Abstract
Stroke is a leading cause of long-term disability. Impairments resulting from stroke lead to persistent difficulties with walking and subsequently, improved walking ability is one of the highest priorities for people living with a stroke. In addition, walking ability has important health implications in providing protective effects against secondary complications common after a stroke such as heart disease or osteoporosis. This paper systematically reviews common gait training strategies (neurodevelopmental techniques, muscle strengthening, treadmill training, intensive mobility exercises) to improve walking ability. The results (descriptive summaries as well as pooled effect sizes) from randomized controlled trials are presented and implications for optimal gait training strategies are discussed. Novel and emerging gait training strategies are highlighted and research directions proposed to enable the optimal recovery and maintenance of walking ability.
Keywords: gait, walking, systematic review, meta-analysis, stroke, CVA, rehabilitation, treadmill, mobility, exercise
I. Walking ability of people with stroke
Stroke is a leading cause of long-term disability which results from brain cell damage due to either an interruption of the blood supply to the brain or hemorrhage into the brain tissue. As a result of an increasing older adult population, coupled with an ever improving acute phase survival rate, the absolute number of persons with stroke is increasing[1]. Of the individuals who survive, approximately 75 to 85% are ultimately discharged home [2,3]. Ninety percent of stroke survivors have some functional disability with mobility being a major impairment [4].
Although some individuals with stroke will have received some rehabilitation during the acute and sub-acute phases, rarely does rehabilitation extend beyond one year post-injury due to the belief that a plateau in functional recovery has been reached by this time and also due to a lack of resources for long term services. Impairments resulting from stroke, such as muscle weakness, pain, spasticity and poor balance can lead to a reduced tolerance to activity and further sedentary lifestyle. Community-dwelling individuals with stroke undertake extremely low levels of physical activity [5].
Although 65% to 85% of stroke survivors learn to walk independently by 6 months post stroke [6], gait abnormalities persist through the chronic stages of the condition. Walking endurance, as measured by the distance walked in 6 minutes (Six Minute Walk Test or 6MWT), remains the most striking area of difficulty among individuals with chronic stroke [7].
Patients with stroke spend more of their rehabilitation time practicing walking compared to all other activities [8]. Improved walking ability is one of the most often stated goals by people with stroke undergoing rehabilitation [9] and with those individuals living with stroke in the community [10].
Walking ability has important implications for health in the older adult population. Newman et al. [11] found that the ability and time to walk 400 meters was an important predictor for mortality, cardiovascular disease and mobility disability in 3075 community-dwelling older adults. Slow walking speed, the inability to walk a mile (1609 m) or inability to walk up a flight of stairs contribute to the transition to greater frailty or disability states in older adults [12]. Similarly, walking is an important predictor in people with stroke. The inability for independent walking is a predictor for discharge to nursing homes following a stroke [3] and increased probability of death [13]. Walking endurance as assessed by the 6MWT has been shown to relate to community reintegration in people with a stroke [7,14]. In addition, walking ability may also provide some protective effects against secondary complications common after a stroke such as osteoporosis and heart disease. For example, poorer walking endurance (6MWT) [15] or lower ground reaction forces during walking in people with stroke [16] correlate to lower paretic hip bone density, a condition which contributes to hip fracture risk. Furthermore, self-selected walking speed and walking endurance (6MWT) correlate to the maximal oxygen uptake (VO2peak) during a treadmill stress test [17] in people with stroke. VO2peak is the criterion measure of cardiovascular fitness and is related to the functional capacity of the heart [18].
II. Major determinants of ambulation function in stroke
Understanding the impairments that primarily determine walking ability of individuals with stroke will help with the development of effective gait training strategies. Of the common impairments, muscle strength, motor control, and balance appear to have the strongest relation with walking. Lower extremity muscle strength, especially that of the ankle plantarflexors, hip flexors, knee extensors, and knee flexors of the paretic leg and that of the knee flexors and ankle plantarflexors of the non-paretic leg, is moderately to highly correlated (r= .5~.8) with self-paced or fast walking speed and self-paced stair-climbing speed [19,20]. Motor control of the paretic lower extremity, measured by the Fugl-Meyer Assessment or Chedoke-McMaster Stroke Assessment, is moderately correlated (r= .5~.75) with self-paced or fast gait speed [19,21].
Standing postural control measures do not seem to be the most significant determinant of walking in regression analysis [22]. However, performance on the Berg Balance Scale, which measures postural control ability while performing functional tasks, is moderately to highly correlated (r= .66~.78) to walking endurance (6MWT) [17,21]. Michael et al. [5] reported that postural control ability while performing functional tasks accounted for 30% of the variance of ambulatory activity as measured by the number of steps performed per day.
Other impairments provide less of a contribution to walking. The contribution of cardiovascular fitness (measured by VO2peak) to walking endurance (6MWT) has been found to be moderate to high (r= .56~.84) in subacute stroke [23,24] and low to moderate (r=.4 to .57) for chronic stroke [15,17,25]. Low or nonsignificant correlations have been found between spasticity and passive joint stiffness of the knee extensors or ankle plantarflexors with walking speed [19,26,27]. Sensory impairment of the paretic lower extremity has low correlations to walking speed [19]. It is possible that walking speed requires more descending central drive than peripheral input for rhythmic movement generation.
III. Using the International Classification of Functioning (ICF) model to guide the development of gait training strategies and the selection of appropriate assessments of training effects
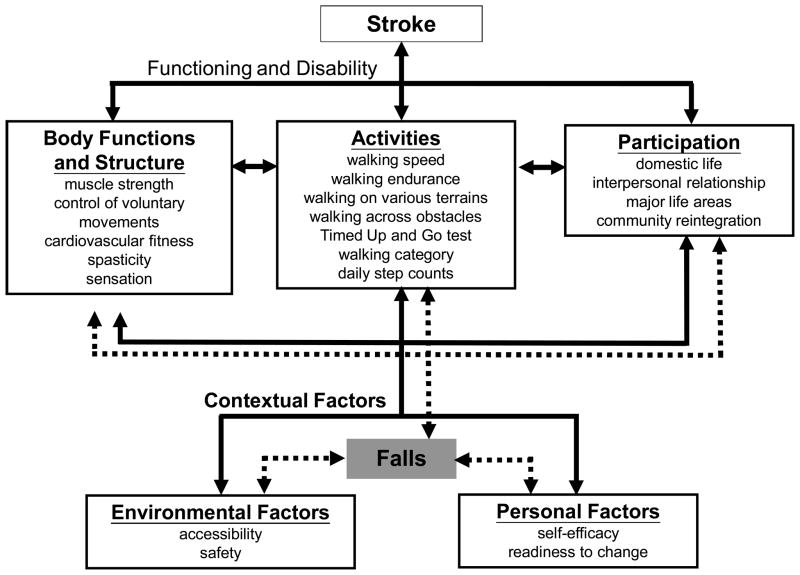
Given the importance of walking ability to people with stroke, we suggest the use of the International Classification of Functioning (ICF) model (Figure 1) [28] as a conceptual framework to assist with (1) the identification of primary factors resulting in particular gait problems post-stroke, (2) the selection of appropriate walking ability-related outcome measures that are reliable, valid and sensitive to changes, (3) the development of tailored training programs to enhance walking ability in individuals with stroke, and (4) the identification of potential environmental or personal factors that facilitate or impede an individual’s goal to improve walking ability. Researchers and clinicians can identify the body functions and structures that are major determinants of walking ability in stroke and select the appropriate outcome measures that reflect an individual’s ability to undertake activities and participation accordingly. Consideration of contextual (including environmental and personal) factors allows the identification of potential barriers and facilitators that may influence an individual to engage in exercise programs to improve walking ability.
Figure 1.
Use of the International Classification of Functioning (ICF) Model to guide the identification of primary factors resulting in particular gait problem, the selection of appropriate walking ability-related outcome measures that are reliable, valid and sensitive to changes, and the identification of potential environmental or personal factors that facilitate or impede an individual’s goal to improve walking ability.
Body Functions and Structures that are major determinants of walking ability in stroke primarily belong to the b7 (neuromusculoskeletal and movement-related functions), s7 (structures related to movement), b2 (sensory functions and pain), s2 (the eye, ear and related structures), b4 (functions of the cardiovascular, haematological, immunological and respiratory systems) and s4 (structures of the cardiovascular, immunological and respiratory systems) categories in the ICF model (Figure 1). When attempting to establish links between frequently used functional outcomes in stroke rehabilitation and the ICF model, Schepers and colleagues [29] noticed that these outcome measures often contain multiple constructs that can fit into different domains of the ICF model. Nevertheless, the outcome measures that reflect an individual’s ability to perform daily activity-related ambulatory and mobility tasks are primarily coded under d4 (mobility) at the Activities domain in the ICF model (Figure 1).
In particular, self-paced gait speed is the most common outcome measure for gait training strategies and reflects the ability to transport the body from one place to another in a timely manner. Perry et al. [30] suggested that individuals with stroke who can walk at a self-paced speed of 25m/min (~0.4 m/s) are more likely to be able to walk in the community. The 6MWT is the second most common walking measure used in clinical trials; it is a convenient tool to measure walking endurance and reflects an individual’s mobility limitation. The distance walked in the 6MWT by individuals with subacute or chronic mild-to-moderate stroke usually ranges from 200 to 300 meters [17,21,23], which is far shorter than that (approximately 400 meters) of healthy age-matched adults [31,32]. Timed stair ascending and descending tests have also been shown to be highly reliable in stroke [33] and are important tasks for community mobility. Although biomechanical studies have shown that people with stroke have an increased risk of falls while walking across obstacles [34], no clinical measures have been developed to rate an individual’s ability to walk across obstacles or over different terrains.
The Timed “Up & Go” Test (TUG) challenges dynamic balance ability in a series of locomotion-related mobility tasks, including rising up from a chair, walking, turning, and sitting down. The TUG is a highly reliable and responsive mobility measure in chronic stroke [32,33]. Individuals with chronic stroke who walk at a self-paced speed of 0.49 m/s require an average of 22.6 seconds to complete the TUG, much longer than that of the age-matched healthy individuals (mean= 9.1 s) [32].
Among the previously mentioned outcome measures, Flansbjer et al. [33] reported that the standard error of the measure (amount of change necessary to detect changes beyond the expected error) was 0.07 m/s for self-paced gait speed, 18.6 m for the 6MWT, 0.67 second for ascent of 12 steps, 0.90 second for descent of 12 steps and 1.14 second for the TUG for individuals with stroke. Self-paced walking speed, stair climbing speed, TUG, and 6MWT performance accounted for 32%, 32%, 40%, and 28% of the variance, respectively, of the participation domain from the Stroke Impact Scale [35].
In addition, walking categories (e.g., Modified Functional Walking Categories [30]), which classify an individual’s walking ability according to independence level, needs of a wheelchair, and the environment (home and community), could also reflect the ICF Activities domain. Approximately 53% to 68% of community-dwelling individuals with chronic stroke can walk in their immediate community environment, assisted or unassisted, whereas only 16% of these individuals can achieve unlimited community ambulation [30,36].
Daily step counts, a new method of assessing the amount of daily walking-related activities by utilizing step counters, also reflect the ICF Activities domain [5]. The number of steps walked per day for community-dwelling mild-to-moderate chronic (> 6 months post onset) stroke has high individual variability (60 to 6000 plus steps/day), but the mean value is approximately 2800 to 3000 steps/day, far below the daily step counts recorded from age-matched sedentary healthy older individuals (5000–6000 steps/day) [5,37].
Walking ability-related outcome measures at the Participation domain of the ICF model are rarely directly employed. However, as mentioned previously, outcome measures at the Activities domain, such as self-paced walking speed, stair climbing speed, TUG, and 6MWT, are all highly related to the participation domain of the Stroke Impact Scale. Thus, it is likely that improvement in these Activities domain outcomes could potentially lead to changes in Participation [35]. Indeed, recently Schmid and colleagues [38] revealed that patients with subacute stroke who gained sufficient gait velocity over a 3 month training period to change to a higher walking category (e.g., improved from household ambulation (< .4 m/s) to limited community ambulation (.4 – .8 m/s) scored better on the Participation domain of the Stroke Impact Scale. This study supports the suggestion made by Perry et al. [30] that an average self-paced walking speed of .4 m/s and of .8 m/s was the minimum criterion for limited and unlimited community ambulation, respectively. In addition, being able to overcome a curb height has also been suggested as one of the criteria of becoming an independent community walker [39].
The measure of daily step count could potentially fit into the Participation domain as well because it does provide an indication of how much an individual with stroke is engaged in walking in the real world situation. It has been found that the daily step counts improved from a mean of 1536 ± 106 steps/day at 2 weeks post rehabilitation discharge to 2765 ± 1677 steps/day 3 months later [40]. It would be of value to assess how the improvement in daily step counts influences an individual’s social participation, such as return to employment or to one’s regular roles in society.
In the Contextual Factors component of the ICF model, identifying personal and environmental factors may help determine which gait training interventions would be better suited for which individual with stroke to improve their walking ability. Two important personal factors which may be considered prior to a gait training intervention are the readiness to change (motivational factor) (e.g., Stages of Change Questionnaire [41]) and self-efficacy (e.g., Activities Balance Confidence score, Ambulatory Self-Confidence [42,43]) (Figure 1). Those who are psychologically ready to change may be more likely to undertake and adhere to an intervention program [44]. Self-efficacy scores could help determine whether an individual has the confidence to undertake an unsupervised, versus a supervised program. Environmental evaluation might measure the accessibility to the training intervention (including the frequency of inclement weather, accessibility to transportation and community services) as well as the safety of the home or intervention setting (Figure 1).
Finally, falls (and their resulting injuries) are an adverse event that relates to all levels of the ICF model (Figure 1). Falls occur frequently during walking in people with stroke [45] and are influenced by the surrounding environment, as well as by impairments and restrictions in the Activities and Participation domain. Improvement of Body Functions such as muscle strength and balance can reduce falls, while a single fall can result in a reduction in self-efficacy and restriction in participation. Falls should be monitored to quantify adverse effects and when sample sizes permit, it would be ideal to assess this variable through methods such as monthly fall diaries and follow-up phone calls.
Given the importance of gait speed and endurance (6MWT), in addition to their frequency of reporting, we would recommend that these two measures be the minimal outcome measures assessed in a gait training intervention. Common outcome measures will facilitate the undertaking of meta-analyses to evaluate the effects of gait training across trials. Some walking category outcome measure is essential for those individuals with stroke who might change from dependent to independent walking status to capture this meaningful transition.
III. Training strategies to improving walking ability in people with stroke
A systematic review of training strategies to improve walking ability in people with stroke was undertaken. A keyword search using Medline (1950 to June 2007), CINAHL (1982 to June 2007), and Cochrane Collaboration was undertaken using the terms gait, locomotion, walking, ambulation combined with stroke or cerebrovascular disease (CVA). In addition, hand searching of references from these articles was undertaken. Primary importance was placed on recent meta-analyses and systematic reviews if they were available. Individual randomized controlled trials (RCTs) were also assessed. For areas with multiple RCTs where current meta-analyses were not available, a pooled standardized effect size with confidence intervals (CI) was constructed across trials based on methods reported previously [46].
Exercise is the most common therapeutic intervention currently used to improve walkings. Thus, the review focused on exercise interventions and did not evaluate the literature pertaining to assistive devices (e.g., canes) or modalities (e.g., functional electrical stimulation, biofeedback). Following the search (n=1482), studies were eliminated if they did not involve a randomized controlled trial, involved populations other than adults with stroke, reported no walking outcome measures, were not journal publications (e.g., abstracts), or were not written in English, There were 9 systematic reviews or meta-analyses which included gait outcomes [47–55]. Common interventions were collated and the literature was sorted into the topics of neurodevelopmental techniques (n=7) [56–62], strength training (n=5) [63–67] and task-specific training (treadmill (n=17) [58,68–83] and intensive mobility training (n=10) [84–93].
A. Neurodevelopmental approaches to improve walking ability
Traditional approaches to stroke recovery have a focus on neurofacilitation or neurodevelopmental techniques (NDT) to inhibit excessive tone, stimulate muscle activity if hypotonia is present and to facilitate normal movement patterns through hands-on techniques. Practice based on the framework advocated by Berta Bobath remains the predominant physical therapy approach to stroke patients in the UK [94], and is also common in many other parts of the world, including Canada, United States, Europe, Australia, Hong Kong and Taiwan. The Bobath framework has evolved from its original foundations, however, therapists surveyed on the core Bobath elements still emphasize normal tone and the necessity of normal movement patterns to perform functional tasks [94].
Paci [47] evaluated 6 trials (of which 3 were controlled trials) which used NDT/Bobath Concept for gait re-training post-stroke and concluded that neurofacilitation programs were equivalent or inferior to other approaches to improving walking ability. One study was a randomized controlled trial [56] which compared NDT to EMG biofeedback but found no differences between groups for walking speed. Two non-randomized controlled trials found NDT to be inferior for improving walking speed and walking category compared to 3 weeks body-weight supported treadmill training (BWSTT) during inpatient rehabilitation [95] or BWSTT with functional electrical stimulation in chronic stroke [96]. Systematic reviews by Pollock et al. [51] and Van Peppen et al. [52] also found no evidence for traditional neurological treatment approaches in terms of walking ability. Recent individual trials have reported similar findings. Thaut et al. [62] reported that 3 weeks of daily rhythmic auditory stimulation improved walking speed more than NDT/Bobath training. van Vliet et al. [61] reported that a Bobath or a motor learning approach to inpatient stroke physical therapy produced equivalent results in walking speed at approximately 1, 3, and 6 months following stroke onset. Table 1 presents the results of 7 randomized controlled trials [56–62] which compared NDT/Bobath to other interventions and measured walking outcomes. Not one of the seven studies showed significant gait improvements with NDT/Bobath over the other interventions. Our meta-analysis of the four studies with gait speed [56,58,59,62] showed that the alternative interventions (e.g. treadmill training, functional training) had a large effect on improving gait speed compared to Bobath/NDT (significant heterogeneous effect size; d=−0.90, 95% CI −1.80 to −0.01, p=0.02).
Table 1.
Effect of neurophysiological techniques (NDT/Bobath) on walking.
| Author, Year | Subjects | Program description | Results |
|---|---|---|---|
| Mulder et al. [56] 1986 |
N=12 Had completed rehabilitation Lacked dorsiflexion function |
5 weeks, 3X/week Group 1 (n=6) NDT/Bobath Group 2 (n=6) EMG visual feedback of tibialis anterior and peroneus longus during lying and standing. |
No group differences in gait speed. |
| Dickstein et al. [57] 1986 |
N=131 Took part and completed 6 weeks inpatient rehabilitation |
Group 1 (n=38) Bobath Group 2 (n=57) Conventional (functional activities) Group 3 (n=36) Proprioceptive neuromuscular facilitation |
No group differences in indoor ambulatory independence at 6 weeks post admission. |
| Richards et al. [58] 1993 |
N=27 Inpatient rehabilitation |
All groups received conventional rehabilitation, plus one of three physiotherapy groups: Group 1: Early and intensive therapy (treadmill, isokinetic device, tilt table, limb-load monitor) (n=10) Group 2: Early neurophysiological physiotherapy (n=8) Group 3: Neurophysiological physiotherapy (n=9) Groups 1 and 2 started mean 8 days and Group 3 started 13 days post-stroke. |
Gait speed: Intensive group had faster (but not significant) speed at 6 weeks post-stroke compared to other 2 groups. Note: percent of time on treadmill was not described. |
| Gelber et al. [59] 1995 |
N=27 < 1 month post-stroke |
Group 1: NDT (n=15): inhibition of abnormal muscle tone, emphasis on normal movement patterns with progression to functional activities Group 2 (n=12): Functional training (passive range of motion, progressive resistive exercises even in the presence of spasticity, early use of assistive device and bracing) |
Gait speed: Bobath Group lost gait speed at discharge (−5%), 6 months (−45%) and 12 months (−62%); Functional Group improved from baseline speed at discharge (+17%), 6 months (+133%) and 12 months (+133%). More than 60% missing data due to inability to walk. Number of days to walk without device was 57% longer for NDT group*. |
| Langhammer and Stranghelle [60] 2000 |
N=61 First-ever stroke |
Physiotherapy, 5X/week, minimum 40 min Interdiscplinary care (doctors, nurses, occupational therapists, etc). Group 1: Bobath (n=28) Group 2: Motor Relearning (n=33) |
At 2 weeks after the first assessment and 3 months post-stroke, there was no group difference in the trunk, balance and gait subscale of the Sodring Motor Evaluation Scale. Motor Relearning had higher Motor Assessment Scale (includes walking) at 3 months compared to Bobath Group |
| Van Vliet et al. [61] 2005 |
N=120 Stroke inpatients, < 2 week post-stroke |
Sessions were a median of 23 minutes for total of 365 min Group 1: Bobath (n=60) Group 2: Motor learning (n=60) Assessment at 1, 3, 6 months post-entry |
No group differences for any outcome including 6MWT, Rivermead Motor Asessment, Motor Asessement Scale (used area under curve analysis) at 1, 3 and 6 months after baseline. |
| Thaut et al. [62] 2007 |
N=78 < 4 weeks post-stroke Can walk 5 strides with arm support by therapist |
3 weeks, 30 min, 5X/week of gait training Group 1: Bobath/NDT (n=35) Group 2: Rhythmic auditory stimulation (n=43): metronome, cueing and music during walking and adaptive gait tasks (e.g., ramps) |
Gait speed*: Bobath +56%; Auditory Group + 145%. |
+/− % indicates percent increase or decrease from baseline.
indicates significant comparison reported in study.
The lack of effect from NDT practice compared to other therapies may be in part from the diverse opinions even among experienced practitioners as to what constitutes NDT [94]. Marsden and Greenwood [97] commented that it would help to have a more rigorous definition of these physical therapy practices which include the exact content, schedule and intensity of the practice. Others have questioned the theoretical foundation of neurodevelopmental practice; Lennon et al. [98] found that the walking patterns of individuals with stroke following outpatient Bobath treatment did not result in more normal movement patterns (a major goal of the Bobath Concept). However, patients did improve in temporal gait parameters.
B. Strength training to improve walking ability
Bobath [99] advocated that decreased muscle function was not due to weakness but to the opposition of spastic antagonists and that strenuous activity would increase spasticity and reinforce abnormal movement. There was a presumed thought that patients with stroke were “fragile neurologically and physiologically and may not tolerate intensive exercise programs” [100]. The last decade has demonstrated that intensive training to improve walking has not been found to be associated with increases in spasticity. A 6 week isokinetic muscle strengthening program of the knee flexors and extensors was found to improve walking speed in people with stroke, but leg muscle spasticity was not increased [101]. In fact, Welmer et al. [102] followed a consecutive sample of 66 people who had a first stroke and noted that few people exhibited leg spasticity 18 months after their stroke. In addition, the importance of muscle strength has been fueled by the many studies which have demonstrated strong correlations between walking speed and lower limb muscle strength of the paretic side.
Several recent systematic reviews or meta-analyses [49,52–55] have evaluated the effect of graded muscle strengthening on walking ability post-stroke using 3 to 6 RCTs. Both subacute inpatient and chronic patients are represented in these studies. These studies generally used weights or machines (e.g., isokinetic dynamometer or leg press machine) to add a resistive component. Thus, these studies do not utilize task-specific muscle strengthening which are described later in this review. In general, the authors of these reviews conclude that graded strength training can improve the ability to generate force, but does not transfer to improvements in walking.
The meta-analysis by Van Peppen et al. [52] reported a nonsignificant effect size (0.32) in favor of strength training on walking speed based on 3 RCTs [63–65]. van de Port et al. [53] used one additional study [67] in their meta-analysis and reported an effect size of −0.13 and concluded that lower limb strengthening did not improve walking speed. Similarly, our meta-analysis of five studies [63–67] showed that strength training did not have an effect on walking speed (non-significant homogeneous effect size; d=−0.11, 95% CI −.46 to 0.24, p=0.28) (Table 2). In addition, two studies using participants with chronic stroke found no effect of muscle strengthening on stair-climbing ability [64,67].
Table 2.
Effect of muscle strengthening on walking.
| Author, Year | Subjects | Program description | Results |
|---|---|---|---|
| Glasser [63] 1986 |
N=20 3–6 months post-stroke |
Strength Group (n=10): 5 week physical therapy with kinetron isokinetic LE exercise (resisted reciprocal hip/knee flexion during semi-sitting posture progressed from 10–30 minutes of the 2 one hour physical therapy sessions/day) Control Group (n=10): 5 week inpatient physical therapy (5X week, 2 one hour sessions/day) |
No group difference in functional ambulation profile (includes speed, number of steps, step length) |
| Kim et al. [64] 2001 |
N=20 > 1 year post-stroke Able to walk independently with or without devices. |
Strength Group (n=10): 6 week isokinetic paretic strengthening (hip, knee, ankle). Control Group (n=10): 6 week passive exercise |
No group difference in gait or stair speed. |
| Bourbonnais et al. [65] 2002 |
N=25 chronic stroke |
6 week, 3X week, visual feedback of multi-joint, multi-directional isometric force generation (coordination exercises). Strength Group (n=12): varying combinations of hip, knee and ankle forces (40–90% MVC). Control Group (n=13): varying combinations of shoulder, elbow, and grip forces (progress from 20–60% MVC). |
Gait speed*: Leg strength group +25%, Control +0%* 2 min walk*: Leg strength group +17%, Control +1%* No group difference in Timed up and Go Test. |
| Moreland et al. [66] 2003 |
N=133 (multi-centre) < 6 months post-stroke Inpatient rehabilitation |
Strength Group (n=54): Conventional + resistive exercises with weights, 30 minutes, 3X/week Control Group (n=52): Inpatient conventional physical therapy |
No group difference in 2 minute walk test at 4 weeks, discharge and 6 months post discharge. |
| Ouellette et al. [67] 2004 |
N=42 6 months to 6 years post-stroke Mild to moderate stroke Community-dwelling Independent ambulator |
12 weeks, 3X/week Strength Group (n=21): 3 sets of 8–10 reps, at 70% at 1 repetition maximum Resisted exercises of unilateral knee extension, ankle dorsiflexion, and plantarflexion of paretic and non-paretic side; Seated bilateral leg press Control Group (n=21): range of motion exercises |
No group difference in 6MWT, stair climb, self-paced and maximal gait speed. |
+/− % indicates percent increase or decrease from baseline.
indicates significant comparison reported in study.
Kim et al. [64] used a particularly rigorous double-blind randomized controlled design. Both groups used an isokinetic dynamometer for 3 times a week for 6 weeks. However, the control group was put on “passive mode” so experienced the same range of motion, but without the resistance. Both the strengthening and passive group improved their walking speed, but there were no group differences. This study highlights the importance of an appropriate control group. Several factors may have contributed to the improvement in walking ability of the control group such as the focus on the leg, attention from the therapist, familiarity with the outcome measures and increased activity from coming three times a week to the centre.
Thus, it appears that graded muscle strengthening can improve the ability to generate force, but this improvement in strength does not appear to transfer to improved walking ability. These results contrast to the older adult literature which has found that graded strengthening can improve walking speed [103] and stair climbing time [104]. Altered motor and sensory coordination following stroke may require that task-specific practice be utilized to take advantage of strength gains from resistive training. An optimal trial may involve strength training plus walking practice or incorporate strengthening within functional tasks. Muscle strengthening should not be disregarded in stroke rehabilitation as there are other positive effects from strength training. For example, muscle strengthening can improve bone strength which is particularly important since osteoporosis is common on the paretic side [105] and there is a seven-fold increase in fracture risk for individuals with stroke within the first year [106].
C. Task-specific training to improve walking ability
Stroke results in a number of deficits which may affect motor unit recruitment and rate coding, proprioception, viscoelastic characteristics of muscle and connective tissues, sense of effort, postural reflexes, vestibular function and vision. Although the debate continues as to how motor representations are coded in the nervous system [107], repetitive practice of a task may facilitate the development of new motor programs or refinement of existing programs necessary to accommodate to these deficits. Repetitive practice may facilitate the integration of remaining and altered sensory and motor systems, given the new state following a stroke.
In the literature, there exists two main approaches to task-specific training to improve walking. One approach is treadmill training with or without a harness system to provide bodyweight support so that walking can be practiced repetitively under controlled conditions. The other approach is intensive practice of a wide variety of functional mobility tasks (e.g., walking, rising from a chair, turning, stepping over an obstacle). Challenge to these mobility tasks are provided by incorporating endurance components, functional strengthening, balance challenges and varying speed requirements.
Treadmill training
The benefits from treadmill training may have neurophysiological underpinnings; spinalized animals demonstrate coordinated activation of spinal neural circuits from the alternating limb movements facilitated from a treadmill [108]. Treadmill practice could also be considered “forced use” which maximizes the use of the paretic limb through a large number of steps, and consequently a greater amount of load-bearing and activation of the paretic muscles, particularly at faster speeds. Lastly, treadmills with a body-weight support system may enable lower functioning individuals who cannot be safely supervised using traditional therapy methods to undertake early walking practice.
Moseley et al. [50] undertook a meta-analysis which included 15 treadmill training RCTs post-stroke and concluded that there was no statistical difference between treadmill training (with or without body weight support) compared to other physical therapy interventions for walking speed or category. There was a non-significant trend for people independent in walking to walk faster following BWSTT. Treadmill training was at least as effective as other gait interventions. Previous reviews concur with the conclusions of Moseley et al. [50]. Van Peppen et al. [52] reported a significant standardized effect size (0.70) for three BWSTT RCTs [68,69,73] for walking endurance (30% mean change), but this was not significant for walking speed or walking category. For treadmill training without BWSTT, Van Peppen et al. [52] reported a significant effect size (1.09) for walking ability, but a non-significant effect size (0.58) for walking speed. From their systematic review of 6 RCTs of BWSTT and 2 RCTs without BWSTT, Teasell et al. [49] concluded that there was conflicting evidence that treadmill training with or without BWSTT resulted in improvements in gait performance over standard treatments. Although the evidence supporting treadmill training appears to be conflicting, two recent clinical practice guidelines [109,110] recommended that BWSTT be included as an intervention for stroke.
Previous meta-analyses have not differentiated between clinical trials involving sub-acute and chronic subjects or the specific type of control comparison. Our search produced 17 treadmill studies (Table 3) and our meta-analysis of nine sub-acute stroke studies [58,69–73,76,78,111] which compared treadmill training (with or without body-weight support) to an equivalent amount of conventional physiotherapy showed that treadmill training did not have a significant effect on walking speed (non-significant heterogeneous effect size; d=0.23, 95% CI −0.14 to 0.59, p=0.11). Note for the studies with three different groups, we combined the early and late conventional physiotherapy group of Richards et al. [58] and also combined the structured speed-dependent treadmill group with the limited progressive treadmill training of Pohl et al. [72] for this meta-analysis.
Table 3.
Effect of treadmill training on walking.
| Author | Subjects | Program description | Results |
|---|---|---|---|
| Early post-stroke (inpatient rehabilitation) | |||
| Richards et al. [58] 1993 |
N=27 Inpatient rehabilitation |
All groups received conventional rehabilitation, plus one of three physiotherapy groups: Group 1: Early and intensive therapy (treadmill without body weight support, isokinetic device, tilt table, limb-load monitor) (n=10) Group 2: Early neurophysiological physiotherapy (n=8) Group 3: Neurophysiological physiotherapy (n=9) Groups 1 and 2 started mean 8 days and Group 3 started 13 days post-stroke. |
Gait speed: Intensive group had faster (but not significant) speed at 6 weeks post-stroke compared to other 2 groups. Note: percent of time on treadmill was not described. |
| Visintin et al. [68] 1998 Barbeau et al. [125] 2003 |
N=100 Inpatient rehab Mod-severe impairment as initial mean gait was < 0.2 m/s |
6 weeks gait training, 20 min, 4X/week in addition to standard of care Group 1 (n=50): BWSTT with 1 therapist for support and 1 therapist to assist with stepping movement. Started body-weight between 0–40%. Task was progressed with increasing treadmill speed and reducing body-weight. Group 2 (n=50): Control Group of Treadmill without body-weight support |
At 6 weeks Gait speed*: BWSTT+79%, control+56% Walking endurance distance*: BWSTT+230%, control+127% BWSTT allowed earlier gait training At 3 months: group differences persisted. |
| Kosak and Reding [69] 2000 |
N=56 Inpatient rehabilitation Moderate assistance for walking Mean 40 days post-stroke |
Both groups had 45 min conventional therapy, 5X/week Group 1 (n=22): additional 45 min BWSTT 5X/week. 2 person assist with leg advancement and foot placement. Group 2 (n=34): additional 45 min aggressive bracing assisted walking |
No group differences for gait speed or distance over 6 weeks. |
| Nilsson et al. [70] 2001 |
N=73 Within 8 weeks post-stroke 10 m walk > 14 seconds |
Group 1 (n=36): BWSTT, 30 min, 5X/day with 1 or 2 person assist with leg advancement plus conventional rehabilitation. Gradual reduction of support and increase in speed. Group 2 (n=37): Control. Individual walking practice (motor re-learning) plus conventional rehabilitation. |
No group difference for gait speed and functional ambulation category at discharge and 10 month follow-up. |
| Laufer el al. [71] 2001 |
N=25 Inpatient rehabilitation < 90 days post-stroke Can walk on treadmill 0.2 km/hr |
Group 1 (n=13): 3 weeks treadmill training without body weight support (hip flexion and foot placement assistance from therapist) + conventional rehabilitation. Group 2 (n=12): 3 weeks overground gait training + conventional rehabilitation Both groups progressed from 4 to 8 min/day of walking. |
Functional ambulation category*: Treadmill+75%, Overground+43% No group difference in walking speed. |
| Pohl et al. [72] 2002 |
N=60 Inpatient rehabilitation > 4 weeks post-stroke No or little spasticity Able to walk without assistance |
4 weeks, 12 sessions Group 1: 30 min sessions of Structured speed-dependent treadmill training (STT) (n=20). 10 sec bouts of maximal waking speed with 10% increases on next bout if they felt safe. Group 2: 30 min sessions of Limited progressive treadmill training (LTT) (n=20) Up to 5% increase in speed each week. Group 3: 45 min sessions of Bobath/NDT gait training (n=20) Up to 10% body weight support provided in first 3 sessions of Group 1 and 2. In addition, all 3 groups received 8 sessions of 45 min conventional physiotherapy. |
Gait speed*: STT+167%, LTT+85%, Bobath+47% Functional ambulation category*: STT+35%, LTT+24%, Bobath+10% |
| Da Cunha Filho et al. [73,126] 2001;2002 |
N=15 (2 drop-outs) Inpatient rehabilitation (< 6 weeks post-stroke) Can take 1 or more steps with or without assistance |
Group 1 (n=6): 20 min, 5X/week BWSTT (up to 30% body support and progressively decreased with increases in speed) + conventional interdisciplinary rehab Group 2 (n=7): conventional rehab Treatment time same in each group. |
No group differences for gait speed, 5MWT, gait energy expenditure or gait energy cost, Locomotor score of FIM or Functional Ambulation Category. |
| Werner et al. [74] 2002 |
N=30 Non-ambulatory 4–12 weeks post-stroke Inpatient rehabilitation |
Cross-over design. 6 weeks of A-B-A (n=15) or B-A-B (n=15) where: RX A: 2 weeks, 15–25 min, 5X/week, Gait trainer with body weight support (has reciprocally moving footplates and 1 person assist) RX B: 2 weeks, 15–25 min, 5X/week, BWSTT with 2 person assist Conventional physiotherapy was also provided to all subjects during the 6 weeks. |
No group effects for gait speed. Less therapist assistance required for gait trainer. |
| Werner et al. [75] 2002 |
N=28 8 weeks to 9 months post-stroke Inpatient rehabilitation Non-ambulatory |
3 weeks of conventional therapy followed by: Group 1 (n=14): 3 weeks BWSTT 30 min, 5X/week, plus 40 min physiotherapy Group 2 (n=14): 3 weeks BWSTT 30 min, 5X/week |
Functional ambulation category*: Combined group+390%; BWSTT only+100% No group effect for gait speed. Note: Therapy hours per group differed. |
| Eich et al. [111] 2004 |
N=50 Inpatient rehabilitation Able to walk 12 m with or without assistance Can pedal at least 50 W |
Group 1 (n=25): 6 weeks, 30 min, treadmill (maximum 15% body-weight) + 30 min Bobath physiotherapy (tone-inhibiting and gait preparatory maneuvers, walking practice on floor and stairs) Group 2 (n=25): 6 weeks, 60 min Bobath physiotherapy |
Gait speed*: Treadmill Group+78%, Control+36% 6MWT*: Treadmill Group+84%, Control+51% Differences persisted at 18 weeks post-admission. |
| Richards et al. [76] 2004 |
N=63 Inpatient rehabilitation Gait speed between .1 and .6 m/s |
Both groups had interdisciplinary rehabilitation. Group 1 (n=32): gait training with tilt table, Kinetron and treadmill (no body weight support). Treadmill use was mean 11 min/session. Group 2 (n=31): conventional physiotherapy (no treadmill) |
No group differences in gait speed at discharge or after 2 months therapy. |
| Yagura et al. [77] 2006 |
N=49 < 3 months post-stroke Inpatient rehabilitation Required physical assistance for gait after 4 weeks rehabilitation. |
6 weeks (week 5–10 of inpatient rehab), 20 min, 3X/week Group 1 (n=23): BWSTT with facilitation (manual facilitation of hip and pelvis movements to ensure stable swing and stance) Group 2 (n=26): BWSTT with assistance to paretic foot |
No group difference in gait speed or FIM gait score at week 10 or week 16. Facilitation group required more resources. |
| Husemann et al. [78] 2007 |
N=30 Need for personal assistance in walking. 28–200 days post-stroke Inpatient rehabilitation |
Group 1 (n=16): Treadmill training with Robotic-driven orthosis (Lokomat), 30 min, 5X/week Group 2 (n=14): Conventional physiotherapy (treadmill training added if possible), 30 min, 5X/week Both groups also had an additional 30 min conventional physiotherapy. |
No group difference in gait speed or Functional ambulatory category after 4 weeks of training. Lokomat group improved duration of single limb support time more than conventional physiotherapy. |
| > 6 months post-stroke | |||
| Sullivan el al. [79] 2002 |
N=24 > 6 months post-stroke Can walk 10 at least with standby physical assistance. |
12 sessions of 20 min (4–5min bouts) BWSTT over 4–5 weeks training Group 1 (n=8): fast (2.0 mph) BWSTT Group 2 (n=8): slow (.5 mph) BWSTT Group 3 (n= 8): variable speed BWSTT |
Gait speed*:fast+26% different from slow+11% and variable+13% Improvement continued at 1 month and maintained at 3 month follow-up. |
| Ada et al. [80] 2003 |
6 months to 5 years post-stroke Walked independently 10 m N=27 |
3X/week, 4 weeks, 45 min Group 1 (n=13): treadmill without body weight support. Challenges to speed, step length, balance and cognitive tasks (80–50% of time). Overground walking included varying directions, speeds and step lengths, stairs, balance challenges, outdoor circuit (curbs, slopes, varying terrain) (20–50% of time) Group 2 (n=14): Control. Low intensity home exercise of stretching and strengthening leg muscles, recommended daily walk, balance exercises. |
Gait speed*: Treadmill Group+21%, Control +6% 6MWT*: Treadmill Group+ 28%, Control +5% At 3 months post-intervention, these group differences persisted. |
| Jaffe et al. [81] 2004 |
N=20 > 6 months post-stroke Could walk independently or with supervision |
Group 1 (n=10): 6-1 hour sessions over 2 weeks treadmill training stepping over foam objects Group 2 (n=10): 2 weeks treadmill training stepping over virtual objects with feedback Neither group used body weight support on the treadmill. |
Maximum gait speed*:Virtual +20%% Foam+12% No group differences for self-selected gait speed or 6MWT Both groups retained improvements 2 weeks post-training. |
| Macko et al. [82] 2005 |
N=61 > 6 months post-stroke Could complete 3 min treadmill walking at 0.22 m/sec |
40 min sessions 3X/week for 6 months Group 1 (n=25): Treadmill aerobic training (without body weight support). Progressed from 40% to 60–70% HRR at 5% heart rate reserve (HRR) every 2 weeks. Group 2 (n=20): Control Group. Stretching and 5 min low intensity treadmill walking (30–40% HRR). |
6MWT*: Treadmill Group+21%, Control+2% Self-report walking distance scale*:Treadmill Group+56%, Control+12% No group differences for usual or fast gait speed |
| Yen et al. [83] 2007 |
N=14 > 6 months post-stroke Could walk 10 m with or without assistance |
Group 1 (n=7): 4 weeks of 50 min, 2–5X/week of general physical therapy and 30 min, 3X/week of BWSTT with 1 or 2 assistants. Body weight support was less than 40% and was decreased with progression. Group 2 (n=7): Control group of general physical therapy (4 weeks, 50 min, 2–5X/week of stretching, strengthening, overground gait) |
Gait speed*: Treadmill Group+34%, Control +11% Note: Therapy hours per group differed. |
+/− % indicates percent increase or decrease from baseline.
indicates significant comparison reported in study.
Our meta-analysis of three chronic stroke studies [79,80,82] which compared treadmill training (with or without body-weight support) to an equivalent amount of physical therapy or slow treadmill training produced a significant effect on walking speed (small homogenous effect size; d=0.31, 95% CI −0.06 to 0.69, p=0.05) (Table 3). Note for the Sullivan et al. study [79], we combined the fast and variable speed treadmill groups for this meta-analysis. The number of the randomized controlled trials with common walking outcome measures in the literature is still scant and this is apparent by the different meta-analyses which change from significant to non-significant status (or vice versa) based on one or two additional studies. Hence, the results from treadmill training are not yet robust.
Four of the sub-acute stroke treadmill studies [69,70,77,112] did not produce any greater improvement over conventional treatment. Such findings echo the systematic review of BWSTT in spinal cord injury which concluded that there is strong evidence that functional ambulation outcomes following BWSTT are comparable to an equivalent intensity of overground gait training in sub-acute spinal cord injury [113]. Perhaps the equivalent effectiveness of treadmill training to other treatments is due to the nature of the control group intervention. For most trials, the standard of care gait training served as a control group. Current physical therapy practices have evolved over time with a greater emphasis on early task-specific gait re-training with more repetition which may provide a comparable stimulus for gait recovery. Given the greater intensity of conventional therapy, perhaps treadmill training requires faster speeds or longer durations to be effective over conventional therapy. There is some evidence that BWSTT applied at fast or maximal walking speeds [72,79] in people with stroke who can walk without physical assistance is more effective than BWSTT at slower speeds or conventional gait training.
An alternative explanation for the equivalent effects is that BWSTT may offer advantages in promoting a larger number of steps, but overground gait training provides a more natural stimulus to challenge different components necessary for walking. Successful overground walking requires anticipatory postural control (e.g., step around another person or over an obstacle), in addition to reactive control (e.g., respond to a slip, trip or push). Furthermore, visual sampling is task-specific and critical for negotiating realistic environments [114]. Treadmill training may not allow participants to experience the normal postural demands or visual flow stimuli that occurs during walking. The many functional variations in gait cannot be easily practiced on the treadmill such as turning, rising from a chair and walking, starting or stopping which are all requirements of common gait outcome measures like the 6MWT or TUG. Lastly, balance confidence has been shown to be an important factor contributing to walking speed [42]. One mechanism for enhancing self-efficacy is through positive experiences and successful execution of relevant tasks [115]. Thus, successful overground practice of the variety of tasks that represent community walking (e.g., stepping up a curb, walking around a person, walking in a crowded hallway) may enhance self-efficacy, and consequently walking ability.
The optimal training might be a combination of treadmill with task-specific practice. In individuals with moderate stroke severity, Eich et al. [111] found that 6 weeks, 5X/day, 30 minutes standard inpatient stroke physical therapy (focused on walking practice) plus 30 minutes treadmill training (maximum of 15% of bodyweight) was superior to 60 minutes of standard physical therapy for walking speed and walking endurance (6MWT). Gait speed for the treadmill combination group improved from a mean of 0.4 to .71 m/sec, but only improved from 0.44 to 0.6 m/sec for the standard physical therapy group. The improvement with the treadmill combination group was maintained at 3 months post intervention. Ada et al. [80] found similar results in people with chronic stroke. A combination of treadmill training and overground gait training (4 weeks, 3X/week) was compared to a low intensity home program (matched for time). There were minimal changes with the home program, but the combination group increased their walking speed 21% (0.62 to 0.75 m/sec) and their 6MWT distance 28% (from 296 to 379 m). These improvements were maintained at a 3 month follow-up.
Intensive mobility training
Given the many mechanisms which contribute to gait and the varying tasks and environments under which gait is utilized, an intervention that addresses different elements underlying walking and the broader framework of mobility might be optimal. A number of intensive training programs have been developed that incorporate repetitive practice of a wide variety of mobility tasks. What makes these programs “intensive” from traditional rehabilitation programs which may have repetitive tasks or gait oriented training? Table 4 summarizes the tasks of published randomized controlled trials which have used intensive mobility training. These programs generally have two, if not all three of the following components: graded strengthening using functional tasks (e.g., repetitive rise from a chair, stepping up and down a stepper), aerobic component (e.g., graded walking activity, stationary bicycle or goal of continuous period of functional tasks at least at a moderate intensity) and a variety of challenging walking activities with substantial postural control demands (e.g., walking backwards, on foam or stepping over obstacles). More importantly, the intensity (amount of activity within a given time) and the challenge of the activity (e.g., balance task) are continually incremented to provide a maximal challenge to the participant. The authors of these studies provide some indication of how the intensity is progressed (e.g., increasing heart rate or perceived exertion at set target zones, increasing number of repetitions, reduction of rest breaks). In contrast to traditional hands-on neurofacilitation programs, these intensive mobility programs often have one therapist or instructor working with multiple participants and thus there is an emphasis for the participant to work independently under supervision. Often a circuit of workstations is used to ensure that participants can eventually continue from one task to another with little rest between. Given the requirement to participate in walking tasks, the ability to walk 10 m with or without assistive devices independently or with supervision is one of the most common inclusion criteria (see Table 4) for these intensive mobility programs.
Table 4.
Effect of intensive mobility training on walking (studies ordered chronologically).
| Author | Subjects | Group Assignment | Mobility Program description | Results |
|---|---|---|---|---|
| Early post-stroke (within 6 months) | ||||
| Duncan et al. [84] 1998 |
N=20 Stroke within 30–90 days Ambulatory with supervision, mild to moderate impairment (Fugl-Meyer Motor score 40–90). |
Group 1 (n=10): Mobility, IP=1:1 12 weeks (8 week supervised and 4 week unsupervised) at home Group 2 (n=10): Control (Usual care) |
Assisted and resisted exercises with theraband Functional exercises Balance exercises Progressive walking program or bicycle ergometer |
No group difference for 6MWT but trend for gait speed (0.1>p>,05) |
| Duncan et al. [85] 2003 |
N=92 Stroke within 30–150 days Able to walk 25 ft independently |
Group 1 (n=44): Mobility, 36 sessions, 90 min, IP=1:1 over 12–14 weeks at home Group 2 (n=48): Control (Usual care) |
Strengthening using theraband Balance: step up and sideways onto a step, chair rise, fall back against wall while standing and bounce upright, marching, rise on toes, kicking ball, simulated golfing/batting, stops and turns while walking Arm and hand use in real-life tasks (washing counters, putting away dishes, folding towels) Stationary bike up to 30 min with increasing speed and resistance |
Gait speed*: Mobility+ 26%, Control +18% (ES=0.23) 6MWT*: Mobility +40%, Control +15% No group difference for TUG |
| Blennerhasset and Dite [86] 2004 |
N=30 Stroke inpatients, mean 36 days since stroke Able to walk 10 m with close supervision, independent ambulators were excluded |
4 weeks, 1 hour, 5 day/week, IP=1:4 in addition to standard therapy in medical center Group 1 (n=15): Mobility Control Group (n=15): Arm exercises (strengthening, reaching, stretching) |
10 stations (5 min each): stationary bikes, treadmills, sit-to-stand, step-ups, obstacle course, standing balance, stretching, strengthening using gym equipment | 6MWT*: Mobility + 121%, Control +59% TUG*: Mobility −52%, Control +18% |
| 6 months post-stroke or later | ||||
| Dean et al. [87] 2000 |
N=12 Community dwelling, > 3 months post-stroke Walk 10 m independently |
4 weeks, 1 hour, 3X/week, IP=2:5 in medical center Group 1 (n=6): Mobility Group 2 (n=6): Control Group Upper extremity program |
10 stations (5 min each): sit at table and reach, rise from chair of different heights, step in different directions to blocks of different heights, rise on toes, standing and reaching (including to floor), Kinetron for resisted leg flexion/extension, rise from chair and walk, walk on treadmill, walk on different surfaces, walk on different slopes and stairs, walking relays | Gait speed without device* Mobility+ 22%, Control +0.1% 6MWT*: Mobility + 20%, Control +0.7% No group difference for TUG or gait speed with device |
| Chu et al. [88] 2004 |
N=12 > 1 year post-stroke Independent in walking, able to reach 60% of age-predicted heart rate on cycle ergometer |
8 weeks, 1 hour, 3 day/week, IP=1:3 to 1:5 Group 1: (n=7): Mobility in community pool Group 2: (n=5): Control arm exercise |
Shallow water walking, running, hopping, side stepping, marching, single leg and double-leg hops with graded aerobic component from 50–80% heart rate reserve. Resistance provided by water. | Gait speed*: Mobility +19%, Control + 3% |
| Salbach et al. [89,122] 2004, 2005 |
N=91 Community dwelling, within 1 year post-stroke (mean about 7–8 months) Walk 10 m independently with or without supervision |
6 weeks, 1 hour, 3X/week in medical center Group 1 (n=44): Mobility, IP=1:1 Group 2 (n=47): Control Upper extremity program |
10 tasks (5 min each, except 10 min treadmill): step-up, walking on narrow line (forward, backward, sideways), kicking and dribbling ball, stand up and walk, treadmill with increasing speed and inclination, walking and carrying, continuous walking with progression to running, walking backwards, going up and down stairs | 6MWT*: Mobility+19%, Control+2% Gait speed*: Mobility+22%, Control+5% TUG*: Mobility−29%, Control−10% |
| Marigold et al. [90] 2005 |
N=61 Community dwelling, > 1 year post-stroke Walk 10 m independently |
10 weeks, 1 hour, 3X/week, IP=1:3 in community setting Group 1(n=30): Mobility Agility program Group 2 (n=31): Control Weight-bearing and stretching |
Agility tasks: weight-shifting, varying step lengths and speeds, tandem walking, figure of 8 walk, alternate stepping on low rises, crossover stepping, repetitive sit-to-stand, knee raises while standing, standing perturbations. Eyes closed and foam surfaces were used for some of the tasks. |
TUG*: Mobility −17%, Control − 8%* Induced falls on translating platform*: Mobility group reduced falls, while Control increased falls. Falls in community*: For those with history of falls, Mobility subjects had less falls in the 1 year following study than Control subjects. |
| Pang et al. [91] 2005 |
N=63 Community dwelling, > 1 year post-stroke Walk 10 m independently Able to raise heart rate to 60% of age-predicted max with a leg cycle ergometer |
5 months, 1 hour, 3X/week, IP=1:4 in community setting Group 1 (n=32): Mobility and fitness Group 2 (n=31): Control, Seated arm program |
Brisk walking, sit-to-stand, alternate stepping on low rises (increment from 40–70% heart rate reserve) Mobility tasks: walking in different directions, tandem walking, obstacle course, sudden stops and turns, walking on different surfaces, standing on foam, kicking a ball Functional strengthening: partial squats, rising on toes |
6MWT*: Mobility+20%, Control+13% |
| Yang et al. [92] 2006 |
N=48 Community dwelling, > 1 year post-stroke Walk 10 m independently |
Group 1 (n=24): Mobility (task-oriented progressive resisted program), 4 weeks, 30 min, 3X/week, IP=1:1, in medical center Group 2 (n=24): Control with no treatment |
6 stations (5 min each): 1. standing and reaching, 2. rising from chair of different heights, 3. step forward/backward onto blocks, 4. step sideways, onto blocks, 5. step up onto blocks, 6. rise on toes | 6MWT*: Mobility+12%, Control+2% Gait speed*: Mobility+10%, Control−0.2 TUG*: Mobility +12%, Control + 0.7% |
| Olney et al. [93] 2006 |
N=72 Chronic stroke (mean 3–4 years post-stroke) Able to walk 15 min with rests |
Group 1 (n=37): Supervised: 10 weeks, 1.5 hour, 3 day/week, IP=1:4 in community setting Group 2 (n=35): Unsupervised: 3 supervised sessions followed by 9 week unsupervised home program |
Graded walking program (50–70% age-adjusted heart rate max) Strength training (theraband, simple weights, functional exercises) | 6MWT: Supervised+12%, Unsupervised+14% (No group difference) |
+/− % indicates percent increase or decrease from baseline.
indicates significant comparison reported in study. IP=Instructor:Participant ratio
We identified 3 trials which evaluated an intensive mobility training program early after stroke (within 6 months) (Table 4). The three trials were not statistically combined due to differences in purpose and protocol. The inpatient study by Blennerhassett and Dite [86] added a 4 week, 50 min group mobility and endurance circuit to standard of care treatment and had large effects with a 120% improvement in 6MWT of the circuit group compared to 60% improvement in the control group and these effects were maintained at 6 months after the extra training ended. However, it is not possible to determine whether the results are from the specific mobility tasks practiced in the circuit program or the increase in the amount of therapy as the circuit group got an additional 20 hours of mobility training. Both studies by Duncan et al. [84,85] evaluated the effects of a therapist-supervised home program which targeted balance, endurance, strength, flexibility and upper extremity function compared to usual care (about half of which received no physical therapy or occupational therapy services). The small sample (n=20) study [84] found a trend towards improvement in gait speed, while the larger study (n=100) [85] found greater improvement in the 6MWT and gait speed with participants in the home program. Intensive mobility programs are feasible and efficacious early after stroke in those individuals with some walking ability.
The majority of these intensity mobility programs have been evaluated for community-dwelling individuals beyond 6 months post-stroke who have mild to moderate severity of stroke and can walk to some extent. Seven RCTS assessed the effect of an intensive mobility training program with individuals greater than 6 months post-stroke (Table 4). We performed a meta-analysis and found that intensive mobility training had a small significant homogeneous effect size (d=0.20, 95% CI −0.03 to 0.44, p=0.04) for the 6WMT [87,89,91–93] and a small homogenous effect size for walking speed (d=0.45, 95% CI 0.14 to 0.77, p< .002) [87–89,92] in favor of the intensive mobility training. The TUG had a non-significant homogeneous effect size (d=0.17, 95% CI −0.11, 0.45, p=0.11) based on 4 studies [87,89,90,92].
The group setting in these mobility studies promotes socialization and collegiality which may be particularly important as stroke can be an isolating condition in which depression is frequent. Peers are generally supportive and encourage each other to attend the classes and do the exercises. Group, versus individual treatment, also facilitates the implementation of the program into the community as it does not require costly one-on-one training and can be done in local recreational centres. In addition, the group setting may provide a positive environment for enhancing self-efficacy. Self-efficacy can be enhanced by an individual having positive experiences in executing walking tasks (mastery experience) or receiving verbal affirmation of their abilities from others (verbal persuasion), and also by the individual observing others successfully practising the tasks (vicarious experience) [115].
The Olney et al. [93] study contrasted from the other trials in that the supervised mobility group had equivalent effects to the control group. However, their control group was unique from the other trials. Their supervised experimental group was compared to a control group that was provided 3 supervised sessions prior to 9 unsupervised weeks. This study raises important issues about what motivates individuals to adhere to exercise. If results from an unsupervised program can produce similar benefits, the cost savings could be substantial since this reduces the cost of instructors and the necessity of finding a facility for the program. In addition, participation may be higher as transportation to a centre is not required and individuals can do the exercise on their own schedule. Olney et al. [93] attribute their improvements to three factors: 1) the exercise equipment could be used at home and exercises could be progressed independently, 2) a high level of social support may have motivated participants and 3) the follow-up assessment may have provided incentive to adhere to the program. Understanding factors which promote adherence to exercise in people with chronic conditions is an area of research with limited literature and we have only begun to comprehend motivators for exercise. For example, Liu-Ambrose et al. [116] found that three groups (stretching control group, resistance exercise group and agility exercise group) of older adults all continued to improve their function in the year after the intervention ended. They attributed this continued improvement to the intervention acting as a catalyst to increase physical activity. More research is required on long-term adherence to exercise, as well as innovative methods to stimulate physical activity such as “report cards” to the client which provide feedback on one’s current walking performance and targets for improvement.
There does appear to be a minimal requirement for intensity; for example, a limited number of low intensity home visits resulted in small, transitory improvements in gait post-stroke [117]. Kwakkel et al. [48] reviewed 6 randomized controlled trials [58,117–121] for the effect of treatment time (total number of hours of therapy) on walking speed in people with stroke and found a significant effect size of 0.19. They concluded that an additional 16 hours was necessary to have a positive effect on activities in daily living (however, this number did not specifically refer to walking). Although one method of quantifying intensity is tracking the number of treatment hours, the type of tasks performed will be critical factors in determining the challenges to muscle, movement, endurance and balance. Although the key components appear to be paretic limb loading, functional strengthening, repetitive movements, balance, agility and aerobics (Table 4), the exercises are individualized to the level of the participant. These protocols are not as easy to replicate as a treadmill program where the intensity can be manipulated by the speed and slope of the treadmill. In addition, training background of the instructors, instructor: participant ratio and how much assistance instructors provide could be influencing factors. Furthermore, often journal articles do not provide sufficient details to implement the program. We have developed a detailed evidence-based group exercise manual for people with stroke at http://www.rehab.ubc.ca/jeng and it is freely available.
Interestingly, one of the control groups which participated in an intensive upper extremity exercise program improved their balance and walking speed, although not as much as the intensity mobility group [91]. The seated upper extremity program encompassed reaching tasks (and consequently weight-bearing through the lower limbs), trunk movements (e.g., push-ups against a table while sitting) and arm and hand strengthening. Such tasks have the potential to improve trunk control and weight-bearing ability which can contribute to improved gait. These findings have important implications as the optimal gait training program should likely involve the practice of upper extremity and trunk mobility tasks.
The benefits from multi-dimensional mobility programs extend beyond walking. These studies have improved cardiorespiratory fitness [88,91], bone density [91], balance confidence [90,122] and muscle strength [88,91]. Marigold et al. [90] also reported that individuals who had fallen prior to the trial were less likely to fall in the year following the intervention. As mobility deteriorates over time post-stroke [123] maintaining walking ability and preventing secondary complications become increasingly important.
IV. Expert commentary
The evidence suggests that the optimal program to improve walking ability involves repetitive and intensive practice which is continually incremented in difficulty according to the tolerance of the participant. Additional intensive mobility practice can enhance recovery of walking ability early after stroke. Furthermore, community-based intensive mobility exercise can maintain or even improve walking abilities in the chronic phase. This review is limited by its exclusion of assistive devices and modalities like electrical stimulation. The appropriate prescription of assistive devices like ankle foot orthoses, canes and walkers should not be overlooked. Assistive technology such as functional electrical stimulation may play an important role in maximizing gait recovery, however, regular use of this modality beyond the hospital setting is currently restricted to a very small number of people.
Paretic limb weight-bearing, aerobic, functional strengthening and balance appear to be critical components to improving walking ability. Strengthening appears to be most effective when incorporated in functional activities (e.g., repetitive rise from a chair, walking uphill on a treadmill). Ideal programs are likely a combination of activities which promote a large number of steps, plus training on a variety of walking tasks. Treadmill training, particularly at faster speeds, is effective for improving walking speed. A body-weight supported treadmill system may reduce the physical strain of the therapist to support a lower functioning client to participate in walking practice. For community ambulation, tasks should also include also curbs, stairs, escalators, turning, quick stops, stepping over obstacles, and walking on different terrains (slopes, uneven ground). If space, safety or resources prevent practice in varying indoor and outdoor environments, then environments need to simulate realistic visual stimuli (crowded walkways, obstacle on the ground, low lighting for an evening effect).
When possible, the chronicity of the stroke should be considered. Individuals admitted to inpatient stroke rehabilitation undertake a full day multi-disciplinary program which could involve a number of physical activities from physical therapy, occupational therapy, recreation therapy or nursing management (e.g., practice of transfers and self-care). Even walking to the many scheduled appointments (e.g., speech therapy, psychology) could provide an exercise stimulus. Thus, an additional 30 minute inpatient treatment must have sufficient effect to outweigh the already intensive schedule of physical activities, subject variability, and large natural recovery in walking ability which occurs in the first few months following a stroke [6]. Kwakkel et al. [124] reported that spontaneous recovery due to time alone accounted for 22% of the change in functional ambulation category in the first 10 week post-stroke. In contrast, individuals with chronic stroke may be leading a relatively sedentary lifestyle and there will be little competing physical activities. In fact, maintaining or reducing deterioration of walking ability (rather than improving) may be of clinical importance in the chronic stage [123].
V. Five-year view
In terms of future research, there is no doubt that the quality of clinical trials in this field will continue to improve. The last ten years has seen a surge of randomized controlled trials to improve walking ability following a stroke. The next 5 years will produce multi-site collaborations with larger sample sizes to enable long term follow-up of walking ability and the evaluation of important secondary complications, like falls, fractures, heart disease and recurrence of stroke. Models like the International Classification of Functioning will help to guide our assessment of walking ability in stroke and evaluation of treatment effects. We will see an increase in use of common outcome measures like gait speed and 6MWT, as well as new meaningful measures relevant to participation in walking activities. Clinical trials will attempt to quantify and manipulate the mechanisms (e.g., aerobic, brain plasticity, postural control, strengthening) which contribute to the gains in walking following an intervention. In addition, there will be pioneering methods to quantify the dose and intensity of the training. For example, the intensity during the intervention to improve walking ability can be monitored and quantified using accelerometers, step counters and/or heart rate monitors. In addition, such outcome measures could also be used to gauge whether the intervention promoted an increase in walking in the home and community setting (i.e., participation measure of walking).
There will be more consideration of real world issues like the cost-benefits, accessibility, long term effects and adherence to the program which are factors that contribute to the feasibility of continuing the program after the research funding has expired. The effect of innovative delivery methods which manipulate the training environment and levels of supervision will be explored (e.g., group versus individual, self-managed home program versus supervised program, community center versus rehabilitation center) to determine candidates who might be most appropriate for which program.
Research which is able to draw conclusions about effects on high impact outcome measures (e.g., recurrence of stroke or fractures), coupled with innovative methods to make these programs accessible, cost-effective and easy to implement will result in more people with stroke engaging in programs to improve and maintain their walking ability long after the initial occurrence of their stroke.
VI. Key issues
Impairments resulting from stroke, such as muscle weakness, incoordination, poor endurance, pain, spasticity and poor balance lead to persistent difficulties with walking.
Improved walking ability is one of the highest priorities for people living with a stroke.
Gait training interventions have potential to improve walking ability across the 3 levels of functioning (Body Functions and Structures, Activities and Participation) and it would be ideal to have outcome measures representing each level.
Gait retraining through different types of exercise is the most common approach to improving walking ability.
Neurodevelopmental approaches were equivalent or inferior to other approaches to improve walking ability.
Graded muscle strengthening (not using functional activities) has been found to improve muscle strength, but not transfer to improved walking ability.
Treadmill training has been found to have equivalent effects to overground gait training in sub-acute rehabilitation, but beneficial effects compared to low intensity control groups in chronic stroke. A combination of treadmill with task-specific practice may be optimal.
Intensive mobility training which incorporates functional strengthening, balance and aerobic exercises and practice on a variety of walking tasks improves gait ability both in sub-acute and chronic stroke.
Acknowledgments
We would like to acknowledge career scientist awards (JJE) from the Canadian Institutes of Health Research (MSH-63617) and the Michael Smith Foundation for Health Research and grant no. NHRI-EX96-9210EC (PFT) from the National Health Research Institutes, Taiwan, ROC.
References
- 1.Petrasovits A, Nair C. Epidemiology of stroke in Canada. Health Rep. 1994;6(1):39–44. [PubMed] [Google Scholar]
- 2.Kelly-Hayes M, Beiser M, Kase CS, Scaramucci A, D’Agostino RB, Wolf PA. The influence of gender and age on disability following ischemic stroke: the Framingham study. J Stroke Cerebrovasc Dis. 2003;12(3):119–126. doi: 10.1016/S1052-3057(03)00042-9. [DOI] [PubMed] [Google Scholar]
- 3.Portelli R, Lowe D, Irwin P, Pearson M, Rudd AG. Intercollegiate Stroke Working Party. Institutionalization after stroke. Clin Rehabil. 2005;19(1):97–108. doi: 10.1191/0269215505cr822oa. [DOI] [PubMed] [Google Scholar]
- 4.Gresham GE, Fitzpatrick TE, Wolf PA, McNamara PM, Kannel WB, Dawber TR. Residual disability in survivors of stroke--the Framingham study. N Engl J Med. 1975;293(19):954–956. doi: 10.1056/NEJM197511062931903. [DOI] [PubMed] [Google Scholar]
- 5.Michael KM, Allen JK, Macko RF. Reduced ambulatory activity after stroke: the role of balance, gait, and cardiovascular fitness. Arch Phys Med Rehabil. 2005;86(8):1552–1556. doi: 10.1016/j.apmr.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 6.Wade DT, Wood VA, Heller A, Maggs J, Langton Hewer R. Walking after stroke. Measurement and recovery over the first 3 months. Scand J Rehabil Med. 1987;19(1):25–30. [PubMed] [Google Scholar]
- 7.Mayo NE, Wood-Dauphinee S, Ahmed S, et al. Disablement following stroke. Disabil Rehabil. 1999;21(5–6):258–268. doi: 10.1080/096382899297684. [DOI] [PubMed] [Google Scholar]
- 8.Latham NK, Jette DU, Slavin M, et al. Physical therapy during stroke rehabilitation for people with different walking abilities. Arch Phys Med Rehabil. 2005;86(12 Suppl 2):S41–S50. doi: 10.1016/j.apmr.2005.08.128. [DOI] [PubMed] [Google Scholar]
- 9.Bohannon RW, Andrews AW, Smith MB. Rehabilitation goals of patients with hemiplegia. Int J Rehabil Res. 1988;11(2):181–183. [Google Scholar]
- 10.Harris JE, Eng JJ. Goal priorities identified by individuals with chronic stroke: Implications for rehabilitation professionals. Physiotherapy Canada. 2004;56:171–176. doi: 10.2310/6640.2004.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newman AB, Simonsick EM, Naydeck BL, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295(17):2018–2026. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- 12.Gill TM, Gahbauer EA, Allore HG, Han L. Transitions between frailty states among community-living older persons. Arch Intern Med. 2006;166(4):418–423. doi: 10.1001/archinte.166.4.418. [DOI] [PubMed] [Google Scholar]
- 13.Wade DT, Skilbeck CE, Wood VA, Langton Hewer R. Long-term survival after stroke. Age Ageing. 1984;13(2):76–82. doi: 10.1093/ageing/13.2.76. [DOI] [PubMed] [Google Scholar]
- 14.Pang MY, Eng JJ, Miller WC. Determinants of satisfaction with community reintegration in older adults with chronic stroke: role of balance self-efficacy. Phys Ther. 2007;87(3):282–291. doi: 10.2522/ptj.20060142. [DOI] [PubMed] [Google Scholar]
- 15.Pang MY, Eng JJ, Dawson AS. Relationship between ambulatory capacity and cardiorespiratory fitness in chronic stroke: influence of stroke-specific impairments. Chest. 2005;127(2):495–501. doi: 10.1378/chest.127.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Worthen LC, Kim CM, Kautz SA, Lew HL, Kiratli BJ, Beaupre GS. Key characteristics of walking correlate with bone density in individuals with chronic stroke. J Rehabil Res Dev. 2005;42(6):761–768. doi: 10.1682/jrrd.2005.02.0036. [DOI] [PubMed] [Google Scholar]
- 17.Patterson SL, Forrester LW, Rodgers MM, et al. Determinants of walking function after stroke: differences by deficit severity. Arch Phys Med Rehabil. 2007;88(1):115–119. doi: 10.1016/j.apmr.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 18.Franklin BA. Normal cardiorespiratory responses to acute aerobic exercise. In: Roitman JL, editor. ASCM’s Resource Manual for Guidelines for Exercise Testing and Prescription. 4. Lippincott Williams and Wilkins; Philadelphia: 2001. pp. 141–149. [Google Scholar]
- 19.Hsu AL, Tang PF, Jan MH. Analysis of impairments influencing gait velocity and asymmetry of hemiplegic patients after mild to moderate stroke. Arch Phys Med Rehabil. 2003;84(8):1185–1193. doi: 10.1016/s0003-9993(03)00030-3. [DOI] [PubMed] [Google Scholar]
- 20.Kim CM, Eng JJ. The relationship of lower-extremity muscle torque to locomotor performance in people with stroke. Phys Ther. 2003;83(1):49–57. [PubMed] [Google Scholar]
- 21.Eng JJ, Chu KS, Dawson AS, Kim CM, Hepburn KE. Functional walk tests in individuals with stroke: relation to perceived exertion and myocardial exertion. Stroke. 2002;33(3):756–761. doi: 10.1161/hs0302.104195. [DOI] [PubMed] [Google Scholar]
- 22.Nadeau S, Arsenault AB, Gravel D, Bourbonnais D. Analysis of the clinical factors determining natural and maximal gait speeds in adults with a stroke. Am J Phys Med Rehabil. 1999;78(2):123–130. doi: 10.1097/00002060-199903000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Kelly JO, Kilbreath SL, Davis GM, Zeman B, Raymond J. Cardiorespiratory fitness and walking ability in subacute stroke patients. Arch Phys Med Rehabil. 2003;84(12):1780–1785. doi: 10.1016/s0003-9993(03)00376-9. [DOI] [PubMed] [Google Scholar]
- 24.Tang A, Sibley KM, Bayley MT, McIlroy WE, Brooks D. Do functional walk tests reflect cardiorespiratory fitness in sub-acute stroke? J Neuroengineering Rehabil. 2006;3:23. doi: 10.1186/1743-0003-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eng JJ, Dawson AS, Chu KS. Submaximal exercise in persons with stroke: test-retest reliability and concurrent validity with maximal oxygen consumption. Arch Phys Med Rehabil. 2004;85(1):113–118. doi: 10.1016/s0003-9993(03)00436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lamontagne A, Malouin F, Richards CL. Contribution of passive stiffness to ankle plantarflexor moment during gait after stroke. Arch Phys Med Rehabil. 2000;81(3):351–358. doi: 10.1016/s0003-9993(00)90083-2. [DOI] [PubMed] [Google Scholar]
- 27.Lin PY, Yang YR, Cheng SJ, Wang RY. The relation between ankle impairments and gait velocity and symmetry in people with stroke. Arch Phys Med Rehabil. 2006;87(4):562–568. doi: 10.1016/j.apmr.2005.12.042. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization. International Classification of Impairments, Disabilities and Health. Geneva: World Health Organization; 2001. [Google Scholar]
- 29.Schepers VP, Ketelaar M, van de Port IG, Visser-Meily JM, Lindeman E. Comparing contents of functional outcome measures in stroke rehabilitation using the International Classification of Functioning, Disability and Health. Disabil Rehabil. 2007;29(3):221–230. doi: 10.1080/09638280600756257. [DOI] [PubMed] [Google Scholar]
- 30.Perry J, Garrett M, Gronley JK, Mulroy SJ. Classification of walking handicap in the stroke population. Stroke. 1995;26(6):982–989. doi: 10.1161/01.str.26.6.982. [DOI] [PubMed] [Google Scholar]
- 31.Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med. 1998;158(5 Pt 1):1384–1387. doi: 10.1164/ajrccm.158.5.9710086. [DOI] [PubMed] [Google Scholar]
- 32.Ng SS, Hui-Chan CW. The timed up & go test: its reliability and association with lower-limb impairments and locomotor capacities in people with chronic stroke. Arch Phys Med Rehabil. 2005;86(8):1641–1647. doi: 10.1016/j.apmr.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 33.Flansbjer UB, Holmback AM, Downham D, Patten C, Lexell J. Reliability of gait performance tests in men and women with hemiparesis after stroke. J Rehabil Med. 2005;37(2):75–82. doi: 10.1080/16501970410017215. [DOI] [PubMed] [Google Scholar]
- 34.Said CM, Goldie PA, Patla AE, Culham E, Sparrow WA, Morris ME. Balance during obstacle crossing following stroke. Gait Posture. 2007 doi: 10.1016/j.gaitpost.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 35.Flansbjer UB, Downham D, Lexell J. Knee muscle strength, gait performance, and perceived participation after stroke. Arch Phys Med Rehabil. 2006;87(7):974–980. doi: 10.1016/j.apmr.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 36.Lord SE, McPherson K, McNaughton HK, Rochester L, Weatherall M. Community ambulation after stroke: how important and obtainable is it and what measures appear predictive? Arch Phys Med Rehabil. 2004;85(2):234–239. doi: 10.1016/j.apmr.2003.05.002. [DOI] [PubMed] [Google Scholar]
- 37.Haeuber E, Shaughnessy M, Forrester LW, Coleman KL, Macko RF. Accelerometer monitoring of home- and community-based ambulatory activity after stroke. Arch Phys Med Rehabil. 2004;85(12):1997–2001. doi: 10.1016/j.apmr.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 38.Schmid A, Duncan PW, Studenski S, et al. Improvements in speed-based gait classifications are meaningful. Stroke. 2007;38(7):2096–2100. doi: 10.1161/STROKEAHA.106.475921. [DOI] [PubMed] [Google Scholar]
- 39.Lord SE, Rochester L. Measurement of community ambulation after stroke: current status and future developments. Stroke. 2005;36(7):1457–1461. doi: 10.1161/01.STR.0000170698.20376.2e. [DOI] [PubMed] [Google Scholar]
- 40.Shaughnessy M, Michael KM, Sorkin JD, Macko RF. Steps after stroke: capturing ambulatory recovery. Stroke. 2005;36(6):1305–1307. doi: 10.1161/01.STR.0000166202.00669.d2. [DOI] [PubMed] [Google Scholar]
- 41.Garner C, Page SJ. Applying the transtheoretical model to the exercise behaviors of stroke patients. Top Stroke Rehabil. 2005;12(1):69–75. doi: 10.1310/YJW0-FK07-TGN7-AVW7. [DOI] [PubMed] [Google Scholar]
- 42.Botner EM, Miller WC, Eng JJ. Measurement properties of the Activities-specific Balance Confidence Scale among individuals with stroke. Disabil Rehabil. 2005;27(4):156–163. doi: 10.1080/09638280400008982. [DOI] [PubMed] [Google Scholar]
- 43.Asano M, Miller WC, Eng JJ. Development and psychometric properties of the ambulatory self-confidence questionnaire. Gerontology. 2007;53(6):135–143. doi: 10.1159/000104830. [DOI] [PubMed] [Google Scholar]
- 44.van der Ploeg HP, van der Beek AJ, van der Woude LH, van Mechelen W. Physical activity for people with a disability: a conceptual model. Sports Med. 2004;34(10):639–649. doi: 10.2165/00007256-200434100-00002. [DOI] [PubMed] [Google Scholar]
- 45.Harris JE, Eng JJ, Marigold DS, Tokuno CD, Louis CL. Relationship of balance and mobility to fall incidence in people with chronic stroke. Phys Ther. 2005;85(2):150–158. [PubMed] [Google Scholar]
- 46.Pang MY, Eng JJ, Dawson AS, Gylfadottir S. The use of aerobic exercise training in improving aerobic capacity in individuals with stroke: a meta-analysis. Clin Rehabil. 2006;20(2):97–111. doi: 10.1191/0269215506cr926oa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paci M. Physiotherapy based on the Bobath concept for adults with post-stroke hemiplegia: a review of effectiveness studies. J Rehabil Med. 2003;35(1):2–7. doi: 10.1080/16501970306106. [DOI] [PubMed] [Google Scholar]
- 48.Kwakkel G, van Peppen R, Wagenaar RC, et al. Effects of augmented exercise therapy time after stroke: a meta-analysis. Stroke. 2004;35(11):2529–2539. doi: 10.1161/01.STR.0000143153.76460.7d. [DOI] [PubMed] [Google Scholar]
- 49.Teasell RW, Bhogal SK, Foley NC, Speechley MR. Gait retraining post stroke. Top Stroke Rehabil. 2003;10(2):34–65. doi: 10.1310/UDXE-MJFF-53V2-EAP0. [DOI] [PubMed] [Google Scholar]
- 50.Moseley AM, Stark A, Cameron ID, Pollock A. Treadmill training and body weight support for walking after stroke. Cochrane Database Syst Rev. 2005;(4):CD002840. doi: 10.1002/14651858.CD002840.pub2. [DOI] [PubMed] [Google Scholar]
- 51.Pollock A, Baer G, Pomeroy V, Langhorne P. Physiotherapy treatment approaches for the recovery of postural control and lower limb function following stroke. Cochrane Database Syst Rev. 2007;(1):CD001920. doi: 10.1002/14651858.CD001920.pub2. [DOI] [PubMed] [Google Scholar]
- 52.Van Peppen RP, Kwakkel G, Wood-Dauphinee S, Hendriks HJ, Van der Wees PJ, Dekker J. The impact of physical therapy on functional outcomes after stroke: what’s the evidence? Clin Rehabil. 2004;18(8):833–862. doi: 10.1191/0269215504cr843oa. [DOI] [PubMed] [Google Scholar]
- 53.van de Port IG, Wood-Dauphinee S, Lindeman E, Kwakkel G. Effects of Exercise Training Programs on Walking Competency After Stroke: A Systematic Review. Am J Phys Med Rehabil. doi: 10.1097/PHM.0b013e31802ee464. in press. [DOI] [PubMed] [Google Scholar]
- 54.Eng JJ. Strength training in individuals with stroke. Physiotherapy Canada. 2004;56:189–201. doi: 10.2310/6640.2004.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morris SL, Dodd KJ, Morris ME. Outcomes of progressive resistance strength training following stroke: a systematic review. Clin Rehabil. 2004;18(1):27–39. doi: 10.1191/0269215504cr699oa. [DOI] [PubMed] [Google Scholar]
- 56.Mulder T, Hulstijn W, van der Meer J. EMG feedback and the restoration of motor control. A controlled group study of 12 hemiparetic patients. Am J Phys Med. 1986;65(4):173–188. [PubMed] [Google Scholar]
- 57.Dickstein R, Hocherman S, Pillar T, Shaham R. Stroke rehabilitation. Three exercise therapy approaches. Phys Ther. 1986;66(8):1233–1238. doi: 10.1093/ptj/66.8.1233. [DOI] [PubMed] [Google Scholar]
- 58.Richards CL, Malouin F, Wood-Dauphinee S, Williams JI, Bouchard JP, Brunet D. Task-specific physical therapy for optimization of gait recovery in acute stroke patients. Arch Phys Med Rehabil. 1993;74(6):612–620. doi: 10.1016/0003-9993(93)90159-8. [DOI] [PubMed] [Google Scholar]
- 59.Gelber DA, Josefczyk PB, Herrmann D, Good DC, Verhulst SJ. Comparison of two therapy approaches in rehabilitation of the pure motor hemiparetic stroke patient. J Neuro Rehab. 1995;9(4):191–196. [Google Scholar]
- 60.Langhammer B, Stanghelle JK. Bobath or motor relearning programme? A comparison of two different approaches of physiotherapy in stroke rehabilitation: a randomized controlled study. Clin Rehabil. 2000;14(4):361–369. doi: 10.1191/0269215500cr338oa. [DOI] [PubMed] [Google Scholar]
- 61.van Vliet PM, Lincoln NB, Foxall A. Comparison of Bobath based and movement science based treatment for stroke: a randomised controlled trial. J Neurol Neurosurg Psychiatry. 2005;76(4):503–508. doi: 10.1136/jnnp.2004.040436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thaut MH, Leins AK, Rice RR, et al. Rhythmic Auditory Stimulation Improves Gait More Than NDT/Bobath Training In Near-Ambulatory Patients Early Post Stroke: A Single-Blind, Randomized Trial. Neurorehabil Neural Repair. doi: 10.1177/1545968307300523. in press. [DOI] [PubMed] [Google Scholar]
- 63.Glasser L. Effects of isokinetic training on the rate of movement during ambulation in hemiparetic patients. Phys Ther. 1986;66(5):673–676. doi: 10.1093/ptj/66.5.673. [DOI] [PubMed] [Google Scholar]
- 64.Kim CM, Eng JJ, MacIntyre DL, Dawson AS. Effects of isokinetic strength training on walking in persons with stroke: A double-blind controlled pilot study. J Stroke Cerebrovasc Dis. 2001;10:265–273. doi: 10.1053/jscd.2001.123775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bourbonnais D, Bilodeau S, Lepage Y, Beaudoin N, Gravel D, Forget R. Effect of force-feedback treatments in patients with chronic motor deficits after a stroke. Am J Phys Med Rehabil. 2002;81(12):890–897. doi: 10.1097/00002060-200212000-00002. [DOI] [PubMed] [Google Scholar]
- 66.Moreland JD, Goldsmith CH, Huijbregts MP, et al. Progressive resistance strengthening exercises after stroke: a single-blind randomized controlled trial. Arch Phys Med Rehabil. 2003;84(10):1433–1440. doi: 10.1016/s0003-9993(03)00360-5. [DOI] [PubMed] [Google Scholar]
- 67.Ouellette MM, LeBrasseur NK, Bean JF, et al. High-intensity resistance training improves muscle strength, self-reported function, and disability in long-term stroke survivors. Stroke. 2004;35(6):1404–1409. doi: 10.1161/01.STR.0000127785.73065.34. [DOI] [PubMed] [Google Scholar]
- 68.Visintin M, Barbeau H, Korner-Bitensky N, Mayo NE. A new approach to retrain gait in stroke patients through body weight support and treadmill stimulation. Stroke. 1998;29(6):1122–1128. doi: 10.1161/01.str.29.6.1122. [DOI] [PubMed] [Google Scholar]
- 69.Kosak MC, Reding MJ. Comparison of partial body weight-supported treadmill gait training versus aggressive bracing assisted walking post stroke. Neurorehabil Neural Repair. 2000;14(1):13–19. doi: 10.1177/154596830001400102. [DOI] [PubMed] [Google Scholar]
- 70.Nilsson L, Carlsson J, Danielsson A, et al. Walking training of patients with hemiparesis at an early stage after stroke: a comparison of walking training on a treadmill with body weight support and walking training on the ground. Clin Rehabil. 2001;15(5):515–527. doi: 10.1191/026921501680425234. [DOI] [PubMed] [Google Scholar]
- 71.Laufer Y, Dickstein R, Chefez Y, Marcovitz E. The effect of treadmill training on the ambulation of stroke survivors in the early stages of rehabilitation: a randomized study. J Rehabil Res Dev. 2001;38(1):69–78. [PubMed] [Google Scholar]
- 72.Pohl M, Mehrholz J, Ritschel C, Ruckriem S. Speed-dependent treadmill training in ambulatory hemiparetic stroke patients: a randomized controlled trial. Stroke. 2002;33(2):553–558. doi: 10.1161/hs0202.102365. [DOI] [PubMed] [Google Scholar]
- 73.da Cunha IT, Jr, Lim PA, Qureshy H, Henson H, Monga T, Protas EJ. Gait outcomes after acute stroke rehabilitation with supported treadmill ambulation training: a randomized controlled pilot study. Arch Phys Med Rehabil. 2002;83(9):1258–1265. doi: 10.1053/apmr.2002.34267. [DOI] [PubMed] [Google Scholar]
- 74.Werner C, Von Frankenberg S, Treig T, Konrad M, Hesse S. Treadmill training with partial body weight support and an electromechanical gait trainer for restoration of gait in subacute stroke patients: a randomized crossover study. Stroke. 2002;33(12):2895–2901. doi: 10.1161/01.str.0000035734.61539.f6. [DOI] [PubMed] [Google Scholar]
- 75.Werner C, Bardeleben A, Mauritz KH, Kirker S, Hesse S. Treadmill training with partial body weight support and physiotherapy in stroke patients: a preliminary comparison. Eur J Neurol. 2002;9(6):639–644. doi: 10.1046/j.1468-1331.2002.00492.x. [DOI] [PubMed] [Google Scholar]
- 76.Richards CL, Malouin F, Bravo G, Dumas F, Wood-Dauphinee S. The role of technology in task-oriented training in persons with subacute stroke: a randomized controlled trial. Neurorehabil Neural Repair. 2004;18(4):199–211. doi: 10.1177/1545968304269397. [DOI] [PubMed] [Google Scholar]
- 77.Yagura H, Hatakenaka M, Miyai I. Does therapeutic facilitation add to locomotor outcome of body weight--supported treadmill training in nonambulatory patients with stroke? A randomized controlled trial. Arch Phys Med Rehabil. 2006;87(4):529–535. doi: 10.1016/j.apmr.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 78.Husemann B, Muller F, Krewer C, Heller S, Koenig E. Effects of locomotion training with assistance of a robot-driven gait orthosis in hemiparetic patients after stroke: a randomized controlled pilot study. Stroke. 2007;38(2):349–354. doi: 10.1161/01.STR.0000254607.48765.cb. [DOI] [PubMed] [Google Scholar]
- 79.Sullivan KJ, Knowlton BJ, Dobkin BH. Step training with body weight support: effect of treadmill speed and practice paradigms on poststroke locomotor recovery. Arch Phys Med Rehabil. 2002;83(5):683–691. doi: 10.1053/apmr.2002.32488. [DOI] [PubMed] [Google Scholar]
- 80.Ada L, Dean CM, Hall JM, Bampton J, Crompton S. A treadmill and overground walking program improves walking in persons residing in the community after stroke: a placebo-controlled, randomized trial. Arch Phys Med Rehabil. 2003;84(10):1486–1491. doi: 10.1016/s0003-9993(03)00349-6. [DOI] [PubMed] [Google Scholar]
- 81.Jaffe DL, Brown DA, Pierson-Carey CD, Buckley EL, Lew HL. Stepping over obstacles to improve walking in individuals with poststroke hemiplegia. J Rehabil Res Dev. 2004;41(3A):283–292. doi: 10.1682/jrrd.2004.03.0283. [DOI] [PubMed] [Google Scholar]
- 82.Macko RF, Ivey FM, Forrester LW, et al. Treadmill exercise rehabilitation improves ambulatory function and cardiovascular fitness in patients with chronic stroke: a randomized, controlled trial. Stroke. 2005;36(10):2206–2211. doi: 10.1161/01.STR.0000181076.91805.89. [DOI] [PubMed] [Google Scholar]
- 83.Yen CL, Wang RY, Liao KK, Huang CC, Yang YR. Gait Training-Induced Change in Corticomotor Excitability in Patients With Chronic Stroke. Neurorehabil Neural Repair. doi: 10.1177/1545968307301875. in press. [DOI] [PubMed] [Google Scholar]
- 84.Duncan P, Richards L, Wallace D, et al. A randomized, controlled pilot study of a home-based exercise program for individuals with mild and moderate stroke. Stroke. 1998;29(10):2055–2060. doi: 10.1161/01.str.29.10.2055. [DOI] [PubMed] [Google Scholar]
- 85.Duncan P, Studenski S, Richards L, et al. Randomized clinical trial of therapeutic exercise in subacute stroke. Stroke. 2003;34(9):2173–2180. doi: 10.1161/01.STR.0000083699.95351.F2. [DOI] [PubMed] [Google Scholar]
- 86.Blennerhassett J, Dite W. Additional task-related practice improves mobility and upper limb function early after stroke: a randomised controlled trial. Aust J Physiother. 2004;50(4):219–224. doi: 10.1016/s0004-9514(14)60111-2. [DOI] [PubMed] [Google Scholar]
- 87.Dean CM, Richards CL, Malouin F. Task-related circuit training improves performance of locomotor tasks in chronic stroke: a randomized, controlled pilot trial. Arch Phys Med Rehabil. 2000;81(4):409–417. doi: 10.1053/mr.2000.3839. [DOI] [PubMed] [Google Scholar]
- 88.Chu KS, Eng JJ, Dawson AS, Harris JE, Ozkaplan A, Gylfadottir S. Water-based exercise for cardiovascular fitness in people with chronic stroke: a randomized controlled trial. Arch Phys Med Rehabil. 2004;85(6):870–874. doi: 10.1016/j.apmr.2003.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Salbach NM, Mayo NE, Wood-Dauphinee S, Hanley JA, Richards CL, Cote R. A task-orientated intervention enhances walking distance and speed in the first year post stroke: a randomized controlled trial. Clin Rehabil. 2004;18(5):509–519. doi: 10.1191/0269215504cr763oa. [DOI] [PubMed] [Google Scholar]
- 90.Marigold DS, Eng JJ, Dawson AS, Inglis JT, Harris JE, Gylfadottir S. Exercise leads to faster postural reflexes, improved balance and mobility, and fewer falls in older persons with chronic stroke. J Am Geriatr Soc. 2005;53(3):416–423. doi: 10.1111/j.1532-5415.2005.53158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pang MY, Eng JJ, Dawson AS, McKay HA, Harris JE. A community-based fitness and mobility exercise program for older adults with chronic stroke: a randomized, controlled trial. J Am Geriatr Soc. 2005;53(10):1667–1674. doi: 10.1111/j.1532-5415.2005.53521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang YR, Wang RY, Lin KH, Chu MY, Chan RC. Task-oriented progressive resistance strength training improves muscle strength and functional performance in individuals with stroke. Clin Rehabil. 2006;20(10):860–870. doi: 10.1177/0269215506070701. [DOI] [PubMed] [Google Scholar]
- 93.Olney SJ, Nymark J, Brouwer B, et al. A randomized controlled trial of supervised versus unsupervised exercise programs for ambulatory stroke survivors. Stroke. 2006;37(2):476–481. doi: 10.1161/01.STR.0000199061.85897.b7. [DOI] [PubMed] [Google Scholar]
- 94.Lennon S, Baxter D, Ashburn A. Physiotherapy based on the Bobath concept in stroke rehabilitation: a survey within the UK. Disabil Rehabil. 2001;23(6):254–262. doi: 10.1080/096382801750110892. [DOI] [PubMed] [Google Scholar]
- 95.Hesse S, Bertelt C, Jahnke MT, et al. Treadmill training with partial body weight support compared with physiotherapy in nonambulatory hemiparetic patients. Stroke. 1995;26(6):976–981. doi: 10.1161/01.str.26.6.976. [DOI] [PubMed] [Google Scholar]
- 96.Hesse S, Malezic M, Schaffrin A, Mauritz KH. Restoration of gait by combined treadmill training and multichannel electrical stimulation in non-ambulatory hemiparetic patients. Scand J Rehabil Med. 1995;27(4):199–204. [PubMed] [Google Scholar]
- 97.Marsden J, Greenwood R. Physiotherapy after stroke: define, divide and conquer. J Neurol Neurosurg Psychiatry. 2005;76(4):465–466. doi: 10.1136/jnnp.2004.053827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lennon S, Ashburn A, Baxter D. Gait outcome following outpatient physiotherapy based on the Bobath concept in people post stroke. Disabil Rehabil. 2006;28(13–14):873–881. doi: 10.1080/09638280500535132. [DOI] [PubMed] [Google Scholar]
- 99.Bobath B. Adult Hemiplegia: Evaluation and Treatment. London, UK: Heinemann Medical Books Ltd; 1978. [Google Scholar]
- 100.Giuliani CA, Duncan PW. Commentary. Phys Ther. 1992;72(11):789–792. [Google Scholar]
- 101.Sharp SA, Brouwer BJ. Isokinetic strength training of the hemiparetic knee: effects on function and spasticity. Arch Phys Med Rehabil. 1997;78(11):1231–1236. doi: 10.1016/s0003-9993(97)90337-3. [DOI] [PubMed] [Google Scholar]
- 102.Welmer AK, von Arbin M, Widen Holmqvist L, Sommerfeld DK. Spasticity and its association with functioning and health-related quality of life 18 months after stroke. Cerebrovasc Dis. 2006;21(4):247–253. doi: 10.1159/000091222. [DOI] [PubMed] [Google Scholar]
- 103.Latham N, Anderson C, Bennett D, Stretton C. Progressive resistance strength training for physical disability in older people. Cochrane Database Syst Rev. 2003;(2):CD002759. doi: 10.1002/14651858.CD002759. [DOI] [PubMed] [Google Scholar]
- 104.Vincent KR, Braith RW, Feldman RA, et al. Resistance exercise and physical performance in adults aged 60 to 83. J Am Geriatr Soc. 2002;50(6):1100–1107. doi: 10.1046/j.1532-5415.2002.50267.x. [DOI] [PubMed] [Google Scholar]
- 105.Pang MY, Eng JJ, McKay HA, Dawson AS. Reduced hip bone mineral density is related to physical fitness and leg lean mass in ambulatory individuals with chronic stroke. Osteoporos Int. 2005;16(12):1769–1779. doi: 10.1007/s00198-005-1925-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kanis J, Oden A, Johnell O. Acute and long-term increase in fracture risk after hospitalization for stroke. Stroke. 2001;32(3):702–706. doi: 10.1161/01.str.32.3.702. [DOI] [PubMed] [Google Scholar]
- 107.Jeannerod M. The origin of voluntary action: history of a physiological concept. C R Biol. 2006;329(5–6):354–362. doi: 10.1016/j.crvi.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 108.Barbeau H, Rossignol S. Recovery of locomotion after chronic spinalization in the adult cat. Brain Res. 1987;412(1):84–95. doi: 10.1016/0006-8993(87)91442-9. [DOI] [PubMed] [Google Scholar]
- 109.Brosseau L, Wells GA, Finestone HM, et al. Clinical practice guidelines for gait training. Top Stroke Rehabil. 2006;2(34):41. [Google Scholar]
- 110.Duncan PW, Zorowitz R, Bates B, et al. Management of Adult Stroke Rehabilitation Care: a clinical practice guideline. Stroke. 2005;36(9):e100–43. doi: 10.1161/01.STR.0000180861.54180.FF. [DOI] [PubMed] [Google Scholar]
- 111.Eich HJ, Mach H, Werner C, Hesse S. Aerobic treadmill plus Bobath walking training improves walking in subacute stroke: a randomized controlled trial. Clin Rehabil. 2004;18(6):640–651. doi: 10.1191/0269215504cr779oa. [DOI] [PubMed] [Google Scholar]
- 112.Jongbloed L, Stacey S, Brighton C. Stroke rehabilitation: sensorimotor integrative treatment versus functional treatment. Am J Occup Ther. 1989;43(6):391–397. doi: 10.5014/ajot.43.6.391. [DOI] [PubMed] [Google Scholar]
- 113.Lam T, Eng JJ, Wolfe DL, Hsieh J, Whittaker M. A systematic review of the efficacy of gait rehabilitation strategies for SCI. Topics in Spinal Cord Rehabilitation. doi: 10.1310/sci1301-32. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Marigold DS, Patla AE. Gaze fixation patterns for negotiating complex ground terrain. Neuroscience. 2007;144(1):302–313. doi: 10.1016/j.neuroscience.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 115.Bandura A. Exercise of personal and collective efficacy in changing societies. In: Bandura A, editor. Self-Efficacy in Changing Societies. Cambridge University Press; Cambridge, United Kingdom: 1995. pp. 145–145. [Google Scholar]
- 116.Liu-Ambrose TY, Khan KM, Eng JJ, Gillies GL, Lord SR, McKay HA. The beneficial effects of group-based exercises on fall risk profile and physical activity persist 1 year postintervention in older women with low bone mass: follow-up after withdrawal of exercise. J Am Geriatr Soc. 2005;53(10):1767–1773. doi: 10.1111/j.1532-5415.2005.53525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Green J, Forster A, Bogle S, Young J. Physiotherapy for patients with mobility problems more than 1 year after stroke: a randomised controlled trial. Lancet. 2002;359(9302):199–203. doi: 10.1016/S0140-6736(02)07443-3. [DOI] [PubMed] [Google Scholar]
- 118.Partridge C, Mackenzie M, Edwards S, et al. Is dosage of physiotherapy a critical factor in deciding patterns of recovery from stroke: a pragmatic randomized controlled trial. Physiother Res Int. 2000;5(4):230–240. doi: 10.1002/pri.203. [DOI] [PubMed] [Google Scholar]
- 119.Kwakkel G, Wagenaar RC, Twisk JW, Lankhorst GJ, Koetsier JC. Intensity of leg and arm training after primary middle-cerebral-artery stroke: a randomised trial. Lancet. 1999;354(9174):191–196. doi: 10.1016/S0140-6736(98)09477-X. [DOI] [PubMed] [Google Scholar]
- 120.Wade DT, Collen FM, Robb GF, Warlow CP. Physiotherapy intervention late after stroke and mobility. BMJ. 1992;304(6827):609–613. doi: 10.1136/bmj.304.6827.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Can augmented physiotherapy input enhance recovery of mobility after stroke? A randomized controlled trial. Clin Rehabil. 2004;18(5):529–537. doi: 10.1191/0269215504cr768oa. [DOI] [PubMed] [Google Scholar]
- 122.Salbach NM, Mayo NE, Robichaud-Ekstrand S, Hanley JA, Richards CL, Wood-Dauphinee S. The effect of a task-oriented walking intervention on improving balance self-efficacy poststroke: a randomized, controlled trial. J Am Geriatr Soc. 2005;53(4):576–582. doi: 10.1111/j.1532-5415.2005.53203.x. [DOI] [PubMed] [Google Scholar]
- 123.van de Port IG, Kwakkel G, van Wijk I, Lindeman E. Susceptibility to deterioration of mobility long-term after stroke: a prospective cohort study. Stroke. 2006;37(1):167–171. doi: 10.1161/01.STR.0000195180.69904.f2. [DOI] [PubMed] [Google Scholar]
- 124.Kwakkel G, Kollen B, Twisk J. Impact of time on improvement of outcome after stroke. Stroke. 2006;37(9):2348–2353. doi: 10.1161/01.STR.0000238594.91938.1e. [DOI] [PubMed] [Google Scholar]
- 125.Barbeau H, Visintin M. Optimal outcomes obtained with body-weight support combined with treadmill training in stroke subjects. Arch Phys Med Rehabil. 2003;84(10):1458–1465. doi: 10.1016/s0003-9993(03)00361-7. [DOI] [PubMed] [Google Scholar]
- 126.Teixeira da Cunha Filho I, Lim PA, Qureshy H, Henson H, Monga T, Protas EJ. A comparison of regular rehabilitation and regular rehabilitation with supported treadmill ambulation training for acute stroke patients. J Rehabil Res Dev. 2001;38(2):245–255. [PubMed] [Google Scholar]