Abstract
Background
Drug resistance poses a significant challenge for the successful application of highly active antiretroviral therapy (HAART) globally. Furthermore, emergence of HIV-1 isolates that preferentially utilize CXCR4 as a coreceptor for cell entry, either as a consequence of natural viral evolution or HAART use may compromise the efficacy of CCR5 antagonists as alternative antiviral therapy.
Methods
We sequenced the pol gene of viruses from 45 individuals failing at least six months of HAART in Durban, South Africa to determine the prevalence and patterns of drug resistance mutations. Coreceptor usage profiles of these viruses and those from 45 HAART-naive individuals were analyzed using phenotypic and genotypic approaches.
Results
Ninety-five percent of HAART-failing patients had at least one drug resistance mutation. Thymidine analog mutations (TAMs) were present in 55% of patients with 9% of individuals possessing mutations indicative of the TAM1 pathway, 44% had TAM2 while 7% had mutations common to both pathways. Sixty percent of HAART-failing subjects had X4/dual//mixed-tropic viruses compared to 30% of HAART-naïve subjects (p<0.02). Genetic coreceptor usage prediction algorithms correlated with phenotypic results with 60% of samples from HAART-failing subjects predicted to possess CXCR4-using (X4/dual/mixed viruses) versus 15% of HAART-naïve patients.
Conclusions
The high proportion of TAMs and X4/dual/mixed HIV-1 viruses among patients failing therapy highlight the need for intensified monitoring of patients taking HAART and the problem of diminished drug options (including CCR5 antagonists) for patients failing therapy in resource-poor settings.
Keywords: Coreceptor usage, viral tropism, antiretroviral drug resistance, HAART-failing patients, HAART-naïve patients
Introduction
HIV/AIDS is the leading cause of death in sub-Saharan Africa and South Africa has the highest number of HIV infections worldwide 1. HIV-1 subtype C (HIV-1C) is responsible for the majority of infected individuals in South Africa and worldwide 2. Access to HAART in South Africa has increased dramatically since the launch of the Operational Plan for Comprehensive HIV and AIDS Care and Treatment in 2003 3. However, antiretroviral drug coverage in sub-Saharan African remains low and it was estimated that in 2009, only 37% of patients eligible for treatment according to the World Health Organization guidelines were receiving it 4.
Despite the suppression of HIV-1 plasma viral load by HAART to undetectable levels, viral transcription persists 5–6 and this can lead to the emergence and persistence of drug resistance, which has significant public health implications. Most developing countries rely on clinical or immunological algorithms to monitor the effectiveness of HAART 7, which may result in a delay in the switching of failing HAART regimens. The delay in the switching of failing HAART regimens in the developing world, where WHO guidelines recommend the use of 2 nucleoside reverse-transcriptase inhibitors (NRTIs) and one non-nucleoside reverse-transcriptase inhibitor (NNRTI) as first line therapy may in turn result in the accumulation of thymidine analog mutations (TAMs) which are associated with broad cross resistance and may therefore limit the options available for alternative regimens. Recent studies have highlighted this emerging problem 8–11.
In addition to concerns regarding the emergence of TAMs in resource-poor settings, there are suggestive data that individuals failing HAART may have a higher proportion of CXCR4-utilizing viruses compared to antiretroviral-naïve patients even after controlling for the level of immunodeficiency between the groups 12–13. CXCR4 (X4) and dual-tropic (R5X4/dual) viruses are associated with rapid disease progression and emerge in the late chronic phase of disease in a significant proportion of patients 14–15. However, the switch to X4 viruses appears to be significantly less common for HIV-1C, even in late stages of disease 16–20. The predominant utilization of CCR5 by HIV-1C could be interpreted to suggest that CCR5 antagonists would be more efficacious for this subtype. Therefore, characterization of viral tropism in HIV-1C could help inform whether CCR5 antagonists should be used as salvage therapy in patients failing current widely used regimens or as part of first-line/early regimens for maximum benefit.
Here we investigated the drug resistance mutational pathways and factors associated with failure of HAART among HIV-1C infected patients in a setting where monitoring relies mainly on clinical and immunological algorithms. Furthermore, we determined coreceptor utilization profiles of HIV-1C viruses from individuals initiating or failing HAART to assess the usefulness of CCR5 antagonists as first-line or salvage therapy. We also explored the accuracy of env sequence based genotypic predictive algorithms in assessing the prevalence of R5 and CXCR4-using viruses in these patients.
Materials and methods
Study participants
Study participants were recruited from the Sinikithemba outpatient HIV/AIDS clinic at McCord Hospital in Durban, South Africa. Patients were included in the ARV-naïve cohort if they were at least 18 years of age, had a known HIV infection and had no prior history of HAART (the use of single dose nevirapine for the prevention of mother to child HIV transmission was not an exclusion criteria). Patients who met these criteria, had CD4+ T-cell counts ≤ 200 cells/µl or displayed AIDS defining clinical features according to WHO staging irrespective of CD4 counts or viral loads were recruited into this study. Patients were included in the ARV-failing arm if they were at least 18 years of age, had a known HIV infection, HIV-1 RNA load of ≥5,000 copies/ml and had at least 6 months of uninterrupted HAART. HAART-failing patients were also recruited into the study if they were clinically assessed to be failing therapy irrespective of viral loads or CD4+ T-cell counts. All study participants gave written informed consent and the study was approved by all participating institutional review boards.
Sample collection, viral load and CD4 measurement
CD4+ T-cell counts were determined from fresh blood from all participants by standard flow cytometry on a FACSCalibur (Becton-Dickinson, Franklin Lakes, NJ, USA) according to the manufacturer’s instructions. Plasma viral loads were measured using the COBAS AmpliPrep/COBAS Amplicor HIV-1 Monitor Test, version 1.5 (Roche Diagnostics, Rotkruez, Switzerland).
Genotypic resistance testing
Genotypic resistance testing was done from plasma samples using the Viroseq HIV-1 Genotyping System (Celera Diagnostics, CA, USA) as directed by the manufacturer.
Phenotypic coreceptor analysis
Coreceptor usage of viruses from patient plasma samples was determined using the enhanced sensitivity Trofile co-receptor tropism assay (Monogram Biosciences Inc., South San Francisco, California, USA) 21–22. The Trofile® assay is a commercial, standardized cell-based approach for determination of coreceptor usage by plasma-derived HIV-1 envelope proteins 23–24.
Envelope sequence analysis
cDNA synthesis, envelope amplification and cloning were done as previously described 25. Full-length env from 20 ARV-failing patients and 20 ARV-naïve patients was cloned into the pcDNA3.1D/V5-His-TOPO vector (Invitrogen). Sequencing was done using the ABI PRISM Big Dye Terminator Cycle Sequencing Ready Reaction kit version 3.1 (Applied Biosystems, CA, USA). Sequences were assembled and edited using Sequencher 4.8 and aligned with Mega 4. Phylogenetic trees were constructed in Paup 4.0 and visualized using Treeview 1.6.6. Coreceptor utilization was predicted using various publicly available or published sequence-based predictive algorithms 26–29.
Statistical analysis
All statistical analysis was done using Graph Pad Prism 5. Factors associated with tropism were assessed using unpaired t tests, Fisher’s exact tests and logistical regression analysis.
Nucleotide sequence accession numbers
The sequence data obtained from this study have been submitted to GenBank under accession numbers GU080160 to GU080199.
Results
Demographic and clinical characteristics
Forty five HAART-naïve and 45 HAART-failing patients were recruited. Patient demographic and clinical data are summarized in Table 1. At the time of analysis, HAART-naïve patients had a lower median CD4+ T-cell count (123 cells/mm3) than HAART-failing (174 cells/mm3) subjects (p=0.036). However, the median CD4+ T-cell count of HAART-naïve patients was higher than the nadir median CD4+ T-cell count (57 cells/mm3) (p=0.0004) of HAART-failing patients. HAART-naïve patients had significantly higher median plasma viral load of 44,042 copies/ml compared to 6,653 copies/ml for HAART-experienced participants (p=0.001). For patients failing treatment, the median duration on therapy was 29 months.
Table 1.
Patient information
| Patient Characteristic | ARV-Experienced Patients failing Treatment (n=45) |
ARV-Naïve Patients (n=45) |
p-value |
|---|---|---|---|
| Age, median years (Q1–Q3) | 36 (24–51) | 36 (20–78) | 0.65 |
| Gender: Female | 28 (65%) | 27 (60%) | |
| Black race | 45 (100%) | 45 (100%) | |
| CD4 cell count, median cells/mm3 (Q1–Q3) | |||
| Current | 174 (9–718) | 123 (8–660) | 0.036 |
| Nadir | 57 (3–197) | 0.0004 | |
| Vial load, median copies/ml | 6, 653 (225-220,010) | 44,042 (1,702–1,167,759) | 0.0010 |
| Current treatment regimen: | Current treatment regimen: | ||
| Regimen 1A (d4T, 3TC, EFV) | 23 (51.1%) | Regimen 1A (d4T, 3TC, EFV) | 23 (51.1%) |
| Regimen 1B (d4T, 3TC, NVP) | 2 (4.4%) | Regimen 1B (d4T, 3TC, NVP) | 2 (4.4%) |
| ZDV, d4T, ddI, NVP | 1 (2.2%) | ZDV, d4T, ddI, NVP | 1 (2.2%) |
| ZDV, d4T, 3TC, EFV | 2 (4.4%) | ZDV, d4T, 3TC, EFV | 2 (4.4%) |
| ZDV, 3TC, EFV | 13 (29.0%) | ZDV, 3TC, EFV | 13 (29.0%) |
| ZDV, 3TC,NVP | 4 (8.9%) | ZDV, 3TC,NVP | 4 (8.9%) |
Of these, 11/27 (40.8%) had previous history of Regimen 1A. 2/27 (7.4%) had a previous history of d4T, 3TC, NVP and EFV; 2/27 (7.4%) had a previous history of d4T and NVP and 1/27 (3.7%) each had previous history of the following: AZT, d4T, 3TC, ddI, EFV; d4T, NVP, EFV; AZT, 3TC, NVP, Lopinivir/Ritonivir; AZT, d4T, EFV; AZT, 3TC, Lopinivir/Ritonivir; AZT, d4T, 3TC, NVP; d4T, 3TC; AZT, d4T, NVP; 3TC, ddI, EFV; AZT, 3TC, EFV; d4T, 3TC, ddI, EFV; and AZT, 3TC,ddI.
Genotypic drug resistance typing
Drug resistance results were obtained for 43 of the 45 HAART-failing patients. Resistance testing was also performed for ten HAART-naïve patients. The only drug resistance mutation observed in the HAART-naïve individuals was in one patient with the NNRTI-associated E138A mutation. Three HAART-naive patients had minor protease inhibitor (PI) resistance mutations, one patient with mutations L10V and T74S/T, another had mutation A71T and the third patient had the T74S/T mutation. Of the 45 HAART-failing patients, 51.1% were on South African national treatment guidelines Regimen 1A (d4T, 3TC and EFV); 4.4% were on Regimen 1B (d4T, 3TC and NVP), 29% were on AZT, 3TC and EFV; 8.9% were on AZT, 3TC and NVP; 2.2% were on AZT, d4T, ddI and NVP and 4.4% were on AZT, d4T, 3TC and EFV. Twenty seven (60%) of the patients were on previous ARV therapy as detailed in table 1.
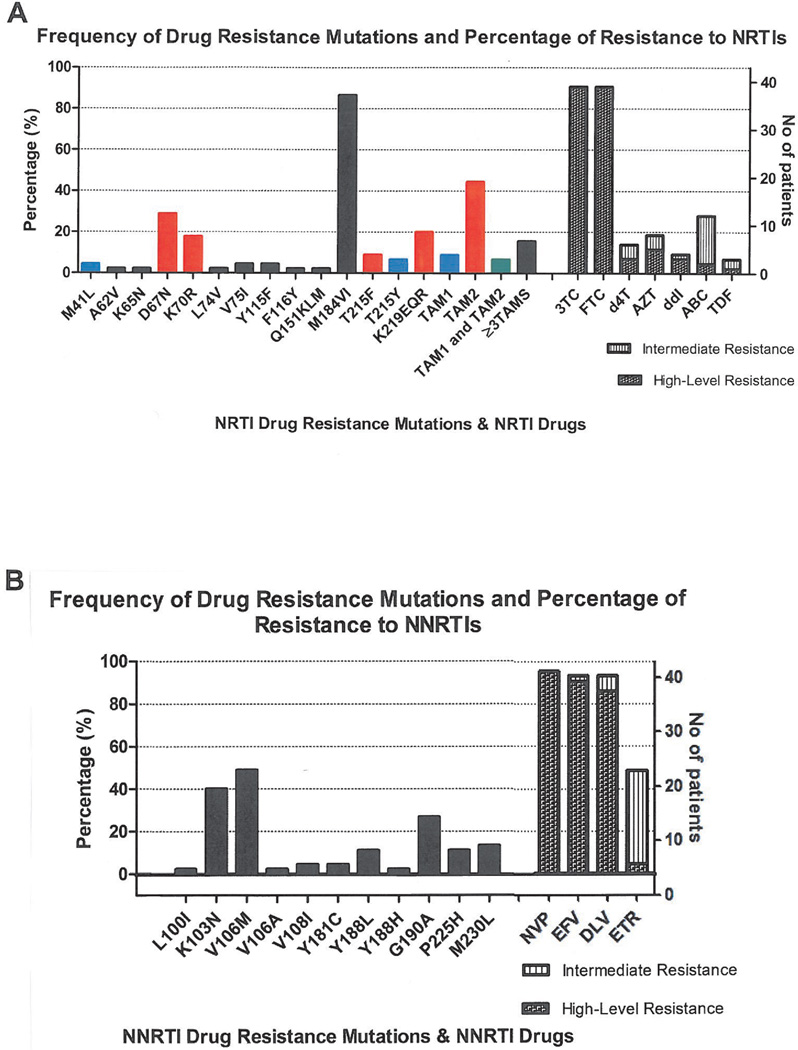
The specific drug resistance mutations detected in HAART-failing patients, and their frequencies are shown in figure 1. Mutations to all three major classes of drugs were noted. Forty one of the 43 (95%) ARV-failing patients possessed at least one drug resistance mutation. Ninety one percent of patients had at least one drug resistance mutation against 2 classes of drugs (NRTI and NNRTI). Nineteen percent had at least one resistance mutation against all 3 classes of drugs (NRTI, NNRTI and PI). For PI, only one minor mutation (T74S) was present in 9 (21%) of the HAART-failing patients (data not shown).
Figure 1. Frequency of drug resistance mutations and analog resistance mutations (TAMs).
A) shows the frequency of NRTI resistance mutations, thymidine analog mutation frequencies and number of patients displaying high and intermediate level resistance to specific NRTIs and B) shows the frequency of NNRTI resistance mutations and the number of patients showing resistance to specific NNRTIs. According to the Stanford drug resistance database algorithms for interpretation of resistance, high-level resistance refers to a genotypic pattern in which isolates have the maximum level of in vitro drug resistance and/or patients infected with isolates having similar genotypes display little or no virologic response to treatment with the drug. In low-level resistance, virus isolates have partial in vitro drug susceptibility and/or patients with viruses of this genotype may have a suboptimal virologic response to treatment compared with the treatment of a wildtype virus. Intermediate resistance suggests a degree of drug resistance greater than low-level resistance but lower than high-level resistance.
M184V/I, present in 87% of HAART-failing patients was the most common NRTI mutation detected. Thymidine analog resistance mutations (TAMs) were detected in 55% of patients. The TAM1 pathway NRTI mutations M41L and T215Y, associated with intermediate to high level resistance to AZT and d4T and low level resistance to ddI, ABC and TDF 30 were present in approximately 9% of patients. Neither an insertion at codon 69 nor the L210W mutation which is also indicative of the TAM1 pathway was noted. The TAM2 pathway mutations present were D67N, K70R, T215F and K219EQR. Forty four percent of patients had TAM2 pathway mutations. Seven percent of patients possessed both TAM1 and TAM2 mutations and 16% had three or more TAMs.
Approximately 91% of patients had resistance to 3TC and FTC, 19% had high or intermediate level resistance to AZT as defined by the Stanford database resistance scores (http://hivdb.stanford.edu/) (see also figure 1 legend). Fourteen percent had high or intermediate level resistance to d4T while 9% had high or intermediate level resistance to ddI. High or intermediate level resistance to ABC was noted in 28% of patients, while only 7% displayed high or intermediate level resistance to TDF (Figure 1A).
NNRTI mutations noted are summarized in Figure 1B. The most common NNRTI resistance mutation was V106M, found in 49% of HAART-failing participants. The K103N (40%) and G190A (27%) mutations were also relatively common but no G190S mutations were present in any of the patients. V106M and K103N both cause high-level resistance to 3 of the 4 NNRTI: nevirapine (NVP), delavirdine (DLV) and efavirenz (EFV) but has no effect on etravirine (ETR). G190A causes high level resistance to NVP, intermediate resistance to EFV and low level resistance to ETR. This mutation also increases susceptibility to DLV 31. Interestingly, the M230L mutation, associated with high level resistance to ETR and uncommon (<5%) in both subtype B and C NNRTI-failing patients, occurred at an unusually high frequency of 13.3% in this cohort. Ninety-five percent of patients had mutations associated with high level resistance to NVP, 93% had high/intermediate level EFV resistance mutations. Ninety three percent displayed high or intermediate level resistance to DLV, with 49% displaying high/intermediate level resistance to ETR (Figure 1B).
Recent studies in southern Africa have highlighted the growing problem of thymidine analog mutations in patients receiving the World Health Organization (WHO) or national antiretroviral programmes recommended first-line therapy 9–10, 32. We hypothesized that these mutations may be increasing as antiretroviral roll-out accelerates, accompanied by mainly clinical and immunological based monitoring of treatment. We therefore compared the proportion and patterns of TAM mutations observed in our study, in which patients had been treated for a median of 29 months, with data reported from an earlier study from the same healthcare facility in patients (median treatment duration of 11 months) also following the South African national antiretroviral treatment and monitoring guidelines. We found that 55% of individuals failing therapy had TAM mutations compared to 32% reported in the earlier study 10 and there was there was a non-significant trend in the present study for association of TAMs with duration of treatment (p=0.08) (data not shown).
Coreceptor utilization
Phenotypic coreceptor analysis
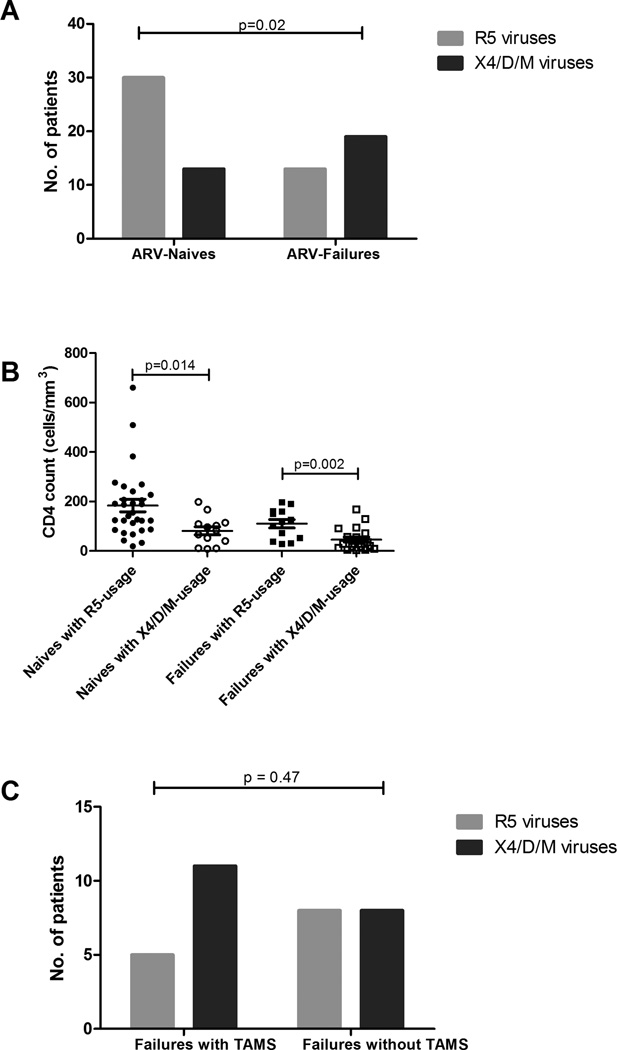
Only 83 of 90 samples were available for phenotypic coreceptor analysis of which 75 samples (32 from ARV-experienced patients failing treatment and 43 from ARV-naive patients) yielded reportable data from the Trofile coreceptor tropism assay. Overall, 31/75 (41%) were dual/mixed viruses, 43/75 (57%) were CCR5-using, and only 1 (1%) was exclusively CXCR4-utilizing. Of the 43 ARV-naive patients, 30 (70%) possessed R5 viruses compared to 13 (30%) with dual/mixed viruses. No ARV-naive patients exhibited exclusive X4 viruses. Among the 32 ARV-experienced patients failing treatment, 13 (41%) possessed R5 viruses, 18 (56%) had dual/mixed infections while one patient (3%) had X4-only viruses (Figure 2A). Thus patients failing treatment had a higher percentage (59%) of X4/dual/mixed viruses compared to ARV-naive patients with 30%, ARV-naive patients had higher proportion of R5 viruses (70%) compared to patients failing therapy with 41% (p=0.02).
Figure 2. Relationship of viral tropism of ARV-naive and ARV-failing patients.
A) Frequency of X4/dual/mixed- and R5-utilizing viruses in patients failing treatment and treatment-naive individuals. Bar graph indicating results from the trofile assay comparing the proportion of patients with R5 viruses to those harbouring X4/dual/mixed viruses in HAART-naive versus HAART-failing patients (p=0.02, Fisher’s exact test). B) CD4 counts and coreceptor usage in HAART-naive patients and HAART-failing patients. Dot graph indicating results from trofile assay. ARV-naive patients with X4/D/M viruses had a lower CD4 count than ARV-naive patients with R5 viruses (p=0.014, unpaired t test). ARV-failing patients with X4/D/M viruses had a lower CD4 count than ARV-failing patients with R5 viruses (p=0.02, unpaired t test). For ARV-failing patients nadir CD4 counts are respresented C) Association of TAMs in X4/D/ M viruses. Bar graph indicates trofile assay results. D/M indicates dual/mixed viruses.
We then sought to determine if there was a relationship between CD4 counts, viral loads and viral tropism. Patients with X4/dual/mixed viruses had significantly lower nadir CD4+ T-cell counts compared to those with R5 viruses in both the ART-naive and ART-failing groups with significant p-values of 0.014 and 0.002 respectively. In logistic regression analysis, lower CD4 counts (p=0.0004) but not age of patient (p=0.29) or duration on HAART (p=0.95) treatment were a significant predictor of X4/dual/mixed infections. We also investigated whether patients with TAMs were more likely to harbour dual/mixed/X4 viruses. Eleven of 16 (69%) HAART-failing patients with TAMs had X4/dual/mixed viruses and 5 (31%) had CCR5-using viruses, compared to the respective proportions of 50% dual/mixed/X4 versus 50% R5 among patients without TAMs (p=0.47) (Figure 2C).
Genotypic analysis of the env gene
HIV-1 envelope sequence determines coreceptor utilization 28, 33–36. We therefore next sought to assess the extent to which viral tropism could be predicted by env sequence characteristics. We randomly selected 20 virologically failing and 20 ARV-naïve patients and analyzed full-length env sequences for predictive coreceptor utilization profiles.
All 40 full-length env clones analyzed phylogenetically clustered with HIV-1 subtype C references (Figure 3A left panel). Phylogenetic analysis of the pol region also showed that all the patients clustered with HIV-1 subtype C with the exception of 704MC012F which is a recombinant of subtypes A and G; 704MC003F which is a recombinant of subtypes A and C and 704MC006F which is a recombinant of subtypes C and J as confirmed using Simplot (Figure 3A right panel). The env V3 loop sequences from HAART-naïve and failing participants are shown in figure 3B. The overall V3 consensus sequence generated for ARV-naive patients (right panel) had 2 more amino acids than the consensus sequence generated for ARV-failing patients (left panel). Thirteen (65%) of failures and seventeen (85%) of ARV-naive patients had a V3 region consisting of 35 amino acids consistent with the consensus subtype C sequence. Thirty one of 40 (78%) clones analyzed contained the consensus subtype C GPGQ crown motif sequence.
Figure 3. Neighbour-Joining phylogenetic trees.
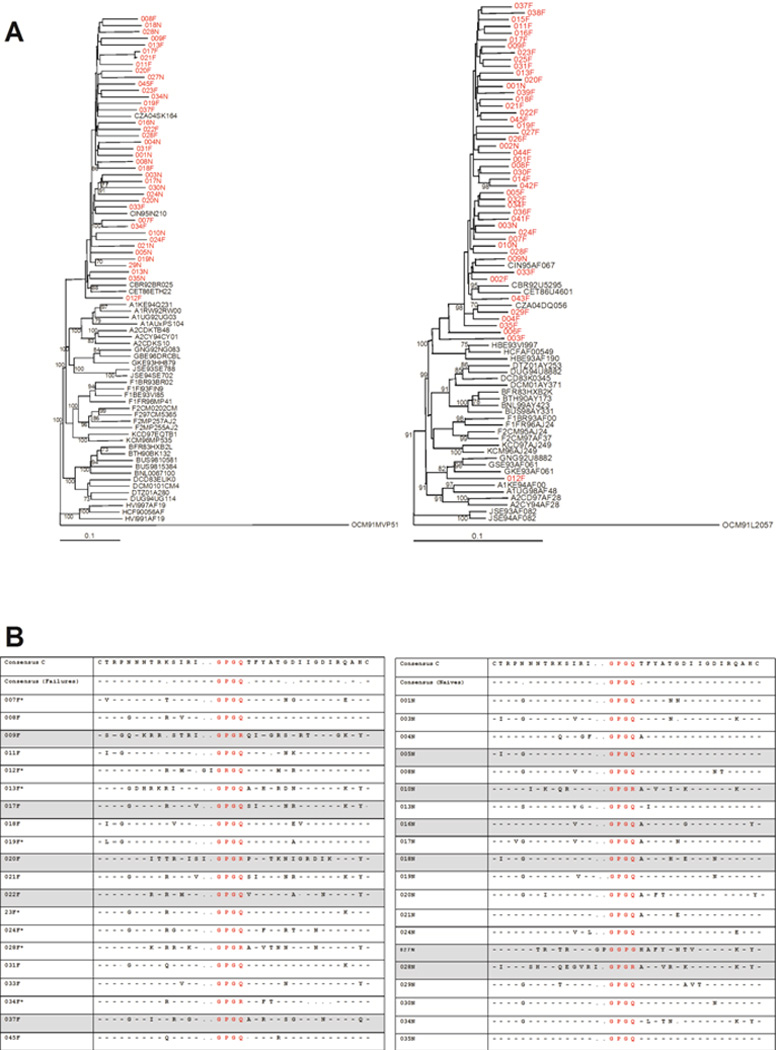
A) Left panel - Neighbour-Joining phylogenetic tree constructed from the env gene sequences. All clones highlighted in red cluster closely with subtype C. Right panel - Neighbour-Joining phylogenetic tree constructed from the pol gene sequences. All patient samples highlighted in red cluster together with the subtype C reference with the exception of 704MC012F which clusters with subtype G. Boostrap values more than 70% are shown. B) Alignment of V3 sequences of clones of ARV-failing and ARV-naïve patients. The panel on the left indicates V3 sequences in patients failing treatment and the panel on the right indicates V3 sequences in ARV-naive patients. The crown motif for each sequence is highlighted in red. All sequences from viruses determined to be X4/dual/mixed by the Trofile assay are highlighted in grey and samples with asterisk next to sample number were not available for analysis in the Trofile assay or did not yield reportable data.
We next investigated the reliability of V3 loop sequence based predictive algorithms against the phenotypic results obtained using the Trofile assay. Coreceptor prediction genotypic methods evaluated here included the 11/25 rule; the overall net V3 charge; the subtype C-specific position specific scoring matrix (C-PSSM), a web-based coreceptor prediction tool (http://indra.mullins.microbiol.washington.edu/pssm/) and another web-based coreceptor prediction tool, Geno2Pheno (http://coreceptor.bioinf.mpi-inf.mpg.de/) 26–29. We also investigated whether a combined algorithm (combining 3 of the above 4 methods) would provide a better correlate of the phenotype data. Overall, the combined algorithm correlated with the Trofile assay results in 87% of cases, compared to 81% for C-PSSM, 78% for the 11/25 rule and 75% for the V3 net charge method and 84% for Geno2Pheno (Table 2). The combined algorithm and the 11/25 rule correctly identified 90% of R5 sequences, C-PSSM correctly predicted 85% and the V3 net charge method predicted 71% of R5 viruses while Geno2Pheno was accurate for 86% of R5 cases. In contrast, the Geno2Pheno method was the best in accurately predicting X4/dual/mixed at 82%, V3 overall charge method accurately predicted 81% X4/dual/mixed, the combined algorithm was at 80%, C-PSSM 72% and the 11/25 rule correctly predicted only 55% of X4/dual/mixed viruses. Sensitivity of these methods was also determined using the Trofile assay as the gold standard. The Geno2Pheno and V3 charge prediction methods were 82% sensitive in predicting X4/dual/mixed virus variants followed by the combined algorithm (80%), C-PSSM (73%) and 11/25 rule (46%).
Table 2.
V3 loop-based methods for coreceptor usage prediction
| Method | % of sequences# correctly predicted |
% of R5 sequences correctly predicted |
% of X4/D/M sequences correctly predicted |
|---|---|---|---|
| 11/25 | 78 | 90 | 55 |
| Overall net V3 charge | 75 | 71 | 81 |
| C-PSSM | 81 | 85 | 72 |
| Geno2Pheno | 84 | 86 | 82 |
| Combined algorithm* | 87 | 90 | 80 |
R5 refers to viruses using CCR5 only, X4/D/M refers to CXCR4-using viruses (CXCR4-only, dual tropic and mixed populations).
In the combined algorithm, concordant results from at least 3 of 4 methods (i.e. the amino acids at positions 11 and/or 25, the overall net V3 charge, C-PSSM prediction and Geno2Pheno prediction) were used.
Discussion
Monitoring the emergence and patterns of antiretroviral drug resistance is crucial for the success and sustainability of treatment programmes. Moreover, as new drugs become available, there is a growing need to better characterize viruses from both drug naïve and virologically failing patients to better understand the suitability of these new drugs either as additional components of the current regimens or as salvage therapy. In this study we investigated the prevalence and pattern of drug resistance mutations in a cohort of HIV-1 subtype C-infected individuals failing therapy. In addition, since it has previously been suggested that suboptimal antiretroviral treatment or certain classes of drugs may select for the more virulent X4 virus variants 13, we sought to identify correlates of viral tropism in HAART-naïve and therapy experienced virologically failing patients.
Our results show that in a South African setting where patients are receiving antiretroviral therapy according to national and WHO guidelines, 95% of patients failing therapy had at least one drug resistance mutation. The most common NRTI mutation was M184V/I in 87% of patients; with V106M/A (51%) and K103N (40%) the commonest NNRTI resistance mutations. These patterns are consistent with data from previous HIV-1C studies 10, 37–39. However, we also found a high prevalence (13.3%) of the ETR-resistance associated M230L mutation, which occurs relatively rarely in NNRTI-failing patients. This finding may require follow-up studies. Data from this study also revealed that 55% of patients failing therapy had thymidine analog mutations (TAMs) compared to 32% of patients studied from the same city in 2005–2006 10. Recent studies from Botswana, Malawi and South Africa have also reported similar high proportions of TAMs in persons failing therapy under public sector antiretroviral programmes with an apparent bias towards the TAM2 pathway in these subtype C studies 32, 40–41. In contrast, a recent subtype B study showed that 65% of virologically failing subjects harboured TAM1 pathway mutations 8 suggesting that there may be subtype-specific differences in thymidine analog mutation pathways. Other reasons for TAM pathway mutation differences may include differences in antiretroviral regimens, nadir CD4+ T-cell count before HAART commencement, duration of treatment before failure and duration on a failing regimen. Overall, we found that more than 90% of patients failing therapy had at least one drug resistance mutation and 55% of patients harbored TAMs. To minimize the loss of future treatment options, improved adherence support mechanisms and better monitoring algorithms for patients on HAART in resource-poor settings will be required.
We also investigated here whether HAART-failing HIV-1C-infected patients had higher proportion of X4/dual/mixed viruses compared to HAART-naïve patients. Although we found this to be the case, the patients failing HAART had lower median (nadir) CD4+ counts compared to HAART-naïve patients, and individuals possessing X4/dual/mixed viruses had significantly lower CD4+ T cell counts compared to those with R5-only viruses, in both the HAART-naïve and HAART-failing arms of this study. These data therefore suggest that low CD4+ T-cell counts (and by extension the length of infection), rather than HAART is the possible main cause of X4/dual/mixed viruses, consistent with data from HIV-1 subtype B studies 12, 40. However, only a longitudinal study can decisively determine whether there is higher proportion of emergence of X4/dual/mixed viruses in treated versus HAART-naïve patients with similar CD4+ T-cell counts. Nevertheless, our study underlines the importance of introducing CCR5 inhibitors relatively early in the course of HIV-1 subtype C infection for possible maximum benefit and to preserve other drugs for salvage use. Clinical trials are needed to determine the equivalence or superiority of CCR5 inhibitors as part of first line or early regimens, rather than as salvage therapy in HIV-1C settings.
Finally, the availability of virus phenotype and genotype data allowed us to assess the utility of V3 loop sequenced based methods for predicting viral tropism. Envelope sequence-based genotypic coreceptor prediction algorithms offer a simpler and less expensive means of analyzing viral tropism in patients and could facilitate easier and less expensive determination of whether a patient can be treated with CCR5 antagonists or not. Our data show that while genotypic methods are reliable for a majority of cases, they failed to correctly predict CXCR4-tropism in a significant minority of cases consistent with previous studies 42. Although the Geno2Pheno and V3 charge methods achieved more than 80% sensitivity in predicting CXCR4/dual-tropism for this dataset, there remains an urgent need to further investigate and develop better predictive algorithms, perhaps taking into account sequences outside of the V3 and more detailed analysis of V3 loop sequences using newer technologies able to better characterize V3 loop quasispecies diversity.
In summary, our observations confirm and extend the body of knowledge in examining coreceptor tropism directly in patients failing currently recommended regimens, and comparing this with ARV-naïve patients in a HIV-1 subtype C setting. The presence of high proportions of patients with TAMs suggests that these mutations may be accumulating over time in this population as a result of inadequate viral suppression, perhaps as a consequence of poor immunological and/or clinically driven monitoring in the absence of viral load testing. These results may suggest that in situations where virologic monitoring is not possible, measures are needed to improve adherence and to develop better monitoring tools. Comparison of the prevalence of CXCR4-utilizing viruses between ARV-naive prior to initiating ART with the prevalence among treated patients revealed that there was a high prevalence of X4/dual/mixed viruses in patients failing treatment, possibly due to lower nadir CD4 counts in these patients, underlining the need to investigate the possible earlier use of CCR5 inhibitors before the development of X4/dual/mixed viruses. Our data also highlight the usefulness and limitations of genotypic coreceptor prediction methods in assessing whether HIV-1C infected patients can be put on regimens that include CCR5 inhibitors. Longitudinal studies on viral coreceptor evolution in HIV-1C infections are warranted.
Acknowledgements
We are grateful to the all the study participants, and to the management and staff of McCord hospital for their cooperation and support of this study. We thank Jennifer Fisher and Wei Huang for assistance with V3 sequence analysis and members of Monogram Biosciences clinical reference laboratory for assistance with Trofile assays. This study was funded by the U.S. National Institutes of Health (grant AI060354-05) through a Harvard University Center for AIDS Research (CFAR) feasibility grant and grant 5R44AT057068 to Monogram Biosciences. Additional funding was provided by the South African Department of Science and Technology/National Research Foundation Research Chair Initiative to TN. AS was supported by the Columbia University-Southern African Fogarty AIDS International Training and Research Programme (AITRP) funded by the Fogarty International Center, National Institutes of Health (grant # D43TW00231) and by a training grant from the East Coast Biotechnology Research Innovation Centre (LIFElab), funded by the South African Department of Science and Technology. We thank Dr Johannes Viljoen and the Africa Centre for providing access to the sequencing facility.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.UNAIDS. AIDS epidemic update. 2007 www.unaids.org.
- 2.Taylor BS, Sobieszczyk ME, McCutchan FE, Hammer SM. The challenge of HIV-1 subtype diversity. N Engl J Med. 2008 Apr 10;358(15):1590–1602. doi: 10.1056/NEJMra0706737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. http://www.info.gov.za/otherdocs/2003/aidsplan/report.pdf. South Africa Government online: the Government and Information System.
- 4.Factsheet. 2010 www.unaids.org.
- 5.Furtado MR, Callaway DS, Phair JP, et al. Persistence of HIV-1 transcription in peripheral-blood mononuclear cells in patients receiving potent antiretroviral therapy. N Engl J Med. 1999;340(21):1614–1622. doi: 10.1056/NEJM199905273402102. [DOI] [PubMed] [Google Scholar]
- 6.Tobin NH, Learn GH, Holte SE, et al. Evidence that low-level viremias during effective highly active antiretroviral therapy result from two processes: expression of archival virus and replication of virus. J Virol. 2005;79(15):9625–9634. doi: 10.1128/JVI.79.15.9625-9634.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO Guidelines. 2006
- 8.Cozzi-Lepri A, Phillips AN, Martinez-Picado J, et al. Rate of accumulation of thymidine analogue mutations in patients continuing to receive virologically failing regimens containing zidovudine or stavudine: implications for antiretroviral therapy programs in resource-limited settings. J Inf Dis. 2009;200(5):687–697. doi: 10.1086/604731. [DOI] [PubMed] [Google Scholar]
- 9.Hosseinipour MC, van Oosterhout JGG, Weigel R, et al. The public health approach to identify antiretroviral therapy failure: high-level nucleoside reverse transcriptase inhibitor resistance among Malawians failing first-line antiretroviral therapy. Aids. 2009;23(9):1127–1134. doi: 10.1097/QAD.0b013e32832ac34e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marconi VC, Sunpath H, Lu Z, et al. Prevalence of HIV-1 drug resistance after failure of a first highly active antiretroviral therapy regimen in KwaZulu Natal, South Africa. Clin Infect Dis. 2008 May 15;46(10):1589–1597. doi: 10.1086/587109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wallis C, Sanne I, Venter F, Mellors J, Stevens WS. Varied patterns of HIV-1 drug resistance on failing first-line antiretroviral therapy in South Africa. J Acquir Immune Defic Syndr. 2009 doi: 10.1097/QAI.0b013e3181bc478b. 00(0) [DOI] [PubMed] [Google Scholar]
- 12.Hunt PW, Harrigan PR, Huang W, et al. Prevelence of CXCR4 Tropism among Antiretroviral-Treated HIV-1-Infected Paitients with Detectable Viremia. J Inf Dis. 2006;194:926–930. doi: 10.1086/507312. [DOI] [PubMed] [Google Scholar]
- 13.Johnston ER, Zijenah LS, Mutetwa S, Kantor R, Kittinunvorakoon C, Katzenstein DA. High frequency of syncytium-inducing and CXCR4-tropic viruses among human immunodeficiency virus type 1 subtype C-infected patients receiving antiretroviral treatment. J Virol. 2003;77(13):7682–7688. doi: 10.1128/JVI.77.13.7682-7688.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Connor RL, Sheridan KE, Ceradini D, Choe S, Landau NR. Change in coreceptor use correlates with disease progression in HIV-1-infected individuals. J Exp Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scarlatti G, Tresoldi E, Bjorndal A. In vivo evolution of HIV-1 coreceptor usage and sensitivity to chemokine-mediated suppression. Nat Med. 1997;3:1259–1265. doi: 10.1038/nm1197-1259. [DOI] [PubMed] [Google Scholar]
- 16.Bjorndal A, Sonnerborg A. Phenotypic characteristics of human immunodeficiency virus type 1 subtype C isolates of Ethiopian AIDS patients. Aids Res Hum Retroviruses. 1999;15(7):647–653. doi: 10.1089/088922299310944. [DOI] [PubMed] [Google Scholar]
- 17.Cecilia D, Kulkarni SS, Tripathy SP, Gangarkhedkar RR, Paranjape RS, Gadkari DA. Absence of coreceptor switch with disease progression in human immunodeficiency virus infections in India. Virology. 2000;271:253–258. doi: 10.1006/viro.2000.0297. [DOI] [PubMed] [Google Scholar]
- 18.Cilliers T, Nhlapo J, Coetzer M, et al. The CCR5 and CXCR4 coreceptors are both used by human immunodeficiency virus type 1 primary isolates from subtype C. J Virol. 2003;77(7):4449–4456. doi: 10.1128/JVI.77.7.4449-4456.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ndung'u T, Sepako E, McLane MF, et al. HIV-1 subtype C in vitro growth and coreceptor utilization. Virology. 2006;347(2):247–260. doi: 10.1016/j.virol.2005.11.047. [DOI] [PubMed] [Google Scholar]
- 20.Tscherning CA, Alaeus RF, Fredriksson A, et al. Differences in chemokine coreceptor usage between genetic subtypes of HIV-1. Virology. 1998;241:181–188. doi: 10.1006/viro.1997.8980. [DOI] [PubMed] [Google Scholar]
- 21.Reeves JD, Coakley E, Petropoulos CJ, Whitcomb JM. An enhanced-sensitivity TrofileTM HIV coreceptor tropism assay for selecting patients for therapy with entry inhibitors targeting CCR5: A review of analytical and clinical studies. J Viral Entry. 2009;3:94–102. [Google Scholar]
- 22.Trinh L, Han D, Huang W, et al. Technical validation of an enhanced sensitivity Trofile HIV co-receptor tropism assay for selecting patients for therapy with entry inhibitors targeting CCR5. Antiviral Ther. 2008;13(A:128) [Google Scholar]
- 23.Coakley E, Petropoulos CJ, Whitcomb JM. Assessing chemokine co-receptor usage in HIV. Curr Opin Infect Dis. 2005;18(1):9–15. doi: 10.1097/00001432-200502000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Westby M, Lewis M, Whitcomb J, et al. Emergence of CXCR4-using human immunodeficiency virus type 1 (HIV-1) variants in a minority of HIV-1-infected patients following treatment with the CCR5 antagonist maraviroc is from a pretreatment CXCR4-using virus reservoir. J Virol. 2006;80:4909–4920. doi: 10.1128/JVI.80.10.4909-4920.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh A, Page T, Moore PL, et al. Functional and genetic analysis of coreceptor usage by dualtropic HIV-1 subtype C isolates. Virology. 2009;393:56–67. doi: 10.1016/j.virol.2009.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coetzer M, Cilliers T, Ping LH, Swanstrom R, Morris L. Genetic characteristics of the V3 region associated with CXCR4 usage in HIV-1 subtype C isolates. Virology. 2006 Dec 5–20;356(1–2):95–105. doi: 10.1016/j.virol.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 27.Fouchier RA, Brouwer M, Broersen SM, Schuitemaker H. Simple determination of human immunodeficiency virus type 1 syncytium-inducing V3 genotype by PCR. J Clin Microbiol. 1995 Apr;33(4):906–911. doi: 10.1128/jcm.33.4.906-911.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fouchier RA, Groenink M, Kootstra NN, et al. Phenotype-associated sequence variationin the third variable domain of the human immunodeficiency virus type 1 gp 120 molecule. J Virol. 1992;66:3183–3187. doi: 10.1128/jvi.66.5.3183-3187.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuiken CL, de Jong JJ, Baan E, Keulen W, Tersmette M, Goudsmit J. Evolution of the V3 envelope domain in proviral sequences and isolates of human immunodeficiency virus type 1 during transition of the viral biological phenotype. J Virol. 1992 Sep;66(9):5704. [PMC free article] [PubMed] [Google Scholar]
- 30.Whitcomb J, Parkin N, Cheppey C, Hellmann N, Petropoulos CJ. Broad NRTI cross-resistance in HIV-1 clinical isolates. J Inf Dis. 2003;188:992–1000. doi: 10.1086/378281. [DOI] [PubMed] [Google Scholar]
- 31.Stanford HIV Drug Resistance Database [Google Scholar]
- 32.Novitsky V, Wester W, DEgruttola V, et al. The reverse transcriptase 67N 70R 215Y genotype is the predominant TAM pathway associated with virologic failure among HIV type 1C-infected adults treated with ZDV/ddI-containing HAART in Southern Africa. Aids Res Hum Retroviruses. 2007;23(7):868–878. doi: 10.1089/aid.2006.0298. [DOI] [PubMed] [Google Scholar]
- 33.Briggs DR, Tuttle DL, Sleasman JW, Goodenow MM. Envelope V3 amino acid sequence predicts HIV-1 phenotype (co-receptor usage and tropism for macrophages) Aids. 2000;14(18):2937–2939. doi: 10.1097/00002030-200012220-00016. [DOI] [PubMed] [Google Scholar]
- 34.Cann AJ, Churcher MJ, Boyd M, et al. The region of the envelope gene of human immunodeficiency virus type 1 responsible for determination of cell tropism. J Virol. 1992;66(1):305–309. doi: 10.1128/jvi.66.1.305-309.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rizzuto C, Wyatt R, Hernandez-Ramos A conserved HIV gp120 glycoprotein structure involved in chemokine receptor binding. Science. 1998;280:1949–1953. doi: 10.1126/science.280.5371.1949. [DOI] [PubMed] [Google Scholar]
- 36.Wu X, Parast AB, Richardson BA, et al. Neutralization escape variants of human immunodeficiency virus type 1 are transmitted from mother to infant. J Virol. 2006 Jan;80(2):835–844. doi: 10.1128/JVI.80.2.835-844.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brenner B, Oliveira M, Moisi D. A V106M mutation in HIV-1 clade C viruses exposed to efavirenz confers cross resistance to nonnucleoside reverse transcriptase inhibitors. Aids. 2003;17(F1–5) doi: 10.1097/00002030-200301030-00001. [DOI] [PubMed] [Google Scholar]
- 38.Kantor R, Katzenstein DA, Effron B. Impact of HIV-1 subtype and antiretroviral therapy on protease and reverse transcriptase genotype: results of a global collaboration. PLoS Med. 2005;2:e112. doi: 10.1371/journal.pmed.0020112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loemba H, Brenner B, Parniak MA. Genetic divergence of human immunodeficiency virus type 1 Ethiopian clade C reverse transcriptase (RT) and rapid development of resistance against nonnucleoside inhibitors of RT. Antimicrob Agents Chemother. 2002;46:2087–2094. doi: 10.1128/AAC.46.7.2087-2094.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Briz V, Poveda E, del Mar Gonzalez M, Martin-Carbonero L, Gonzalez-Gonzalez R, Soriano V. Impact of antiretroviral therapy on viral tropism in HIV-infected patients followed longitudinally for over 5 years. Journal of Antimicrobial Chemotherapy. 2008;65:405–410. doi: 10.1093/jac/dkm469. [DOI] [PubMed] [Google Scholar]
- 41.Irlbeck DM, Amrine-Madsen H, Kitrinos KM, Labranche CC, Demarest JF. Chemokine (C-C motif) receptor 5-using envelopes predominate in dual/mixed-tropic HIV from the plasma of drug-naive individuals. Aids. 2008;22(12):1425–1431. doi: 10.1097/QAD.0b013e32830184ba. [DOI] [PubMed] [Google Scholar]
- 42.Low AJ, Dong W, Chan D, et al. Current V3 genotyping algorithms are inadequate for predicting X4 co-receptor usage in clinical isolates. Aids. 2007 Sep 12;21(14):F17–F24. doi: 10.1097/QAD.0b013e3282ef81ea. [DOI] [PubMed] [Google Scholar]