Abstract
Homeostasis of selenium (Se), a critical antioxidant incorporated into amino acids and enzymes, is disrupted by exposure to aryl hydrocarbon receptor (AhR) agonists. Here we examined the importance of dietary Se in preventing the toxicity of the most toxic polychlorinated biphenyl congener, 3,3′,4,4′,5-pentachlorobiphenyl (PCB 126), a potent AhR agonist. Male Sprague-Dawley rats were fed a modified AIN-93 diet with differing dietary Se levels (0.02, 0.2, and 2 ppm). Following 3 weeks of acclimatization, rats from each dietary group were given a single ip injection of corn oil (vehicle), 0.2, 1, or 5 μmol/kg body weight PCB 126, followed 2 weeks later by euthanasia. PCB exposure caused dose-dependent increases in liver weight and at the highest PCB 126 dose decreases in whole body weight gains. Hepatic cytochrome P-450 (CYP1A1) activity was significantly increased even at the lowest dose of PCB 126, indicating potent AhR activation. PCB exposure diminished hepatic Se levels in a dose-dependent manner, and this was accompanied by diminished Se-dependent glutathione peroxidase activity. Both these effects were partially mitigated by Se supplementation. Conversely, thioredoxin (Trx) reductase activity and Trx oxidation state, although significantly diminished in the lowest dietary Se groups, were not affected by PCB exposure. In addition, PCB 126–induced changes in hepatic copper, iron, manganese, and zinc were observed. These results demonstrate that supplemental dietary Se was not able to completely prevent the toxicity caused by PCB 126 but was able to increase moderately the levels of several key antioxidants, thereby maintaining them roughly at normal levels.
Keywords: polychlorinated biphenyls, glutathione, glutathione peroxidase, redox status, selenium, copper, zinc, superoxide dismutase, thioredoxin, hepatotoxicity
Selenium (Se) is an essential trace element, a key component of the antioxidant defense against reactive oxygen species (Rayman 2000). Se is unusual in that it is cotranslationally incorporated into the amino acid selenocysteine (SeCys) (Papp et al., 2007). SeCys becomes part of the active site of antioxidant enzymes, including the Se-dependent family of glutathione peroxidases (SeGPx) responsible for reducing free hydrogen peroxide to water (Flohe et al., 1973; Rotruck et al., 1973) and thioredoxin reductase (TrxR) responsible for reducing thiol-donating thioredoxin (Trx). Se deficiency has been associated with congestive cardiomyopathy and osteoarthropathy in humans (Brenneisen et al., 2005), white muscle disease in animals (Oldfield, 1987), and downregulation of antioxidant defense genes in rats (Fischer et al., 2002). In recent studies, supplemental Se was shown to reduce oxidative stress in animals (Menéndez-Carreño et al., 2008; Singh et al., 2006) and in humans (Bardia et al., 2008; Seyedrezazadeh et al., 2008).
Polychlorinated biphenyls (PCBs), a family of 209 different congeners, have been produced commercially as mixtures and were widely used in industrial settings because of their stability under a broad range of chemical, thermal, and electrical conditions (Safe, 1994). However, that same stable nature of PCBs also allows them to persist in the environment, despite the decline in production since the 1970’s. Because of their lipophilic nature, PCBs also bioaccumulate and biomagnify in the food chain (Hansen, 1987; La Rocca and Mantovani, 2006). Biologically, individual PCB congeners were observed to have very different toxic effects, necessitating studies of individual congeners (Safe, 1984).
Of the 209 congeners, one of the most studied group of PCBs are the non–ortho substituted, more coplanar PCBs, which are able to bind avidly to the aryl hydrocarbon receptor (AhR) and effect the induction of cytochrome P-450 (CYP) enzymes. The most potent of these is PCB 126, with a toxicity equivalency factor of 0.1 relative to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) (Bandiera et al., 1982; Birnbaum and DeVito, 1995; Sawyer and Safe, 1982). PCB 126 is found in commercial Aroclor mixtures, most notably Aroclor 1254, and is a by-product of combustion emissions (Alcock et al., 1998). Despite the end of intentional PCB production in the United States, PCB 126 has been found in city air and in buildings (Zhao et al., 2010). Human adipose tissue shows PCB 126 levels from 120 to 730 pg/g wet weight (Kannan et al., 1988). Dioxins and dioxin-like PCBs disrupt the homeostasis of antioxidant enzymes, including the SeGPx (Hassan et al., 1985; Twaroski et al., 2001). Exposure to various dietary factors or environmental contaminants may increase oxidative stress. In such a situation, severe liver injury may occur if antioxidant enzymes, such as GPx, are not sufficiently present to remove the toxic intermediates (Polavarapu et al., 1998).
We hypothesized that supplemental Se would be able to mitigate some or all the hepatic alterations in enzyme activities and oxidative stress caused by a PCB congener of potent toxicity, PCB 126, whereas on the other hand, low dietary intake of Se should increase the adverse effects of PCB 126 exposure. Male rats were fed Se-controlled diets with low (0.02 ppm), adequate (0.2), or supplemental (2 ppm) levels of Se and administered a single low (0.2), medium (1), or high (5 μmol/kg) dose of PCB 126, and their hepatic activity of CYP1A1, GPx, and TrxR, as well as the hepatic levels of Se, Fe, Cu, Zn, and GSH, were determined 2 weeks after the treatment with PCB 126 to test this hypothesis.
MATERIALS AND METHODS
Chemicals.
All chemicals were obtained from Sigma-Aldrich Chemical Company (St Louis, MO) unless otherwise stated. 3,3′,4,4′,5-pentachlorobiphenyl (PCB 126) was prepared as described (Luthe et al., 2006). The crude product was purified by aluminum oxide column and flash silica gel column chromatography and recrystallized from methanol. The final product purity was determined by gas chromatography-mass spectrometry analysis to be > 99.8% and its identity confirmed by 13C nuclear magnetic resonance. “Caution: PCBs and their metabolites should be handled as hazardous compounds in accordance with National Institutes of Health guidelines.”
Animals, diet, and PCB 126 exposure.
Male Sprague-Dawley rats weighing 75–100 grams from Harlan Laboratories (Indianapolis, IN) were housed in individual wire cages in a controlled environment maintained at 22°C with a 12-h light-dark cycle and water ad libitum. Animals were randomly divided into three dietary groups and were fed ad libitum an AIN-93–based diet (Supplementary table S-1) containing 0.02, 0.2, or 2 ppm sodium selenate obtained from Harlan Teklad (Madison, WI). Following 3 weeks of acclimatization, animals were given a single ip injection of vehicle (stripped corn oil; 5 ml/kg body weight [b.w.]; Acros Chemical Company, Pittsburgh, PA) or vehicle containing a low (0.2 μmol/kg, 65 μg/kg), medium (1 μmol/kg, 326 μg/kg), or high (5 μmol/kg, 1.63 mg/kg) dose of PCB 126. All groups contained 4–6 rats. Feed consumption was monitored by determining the weight of the remaining food per cage every other day. Two weeks after the injection, animals were euthanized using carbon dioxide asphyxiation followed by cervical dislocation. We found that a 2-week exposure period allows sufficient time for the manifestation of the adverse effects of PCB 126 (Lai et al., 2010). Because of its potency, only a small dose of PCB 126 is required for significant toxic effects. In rodents, doses as low as 1 μmol (326 μg) per kg b.w. have been shown to cause significant liver changes and CYP1A1 induction (Fisher et al., 2006; Lai et al., 2010). Liver and other organs were excised, weighed, and further processed as described below. All animal protocols were approved by the Institutional Animal Care and Use Committee of the University of Iowa.
Preparation of hepatic subcellular fractions.
Parts of the liver tissues were immediately minced and homogenized in ice-cold 0.25M sucrose solution containing 0.1mM EDTA, pH 7.4. The homogenates were centrifuged at 10,000 × g for 20 min. The resulting supernatants were then centrifuged at 100,000 × g for 1 h. These supernatants, containing the cytosolic fractions, were dispensed and aliquoted. The pellets, containing the microsomes, were washed twice with cold sucrose/EDTA solution, resuspended in this solution, and aliquoted. Protein concentrations of the microsomal solutions were determined by the method of Lowry et al. (1951). All tissue fractions were frozen and stored at −80°C until further analysis.
Measurement of CYP (CYP1A1) activity.
CYP1A1 activity in hepatic microsomal fractions was determined according to a slightly modified method of Burke and Mayer (1974) by measuring the ethoxyresorufin deethylase (EROD) activity with ethoxyresorufin as the substrate. The resulting fluorescent resorufin product from the monooxygenase reaction was detected using a PerkinElmer LS 55 Fluorescence Spectrometer at excitation wavelength of 550 nm and emission wavelength of 585 nm.
Measurement of superoxide dismutase activities.
Superoxide dismutase (SOD) activities were determined following the method of Spitz and Oberley (1989). SOD activities are expressed as units of SOD activity per milligram protein.
Trace element determinations.
Metal concentrations in different rat tissues were quantitatively determined with an elemental mass spectrometer: inductively coupled plasma MS (ICP-MS). ICP-MS was selected because of its low detection limits and multielement capacity (Entwisle and Hearn, 2006). Liver tissues were pretreated with microwave-assisted close-vessel acid (HNO3) digestion prior to instrument measurement, and metal concentrations in the treated tissues were determined with an Agilent 7500ce ICP-MS equipped with a CETAC AS520 auto sampler.
Measurement of glutathione peroxidase (SeGPx, total GPx, and glutathione transferase) activities.
SeGPx and total GPx activities were measured in hepatic cytosolic fractions by the methods of Lawrence and Burk (1976) using hydrogen peroxide and cuemene hydroperoxide as the substrates, respectively. Glutathione transferase (GST) activity was determined by the method of Habig et al. (1974) using 1-chloro-2,4-dinitrobenzene (CDNB) as the substrate. An absorbance change at 340 nm caused by the conjugation of CDNB to reduced glutathione (GSH) was followed in a Beckman DU-650 spectrophotometer for 5 min. One unit of enzymatic activity is defined as the amount of protein that oxidizes 1μM of nicotinamide adenine dinucleotide phosphate per min, expressed as milliunits per mg protein.
Measurement of TrxR activity.
TrxR activity was measured in hepatic cytosolic fractions by the methods of Holmgren and Bjornstedt (1995) using 5,5′-dithio-bis (2-nitrobenzoic acid) (DTNB) as the substrate (Holmgren and Bjornstedt 1995). The formation of the resulting product, 2-nitro-5-thiobenzoate (TNB), was determined spectrometrically at an absorbance of 412 nm. TrxR activity was estimated by subtracting the time-dependent increase in absorbance in the presence of a TrxR activity inhibitor. One unit of activity was defined as 1μM TNB formed (min × mg protein).
Measurement of the redox state of thioredoxin-1 and thioredoxin-2.
The redox states of Trx1 and Trx2 were determined by the redox Western blot technique, as described previously (Halvey et al., 2005). After derivatization of reduced Trx1 and Trx2 with 4-acetoamido-4-maleimidylstilbene-2,2-disulphonic acid (Molecular Probes), oxidized and reduced forms were separated by SDS-polyacrylamide gel electrophoresis and detected by Western blotting using a commercially available Trx1-specific antibody (American Diagnostica, Stamford, CT) or a Trx2-specific antibody (a kind gift from Dean P. Jones, Emory University, GA). Densitometry was performed using LI-COR imaging software. The redox state of Trx1 was calculated by dividing the sum of the 1 disulfide and 2 disulfide forms by the sum of all forms (oxidized and reduced) and expressed as percent oxidized. Trx2 contains only one pair of cysteines. Therefore, the redox state of Trx2 was calculated as percent oxidized according to the equation: % oxidized = oxidized/(oxidized + reduced) × 100.
Total glutathione (GSH and GSSG) analysis.
For the determination of glutathione, liver samples were homogenized in 5% salicylic acid. GSH and oxidized glutathione (GSSG) levels were determined by the methods of Griffith (1980) and Anderson (1985) using glutathione reductase as the substrate. Absorbance change at 412 nm was followed in a Beckman DU-670 spectrophotometer for 5 min. The rate of yellow color accumulation is the result of 5-thio-2-nitrobenzoate formation from DTNB proportional to the amount of total glutathione in the sample. GSSG was measured independently by incubating the tissue in 2-vinylpyridine, which conjugates GSH. GSH was determined by subtracting GSSG from total glutathione. GSH levels are expressed as per mg protein.
Histology.
Formalin-fixed tissues (liver, spleen, and thymus) were processed routinely, embedded in paraffin, sectioned at 3–4 μm, and stained with hematoxylin and eosin for light microscopic examination. Frozen sections were prepared from selected liver samples and stained with oil-red-O for determination of the presence of lipid.
Statistics.
The effect of PCB 126 treatment and dietary Se level on various responses was studied using ANOVA analysis via procedure PROC general linear models (GLM) in the statistical analysis package SAS (version 9.2). The Dunnett's test was used to compare PCB 126 treatment with the corn oil control and other Se levels with 0.2 ppm Se level. This comparison was conducted separately to PCB 126 treatment and Se level (one-way ANOVA) and jointly (two-way ANOVA). In two-way ANOVA, the interaction term was removed if it was not significant at 0.05 level. The effect of Se level is controlled when applying Dunnett's test to PCB 126 treatment by using LSMEANS statement in PROC GLM. The same was done when applying the Dunnett's test to Se level.
RESULTS
Growth and Organ Weights
The growth rate, indicated by weight gain during the 2 weeks after PCB 126 injection, was not significantly changed in rats treated with low (0.2 μmol/kg) and mid (1 μmol/kg) doses of PCB 126 in any of the Se groups (Table 2). However, growth was significantly slowed, and some animals even lost weight at the high (5 μmol/kg) dose of PCB 126. This effect was slightly ameliorated by high dietary Se levels. Feed consumption was also significantly diminished overall by PCB 126 exposure (data not shown) and correlated with the growth effects (the correlation effect is indicated in Table 1).
TABLE 2.
Weight Gain (%) and Relative Organ Weights (%) under Each Experiment Condition and Significance of Various Comparisons
| Treatment | Dietary Se level |
||||
| Low (0.02 ppm) | Adequate (0.2 ppm) | Supplemental (2 ppm) | 2-Way ANOVA | ||
| Growth (%) | Corn oil | 21.1 ± 1.2 | 21.1 ± 1.7 | 21.4 ± 1.7 | n/a |
| 0.2 μmol/kg PCB 126 | 19.3 ± 1.4 | 16.1 ± 1.9 | 16.9 ± 2.0 | * | |
| 1 μmol/kg PCB 126 | 16.6 ± 2.1 | 15.7 ± 2.2 | 14.1 ± 1.2* | * | |
| 5 μmol/kg PCB 126 | −5.1 ± 2.5* | −4.2 ± 1.9* | −0.6 ± 1.1* | * | |
| 2-Way ANOVA | — | n/a | — | ||
| Relative liver weight (%) | Corn oil | 3.86 ± 0.07 | 4.11 ± 0.06 | 4.36 ± 0.10 | n/a |
| 0.2 μmol/kg PCB 126 | 5.00 ± 0.19* | 5.57 ± 0.17* | 4.84 ± 0.17# | * | |
| 1 μmol/kg PCB 126 | 6.02 ± 0.10* | 6.27 ± 0.17* | 6.15 ± 0.17* | * | |
| 5 μmol/kg PCB 126 | 6.91 ± 0.43* | 7.01 ± 0.31* | 7.52 ± 0.14* | * | |
| 2-Way ANOVA | — | n/a | — | ||
| Relative lung | Corn oil | 0.459 ± 0.012 | 0.464 ± 0.009 | 0.493 ± 0.018 | n/a |
| Weight (%) | 0.2 μmol/kg PCB 126 | 0.526 ± 0.015 | 0.573 ± 0.023* | 0.514 ± 0.022 | * |
| 1 μmol/kg PCB 126 | 0.622 ± 0.031* | 0.557 ± 0.024* | 0.611 ± 0.038 | * | |
| 5 μmol/kg PCB 126 | 0.596 ± 0.026* | 0.653 ± 0.027* | 0.604 ± 0.040 | * | |
| 2-Way ANOVA | — | n/a | — | ||
| Relative | Corn oil | 0.228 ± 0.012 | 0.197 ± 0.012 | 0.213 ± 0.006 | n/a |
| Thymus | 0.2 μmol/kg PCB 126 | 0.161 ± 0.003* | 0.151 ± 0.009* | 0.171 ± 0.012* | * |
| Weight (%) | 1 μmol/kg PCB 126 | 0.101 ± 0.008* | 0.089 ± 0.006* | 0.084 ± 0.004* | * |
| 5 μmol/kg PCB 126 | 0.057 ± 0.004* | 0.046 ± 0.002* | 0.038 ± 0.004* | * | |
| 2-Way ANOVA | # | n/a | — | ||
Note. Results are expressed as mean ± SEM. Each group contained four to six animals. One-way ANOVA was used to examine statistically significant differences (p < 0.05) between each PCB 126 level and the corn oil treatment (indicated by “*” if significant) and between low or supplemental and the adequate dietary Se level (indicated by “#” if significant). Significance for each factor based on two-way ANOVA is indicated in the “2-way ANOVA” rows by “#” for Se level and the “2-way ANOVA” column by “*” for PCB 126 level. All p values have been adjusted for multiple testing using Dunnett’s test. n/a, not applicable.
TABLE 1.
Two-Way ANOVA Analysis of the Effects of PCB 126, Dietary Se, and Interaction
| PCB 126 | Dietary Se | Interaction effect | |
| Growth rate (%) | < 0.001 | — | — |
| Feed consumption | < 0.001 | — | 0.014 |
| Relative liver weight (%) | < 0.001 | — | — |
| Relative thymus weight (%) | < 0.001 | 0.013 | — |
| Relative lung weight (%) | < 0.001 | — | — |
| Relative kidney weight (%) | — | — | — |
| Relative testes weight (%) | 0.021 | — | — |
| EROD activity | < 0.001 | — | — |
| CuZnSOD activity | — | — | — |
| Total SOD activity | — | — | — |
| Hepatic Se | < 0.001 | < 0.001 | 0.004 |
| Hepatic copper | < 0.001 | — | — |
| Hepatic iron | < 0.001 | — | — |
| Hepatic manganese | < 0.001 | 0.047 | — |
| Hepatic zinc | 0.013 | — | — |
| SeGPx activity | < 0.001 | < 0.001 | 0.002 |
| GST activity | < 0.001 | — | — |
| Total GPx activity | < 0.001 | < 0.001 | 0.043 |
| TrxR activity | — | < 0.001 | 0.030 |
| Trx1 oxidation (%) | — | 0.003 | — |
| Trx2 oxidation (%) | — | < 0.001 | — |
| GSH (protein) | — | — | — |
| GSSG (protein) | — | 0.001 | — |
| GSSG/GSH (protein) | — | 0.002 | — |
Note. Only p values < 0.05 are reported.
Relative liver weight (as percentage of body weight) was increased dose dependently by PCB 126 from 11 to 35% with 0.2 μmol/kg PCB 126 to over 70% in all three Se groups with 5 μmol/kg PCB 126 (Table 2). Dose-dependent thymic involution of 20–29% at the low dose and around 80% at the high dose of PCB 126 was observed (Table 2). Two-way ANOVA analysis revealed that low (0.02 ppm) dietary Se significantly reduced thymic involution. All doses of PCB 126 significantly increased relative lung weights, but not in a dose-dependent manner nor was an effect of Se diets apparent (Table 2). No consistent significant effect on relative kidney weight was seen by PCB 126 or dietary Se (data not shown). The relative testes weight was significantly affected overall only by the high dose of PCB 126, where an increase compared with the solvent control was seen (Supplementary table S-2).
Effects on CYP1A1 (EROD) Activity
As depicted in Table 3, EROD activity was significantly increased (17- to 27-fold) by low (0.2 μmol/kg) and medium (1 μmol/kg) doses of PCB 126. At a 5-μmol/kg dose of PCB 126, EROD activity was also significantly increased, but at a lower magnitude (8- to 13-fold) compared with the two lower doses. The EROD activity was not significantly affected by different dietary levels of Se.
TABLE 3.
EROD Activity (nmol/min/mg Protein) under Each Experiment Condition and Significance of Various Comparisons (Adjusted Using Dunnett’s Test)
| Treatment | Dietary Se level |
|||
| Low (0.02 ppm) | Adequate (0.2 ppm) | Supplemental (2 ppm) | 2-Way ANOVA | |
| Corn oil | 0.10 ± 0.02 | 0.13 ± 0.02 | 0.11 ± 0.02 | n/a |
| 0.2 μmol/kg PCB 126 | 2.68 ± 0.12* | 2.70 ± 0.08* | 2.72 ± 0.09* | * |
| 1 μmol/kg PCB 126 | 2.38 ± 0.22* | 2.29 ± 0.16* | 2.28 ± 0.10* | * |
| 5 μmol/kg PCB 126 | 1.26 ± 0.17* | 1.08 ± 0.07* | 1.22 ± 0.12* | * |
| 2-Way ANOVA | — | n/a | — | |
Note. Results are expressed as mean ± SEM. Each group contained four to nine animals. One-way ANOVA was used to examine the difference between each PCB 126 level and the corn oil treatment (*). No significant differences between low or supplemental and the adequate dietary Se level were observed. Significance for each factor based on two-way ANOVA is indicated in the bottom margin (Se, #) and in the right margin (PCB, *). The level for significance is 0.05. n/a, not applicable.
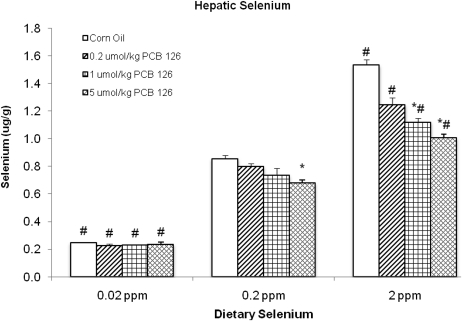
Effects on Hepatic Se
Hepatic Se levels were significantly (65–75%) lower in the low–dietary Se groups compared with the adequate Se groups (Fig. 1). The higher Se level in the supplemental (2 ppm) Se group resulted in a significantly 50–80% increased hepatic Se levels compared with the adequate Se groups (Fig. 1). PCB 126 produced no significant change in hepatic Se in the low- or adequate-Se animals. However, overall PCB 126 significantly diminished hepatic Se by 8–35% at all doses, and this reduction reached statistical significance with the mid (1 μmol/kg) and high (5 μmol/kg) PCB doses in the supplemental dietary Se groups relative to the corn oil control (Fig. 1). This resulted in a highly significant (p < 0.004) interaction effect of PCB 126 and dietary Se (Table 1).
FIG. 1.
Liver Se (μg/g tissue) under each experiment condition and significance of various comparisons (adjusted using Dunnett’s test). Results are expressed as mean ± SEM (n = 4–6 animals/group). One-way ANOVA was used to examine the difference between each PCB 126 level and the corresponding corn oil treatment (*) and low (0.02) or supplemented (2 ppm)-Se level versus adequate (0.2 ppm) Se level (#). p < 0.05.
Effects on Hepatic Copper, Iron, Manganese, and Zinc
The liver tissue levels of several metals were analyzed to identify the effects of PCB 126 and/or different dietary Se levels. Hepatic iron was diminished by PCB 126, significantly at the mid and high PCB doses and overall (Table 4). This effect was most pronounced in the supplemented Se group where the reduction reached 49% and least pronounced in the low-Se group, where it only reached a 15% reduction with 5 μmol/kg PCB 126. However, overall, the dietary level of Se had no effect on hepatic iron. Copper levels were not affected by dietary Se but increased in a dose-dependent manner by PCB 126 treatment, which reached significance at the mid (1 μmol/kg) and high (5 μmol/kg)-dose groups of PCB 126 in all dietary Se groups (Table 4). PCB 126 reduced hepatic Zn levels by 5–20%, which was significant overall at the high PCB dose (Table 4). No significant differences in zinc levels were seen among the different dietary Se groups. Hepatic manganese was diminished 8–33% by PCB 126, and this effect was dose dependent, although statistically significant only in all mid-PCB dose groups and at all doses overall and in the supplemental (2 ppm) dietary Se groups. Although different Se levels did not cause a significant difference individually, a significant overall higher Mn level was seen in the low-Se group compared with the adequate Se group (Table 4).
TABLE 4.
Liver Copper, Iron, Manganese, and Zinc (μg/g) under Each Experiment Condition and Significance of Various Comparisons
| Hepatic level of metals (μg/g) | Treatment | Dietary Se level |
|||
| Low (0.02 ppm) | Adequate (0.2 ppm) | Supplemental (2 ppm) | 2-Way ANOVA | ||
| Copper | Corn oil | 5.41 ± 0.39 | 4.87 ± 0.33 | 5.70 ± 0.25 | n/a |
| 0.2 μmol/kg PCB 126 | 6.24 ± 0.46 | 5.81 ± 0.19 | 5.93 ± 0.32 | — | |
| 1 μmol/kg PCB 126 | 8.51 ± 0.90* | 7.31 ± 0.62* | 7.99 ± 0.36* | * | |
| 5 μmol/kg PCB 126 | 10.77 ± 0.88* | 10.51 ± 0.60* | 9.67 ± 0.46* | * | |
| 2-Way ANOVA | — | n/a | — | ||
| Iron | Corn oil | 165 ± 14 | 154 ± 14 | 191 ± 14 | n/a |
| 0.2 μmol/kg PCB 126 | 138 ± 14 | 158 ± 14 | 150 ± 12* | — | |
| 1 μmol/kg PCB 126 | 137 ± 11 | 119 ± 7 | 125 ± 3* | * | |
| 5 μmol/kg PCB 126 | 141 ± 14 | 119 ± 11 | 98 ± 10* | * | |
| 2-Way ANOVA | — | n/a | — | ||
| Manganese | Corn oil | 3.00 ± 0.25 | 2.50 ± 0.17 | 2.91 ± 0.18 | n/a |
| 0.2 μmol/kg PCB 126 | 2.49 ± 0.26 | 2.29 ± 0.18 | 2.26 ± 0.16* | * | |
| 1 μmol/kg PCB 126 | 2.17 ± 0.18* | 1.83 ± 0.18* | 2.09 ± 0.13* | * | |
| 5 μmol/kg PCB 126 | 2.45 ± 0.20 | 2.17 ± 0.14 | 1.95 ± 0.15* | * | |
| 2-Way ANOVA | # | n/a | — | ||
| Zinc | Corn oil | 37.5 ± 3.0 | 35.2 ± 2.6 | 40.7 ± 1.9 | n/a |
| 0.2 μmol/kg PCB 126 | 35.5 ± 2.9 | 34.3 ± 2.7 | 34.6 ± 2.1 | — | |
| 1 μmol/kg PCB 126 | 35.7 ± 2.2 | 31.8 ± 1.2 | 35.1 ± 1.5 | — | |
| 5 μmol/kg PCB 126 | 31.7 ± 2.6 | 29.8 ± 1.6 | 31.0 ± 1.5* | * | |
| 2-Way ANOVA | — | n/a | — | ||
Note. Results are expressed as mean ± SEM. Each group contained four to six animals. One-way ANOVA was used to examine statistically significant differences (p < 0.05) between each PCB 126 level and the corn oil treatment (indicated by “*” if significant) and between low or supplemental and the adequate dietary Se level (indicated by “#” if significant). Significance for each factor based on two-way ANOVA is indicated in the “2-way ANOVA” rows by “#” for Se level and the “2-way ANOVA” column by “*” for PCB 126 level. All p values have been adjusted for multiple testing using Dunnett’s test. n/a, not applicable.
CuZnSOD and Total SOD Activities
The cytoplasmic SOD has an atom of Cu and Zn in its active site; activities could potentially be influenced by changes in the cellular levels of these metals. CuZnSOD and total SOD activities were not significantly affected by PCB treatment or dietary Se levels (Supplementary table S-3).
Effects on Glutathione Peroxidase and GST Activities
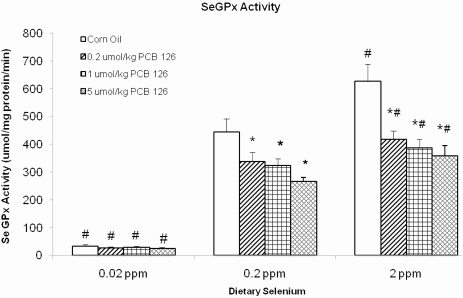
SeGPx activities were strongly influenced by the dietary Se levels. In the solvent control animals, 0.02 ppm Se in the diet resulted in 92% lower SeGPx activity compared with the adequate (0.2 ppm) Se diet, whereas a supplementation of the diet with 2 ppm Se resulted in a 41% increase of SeGPx (Fig. 2). In the adequate (0.2 ppm) and supplemental (2 ppm) dietary Se groups, PCB 126 caused a dose-dependent reduction of SeGPx activity by 25–40% (Fig. 2). No effect of PCB 126 treatment was apparent in the low–Se diet groups. Nevertheless, overall, the effects of dietary Se and PCB 126 were strongly interactive (p < 0.002; Table 1). GST activities were not affected by dietary Se but were increased in a dose-dependent fashion (1.4- to 2.5-fold) by PCB 126 in all three Se groups (Table 5). The total GPx activity was 73% lower in the low-Se control group and 24% higher in the supplemented Se controls compared with the adequate Se control group (Table 5). PCB 126 exposure caused a dose-dependent (1.3- to 2-fold) increase in total GPx activity in the lowest (0.02 ppm) dietary Se groups, but dose-dependent increase was not observed in the adequate (0.2 ppm) and supplemental (2 ppm) dietary Se groups. In the adequate Se group, total GPx activities were decreased by low (0.2 μmol/kg) dose PCB 126, reached control level in the 1 μmol/kg doses of PCB 126, and was increased, although nonsignificantly, in the high (5 μmol/kg)-dose group (Table 5). With the supplemental Se diet, PCB 126 caused a (nonsignificant) decrease in total GPx activity at the two lower doses, and a return to control levels was seen with the high dose of PCB 126. Overall, both, dietary Se levels and PCB 126, had a significant effect on total GPx and together a significant interaction (p < 0.043, Table 1).
FIG. 2.
SeGPx activity (μmol/min/mg protein) and significance of various comparisons (adjusted using Dunnett’s test). Results are expressed as mean ± SEM. Each group contained four to six animals. One-way ANOVA was used to examine the difference between each PCB 126 level and the corn oil treatment (*) and between low/high Se and the adequate Se control (#). p < 0.05.
TABLE 5.
GST (μmol/min/mg Protein) and Total Glutathione Peroxidase Activities (μmol/min/mg Protein) under Each Experiment Condition and Significance of Various Comparisons (Adjusted Using Dunnett’s Test)
| PCB | Glutatione transferase activity (μmol/min/mg protein) |
Total GPx activity (μmol/min/mg protein) |
||||||
| Dietary Se level |
Dietary Se level |
|||||||
| Low (0.02 ppm) | Adequate (0.2 ppm) | Supplemental (2 ppm) | 2-Way ANOVA | Low (0.02 ppm) | Adequate (0.2 ppm) | Supplemental (2 ppm) | 2-Way ANOVA | |
| 0 μmol/kg | 211 ± 16 | 225 ± 11 | 205 ± 37 | n/a | 246 ± 15# | 670 ± 55 | 832 ± 25# | n/a |
| 0.2 μmol/kg | 295 ± 27* | 301 ± 32 | 305 ± 20* | * | 323 ± 28# | 640 ± 48 | 723 ± 35 | — |
| 1 μmol/kg | 354 ± 22* | 382 ± 23* | 331 ± 22* | * | 384 ± 23*# | 707 ± 41 | 719 ± 20 | # |
| 5 μmol/kg | 475 ± 41* | 562 ± 68* | 461 ± 45* | * | 500 ± 40*# | 830 ± 80 | 821 ± 55 | * |
| 2-Way ANOVA | — | n/a | — | # | n/a | — | ||
Note. Results are expressed as mean ± SEM. Each group contained four to six animals. One-way ANOVA was used to examine statistically significant differences (p < 0.05) between each PCB 126 level and the corn oil treatment (*) and between low or supplemental and the adequate dietary Se level (#). Significance for each factor based on two-way ANOVA is indicated in the bottom margin (#, Se) and in the right margin (*, PCB). n/a, not applicable.
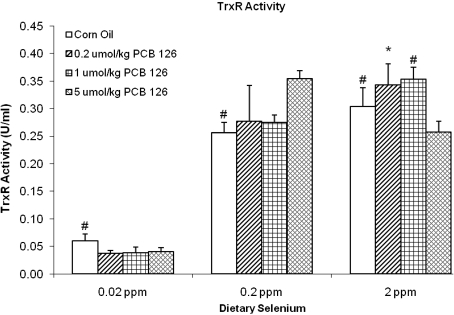
Effects on TrxR Activity and Trx Oxidation States
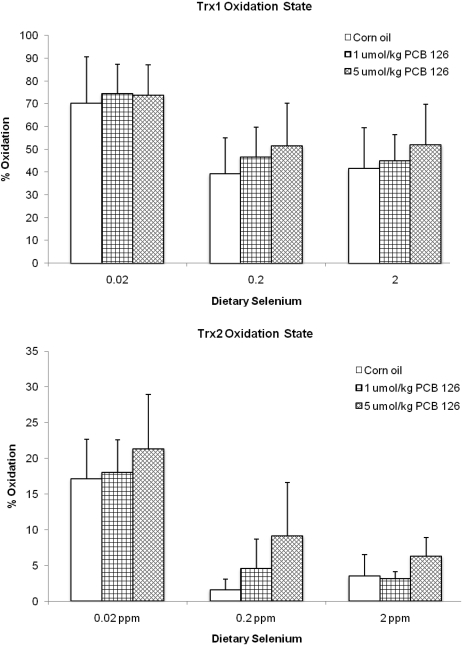
The TrxR activity was only influenced by low dietary Se levels (Fig. 3). A reduction of Se to 0.02 ppm in the diet caused a significantly (76–89%) diminished TrxR activity compared with the adequate Se groups. No overall effect on TrxR was observed in rats fed supplemental (2 ppm) Se (Fig. 3). Different concentrations of PCB 126 did not have a significant effect on TrxR activity at any of the dietary Se levels, but an overall significant decrease of TrxR activity was seen in the low-Se groups. This effect resulted in a significant interaction effect between PCB 126 and Se (Table 1). Thioredoxin-1 (Trx1) and thioredoxin-2 (Trx2) were significantly more oxidized, 79 and 107%, respectively, in the controls with a diet that was low in Se compared with the adequate Se group (Fig. 4a). PCB 126 had no significant effect on the oxidation state of Trx1 or Trx2.
FIG. 3.
TrxR activity (U/ml) under each experiment condition and significance of various comparisons (adjusted using Dunnett’s test). Results are expressed as mean ± SEM. Each group contained four to six animals. One-way ANOVA was used to examine the difference between each PCB 126 level and the corn oil treatment. “*” Indicates significant differences of PCB treatments versus corn oil vehicle control; “#” indicates significant difference of low/high Se versus adequate Se. p < 0.05.
FIG. 4.
Trx1 (top) and Trx2 (bottom) oxidation state and significance of various comparisons (adjusted using Dunnett’s test). Results are expressed as mean ± SEM. Each group contained three animals. One-way ANOVA was used to examine the difference between each PCB 126 level and the corn oil treatment (*) and low/supplemented Se versus 0.2 ppm dietary Se (#); p < 0.05.
Effects on Hepatic Glutathione
No significant effects by PCB 126 or dietary Se on hepatic GSH levels were observed, although Se had an overall significant effect on hepatic GSSG and the GSSG/GSH ratio, both of which were diminished by low dietary Se (Table 6, see Table 1 for statistics).
TABLE 6.
GSH (nmoles GSH/mg Liver Wet Weight), GSSG (nmoles GSSG/mg Liver Wet Weight), and GSSG/GSH Ratio under Each Experiment Condition and Significance of Various Comparisons
| Treatment | Dietary Se level |
||||
| Low (0.02 ppm) | Adequate (0.2 ppm) | Supplemental (2 ppm) | 2-Way ANOVA | ||
| GSH (nmoles GSH/mg liver wet weight) | Corn oil | 8.15 ± 0.93 | 6.86 ± 0.83 | 7.00 ± 0.82 | n/a |
| 1 μmol/kg PCB 126 | 8.57 ± 1.18 | 6.71 ± 0.53 | 7.12 ± 1.93 | — | |
| 5 μmol/kg PCB 126 | 8.45 ± 0.67 | 7.26 ± 0.93 | 7.60 ± 0.87 | — | |
| 2-Way ANOVA | — | n/a | — | ||
| GSSG (nmoles GSSG/mg liver wet weight) | Corn oil | 0.052 ± 0.016 | 0.062 ± 0.005 | 0.060 ± 0.009) | n/a |
| 1 μmol/kg PCB 126 | 0.042 ± 0.004 | 0.058 ± 0.010 | 0.055 ± 0.009 | — | |
| 5 μmol/kg PCB 126 | 0.043 ± 0.006 | 0.057 ± 0.007 | 0.051 ± 0.015 | — | |
| 2-Way ANOVA | # | n/a | — | ||
| GSSG/GSH | Corn oil | 0.638 ± 0.123 | 0.902 ± 0.166 | 0.853 ± 0.061 | n/a |
| Ratio (%) | 1 μmol/kg PCB 126 | 0.494 ± 0.112 | 0.863 ± 0.087 | 0.770 ± 0.183 | — |
| 5 μmol/kg PCB 126 | 0.511 ± 0.060 | 0.780 ± 0.184 | 0.667 ± 0.019 | — | |
| 2-Way ANOVA | # | n/a | — | ||
Note. Results are expressed as mean ± SEM. Each group contained four to six animals. One-way ANOVA was used to examine statistically significant differences (p < 0.05) between each PCB 126 level and the corn oil treatment (indicated by “*” if significant) and between low or supplemental and the adequate dietary Se level (indicated by “#” if significant). Significance for each factor based on two-way ANOVA is indicated in the “2-way ANOVA” rows by “#” for Se level and the “2-way ANOVA” column by “*” for PCB 126 level. All p values have been adjusted for multiple testing using Dunnett’s test. n/a, not applicable.
Histology
Dose-related histological changes were present in all PCB 126–treated rat livers. PCB 126 caused an increase in the amount and density of cytoplasm in centrilobular hepatocytes, suggestive of smooth endoplasmic reticulum induction. No additional histological changes were observed in rats that received 0.02 μmol PCB/kg. In rats that were treated with higher doses of PCB 126 (1 and 5 μmol), dose-dependent cytoplasmic vacuolation and degeneration were present in all groups except for the group exposed to 1 μmol PCB and the low 0.02 ppm Se diet. In addition, dose-dependent scattered apoptosis and karyomegaly were observed in all mid- and high-PCB dose groups. Oil-red-O staining indicated that the vacuolation was due to lipid accumulation (lipidosis/steatosis). Dose-related thymic atrophy was observed in rats given higher doses (1 and 5 μmol) of PCB 126, independent of dietary Se level. No changes were observed in the spleen.
DISCUSSION
We previously observed a significant decrease in liver Se levels and SeGPx activity after exposure of rats to higher doses of PCB 77 (Schramm et al., 1985; Twaroski et al., 2001) and to 1 μmol/kg PCB 126 (Lai et al., 2010). Se and Se-containing enzymes are important for health and for antioxidant defense (Oldfield, 1987; Stadtman, 2000). Se supplementation was shown to inhibit high fat-induced serum cholesterol oxidation (Menéndez-Carreño et al., 2008), to decrease the toxicity of cadmium in rats (Banni et al., 2010), and to be promising in protecting against cancer in animals (Menéndez-Carreño et al., 2008; Singh et al., 2006) and humans (Bardia et al., 2008; Seyedrezazadeh et al., 2008). Even modest deficiency in Se may increase the risk of diseases (McCann and Ames, 2011). Thus, a decrease in Se and Se-containing enzymes such as the one observed with PCB 126 (Lai et al., 2010), PCB 77 (Schramm et al., 1985; Stemm et al., 2008; Twaroski et al., 2001), and TCDD (Hassan et al., 1985) may have unexpected negative health consequences. However, high dietary Se levels are toxic and may increase the risk of type II diabetes and cancer, including PCB-induced hepatocarcinogenesis (Stemm et al., 2008; Stranges et al., 2007). Therefore, to examine the effect of different dietary Se levels, we fed rats with a diet containing low (0.02 ppm), adequate (0.2 ppm), or supplemented (2 ppm) levels of Se for 5 weeks with one ip injection of corn oil or PCB 126 (0.2, 1, and 5 μmol/kg) at the end of the third week.
All Se diets were very well tolerated by the animals with respect to growth and organ weights. PCB 126 on the other hand inhibited growth and even resulted in weight loss at the highest (5 μmol/kg) dose. This was due most likely to decreased food intake (wasting syndrome), similar to the effects of acute TCDD toxicity (Seefeld et al., 1984). Even though Se alone had no effect on feed intake, a significant interaction was seen with PCB 126 and Se diet. This could indicate that Se ameliorates PCB 126–induced wasting, but more experiments are needed to confirm the small effect seen in these studies.
As expected, relative liver weights were dose dependently increased by PCB 126, an effect that was also seen to a smaller extent in the lungs of the animals (Table 2). The increase in relative lung weights was consistent, but not clearly dose dependent, and is therefore considered a treatment effect and not reflective of weight loss. PCBs induce liver cancer in rodents and possibly in humans (Mayes et al., 1998; Ward et al. 2010). Although their mechanism of carcinogenicity is not known, it is assumed that AhR activation may be involved, at least in the promoting activity of certain PCBs (Brown et al., 2007). PCB 126 is by far the most potent AhR agonist of all 209 PCB congeners. Increase in liver weight is a well-known consequence of AhR-mediated increase in endoplasmic reticulum and may be the cause for the increase in lung weights. Histological analysis confirmed this effect on the liver with all PCB doses. Se had no effect on these parameters either alone or in combination with PCB 126. The slight increase in relative testes weights with high dose PCB is most likely a consequence of reduced body weights in this group.
Thymic involution is another well-known effect of AhR agonists. Macroscopic and histological evaluation confirmed the dose-related thymic toxicity of PCB 126. Animals receiving the low-Se diet had overall smaller reduction in thymus weights compared with the normal Se group (Table 2), not enough, however, to produce visible histological differences. We do not know the mechanism or consequence of this small protective effect of the low-Se diet.
The 5 weeks on low- or supplemented-Se diets produced a significant 70% decrease and 80% increase in hepatic Se levels, respectively, compared with the adequate Se diet. These changes in Se were more pronounced than in our previous 10-week promotion study on the same Se diets (Stemm et al., 2008). It is possible that the longer time on the diets allowed for adaptations in the Se kinetics in the body. As before (Lai et al., 2010), PCB 126 lowered hepatic Se levels in the adequate dietary Se group, and this effect was dose dependent (Fig. 1). Whereas supplemental dietary Se was unable to prevent the PCB 126–induced loss of Se from the liver, the remaining hepatic Se levels were still significantly higher than those in rats receiving adequate Se. Thus, supplementing the diet with Se was chemoprotective because it prevented hepatic Se to fall below the normal tissue levels. This is similar to the protective effect seen in cadmium-exposed rats on a Se-supplemented diet (Banni et al., 2010). Rats fed the low-Se diet showed hepatic Se levels that were already very low, and PCB 126 did not produce a significant further reduction. Interestingly, this level (∼0.22 to 0.24 μg/g tissue) is the same as the one observed with PCB 77 treatment of rats after 10 weeks on a low-Se diet (Stemm et al., 2008). This may be the lowest hepatic Se level that the rat physiology will tolerate. Overall, the interaction between PCB 126 and dietary Se levels was highly statistically significant (Table 1), confirming the observed effect of this strong AhR agonist but also the modulation of this effect by different dietary Se levels.
PCB 126 is known to induce CYPs, particularly CYP1A. CYP1A1 is not constitutively expressed, but its synthesis can be greatly enhanced by AhR-driven upregulation of gene expression (Parkinson et al., 1983). PCB 126 caused an increase in CYP1A1 activity (Table 3), consistent with the higher liver weight and induction of smooth endoplasmic reticulum. The lower CYP activity in the high–PCB 126 dose groups may be due to oxidative inactivation of the enzyme by reactive oxygen species or diminished cellular resources available to support protein synthesis. Important in this study is that the different Se diets did not influence this effect of PCB 126 on CYP1A1 activity in any way.
High CYP levels are potentially dangerous because uncoupling of their catalytic cycle can cause a release of superoxide and hydrogen peroxides (Schlezinger et al., 1999). Thus, this increase in CYP activity by PCB 126 is believed to increase oxidative stress in the liver. Surprisingly, in this study, we did not observe an effect of PCB 126 on hepatic glutathione (GSH) as was seen with PCB 77 (Twaroski et al., 2001). Even more surprising, the low-Se diet slightly increased the hepatic total GSH level and decreased GSSG and the GSSG/GSH ratio, suggesting less oxidative stress. In wild birds, high tissue Se levels were shown to correlate with high GSSG levels and GSSG/GSH ratios (Franson et al., 2002; Hoffman, 2002). The authors suggest that an increased SeGPx activity caused an increase in oxidative stress (thiobarbituric acid reactive substances) and GSSG, which could explain the increased carcinogenicity of PCBs at high Se levels (Oldfield, 1987; Stemm et al., 2008). However, Se-related toxicity in birds was seen at tissue levels of 3 ppm, 200% higher than those achieved in the rats on supplemented Se diet.
A metal with possible chemoprotective activity is zinc. Hepatic zinc levels were significantly decreased by the high (5 μmol/kg) dose and slightly but not significantly with 1 μmol/kg PCB 126, similar to our previous findings (Lai et al., 2010). TCDD reportedly produced no change (Wahba et al., 1988) or increases (Nishimura et al., 2001) in hepatic Zn levels. The reason for this difference between PCB 126 and TCDD is not known. The Se diets did not influence Zn levels, indicating that PCB-induced changes in Se levels were not the cause of the lower hepatic Zn content. In addition, hepatic manganese was decreased by all PCB 126 treatments. Interestingly, liver manganese levels were slightly but overall significantly higher in the low-Se group compared with adequate or supplemented Se, possibly a compensatory mechanism.
Despite the induction of CYP, a hemoprotein, hepatic iron levels were decreased as the dose of PCB 126 increased (Table 4). This is most likely a diet effect because 2 weeks exposure to PCB 126 seems too short to produce uroporphyria. TCDD increased hepatic iron levels (Nishimura et al., 2001), a second dissimilarity with PCB 126 effects. Dietary Se did not influence hepatic iron levels, indicating that these parameters are independent from each other.
Hepatic copper was significantly increased by PCB 126 in a dose-dependent manner, in agreement with our previous findings and similar findings with TCDD (Elsenhans et al., 1991; Lai et al., 2010; Nishimura et al., 2001; Wahba et al., 1988). Even a single injection of 0.2 μmol/kg PCB 126 produced an apparent, although not significant, increase in Cu, whereas the high-dose doubled hepatic copper levels. The mechanism of this hepatic Cu increase by PCB 126 or TCDD is not known, but an impairment of biliary excretion was suggested (Elsenhans et al., 1991). Cu is a very efficient Fenton reagent, which can convert H2O2 to the highly reactive hydroxyl radical, which immediately oxidizes proteins and cellular DNA (Buettner and Jurkiewicz, 1996). Copper supplementation was shown to decrease Se levels in muscles of cattle (García-Vaquero et al., 2011), indicating that these two trace metals influence each other. Our major question in this study was whether the Se diet would influence copper levels. As shown in Tables 1 and 4, Se did not affect hepatic Cu levels alone or in combination with PCB 126.
Cu is a constituent of the CuZnSOD, an important cytoplasmic antioxidant enzyme, and believed to be rate limiting. However, the higher availability of Cu as a result of PCB exposure did not cause an increase in CuZnSOD activity, neither did dietary Se. Dietary Se has been reported to influence other antioxidant enzymes, notable are the selenoenzymes glutathione peroxidase (SeGPx) and TrxR (Stadtman, 2000). SeGPx is a major peroxidase that detoxifies H2O2 and cytosolic hydroperoxides. Rats receiving the supplemented Se diet had higher SeGPx activity, whereas animals on low Se had significantly diminished SeGPx activities compared with the adequate group (Fig. 2). In addition, PCB 126 decreased SeGPx activity in a dose-dependent manner; this effect was more pronounced when the hepatic Se level was higher—even the low dose of PCB 126 significantly lowered SeGPx in the supplemental Se group, whereas the mid-PCB 126 dose was needed in the adequate Se group and no statistical significance effect of PCB 126 was apparent in the low-Se group. However, in Se-supplemented rats, the SeGPx activity after PCB 126 treatment remained in the baseline range of the control animals on adequate Se diet. Thus, supplementation of the diet with Se prevented a significant decrease of this important antioxidant enzyme below normal levels, a chemoprotective effect against PCB 126 toxicity. Low dietary Se had a much stronger effect on SeGPx than PCB 126, but it was hypothesized that even a small decline in nonessential selenoproteins like the SeGPX may cause aging-related diseases such as cancer, loss of bone density, and resistance to infections, heart diseases, and others (McCann and Ames, 2011). In addition, PCB 126 and Se diet had an interactive effect (Table 1), raising the concerns that even small dietary deficiency of Se, if combined with exposure to AhR agonists like PCB 126 and TCDD, may be enough to cross the threshold of the no observable effect limit.
Total GPx was significantly affected by PCB 126, dietary Se, and in interaction between these two factors. Total GPx activity increased with increasing dietary Se levels, but PCB 126 did not have a significant effect in the adequate and supplemental Se groups and even caused an increase in the low-Se groups. This seems contradictory, considering the decrease in SeGPx. One component of total GPx activity is GST activity. PCB 126 caused a dose-dependent increase in GST activity (Table 5), similar to the effect of Aroclor 1254 (Schramm et al., 1985; Steinberg et al., 1989). This induction of GST by PCB 126 was not Se dependent and sufficient to more than compensate for the decrease of SeGPx by PCB 126.
The second major Se-dependent antioxidant system includes TrxR. The low-Se diet caused about 75–85% reduction in TrxR activity (Fig. 3), similar to effects reported by others (Hill et al., 1997). A small, nonsignificant increase in TrxR was seen in the Se-supplemented group. The TrxR activity was not affected by PCB 126 (Fig. 3), which is in agreement with reports that SeGPx activity declines before TrxR activity, possibly because the GPx messenger RNA loses stability during Se deficiency (Bermano et al., 1996). However, a small, but significant, overall interaction effect between Se and PCB 126 was observed by ANOVA analysis (Table 1).
The significant increase in the oxidation level of Trx1 and Trx2 in the low-Se group (Fig. 4) was most likely a consequence of the very low TrxR activity levels in this group. Although several PCB treatment groups showed higher oxidized Trx levels than the controls, this did not reach significance, indicating that the Trx system may be stable enough to resist insults from AhR agonists even under low-Se conditions.
These results demonstrate that although dietary Se supplementation did not prevent Se depletion in the liver following PCB 126 exposure, added dietary Se was able to increase hepatic Se levels and SeGPx activity moderately, keeping both above or within the range of the control livers. This modulating effect could be the explanation for the lower levels of 8-oxodG and DNA adducts in Inuits who, due to their diet, have high PCBs but also very high Se levels in their blood (Ravoori et al., 2010). Thus, considering the bioaccumulation of ubiquitous environmental contaminants like PCBs, dioxins, and furans and the possibility that even a small diet-related reduction of selenoproteins like SeGPx may increase the risk of age-related diseases like cancer, more emphasis should be placed in understanding the complex interactions between/among these contaminants and our diet.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
National Institute of Environmental Health Sciences; National Cancer Institute of the National Institutes of Health (P42 ES013661, P30 ES05605, P30 CA086862).
Supplementary Material
Acknowledgments
The authors thank Dr Gregor Luthe for the synthesis of PCB 126 and the Radiation and Free Radical Research Core Facility and members of laboratory for help with the animal studies. I.K.L. gratefully acknowledges support from the Iowa Superfund Research Program Training Core.
References
- Alcock RE, Behnisch PA, Jones KC, Hagenmaier H. Dioxin-like PCBs in the environment-human exposure and the significance of sources. Chemosphere. 1998;37:1457–1472. doi: 10.1016/s0045-6535(98)00136-2. [DOI] [PubMed] [Google Scholar]
- Anderson ME. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985;113:548–555. doi: 10.1016/s0076-6879(85)13073-9. [DOI] [PubMed] [Google Scholar]
- Bandiera S, Safe S, Okey AB. Binding of polychlorinated biphenyls classified as either phenobarbitone-, 3-methylcholanthrene- or mixed-type inducers to cytosolic Ah receptor. Chem. Biol. Interact. 1982;39:259–277. doi: 10.1016/0009-2797(82)90045-x. [DOI] [PubMed] [Google Scholar]
- Banni M, Messaoudi I, Said L, El Heni J, Kerkeni A, Said K. Metallothionein gene expression in liver of rats exposed to cadmium and supplemented with zinc and selenium. Arch. Environ. Contam. Toxicol. 2010;59:513–519. doi: 10.1007/s00244-010-9494-5. [DOI] [PubMed] [Google Scholar]
- Bardia A, Tleyjeh IM, Cerhan JR, Sood AK, Limburg PJ, Erwin PJ, Montori VM. Efficacy of antioxidant supplementation in reducing primary cancer incidence and mortality: systematic review and meta-analysis. Mayo Clin. Proc. 2008;83:23–34. doi: 10.4065/83.1.23. [DOI] [PubMed] [Google Scholar]
- Bermano G, Arthur JR, Hesketh JE. Selective control of cytosolic glutathione peroxidase and phospholipid hydroperoxide glutathione peroxidase mRNA stability by selenium supply. FEBS Lett. 1996;387:157–160. doi: 10.1016/0014-5793(96)00493-0. [DOI] [PubMed] [Google Scholar]
- Birnbaum LS, DeVito MJ. Use of toxic equivalency factors for risk assessment for dioxins and related compounds. Toxicology. 1995;105:391–401. doi: 10.1016/0300-483x(95)03237-a. [DOI] [PubMed] [Google Scholar]
- Brenneisen P, Steinbrenner H, Sies H. Selenium, oxidative stress, and health aspects. Mol. Aspects Med. 2005;26:256–267. doi: 10.1016/j.mam.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Brown JF, Jr, Mayes BA, Silkworth JB, Hamilton SB. Polychlorinated biphenyls modulated tumorigenesis in Sprague Dawley rats: correlation with mixed function oxidase activities and superoxide (O2*) formation potentials and implied mode of action. Toxicol. Sci. 2007;98:375–394. doi: 10.1093/toxsci/kfm122. [DOI] [PubMed] [Google Scholar]
- Buettner GR, Jurkiewicz BA. Catalytic metals, ascorbate and free radicals: combinations to avoid. Radiat. Res. 1996;145:532–541. [PubMed] [Google Scholar]
- Burke MD, Mayer RT. Ethoxyresorufin: direct fluorimetric assay of a microsomal O-dealkylation which is preferentially inducible by 3-methylcholanthrene. Drug Metab. Dispos. 1974;2:583–588. [PubMed] [Google Scholar]
- Elsenhans B, Forth W, Richter E. Increased copper concentrations in rat tissues after acute intoxication with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Arch. Toxicol. 1991;65:429–432. doi: 10.1007/BF02284268. [DOI] [PubMed] [Google Scholar]
- Entwisle J, Hearn R. Development of an accurate procedure for the determination of arsenic in fish tissues of marine origin by inductively coupled plasma mass spectrometry. Spectrochim. Acta Part B At. Spectrosc. 2006;61:438–443. [Google Scholar]
- Fischer A, Pallauf J, Rimbach G. Selenium- and vitamin E-dependent gene expression in rats: analysis of differentially expressed mRNAs. Methods Enzymol. 2002;347:267–276. doi: 10.1016/s0076-6879(02)47026-7. [DOI] [PubMed] [Google Scholar]
- Fisher JW, Campbell J, Muralidhara S, Bruckner JV, Ferguson D, Mumtaz M, Harmon B, Hedge JM, Crofton KM, Kim H, et al. Effect of PCB 126 on hepatic metabolism of thyroxine and perturbations in the hypothalamic-pituitary-thyroid axis in the rat. Toxicol. Sci. 2006;90:87–95. doi: 10.1093/toxsci/kfj069. [DOI] [PubMed] [Google Scholar]
- Flohe L, Gunzler WA, Schock HH. Glutathione peroxidase: a selenoenzyme. FEBS Lett. 1973;32:132–134. doi: 10.1016/0014-5793(73)80755-0. [DOI] [PubMed] [Google Scholar]
- Franson JC, Hoffman DJ, Schmutz JA. Blood selenium concentrations and enzyme activities related to glutathione metabolism in wild emperor geese. Environ. Toxicol. Chem. 2002;21:2179–2184. [PubMed] [Google Scholar]
- García-Vaquero M, Miranda M, Benedito JL, Blanco-Penedo I, López-Alonso M. Effect of type of muscle and Cu supplementation on trace element concentrations in cattle meat. Food Chem. Toxicol. 2011;49:1443–1449. doi: 10.1016/j.fct.2011.03.041. [DOI] [PubMed] [Google Scholar]
- Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980;106:207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- Halvey PJ, Watson WH, Hansen JM, Go YM, Samali A, Jones DP. Compartmental oxidation of thiol-disulphide redox couples during epidermal growth factor signalling. Biochem. J. 2005;386:215–219. doi: 10.1042/BJ20041829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen LG. Food chain modification of the composition and toxicity of PCB residues. Rev. Environ. Toxicol. 1987;3:149–212. [Google Scholar]
- Hassan MQ, Stohs SJ, Murray WJ, Birt DF. Dietary selenium, glutathione peroxidase activity, and toxicity of 2,3,7,8-tetrachloro-dibenzo-p-dioxin. J. Toxicol. Environ. Health. 1985;15:405–415. doi: 10.1080/15287398509530668. [DOI] [PubMed] [Google Scholar]
- Hill KE, McCollum GW, Boeglin ME, Burk RF. Thioredoxin reductase activity is decreased by selenium deficiency. Biochem. Biophys. Res. Commun. 1997;234:293–295. doi: 10.1006/bbrc.1997.6618. [DOI] [PubMed] [Google Scholar]
- Hoffman DJ. Role of selenium toxicity and oxidative stress in aquatic birds. Aquat. Toxicol. 2002;57:11–26. doi: 10.1016/s0166-445x(01)00263-6. [DOI] [PubMed] [Google Scholar]
- Holmgren A, Björnstedt M. Thioredoxin and thioredoxin reductase. Methods Enzymol. 1995;252:199–208. doi: 10.1016/0076-6879(95)52023-6. [DOI] [PubMed] [Google Scholar]
- Kannan N, Tanabe S, Tatsukawa R. Potentially hazardous residues of non-ortho chlorine substituted coplanar PCBs in human adipose tissue. Arch. Environ. Health. 1988;43:11–14. doi: 10.1080/00039896.1988.9934366. [DOI] [PubMed] [Google Scholar]
- La Rocca C, Mantovani A. From environment to food: the case of PCB. Ann. Ist. Super Sanita. 2006;42:410–416. [PubMed] [Google Scholar]
- Lai I, Chai Y, Simmons D, Luthe G, Coleman MC, Spitz D, Haschek WM, Ludewig G, Robertson LW. Acute toxicity of 3,3',4,4',5-pentachlorobiphenyl (PCB 126) in male Sprague-Dawley rats: effects on hepatic oxidative stress, glutathione and metals status. Environ. Int. 2010;36:918–923. doi: 10.1016/j.envint.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence RA, Burk RF. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem. Biophys. Res. Commun. 1976;71:952–958. doi: 10.1016/0006-291x(76)90747-6. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Luthe GM, Schut BG, Aaseng JE. Monofluorinated analogues of polychlorinated biphenyls (F-PCBs): synthesis using the Suzuki-coupling, characterization, specific properties and intended use. Chemosphere. 2009;77:1242–1248. doi: 10.1016/j.chemosphere.2006.02.029. [DOI] [PubMed] [Google Scholar]
- Mayes BA, McConnell EE, Neal BH, Brunner MJ, Hamilton SB, Sullivan TM, Peters AC, Ryan MJ, Toft JD, Singer AW, et al. Comparative carcinogenicity in Sprague-Dawley rats of the polychlorinated biphenyl mixtures Aroclors 1016, 1242, 1254, and 1260. Toxicol. Sci. 1998;41:62–76. doi: 10.1093/toxsci/41.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann JC, Ames BN. Adaptive dysfunction of selenoproteins from the perspective of the triage theory: why modest selenium deficiency may increase risk of diseases of aging. FASEB J. 2011;25:1793–1814. doi: 10.1096/fj.11-180885. [DOI] [PubMed] [Google Scholar]
- Menéndez-Carreño M, Ansorena D, Milagro FI, Campión J, Martínez JA, Astiasarán I. Inhibition of serum cholesterol oxidation by dietary vitamin C and selenium intake in high fat fed rats. Lipids. 2008;43:383–390. doi: 10.1007/s11745-008-3163-8. [DOI] [PubMed] [Google Scholar]
- Nishimura N, Miyabara Y, Suzuki JS, Sato M, Aoki Y, Satoh M, Yonemoto J, Tohyama C. Induction of metallothionein in the livers of female Sprague-Dawley rats treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Life Sci. 2001;69:1291–1303. doi: 10.1016/s0024-3205(01)01212-7. [DOI] [PubMed] [Google Scholar]
- Oldfield JE. The two faces of selenium. J. Nutr. 1987;117:2002–2008. doi: 10.1093/jn/117.12.2002. [DOI] [PubMed] [Google Scholar]
- Papp LV, Lu J, Holmgren A, Khanna KK. From selenium to selenoproteins: synthesis, identity, and their role in human health. Antioxid. Redox Signal. 2007;9:775–806. doi: 10.1089/ars.2007.1528. [DOI] [PubMed] [Google Scholar]
- Parkinson A, Safe SH, Robertson LW, Thomas PE, Ryan DE, Reik LM, Levin W. Immunochemical quantitation of cytochrome P-450 isozymes and epoxide hydrolase in liver microsomes from polychlorinated or polybrominated biphenyl-treated rats. A study of structure-activity relationships. J. Biol. Chem. 1983;258:5967–5976. [PubMed] [Google Scholar]
- Polavarapu R, Spitz DR, Sim JE, Follansbee MH, Oberley LW, Rahemtulla A, Nanji AA. Increased lipid peroxidation and impaired antioxidant enzyme function is associated with pathological liver injury in experimental alcoholic liver disease in rats fed diets high in corn oil and fish oil. Hepatology. 1998;27:1317–1323. doi: 10.1002/hep.510270518. [DOI] [PubMed] [Google Scholar]
- Ravoori S, Srinivasan C, Pereg D, Robertson LW, Ayotte P, Gupta RC. Protective effects of selenium against DNA adduct formation in Inuit environmentally exposed to PCBs. Environ. Int. 2010;36:980–986. doi: 10.1016/j.envint.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayman MP. The importance of selenium to human health. Lancet. 2000;356:233–241. doi: 10.1016/S0140-6736(00)02490-9. [DOI] [PubMed] [Google Scholar]
- Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- Safe S. Polychlorinated biphenyls (PCBs) and polybrominated biphenyls (PBBs): biochemistry, toxicology, and mechanism of action. Crit. Rev. Toxicol. 1984;13:319–395. doi: 10.3109/10408448409023762. [DOI] [PubMed] [Google Scholar]
- Safe SH. Polychlorinated biphenyls (PCBs): environmental impact, biochemical and toxic responses, and implications for risk assessment. Crit. Rev. Toxicol. 1994;24:87–149. doi: 10.3109/10408449409049308. [DOI] [PubMed] [Google Scholar]
- Sawyer T, Safe S. PCB isomers and congeners: induction of aryl hydrocarbon hydroxylase and ethoxyresorufin O-deethylase enzyme activities in rat hepatoma cells. Toxicol. Lett. 1982;13:87–93. doi: 10.1016/0378-4274(82)90142-4. [DOI] [PubMed] [Google Scholar]
- Schlezinger JJ, White RD, Stegeman JJ. Oxidative inactivation of cytochrome P-450 1A (CYP1A) stimulated by 3,3′,4,4′-tetrachlorobiphenyl: production of reactive oxygen by vertebrate CYP1As. Mol. Pharmacol. 1999;56:588–597. doi: 10.1124/mol.56.3.588. [DOI] [PubMed] [Google Scholar]
- Schramm H, Robertson LW, Oesch F. Differential regulation of hepatic glutathione transferase and glutathione peroxidase activities in the rat. Biochem. Pharmacol. 1985;34:3735–3739. doi: 10.1016/0006-2952(85)90239-4. [DOI] [PubMed] [Google Scholar]
- Seefeld MD, Corbett SW, Keesey RE, Peterson RE. Characterization of the wasting syndrome in rats treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Appl. Pharmacol. 1984;73:311–322. doi: 10.1016/0041-008x(84)90337-5. [DOI] [PubMed] [Google Scholar]
- Seyedrezazadeh E, Ostadrahimi A, Mahboob S, Assadi Y, Ghaemmagami J, Pourmogaddam M. Effect of vitamin E and selenium supplementation on oxidative stress status in pulmonary tuberculosis patients. Respirology. 2008;13:294–298. doi: 10.1111/j.1440-1843.2007.01200.x. [DOI] [PubMed] [Google Scholar]
- Singh H, Sodhi S, Kaur R. Effects of dietary supplements of selenium, vitamin E or combinations of the two on antibody responses of broilers. Br. Poult. Sci. 2006;47:714–719. doi: 10.1080/00071660601040079. [DOI] [PubMed] [Google Scholar]
- Spitz DR, Oberley LW. An assay for superoxide dismutase activity in mammalian tissue homogenates. Anal. Biochem. 1989;179:8–18. doi: 10.1016/0003-2697(89)90192-9. [DOI] [PubMed] [Google Scholar]
- Stadtman TC. Selenium biochemistry. Mammalian selenoenzymes. Ann. N Y Acad. Sci. 2000;899:399–402. doi: 10.1111/j.1749-6632.2000.tb06203.x. [DOI] [PubMed] [Google Scholar]
- Steinberg P, Schramm H, Schladt L, Robertson LW, Thomas H, Oesch F. The distribution, induction and isoenzyme profile of glutathione S-transferase and glutathione peroxidase in isolated rat liver parenchymal, Kupffer and endothelial cells. Biochem. J. 1989;264:737–744. doi: 10.1042/bj2640737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemm DN, Tharappel JC, Lehmler HJ, Srinivasan C, Morris JS, Spate VL, Robertson LW, Spear BT, Glauert HP. Effect of dietary selenium on the promotion of hepatocarcinogenesis by 3,3', 4,4'-tetrachlorobiphenyl and 2,2', 4,4', 5,5'-hexachlorobiphenyl. Exp. Biol. Med. (Maywood) 2008;233:366–376. doi: 10.3181/0708-RM-211. [DOI] [PubMed] [Google Scholar]
- Stranges S, Marshall JR, Natarajan R, Donahue RP, Trevisan M, Combs GF, Cappuccio FP, Ceriello A, Reid ME. Effects of long-term selenium supplementation on the incidence of type 2 diabetes: a randomized trial. Ann. Intern. Med. 2007;147:217–223. doi: 10.7326/0003-4819-147-4-200708210-00175. [DOI] [PubMed] [Google Scholar]
- Twaroski TP, O'Brien ML, Robertson LW. Effects of selected polychlorinated biphenyl (PCB) congeners on hepatic glutathione, glutathione-related enzymes, and selenium status: implications for oxidative stress. Biochem. Pharmacol. 2001;62:273–281. doi: 10.1016/s0006-2952(01)00668-2. [DOI] [PubMed] [Google Scholar]
- Wahba ZZ, al-Bayati ZA, Stohs SJ. Effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin on the hepatic distribution of iron, copper, zinc, and magnesium in rats. J. Biochem. Toxicol. 1988;3:121–129. doi: 10.1002/jbt.2570030206. [DOI] [PubMed] [Google Scholar]
- Ward EM, Schulte PA, Straif K, Hopf NB, Caldwell JC, Carreon T, DeMarini DM, Fowler BA, Goldstein BD, Hemminki K, et al. Research recommendations for selected IARC-classified agents. Environ. Health Perspect. 2010;118:1355–1362. doi: 10.1289/ehp.0901828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao HX, Adamcakova-Dodd A, Hu D, Hornbuckle KC, Just CL, Robertson LW, Thorne PS, Lehmler HJ. Development of a synthetic PCB mixture resembling the average polychlorinated biphenyl profile in Chicago air. Environ. Int. 2010;36:819–827. doi: 10.1016/j.envint.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.