Abstract
Calcineurin inhibitor (CI) therapy has been associated with chronic nephrotoxicity, which limits its long-term utility for suppression of allograft rejection. In order to understand the mechanisms of the toxicity, we analyzed gene expression changes that underlie the development of CI immunosuppressant–mediated nephrotoxicity in male Sprague-Dawley rats dosed daily with cyclosporine (CsA; 2.5 or 25 mg/kg/day), FK506 (0.6 or 6 mg/kg/day), or rapamycin (1 or 10 mg/kg/day) for 1, 7, 14, or 28 days. A significant increase in blood urea nitrogen was observed in animals treated with CsA (high) or FK506 (high) for 14 and 28 days. Histopathological examination revealed tubular basophilia and mineralization in animals given CsA (high) or FK506 (low and high). We identified a group of genes whose expression in rat kidney is correlated with CI-induced kidney injury. Among these genes are two genes, Slc12a3 and kidney-specific Wnk1 (KS-Wnk1), that are known to be involved in sodium transport in the distal nephrons and could potentially be involved in the mechanism of CI-induced nephrotoxicity. The downregulation of NCC (the Na-Cl cotransporter coded by Slc12a3) in rat kidney following CI treatment was confirmed by immunohistochemical staining, and the downregulation of KS-Wnk1 was confirmed by quantitative real-time-polymerase chain reaction (qRT-PCR). We hypothesize that decreased expression of Slc12a3 and KS-Wnk1 could alter the sodium chloride reabsorption in the distal tubules and contribute to the prolonged activation of the renin-angiotensin system, a demonstrated contributor to the development of CI-induced nephrotoxicity in both animal models and clinical settings. Therefore, if validated as biomarkers in humans, SLC12A3 and KS-WNK1 could potentially be useful in the early detection and reduction of CI-related nephrotoxicity in immunosuppressed transplant patients when monitoring the health of kidney xenographs in clinical practice.
Keywords: nephrotoxicity, cyclosporine, FK506 (tacrolimus), rapamycin, gene expression, biomarker, renin-angiotensin system
Calcineurin inhibitors (CIs) cyclosporine (CsA) and FK506 are the backbone of current immunosuppressive therapy, widely used in organ transplant patients and patients with autoimmune disease (Meier-Kriesche et al., 2006). Both compounds suppress a number of immune genes by inhibiting calcineurin. Since the introduction of CsA in the early 1980’s, early allograft survival rate has improved significantly, due to reduced acute rejection (Mayer et al., 1997; Williams and Haragsim, 2006). However, concerns have been raised about adverse side effects, mainly nephrotoxicity, caused by long-term use of CIs. CI therapy has been associated with acute kidney dysfunction defined as decreased glomerular filtration rate and renal blood flow and chronic structural changes, such as stripped interstitial fibrosis, arteriolar hyalinosis, or severe tubular microcalcification (Duvoux and Pageaux, 2011; Kivela et al., 2011; Nankivell et al., 2004; Olyaei et al., 2001; Solez et al., 1998; Williams and Haragsim, 2006). These side effects may cause late allograft loss in renal transplant recipients or kidney failure in nonrenal transplant patients, limiting the long-term utility of CI drugs. Although the combination of a low-dose CI and a mammalian target of rapamycin (mTOR) inhibitor has been an effective approach to reduce CI-related chronic nephrotoxicity, the new approach itself has complications and adverse effects, such as synergistic nephrotoxicity, posttransplant diabetes mellitus, delayed graft function, and wound-healing problems, as well as CI-related nephrotoxicity cannot be avoid completely (Campistol, 2010). Although most acute toxicity caused by CI can be resolved by reducing drug dose or complete withdrawal, the long-term effects are irreversible and can only be diagnosed by histology. Therefore, alternative diagnostic tools and biomarkers for early detection of CI-related nephrotoxicity prior to the generation of irreversible damage are needed.
The mechanisms that underlie the toxicity caused by CI are not completely understood, although several mechanisms have been proposed (Lustig et al., 1987; Nankivell et al., 2004; Olyaei et al., 2001; Pallet and Legendre, 2010; Solez et al., 1998; Williams and Haragsim, 2006). These include CI-induced imbalance between renal vasoconstrictor factors and vasodilator factors, activation of sympathetic system, and activation of the renin-angiotensin system (RAS). Activation of RAS following CI treatment has been demonstrated in both animal models and clinical settings (Iijima et al., 2000; Lassila, 2002; Lee, 1997). RAS plays important roles in both acute kidney dysfunction and the development of chronic nephrotoxicity due to its ability to promote vasoconstriction and transforming growth factor-β expression (Nakatani et al., 2003; Shihab et al., 1997). A number of studies have also demonstrated that CI-induced renal impairment was attenuated by the blockage of renin-angiotensin in both rats and organ transplant patients, supporting the critical role of RAS activation in the development of CI-related nephrotoxicity (Langham et al., 2001; Pichler et al., 1995; Sun et al., 2005). The CI activation of RAS is mediated by CI-induced renin overexpression in the kidney, although the mechanism is not clear.
The current study was undertaken in order to gain a better understanding of the mechanisms underlying CI-induced nephrotoxicity and to identify potential biomarkers for early detection of toxicity. Nephrotoxicity was induced in rats through ip injection of CsA (2.5 or 25 mg/kg/day) and FK506 (0.6 or 6 mg/kg/day). Rapamycin (Rapa), an immunosuppressive drug that does not cause the same nephrotoxicity as CI, was administered to another set of animals (1 or 10 mg/kg/day) to help distinguish CI-specific nephrotoxic effects from immunosuppressive effects. Our dosing model generated pathological changes and functional toxicity that resemble typical CI-induced toxicity such as tubular mineralization (after 7-day treatment) and increase in blood urea nitrogen (BUN; after 14 and 28 days) in animals given CsA or FK506. Necrosis was also observed in CsA-treated animals. By anchoring the gene expression changes induced by CI drugs to the nephrotoxicity elicited, the current study identified a gene expression signature in rat kidney that quantitatively correlated with the progression of kidney injury. The downregulation of these genes precedes pathological and/or functional changes, and these gene expression changes may be predictive of CI immunosuppressant–mediated kidney injury in rats. In addition, genes and pathways not previously associated with CI-related injury were discovered, which provide new mechanistic insights into this toxicity.
MATERIALS AND METHODS
Materials.
CsA (Chemical Abstract Service registry number 59865-13-3), FK506 (Chemical Abstract Service registry number 104987-11-3), and Rapa (Chemical Abstract Service registry number 53123-88-9) were obtained from LC Laboratories (Woburn, MA). Rat genome chips (RAE 230 2.0) and One-Cycle Target Labeling and Controls Kit were the products of Affymetrix Inc. (Santa Clara, CA).
Animal treatment.
Male Sprague-Dawley VAF/Plus (Virus Antibody Free) albino rats [Crl:CD(SD) BR; Charles River, Kingston, NY] approximately 5–7 weeks old were maintained on certified rodent chow (PMI Feeds, Inc., Brentwood, MO) ad libitum in individual stainless steel wire bottom cages suspended on racks. The animals were kept under controlled conditions of 12 h light-dark cycle, 72°F ± 5°F, and 50 ± 20% relative humidity. The animals were acclimated to this environment for 4–7 days prior to the start of the study. Rats were randomly assigned to treatment groups and dosed ip with olive oil vehicle or CsA (2.5 and 25 mg/kg/day), FK506 (0.6 and 6 mg/kg/day), or Rapa (1 and 10 mg/kg/day) for 1, 7, 14, and 28 consecutive days (4 animals/group for most treatments and 6/group for 28-day exposure). The high doses of CsA and FK506 were selected based on a review of literature that produced nephrotoxicity in rats without lethality after the desired exposure period. For Rapa, a dose that was higher than pharmacological doses was picked as the high dose. The low doses (1/10 of the high dose) were selected to target the pharmacology doses that do not produce apparent nephrotoxicity. Following treatment, blood was collected at the terminal necropsy (24 h after 1, 7, 14, or 28 days of treatment) for clinical chemistry analysis, and kidneys were examined macroscopically. Each kidney was cut in half (one cross-sectional and the other longitudinal). One half of the left kidney and one half of the right kidney were used for histopathological evaluation, and the remaining portions of the kidney were snap frozen in liquid nitrogen for RNA isolation. Experiments were performed according to the guidelines established in the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Histopathology.
Rat kidney specimens from animals treated with CsA, FK506, Rapa, or olive oil vehicle were collected at necropsy, fixed in 10% neutral buffered formalin, paraffin embedded, sectioned at 5μ thickness, and stained with hematoxylin and eosin (H&E). Histopathologic examinations of the tissue sections were conducted by veterinary pathologists and peer reviewed. Trichrome stain was performed to evaluate interstitial fibrosis in the kidney sections.
mRNA gene expression measurements.
Total RNA was isolated from rat kidney using QIAGEN RNeasy kits (QIAGEN Inc., Valencia, CA). RNA samples were processed for gene expression analysis by Icoria Inc. (Research Triangle Park, NC). The GeneChip Rat Genome 230 2.0 Array (Affymetrix) was used for gene expression profiling. The probe synthesis was performed using One-Cycle Target Labeling protocol by Affymetrix. Chip hybridization, washing, and staining were done according to the standard Affymetrix protocol. The gene chips were scanned with the Affymetrix GeneChip 3000 Scanner, and the expression signals were detected and quantitated by the algorithms in MAS 5.0. All the expression data have been deposited to Gene Expression Omnibus and is accessible via accession number GSE19366.
Gene expression analysis.
Kidney gene expression data were preprocessed by array-based systematic variation normalization (Chou et al., 2005). “Extracting gene expression Patterns and Identifying coexpressed Genes” (EPIG) was then performed on all treated and control groups (Chou et al., 2007). EPIG analysis identified 4 gene expression patterns and 729 coexpressed probes in rat kidney (signal:noise ratio > 2 and r > 0.8). Principle component analysis (PCA) of all animals was performed in Partek Genomic Suite using the 18 probes in the center node of the hierarchical cluster.
Immunohistochemical staining.
For both NCC (the protein coded by Slc12a3) and renin, immunohistochemical staining was performed for kidney samples from animals treated for 7 or 28 days. The rabbit anti-rat NCC antibody (Rabbit Anti–Thiazide-sensitive NaCl Cotransporter Antibody, AB3553) was purchased from Millipore, Billerica, MA. Staining was performed using the Ventana Discovery XT Autostainer (protocol #79; Ventana, Tucson, AZ). The primary antibody was applied at a 1:250 dilution, followed by secondary probing with reagents from the anti-rabbit multimer detection kit. The slides were counterstained with hematoxylin. The NCC staining foci in the rat kidney cortex were counted under the microscope at a ×20 magnification. For each sample, 10 fields were counted and averages for NCC staining foci were used to calculate the mean of all samples in each treatment group. The results are presented as mean staining foci per field ± SE (n = 4–6). All treatments were compared with time-matched controls. ANOVA-protected t-test was used for significance tests. The rabbit anti-rat renin polyclonal antibody was a kind gift from Dr Tadashi Inagami at Vanderbilt University. The high specificity of this antibody was documented previously, as part of the full characterization of the antibody (Naruse et al., 1981). For immunohistochemical staining of rat renin, each slide was blocked with 10% normal donkey serum for 20 min at room temperature. The primary antibody was used at a 1:2500 dilution, whereas the secondary antibody was diluted 1:500. The labeled secondary antibody was from the Vector Rabbit Elite Kit. Each slide was counterstained with hematoxylin.
Quantitative RT-PCR.
Rat kidney total RNA was analyzed for the messenger RNA (mRNA) levels of rat Wnk1 and kidney-specific Wnk1 (KS-Wnk1) using quantitative real-time-polymerase chain reaction (qRT-PCR). Rat beta-actin (Actb) was used as an endogenous control. The sequences of the oligonucleotide primers used for Wnk1 (forward primer 3′-ACTCCGGAATTGGCAGGA-5′, reverse primer 3′-AGTCGCAGATGACGCTTCG-5′), KS-Wnk1 (forward primer 3′-GTTTTGCCTTTTCTGATGGAT-5′, reverse primer 3′-TCCTTCACTTCAGGAATTGCT-5′), and Actb (forward primer, 3′-TAAGGCCAACCGTGAAAAGAT-5′, reverse primer 3′-TCCATCACAATGCCAGTGGT-5′) were designed using Web Primer (http://seq.yeastgenome.org/cgi-bin/web-primer). qRT-PCR was performed using QuantiTect SYBR Green RT-PCR Kit (Qiagen, Chatsworth, CA) following the manufacturer’s instructions in an ABI Prism 7000 Sequence Detection System (Applied Biosystems, Foster City, CA). Three to four biological replicates of RNA were prepared for each treatment, and each biological replicate was measured three times. Equal amounts of total RNA from all control samples of the same time point were pooled together. All measurements were normalized to rat Actb of the same sample, and fold change of each gene following treatment was calculated by comparing the normalized gene expression level with that observed in the untreated, time-matched pooled control. Final results are presented as mean log2fold ± SE (n = 4). ANOVA-protected t-test was performed to identify treatment significance (p < 0.05).
RESULTS
Gross Pathology and Serum Chemistry
All but six animals survived until the scheduled necropsy. The early demise of the six rats was most likely related to complications associated with the ip injections. The effects of CsA, FK506, and Rapa on body weight (BW), BUN, and serum creatinine are presented in Table 1. Significant reduction of BW gain was produced by the administration of CsA (25 mg/kg), FK506 (0.6 and 6 mg/kg), or Rapa (1 and 10 mg/kg) for 7, 14, and 28 days, compared with time-matched controls. CsA (25 mg/kg/day) and FK506 (6 mg/kg) treatment caused prominent elevation of BUN following 14 and 28 days of drug administration. Increase of BUN was also observed in the rats from FK506 (0.6 mg/kg) and Rapa (1 and 10 mg/kg), albeit to a lesser extent. No effect on serum creatinine levels was observed associated with the treatment of any immunosuppressant. Neither CsA nor FK506 treatment caused any change in electrolytes in serum (data not shown). Interestingly, Rapa (1 and 10 mg/kg) treatment caused significant hypocalcaemia (∼10% decrease in calcium), hypokalemia (∼25% decrease in potassium), and hypophosphatemia (∼25% decrease in inorganic phosphorous; data not shown), consistent with previously reported hypokalemia (Johnson et al., 2001) and hypophosphatemia (Schwarz et al., 2001) associated with Rapa treatment for renal allografts in clinic.
TABLE 1.
Effects of CsA, FK506, and Rapa on BW, Serum BUN, and Serum Creatinine (Cr)
| Time (days) | Olive oil, 4 ml/kg | CsA, 2.5 mg/kg | CsA, 25 mg/kg | FK506, 0.6 mg/kg | FK506, 6 mg/kg | Rapa, 1 mg/kg | Rapa, 10 mg/kg |
| BW | |||||||
| 1 | 246 ± 5 | 245 ± 4 | 243 ± 4 | 243 ± 4 | 245 ± 3 | 248 ± 17 | 251 ± 3 |
| 7 | 313 ± 8 | 295 ± 8 | 274 ± 6* | 270 ± 3* | 250 ± 6* | 264 ± 6* | 254 ± 4* |
| 14 | 371 ± 6 | 364 ± 12 | 313 ± 10* | 303 ± 6* | 289 ± 7* | 279 ± 8* | 271 ± 5* |
| 28 | 456 ± 7 | 448 ± 19 | 361* | 351 ± 8* | 344 ± 14* | 286 ± 10* | 294 ± 9* |
| BUN | |||||||
| 1 | 12 ± 1 | 14 | 13 | 11 ± 1 | 11 ± 1 | 13 ± 2 | 12 ± 1 |
| 7 | 13 ± 1 | 15 | 18 ± 2 | 17 ± 2 | 21 ± 2* | 16 ± 1 | 17 ± 1 |
| 14 | 16 ± 1 | 15 ± 1 | 32 ± 2* | 20 ± 1 | 28 ± 2* | 22 ± 2* | 19 ± 3 |
| 28 | 18 ± 2 | 18 ± 1 | 44 ± 4* | 26 ± 2 | 47 ± 18* | 25 ± 7 | 20 ± 1 |
| Cr | |||||||
| 1 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| 7 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.6 | 0.5 |
| 14 | 0.6 | 0.5 | 0.6 | 0.5 | 0.6 | 0.6 | 0.5 |
| 28 | 0.6 | 0.6 | 0.7 | 0.6 | 0.7 ± 0.1 | 0.5 | 0.6 |
*Significantly different from time-matched controls, p < 0.05 (analyzed by ANOVA, followed by Dunnett’s test).
Histopathology
Histopathological evaluation of stained kidney sections revealed adverse histopathological findings associated with CsA and FK506. Although the current study adopted the dosing regimen reported previously (Gillum et al., 1988), we did not observe arteriolopathy and interstitial fibrosis as reported in that study. Three drug-related findings observed in CsA-treated kidneys were tubular basophilia, tubular mineralization, and tubular necrosis (Figs. 1 and 2). Proximal and distal tubular epithelial basophilia (minimal to mild, three animals in total) and tubular mineralization (minimal, one animal) were present at low incidence in the control animals. The degree of severity and incidence of tubular basophilia were substantially increased in the high-dose-CsA and low- and high-dose-FK506 group animals following 14 and 28 days of treatment, compared with earlier treatment (Table 2). Tubular mineralization was seen in collecting ducts within the corticomedullary region of the kidney (Fig. 2). Tubular mineralization was present in high-dose-CsA–treated (7, 14, and 28 days), low- (14 and 28 days) and high-dose-FK506–treated (14 and 28 days) kidneys (Table 2). The degree of severity and incidence of tubular mineralization increased with prolonged treatment. Animals in the high-dose-CsA group had slightly more severe mineralization compared with those animals that received FK506 treatment. Animals treated with CsA exhibited minimal tubular epithelial cell necrosis within proximal convoluted tubules (two in the low-dose group and seven in the high-dose group). Besides drug-related histopathological changes, renal capsulitis was observed and considered related to ip injection. The capsulitis was characterized by the infiltration of capsule by neutrophils and macrophages with increased number of fibroblasts. This finding was absent in the control group, presented in low incidence (one to two rats) in the CsA and FK506 treatment groups. Interestingly, higher incidence (three to five rats) of capsulitis was observed in the low- and high-dose Rapa groups.
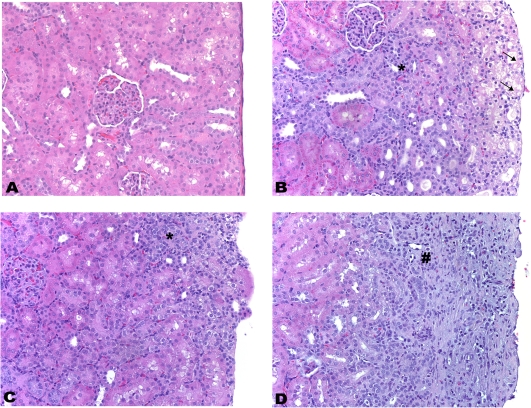
FIG. 1.
Histopathology of kidney from control male rats and rats treated with CsA (25 mg/kg/day), FK506 (6 mg/kg/day), and Rapa (10 mg/kg/day) for 28 days. Histopathological evidence of tubular basophilia (H&E, ×200). (A) Control. (B) CsA. Tubular cell necrosis within the outer cortex (↑) and basophilia of tubular epithelial cells lining the proximal convoluted tubules and DCTs (*). (C) FK506. Basophilia of tubular epithelial cells lining the proximal convoluted tubules and DCTs (*). (D) Rapa. Chronic interstitial nephritis, characterized by neutrophil and macrophage infiltrates mixed with hemorrhage and edema fluid (#). This finding is related to nephritis/capsulitis induced by ip injection.
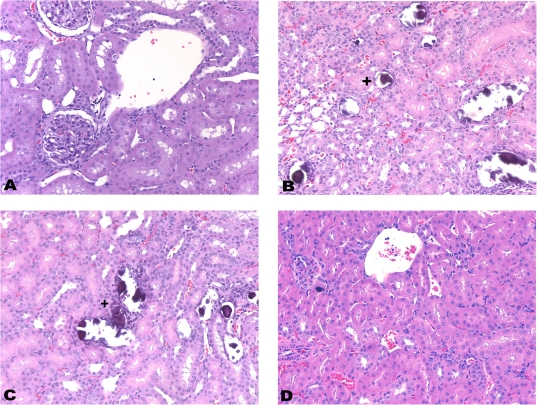
FIG. 2.
Histopathology of kidney from control male rats and rats treated with CsA (25 mg/kg/day), FK506 (6 mg/kg/day), and Rapa (10 mg/kg/day) for 28 days. Histopathological evidence of tubular mineralization (H&E, ×200). (A) Control. (B) CsA. Mineralization of collecting ducts within the corticomedullary region (+). (C) FK506. Mineralization of collecting ducts within the corticomedullary region (+). (D) Rapa.
TABLE 2.
Rat Kidney Histopathology Following CsA, FK506, and Rapa Treatments
| Treatment | Lesions | Number of incidences (average scorea) |
|||
| 1 Day | 7 Days | 14 Days | 28 Days | ||
| Olive oil 4 ml/kg | Basophilia, tubular | 1/4 (2.0) | 1/4 (1.0) | 1/5b (1.0) | |
| Mineralization, tubular | 1/4 (1.0) | ||||
| Necrosis, tubular | |||||
| CsA 2.5 mg/kg | Basophilia, tubular | 2/4 (1.0) | 2/4 (1.0) | 1/5b (1.0) | |
| Mineralization, tubular | |||||
| Necrosis, tubular | 1/4 (1.0) | 1/5b (1.0) | |||
| CsA 25 mg/kg | Basophilia, tubular | 1/4 (1.0) | 4/4 (1.0) | 4/4 (2.0) | 4/4c (2.5) |
| Mineralization, tubular | 2/4 (1.0) | 4/4 (1.8) | 4/4c (2.8) | ||
| Necrosis, tubular | 3/4 (1.0) | 4/4 (1.0) | |||
| FK506 0.6 mg/kg | Basophilia, tubular | 2/4 (1.0) | 4/4 (1.0) | 6/6 (2.0) | |
| Mineralization, tubular | 3/4 (1.0) | 6/6 (2.0) | |||
| Necrosis, tubular | |||||
| FK506 6 mg/kg | Basophilia, tubular | 4/4 (1.0) | 3/3b (1.3) | 5/5b (1.8) | |
| Mineralization, tubular | 1/3b (1.0) | 5/5b (1.6) | |||
| Necrosis, tubular | |||||
| Rapa 1 mg/kg | Basophilia, tubular | 1/4 (1.0) | 1/4 (1.0) | 2/6 (2.5) | |
| Mineralization, tubular | |||||
| Necrosis, tubular | |||||
| Rapa 10 mg/kg | Basophilia, tubular | 1/4 (1.0) | 3/6 (1.3) | ||
| Mineralization, tubular | |||||
| Necrosis, tubular | |||||
Average score: 1, minimal; 2, mild; 3, moderate; 4, marked.
One animal failed to survive the treatment.
Two animals failed to survive the treatment.
Based on the traditional indicators of kidney injury, we classified animals into three groups: a group with nephrotoxicity (“toxicity” group, usually with both tubular lesions and significantly elevated BUN comparing with time-matched control), a group without nephrotoxicity (“no toxicity” group, with neither major changes in histopathology nor significantly elevated BUN comparing with time-matched control) and a group with mild nephrotoxicity (“intermediate toxicity” group, with either tubular lesions or significantly elevated BUN; Supplementary Table 1). This classification was incorporated into subsequent gene expression analyses in order to build relationships between gene expression changes and CI-related kidney injury.
Gene Expression Changes Associated with CI-Induced Nephrotoxicity
An unsupervised clustering of all treated animals using differentially expressed genes from all treatment groups (twofold or more, p < 0.01) resulted in four major clusters: two clusters from the earlier time point segregated by time (the 1-day treatment cluster and the 7-day treatment cluster) and two clusters from later time point segregated by treatment (the CI-treated cluster and the Rapa-treated cluster; Supplementary figure 1). Clusters that segregated based on treatment correlated with animals showing changes in traditional indicators of kidney injury, demonstrating that a different set of biological events were taking place in the injured animals (Supplementary table 2).
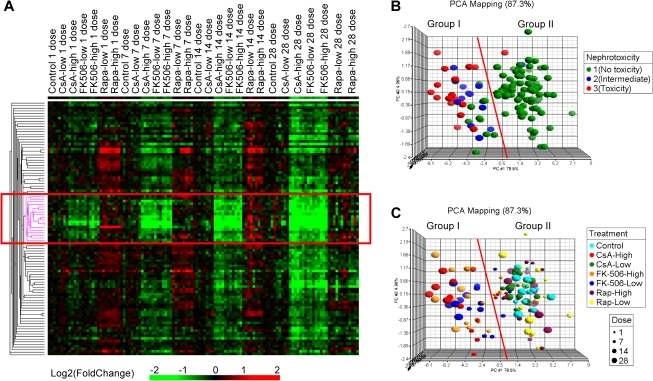
EPIG is a software that utilizes the underlying structure of gene expression data to extract patterns and identify coexpressed genes that are responsive to experimental conditions (Chou et al., 2007). It was used in order to extract kidney genes that drive the separation of CI-treated animals from control and Rapa-treated animals. One pattern contained 98 probes (Supplementary table 3) that were downregulated by CI drugs (CsA high, FK506 low and high) at later time points, a pattern that was consistent with the progression of histopathological changes and elevated BUN levels. Hierarchical clustering of these 98 probes revealed a subgroup (the center node containing 18 probes; Supplementary table 3) that was inhibited by CI drugs at the 1-day time point (Fig. 3A). Because no histopathological changes and/or kidney dysfunction was observed at 1 day, these genes may be predictors of CI-induced kidney injury. Indeed, PCA of all samples using the 18 center node probes resulted in two main groupings (Figs. 3B and 3C). Group I contained all of the animals with CI-related nephrotoxicity (toxicity group and intermediate toxicity group), whereas group II contained animals with no toxicity along with two animals that had Rapa-induced toxicity. Group I also contained CI-treated animals from the earlier time point (7 days) that showed no toxicity, indicating the capacity of early prediction. The two animals with non–CI-related nephrotoxicity (caused by Rapa treatment) did not fall into the same grouping as animals with CI-related kidney injury, suggesting that this gene set shows specificity for correlation with CI-induced nephrotoxicity.
FIG. 3.
A kidney gene expression signature correlates with CI-induced nephrotoxicity in the rat. (A) A gene expression signature identified by EPIG (boxed area). Each vertical bar represents an experiment animal, and each horizontal bar represents a probe on the array. Probes used for clustering were identified by EPIG. Color scale is indicated in the bottom. Fold change of each gene was calculated by comparing with time-matched controls. (B, C) PCA of rat kidney gene expression. Each sphere in the graph represents an experimental animal, and all experimental animals were mapped based on the expression of 18 probes in the center node of (A) (boxed area). Animals in (B) are colored by the degree of nephrotoxicity. Animals in (C) are colored based on the treatment and sized by the duration of treatment.
Calbindin-1(Calb1) and epidermal growth factor (Egf) were among the genes that appear to be predictive of CI-induced kidney injury in rats, and both are well known to be involved in the CI-related nephrotoxicity (Aicher et al., 1997; Lee et al., 2002; Nijenhuis et al., 2004; Sairanen et al., 2008). These studies support the current results and suggest that the coexpression of these genes may play important roles in the mechanism of CI-related nephrotoxicity. Therefore, additional experiments were carried out to understand the functional relationship between other genes in this node and nephrotoxicity. Slc12a3 and Wnk1 were the initial focus because these are recognized to have important roles in normal renal function.
NCC Expression Was Inhibited by CI Treatment
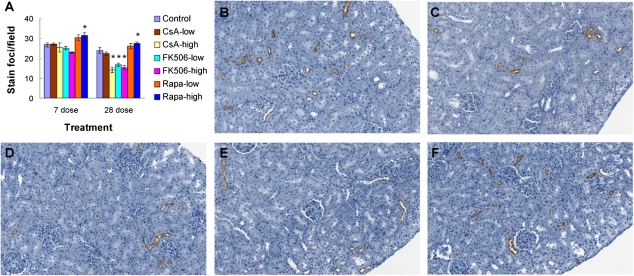
Slc12a3 encodes a sodium chloride cotransporter that is exclusively expressed in the kidney distal convoluted tubule (DCT). It is one of the major transporters responsible for sodium reabsorption in distal tubules of the kidney (Yang et al., 2005). Mutations in NCCT (human homolog of rat NCC) cause Gitelman’s syndrome in humans (Naesens et al., 2004; Simon et al., 1996). NCC has not been associated with CI-induced nephrotoxicity, although decreased expression of NCC in rat kidney following CsA treatment has been reported in one study (Lim et al., 2007). In order to determine if NCC protein changes correlated with mRNA changes, immunohistochemical staining of NCC was performed on kidney samples from animals treated for 7 and 28 days. NCC protein expression was significantly decreased in a dose-dependent manner following CI treatment (CsA high, FK506 low and high) for both time points (Figs. 4A–E). Furthermore, the number of NCC staining foci in the kidney cortex was also significantly decreased (Fig. 4A). This suggests that NCC expression is not only decreased but also absent in some parts of the DCT, which may significantly reduce the reabsorption of sodium chloride. Treatment with Rapa did not decrease NCC expression in rat kidney at any time point (Figs. 4A and 4F).
FIG. 4.
NCC expression in rat kidney cortex following CI or Rapa treatment. (A) Quantitative assessment of NCC staining foci in rat kidney cortex (7 and 28 days). The number of stained foci per field was counted at ×10 magnification. Results are presented as mean stain foci per field ± SE (n = 4–6). *p < 0.05. (B–F) Representative images of NCC immunohistochemical staining in kidney cortex of rats treated for 28 days with vehicle alone (B), high-dose CsA (C), low-dose FK506 (D), high-dose FK506 (E), or high-dose Rapa (F) (×200).
KS-Wnk1 Expression Was Decreased by CI Treatment
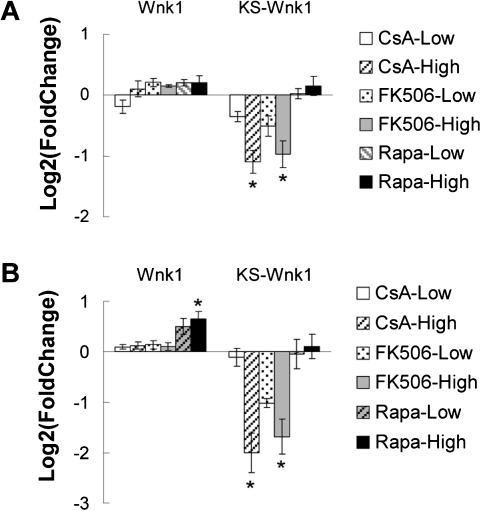
WNK1, a member of the WNK (with-no-lysine [K]) family, is an essential regulator of ion transport in the kidney (McCormick et al., 2008; Xu et al., 2000). In humans, mutations in WNK1 cause familial hyperkalemic hypertension (Wilson et al., 2001). The Wnk1 gene has two products in the kidney, a full-length product (Wnk1) that is widely expressed as well as a kidney-specific truncated product (KS-Wnk1) that lacks the kinase domain and is predominantly expressed in the DCT and the connecting tubules (CNT). In order to determine which form of Wnk1 was downregulated by CI drugs, two pairs of primers were used in qRT-PCR to specifically target different transcripts. Consistent with Affymetrix gene expression data, KS-Wnk1 was significantly downregulated by high-dose CI drug treatment at both 7 and 28 days (Fig. 5). In contrast, expression of full-length Wnk1 remained unchanged following CI treatment.
FIG. 5.
mRNA levels of Wnk1 (full length) and KS-Wnk1 in rat kidney after 7 and 28 days. (A) Gene expression following 7 days of treatment. (B) Gene expression following 28 days of treatment. Rat kidney total RNA was isolated from animals treated with CsA, FK506, Rapa, or vehicle alone. Fold change was computed by comparing with time-matched control group. Results are presented in mean log2(fold change) ± SE (n = 4). *p < 0.05 when compared with 0 (no change in expression).
RAS Was Activated following CI Treatment
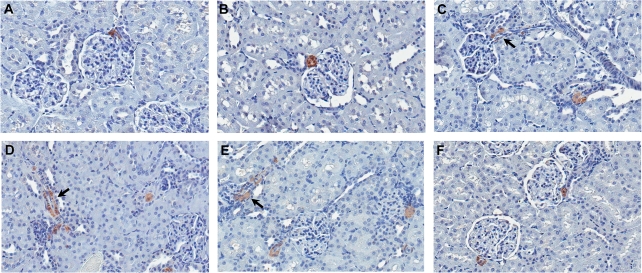
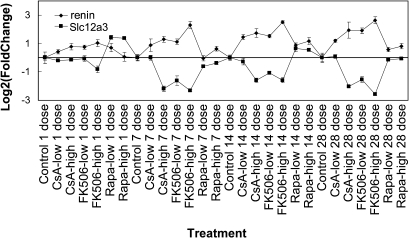
It is believed that the CI-induced activation of RAS is mediated by renin overexpression in the kidney. Immunostaining for renin on kidney samples from animals treated by CI for 7 or 28 days showed an increase in numbers of staining foci in the kidney cortex and elongated expression of renin along the afferent arterioles, suggesting that CI treatment resulted in the recruitment of renin-containing cells (Figs. 6C–E, 7-day data not shown; Iijima et al., 2000; Tufromcreddie et al., 1993). Renin staining in the kidney juxtaglomerular cells of CI-treated animals is less intense than in the control or Rapa-treated animals, probably due to increased renin release from these cells, which supports the activation of RAS following CI treatment. Several studies have previously demonstrated that sodium depletion can promote CsA- or FK506-induced tubulointerstitial injury in rat and mouse kidney (Andoh et al., 1995, 1997; Elzinga et al., 1993; Shihab et al., 1997). In addition, the enhancing effect of low sodium on chronic structural changes has been attributed to the activation of the RAS by low-sodium diet. In the current study, the NCC protein expression decrease may result in a reduction in sodium reabsorption in the DCT and subsequently contribute to the prolonged overexpression of renin. Interestingly, microarray data indicated a strong correlation between renin mRNA level induction and Slc12a3 mRNA level decrease (Fig. 7). This suggests that reduced NCC expression may contribute to the overexpression of renin and the stimulation of RAS.
FIG. 6.
Renin expression in rat kidney cortex following CI or Rapa treatment. (A–F) Representative images of renin immunohistochemical staining in kidneys of rats treated for 28 days with vehicle alone (A), low-dose CsA (B), high-dose CsA (C), low-dose FK506 (D), high-dose FK506 (E), or high-dose Rapa (F) (×400). Arrows demarcate areas of elongated renin expression along blood vessels.
FIG. 7.
Renin mRNA levels are inversely correlated with the expression of Slc12a3 in rat kidney. Affymetrix rat genome array (RAT 230 2.0) GeneChip data. Fold change was calculated by comparing with time-matched control group. Results are presented in mean log2(fold change) ± SE (n = 4–6).
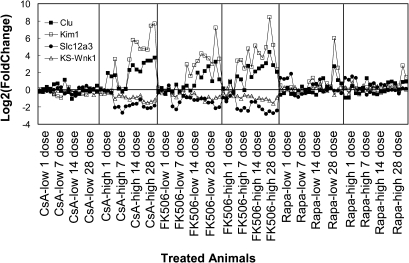
Over the past few years, considerable effort has been devoted to searching for protein markers associated with kidney disease and drug-induced nephrotoxicity (Perco et al., 2006; Thukral et al., 2005; Wang et al., 2008). Among the candidate markers reported, kidney injury molecule-1 (Kim1) and clusterin (Clu) are two of the most promising renal injury markers and have been associated with many forms of renal toxicity and diseases (Abulezz, 2008; Zhang et al., 2008). Both Kim1 and Clu were upregulated in injured animals in the current study (Fig. 8). We compared the expression of Kim1 and Clu with Slc12a3 and KS-Wnk1, as measured by the Affymetrix GeneChip for each individual treated animal relative to levels in control animals. We found that Slc12a3 and KS-Wnk1 were strongly correlated with Kim1 and Clu and that Slc12a3 and KS-Wnk1 appear to be more specific to CI-related injuries. Both Slc12a3 and KS-Wnk1 were not changed in Rapa-treated animals, whereas both Kim1 and Clu were upregulated in later time point Rapa-treated animals (Fig. 8). This probably is due to Slc12a3 and KS-Wnk1 downregulation being associated with the mechanism of CI-induced nephrotoxicity.
FIG. 8.
mRNA levels of potential genomic biomarkers in rat kidney from animals treated with CI or Rapa. mRNA levels of Kim1, Clu, Slc12a3, and KS-Wnk1 in each individual treated animal was normalized to time-matched untreated animals, respectively. For each individual animal, four mRNA level values (on log scale) were listed (Clu, solid square; Kim1, empty square; Slc12a3, solid circle; KS-Wnk1, empty triangle). Results were from Affymetrix rat genome array (RAT 230 2.0) GeneChip data.
DISCUSSION
In the current study, a rat model was used to study the effects of CI treatment on global gene expression of kidney, in order to understand the mechanisms underlying CI-related nephrotoxicity and to search for biomarkers of toxicity. CI treatment induced microcalcification and isometric vacuolization in rat kidney, which resemble the pathological findings in CI toxicity in human patients (Jennette et al., 2007). Although we did not observe arteriolopathy and interstitial fibrosis, gene expression analysis revealed significant upregulation of genes involved in tissue repair, extracellular matrix/fibrosis, and immune and inflammation following 28 days of treatment with high-dose CsA and FK506, all of which have been reported to be activated prior to development of tubulointerstitial damage in humans (Vitalone et al., 2010; Supplementary table 2). Therefore, both pathological and gene expressional findings suggest the current model is suitable for the purpose of the study.
Biomarkers for Early Detection of CI-Related Nephrotoxicity
CI-related chronic nephrotoxicity is the Achilles’ heel of the current immunosuppressive regimens (Naesens et al., 2009). Although the histological lesions caused by CI drugs are well defined, the histological diagnosis remains problematic due to difficulties in the differential diagnosis because other injurious processes in renal transplant patients can cause similar pathological changes (Naesens et al., 2009). Using a genomewide approach, this study identified a group of genes that are specifically regulated by CI immunosuppressants. These genes include both upregulated genes, such as renin and Klks3, and downregulated genes, such as Slc12a3 and KS-Wnk1. A number of the identified genes have previously been associated with CI-related nephrotoxicity, such as renin, Calb1, and Egf (Nakatani et al., 2003; Nijenhuis et al., 2004; Yang et al., 2002); however, a longitudinal assessment of the relationship between expression and the development of CI-related nephrotoxicity has been lacking. By gene expression profiling of rat kidney samples collected over a 4-week period of time, we were able to show that the regulation of these genes by CI is time and dose dependent and is quantitatively correlated with the progression of kidney injury. The transcriptional changes in rat kidney had an earlier onset (1–7 days; shown in Fig. 3A) than changes in BUN (14 days) and kidney histopathology (7–14 days). In fact, using only a subgroup of downregulated genes, it was possible to identify not only all animals with significant CI-related nephrotoxicity but also CI-treated animals with mild and no overt toxicity (mostly animals from earlier time points), demonstrating that these genes could be potential biomarkers for early detection of CI-induced nephrotoxicity (Fig. 3B).
Over the past few years, considerable effort has been devoted to searching for protein markers associated with kidney disease and drug-induced nephrotoxicity (Perco et al., 2006; Thukral et al., 2005; Wang et al., 2008). In one of the studies, Wang et al. (2008) evaluated changes of a panel of 48 putative genomic markers of nephrotoxicity in rats using a variety of nephrotoxicants. In most cases, the expression of an individual genetic marker in rat kidney was changed by multiple types of nephrotoxicants. This suggests the necessity of using the profile of multiple genetic markers for the detection of specific types of kidney injury. In a recent study, increased KIM1 mRNA level has been observed in biopsies of CI nephrotoxicity or interstitial fibrosis and tubular atrophy patients (Nogare et al., 2010). Thus, in combination with some of the general kidney injury markers such as KIM1, the mRNA levels of SLC12A3 and KS-WNK1 may serve as specific genomic markers for CI-related kidney injury in humans. In fact, together, these genes successfully separated animals with CI-related kidney injury from animals with Rapa-related kidney injury (Fig. 3).
RAS Activation and Sodium Transport in the Distal Nephron
RAS activation following CI treatment is a consistent finding in animal models (Lassila, 2002). CI can cause elevated plasma renin activity by increasing the synthesis and excretion of renin in kidney juxtaglomerular cells (Andoh et al., 1995; Lee, 1997; Nakatani et al., 2003; Shihab et al., 1997; Stillman et al., 1995). In humans, although CI therapy usually does not cause significant increase in plasma renin activity, it activates the intrarenal RAS, which plays an important role in the development of CI-related interstitial fibrosis (Iijima et al., 2000; Kobori et al., 2007; Shang et al., 2008). How CIs induce renin synthesis remains unclear due to the complexity of the feedback mechanism between the RAS and kidney hemodynamics. On the other hand, it is well established that kidney juxtaglomerular cells are the main source of renin and that reduced sodium chloride concentration in the macula densa can signal these cells to increase renin production (Harris, 1996; Lorenz et al., 1991; Skott and Briggs, 1987).
The DCT is responsible for approximately 5–7% of the reabsorption of filtered sodium and plays an important role in electrolyte balance and ion homeostasis (Ecelbarger and Tiwari, 2006). In this study, NCC, one of the major sodium transporters in the DCT, was found to be downregulated following treatment of CsA and FK506. Loss-of-function mutations in NCCT cause a human disease known as Gitelman’s syndrome, characterized by hypomagnesemia, hypocalciuria, and increased renin activity. Some of these features are also associated with CI use (Naesens et al., 2004; Simon et al., 1996). The results of the current study demonstrate that NCC membrane expression was significantly decreased following CI treatment in a time- and dose-dependent fashion, and renin was overexpressed, suggesting activation of RAS. Based on the strong correlation between Slc12a3 decrease and renin increase, we propose that decreased expression of NCC in the DCT will result in reduced sodium reabsorption in the distal nephrons and contribute to increased expression of renin in rat kidney. In addition, the mechanistic relevance of a decrease in NCC expression in distal tubules to the CI-induced nephrotoxicity appears to be supported by the observed phenotype of NCC null mice (Schultheis et al., 1998). NCC null mice showed no significant change in sodium levels in serum or urine but exhibited increased renin mRNA levels in the kidney and were unable to adequately compensate blood pressure in response to a sodium-depleted diet. Our hypothesis is supported further by previous observations in rats that low sodium intake can enhance chronic nephrotoxicity caused by CIs due to the activation of the RAS on low-sodium diet (Andoh et al., 1995, 1997; Elzinga et al., 1993; Shihab et al., 1997).
However, factors other than NCC cannot be ruled out as contributing to CI-induced activation of RAS. Reduced expression of Na-K-2Cl cotransporter (NKCC2) in tubular epithelial cells has also been associated with CI treatment–induced change in renin expression (Naesens et al., 2009). The regulation of NKCC2 by CI appears to be complex with differing effects reported and variance based on exposure time and/or tissue section (Lim et al., 2007). This complexity may explain why we did not see significant reduction in the overall mRNA level of NKCC2 in the current study. Interestingly, as seen in both patients with Gitelman’s syndrome (NCCT mutations) and patients with CI-related nephrotoxicity, polyuria, hypomagnesemia, and hyperreninemic hyperaldosteronism are also features of type I Bartter’s syndrome (NKCC2 mutations; Naesens et al., 2004, 2009). This suggests that both transporters may play a role in prolonged activation of RAS induced by CI.
The activity of NCC is also regulated by a signaling pathway involving the WNK kinase family, which is essential for blood pressure regulation in humans (Wilson et al., 2001; Xu et al., 2000). WNK1 affects NCC indirectly by inhibiting WNK4’s ability to reduce NCC surface expression. KS-WNK1, the renal isoform of WNK1, is expressed in epithelial cells predominately along the DCT and CNT of the kidney and is a negative regulator of WNK1 (McCormick et al., 2008; Richardson et al., 2008; Subramanya et al., 2006). Decrease in KS-WNK1 activates NCC in DCT by no longer inhibiting WNK1, allowing WNK1 to inhibit WNK4. However, our results suggest both KS-Wnk1 and NCC are downregulated by CI treatment as confirmed by qRT-PCR and immunohistochemistry, respectively. The consequence of KS-Wnk1 downregulation remains unclear given the fact that WNK1 and KS-WNK1 also regulate the activity of other transport proteins along the distal nephron in addition to NCC. More studies are needed to examine the effects of CI treatment on the WNK regulatory network.
CONCLUSIONS
The current study identified a group of genes with expression changes quantitatively correlated with the progression of rat kidney injury. The downregulation of these genes precedes pathological and/or functional changes; therefore, the expression pattern may be predictive of CI immunosuppressant–mediated kidney injury in rats. Combining expression changes in these CI-specific kidney injury–responsive genes with expression changes in general kidney injury–associated genes such as KIM1 or clusterin may provide a diagnostic signature or biomarker of CI-specific kidney injury. Among the genes, Slc12a3 and KS-Wnk1, which are important components of the sodium transport regulation network in the DCT, are potentially involved in the mechanism of CI-related RAS activation. How the activity of NCC and other transporters, under the regulation of the WNK kinase network, contribute to renin overexpression is not clear. However, our findings support the conclusion that sodium transport may be important in the mechanism of CI-induced chronic nephrotoxicity. Further study of transporter activities and the WNK kinase signaling cascade following CI treatment may provide for a more detailed understanding of the CI-related nephrotoxicity.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
Intramural Research Program of the National Institutes of Health; National Institute of Environmental Health Sciences; Cooperative Research and Development Agreement between National Institute of Environmental Health Sciences and Boehringer Ingelheim Pharma., Inc. (CRADA Z01ES023026).
Supplementary Material
Acknowledgments
The authors would like to thank Drs John Pritchard, Richard Irwin, and Scott Auerbach for their helpful comments.
References
- Abulezz S. KIM-1 expression in kidney allograft biopsies: improving the gold standard. Kidney Int. 2008;73:522–523. doi: 10.1038/sj.ki.5002772. [DOI] [PubMed] [Google Scholar]
- Aicher L, Meier G, Norcross AJ, Jakubowski J, Varela MD, Cordier A, Steiner S. Decrease in kidney calbindin-D 28kDa as a possible mechanism mediating cyclosporine A- and FK-506-induced calciuria and tubular mineralization. Biochem. Pharmacol. 1997;53:723–731. doi: 10.1016/s0006-2952(96)00772-1. [DOI] [PubMed] [Google Scholar]
- Andoh TF, Burdmann EA, Lindsley J, Houghton DC, William M, Bennett WM. Functional and structural characteristics of experimental FK 506 nephrotoxicity. Clin. Exp. Pharmacol. Physiol. 1995;22:646–654. doi: 10.1111/j.1440-1681.1995.tb02082.x. [DOI] [PubMed] [Google Scholar]
- Andoh TF, Lam TT, Lindsley J, Alpers CE, Bennett WM. Enhancement of chronic cyclosporine nephrotoxicity by sodium depletion in an experimental mouse model. Nephrology. 1997;3:471–478. [Google Scholar]
- Campistol JM. Long-term maintenance therapy with calcineurin inhibitors: an update. Transplant. Proc. 2010;42:S21–S24. doi: 10.1016/j.transproceed.2010.09.015. [DOI] [PubMed] [Google Scholar]
- Chou JW, Paules RS, Bushel PR. Systematic variation normalization in microarray data to get gene expression comparison unbiased. J. Bioinform. Comput. Biol. 2005;3:225–241. doi: 10.1142/s0219720005001028. [DOI] [PubMed] [Google Scholar]
- Chou JW, Zhou T, Kaufmann WK, Paules RS, Bushel PR. Extracting gene expression patterns and identifying co-expressed genes from microarray data reveals biologically responsive processes. BMC Bioinformatics. 2007;8:427. doi: 10.1186/1471-2105-8-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvoux C, Pageaux G. Immunosuppression in liver transplant recipients with renal impairment. J. Hepatol. 2011;54:1041–1054. doi: 10.1016/j.jhep.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Ecelbarger CA, Tiwari S. Sodium transporters in the distal nephron and disease implications. Curr. Hypertens. Rep. 2006;8:158–165. doi: 10.1007/s11906-006-0013-z. [DOI] [PubMed] [Google Scholar]
- Elzinga LW, Rosen S, Bennett WM. Dissociation of glomerular filtration rate from tubulointerstitial fibrosis in experimental chronic cyclosporine nephropathy: role of sodium intake. J. Am. Soc. Nephrol. 1993;4:214–221. doi: 10.1681/ASN.V42214. [DOI] [PubMed] [Google Scholar]
- Gillum DM, Truong L, Tasby J, Migliore P, Suki WN. Chronic cyclosporine nephrotoxicity—A rodent model. Transplantation. 1988;46:285–292. doi: 10.1097/00007890-198808000-00019. [DOI] [PubMed] [Google Scholar]
- Harris RC. The macula densa: recent developments. J. Hypertens. 1996;14:815–822. doi: 10.1097/00004872-199607000-00003. [DOI] [PubMed] [Google Scholar]
- Iijima K, Hamahira K, Kobayashi A, Nakamura H, Yoshikawa N. Immunohistochemical analysis of renin activity in chronic cyclosporine nephropathy in childhood nephrotic syndrome. J. Am. Soc. Nephrol. 2000;11:2265–2271. doi: 10.1681/ASN.V11122265. [DOI] [PubMed] [Google Scholar]
- Jennette JC, Olson JL, Schwartz MM, Silva FG. Heptinstall's Pathology of the Kidney. Philadelphia, PA: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- Johnson RWG, Kreis H, Oberbauer R, Brattstrom C, Claesson K, Eris J. Sirolimus allows early cyclosporine withdrawal in renal transplantation resulting in improved renal function and lower blood pressure. Transplantation. 2001;72:777–786. doi: 10.1097/00007890-200109150-00007. [DOI] [PubMed] [Google Scholar]
- Kivela JM, Raisanen-Sokolowski A, Pakarinen MP, Makisalo H, Jalanko H, Holmberg C, Lauronen J. Long-term renal function in children after liver transplantation. Transplantation. 2011;91:115–120. doi: 10.1097/tp.0b013e3181fa94b9. [DOI] [PubMed] [Google Scholar]
- Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol. Rev. 2007;59:251–287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- Langham RG, Egan MK, Dowling JP, Gilbert RE, Thomson NM. Transforming growth factor-beta1 and tumor growth factor-beta-inducible gene-H3 in nonrenal transplant cyclosporine nephropathy. Transplantation. 2001;72:1826–1829. doi: 10.1097/00007890-200112150-00019. [DOI] [PubMed] [Google Scholar]
- Lassila M. Interaction of cyclosporine A and the renin-angiotensin system; new perspectives. Curr. Drug Metab. 2002;3:61–71. doi: 10.2174/1389200023337964. [DOI] [PubMed] [Google Scholar]
- Lee CT, Huynh VM, Lai LW, Lien YHH. Cyclosporine A-induced hypercalciuria in calbindin-D28k knockout and wild-type mice. 2002. Kidney Int.62, 2055–2061. [DOI] [PubMed] [Google Scholar]
- Lee DBN. Cyclosporine and the renin-angiotensin axis. Kidney Int. 1997;52:248–260. doi: 10.1038/ki.1997.328. [DOI] [PubMed] [Google Scholar]
- Lim SW, Ahn KO, Sheen MR, Jeon US, Kim J, Yang CW, Kwon HM. Downregulation of renal sodium transporters and tonicity-responsive enhancer binding protein by long-term treatment with cyclosporin A. J. Am. Soc. Nephrol. 2007;18:421–429. doi: 10.1681/ASN.2006060664. [DOI] [PubMed] [Google Scholar]
- Lorenz JN, Weihprecht H, Schnermann J, Skott O, Briggs JP. Renin release from isolated juxtaglomerular apparatus depends on macula densa chloride transport. Am. J. Physiol. 1991;260:F486–F493. doi: 10.1152/ajprenal.1991.260.4.F486. [DOI] [PubMed] [Google Scholar]
- Lustig S, Stern N, Eggena P, Tuck ML, Lee DBN. Effect of cyclosporin on blood pressure and renin-aldosterone axis in rats. Am. J. Physiol. 1987;253:H1596–H1600. doi: 10.1152/ajpheart.1987.253.6.H1596. [DOI] [PubMed] [Google Scholar]
- Mayer AD, Dmitrewski J, Squifflet JP, Besse T, Grabensee B, Klein B, Eigler FW, Heemann U, Pichlmayr R, Behrend M, et al. Multicenter randomized trial comparing tacrolimus (FK506) and cyclosporine in the prevention of renal allograft rejection—a report of the European Tacrolimus Multicenter Renal Study Group. Transplantation. 1997;64:436–443. doi: 10.1097/00007890-199708150-00012. [DOI] [PubMed] [Google Scholar]
- McCormick JA, Yang CL, Ellison DH. WNK kinases and renal sodium transport in health and disease—an integrated view. Hypertension. 2008;51:588–596. doi: 10.1161/HYPERTENSIONAHA.107.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier-Kriesche HU, Li S, Gruessner RWG, Fung JJ, Bustami RT, Barr ML, Leichtman AB. Immunosuppression: evolution in practice and trends, 1994–2004. Am. J. Transplant. 2006;6:1111–1131. doi: 10.1111/j.1600-6143.2006.01270.x. [DOI] [PubMed] [Google Scholar]
- Naesens M, Kuypers DRJ, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin. J. Am. Soc. Nephrol. 2009;4:481–508. doi: 10.2215/CJN.04800908. [DOI] [PubMed] [Google Scholar]
- Naesens M, Steels P, Verberckmoes R, Vanrenterghem Y, Kuypers D. Bartter's and Gitelman's syndromes: from gene to clinic. Nephron Physiol. 2004;96:p65–p78. doi: 10.1159/000076752. [DOI] [PubMed] [Google Scholar]
- Nakatani T, Uchida J, Iwai T, Matsumura K, Naganuma T, Kuratsukuri K, Sugimura K. Renin mRNA expression and renal dysfunction in tacrolimus-induced acute nephrotoxicity. Int. J. Mol. Med. 2003;11:75–78. [PubMed] [Google Scholar]
- Nankivell BJ, Borrows RJ, Fung CLS, O'Connell PJ, Chapman JR, Allen RDM. Calcineurin inhibitor nephrotoxicity: longitudinal assessment by protocol histology. Transplantation. 2004;78:557–565. doi: 10.1097/01.tp.0000128636.70499.6e. [DOI] [PubMed] [Google Scholar]
- Naruse K, Takii Y, Inagami T. Immunohistochemical localization of renin in luteinizing hormone-producing cells of rat pituitary. Proc. Natl. Acad. Sci. U.S.A. 1981;78:7579–7583. doi: 10.1073/pnas.78.12.7579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijenhuis T, Hoenderop JGJ, Bindels RJM. Downregulation of Ca2+ and Mg2+ transport proteins in the kidney explains tacrolimus (FK506)-induced hypercalciuria and hypomagnesemia. J. Am. Soc. Nephrol. 2004;15:549–557. doi: 10.1097/01.asn.0000113318.56023.b6. [DOI] [PubMed] [Google Scholar]
- Nogare AL, Joelsons G, Pedrosa JAR, Veronese FJV, Goncalves LF, Manfro RC. Quantitative analyses of kidney injury molecule-1 messenger RNA in kidney transplant recipients with graft dysfunction. Transplant. Proc. 2010;42:473–474. doi: 10.1016/j.transproceed.2010.01.042. [DOI] [PubMed] [Google Scholar]
- Olyaei AJ, de Mattos AM, Bennett WM. Nephrotoxicity of immunosuppressive drugs: new insight and preventive strategies. Curr. Opin. Crit. Care. 2001;7:384–389. doi: 10.1097/00075198-200112000-00003. [DOI] [PubMed] [Google Scholar]
- Pallet N, Legendre C. Deciphering calcineurin inhibitor nephrotoxicity: a pharmacological approach. Pharmacogenomics. 2010;11:1491–1501. doi: 10.2217/pgs.10.137. [DOI] [PubMed] [Google Scholar]
- Perco P, Pleban C, Kainz A, Lukas A, Mayer G, Mayer B, Oberbauer R. Protein biomarkers associated with acute renal failure and chronic kidney disease. Eur. J. Clin. Invest. 2006;36:753–763. doi: 10.1111/j.1365-2362.2006.01729.x. [DOI] [PubMed] [Google Scholar]
- Pichler RH, Franceschini N, Young BA, Hugo C, Andoh TF, Burdmann EA, Shankland SJ, Alpers CE, Bennett WM, Couser WG, et al. Pathogenesis of cyclosporine nephropathy—roles of angiotensin-II and osteopontin. J. Am. Soc. Nephrol. 1995;6:1186–1196. doi: 10.1681/ASN.V641186. [DOI] [PubMed] [Google Scholar]
- Richardson C, Rafiqi FH, Karlsson HKR, Moleleki N, Vandewalle A, Campbell DG, Morrice NA, Alessi DR. Activation of the thiazide-sensitive Na+-Cl- cotransporter by the WNK-regulated kinases SPAK and OSR1. J. Cell Sci. 2008;121:675–684. doi: 10.1242/jcs.025312. [DOI] [PubMed] [Google Scholar]
- Sairanen J, Hotakainen K, Tammela TLJ, Stenman UH, Ruutu M. Urinary epidermal growth factor and interleukin-6 levels in patients with painful bladder syndrome/interstitial cystitis treated with cyclosporine or pentosan polysulfate sodium. Urology. 2008;71:630–633. doi: 10.1016/j.urology.2007.11.055. [DOI] [PubMed] [Google Scholar]
- Schultheis PJ, Lorenz JN, Meneton P, Nieman ML, Riddle TM, Flagella M, Duffy JJ, Doetschman T, Miller ML, Shull GE. Phenotype resembling Gitelman's syndrome in mice lacking the apical Na+-Cl- cotransporter of the distal convoluted tubule. J. Biol. Chem. 1998;273:29150–29155. doi: 10.1074/jbc.273.44.29150. [DOI] [PubMed] [Google Scholar]
- Schwarz C, Bohmig GA, Steininger R, Mayer G, Oberbauer R. Impaired phosphate handling of renal allografts is aggravated under rapamycin-based immunosuppression. Nephrol. Dial. Transplant. 2001;16:378–382. doi: 10.1093/ndt/16.2.378. [DOI] [PubMed] [Google Scholar]
- Shang MH, Yuan WJ, Zhang SJ, Fan Y, Zhang Z. Intrarenal activation of renin angiotensin system in the development of cyclosporine A induced chronic nephrotoxicity. Chin. Med. J. 2008;121:983–988. [PubMed] [Google Scholar]
- Shihab FS, Andoh TF, Tanner AM, Bennett WM. Sodium depletion enhances fibrosis and the expression of TGF-beta1 and matrix proteins in experimental chronic cyclosporine nephropathy. Am. J. Kidney Dis. 1997;30:71–81. doi: 10.1016/s0272-6386(97)90567-9. [DOI] [PubMed] [Google Scholar]
- Simon DB, Nelson-Williams C, Bia MJ, Ellison D, Karet FE, Molina AM, Vaara I, Iwata F, Cushner HM, Koolen M, et al. Gitelman's variant of Bartter's syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na-Cl cotransporter. Nat. Genet. 1996;12:24–30. doi: 10.1038/ng0196-24. [DOI] [PubMed] [Google Scholar]
- Skott O, Briggs JP. Direct demonstration of macula densa-mediated renin secretion. Science. 1987;237:1618–1620. doi: 10.1126/science.3306925. [DOI] [PubMed] [Google Scholar]
- Solez K, Vincenti F, Filo RS. Histopathologic findings from 2-year protocol biopsies from a US multicenter kidney transplant trial comparing tacrolimus versus cyclosporine: a report of the FK506 Kidney Transplant Study Group. Transplantation. 1998;66:1736–1740. doi: 10.1097/00007890-199812270-00029. [DOI] [PubMed] [Google Scholar]
- Stillman IE, Andoh TF, Burdmann EA, Bennett WM, Rosen S. FK506 nephrotoxicity: morphologic and physiologic characterization of a rat model. Lab. Invest. 1995;73:794–803. [PubMed] [Google Scholar]
- Subramanya AR, Yang CL, Zhu XM, Ellison DH. Dominant-negative regulation of WNK1 by its kidney-specific kinase-defective isoform. Am. J. Physiol. Renal Physiol. 2006;290:F619–624. doi: 10.1152/ajprenal.00280.2005. [DOI] [PubMed] [Google Scholar]
- Sun BK, Li C, Lim SW, Choi BS, Lee SH, Kim IS, Kim YS, Bang BK, Yang CW. Blockade of angiotensin II with losartan attenuates transforming growth factor-beta inducible gene-h3 (beta ig-h3) expression in a model of chronic cyclosporine nephrotoxicity. Nephron Exp. Nephrol. 2005;99:e9–e16. doi: 10.1159/000081793. [DOI] [PubMed] [Google Scholar]
- Thukral SK, Nordone PJ, Hu R, Sullivan L, Galambos E, Fitzpatrick VD, Healy L, Bass MB, Cosenza ME, Afshari CA. Prediction of nephrotoxicant action and identification of candidate toxicity-related biomarkers. Toxicol. Pathol. 2005;33:343–355. doi: 10.1080/01926230590927230. [DOI] [PubMed] [Google Scholar]
- Tufromcreddie A, Gomez RA, Norling LL, Omar AA, Moore LC, Kaskel FJ. Effect of CsA on the expression of renin and angiotensin type 1 receptor genes in the rat kidney. Kidney Int. 1993;43:615–622. doi: 10.1038/ki.1993.90. [DOI] [PubMed] [Google Scholar]
- Vitalone MJ, O'Connell PJ, Wavamunno M, Fung CLS, Chapman JR, Nankivell BJ. Transcriptome changes of chronic tubulointerstitial damage in early kidney transplantation. Transplantation. 2010;89:537–547. doi: 10.1097/TP.0b013e3181ca7389. [DOI] [PubMed] [Google Scholar]
- Wang EJ, Snyder RD, Fielden MR, Smith RJ, Gu YZ. Validation of putative genomic biomarkers of nephrotoxicity in rats. Toxicology. 2008;246:91–100. doi: 10.1016/j.tox.2007.12.031. [DOI] [PubMed] [Google Scholar]
- Williams D, Haragsim L. Calcineurin nephrotoxicity. Adv. Chronic Kidney Dis. 2006;13:47–55. doi: 10.1053/j.ackd.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Willams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, et al. Human hypertension caused by mutations in WNK kinases. Science. 2001;293:1107–1112. doi: 10.1126/science.1062844. [DOI] [PubMed] [Google Scholar]
- Xu BE, English JM, Wilsbacher JL, Stippec S, Goldsmith EJ, Cobb MH. WNK1, a novel mammalian serine/threonine protein kinase lacking the catalytic lysine in subdomain II. J. Biol. Chem. 2000;275:16795–16801. doi: 10.1074/jbc.275.22.16795. [DOI] [PubMed] [Google Scholar]
- Yang CL, Zhu XM, Wang ZH, Subramanya AR, Ellison DH. Mechanisms of WNK1 and WNK4 interaction in the regulation of thiazide-sensitive NaCl cotransport. J. Clin. Invest. 2005;115:1379–1387. doi: 10.1172/JCI22452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CW, Lee SH, Lim SW, Jung JY, Kim WY, Kim HW, Choi BS, Li C, Cha JH, Kim YS, et al. Cyclosporine or FK506 decrease mature epidermal growth factor protein expression and renal tubular regeneration in rat kidneys with ischemia/reperfusion injury. Nephron. 2002;92:914–921. doi: 10.1159/000065435. [DOI] [PubMed] [Google Scholar]
- Zhang PL, Rothblum LI, Han WK, Blasick TM, Potdar S, Bonventre JV. Kidney injury molecule-1 expression in transplant biopsies is a sensitive measure of cell injury. Kidney Int. 2008;73:608–614. doi: 10.1038/sj.ki.5002697. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.