Abstract
Intranasal aspiration of satratoxin G (SG), a mycotoxin produced by the black mold Stachybotrys chartarum, selectively induces apoptosis in olfactory sensory neurons (OSNs) in mouse olfactory epithelium (OE) through unknown mechanisms. Here, we show a dose-dependent induction of apoptosis 24 h post-SG exposure in vitro as measured by increased activated caspases in the OP6 olfactory placodal cell line and increased propidium iodide staining in primary OE cell cultures. Intranasal aspiration of SG increased TUNEL (Terminal dUTP Nick End Labeling) staining in the neuronal layer of the OE and significantly increased the latency to find a buried food pellet, confirming that SG selectively induces neuronal apoptosis and demonstrating that SG impairs the sense of smell. Next, we investigated whether ATP can prevent SG-induced OE toxicity. ATP did not decrease apoptosis under physiological conditions but significantly reduced SG-induced OSN apoptosis in vivo and in vitro. Furthermore, purinergic receptor inhibition significantly increased apoptosis in OE primary cell culture and in vivo. These data indicate that ATP is neuroprotective against SG-induced OE toxicity. The number of cells that incorporated 5′-bromodeoxyuridine, a measure of proliferation, was significantly increased 3 and 6 days post-SG aspiration. Treatment with purinergic receptor antagonists significantly reduced SG-induced cell proliferation, whereas post-treatment with ATP significantly potentiated SG-induced cell proliferation. These data indicate that ATP is released and promotes cell proliferation via activation of purinergic receptors in SG-induced OE injury. Thus, the purinergic system is a therapeutic target to alleviate or restore the loss of OSNs.
Keywords: apoptosis, neuroregeneration, toxicity
Satratoxin G (SG) is macrocyclic trichothecene mycotoxin produced by indoor air mold Stachybotrys chartarum growing on cellulose-containing building materials (Pestka et al., 2008). This mold can be detected in indoor air in water-damaged housing and is suggested to play a role in the etiology of damp building-related illnesses, including upper respiratory tract symptoms, wheezing, cough, and exacerbation of asthma (Institute of Medicine, 2004; Miller et al., 2003; Straus, 2009). An impaired sense of smell was also reported in subjects living in moldy environments (Koskinen et al., 1999a). Exposure to either black mold spores or associated satratoxins initiates acute inflammatory responses in the rodent lung (Lichtenstein et al., 2010; Nikulin et al., 1996; Rao et al., 2000; Rosenblum Lichtenstein et al., 2006).
Intranasal administration of SG to the mouse reduces the thickness of olfactory epithelium (OE) (Islam et al., 2008), the site where odorant transduction occurs. The OE comprises an apicial non-neuronal cell layer, a middle layer of olfactory sensory neurons (OSNs), and a basal stem cell layer. SG administration selectively induced apoptosis in OSNs (Islam et al., 2008), which send axons to olfactory bulb and are responsible for the sense of smell. The OSNs are in direct contact with airborne pollutants, toxicants, and microbes and are often damaged. However, the OE is able to regenerate by proliferation and differentiation of the basal stem cells (Schwob, 2002). Normally, in the mature OE, basal stem cells proliferate into neuronal precursor cells and then differentiate into immature followed by mature OSNs to replace dying and dead OSNs (Graziadei and Graziadei, 1979; Graziadei and Monti-Graziadei, 1978). The rate of cell proliferation and neuronal differentiation in the OE is tightly regulated by multiple chemical signals produced by the different cell types in the OE (Kawauchi et al., 2004; Mackay-Sim and Chuah, 2000). When there is significant chemical, infectious, or traumatic damage to the OE, the rate of neuroregeneration accelerates (Calof et al., 2002; Sultan-Styne et al., 2009).
Extracellular ATP is released upon injury and exerts neuroproliferative and neuroprotective actions in the central nervous system (CNS) (Chorna et al., 2004; Neary et al., 1996; Rathbone et al., 1992b, 1999). The neurotrophic functions of ATP are mediated via P2Y purinergic receptors that are coupled to G proteins and phospholipase C or other second messengers (Rathbone et al., 1992a). P2X and P2Y purinergic receptors are functionally expressed in the mouse OE (Hegg et al., 2003). Activation of P2 purinergic receptors by intranasal administration of ATP induces cell proliferation and neuroregeneration in normal OE. However, the neuroprotective role of ATP in normal OE and following SG toxicity has not been investigated.
The aims of the present study were (1) to examine the toxic effects of SG in the OE using olfactory placodal cell line, OP6, OE primary cell culture, and an in vivo mouse model and (2) to determine whether ATP has neuroprotective and neuroproliferative roles in these models of SG-induced injury. The results from these models suggest potential therapeutic strategies to alleviate, prevent, and cure the loss of olfactory function associated with water-damaged buildings.
MATERIALS AND METHODS
Animals.
Adult (6–8 weeks) and young (3 weeks) male Swiss Webster mice (Charles River, Portage, MI) were used. All procedures were designed to minimize the number of animals used and their suffering. All animal procedures were approved by Michigan State University Institutional Animal Care and Use Committee and conducted following the guidelines from National Institutes of Health.
Olfactory placodal cell line OP6 and CaspACE FITC-VAD-FMK assay.
The OP6 cell line was obtained from the laboratory of Dr Mary T. Lucero (University of Utah, Salt Lake City, UT) and is a clonal temperature-sensitive cell line derived from the E10 mouse olfactory placode (Illing et al., 2002). Undifferentiated cells resemble process-bearing immature OSNs and express the immature OSN marker growth-associated protein 43 (GAP43). Upon differentiation, the cells become bipolar and express the mature OSN marker olfactory marker protein (OMP) and essential proteins for chemosensory signal transduction, such as the olfactory Golf protein, adenylate cyclase III, olfactory cyclic nucleotide-gated channel, and putative odorant receptors. In addition, differentiated cells express functional voltage-gated sodium and potassium channels. OP6 cells were grown in laminin-coated 8-well glass slides (Nalge Nunc International, Naperville, IL) at 33°C in Dulbecco's modified Eagle medium: nutrient mixture F-12 (DMEM/F-12) growth media supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin (Invitrogen, Carlsbad, CA; 50 U/ml and 50 μg/ml, respectively). Cells were differentiated at ∼90% confluency by supplementing the growth media with 1% N-2 (100×; Invitrogen), 0.1% retinonic acid, and 0.0176% ascorbic acid. The cells were incubated at 33°C overnight and then at 39°C for 5–7 days. The differentiation of OP6 cells was analyzed by the appearance of mature OSN morphology with processes and immunoreactivity for OMP.
Differentiated and undifferentiated OP6 cells were incubated with vehicle (saline) or SG (5, 10, or 20 ng/ml) for 24 h and then incubated with CaspACE FITC-VAD-FMK, a fluorescein isothiocyanate (FITC) conjugate of the cell permeable caspase inhibitor that binds to activated caspase (10μM for 30 min; Promega Corporation, Madison, WI). After washing with PBS, the cells were incubated with Hoechst 33342, used as a nuclear marker (5 μg/ml, 1 h; Invitrogen), fixed with 4% paraformaldhyde, and mounted with Vectashield mounting medium for fluorescence (Vector, Burlingame, CA). FITC and Hoechst were excited at 465–495 and 340–380 nm, respectively, and emissions were collected at 505–555 and 435–485 nm, respectively. The number of Hoechst-positive cells, i.e., total number of cells, and the extent of co-localization of FITC with Hoechst were analyzed using Metamorph 7.5 from two non-overlapping fields per well taken using ×20 objective (0.5 n.a.) on a Nikon TE2000-U inverted fluorescence microscope (Nikon, Melville, NY). Data were expressed as Hoechst-positive cells per field and the percentage of FITC-positive cells to Hoechst-positive cells. The average of each treatment (n = 3 separate wells) was compared.
OE primary cell culture, flow cytometry, and propidium iodide exclusion assay.
SG was prepared from S. chartarum culture and purified as described previously (Islam et al., 2009). The OEs were obtained from young Swiss Webster mouse (postnatal day 21) at an age when ∼8% of the cells isolated are basal progenitor cells (unpublished data), providing cell cultures that are long-lived. We have verified that there are no differences between neonates and adults in respect to the effect of ATP on proliferation in the OE (Jia et al., 2009). The primary OE cells were isolated as described previously (Hegg et al., 2003). The cells were plated onto concanavalin A-coated 6-well plates at 2 × 106 cells per milliliter and cultured in DMEM media supplemented with 5% FBS, 1% penicillin/streptomycin (50 U/ml and 50 μg/ml, respectively), 1% insulin, and 0.1% ascorbic acid at 37°C for 5–7 days. At day 6, OE primary cells were incubated with vehicle (saline), SG (10 ng/ml), ATP (100μM), or the combination of ATP (100μM) and SG (10 ng/ml) for 24 h. Some cells were pretreated with vehicle or P2 purinergic receptor antagonists pyridoxalphosphate-6-azophenyl-20, 40-disulfonic acid (PPADS, 25μM) + suramin (100μM) for 30 min before ATP incubation. Co-administration of both antagonists has been used previously to inhibit both calcium responses and cell proliferation induced by ATP (Hegg et al., 2003, 2009) and was used to ensure inhibition of the multiple purinergic receptors expressed in the OE.
Adherent OE cells were prepared for flow cytometric analysis as previously described (Islam et al., 2008). Briefly, cells were trypsinized (0.05% trypsin–EDTA; Invitrogen), centrifuged at 400 × g for 10 min at 4°C, resuspended in PBS with 50% heat-inactivated FBS (1 ml), fixed immediately by dropwise addition of 2.5 ml ice-cold 70% ethanol with gentle mixing, and stored at −20°C until further study. Apoptosis was detected by flow cytometric cell cycle analysis. Unthawed cells were washed with 5% heat-inactivated FBS in PBS and incubated with 5% heat-inactivated FBS, 50 μg/ml propidium iodide (PI), and 200 μg/ml RNase A for 30 min at room temperature. Cell cycle distribution for single cells was measured by exciting PI (488 nm), and fluorescence was detected at 615–645 nm with a Becton Dickinson FACS Vantage (San Jose, CA). The cell cycle of individual cells was performed using doublet discrimination gating to eliminate doublet and cell aggregates based on DNA fluorescence. A gate including hypofluorescent cells was selected. Cells with hypofluorescence in the DNA histogram were designated apoptotic. All other cells were distributed normally in a cell cycle profile. Data from 8000 to 10,000 cells were collected for each sample. The data are expressed as percentage of vehicle.
For the PI exclusion assay, primary OE cultured cells were incubated with ATP (100μM) or UTP (100μM) in media without FBS for 2 h following 30 min pre-incubation with PPADS (25μM) and suramin (100μM). PI (400 μg/ml) was added and incubated for 30 min at 37°C, cells were washed with PBS, and nuclei were counterstained with 4',6-diamidino-2-phenylindole (DAPI) (7 pg/ml). PI and DAPI were excited at 530–560 and 340–380 nm, respectively, and emissions were collected at 573–648 nm and 435–485 nm, respectively. The extent of co-localization of PI with DAPI staining was analyzed using Metamorph 7.5 (MDS Analytical Technologies, Sunnyvale, CA) from two to seven non-overlapping fields per well collected using a ×10 objective (0.3 n.a.) on a Nikon TE2000-U inverted fluorescence microscope (Nikon). The co-localization data were expressed as percentage of vehicle, and the average of each treatment was compared (n = 3 wells).
In vivo apoptosis and proliferation studies.
Anesthetized adult mice (4% isoflurane) aspirated saline or SG (100 μg/kg) followed by daily aspiration of saline or ATP (400 nmol/kg) for 2 or 5 days. Some mice aspirated P2 purinergic receptor antagonists, PPADS (50 nmol/kg), and suramin (200 nmol/kg) 30 min prior to saline or SG treatment followed by daily aspiration of the same antagonists for 2 or 5 days. Mice in the proliferation study received three 5′bromodeoxyuridine (BrdU) injections (ip, 180 mg/kg total) at 18, 20, and 22 h before tissue collection. Twenty-four hours after the last aspiration, the OE tissue was fixed via transcardiac infusion, decalcified in EDTA (0.5M, pH = 8) for 5 days, cryoprotected with 30% sucrose, and embedded in Tissue Tek OCT (Sakura Finetek, Torrance, CA) as previously described (Jia et al., 2010). Frozen coronal sections of OE (20 μm) were collected to detect the levels of apoptosis by Terminal dUTP Nick End Labeling (TUNEL) from level 3 of the mouse nasal cavity taken at the level of the second palatal ridge (Young, 1981). TUNEL was performed with an In Situ Cell Death Detection Kit (TMR Red; Roche Applied Science, Indianapolis, IN) following the manufacturer’s instructions. BrdU immunoreactivity was assessed as described previously (Jia et al., 2010). The number of BrdU+ or TUNEL+ cells in ectoturbinate 2 and endoturbinate II from three tissue sections of each mouse (three to four mice per group) were manually counted by an experimenter blinded to the treatments. The linear length of OE was measured using Metamorph software. Data are expressed as a ratio of TUNEL-positive cells to the linear length of OE scored.
Buried food test behavioral assay.
An adult mouse, fasted 16 h prior to testing, was placed in a cage filled only with fresh wood chip bedding for 5 min, transferred to a second cage for 5 min, and then to a third cage that contains a piece of sugary cereal buried beneath the bedding. Upon placement in this third cage, the latency to uncovering and eating the buried pellet was measured. Trials were performed every 2 days until latencies were consistent for three trials in a row. Then, anesthetized mice (4% isoflurane) intranasally aspirated saline (control) or SG (100 μg/kg mouse) 1 day prior to performing three additional trials as described above. Data are expressed as mean latency ± SEM for three to four mice per treatment. All mice were able to find and consume a piece of sugary cereal when placed on the top of the bedding.
Data and statistical analysis.
Student’s t-test or one- or two-way ANOVA was performed followed by Bonferroni’s multiple comparison test using Prism 5 (GraphPad Software, San Diego, CA).
RESULTS
SG Induces Apoptosis in OP6 Cells, OE Primary Cell Culture, and In Vivo
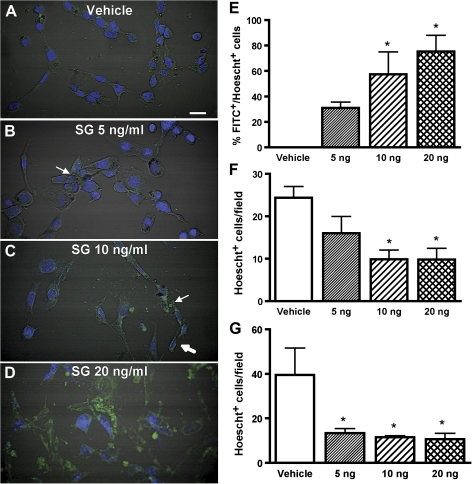
The toxic effects of SG were characterized in multiple models of OE cells in addition to in vivo studies. The olfactory placodal OP6 cell line, in the undifferentiated state, consists of cells that are morphologically and biochemically similar to immature neurons. Differentiated OP6 cells, however, represent mature neuronal-like cells (Illing et al., 2002). The differentiated and undifferentiated OP6 cells were incubated with saline or SG (5, 10, or 20 ng/ml) for 24 h. Apoptosis was detected by the combination of morphological alternations (cell clumping, shrinkage, and induction of vesicles), presence of activated caspases (measured by FITC-VAD-FMK fluorescence), and reduction of cell numbers (measured by Hoechst nuclear staining). In vehicle-treated differentiated OP6 cells, there were no changes in morphology, and FITC-VAD-FMK fluorescence was barely observed (Fig. 1A). However, morphological changes were evident 24 h after differentiated OP6 cells were incubated with 5 ng/ml SG with noticeable cell clumping (Fig. 1B). Differentiated OP6 cells treated with 10 or 20 ng/ml SG for 24 h had obvious cell shrinkage, induction of vesicles, and FITC-VAD-FMK fluorescence (Figs. 1C and 1D). SG dose-dependently increased the FITC fluorescence that was co-localized with Hoechst nuclear staining (Fig. 1E). Furthermore, 24-h SG treatment significantly decreased the total cell numbers (Fig. 1F). These data indicate that SG induces caspase-dependent apoptosis in differentiated OP6 cells. SG treatment induced morphological changes in undifferentiated OP6 cells that included cell clumping, cell shrinkage, and chromatin clumping (data not shown, similar to Figs. 1B–D) and significantly reduced the total number of cells (Fig. 1G). These data indicate that SG also induces apoptosis in undifferentiated OP6 cells. The dose of 10 ng/ml was chosen for use in subsequent in vitro experiments with SG.
FIG. 1.
SG induces apoptosis in OP6 cells in a dose-dependent manner. Differentiated and undifferentiated OP6 cells were incubated with vehicle saline or SG (5, 10, or 20 ng/ml) for 24 h. Apoptotic cells were identified by the presence of activated caspase using the FITC-VAD-FMK assay (green) and nuclei were counterstained with Hoechst 33342 (blue). (A–D) Representative images from the FITC-VAD-FMK assay using differentiated OP6 cells treated with vehicle (saline) and 5, 10, or 20 ng/ml SG. Arrow indicates cell clumping (B) and vesicle induction (C), and thick arrow indicates cell shrinkage (C). Scale bar = 25 μm. (E–G) (E) SG dose-dependently increases the number of differentiated OP6 cells that are apoptotic (FITC+) with a Hoechst+ nuclei. In differentiated (F) and undifferentiated (G) OP6 cells, SG significantly reduces the total number of cells (Hoechst+). *p < 0.05 versus vehicle (one-way ANOVA followed by Bonferroni’s multiple comparison test).
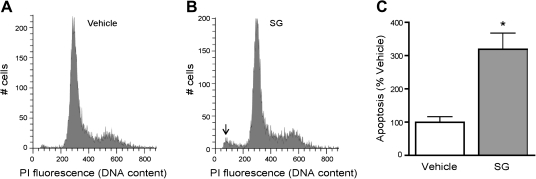
The toxic effects of SG were next investigated in OE primary cell cultures, which consist of mature OSNs, basal progenitor cells, sustentacular cells, and microvillous cells of varying sizes and morphology. Thus, rather than assessing apoptosis based on morphological alterations as with the OP6 cells, we quantified the frequencies of apoptotic cells by measurement of hypodiploid cell fluorescence following PI staining using flow cytometry. Consistent with the OP6 model, incubation with 10 ng/ml SG for 24 h significantly increased the level of apoptosis in OE primary cells as compared with vehicle-treated cells (Fig. 2).
FIG. 2.
SG induces apoptosis in OE primary cell culture. Primary OE cell cultures were incubated with vehicle (saline) or SG (10 ng/ml) for 24 h, and the cells were stained with PI and subjected to flow cytometric analysis. (A–B) Representative flow cytograms for each treatment group are shown. Arrow indicates the hypodiploid peak associated with apoptosis. (C) The percentages of apoptosis were quantified and normalized to vehicle (n = 6 and 3, respectively). *p = 0.008 versus vehicle (unpaired Student’s t-test).
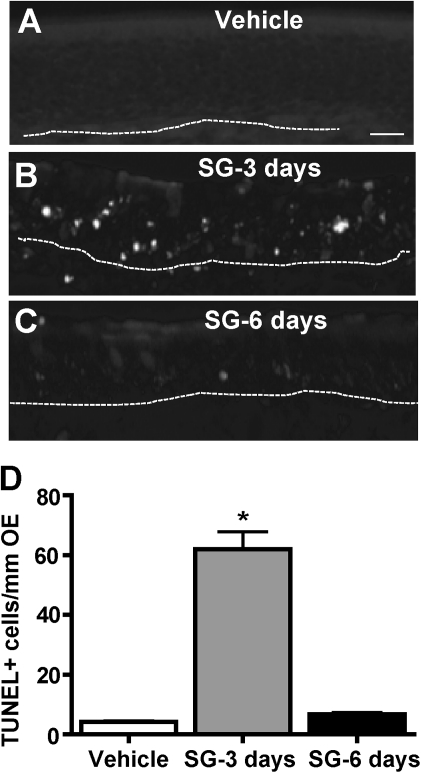
SG toxicity was further assessed in the mouse OE. SG (100 μg/kg) intranasally administered in vivo increased the number of TUNEL-positive cells located in the middle neuronal layer of the OE compared with vehicle at 3 days but not at 6 days post-administration (Figs. 3A–C). When the number of TUNEL+ cells was quantified in the ectoturbinate 2 and endoturbinate II, there was significantly more TUNEL+ staining in SG-treated animals 3 days post-administration, but TUNEL staining returned to control levels at 6 days post-administration (Fig. 3D). These data indicate that intranasal administration of SG induces apoptosis in the OE, consistent with previous reports that SG selectively induces OSN apoptosis (Islam et al., 2006, 2009).
FIG. 3.
SG induces apoptosis in the OE in vivo. Mice intranasally aspirated vehicle (saline) or SG (100 μg/kg) and TUNEL staining was performed 3 and 6 days post-administration. (A–C) Representative images of TUNEL staining in each group. Dotted white line depicts the basement membrane. Scale bar = 20 μm. (D) SG significantly induces apoptosis at 3 days post-administration. TUNEL-positive staining was quantified in ectoturbinate 2 and endoturbinate II OE (n = 6–9 sections from 3 to 4 mice). *p < 0.001 versus vehicle (one-way ANOVA followed by Bonferroni's post hoc test).
SG Impairs the Sense of Smell in Adult Mice
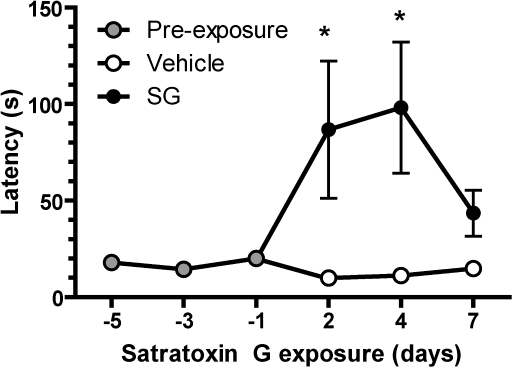
The buried food behavioral test (Yang and Crawley, 2009) was performed to determine the ability to detect a food odorant following SG administration. Before mice were administered saline or SG (100 μg/kg), the latency to find a buried sugary cereal was 17.4 ± 5.5 s over three trials (Fig. 4). The latency increased significantly for mice 2–4 days after SG administration compared with mice administered saline. These data suggest that SG administration impairs the ability to smell shortly following SG exposure.
FIG. 4.
SG reduces the ability to detect food odorants. Fasted mice (16 h) intranasally aspirated vehicle (saline) or SG (100 μg/kg) and were immediately administered the buried food test. Shown are average (±SEM) latencies from buried food trials. Latencies increased 2–4 days post-administration of SG (100 μg/kg) but decreased to control levels 7 days later (n = 4 mice per treatment). *p < 0.001 versus vehicle (two-way ANOVA followed by Bonferroni's post hoc test).
ATP Reduces SG-Induced Apoptosis in the OE In Vitro and In Vivo
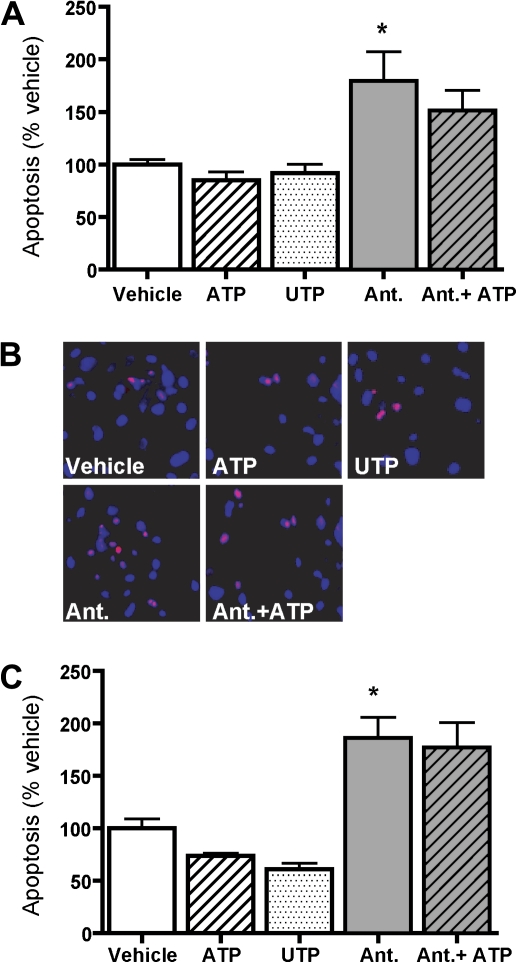
ATP is neuroprotective in the CNS (Chorna et al., 2004; Neary et al., 1996; Rathbone et al., 1992b, 1999). We thus hypothesized that ATP may play a similar role in the OE following SG toxicity. We first ascertained whether ATP has a protective function under normal conditions by examining apoptosis via flow cytometry (Fig. 5A). ATP, a P2X and P2Y receptor agonist, and UTP, a P2Y receptor agonist, (2 h, 100μM each) had no effect on the number of cells undergoing apoptosis compared with the vehicle (saline) in primary OE cell cultures. These results suggest that, contrary to that which occurs in the CNS, purinergic receptor activation does not provide protection against cell death in the OE beyond that of control conditions. Interestingly, however, treatment of cell cultures with nonspecific P2 receptor antagonists PPADS (25μM) and suramin (100μM) alone or co-treatment of ATP and the P2 receptor antagonists significantly increased the percentage of apoptotic cells compared with vehicle control. In order to confirm these flow cytometry data, we performed the PI exclusion assay used previously to measure the levels of dead or dying cells in OE primary cell culture (Figs. 5B and 5C; Gangadhar et al., 2008). There was no significant difference in the number of PI-positive cells among vehicle, ATP, and UTP treatments. However, P2 receptor antagonists or co-administration of P2 receptor antagonists with ATP significantly increased the number of PI-positive cells compared with the vehicle. Collectively, both assays reveal that blockade of P2 purinergic receptors increases cell death or apoptosis, suggesting that activation of P2 purinergic receptors provides protection. Accordingly, P2 purinergic receptors appear to be involved in the regulation of cell survival in OE primary cell culture.
FIG. 5.
P2 purinergic receptor antagonists increase apoptosis in OE primary cell culture. Primary OE cell cultures were pre-incubated with vehicle (saline) or P2 purinergic receptor antagonists PPADS (25μM) and suramin (100μM) for 30 min. Cells were then incubated with vehicle (saline), ATP (100μM), or UTP (100μM) for 2 h. (A) The cells were stained with PI and subjected to flow cytometric analysis. The levels of apoptosis were normalized to vehicle (n = 3–9). *p < 0.01 versus vehicle (one-way ANOVA followed by Bonferroni's post hoc test). (B) PI exclusion assay was performed. Apoptotic cells stained positive for PI (red) and nuclei were counterstained with DAPI (blue). Scale bar = 25 μm. (C) Co-localization of PI and DAPI staining was quantified and normalized to vehicle (n = 3). *p < 0.05 versus vehicle (one-way ANOVA followed by Bonferroni's post hoc test).
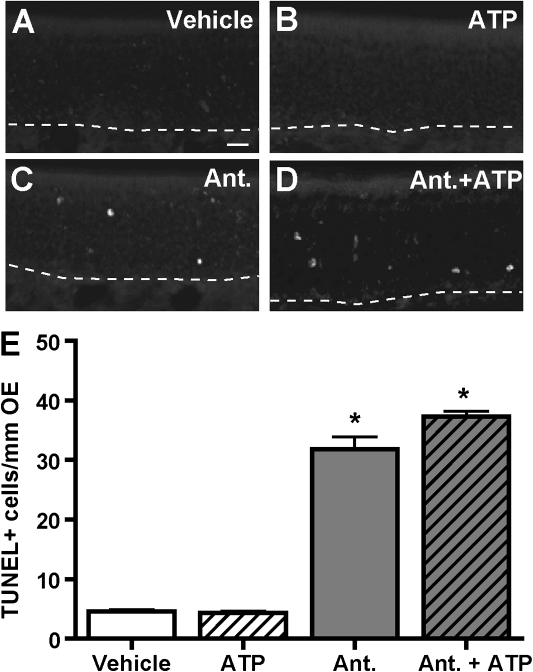
Mice intranasally aspirated vehicle (saline) or ATP (400 nmol/kg) in the presence or absence of P2 receptor antagonists, PPADS (50 nmol/kg) and suramin (200 nmol/kg), and the number of TUNEL-positive cells in ectoturbinate 2 and endoturbinate II of the OE were quantitated. Consistent with in vitro results, the numbers of TUNEL-positive cells in vehicle- and ATP-administered groups were comparable (Figs. 6A, 6B, and 6E). Intranasal aspiration of P2 receptor antagonists significantly increased the number of TUNEL-positive cells (Figs. 6C, 6D, and 6E), indicating that activation of P2 purinergic receptors have a protective function and blockade of these receptors increases apoptosis in the OE in vivo.
FIG. 6.
P2 purinergic receptor antagonists increase apoptosis in the OE in vivo. Mice intranasally aspirated vehicle (saline) or P2 antagonists PPADS (50 nmol/kg) and suramin (200 nmol/kg), followed by vehicle (saline) or ATP (400 nmol/kg) 30 min later. The levels of apoptosis were measured by TUNEL 48 h after ATP administration. (A–D) Representative TUNEL staining for each group is shown. Dotted white line depicts the basement membrane. Scale bar = 20 μm. (E) Quantification of TUNEL+ staining in ectoturbinate 2 and endoturbinate II (n = 9–12 sections from 3 to 4 mice). *p < 0.001 versus vehicle (two-way ANOVA followed by Bonferroni's post hoc test).
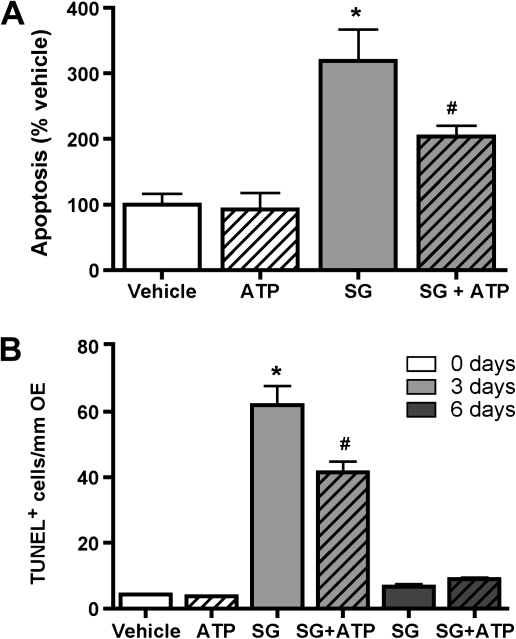
To determine the effect of ATP on SG-induced apoptosis in the OE, we incubated OE primary cell culture with vehicle (saline) or SG (10 ng/ml) in the presence or absence of ATP (100μM) for 24 h and measured the levels of apoptosis by flow cytometry. In the absence of SG, the percentages of apoptotic cells following vehicle and ATP treatment were comparable, whereas co-incubation of ATP with SG significantly reduced SG-induced apoptosis (Fig. 7A). In vivo, mice intranasally aspirated vehicle (saline) or SG (100 μg/kg) followed by daily aspiration of ATP (400 nmol/kg) for 2 or 5 days. Numbers of TUNEL-positive cells in vehicle- and ATP-treated animals were comparable in the absence of SG. However, post-treatment with ATP significantly reduced SG-induced increases in TUNEL-positive staining at 3 days but not at 6 days post-aspiration of SG (Fig. 7B). Taken together, these data indicate that ATP has a neuroprotective function in SG-induced OE injury.
FIG. 7.
ATP reduces SG-induced apoptosis in OE primary cell culture and in vivo. (A) Primary OE cell cultures were incubated with vehicle (saline), ATP (100μM), SG (10 ng/ml), or ATP + SG for 24 h, and apoptosis was measured by flow cytometry and normalized to vehicle (n = 3–6). *p < 0.001 versus vehicle. #p < 0.05 versus SG (two-way ANOVA followed by Bonferroni's post hoc test). (B) Mice intranasally aspirated vehicle (saline) or SG (100 μg/kg) followed by daily aspiration of vehicle (saline) or ATP (400 nmol/kg) for 2 or 5 days. Apoptosis was measured by TUNEL 24 h after last administration (n = 6–9 sections from three to four mice). *p < 0.001 versus vehicle and #p < 0.001 versus SG (two-way ANOVA followed by Bonferroni's post hoc test).
ATP Potentiates OE Proliferation following SG Toxicity via Purinergic Receptor Activation
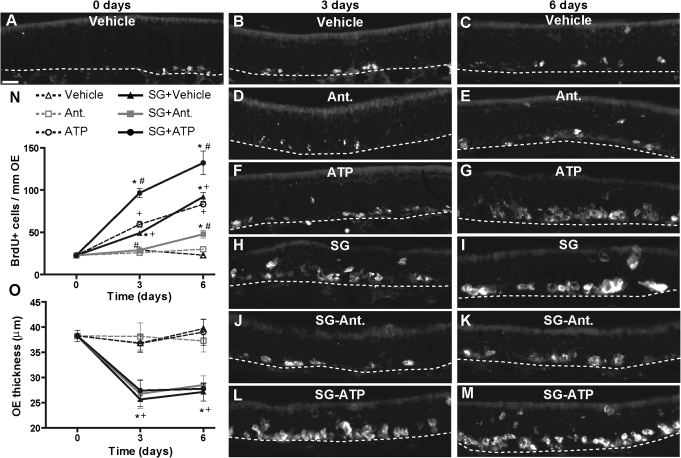
Following injury and toxicity, OE cells undergo increased cell turnover via apoptosis and subsequent proliferation of basal cells. We used BrdU incorporation to measure cell proliferation in turbinates of mice at 3 and 6 days after intranasal administration of vehicle (saline) or SG (100 μg/kg). SG treatment significantly increased BrdU incorporation at 3 and 6 days compared with the vehicle-aspirated animals (Figs. 8A–C, 8H, 8I, and 8N). Furthermore, BrdU incorporation in SG-treated mice at 6 days post-aspiration of SG was significantly higher than that at 3 days. These data indicate that SG-induced toxicity significantly increases OE proliferation.
FIG. 8.
Effects of ATP and P2 purinergic receptor antagonists on SG-induced OE proliferation in vivo. Mice were pretreated intranasally with vehicle (saline) or P2 receptor antagonists PPADS (50 nmol/kg) and suramin (200 nmol/kg) 30 min prior to intranasal administration with vehicle (saline) or SG (100 μg/kg). Mice received daily treatment with vehicle (saline), P2 receptor antagonists, or ATP (400 nmol/kg) via intranasal aspiration for 2 or 5 days. The levels of cell proliferation were measured via BrdU incorporation 24 h after last administration. (A–M) Representative images of BrdU immunoreactivity for each treatment group are shown. Dotted white line depicts the basement membrane. Scale bar = 20 μm. (N) Quantification of BrdU+ cells in ectoturbinate 2 and endoturbinate II (n = 6–12 sections from 3 to 4 mice). *p < 0.001 versus day 0; #p < 0.01 versus SG at 3 and 6 days; +p < 0.01 versus vehicle at 3 and 6 days (two-way ANOVA followed by Bonferroni's post hoc test). (O) Quantification of the thickness of ectoturbinate 2 and endoturbinate II OE (n = 6–12 sections from three to four mice).*p < 0.05 versus 0 day; +p < 0.001 versus vehicle at 3 and 6 days (two-way ANOVA followed by Bonferroni's post hoc test).
As ATP is known to induce neuroproliferation, it could promote proliferation following SG-induced OE injury. To test this hypothesis, mice intranasally aspirated vehicle (saline) or ATP (400 nmol/kg) daily for 2 or 5 days following vehicle or SG treatment. In vehicle-treated animals, ATP administration alone significantly elevated BrdU incorporation at both 3 and 6 days post-administration (Figs. 8F, 8G, and 8N). In SG-treated animals, ATP administration significantly potentiated BrdU incorporation compared with SG treatment at both 3 and 6 days (Figs. 8L–N). These data indicate that intranasal administration of exogenous ATP potentiates SG-induced increases in OE proliferation.
To ascertain whether ATP mediates SG-induced increases in OE proliferation via purinergic receptors, mice intranasally aspirated vehicle (saline) or P2 receptor antagonists PPADS (50 nmol/kg) and suramin (200 nmol/kg) 30 min prior to SG treatment and then daily for an additional 2 or 5 days. Administration of P2 receptor antagonists alone did not affect the BrdU incorporation levels compared with vehicle (Figs. 8D, 8E, and 8N). However, administration of P2 receptor antagonists significantly blocked SG-induced increases in BrdU incorporation compared with SG alone (Figs. 8J, 8K, and 8N). These data suggest that P2 purinergic receptors mediate SG-induced increases in OE proliferation.
Intranasal aspiration of SG significantly reduced OE thickness at 3 and 6 days post-administration (Fig. 8O), and these responses were not altered by treatments with ATP and P2 receptor antagonist treatments. Collectively, these data strongly suggest that ATP is released and promotes cell proliferation via activation of P2 purinergic receptors in the OE after intranasal administration of SG.
DISCUSSION
Stachybotrys chartarum grows well at room temperature on wet surfaces composed of cellulose-containing materials, such as paper, ceiling tiles, and wallboard (Institute of Medicine, 2004; Murtoniemi et al., 2003), and macrocyclic trichothecenes including satratoxins are detectable in air and dust samples taken from buildings heavily contaminated by this fungus (Nielsen, 2002; Vesper et al., 2000). These mycotoxins can be associated with conidiospores, mycelial fragments, and debris (Brasel et al., 2005). Interestingly, an impaired sense of smell has been reported anecdotally in individuals living in moldy environments (Koskinen et al., 1999a, 1999b). Although most rodent studies of Stachybotrys and associated toxins focus on pulmonary effects, nasal damage occurs as the nose serves as a filter for the lower respiratory tract and lung (Pestka et al., 2008). A significant amount of the SG is retained in the mouse nasal turbinate following intranasal exposure with an estimated half-life in this tissue of 7.6 h (Amuzie et al., 2010). Here, we show that a single SG administration decreases the ability of mice to find a hidden food pellet, a task that requires an intact sense of smell. The impairment occurs 2 and 4 days post-administration of SG, the period of elevated neuronal apoptosis; no significant loss of sense of smell occurs at day 7 when neuronal apoptosis has subsided. Further study with repeated administration of SG is warranted to ascertain the effect of chronic exposure of SG to the sense of smell. This is the first demonstration of an olfactory behavioral effect associated with a mold component. Smell loss needs to be systematically addressed in buildings with damp indoor air as other molds may have similar anosmic effects (Epstein et al., 2008). Overall, these data indicate that SG accumulates in the nose and causes an impaired sense of smell.
The results presented herein demonstrate that SG induces apoptosis in the middle OSN layer of OE in vivo and are consistent with previous reports that SG selectively induces activation of caspase-3 in OSNs of OE (Islam et al., 2006, 2009). In addition, SG induced apoptosis in both mature and immature neuronal-like OP6 cells in vitro, suggesting that neurons at early stages of development are susceptible to SG toxicity. SG induced caspase-dependent apoptosis in OP6 cells in this study, in contrast to our previous report that SG-induced apoptosis was caspase independent in the PC-12 neuroblastoma cell line (Islam et al., 2008). This suggests that while the PC-12 cell line is routinely used as a neuronal model to study differentiation, it is not a good model for OSNs. Additionally, this study indicates that the OP6 cell is a valuable in vitro model for olfactory toxicity. Notably, activation of P2 purinergic receptors by intranasal administration of ATP following SG toxicity markedly reduced toxin-induced cell apoptosis and increased cell proliferation. Furthermore, inhibition of purinergic receptors significantly increased toxin-induced apoptosis and reduced SG-induced cell proliferation. These data indicate that ATP is released in SG-induced OE injury and promotes cell proliferation and prevents apoptosis via activation of P2 purinergic receptors.
We found that SG significantly reduced the thickness of OE, and ATP and P2 purinergic receptors had no effect on the SG-induced reduction of OE thickness at 3 and 6 days post-administration. However, basal progenitor cells require 2–3 weeks to undergo proliferation, differentiation, and maturation into OSNs, and an even longer time is needed for newly differentiated OSNs to send axons to the olfactory bulb (Calof and Chikaraishi, 1989; Leung et al., 2007; Schwartz Levey et al., 1991). Therefore, further experimentation at later time points are required to ascertain the effect of ATP on recovery from SG-induced atrophy of OE.
In the CNS, ATP is released upon injury and exerts multiple trophic actions via activation of purinergic receptors, including proliferation, neuronal differentiation, survival, and apoptosis (Neary and Zimmermann, 2009). ATP upregulates heat-shock protein expression (Hegg and Lucero, 2006), suggesting that activation of P2 purinergic receptors initiates a stress signaling cascade to facilitate cell survival. In the present study, contrary to our predictions, neither ATP nor UTP significantly altered the levels of apoptosis compared with control. P2 receptor antagonists, however, significantly increased apoptosis in the OE. This result suggests that activation of P2 purinergic receptors does indeed have a neuroprotective function. Co-administration of ATP and P2 purinergic receptor antagonists did significantly alter apoptosis levels compared with antagonists alone, suggesting that, in these assays, purinergic receptors were sufficiently inhibited. A further implication of these results is that ATP may be tonically released in small amounts to prevent apoptosis; inhibition of purinergic receptors prevents extracellular ATP from tonically inhibiting cellular apoptosis. Notably, however, is that exogenous addition of ATP does not reduce the levels of apoptosis lower than control, suggesting that other protective mechanisms also function in the OE.
Purinergic receptors P2X1-5, P2X7, and P2Y2 are expressed in the OE (Gayle and Burnstock, 2005; Hegg et al., 2003). P2Y2 receptors, activated by both UTP and ATP, most likely do not have a role in P2 purinergic receptor-mediated neuroprotection as UTP and ATP did not reduce the levels of apoptosis. P2X1-6 purinergic receptors are activated by low concentrations of ATP (1–10μM), whereas the P2X7 purinergic receptors have an EC50 of ∼100μM. Recently, P2X7 receptors have been linked to apoptosis in the OE and CNS (Gayle and Burnstock, 2005; Orellano et al., 2010; Sugiyama et al., 2010). Thus, the neuroprotective role of ATP may be counteracted by activation of P2X7 receptors. In addition, we cannot rule out the possibility that the effects of P2 receptor antagonists may be attributed to nonspecific effects of suramin. Suramin can interact with a number of growth factor receptors, such as platelet-derived growth factor, epidermal growth factor, basic fibroblast growth factor, insulin-like growth factor, and transforming growth factor β (Walz et al., 1991) that could contribute to the observed effects. Further experimentation is required to identify the purinergic receptor subtype involved in neuroprotection.
One critical question in this work relates to the rationale for the SG doses employed. Based on our prior studies demonstrating robust OSN death in the mouse exposed to SG at 25–500 μg/kg (Islam et al., 2006), we chose to use 100 μg/kg to investigate the effects of the toxin on the OE, olfaction, and intervention with ATP. Relatedly, following intranasal dosing of mice with 500 μg SG/kg, the toxin selectively concentrates in nasal turbinates at levels between 410 and 480 ng/g within 15–60 min of exposure and with 48 ng/g still detectable after 24 h (Amuzie et al., 2010). Assuming a proportional rate of clearance, it is reasonable to predict that mice exposed to 100 μg SG/kg, as described here, would contain toxin levels in their nasal turbinates ranging from 10 to 100 ng/g over a similar 24 h period. Thus, incubation of cell cultures with 5–20 ng/ml SG over 24 h can be conservatively expected to reflect in vivo concentrations encountered upon a single dose of 100 μg/kg in vivo.
The translational relevance of the 100 μg SG/kg dose to humans might also be questioned. Although reliable analytical data on the extent of human exposure to satratoxins are lacking (Pestka et al., 2008), two studies of highly contaminated buildings reported macrocyclic trichothecene concentrations of 100 pg/m3 (Nielsen, 2002) and 1300 pg/m3 (Brasel et al., 2005). Considering these latter data in the context of the tidal volumes of mice, it is evident that human exposure is likely to be much more modest than that modeled in this study. Furthermore, satratoxin levels in Stachybotrys conidiospores have been estimated to be 1 picogram per spore (Pestka et al., 2008), suggesting that an extraordinarily high spore level (108 spores per kilogram) would be necessary to replicate the 100 μg SG/kg dose in the mouse or human. However, it is critical to recognize that in the environment, SG would not exist as an aerosolized droplet or gas, but rather in association with particulates, most notably spores (Brasel et al., 2005). Using the aforementioned 1 picogram per spore estimate and assuming a 5-μm spore diameter and spherical shape, the actual concentration of satratoxins within a conidiospore (i.e., based on volume) is 15 μg/cm3. Thus, when a spore becomes lodged within the nasal compartment, the toxin concentration within its immediate microenvironment would be extremely high and capable of causing OSN apoptosis. Such localized death might result in punctate regions of cell loss within the OE. It might be further speculated that prolonged repeated exposures to low spore levels could lead to cumulative cell death ultimately evoking reduced olfactory function. Consistent with this latter contention, repeated daily intranasal exposure to SG at 5 μg over 4 days evokes OSN damage in juvenile rhesus monkeys equivalent to that occurring from a single 20 μg dose (Carey et al., 2008). Accordingly, the data presented here represent an initial step in understanding the toxicity mechanisms in the nasal turbinate environment and more importantly, how injury might be reversed. Further research is needed on the effect of toxin-laden spores on the OE at region-specific levels, the effects of prolonged repeated exposures to such spores, and how these adverse effects might reversed by ATP.
Taken together, the data presented herein demonstrate that ATP has neuroproliferative and neuroprotective roles in SG-induced OE injury. This suggests that the purinergic system is a potential therapeutic target to alleviate or restore the loss of olfactory function associated with water-damaged buildings. The results for this SG-induced olfactory dysfunction model are consistent with our previous findings that ATP has a neuroprotective role in NiSO4-induced OE injury (Jia et al., 2010). Notably, both studies indicate that ATP is released and promotes cell proliferation via activation of P2 purinergic receptors in both SG- and NiSO4-induced OE injury. Although both toxicants induce apoptosis, SG and nickel have different cellular targets and toxicity mechanisms. The observations that ATP has a neuroprotective role following both toxicities suggest that ATP may represent a universal pharmaceutical tool to alleviate apoptotic neurotoxicity in the nose.
FUNDING
Royal Thai Government (doctoral scholarship to S.S.); Merck-Merial (Veterinary Scholars Award to B.B.); Michigan State University Graduate School (B.B.); Michigan State University Respiratory Research Initiative (J.J.P.); Michigan State University Foundation Strategic Partnership Grant (J.J.P.); Public Health Service Grant (ES03358 to J.J.P.); National Institutes of Health (T32 NS44928 to T.I.; DC006897 to C.C.H.).
References
- Amuzie CJ, Islam Z, Kim JK, Seo JH, Pestka JJ. Kinetics of satratoxin G tissue distribution and excretion following intranasal exposure in the mouse. Toxicol. Sci. 2010;116:433–440. doi: 10.1093/toxsci/kfq142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasel TL, Douglas DR, Wilson SC, Straus DC. Detection of airborne Stachybotrys chartarum macrocyclic trichothecene mycotoxins on particulates smaller than conidia. Appl. Environ. Microbiol. 2005;71:114–122. doi: 10.1128/AEM.71.1.114-122.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calof AL, Bonnin A, Crocker C, Kawauchi S, Murray RC, Shou J, Wu HH. Progenitor cells of the olfactory receptor neuron lineage. Microsc. Res. Tech. 2002;58:176–188. doi: 10.1002/jemt.10147. [DOI] [PubMed] [Google Scholar]
- Calof AL, Chikaraishi DM. Analysis of neurogenesis in a mammalian neuroepithelium: proliferation and differentiation of an olfactory neuron precursor in vitro. Neuron. 1989;3:115–127. doi: 10.1016/0896-6273(89)90120-7. [DOI] [PubMed] [Google Scholar]
- Carey S, Plopper C, Islam Z, Pestka J, Harkema J. Satratoxin-G from black mold induces rhinitis and apoptosis of olfactory sensory neurons in the nasal airways of rhesus monkeys. FASEB J. 2008;22:897.10. doi: 10.1177/0192623312444028. [DOI] [PubMed] [Google Scholar]
- Chorna NE, Santiago-Perez LI, Erb L, Seye CI, Neary JT, Sun GY, Weisman GA, Gonzalez FA. P2Y receptors activate neuroprotective mechanisms in astrocytic cells. J. Neurochem. 2004;91:119–132. doi: 10.1111/j.1471-4159.2004.02699.x. [DOI] [PubMed] [Google Scholar]
- Epstein VA, Bryce PJ, Conley DB, Kern RC, Robinson AM. Intranasal Aspergillus fumigatus exposure induces eosinophilic inflammation and olfactory sensory neuron cell death in mice. Otolaryngol. Head Neck Surg. 2008;138:334–339. doi: 10.1016/j.otohns.2007.11.029. [DOI] [PubMed] [Google Scholar]
- Gangadhar NM, Firestein SJ, Stockwell BR. A novel role for jun N-terminal kinase signaling in olfactory sensory neuronal death. Mol. Cell. Neurosci. 2008;38:518–525. doi: 10.1016/j.mcn.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayle S, Burnstock G. Immunolocalisation of P2X and P2Y nucleotide receptors in the rat nasal mucosa. Cell Tissue Res. 2005;319:27–36. doi: 10.1007/s00441-004-0979-2. [DOI] [PubMed] [Google Scholar]
- Graziadei PP, Graziadei GA. Neurogenesis and neuron regeneration in the olfactory system of mammals. I. Morphological aspects of differentiation and structural organization of the olfactory sensory neurons. J. Neurocytol. 1979;8:1–18. doi: 10.1007/BF01206454. [DOI] [PubMed] [Google Scholar]
- Graziadei PPC, Monti-Graziadei GA. Continuous nerve cell renewal in the olfactory system. In: Jacobson M, editor. Handbook of Sensory Physiology. New York, NY: Springer; 1978. pp. 55–83. [Google Scholar]
- Hegg CC, Greenwood D, Huang W, Han P, Lucero MT. Activation of purinergic receptor subtypes modulates odor sensitivity. J. Neurosci. 2003;23:8291–8301. doi: 10.1523/JNEUROSCI.23-23-08291.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegg CC, Irwin M, Lucero MT. Calcium store-mediated signaling in sustentacular cells of the mouse olfactory epithelium. Glia. 2009;57:634–644. doi: 10.1002/glia.20792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegg CC, Lucero MT. Purinergic receptor antagonists inhibit odorant-induced heat shock protein 25 induction in mouse olfactory epithelium. Glia. 2006;53:182–190. doi: 10.1002/glia.20258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illing N, Boolay S, Siwoski JS, Casper D, Lucero MT, Roskams AJ. Conditionally immortalized clonal cell lines from the mouse olfactory placode differentiate into olfactory receptor neurons. Mol. Cell. Neurosci. 2002;20:225–243. doi: 10.1006/mcne.2002.1106. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. Damp Indoor Spaces and Health. Washington, DC: National Academies Press; 2004. [PubMed] [Google Scholar]
- Islam Z, Harkema JR, Pestka JJ. Satratoxin G from the black mold Stachybotrys chartarum evokes olfactory sensory neuron loss and inflammation in the murine nose and brain. Environ. Health Perspect. 2006;114:1099–1107. doi: 10.1289/ehp.8854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam Z, Hegg CC, Bae HK, Pestka JJ. Satratoxin G-induced apoptosis in PC-12 neuronal cells is mediated by PKR and caspase independent. Toxicol. Sci. 2008;105:142–152. doi: 10.1093/toxsci/kfn110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam Z, Shinozuka J, Harkema JR, Pestka JJ. Purification and comparative neurotoxicity of the trichothecenes satratoxin G and roridin L2 from Stachybotrys chartarum. J. Toxicol. Environ. Health A. 2009;72:1242–1251. doi: 10.1080/15287390903129234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia C, Doherty JD, Crudgington S, Hegg CC. Activation of purinergic receptors induces proliferation and neuronal differentiation in Swiss Webster mouse olfactory epithelium. Neuroscience. 2009;163:120–128. doi: 10.1016/j.neuroscience.2009.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia C, Roman C, Hegg CC. Nickel sulfate induces location-dependent atrophy of mouse olfactory epithelium: protective and proliferative role of purinergic receptor activation. Toxicol. Sci. 2010;115:547–556. doi: 10.1093/toxsci/kfq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawauchi S, Beites CL, Crocker CE, Wu HH, Bonnin A, Murray R, Calof AL. Molecular signals regulating proliferation of stem and progenitor cells in mouse olfactory epithelium. Dev. Neurosci. 2004;26:166–180. doi: 10.1159/000082135. [DOI] [PubMed] [Google Scholar]
- Koskinen OM, Husman TM, Meklin TM, Nevalainen AI. Adverse health effects in children associated with moisture and mould observations in houses. Int. J. Environ. Health Res. 1999a;9:143–156. [Google Scholar]
- Koskinen OM, Husman TM, Meklin TM, Nevalainen AI. The relationship between moisture or mould observations in houses and the state of health of their occupants. Eur. Respir. J. 1999b;14:1363–1367. [PubMed] [Google Scholar]
- Leung CT, Coulombe PA, Reed RR. Contribution of olfactory neural stem cells to tissue maintenance and regeneration. Nat. Neurosci. 2007;10:720–726. doi: 10.1038/nn1882. [DOI] [PubMed] [Google Scholar]
- Lichtenstein JH, Molina RM, Donaghey TC, Amuzie CJ, Pestka JJ, Coull BA, Brain JD. Pulmonary responses to Stachybotrys chartarum and its toxins: mouse strain affects clearance and macrophage cytotoxicity. Toxicol. Sci. 2010;116:113–121. doi: 10.1093/toxsci/kfq104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay-Sim A, Chuah MI. Neurotrophic factors in the primary olfactory pathway. Prog. Neurobiol. 2000;62:527–559. doi: 10.1016/s0301-0082(00)00009-5. [DOI] [PubMed] [Google Scholar]
- Miller JD, Rand TG, Jarvis BB. Stachybotrys chartarum: cause of human disease or media darling? Med. Mycol. 2003;41:271–291. doi: 10.1080/1369378031000137350. [DOI] [PubMed] [Google Scholar]
- Murtoniemi T, Nevalainen A, Hirvonen MR. Effect of plasterboard composition on Stachybotrys chartarum growth and biological activity of spores. Appl. Environ. Microbiol. 2003;69:3751–3757. doi: 10.1128/AEM.69.7.3751-3757.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary JT, Rathbone MP, Cattabeni F, Abbracchio MP, Burnstock G. Trophic actions of extracellular nucleotides and nucleosides on glial and neuronal cells. Trends Neurosci. 1996;19:13–18. doi: 10.1016/0166-2236(96)81861-3. [DOI] [PubMed] [Google Scholar]
- Neary JT, Zimmermann H. Trophic functions of nucleotides in the central nervous system. Trends Neurosci. 2009;32:189–198. doi: 10.1016/j.tins.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Nielsen FK. Dissertation, The Mycology Group. Technical University of Denmark; 2002. Mold growth on building materials: secondary metabolites, mycotoxins and biomarkers. Available at: http://alcor.concordia.ca/∼raojw/crd/reference/reference002200.html. [Google Scholar]
- Nikulin M, Reijula K, Jarvis BB, Hintikka EL. Experimental lung mycotoxicosis in mice induced by Stachybotrys atra. Int. J. Exp. Pathol. 1996;77:213–218. doi: 10.1046/j.1365-2613.1996.9250323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orellano EA, Rivera OJ, Chevres M, Chorna NE, Gonzalez FA. Inhibition of neuronal cell death after retinoic acid-induced down-regulation of P2X7 nucleotide receptor expression. Mol. Cell. Biochem. 2010;337:83–99. doi: 10.1007/s11010-009-0288-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka JJ, Yike I, Dearborn DG, Ward MD, Harkema JR. Stachybotrys chartarum, trichothecene mycotoxins, and damp building-related illness: new insights into a public health enigma. Toxicol. Sci. 2008;104:4–26. doi: 10.1093/toxsci/kfm284. [DOI] [PubMed] [Google Scholar]
- Rao CY, Burge HA, Brain JD. The time course of responses to intratracheally instilled toxic Stachybotrys chartarum spores in rats. Mycopathologia. 2000;149:27–34. doi: 10.1023/a:1007239017018. [DOI] [PubMed] [Google Scholar]
- Rathbone MP, Christjanson L, Deforge S, Deluca B, Gysbers JW, Hindley S, Jovetich M, Middlemiss P, Takhal S. Extracellular purine nucleosides stimulate cell division and morphogenesis: pathological and physiological implications. Med. Hypotheses. 1992a;37:232–240. doi: 10.1016/0306-9877(92)90193-g. [DOI] [PubMed] [Google Scholar]
- Rathbone MP, Deforge S, Deluca B, Gabel B, Laurenssen C, Middlemiss P, Parkinson S. Purinergic stimulation of cell division and differentiation: mechanisms and pharmacological implications. Med. Hypotheses. 1992b;37:213–219. doi: 10.1016/0306-9877(92)90190-n. [DOI] [PubMed] [Google Scholar]
- Rathbone MP, Middlemiss PJ, Gysbers JW, Andrew C, Herman MA, Reed JK, Ciccarelli R, Di Iorio P, Caciagli F. Trophic effects of purines in neurons and glial cells. Prog. Neurobiol. 1999;59:663–690. doi: 10.1016/s0301-0082(99)00017-9. [DOI] [PubMed] [Google Scholar]
- Rosenblum Lichtenstein JH, Molina RM, Donaghey TC, Brain JD. Strain differences influence murine pulmonary responses to Stachybotrys chartarum. Am. J. Respir. Cell. Mol. Biol. 2006;35:415–423. doi: 10.1165/rcmb.2005-0483OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz Levey M, Chikaraishi DM, Kauer JS. Characterization of potential precursor populations in the mouse olfactory epithelium using immunocytochemistry and autoradiography. J. Neurosci. 1991;11:3556–3564. doi: 10.1523/JNEUROSCI.11-11-03556.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwob JE. Neural regeneration and the peripheral olfactory system. Anat. Rec. 2002;269:33–49. doi: 10.1002/ar.10047. [DOI] [PubMed] [Google Scholar]
- Straus DC. Molds, mycotoxins, and sick building syndrome. Toxicol. Ind. Health. 2009;25:617–635. doi: 10.1177/0748233709348287. [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Oku H, Shibata M, Fukuhara M, Yoshida H, Ikeda T. Involvement of P2X7 receptors in the hypoxia-induced death of rat retinal neurons. Invest. Ophthalmol. Vis. Sci. 2010;51:3236–3243. doi: 10.1167/iovs.09-4192. [DOI] [PubMed] [Google Scholar]
- Sultan-Styne K, Toledo R, Walker C, Kallkopf A, Ribak CE, Guthrie KM. Long-term survival of olfactory sensory neurons after target depletion. J. Comp. Neurol. 2009;515:696–710. doi: 10.1002/cne.22084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesper S, Dearborn DG, Yike I, Allan T, Sobolewski J, Hinkley SF, Jarvis BB, Haugland RA. Evaluation of Stachybotrys chartarum in the house of an infant with pulmonary hemorrhage: quantitative assessment before, during, and after remediation. J. Urban Health. 2000;77:68–85. doi: 10.1007/BF02350963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz TM, Abdiu A, Wingren S, Smeds S, Larsson SE, Wasteson A. Suramin inhibits growth of human osteosarcoma xenografts in nude mice. Cancer Res. 1991;51:3585–3589. [PubMed] [Google Scholar]
- Yang M, Crawley JN. Simple behavioral assessment of mouse olfaction. Curr. Protoc. Neurosci. 2009;8:24.21–24.12. doi: 10.1002/0471142301.ns0824s48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JT. Histopathologic examination of the rat nasal cavity. Fundam. Appl. Toxicol. 1981;1:309–312. doi: 10.1016/s0272-0590(81)80037-1. [DOI] [PubMed] [Google Scholar]