeling pattern as the sclerotic specimen, except that the terminal area of sprouted mossy fibers in the inner dentate molecular layer lacks GAD67-IR. Presumably due to the lack of neurodegeneration, labeling of GABA neurons in the sector CA1 is also more prominent than in the sclerotic specimen. Arrowheads in A, B, D, E, and F denote the borders between the stratum lucidum CA3 and stratum pyramidale CA2. Scale bar: 1 mm.
FIGURE 3.

Distribution of GAD67-IR, dynorphin-IR, and VGAT-IR in the molecular layer of the dentate gyrus of TLE patients with (A–C; patient E41) and without (D–F; patient E27) Ammon’s horn sclerosis. Dynorphin-IR serves as a specific marker for mossy fibers. VGAT labels the axons of GABA interneurons. In the specimen with Ammon’s horn sclerosis, the inner (iml) but not the outer molecular layer (oml) is intensely labeled for GAD67 (A) and dynorphin (B), whereas VGAT-IR labels the inner and the outer molecular layer equally. In the nonsclerotic specimen, labeling of the iml by GAD67 and dynorphin is absent (D, E). Dynorphin-IR in the middle and outer molecular layer (oml) presumably labels axon terminals of the perforant path (E). In the outer molecular layer, VGAT-IR axons of hilar GABA neurons terminate and can be well seen in the nonsclerotic specimen (F). Abbreviation: h, hilus, gc, granule cells. Scale bar: 100 μm.
With the exception of four specimens, the distribution of GAD67-IR closely resembled that obtained for dynorphin-IR in adjacent sections. Labeling of mossy fibers in the hilus of the dentate gyrus, along the pyramidal layer in sector CA3 and the terminal field of mossy fibers in the stratum lucidum was strikingly similar for both markers (Figs. 2A,B). In addition, bundles of mossy fibers and plaque-like structures in the dentate hilus presumably originating from degenerating mossy fibers were immunoreactive for GAD67 and dynorphin (Figs. 2A,B and 4D–F). In all specimens with Ammon’s horn sclerosis, dynorphin-IR was also observed in the inner molecular layer labeling terminals of sprouted mossy fibers (Figs. 2B, 3B, and 4H; Houser et al., 1990). In nonsclerotic specimens, dynorphin-IR was restricted to the middle and outer segment of the dentate molecular layer, where it presumably labeled afferent fibers of the perforant path (Fig. 3E). Faint GAD67-IR was observed also in the terminal field of mossy fibers in post mortem controls (not shown). To find possible differences in the labeling of mossy fibers by GAD67 and to correlate these with the clinical data, we had assessed a rating of the codistribution of GAD67-IR with the mossy fiber marker dynorphin (Table 1).
FIGURE 4.
Double immunofluorescence for GAD67 and the mossy fiber marker dynorphin in terminal areas of mossy fibers (A–I) and of GAD67 and VGAT in GABAergic fibers of the dentate gyrus (J–L) in sections from patients with hippocampal sclerosis. A–C shows colabeling of the stratum lucidum CA3 with GAD67- and dynorphin-IR (patient E18); D–F depicts colabeling of mossy fiber bundles in the dentate hilus (h) with GAD67-IR and dynorphin-IR (patient E18). In G–I labeling of the dentate molecular layer of a sclerotic specimen is shown for GAD67- and dynorphin-IR (case E41). Note colabeling of GAD67 with the mossy fiber marker dynorphin in the inner but not outer molecular layer (this is the terminal area of sprouted mossy fibers in hippocampal sclerosis). On the other hand, VGAT a marker of GABA-ergic neurons labels the outer molecular layer (K, L). Labeling for GAD67-IR again is strong in the inner (J, L) and faint in the outer (J) molecular layer. Scale bars: in C for A–C: 200 μm; in F for D–F: 50 μm, in I and L for G–L: 200 μm.
Clinical variables were compared with the GAD67/dynorphin score by ANCOVA. Controlling for the patient group (sclerotic, nonsclerotic) rating for GAD67/dynorphin colabeling did not correlate with age (F(1,35) = 0.08; P = 0.78), duration of epilepsy (F(1,35) = 2.50; P = 0.12), age of seizure onset (F(1,35) = 1.88; P = 0.18), number of medications (F(1,35) = 2.17; P = 0.15), granule cell dispersion (F(1,35) = 2.18; P = 0.15) or degeneration rate (F(1,35) = 0.44; P = 0.51). There was, however, a significant correlation between GAD67/dynorphin colabeling and number of seizures per month (F(1,35) = 4.91; P = 0.03).
Vesicular GABA Transporter (VGAT) and GAD65
We used VGAT-IR to label GABA-ergic nerve fibers. VGAT-IR was present throughout the hippocampal formation of TLE specimens (Fig. 2E). It labeled thin varicose fibers in the molecular layer (preferentially in the outer molecular layer), the hilus of the dentate gyrus, and throughout all segments of the hippocampus (Figs. 2E and 3C). Axons passing through the dentate granule cell layer and through residual parts of the pyramidal cell layers were labeled with the VGAT antibody in sclerotic specimens (Fig. 2E). In the dentate molecular layer of sclerotic specimens the inner and outer molecular layer were equally labeled for VGAT, presumably reflecting the labeling pattern of sprouted interneurons (Mathern et al., 1995; Furtinger et al., 2001). In nonsclerotic specimens, VGAT-IR was mostly restricted to the outer segment, where it labeled terminals of GABA-ergic interneurons of the dentate hilus (Fig. 3F). In none of the specimens investigated, VGAT-IR resembled that of dynorphin-IR outlining the terminal field of mossy fibers in the stratum lucidum or mossy fiber bundles in the dentate hilus. GAD65-IR was weak and followed the pattern of VGAT-IR and no labeling was observed in mossy fibers (Fig. 2F).
Double Immunofluorescence for GAD67 and Dynorphin, and GAD67 and VGAT
For unequivocally demonstrating expression of GAD67 in mossy fibers, we performed double immunofluorescence labeling of GAD67 with the mossy fiber marker dynorphin and with VGAT as a marker for GABA-ergic nerve fibers. As shown in Figure 4, GAD67-IR was expressed together with dynorphin-IR in mossy fiber terminals of the stratum lucidum of CA3 (Figs. 4A–C) and in bundles of mossy fibers in the hilus of the dentate gyrus (Figs. 4D–F). In specimens with Ammon’s horn sclerosis, GAD67-IR and dynorphin-IR also colabeled fibers in the inner but not in the outer molecular layer of the dentate gyrus (Figs. 4G–I). In contrast, VGAT-IR was strong in the inner and outer molecular layer (labeling terminals of GABA-ergic interneurons arising from the hilus) (Fig. 4K), whereas GAD67-IR was enriched in the inner molecular layer compared with the outer segment (Fig. 4J). GAD67, present in mossy fibers, also demonstrated strong labeling in the hilus (Fig. 4J), which contrasted with the weak VGAT-IR in this area (Fig. 4K).
GAD67 Immunoblotting
To characterize GAD67-IR in the brain specimen and for obtaining a quantitative estimate of the increase in GAD67 levels in TLE specimens, we performed immunoblotting experiments comparing GAD67 levels in the dentate gyrus of eight TLE and eight post mortem specimens. Using the same monoclonal GAD67 antibody as for the immunohistochemical studies, we observed single bands migrating around 67 kDa both in control and epileptic tissue, demonstrating the specificity of immunolabeling (Fig. 5A). NSE was used as a reference protein. GAD67/NSE ratios were about 20 times higher (P < 0.0001) in TLE specimens (n = 8) than in controls (n = 8), gyrus (Fig. 5B).
FIGURE 5.
Immunoblots of dentate gyrus tissue from TLE patients. In A, a representative immunoblot of four arbitrarily selected, snap-frozen specimens from TLE patients (coded with E1 to E4) and post mortem controls (C1 to C4) is shown. The blots were consecutively incubated with a monoclonal GAD67 antibody and an antibody for neuron-specific enolase (NSE). Single bands were observed for GAD67 both in control and in TLE tissue. Panel B depicts the ratios of ROD values obtained for GAD67 and NSE. ***Statistically different from controls (P < 0.0001) using the two-tailed Student’s t test.
In Situ Hybridization of GAD67 mRNA
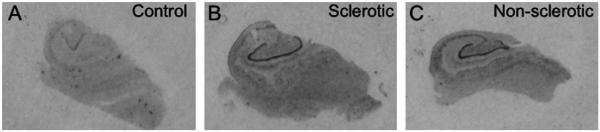
For obtaining further evidence for GAD67 expression in granule cells/mossy fibers we performed in situ hybridization for GAD67 mRNA. As shown in Figure 6, GAD67 mRNA was detected in the granule cell layer of all TLE and postmortem specimens investigated. In representative samples of sclerotic TLE (n = 33) and nonsclerotic specimens (n = 5), concentrations of GAD67 mRNA were significantly increased compared to post mortem control specimens (n = 9): Mean % of control ± SEM of sclerotic specimens: 179.0% ± 18.25%; P = 0.0002; range 107–328%; nonsclerotic specimens: 163% ± 25.9%; P = 0.022; range 92–250%. When correcting in the sclerotic specimens the values to account for cell loss in the granule cell layer, mRNA levels were 361.9% ± 31.38% of control (P = 0.0002; range: 180–558%). There was no significant difference between the increases in mRNA levels in the sclerotic vs. nonsclerotic cases (P = 0.74).
FIGURE 6.
GAD67 mRNA. Autoradiographs of sections from the hippocampus of a postmortem control (A), a TLE specimen with (B, case N 10/99) and one without hippocampal sclerosis (C, case G1/00). Note the pronounced up-regulation of GAD67 mRNA in dentate granule cells equally in the sclerotic and in the nonsclerotic specimen. For quantification see text.
Increased Concentrations of GABA in the Dentate Gyrus
For obtaining evidence for a change in GABA synthesis in the area of mossy fibers, we determined the concentrations of ten amino acids including GABA in sections of the dentate gyrus. To correct for variations in tissue concentrations, we calculated GABA levels as the ratio of GABA/alanine, using alanine as an internal standard. Mean ratios ±SEM were 1.65 ± 0.075 (n = 10; range: 1.35 to 2.00) in TLE and 0.66 ± 0.088 (n = 6) in autopsy control specimens, reflecting a highly significant (P < 0.0001) increase (152%, range: 0.32–0.90) in GABA levels in the TLE specimens. GABA/amino acid ratios were also significantly increased when calculated for either of the other amino acids determined concomitantly (serine, threonine, glycine, taurine, glutamate, glutamine, aspartate, or asparagine).
DISCUSSION
Our study provides the first evidence for pronounced expression of one of the two key enzymes in GABA synthesis, GAD67, in mossy fibers, a prominent glutamatergic fiber tract of the hippocampus. This expression was demonstrated in specimens obtained at surgery from patients with TLE using conventional immunohistochemistry and double immunofluorescence. GAD67 was colocalized together with the mossy fiber marker dynorphin to bundles of mossy fibers of the dentate hilus, to mossy fiber terminals in the stratum lucidum, and to the inner molecular layer aberrantly innervated by mossy fiber sprouting in TLE patients with Ammon’s horn sclerosis. In addition we observed expression of GAD67 mRNA in dentate granule cells. Our findings also indicate an increase in the expression of GAD67 protein, accompanied by increases in GAD67 mRNA and GABA levels in the area of the dentate gyrus. Direct comparisons, however, of freshly removed surgical tissue with post mortem controls have certainly to be done with caution. In particular, GABA levels may be sensitive to post mortem metabolism. Indeed, an experiment in rats mimicking the post mortem autolysis of the human tissue revealed a roughly 25–30% decrease in GAD67 mRNA and protein within 24 h (the longest post mortem interval in the human specimens was 16 h). In spite, taken the 80–260% increases in granule cell mRNA levels and the 2000% increases in GAD67 protein in the dentate gyrus an induction of GAD67 expression in TLE is still likely. This assumption for the epileptic human hippocampus is supported by the recent studies in animal models of TLE showing pronounced expression (and upregulation) of GAD67 but not of VGAT mRNA and protein in granule cells/mossy fibers of the dentate gyrus (Szabo et al., 2000; Sperk et al., 2003).
Interestingly, GAD67 mRNA expression is rapid but transient in the rat epilepsy model (Schwarzer and Sperk, 1995; Szabo et al., 2000). This may explain the relatively modest increases in mRNA levels compared with the strong increases in GAD67 protein in the human specimens. The expression of GAD67 protein may also be triggered by repeated seizures. This is indicated by the correlation of the number of seizures/month and the GAD67/dynorphin colabeling. It implies that GAD67 expression in mossy fibers may be a compensatory mechanism or a long-term effect of repeated seizures.
The relatively poor colabeling of GAD67-IR with VGAT-IR in GABA-ergic neuron terminals was also interesting, but may be explained by a specific and dramatic upregulation of GAD67-IR in mossy fibers with a concurrent loss of GABA interneurons. In contrast to GAD67, GAD65 appears not to be expressed in mossy fibers. Thus, using an antibody that preferentially detects GAD65, Mathern et al. (1999) reported a distribution of GAD65 that correlated with the distribution of GABA-ergic axons and neurons (e.g., labeling the outer molecular layer of the dentate gyrus) but not with that of mossy fibers. In our specimens, we observed a similar pattern of GAD65-IR, corresponding to the distribution of VGAT-IR but not that of dynorphin-IR (see Pirker et al., 2001, not shown here) and indicating that GAD65 is expressed in the axons of GABA-ergic neurons but not in mossy fibers. Since GAD65 seems to be more closely related to synaptic transmission than GAD67 (Soghomonian and Martin, 1998), the finding that GAD67 but not GAD65 is up-regulated in mossy fibers points to a preferentially nonsynaptic role of this mechanism.
Although GAD67 and GAD65 are generally expressed together in GABA-ergic neurons, the two enzymes also present distinct differences (Mathern et al., 1999). Whereas the activity of GAD65 is regulated by its affinity to the cofactor pyridoxal phosphate, GAD67 has the cofactor tightly bound, and its activity is regulated by changes in mRNA expression. GAD65 has also been suggested to be preferentially targeted to nerve endings and to be responsible for synthesis of GABA stored in vesicles, whereas GAD67 is more ubiquitously distributed within neurons and may be associated with the synthesis of GABA released by nonvesicular mechanisms (Esclapez et al., 1994; Soghomonian and Martin, 1998).
The coexistence of GABA with other transmitters is not unique. Neurons expressing GAD67, VGAT, and the vesicular glutamate transporter have been recently identified in the anterioventral periventricular nucleus of female rats, indicating that these neurons have a dual glutamatergic/GABA-ergic phenotype that preconditions them for exocytotic release of both neurotransmitters (Ottem et al., 2004).
Retinal amacrine cells provide another well-characterized example of GABA colocalizing with another classical neurotransmitter in the same neurons. Such cells contain GAD67, but not GAD65, in addition to choline acetyltransferase (Brandon and Criswell, 1995). Whereas acetylcholine (ACh) release from amacrine cells is highly Ca2+-dependent and involves exocytosis, GABA is released from the same cells by Ca2+-independent reverse transport (O’Malley et al., 1992). In contrast to acetylcholine, radiolabeled GABA also does not accumulate in the synaptic vesicles of amacrine cells, indicating that although the cells express GAD67, they do not contain a vesicular GABA transporter (O’Malley and Masland, 1989; O’Malley et al., 1992).
A possible route of GABA release from mossy fibers may also involve reverse transport. Granule cells contain the cell membrane GABA transporter GAT-1 (Frahm et al., 2000; Sperk et al., 2003), and no immunohistochemical evidence for expression of VGAT in granule cells has been reported to date (Sperk et al., 2003; Boulland et al., 2007). The direction of GABA transport has been well documented to be highly dependent on actual intracellular concentrations of Na+ and GABA (Schwartz, 1987; Attwell et al., 1993; Koch and Magnusson, 2009). Thus, even minute increases in intracellular GABA concentration, caused by the extremely high expression of GAD67 in TLE or a rise in intracellular Na+ concentration during epileptiform discharges, will obligatorily induce outward GABA transport in the epileptic hippocampus (During et al., 1995; Wu et al., 2001). GABA released from granule cell dendrites may then act on inhibitory GABAA receptors present at high concentrations especially in the molecular layer of the epileptic hippocampus. Clearly this process may represent an endogenous anticonvulsive mechanism that would be especially active during acute epileptic events.
There is, however, also clear evidence for Ca2+ dependent release of GABA at mossy fiber terminals. Stimulation of granule cells results in monosynaptic GABAA receptor-mediated synaptic signals in the CA3 pyramidal neurons of normal guinea pigs (Walker et al., 2001) and kindled rats (Gutierrez and Heinemann, 2001). These signals have properties typical of those of mossy fibers; they are NMDA-receptor independent and induce long-term potentiation upon repeated stimulation (Walker et al., 2001). On the other hand, these findings have not been replicated in 3-week-old rats in which exocytotic GABA release from mossy fibers could not be provoked (Uchigashima et al., 2007). Irrespective of the actual mechanism (exocytotic release of GABA at mossy fiber-CA3 synapses or reverse transport of GABA, e.g., at granule cell dendrites, or both), increased extracellular availability of GABA may serve as an endogenous anticonvulsive principle that helps terminate seizures.
Another function of the extremely high concentration of GAD67 within granule cells/mossy fibers may be to convert excessive glutamate released from and taken up again by mossy fiber terminals during epileptic seizures to nontoxic GABA. This mechanism would prevent reverse transport of glutamate from mossy fibers and diminish excitotoxic cell death.
Further insight into the expression and functional role of GAD67 in mossy fibers has still to be obtained by electron microscopic studies in tissues obtained at surgery. It would be important to investigate in human TLE tissue a possible role of GABA secreted from mossy fibers or from granule cell dendrites on GABAA and GABAB receptors by elecrophysiology. A major drawback of this study is also the comparison of histochemical data obtained from fresh surgical tissue with that of post mortem tissue. Although we attempted to control for decay of histochemical markers by mimicking post mortem changes in the rat brain, changes in rat brain and human tissue could vary considerably.
Acknowledgments
The authors thank Lisi Gasser for technical assistance and Prof. H. Hörtnagl for discussion.
Grant sponsor: Austrian Science Foundation; Grant numbers: P 19 464, SFB 35-12; Grant sponsor: European Union Grant FP6 EPICURE; Grant number: LSH-CT-2006-037315.
REFERENCES
- Attwell D, Barbour B, Szatkowski M. Nonvesicular release of neurotransmitter. Neuron. 1993;11:401–47. doi: 10.1016/0896-6273(93)90145-h. [DOI] [PubMed] [Google Scholar]
- Boulland JL, Ferhat L, Solbu T Tallak, Ferrand N, Chaudhry FA, Storm-Mathisen J, Esclapez M. Changes in vesicular transporters for gamma-aminobutyric acid and glutamate reveal vulnerability and reorganization of hippocampal neurons following pilocarpine-induced seizures. J Comp Neurol. 2007;503:466–485. doi: 10.1002/cne.21384. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Torp R, Zhang N, Storm-Mathisen J, Ottersen OP. Distribution of glutamate-like immunoreactivity in excitatory hippocampal pathways: A semiquantitative electron microscopic study in rats. Neuroscience. 1990;39:405–417. doi: 10.1016/0306-4522(90)90277-b. [DOI] [PubMed] [Google Scholar]
- Brandon C, Criswell MH. Displaced starburst amacrine cells of the rabbit retina contain the 67-kDa isoform, but not the 65-kDa isoform, of glutamate decarboxylase. Vis Neurosci. 1995;12:1053–1061. doi: 10.1017/s0952523800006714. [DOI] [PubMed] [Google Scholar]
- Bu DF, Erlander MG, Hitz BC, Tillakaratne NJ, Kaufman DL, Wagner-McPherson CB, Evans GA, Tobin AJ. Two human glutamate decarboxylases, 65-kDa GAD and 67-kDa GAD, are each encoded by a single gene. Proc Natl Acad Sci U S A. 1992;89:2115–2119. doi: 10.1073/pnas.89.6.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry FA, Reimer RJ, Bellocchio EE, Danbolt NC, Osen KK, Edwards RH, Storm-Mathisen J. The vesicular GABA transporter, VGAT, localizes to synaptic vesicles in sets of glycinergic as well as GABAergic neurons. J Neurosci. 1998;18:9733–9950. doi: 10.1523/JNEUROSCI.18-23-09733.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KG, Hermann BP, Dohan FCJ, Foley KT, Bush AJ, Wyler AR. Relationship of hippocampal sclerosis to duration and age of onset of epilepsy, and childhood febrile seizures in temporal lobectomy patients. Epilepsy Res. 1996;24:119–126. doi: 10.1016/0920-1211(96)00008-3. [DOI] [PubMed] [Google Scholar]
- During MJ, Ryder KM, Spencer DD. Hippocampal GABA transporter function in temporal-lobe epilepsy. Nature. 1995;376:174–177. doi: 10.1038/376174a0. [DOI] [PubMed] [Google Scholar]
- Esclapez M, Tillakaratne NJ, Kaufman DL, Tobin AJ, Houser CR. Comparative localization of two forms of glutamic acid decarboxylase and their mRNAs in rat brain supports the concept of functional differences between the forms. J Neurosci. 1994;14:1834–1855. doi: 10.1523/JNEUROSCI.14-03-01834.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frahm C, Engel D, Piechotta A, Heinemann U, Draguhn A. Presence of gamma-aminobutyric acid transporter mRNA in interneurons and principal cells of rat hippocampus. Neurosci Lett. 2000;288:175–178. doi: 10.1016/s0304-3940(00)01217-9. [DOI] [PubMed] [Google Scholar]
- Furtinger S, Pirker S, Czech T, Baumgartner C, Ransmayr G, Sperk G. Plasticity of Y1 and Y2 receptors and neuropeptide Y fibers in patients with temporal lobe epilepsy. J Neurosci. 2001;21:5804–5812. doi: 10.1523/JNEUROSCI.21-15-05804.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez R, Heinemann U. Kindling induces transient fast inhibition in the dentate gyrus–CA3 projection. Eur J Neurosci. 2001;13:1371–1379. doi: 10.1046/j.0953-816x.2001.01508.x. [DOI] [PubMed] [Google Scholar]
- Houser CR, Esclapez M. Localization of mRNAs encoding two forms of glutamic acid decarboxylase in the rat hippocampal formation. Hippocampus. 1994;4:530–545. doi: 10.1002/hipo.450040503. [DOI] [PubMed] [Google Scholar]
- Houser CR, Miyashiro JE, Swartz BE, Walsh GO, Rich JR, Delgado-Escueta AV. Altered patterns of dynorphin immunoreactivity suggest mossy fiber reorganization in human hippocampal epilepsy. J Neurosci. 1990;10:267–282. doi: 10.1523/JNEUROSCI.10-01-00267.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch U, Magnusson AK. Unconventional GABA release: Mechanisms and function. Curr Opin Neurobiol. 2009;19:305–310. doi: 10.1016/j.conb.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Mathern GW, Babb TL, Pretorius JK, Leite JP. Reactive synaptogenesis and neuron densities for neuropeptide Y, somatostatin, and glutamate decarboxylase immunoreactivity in the epileptogenic human fascia dentata. J Neurosci. 1995;15:3990–4004. doi: 10.1523/JNEUROSCI.15-05-03990.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathern GW, Mendoza D, Lozada A, Pretorius JK, Dehnes Y, Danbolt NC, Nelson N, Leite JP, Chimelli L, Born DE, Sakamoto AC, Assirati JA, Fried I, Peacock WJ, Ojemann GA, Adelson PD. Hippocampal GABA and glutamate transporter immunoreactivity in patients with temporal lobe epilepsy. Neurology. 1999;52:453–472. doi: 10.1212/wnl.52.3.453. [DOI] [PubMed] [Google Scholar]
- Mikkonen M, Soininen H, Kalvianen R, Tapiola T, Ylinen A, Vapalahti M, Paljarvi L, Pitkanen A. Remodeling of neuronal circuitries in human temporal lobe epilepsy: Increased expression of highly polysialylated neural cell adhesion molecule in the hippocampus and the entorhinal cortex. Ann Neurol. 1998;44:923–934. doi: 10.1002/ana.410440611. [DOI] [PubMed] [Google Scholar]
- O’Malley DM, Masland RH. Co-release of acetylcholine and gamma-aminobutyric acid by a retinal neuron. Proc Natl Acad Sci USA. 1989;86:3414–3418. doi: 10.1073/pnas.86.9.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley DM, Sandell JH, Masland RH. Co-release of acetylcholine and GABA by the starburst amacrine cells. J Neurosci. 1992;12:1394–1408. doi: 10.1523/JNEUROSCI.12-04-01394.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottem EN, Godwin JG, Krishnan S, Petersen SL. Dual-phenotype GABA/glutamate neurons in adult preoptic area: Sexual dimorphism and function. J Neurosci. 2004;24:8097–8105. doi: 10.1523/JNEUROSCI.2267-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirker S, Czech T, Baumgartner C, Maier H, Novak K, Furtinger S, Fischer-Colbrie R, Sperk G. Chromogranins as markers of altered hippocampal circuitry in temporal lobe epilepsy. Ann Neurol. 2001;50:216–226. doi: 10.1002/ana.1079. [DOI] [PubMed] [Google Scholar]
- Pirker S, Gasser E, Czech T, Baumgartner C, Schuh E, Feucht M, Novak K, Zimprich F, Sperk G. Dynamic up-regulation of prodynorphin transcription in temporal lobe epilepsy. Hippocampus. 2009;19:1051–1054. doi: 10.1002/hipo.20633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler R, Smith AD. Coexistence of GABA and glutamate in mossy fiber terminals of the primate hippocampus: An ultrastructural study. J Comp Neurol. 1991;303:177–192. doi: 10.1002/cne.903030202. [DOI] [PubMed] [Google Scholar]
- Schwartz EA. Depolarization without calcium can release gamma-aminobutyric acid from a retinal neuron. Science. 1987;238:350–335. doi: 10.1126/science.2443977. [DOI] [PubMed] [Google Scholar]
- Schwarzer C, Sperk G. Hippocampal granule cells express glutamic acid decarboxylase-67 after limbic seizures in the rat. Neuroscience. 1995;69:705–779. doi: 10.1016/0306-4522(95)00348-m. [DOI] [PubMed] [Google Scholar]
- Sloviter RS, Dichter MA, Rachinsky TL, Dean E, Goodman JH, Sollas AL, Martin DL. Basal expression and induction of glutamate decarboxylase and GABA in excitatory granule cells of the rat and monkey hippocampal dentate gyrus. J Comp Neurol. 1996;373:593–618. doi: 10.1002/(SICI)1096-9861(19960930)373:4<593::AID-CNE8>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Soghomonian JJ, Martin DL. Two isoforms of glutamate decarboxylase: Why? Trends Pharmacol Sci. 1998;19:500–505. doi: 10.1016/s0165-6147(98)01270-x. [DOI] [PubMed] [Google Scholar]
- Sperk G, Schwarzer C, Heilman J, Furtinger S, Reimer RJ, Edwards RH, Nelson N. Expression of plasma membrane GABA transporters but not of the vesicular GABA transporter in dentate granule cells after kainic acid seizures. Hippocampus. 2003;13:806–815. doi: 10.1002/hipo.10133. [DOI] [PubMed] [Google Scholar]
- Sutula T, Cascino G, Cavazos J, Parada I, Ramirez L. Mossy fiber synaptic reorganization in the epileptic human temporal lobe. Ann Neurol. 1989;26:321–330. doi: 10.1002/ana.410260303. [DOI] [PubMed] [Google Scholar]
- Szabo G, Kartarova Z, Hoertnagl B, Somogyi R, Sperk G. Differential regulation of adult and embryonic glutamate decarboxylases in rat dentate granule cells after kainate-induced limbic seizures. Neuroscience. 2000;100:287–295. doi: 10.1016/s0306-4522(00)00275-x. [DOI] [PubMed] [Google Scholar]
- Uchigashima M, Fukaya M, Watanabe M, Kamiya H. Evidence against GABA release from glutamatergic mossy fiber terminals in the developing hippocampus. J Neurosci. 2007;27:8088–8100. doi: 10.1523/JNEUROSCI.0702-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MC, Ruiz A, Kullmann DM. Monosynaptic GABAergic signaling from dentate to CA3 with a pharmacological and physiological profile typical of mossy fiber synapses. Neuron. 2001;29:703–715. doi: 10.1016/s0896-6273(01)00245-8. [DOI] [PubMed] [Google Scholar]
- Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345:311–318. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- Wu Y, Wang W, Richerson GB. GABA transaminase inhibition induces spontaneous and enhances depolarization-evoked GABA efflux via reversal of the GABA transporter. J Neurosci. 2001;21:2630–269. doi: 10.1523/JNEUROSCI.21-08-02630.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]